Abstract
Background:
The purpose of this study was to characterize the pharmacokinetics of the phosphatidylethanol 16:0/20:4 homolog in uncoagulated human blood samples taken from 18 participants in a clinical laboratory setting after consumption of two standard doses of ethanol.
Methods:
Male and female participants received either 0.4 or 0.8 g/kg oral doses of ethanol during a 15-minute period. Blood samples were collected before and throughout six hours immediately after alcohol administration, then again at days 2, 4, 7, 11 and 14 of the follow-up period. Phosphatidylethanol 16:0/20:4 levels were quantified by HPLC with tandem mass spectrometry detection.
Results:
1) The increase of phosphatidylethanol 16:0/20:4 from baseline to maximum concentration was less than that of phosphatidylethanol 16:0/18:1 or phosphatidylethanol 16:0/18:2 homologs during the 6-hour period after ethanol administration; 2) the mean half-life of phosphatidylethanol 16:0/20:4 was 2.1 ± 3 (SD) days, which was shorter than the mean half-life of either phosphatidylethanol 16:0/18:1 or phosphatidylethanol 16:0/18:2, 7.6 ± 3 (SD) or 6.8 ± 4 (SD) days respectively.
Conclusions:
The pharmacokinetics of phosphatidylethanol 16:0/20:4 in whole blood samples is detectable after alcohol consumption and differs in amount synthesized and rate of elimination versus phosphatidylethanol 16:0/18:1 and 16:0/18:2. Measuring the concentrations of these three homologs has the potential to provide more information about the amount and time frame of alcohol consumption than any one alone.
Keywords: Phosphatidylethanol, Pharmacokinetics, Blood, Alcohol, HPLC/MS/MS
Introduction
Phosphatidylethanol (PEth) is an abnormal cell membrane phospholipid generated only in the presence of ethanol (Alling et al., 1983; Gustavsson and Alling, 1987; Kobayashi and Kanfer, 1987). At normal physiological conditions, phospholipase D (PLD) hydrolyzes phosphatidylcholine (PC) into phosphatidic acid (PA) and choline, but in the presence of ethanol, PLD favors transphosphatidylation of PC into the corresponding PEth species (Kobayashi and Kanfer, 1987). PEth is synthesized in the membranes of most types of cells in humans and animals analyzed to date (Aradottir et al., 2002) including in blood erythrocyte cell membranes (Varga et al., 2000). While PEth is rapidly degraded in all other tissues, human red blood cells lack an enzyme (phosphatidylcholine phospholipase) responsible for the degradation of PEth (Aradottir et al., 2004) and thus, its half-life in whole blood is much longer than in other tissues.
PEth has become well accepted as a direct biomarker of alcohol consumption (Aradottir et al., 2006; Beck et al., 2018; Simon, 2018; Varga et al., 1998; Walther et al., 2018) for the following reasons: First, PEth is highly specific because, as mentioned above, it is only generated in the presence of ethanol, and therefore is a direct biomarker of alcohol intake. Second, PEth is highly sensitive because it is detectable after consuming alcohol equivalent to just one or two standard drinks (Javors et al., 2016). Third, its half-life ranges from 3 to 12 days for combined PEth (Gnann et al., 2012; Javors et al., 2016; Schröck et al., 2017; Varga et al., 2000), about 8 days for PEth 16:0/18:1 and about 6 days for PEth 16:0/18:2 (Hill‐Kapturczak et al., 2018).
Compared to other direct biomarkers of alcohol consumption, the longer PEth half-lives are more useful than the short half-lives of other biomarkers such as breath (BrAC) and blood (BAC) alcohol concentrations, 2 to 3 hours, urinary ethyl glucuronide (uEtG), 2 to 3 hours (Schmitt et al., 1997); and urinary ethyl sulfate (uEtS), 2 to 4 hours (Høiseth et al., 2007; Høiseth et al., 2009). When compared against indirect biomarkers, PEth has intermediate half-lives, which may better detect more recent or moderate drinking than the longer half-lives of gamma-glutamyltransferase (GGT), 28 days (Orrego et al., 1985), and carbohydrate deficient transferrin (%CDT), 15 days (Stibler, 1991).
To date, 48 homologs of PEth have been identified (Gnann et al., 2010). These homologs are differentiated by the number of carbons and double bonds present in their fatty acid moieties, which mirror the PC species from which they are derived. Of the 48 PEth homologs, the two most abundant in whole blood of alcohol drinkers are PEth 16:0/18:1 (palmitoyl/oleoyl) and PEth 16:0/18:2 (palmitoyl/linoleyl), representing 37% and 26%, respectively, of total PEth (Helander and Zheng, 2009). A third homolog, PEth 16:0/20:4 (palmitoyl/arachidonoyl) accounts for an additional 13% of total PEth (Helander and Zheng, 2009), making it an appealing candidate to combine with PEth 16:0/18:1 and PEth 16:0/18:2 for clinical applications.
Our previous studies characterized the pharmacokinetics of PEth 16:0/18:1 and PEth 16:0/18:2 in human blood after alcohol intake (Javors et al., 2016; Hill‐Kapturczak et al., 2018) as well as in postmortem brain and serum samples at the time of death (Thompson et al., 2016). Other research groups have reported results that conform to our findings and form the basis of support for the use of PEth as a direct biomarker of alcohol consumption (Gnann et al., 2014; Schröck et al., 2016; Ullah et al., 2017; Wang et al., 2017; Zheng et al., 2011). Taken together, these studies indicate that PEth 16:0/18:1 and PEth 16:0/18:2 are sensitive enough to detect drinking even at the social level (<2 drinks per day) and have a wider window of detection than other biomarkers for alcohol consumption based on half-lives ranging from 4 to 12 days.
In the present study, we assessed the pharmacokinetics of PEth 16:0/20:4, the third most abundant PEth homolog in human whole blood, and compared its synthesis and elimination with those of PEth 16:0/18:1 and PEth 16:0/18:2. PEth 16:0/20:4 in whole blood samples has the potential to provide additional pharmacokinetic information to improve estimation of alcohol consumption when measured simultaneously with PEth 16:0/18:1 and 16:0/18:2.
Materials and Methods
Participants, Study Design, Blood Collection and BrAC Measurements
The present study was performed as a sub study that was part of a larger study designed to characterize the pharmacokinetics of PEth 16:0/18:1 and PEth 16:0/18:2 in a laboratory setting to determine their clinical usefulness. The main study research participants, inclusion-exclusion criteria, study design, blood collection and BrAC monitoring have been described previously (Hill‐Kapturczak et al., 2018). The human experimental protocol was approved by The Institutional Review Board at The University of Texas Health Science Center at San Antonio. From the participants in the main study, only PEth 16:0/20:4 was measured in a subset of 18 participants (8 females and 10 males) for whom samples for all time points remained after finishing the main study examining PEth 16:0/18:1 and PEth 16:0/18:2 completed more than one year earlier (Helander and Zheng, 2009). Participants between the ages 21 to 54 were recruited and randomly assigned to drink either 0.4 g/kg or and 0.8 g/kg of alcohol in three equal volumes during the first 15 min. They were asked to abstain from drinking alcohol for seven days before alcohol administration and wore a transdermal alcohol concentration (TAC) monitor throughout the study to promote abstinence. Blood samples and BrAC were obtained at 0, 15, 30, 45, 60, 90, 120 and 360 minutes during administration day and on the 2nd, 4th, 7th, 11th and 14th days during the follow up time period and upon collection immediately frozen in two sets of tubes, A and B. Tubes A were used to analyze PEth 16:0/18:1 and PEth 16:0/18:2 in the main study. For the analysis of PEth 16:0/20:4, tubes B were defrosted and used.
Measurement of PEth 16:0/20:4 in Human Whole Blood.
PEth 16:0/20:4 was quantified in EDTA-treated whole blood samples using high performance liquid chromatography (HPLC) with tandem mass spectroscopic detection (MS/MS). All reagents were HPLC grade. Milli-Q Plus water, used to prepare all analytical solutions, and reagents were acquired from Millipore Sigma (St. Louis, MO, US). PEth 16:0/20:4 analyte and deuterated PEth 16:0/20:4-d5 internal standard were purchased from Echelon Biosciences (Salt Lake City, UT, US).
On the analysis day, blood samples were thawed at room temperature for a maximum of 15 min, then 2 ml of isopropanol spiked with 10 μl of the PEth 16:0/20:4-d5 internal standard solution was added to 300 μl of each sample. The samples were vortexed for 1 min and then 3 ml of hexane was added followed by shaking for 30 min, and then centrifuged for 30 min at 3,200 g at 4oC. The clear supernatants were transferred to new tubes and evaporated to residues with a gentle stream of nitrogen at 30oC. The residues were dissolved in 100 μl mobile phase A (40% 2 mM ammonium acetate with 60% acetonitrile), and then transferred to microfilter tubes and centrifuged at 1,000 g for 10 min. These final eluted samples were transferred to 300 μl polypropylene autosampler vials and then 10 μl were injected into the HPLC/MS/MS system.
HPLC with Mass Spectrometry Detection
The HPLC system consisted of a Shimadzu SCL controller, two LC-20AD pumps with a DGU20A degassing unit and mixing chamber, SIL-20ACHT autosampler, and an AB Sciex API 4000 Q-TRAP mass spectrometer with turbo ion spray. The analytical column was a High Purity C4 (Thermo Fisher Scientific, Waltham, Massachusetts, USA). Mobile phase A was 40% 2 mM ammonium acetate with 60% acetonitrile and mobile phase B was 100% isopropanol. The solvent program was at 1 min 65% mobile phase A and 35% mobile phase B, at 5.0 min 1% mobile phase A and 99% mobile phase B, at 8.0 min 1% mobile phase A and 99% mobile phase B, at 8.1 min 65% mobile phase A and 35% mobile phase B. Stop time at 10 min.
Mass spectrometer (MS) parameters: ESI interface operated in negative ion mode using selected reaction monitoring to detect the major product ions from the deprotonated molecules of PEth 16:0/20:4 (m/z 723→303) and deuterated PEth 16:0/20:4 (m/z 728→303). The MS gas settings were CUR (curtain gas) 15 psi, CAD (collision gas) 8 psi, IS (ion spray voltage) −4500 V, temperature 550°C, Gas 1 (nebulizer gas) 60 psi and Gas 2 (auxiliary gas) 60 psi. The MS parameters for the PEth 16:0/20:4 analyte were DP (declustering potential) −50 V, Ep (entrance potential) −10 V, CE (collision energy) −42 V and CXP (collision cell exit potential) −15 V. The MS parameters for the deuterated PEth 16:0/20:4 D5 were DP −101 V, Ep −10 V, CE −40 V and CXP −18 V. The flow rate was 350 μl/min and the injection volume 10 μl. The ratio of peak areas of PEth 16:0/20:4 to deuterated PEth 16:0/20:4 were compared against a linear regression of ratios of calibrators from 0 to 2000 ng/ml. Concentrations were expressed in ng/ml.
A partial validation was performed for PEth 16:0/20:4 only because PEth 16:0/18:1 and PEth 16:0/18:2 were validated previously (Javors et al., 2016 and Hill-Kapturczak et al., 2018). PEth 16:0/20:4 limit of detection and lower limit of quantitation were estimated to be 0.8 and 4.0 ng/ml respectively. The upper limit of linearity was not detected at the largest calibrator concentration of 1,000 ng/ml, indicating that saturation is reached at higher concentrations. Matrix effect samples from six non-drinking subjects were tested and no interference was observed. Percent recovery between extracted and unextracted samples was determine to be 90%. PEth 16:0/20:4 analytical and in internal standards were determined to be stable when using aliquots of 1 mg/ml superstock and working solutions prepared at 1 μg/ml stored at −80°C and freshly obtained in every run.
Statistical Analysis
Experimental results obtained for the baselines of the three homologs before alcohol administration were statistically analyzed to see if there was any difference in levels of homolog using parametric ordinary 1-way ANOVA, after testing for normality. Results for the maximum concentration (Cmax), area under the curve for the 360 minutes after alcohol dosing (AUC 360) and half-life of each of the PEth homologs at the two alcohol dosages were statistically analyzed for differences among doses and homologs using 2-way repeated measures ANOVA. The two factors were dose (between factor) and PEth homolog (within factor, repeated measure). Repeated measures were only established for the homolog factor because the homologs were measured in the same participant. AUC 360s were calculated for the increase of PEth levels above baseline using the trapezoid rule with Prism 7.04 software. Tukey’s multiple comparisons test was performed to identify which group means were significantly different. Because of the small sample size, 95% confidence intervals (CI) were computed as well. Data are expressed as mean ± SD. Statistical analyses were performed using GraphPad Prism software, version 7.04 (GraphPad Software Inc., La Jolla, California, USA).
Results
Demographics
Of the 18 participants in this study, 8 (5 males and 3 females) individuals were from the group that consumed 0.4 g/kg of alcohol and 10 (5 males and 5 females) individuals were from the group that consumed 0.8 g/kg of alcohol. The mean age of participants in the low dose was 29 ± 7 years old (27 ± 5 for males and 31 ± 10 for females). The range age was 22 to 34 years old (22 to 34 for males and 22 to 31 for females). The mean age of participants in the high dose was 27 ± 6 years old (25 ± 4 for males and 29 ± 7 for females). The range age was 22 to 37 years old (22 to 33 for males and 22 to 37 for females). The mean weight of participants in the low dose was 79 ±11 kg (85 ± 6 for males and 69 ± 10 for females). The range weight was 57 to 93 kg (79 to 93 for males and 57 to 77 for females). The mean weight of participants in the high dose was 72 ± 16 kg (86 ± 12 for males and 58 ± 9 for females). The range age was 47 to 100 kg (75 to 100 for males and 47 to 70 for females). In the 0.4 g/kg dose group, there were 7 whites (4 males and 3 females) and 1 participant selected to disclose his race. In this same category, there were 5 Hispanics (3 males and 2 females) and 3 Nos-Hispanics (2 males and 1 female). In the 0.8 g/kg dose group, there were 1 female Asian, 2 male blacks, 5 whites (1 male and 4 females) and 2 male multiracial participants. In this same category, there were 5 Hispanics (3 males and 2 females) and 5 Nos-Hispanics (2 males and 3 females).
Description of BrAC Measurements
Proportional increases to the 0.4 and 0.8 g/kg alcohol doses consumed were observed in all participants. Mean maximum concentration levels were reached at 45 min after the 0.4 g/kg initial alcohol dose, 0.0390 ± 0.007 g/dl, and at 60 min after the 0.8 g/kg dose, 0.0899 ± 0.016 g/dl. The BrAC values for the doses were significantly different between doses at every time point (P < 0.0001), except at 0 and 360 min, which there was no difference in BrAC concentration levels. BrAC AUCs were higher in participants that consumed 0.8 g/kg of alcohol than those that drank the 0.4 g/kg dose, (21.0 ± 3.2 vs 6.75 ± 0.6; 95% CI for the difference = 14.7 to 27.4 and 5.48 to 8.01 respectively; P < 0.0001).
Pharmacokinetics of PEth 16:0/20:4
The pharmacokinetic profiles of PEth 16:0/20:4 (Fig. 1A), PEth 16:0/18:1 (Fig. 1B, upper panel) and PEth 16:0/18:2 (Fig. 1B, lower panel) in the 18 participants after either 0.4 and 0.8 g/kg doses of ethanol indicated that PEth 16:0/20:4 had a profile of synthesis onset after alcohol dosing similar to that seen with PEth 16:0/18:1 and PEth 16:0/18:2 (measured previously by Hill-Kapturczak et al., 2018), but that there were differences in the amount of PEth 16:0/20:4 synthesized and the time frame of elimination.
Fig. 1:
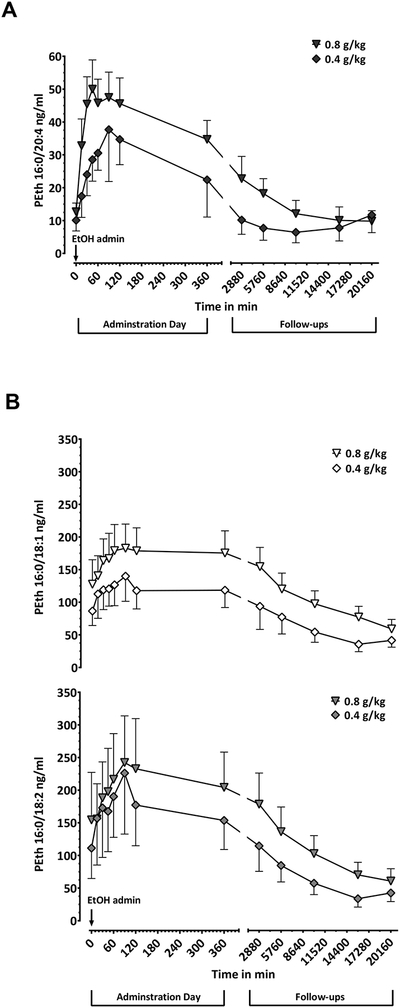
A)PEth 16:0/20:4 time course profile in blood samples from participants after given two initial doses of 0.4 g/kg (diamonds) and 0.8 g/kg (triangles) of alcohol. Blood samples were collected at 0, 15, 30, 45, 60, 90, 120 and 360 minutes after alcohol intake and at 2880, 5760, 10080, 15480 and 20160 minutes during follow-ups (2nd, 4th, 7th, 11th and 14th day respectively). B) PEth 16:0/18:1 (top curves) and PEth 16:0/18:2 (bottom curves) levels from the same blood samples, same time points and same doses as A) (0.4 g/kg, rhomboids and 0.8 g/kg triangles). PEth concentrations were not adjusted for background. Error bars are shown as SEM for easy visualization of the curves.
The mean baseline values, before administering alcohol doses, of the three homologs differed significantly [F (1, 21) = 8.1; P = 0.0070]. Significantly lower levels of PEth 16:0/20:4 were found in the 18 participants than PEth 16:0/18:1 (12 ± 9 ng/ml vs. 109 ± 98 ng/ml; 95% CI for the difference = 42 to 154; P = 0.0009) and PEth 16:0/18:2 (12 ± 9 ng/ml vs. 135 ± 189 ng/ml; 95% CI for the difference = 13 to 235; P = 0.0277), whereas the values of PEth 16:0/18:1 and PEth 16:0/18:2 were not different (109 ± 98 ng/ml vs. 135 ± 189 ng/ml; 95% CI for the difference = −47 to 99; P = 0.6398).
PEth 16:0/20:4, PEth 16:0/18:1 and PEth 16:0/18:2 levels increased to their maximum concentrations (Cmax) at 45 to 90 min after ethanol intake. Each of the three homologs reached the same Cmax at the two alcohol doses [F (1, 16) = 0.15; P = 0.7075]. The average increase from baseline to Cmax for the three homologs [F (2, 32) = 11; P =0.0003] was the lowest for PEth 16:0/20:4 when compared to that of PEth 16:0/18:1 (55 ± 34 ng/ml vs. 181 ± 115 ng/ml in n = 18; 95% CI for the difference = 18 to 235; P = 0.0199) and that of PEth 16:0/18:2 (55 ± 34 ng/ml vs. 261 ± 243 ng/ml in n = 18; 95% CI for the difference = 98 to 315; P = 0.0002). There was no statistically significant difference between maximum concentrations of PEth 16:0/18:1 and PEth 16:0/18:2 (181 ± 115 ng/ml vs 261 ± 243 ng/ml in n = 18; 95% CI for the difference = −29 to 189; P = 0.1818).
PEth 16:0/20:4, as well as PEth 16:0/18:1 and PEth 16:0/18:2 decreased back to baseline levels in participants that abstained from drinking during the 2-week follow up period.
PEth 16:0/20:4 AUC 360
The overall amount of synthesis of each of the three PEth homologs (Fig. 2), determined by AUC 360 for the increase above baseline levels during alcohol administration day, was approximately the same across alcohol dose [F (1, 16) = 2.1; P = 0.1686], but the levels were different among the three homologs [F (2, 32) = 20; P < 0.0001].
Fig. 2. AUC 360 of PEth 16:0/20:4.
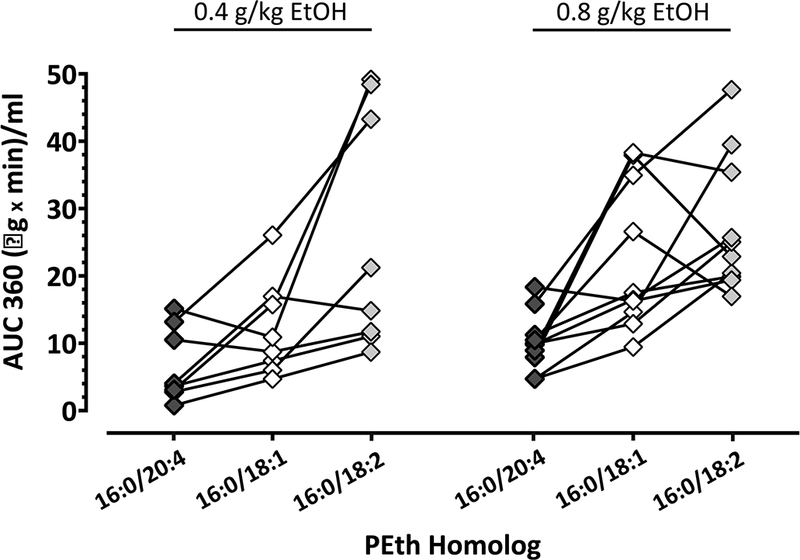
Individual values of the area under the curve (AUC 360) shown in a scatter plot measured from 0 to 360 minutes during the day of alcohol administration at 0.4 g/kg and 0.8 g/kg EtOH doses. Graph compares AUC values between PEth 16:0/20:4 (black diamonds), PEth 16:0/18:1 (white diamonds) and PEth 16:0/18:2 (grey diamonds) with a line connecting the three homologs measured in the same participant.
Collapsing across alcohol dose, formation of PEth 16:0/20:4 was significantly lower than that of PEth 16:0/18:1 (8.62 ± 5.0 μg x min/ml vs. 17.9 ± 11 μg x min/ml in n = 18; 95% CI for the difference = 2.3 to 16; P = 0.0073) and also that of PEth 16:0/18:2 (8.62 ± 5.0 μg x min/ml vs. 26.7 ± 14 μg x min/ml in n = 18; 95% CI for the difference = 11 to 25; P < 0.0001). In addition, synthesis of PEth 16:0/18:1 was lower than that of PEth 16:0/18:2 (17.9 ± 11 μg x min/ml vs. 26.7 ± 14 μg x min/ml in n = 18; 95% CI for the difference = 1.9 to 16; P = 0.0102).
PEth 16:0/20:4 Half-Life
The half-lives of PEth 16:0/20:4, PEth 16:0/18:1 and PEth 16:0/18:2 were determined over the two-week follow-up after alcohol consumption (Fig. 3). The half-life of the each of the three homologs was unaffected by alcohol dose administered [F (1, 16) = 2.1; P = 0.1673], however, the half-lives differed significantly among the homologs [F (2, 32) = 16; P < 0.0001].
Fig 3. PEth 16:0/20:4 Half-Life.
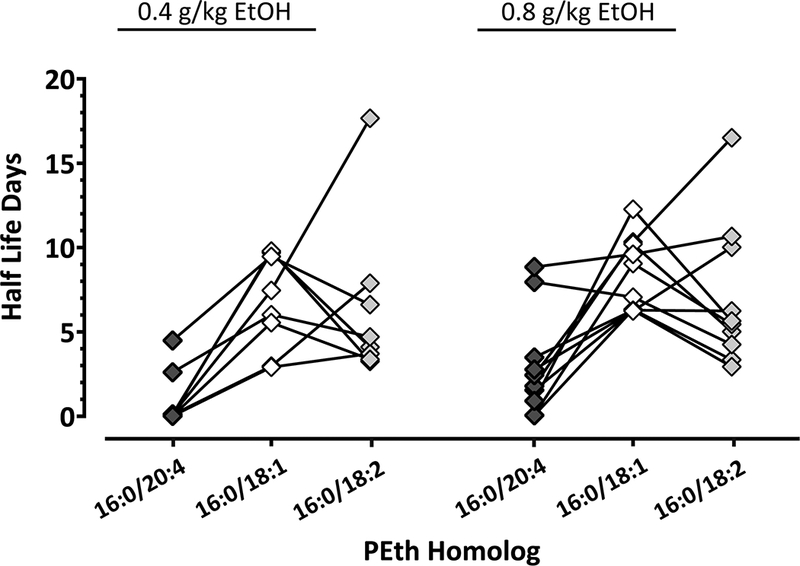
Individual half-lives were measured from the highest peak at administration time (Cmax) to the last follow-up time point and values shown in an individual scatter plot graph. PEth 16:0/20:4 (black diamonds) half-life is compared to the half-lives of PEth 16:0/18:1 (white diamonds) and PEth 16:0/18:2 (grey diamonds). The homologs corresponding to the same participant are connected for a line.
The elimination of PEth 16:0/20:4 was faster than that of PEth 16:0/18:1 (2.1 ± 3 days vs. 7.6 ± 3 days in n = 18; 95% CI for the difference = 3.0 to 8.2; P < 0.0001) and that of PEth 16:0/18:2 (2.1 ± 2 days vs. 6.8 ± 4 days in n = 18; 95% CI for the difference = 2.1 to 7.3; P = 0.0003). In contrast, no difference was found between the half-life of PEth 16:0/18:1 and 16:0/18:2 (7.6 ± 3 days vs. 6.8 ± 4 days in n = 18; 95% CI for the difference = −1.7 to 3.5; P = 0.6757).
Discussion
The present study is the first report of the pharmacokinetics of PEth 16:0/20:4 in human blood samples in a controlled clinical lab study. The principal conclusions of this study are that PEth 16:0/20:4 in blood after consumption of alcohol doses of 0.4 or 0.8 g/kg (1) is synthesized at lower levels than either PEth 16:0/18:1 or PEth 16:0/18:2 and (2) exhibits shorter half-lives than either of the other two homologs.
PEth 16:0/20:4 seems to peak earlier, similar to PEth 16:0/18:2, and there seems to be more variability in these two homologs compared to the PEth 16:0/18:1 homolog. There are multiple sources of variability among humans related to the synthesis of PEth homologs, including differences in PLD levels, amount of hematocrits and rate of ethanol absorption affected by gender, percent body fat and genetics (Hahn et al 2016), among others. These are likely the basis of the observed variability.
Only a few reports have been published that compare the pharmacokinetics of synthesis and elimination of both PEth 16:0/18:1 and 16:0/18:2 (Hill‐Kapturczak et al., 2018; Javors et al., 2016; Schröck et al., 2016). The results of those studies indicate that more PEth 16:0/18:2 than 16:0/18:1 is synthesized immediately after ethanol consumption and PEth 16:0/18:2 is eliminated more rapidly than PEth 16:0/18:1 in the majority of participants tested. The results of the current study show that less PEth 16:0/20:4 is synthesized compared to PEth 16:0/18:1 and 16:0/18:2 and that its short elimination half-life (2.1 days) is new information.
We suggest that knowledge of the relative rates of formation and elimination of various PEth homologs might allow the estimation of recency of alcohol consumption from a single blood sample. For example, if PEth 16:0/18:1 and 16:0/18:2 were present in a blood sample, but PEth 16:0/20:4 was not detectable, it would suggest that alcohol had been consumed during the past week, but not in the last couple of days. Further, if only PEth 16:0/18:1 were detectable in a blood sample, but not either of the other two homologs, it would suggest that alcohol consumption had not occurred within the past week or longer. Also, if the three homologs were detected, it would indicate that alcohol was consumed regularly up to within a couple of days. The simultaneous measurement of all three PEth homologs with different rates of formation and elimination should be useful to increase the accuracy of PEth as a biomarker of alcohol consumption and therefore, provide a useful tool in clinical settings for screening patients for potential alcohol abuse and test treatment efficacy.
Limitations.
This is a preliminary study with limitations mostly based on small sample size of 18 subjects. Nevertheless, the mean AUC360 above baseline and the mean half-life of PEth 16:0/20:4 were lower than PEth 16:0/18:1 and 16:0/18:2, statistically significant and convincing differences. Differences among the PEth homologs based on alcohol dose and sex were not observed using this data set. It should be noted that these parameters were also not statistically significantly different between PEth 16:0/18:1 and PEth 16:0/18:2 in our larger study (Hill‐Kapturczak et al., 2018). Finally, PEth 16:0/20:4 was quantified in uncoagulated, whole blood samples (0oC) that had been thawed and re-frozen a little more than one year earlier when testing for PEth 16:0/18:1 and 16:0/18:2 was performed. PEth 16:0/18:1 and 16:0/18:2 have been shown to be stable when stored at −80oC for several months and during freeze/thaw cycles (Helander and Zheng, 2009) and we can only presume the same is true for PEth 16:0/20:4. Nevertheless, even this reality indicates that PEth 16:0/20:4 is eliminated faster than the other two homologs and this finding would not be affected by sample stability factors.
Future directions
One of the goals of our PEth homolog research is to evaluate the measurement of multiple isoforms in single samples to more accurately estimate the recentness and amount of recent alcohol consumption. Also, we intend to evaluate the possible combined utility of adding direct biomarkers such as ethyl glucuronide in urine or serum CDT and GGT levels to allow a more accurate assessment of recent alcohol consumption and its toxic effects. These biomarkers individually reflect different levels, time frames of consumption, and possible more serious toxic effects of alcohol use.
Acknowledgements
This publication was supported by a grant from the NIH National Center for Advancing Translational Sciences grant supplement to Dr. Marisa Lopez-Cruzan (UL1TR001120-S1). Also, this publication was supported by the National Institute on Alcohol Abuse and Alcoholism of the National Institutes of Health [R01AA022361and R01AA14988] and, in part, by the National Institute on Drug Abuse [T32DA031115] for postdoctoral training for Tara E. Karns-Wright. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Dr. Dougherty also gratefully acknowledges support from a research endowment, the William and Marguerite Wurzbach Distinguished Professorship. Dr. Javors gratefully acknowledges support from the Nancy U. Karren Professorship Endowment.
Footnotes
Conflict of Interest
None of the authors have conflict of interests concerning this manuscript.
References
- Alling C, Gustavsson L, Anggard E (1983) An abnormal phospholipid in rat organs after ethanol treatment. FEBS Lett 152:24–28. [DOI] [PubMed] [Google Scholar]
- Aradottir S, Asanovska G, Gjerss S, Hansson P, Alling C (2006) PHosphatidylethanol (PEth) concentrations in blood are correlated to reported alcohol intake in alcohol-dependent patients. Alcohol and alcoholism (Oxford, Oxfordshire) 41:431–437. [DOI] [PubMed] [Google Scholar]
- Aradottir S, Lundqvist C, Alling C. Phosphatidylethanol in rat organs after ethanol exposure. Alcohol Clin Exp Res. 2002. April;26(4):514–8. [PubMed] [Google Scholar]
- Aradóttir S, Moller K, Alling C. Phosphatidylethanol formation and degradation in human and rat blood. Alcohol Alcohol. 2004. Jan-Feb;39(1):8–13. [DOI] [PubMed] [Google Scholar]
- Beck O, Kenan Modén N, Seferaj S, Lenk G, Helander A (2018) Study of measurement of the alcohol biomarker phosphatidylethanol (PEth) in dried blood spot (DBS) samples and application of a volumetric DBS device. Clinica Chimica Acta 479:38–42. [DOI] [PubMed] [Google Scholar]
- Gnann H, Engelmann C, Skopp G, Winkler M, Auwärter V, Dresen S, Ferreirós N, Wurst FM, Weinmann W (2010) Identification of 48 homologues of phosphatidylethanol in blood by LC-ESI-MS/MS. Analytical and Bioanalytical Chemistry 396:2415–2423. [DOI] [PubMed] [Google Scholar]
- Gnann H, Thierauf A, Hagenbuch F, Rohr B, Weinmann W (2014) Time dependence of elimination of different PEth homologues in alcoholics in comparison with social drinkers. Alcohol Clin Exp Res 38:322–326. [DOI] [PubMed] [Google Scholar]
- Gnann H, Weinmann W, Thierauf A (2012) Formation of Phosphatidylethanol and Its Subsequent Elimination During an Extensive Drinking Experiment Over 5 Days. Alcoholism: Clinical and Experimental Research 36:1507–1511. [DOI] [PubMed] [Google Scholar]
- Gustavsson L, Alling C (1987) Formation of phosphatidylethanol in rat brain by phospholipase D. Biochemical and Biophysical Research Communications 142:958–963. [DOI] [PubMed] [Google Scholar]
- Helander A, Zheng Y (2009) Molecular species of the alcohol biomarker phosphatidylethanol in human blood measured by LC-MS. Clin Chem 55:1395–1405. [DOI] [PubMed] [Google Scholar]
- Hill-Kapturczak N, Dougherty DM, Roache JD, Karns‐Wright TE, Javors MA (2018) Differences in the Synthesis and Elimination of Phosphatidylethanol 16:0/18:1 and 16:0/18:2 after Acute Doses of Alcohol. Alcohol Clin Exp Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Høiseth G, Bernard JP, Karinen R, Johnsen L, Helander A, Christophersen AS, Mørland J (2007) A pharmacokinetic study of ethyl glucuronide in blood and urine:Applications to forensic toxicology. Forensic Science International 172:119–124. [DOI] [PubMed] [Google Scholar]
- Høiseth G, Morini L, Polettini A, Christophersen A, Mørland J (2009) Blood kinetics of ethyl glucuronide and ethyl sulphate in heavy drinkers during alcohol detoxification. Forensic Science International 188:52–56. [DOI] [PubMed] [Google Scholar]
- Javors MA, Hill-Kapturczak N, Roache JD, Karns-Wright T, Dougherty DM (2016) Characterization of the Pharmacokinetics of Phosphatidylethanol 16:0/18:1 and 16:0/18:2 in Human Whole Blood After Alcohol Consumption in a Clinical Laboratory Study. Alcohol Clin Exp Res 40:1228–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M, Kanfer JN (1987) Phosphatidylethanol formation via transphosphatidylation by rat brain synaptosomal phospholipase D. J Neurochem 48:1597–1603. [DOI] [PubMed] [Google Scholar]
- Orrego H, Blake JE, Israel Y (1985) Relationship between gamma-glutamyl transpeptidase and mean urinary alcohol levels in alcoholics while drinking and after alcohol withdrawal. Alcoholism, clinical and experimental research 9:10–13. [DOI] [PubMed] [Google Scholar]
- Schmitt G, Droenner P, Skopp G, Aderjan R (1997) Ethyl glucuronide concentration in serum of human volunteers, teetotalers, and suspected drinking drivers. J Forensic Sci 42:1099–102. [PubMed] [Google Scholar]
- Schröck A, Pfäffli M, König S, Weinmann W (2016) Application of phosphatidylethanol (PEth) in whole blood in comparison to ethyl glucuronide in hair (hEtG) in driving aptitude assessment (DAA). Int J Legal Med 130:1527–1533. [DOI] [PubMed] [Google Scholar]
- Schröck A, Thierauf-Emberger A, Schürch S, Weinmann W (2017) Phosphatidylethanol (PEth) detected in blood for 3 to 12 days after single consumption of alcohol—a drinking study with 16 volunteers. Int J Legal Med 131:153–160. [DOI] [PubMed] [Google Scholar]
- Simon TW (2018) Providing context for phosphatidylethanol as a biomarker of alcohol consumption with a pharmacokinetic model. Regulatory Toxicology and Pharmacology 94:163–171. [DOI] [PubMed] [Google Scholar]
- Stibler H (1991) Carbohydrate-Deficient Transferrin in Serum: a New Marker of Potentially Harmful Alcohol Consumption Reviewed Clin. Chem 37:2029–2037. [PubMed] [Google Scholar]
- Thompson PM, Hill‐Kapturczak N, Lopez‐Cruzan M, Alvarado LA, Dwivedi AK, Javors MA (2016) Phosphatidylethanol in Postmortem Brain and Serum Ethanol at Time of Death. Alcoholism: Clinical and Experimental Research 40:2557–2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah S, Helander A, Olof Beck O (2017) Identification and quantitation of phosphatidylethanols in oral fluid by liquid chromatography-tandem mass spectrometry. Clinical Chemistry and Laboratory Medicine (CCLM) 55:1332–1339. [DOI] [PubMed] [Google Scholar]
- Varga A, Hansson P, Johnson G, Alling C (2000) Normalization rate and cellular localization of phosphatidylethanol in whole blood from chronic alcoholics. Clinica Chimica Acta 299:141–150. [DOI] [PubMed] [Google Scholar]
- Varga A, Hansson P, Lundqvist C, Alling C (1998) Phosphatidylethanol in Blood as a Marker of Ethanol Consumption in Healthy Volunteers: Comparison with Other Markers. Alcoholism: Clinical and Experimental Research 22:1832–1837. [PubMed] [Google Scholar]
- Walther L, Brodén C, Isaksson A, Hedenbro JL (2018) Alcohol Consumption in Obese Patients Before and After Gastric Bypass as Assessed with the Alcohol Marker Phosphatidylethanol (PEth). Obesity Surgery 1–7. [DOI] [PubMed] [Google Scholar]
- Wang S, Yang R, Ji F, Li H, Dong J, Chen W (2017) Sensitive and precise monitoring of phosphatidylethanol in human blood as a biomarker for alcohol intake by ultrasound-assisted dispersive liquid-liquid microextraction combined with liquid chromatography tandem mass spectrometry. Talanta 166:315–320. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Beck O, Helander A (2011) Method development for routine liquid chromatography–mass spectrometry measurement of the alcohol biomarker phosphatidylethanol (PEth) in blood. Clinica Chimica Acta 412:1428–1435. [DOI] [PubMed] [Google Scholar]


