Abstract
Background:
Implementation of screening recommendations for chronic hepatitis B (CHB) among foreign-born persons at risk has been sub-optimal. The use of alerts and reminders in the electronic health record (EHR) has led to increased screening for other common conditions. The aim of our study was to measure the effectiveness of an EHR alert on the implementation of hepatitis B surface antigen (HBsAg) screening of foreign-born Asian and Pacific Islander (API) patients.
Methods:
We used a novel technique to identify API patients by self-identified ethnicity, surname, country of origin, and language preference, and who had no record of CHB screening with HBsAg within the EHR. Patients with Medicare and/or Medicaid insurance were excluded due to lack of coverage for routine HBsAg screening at the time of this study. At-risk API patients were randomized to alert activation in their EHR or not (control).
Results:
A total of 2,987 patients met inclusion criteria and were randomized to the alert (n = 1,484) or control group (n = 1,503). In the alert group, 119 patients were tested for HBsAg, compared to 48 in the control group (odds ratio [OR] = 2.64 [95% CI = 1.88–3.73]; P< .001). In the alert group, 4 of 119 (3.4%) tested HBsAg-positive compared to 5 of 48 (10.4%) in the control group (P = .12).
Conclusions:
An EHR alert significantly increased HBsAg testing among foreign-born APIs.
Impact:
Utilization of EHR alerts has the potential to improve implementation of hepatitis B screening guidelines.
Keywords: Asian Americans, Electronic Health Record, Hepatitis B, Mass Screening, Preventative Care
INTRODUCTION
One in four patients with chronic hepatitis B (CHB) will die prematurely from liver cirrhosis, hepatocellular carcinoma (HCC), or decompensated liver failure if left untreated. (1) In the United States in 2012, there were approximately 847,000 persons infected with hepatitis B virus (HBV), and there are approximately 14,000 CHB-associated annual deaths. (2,3) Foreign-born persons have the highest CHB prevalence in the United States, between 4.5–10.3%, (4) and the majority of foreign-born persons with CHB living in the United States originated from Asia. (4) As a result of the high prevalence of CHB, Asian and Pacific Islanders (APIs) have the highest incidence rates of HCC and HCC-related mortality. (5)
Despite a high burden of disease, CHB screening among foreign-born persons in the United States has been sub-optimal. (6,7) Current guidelines from the Centers for Disease Control and Prevention, United States Preventive Services Task Force, and the American Association for the Study of Liver Diseases, recommend screening patients born in countries with intermediate-high CHB endemicity (>2%).(8–10) Less than one-third of API persons in the United States have been screened for CHB. (11) It is likely that deficiencies in patient and physician knowledge are contributing to these low screening rates. (12–14)
Utilization of health information technology provides an opportunity to overcome these gaps. The modern electronic health record (EHR) has previously been used to increase the likelihood of CHB screening via message prompts to providers 24 hours prior to appointments involving at-risk patients. (15) However, CHB screening gaps remain and more automated approaches may work better. The majority of patients at risk for CHB are foreign-born; however, country of origin is not systematically captured in the EHR. In a previous project we addressed this challenge by applying a technique focused on selecting surnames associated with various Asian ethnicities and demonstrated statistically significant increased ordering of HBsAg tests. (15) Leveraging these findings, the aim of this study was to demonstrate the effectiveness of an automated, randomized EHR alert on CHB screening within an integrated, academic health system using a novel method to identify at-risk patients.
MATERIALS AND METHODS
Study population.
Patients aged 18 years and older with an established primary care provider within the UC Davis health system as of September 17, 2015 with either a self-identified API race or ethnicity or an imputed API race or ethnicity based upon surname, language preference, or country of origin (Supplementary Materials) were enrolled. Patients meeting any of these criteria for API ethnicity had their electronic medical charts tagged to receive the EHR alert. Surname lists used to identify persons of Asian ethnicities have previously been validated. (16–18) Patients with any private insurance were included, but patients with only Medicare and/or Medicaid coverage were excluded because the Centers for Medicare and Medicaid Services (CMS) had not made a determination for coverage for CHB screening based upon nativity during the study period. The decision for CMS to cover screening for CHB was made September 2016, approximately 8 months after our study began. (19) Other exclusion criteria included prior testing for hepatitis B surface antigen (HBsAg) or an ICD-9 code for chronic hepatitis B (070.2–070.3). The study was approved by the University of California Davis Institutional Review Board to proceed without informed consent. The need for informed consent was waived because the study was deemed minimal risk and consenting patients would break the blinding of our intervention.
EHR Alert.
The EHR alert was deployed through the health system’s electronic record (Epic Systems, Verona, WI) under the “Health Maintenance” functionality, which provides reminders for periodic health screenings and preventive care, between January 28, 2016 and January 27, 2017. A completed HBsAg test automatically changed the alert status from “Due” to “Done.” The status could have been manually changed if a patient reported having the test done in another health care system or if a patient refused testing to “previously done” or “patient declined,” respectively. A computer-generated randomized method was used to assign each eligible patient in a 1:1 fashion to either have the HBsAg alert activated or not activated (control). If the alert was not activated, the “HBV screening” icon did not appear in the “Health Maintenance” section of the patient’s electronic medical chart. Patients and providers were blinded and no new interventions to increase CHB testing were implemented during the study period. The primary outcome of the study was HBsAg test completion within our health system during the study period. Secondary outcomes included the difference in HBsAg positivity between the alert and control groups.
Statistical Analysis.
Proportions between the EHR alert and control groups were compared using Fisher’s exact test. The Wilcoxon rank-sum test was used to compare numerical variables between the EHR alert group and control group. Multivariable logistic regression was used to study the association between receiving the HBsAg test, as a binary outcome variable, and the binary predictor, alert (EHR alert vs. control), in order to adjust for confounders, including number of office visits, age, sex, and language. A P-value < .05 was considered statistically significant. Statistical analyses were performed with SAS v 9.4 (SAS Institute Inc., Cary, NC, USA).
RESULTS
The total number of patients within the health system at study initiation was 321,721. Of these, 2,640 had documentation that they had been previously tested (353 HBsAg positive and 2,287 HBsAg negative) and 306,663 were not considered to be at risk for CHB due to their ethnicity as determined by information available in the EHR. Thus, 12,418 at-risk patients had no documentation of having been tested. After 9,431 Medicare and/or Medicaid patients were excluded, a study population of 2,987 patients were randomized (Figure 1). Of these 2,987 patients, 2045 (68.5%) were identified as API based on self-identification, language preference, or country of origin as recorded in the EHR. Therefore, 942 patients (31.5%) were identified as API based on imputed ethnicity or race from the surname list.
Figure 1:
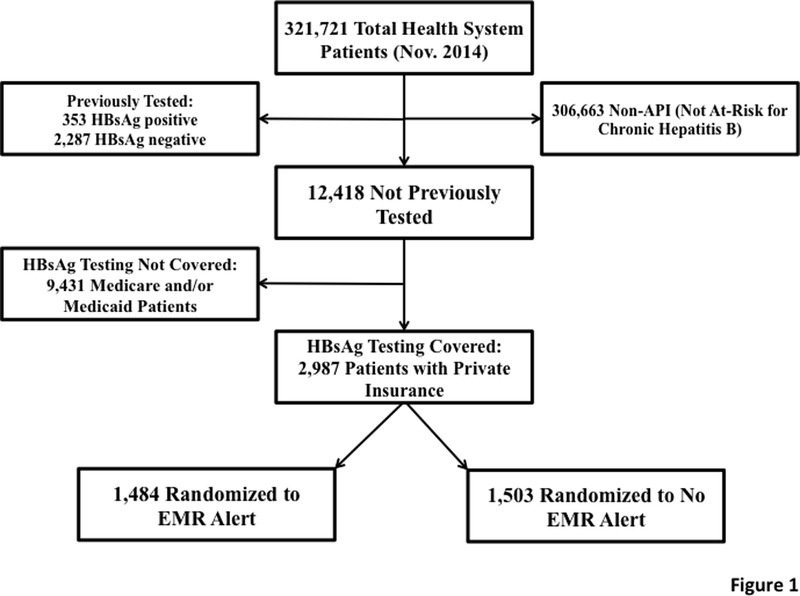
CONSORT Diagram for Patients in the Chronic Hepatitis B (CHB) Screening Alert Study. shows that our method identified 12,418 at-risk patients who had not yet been screened for CHB. After excluding patients with Medicare and/or Medicaid, 2,987 patients were randomized to either receive the alert or not.
There were 1,484 patients randomized to the alert group and 1,503 patients randomized to the control group; there were no significant differences between baseline characteristics in the alert and control groups (Table 1).
Table 1:
Baseline Characteristics of Randomized Electronic Health Record Alert and Control Groups Among Asian and Pacific Islander Patients in UC Davis Health System, January 2016–January 2017
| Characteristic | Alert N=1484 (%) |
Control N=1503 (%) |
P-value |
|---|---|---|---|
| Male | 717 (48.3%) | 697 (46.4%) | .30 |
| English Language (Primary) | 1150 (77.5%) | 1170 (77.8%) | .94 |
| Age (years) ± SD | 38.5 ± 14.7 | 39 ± 14.9 | .39 |
| Private Insurance | 1459 (98.3%) | 1473 (98.0%) | .59 |
| Number of Office Visits (Mean) | 1.5 ± 2.8 | 1.8 ± 3.5 | .39 |
SD: Standard Deviation
HBsAg testing was completed in 119 patients in the alert group compared to 48 in the control group (8.0% vs. 3.2%; P<.001) (Table 2). There was an increased odds of HBsAg testing in the alert group (odds ratio [OR] = 2.64, 95% CI = 1.88–3.73). There was a lower proportion of HBsAg positivity in the alert group compared to the control group, but this was not statistically significant (3.4% vs. 10.4%; P=.12) (Table 2).
Table 2:
Effect of the Electronic Health Record Alert on HBsAg Testing and HBsAg Positivity among Asian and Pacific Islander Patients in UC Davis Health System, January 2016–January 2017
| HBsAg Testing Status | Alert N=1484 (%) |
Control N=1503 (%) |
OR (95% CI) | P-value |
|---|---|---|---|---|
| HBsAg test completed | 119 (8.0%) | 48 (3.2%) | 2.64 (1.88–3.73) | <.001 |
| HBsAg-positive | 4/119 (3.4%) | 5/48 (10.4%) | 0.30 (0.08–1.17) | .12 |
HBsAg: Hepatitis B surface antigen
The multivariable logistic regression analysis showed increased HBsAg testing in the alert group (adjusted odds ratio [aOR] = 3.13 [95% CI = 2.18– 4.48]; P<.001) and an increased number of office visits attended during the study period (aOR = 1.16 [95% CI = 1.12–1.20]; P<.001). Age, sex, and language preference were not found to significantly affect the rate of HBsAg screening (Table 3). The 2,987 evaluable patients (1484 and 1503 patients in the alert and control (i.e., no alert) group, respectively) provide a power of 80% to detect a ratio of 1.53 of the odds of HBsAg testing given the alert group to that given the control group controlling for office visit, age, sex, and language at a significance level of 5%. The power analysis was performed with SAS PROC POWER.
Table 3:
Multivariate Analysis of Characteristics Associated with Receiving an HBsAg Test Among Asian and Pacific Islander Patients in UC Davis Health System, January 2016–January 2017
| Characteristic | aOR (95% CI)* | P-value |
|---|---|---|
| Alert (EMR alert vs. Control) | 3.13 (2.18–4.48) | <.001 |
| Number of Office Visits | 1.16 (1.12–1.20) | <.001 |
| Age | 1.01 (1.00–1.02) | .06 |
| Sex (Male vs. Female) | 0.76 (0.55–1.06) | .11 |
| Language | ||
| English vs. Non-English | 1.31 (0.82–2.10) | .26 |
| Unknown vs. Non-English | 0.50 (0.10–2.39) | .38 |
HBsAg: Hepatitis B surface antigen
Adjusted odds ratio (aOR): model controls for office visit, age, sex, and language
HBsAg screening indications for the EMR alert and control groups are compared in Table 4. There were significantly more asymptomatic patients screened in the alert group compared to the control group (83.2% vs. 58.3%; P=.005). Patients who received HBsAg testing for the indication of “Known CHB” were tested to confirm a self-report diagnosis of CHB. Fisher’s exact test was used to compare the distributions of the different HBsAg screening indications between the alert and control groups so only one P-value is reported. The number of tests ordered in the alert and control groups over the study period is shown in Figure 2.
Table 4:
HBsAg Screening Indications for Asian and Pacific Islanders in UC Davis Health System, January 2016–January 2017
| HBsAg | EMR Alert | Control | P-value |
|---|---|---|---|
| HBsAg ordered | N=119 (%) | N=48 (%) | .005 |
| Asymptomatic | 99 (83.2%) | 28 (58.3%) | |
| Abnormal Liver Test | 7(5.9%) | 9 (18.8%) | |
| Prenatal Screening | 11 (9.2%) | 9 (18.8%) | |
| Known CHB infection | 2 (1.7%) | 2 (4.2%) |
HBsAg: Hepatitis B surface antigen
CHB: Chronic hepatitis B
Figure 2:
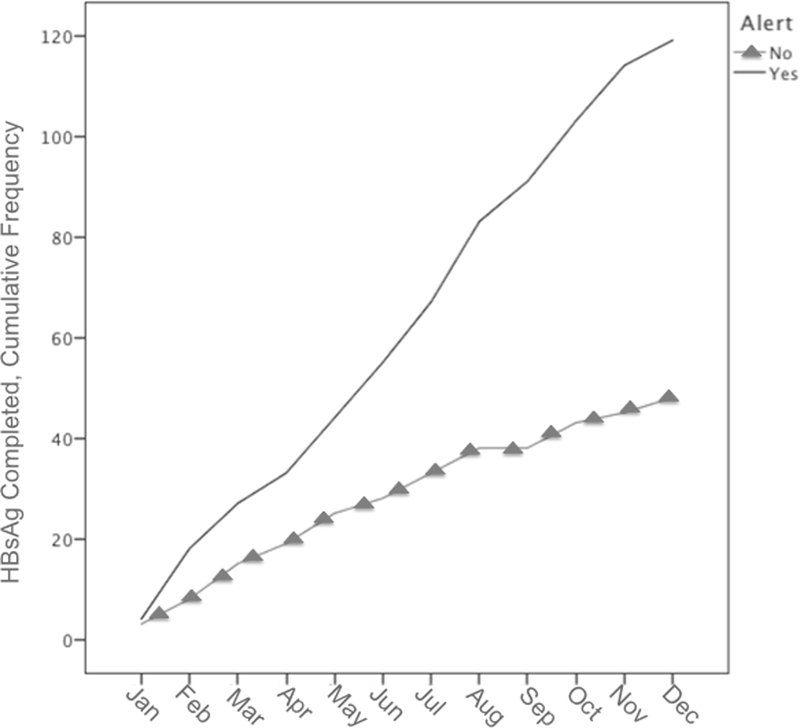
Hepatitis B Surface Antigen Testing among Asian Pacific Islander Americans in the alert group and control groups, January 2016–2017. shows the cumulative frequency of hepatitis B surface antigen tests ordered in the alert and control groups over the study period. The number of tests did not decrease over time suggesting that electronic alert fatigue did not occur.
DISCUSSION
The purpose of this study was to measure the effect of an EHR alert on screening for chronic hepatitis B. Utilization of EHR alerts has the potential to improve implementation of hepatitis B screening guidelines. Our study demonstrated a more than 2-fold increase in HBsAg testing among APIs who were assigned to the alert group and found a 3.6% prevalence of CHB in the alert group overall (Table 2). While it is challenging to identify screening indications with the EHR, we applied a novel method to identify at-risk persons based on surname, ethnicity or race, language preference, and country of origin.
CHB differs from other conditions, such as chronic hepatitis C, colon cancer, breast cancer, and prostate cancer, in which screening guidelines are based upon age/date of birth and sex, which are generally accessible in every medical record. In contrast, HBsAg testing is recommended for patients based on country of birth and other risk factors such as history of intravenous drugs use and being a man who has sex with men. Data on country of birth, race, and ethnicity is frequently incomplete or inaccurately recorded in the EHR. Thus, a novel method to find at risk patients was needed. Our EHR alert was deployed in Epic Systems, which is a commonly used EHR in the American healthcare system. Therefore, we suggest the utilization of clinical decision support tools, such as this EHR alert, to identify patients that may benefit from HBV testing.
CHB remains an important health disparity in the API population, which may be addressed by increased screening and linkage of those identified with CHB to appropriate care and treatment. Anti-viral therapy has been shown to suppress viral replication, prevent decompensation in liver cirrhosis, and reduce the risk of hepatocellular cancer among those with advanced fibrosis. (20–22) In addition, increasing evidence supports the impact of anti-viral therapy on reducing hepatocellular cancer even in those without cirrhosis. (23)
There remains an unmet need for screening those at greatest risk of CHB. One reason may be gaps in hepatitis B knowledge among the API community and healthcare providers. In surveys administered to at-risk Asian Americans prior to educational interventions, many had incorrect beliefs regarding modes of HBV transmission (e.g., through contaminated food) and sub-optimal numbers reported having received the HBV vaccine series. (12,13) Similarly, in a questionnaire administered to interns, residents, and attending physicians at an academic medical center, only 24% correctly identified the lab tests used to screen for CHB. (14) In a survey of primary care providers, only 33% reported universal screening of API patients and 47% reported screening patients based on country of origin. (24) Thus, both patient and physician knowledge gaps may contribute to a lack of awareness of CHB leading to sub-optimal screening. The EHR alert can be deployed to properly identify API at risk for CHB so that these patients may know their status, and those who test positive can be linked to appropriate HBV-directed care.
Utilization of the EMR has been used in a variety of settings to improve implementation of clinical guidelines. Automated electronic alerts have been used to reduce drug interactions, (25,26) increase rates of deep vein thrombosis prophylaxis, (27) and increase rates of preventive measures such as routine vaccinations (28). More recently, EHR “Best Practices” alerts have been shown to increase screening for chronic hepatitis C (29,30) when different clinical sites were cluster randomized to receive these alerts. Using a 1:1 randomized, double-blind, alert mechanism, we demonstrate how EHR can be used to improve hepatitis B testing, adding scientific rigor to our results.
Electronic alert fatigue has been reported as an unintended side effect of automated clinical decision support. (31,32) When providers are inundated by electronic messages and alerts, it is easy to imagine that some of these will be ignored. Based on the number of HBsAg tests ordered over the course of the 12-month study period (Figure 2), the number of tests did not decrease over time suggesting that physicians did not experience electronic alert fatigue and that the effect of the alert would last beyond the study period.
While the EHR alert did increase the rate of HBsAg tests completed, it did not lead to a statistically significant increase in HBsAg positive cases identified compared to the control group. There was a trend towards more HBsAg positive results in the control group although this result was not statistically significant (Table 2). The most likely reason for this disparity is the exclusion of Medicare and Medicaid patients from our study, which could have lowered the prevalence of CHB in our study population. Medicare patients are older (and therefore less likely to have been vaccinated against HBV). Medicaid patients are underinsured and may be at increased risk for CHB given their lower socioeconomic status and decreased access to routine medical care such as vaccination. The control group patients were more likely to have had an HBsAg test completed due to symptoms or abnormal laboratory findings (Table 4). Thus, patients in the control group would have had a higher pre-test probability for being HBsAg-positive than those in the alert group, which may partially explain the trend of more HBsAg positives in the control group.
While the EHR alert more than doubled an at-risk patient’s chance of completing a HBsAg test, the overall rate of test completion was low (8%). This likely occurred because patients would have to have been seen in person by their primary care physician (PCP) who would then see the alert and order the HBsAg test. If a patient did not present to their PCP during the study period, the alert would not be seen, and the HBsAg test would not have been ordered. Future screening efforts may consider using an EHR alert in addition to a more active outreach component (such as electronic messages, telephone calls, or letters to at-risk patients) encouraging at-risk patients to present to their PCPs to receive testing.
Our study was subject to at least four limitations. First, Medicare and Medicaid patients were excluded due to screening coverage concerns and this decreased our overall sample size. Exclusion of Medicare and Medicaid patients also skewed our study towards a younger patient population, which was more likely to have been vaccinated against HBV. Now that CMS is covering screening for CHB we have a follow-up study among Medicare and Medicaid patients on-going to measure the effectiveness of EHR alerts on HBsAg screening within this population. We suspect that more HBsAg positive results will be found as these patients are presumably at higher risk for CHB. Second, our method to identify API may have missed screening eligible patients. We used a surname list in part to impute API ethnicity in cases that otherwise would not have been identified. However, surnames that are associated with non-API ethnic groups, for example “Lee,” were excluded. Adopted APIs, APIs that have taken on a non-API married surname, or non-APIs that have taken on API surnames may also have been misclassified. Third, we did not screen other risk groups such as African born, persons who inject drugs, and men who have sex with men. Lastly, our EHR alert was unable to screen patients outside of our health system.
In conclusion, an automated EHR alert directed towards screening for CHB effectively increased the number of HBsAg tests completed. This study offers empirical evidence for the efficacy of employing EHR alerts for CHB testing for at-risk APIs in a clinical setting and lends itself to the reduction of HBV-induced health disparities. Future research should include identifying other barriers providers face when ordering CHB tests, linking those with CHB to care, and expanding these interventions to include other at-risk patient groups.
Supplementary Material
ACKNOWLEDGEMENTS
The authors are grateful for the financial support of this project provided by:
Centers for Disease Control and Prevention (1U51PS004633–01)
National Center for Advancing Translational Sciences (NCATS), National Institutes of Health (NIH) (UL1TR001860)
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Abbreviations:
- API
Asian and Pacific Islander
- CHB
Chronic hepatitis B
- CMS
Centers for Medicare and Medicaid
- EHR
Electronic Health Record
- HBsAg
Hepatitis B surface antigen
- HBV
Hepatitis B virus
- HCC
Hepatocellular carcinoma
- PCP
Primary care provider
Footnotes
Conflict of Interest Disclosure Statement: Eric Chak MD, MPH: None Declared. Amir Taefi MD: None Declared. Chin-Shang Li PhD: None Declared. Moon S. Chen, Jr., PhD, MPH: Dr. Chen reports receiving consulting and lecture fees from Gilead Sciences. Aaron Harris MD, MPH: None Declared. Scott MacDonald MD: None Declared. Christopher Bowlus MD: None Declared.
REFERENCES
- [1].Centers for Disease Control and Prevention (CDC). Vaccine Information Statement. https://www.cdc.gov/vaccines/hcp/vis/vis-statements/hep-b.html July 7, 2016; December 27, 2017. [Google Scholar]
- [2].Roberts H, Kruszon-Moran D, Ly KN, et al. Prevalence of chronic hepatitis B virus (HBV) infection in U.S. households: National Health and Nutrition Examination Survey (NHANES), 1988–2012. Hepatology 2016; 63: 388–97. [DOI] [PubMed] [Google Scholar]
- [3].Mitchell T, Armstrong GL, Hu DJ, Wasley A, Painter JA. The increasing burden of imported chronic hepatitis B--United States, 1974–2008. PLoS One 2011; 6: e27717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kowdley KV, Wang CC, Welch S, Roberts H, Brosgart CL. Prevalence of chronic hepatitis B among foreign-born persons living in the United States by country of origin. Hepatology 2012; 56: 422–33. [DOI] [PubMed] [Google Scholar]
- [5].Altekruse SF, Henley SJ, Cucinelli JE, McGlynn KA. Changing hepatocellular carcinoma incidence and liver cancer mortality rates in the United States. Am J Gastroenterol 2014; 109: 542–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Waldorf B, Gill C, Crosby SS. Assessing adherence to accepted national guidelines for immigrant and refugee screening and vaccines in an urban primary care practice: a retrospective chart review. J Immigr Minor Health 2014; 16: 839–45. [DOI] [PubMed] [Google Scholar]
- [7].Vijayadeva V, Spradling PR, Moorman AC, et al. Hepatitis B virus infection testing and prevalence among Asian and Pacific Islanders. Am J Manag Care 2014; 20: e98–e104. [PMC free article] [PubMed] [Google Scholar]
- [8].LeFevre M L US Preventive Services Task Force. Screening for hepatitis B virus infection in nonpregnant adolescents and adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2014; 161: 58–66. [DOI] [PubMed] [Google Scholar]
- [9].Weinbaum CM, Williams I, Mast EE, et al. Recommendations for identification and public health management of persons with chronic hepatitis B virus infection. MMWR Recomm Rep 2008; 57: 1–20. [PubMed] [Google Scholar]
- [10].Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology 2007; 45: 507–39. [DOI] [PubMed] [Google Scholar]
- [11].Vijayadeva V, Spradling PR, Moorman AC, et al. Hepatitis B virus infection testing and prevalence among Asian and Pacific Islanders. Am J Manag Care 2014; 20: e98–e104. [PMC free article] [PubMed] [Google Scholar]
- [12].Chen H, Tu SP, Teh CZ, et al. Lay beliefs about hepatitis among North American Chinese: implications for hepatitis prevention. J Community Health 2006; 31: 94–112. [DOI] [PubMed] [Google Scholar]
- [13].Wu CA, Lin SY, So SK, Chang ET. Hepatitis B and liver cancer knowledge and preventive practices among Asian Americans in the San Francisco Bay Area, California. Asian Pac J Cancer Prev 2007; 8: 127–34. [PubMed] [Google Scholar]
- [14].Chao SD, Wang BM, Chang ET, Ma L, So SK. Medical training fails to prepare providers to care for patients with chronic hepatitis B infection. World J Gastroenterol 2015; 21: 6914–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hsu L, Bowlus CL, Stewart SL, et al. Electronic messages increase hepatitis B screening in at-risk Asian American patients: a randomized, controlled trial. Dig Dis Sci 2013; 58: 807–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lauderdale D, Kestenbaum B. Asian American Ethnic Identification by Surname. Population Research and Policy Review 2000; 19: 283–300. [Google Scholar]
- [17].Wong EC, Palaniappan LP, Lauderdale DS. Using name lists to infer Asian racial/ethnic subgroups in the healthcare setting. Med Care 2010; 48: 540–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Shah BR, Chiu M, Amin S, Ramani M, Sadry S, Tu JV. Surname lists to identify South Asian and Chinese ethnicity from secondary data in Ontario, Canada: a validation study. BMC Med Res Methodol 2010; 10: 42,2288-10–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Jensen T, Chin J, Ashby L, Paserchia L, Issa M. Decision Memo for Screening for Hepatitis B Virus (HBV) Infection. The Centers for Medicare and Medicaid Services (CMS), September 28, 2016. ; 2017: 43. [Google Scholar]
- [20].Marcellin P, Gane E, Buti M, et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet 2013; 381: 468–75. [DOI] [PubMed] [Google Scholar]
- [21].Chen CJ, Yang HI, Su J, et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA 2006; 295: 65–73. [DOI] [PubMed] [Google Scholar]
- [22].Sung JJ, Tsoi KK, Wong VW, Li KC, Chan HL. Meta-analysis: Treatment of hepatitis B infection reduces risk of hepatocellular carcinoma. Aliment Pharmacol Ther 2008; 28: 1067–77. [DOI] [PubMed] [Google Scholar]
- [23].Gordon SC, Lamerato LE, Rupp LB, et al. Antiviral therapy for chronic hepatitis B virus infection and development of hepatocellular carcinoma in a US population. Clin Gastroenterol Hepatol 2014; 12: 885–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Upadhyaya N, Chang R, Davis C, Conti MC, Salinas-Garcia D, Tang H. Chronic hepatitis B: perceptions in Asian American communities and diagnosis and management practices among primary care physicians. Postgrad Med 2010; 122: 165–75. [DOI] [PubMed] [Google Scholar]
- [25].Strom BL, Schinnar R, Aberra F, et al. Unintended effects of a computerized physician order entry nearly hard-stop alert to prevent a drug interaction: a randomized controlled trial. Arch Intern Med 2010; 170: 1578–83. [DOI] [PubMed] [Google Scholar]
- [26].Strom BL, Schinnar R, Bilker W, Hennessy S, Leonard CE, Pifer E. Randomized clinical trial of a customized electronic alert requiring an affirmative response compared to a control group receiving a commercial passive CPOE alert: NSAID--warfarin co-prescribing as a test case. J Am Med Inform Assoc 2010; 17: 411–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kucher N, Koo S, Quiroz R, et al. Electronic alerts to prevent venous thromboembolism among hospitalized patients. N Engl J Med 2005; 352: 969–77. [DOI] [PubMed] [Google Scholar]
- [28].Fiks AG, Grundmeier RW, Biggs LM, Localio AR, Alessandrini EA. Impact of clinical alerts within an electronic health record on routine childhood immunization in an urban pediatric population. Pediatrics 2007; 120: 707–14. [DOI] [PubMed] [Google Scholar]
- [29].Brady JE, Liffmann DK, Yartel A, et al. Uptake of hepatitis C screening, characteristics of patients tested, and intervention costs in the BEST-C study. Hepatology 2017; 65: 44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Federman AD, Kil N, Kannry J, et al. An Electronic Health Record-based Intervention to Promote Hepatitis C Virus Testing Among Adults Born Between 1945 and 1965: A Cluster-randomized Trial. Med Care 2017; 55: 590–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Weingart SN, Toth M, Sands DZ, Aronson MD, Davis RB, Phillips RS. Physicians’ decisions to override computerized drug alerts in primary care. Arch Intern Med 2003; 163: 2625–31. [DOI] [PubMed] [Google Scholar]
- [32].Isaac T, Weissman JS, Davis RB, et al. Overrides of medication alerts in ambulatory care. Arch Intern Med 2009; 169: 305–11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


