Abstract
Background
Pediatric cancer-related fatigue is prevalent and significantly impairs health-related quality of life, yet its patterns and correlates are poorly understood. We aimed to describe fatigue prospectively reported by children with advanced cancer, and to identify factors associated with fatigue and associated distress.
Methods
Children (≥2 years) with advanced cancer (N=104) or a parent at three academic hospitals reported their symptoms at most weekly, over nine months, using the computer-based Pediatric Quality of Life Evaluation of Symptoms Technology (PediQUEST) system. PediQUEST administered a modified version of the Memorial Symptom Assessment Scale (PQ-MSAS) as part of a randomized controlled trial. Clinical information was abstracted from medical records. Primary outcomes were 1) fatigue prevalence (yes/no response to PQ-MSAS fatigue item) and 2) fatigue distress (composite score of severity, frequency and bother). Multivariable models were constructed to identify factors independently associated with fatigue prevalence and scores reflecting fatigue distress (i.e., burden).
Results
Of 920 reports, 46% (n=425) noted fatigue. When reported, fatigue was of high frequency in 41% (n=174), severity in 25% (n=107) and bother in 34% (n=143). Most reports (84%, n=358) were associated with scores indicating fatigue distress. In multivariable analyses, fatigue was associated with older age, lower hemoglobin and distress from particular symptoms (anorexia, nausea, sleep disturbance, sadness and irritability). In contrast, fatigue distress was associated with distress from nausea, cough and pain.
Conclusions
Fatigue is common among children with advanced cancer, and is often highly distressing. Interventions focused on uncontrolled symptoms may ease fatigue distress in children with advanced cancer.
Keywords: fatigue, pediatric cancer, symptoms, palliative care, patient-reported outcomes
Condensed abstract
Fatigue is common among children with advanced cancer, and is often distressing. While fatigue is associated with older age, lower hemoglobin and distress from particular symptoms (anorexia, nausea, sleep disturbance, sadness and irritability) fatigue distress was associated with distress from nausea, cough and pain.
Introduction
Fatigue is one of the most common symptoms that children with advanced (relapsed, progressive) cancer experience.1–8 They describe fatigue as a debilitating symptom with physical, cognitive and emotional components that is detrimental to their health-related quality of life (HRQL).9–15 For many, fatigue is one of the most distressing symptoms they experience.14,16–18 Parents of children with advanced cancer view it as a source of significant suffering for their children,6,7,19 and identify it as one of the symptoms of most concern to them.4,20
Despite the significant impact of fatigue, our understanding of its patterns, correlates and potential causes among children with advanced cancer is limited. As a result, strategies with proven efficacy to mitigate this complex symptom are lacking and it remains undertreated.6–8 To develop effective interventions aimed at treating fatigue and easing suffering in this population it is imperative to understand the factors potentially contributing to fatigue that may be targeted.
Studies of pediatric cancer-related fatigue to date are largely limited by retrospective design,1–4,6–8 reliance on proxy report1,3,4,6–8,19 and a focus on the end-of-life period as opposed to earlier stages of advanced cancer.1–4,6–8 Quantitative studies, especially fatigue as reported by children themselves, are lacking. Research addressing the difference between the presence of fatigue and fatigue distress (i.e., high fatigue burden, or impact on the child) is similarly limited. Given the complex, multifactorial nature of fatigue, studies addressing how patient-reported factors (e.g. symptoms) child factors (e.g. age) and clinical factors (e.g. cancer-directed therapy), taken together, contribute to fatigue and fatigue distress are especially needed. Elucidating these factors may inform the development of effective interventions to mitigate fatigue. Understanding the prevalence and factors associated with fatigue may also create a better framework in which to test the effectiveness of future fatigue interventions.
We therefore sought to describe patterns of both fatigue and distress associated with fatigue in pediatric advanced cancer. We utilized a multicenter cohort of children with advanced cancer who primarily self-reported symptoms to comprehensively evaluate factors associated with fatigue during nine months of follow-up.
Methods
Design and Setting
The setting and data collection methods have been previously detailed.21–23 Briefly, Pediatric Quality of Life Evaluation of Symptoms Technology (PediQUEST) is a computer-based data collection system that prospectively collects child- (or parent proxy-, when necessary) reported symptom and HRQL outcomes, and can generate feedback reports for clinicians and families. Data were collected in the context of a pilot randomized controlled trial assessing the effect of PediQUEST reports on symptoms and HRQL.21 The study (clinicaltrials.gov identifier NCT01838564). was conducted at three large pediatric cancer centers, Boston Children’s Hospital/Dana Farber Cancer Institute, Seattle Children’s Hospital and the Children’s Hospital of Pennsylvania. The institutional review board of each participating site approved the study.
Participants
Eligible children were at least two years of age, receiving cancer care at a study site, with at least a two-week history of advanced cancer (i.e., not responsive to therapy, progressive, or recurrent) or for whom there was a decision to not pursue cancer-directed therapy. Children with an isolated relapsed solid tumor treated with radiation or surgery alone, or a first relapse of hematologic malignancy and proceeding to stem cell transplant were excluded. One eligible parent per enrolled child was selected by the family to participate. Eligible parents had written command of English and the ability to complete self-administered surveys.
Study Instruments
Participants were regularly presented age and respondent-specific PediQUEST surveys via tablet computers. PediQUEST-surveys included the PediQUEST Memorial Symptom Assessment Scale (PQ-MSAS). PQ-MSAS is an adapted version of the Memorial Symptom Assessment Scale (MSAS), the only multidimensional and multi-symptom instrument that has been validated for use in children with cancer.25–27 Three PQ-MSAS versions allowed the assessment of 24 physical and psychological symptoms across the study age range: PQ-MSAS 7-12 for children 7-12 years old, comprised of a self-report version assessing eight symptoms, including fatigue, and a parent supplemental version asking about the remaining 16 symptoms; PQ-MSAS 13-18 for adolescents ≥13 years; and PQ-MSAS proxy-full for parents whose child was too young (<7 years old) or not able/willing to respond.
PQ-MSAS asks about the presence, frequency, severity and bother for symptoms that the child experienced in the preceding week. Response options use a 4-point (PQ-MSAS 7-12) or 5-point (PQ-MSAS 13-18, PQ-MSAS proxy-full) Likert-type scales.25,26 PQ-MSAS symptom scores reflecting burden/distress for individual symptoms were calculated as the average of the three sub-items, per the authors’ recommendations. Sub-item scores were standardized using a 0-100 scale (100 worst). Equivalence across age groups and respondents was assumed. Whenever a child answered, the administration was considered self-report, even if it was a combined child-parent report. A full description of PQ-MSAS is available elsewhere.21,22,27
Study Procedures
Eligible children who assented (if developmentally able) and had informed permission (consent) from a parent who also consented to participate were enrolled sequentially. One hundred four children were enrolled from December 2004 to June 2009 and were followed until death or the end of data collection. Children completed PediQUEST in clinic or the inpatient ward at most once a week, and at least once a month. Children at least 7 years of age were asked to complete PediQUEST using the corresponding PQ-MSAS version. If the child was unable or unwilling to do so, the parent completed a proxy version on the child’s behalf.
Demographic and clinical data were abstracted from medical records. For each PediQUEST administration, detailed data were collected from the corresponding clinical encounter and the preceding ten days. Abstracted encounter data included disease status, receipt and type of cancer-directed treatment (chemotherapy, procedures, radiation and surgery) laboratory data (Hb) and treatment for symptoms (e.g. opioids).
Statistical Analysis
All analyses were conducted using the SAS statistical package (version 9.4 SAS Institute, Inc., Cary, N.C.). Because the intervention did not significantly affect PQ-MSAS scores, data from the arms were pooled.21 These analyses include reports generated over 9 months of follow-up. Because PediQUEST assessments were not tied to specific clinical events nor precisely defined intervals of time, and anchors for measurements over time were not available, an analytic approach in which PediQUEST administrations were considered the unit of analysis was chosen.
For the purpose of analysis, fatigue sub-item scores were classified as follows: high frequency (≥66; medium amount/a lot/almost always), high severity (≥66; medium amount/severe/very severe) and high bother (≥50; somewhat/quite a bit/very much). Fatigue distress was defined as a fatigue score (composite of severity, frequency and bother sub-item scores) ≥44 (PQ-MSAS 7-12), and ≥33 (PQ-MSAS-13-18, PQ-MSAS-proxy-full). Symptom distress was defined similarly for other symptoms. The rationale for these cut points and dichotomization is presented in detail elsewhere.21,22
The primary outcomes of interest were report of fatigue (as indicated by the response to the PQ-MSAS fatigue item) and among the reports of fatigue, fatigue distress (i.e. high degree of fatigue burden). High fatigue frequency, severity and bother and fatigue distress frequencies were also reported. Factors hypothesized to be associated with fatigue outcomes (including age [continuous variable], diagnosis, disease status, time since diagnosis, receipt of cancer-directed treatment, cancer treatment (chemotherapy, radiation, surgery, other procedures part of cancer treatment), opioid therapy, hemoglobin [continuous variable], distress from other specific symptoms) were selected a priori based on existing cancer fatigue literature and clinical experience.7,28–32 Generalized mixed linear models (logit link and binomial distribution) were used to evaluate associations between these factors and fatigue or fatigue distress, taking into account intervention arm and patient-level clustering. All factors associated with fatigue outcomes on univariate analysis were entered into multivariable models (p≤0.1 for variable entry) and then eliminated by backward selection (retention criterion p≤0.1). All models included patient as a random effect (to account for the repeated measurements from single patients) and study arm as fixed to account for a potential small intervention effect.
Results
Over the course of the study, 147 eligible children were approached and 104 (71%) enrolled. Among those who enrolled, nearly one half were adolescents (48% were ≥13 years) and nearly one half were female (49%, n=51). (Table 1) Slightly over half (56%, n=58) had a diagnosis of solid tumor and relatively few (10%, n=10) had a brain tumor. Twenty-six children (25%) died during follow-up.
TABLE 1.
Baseline characteristics of study sample (n=104)
| Site of care | |
| Site 1, n (%) | 24 (23) |
| Site 2, n (%) | 59 (57) |
| Site 3, n (%) | 21 (20) |
| Female, n (%) | 51 (49) |
| Age, median (IQR) years | 12.1 (6.8-17.1) |
| White non-Hispanic | 93 (89) |
| Diagnosis | |
| Hematologic malignancy, n (%) | 36 (34) |
| Solid tumor, n (%) | 58 (56) |
| Brain tumor, n (%) | 10 (10) |
| Months from diagnosis to enrollment, median (IQR) | 27 (17-51) |
| Months from last disease progression to enrollment, median (IQR) | 5.9 (3.4-9.4) |
| Intervention arm, n (%) | 51 (49) |
Abbreviations:SD, standard deviation; IQR, interquartile range
Over the course of 9 months of follow-up, 920 PediQUEST administrations were completed, with a median (inter-quartile range, [IQR]) of 8 (4-12) administrations per child. The vast majority of administrations for children ≥7 years of age (i.e. children able to self-report) were completed by the child. Specifically, among reports representing the 7-12 age group, 238 of 248 (96%) were self-reports, and in the ≥13 age group 453 of 456 (99%) were self-reports.
Patterns of Fatigue and Fatigue Distress
Among the 104 children, 90 reported fatigue at least once. And, of those 90 children, 87 reported fatigue distress at least once. Among the 920 PediQUEST administrations, fatigue was reported in almost half (46%, n=425). Among those 425 reporting fatigue, 41% (n=174) reported fatigue of high frequency, 25% (n=107) high severity and 34% (n=143) high bother. The average fatigue distress score was 46.8 (standard deviation [SD] 16.6). The majority (84%, n=358) had a fatigue score above the pre-specified cut point indicating fatigue distress. (Fig. 1)
Figure 1.
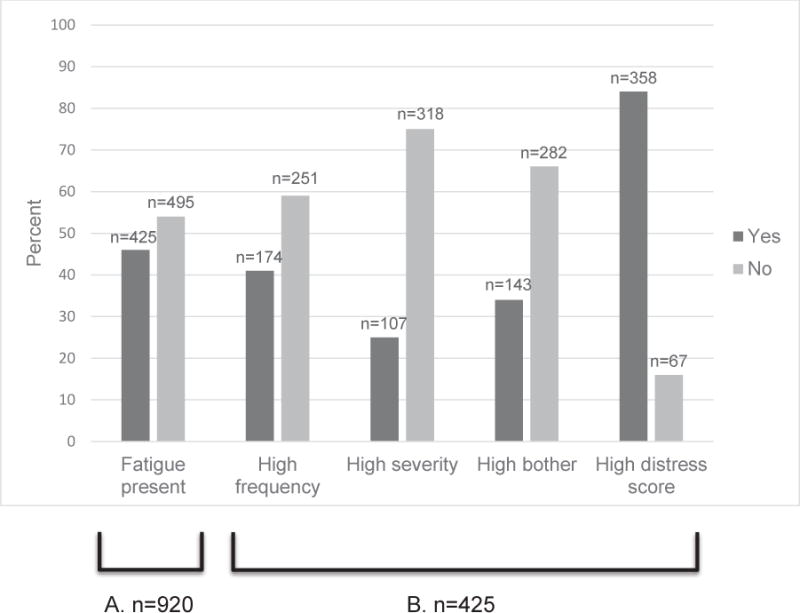
Frequencies of reports of fatigue, fatigue dimensions, and fatigue distress.
A. Surveys reporting fatigue among all n=920 surveys
B. Surveys reporting high frequency, high severity, high bother and high distress score among all n=425 surveys reporting fatigue. Fatigue sub-item scores were classified as follows: high frequency (≥66; medium amount/a lot/almost always), high severity (≥66; medium amount/severe/very severe) and high bother (≥50; somewhat/quite a bit/very much). High fatigue distress scores (composite of severity, frequency and bother sub-item scores) were defined as ≥44 for PQ-MSAS 7-12, and ≥33 for PQ-MSAS-13-18 and PQ-MSAS-proxy-full.
Factors Associated with Fatigue
Some variables representing clinical factors (including receipt of opioids in the prior 10 days, and lower Hb) and reports of distressing symptoms (except cough) were significantly associated with fatigue on univariate analysis while other variables (diagnosis, time from diagnosis, disease status) were not. (Table 2) Older child age and undergoing a procedure were marginally associated with fatigue. In the multivariable model, older age, lower Hb, other distressing physical (anorexia, nausea, difficulty sleeping) and psychological symptoms (sadness, irritability) were associated with fatigue. (Table 3)
TABLE 2.
Factors associated with reports of fatigue and high fatigue distress (univariate analysis)
| All Reports (n=920) | Fatigue Reports (n=425) | |||||
|---|---|---|---|---|---|---|
| No Fatigue (n=495) |
Fatigue (n=425) |
p* | Low Distress* (n=67) |
High Distress* (n=358) |
p* | |
| Demographic characteristics | ||||||
| Site of care | 0.31 | 0.53 | ||||
| Site 1, n (%) | 105 (61) | 67 (39) | 15 (22) | 52 (78) | ||
| Site 2, n (%) | 257 (51) | 248 (49) | 38 (15) | 210 (85) | ||
| Site 3, n (%) | 133 (55) | 110 (45) | 14 (13) | 96 (87) | ||
| Female, n (%) | 215 (49) | 222 (51) | 0.32 | 24 (11) | 198 (89) | 0.03 |
| Age at enrollment, median years (IQR) | 11.7 (6.7-16.7) | 14.7 (8.7-17.2) | 0.05 | 10.4 (6.5-16.4) | 15.4 (8.7-17.6) | 0.05 |
| White non-Hispanic, n (%) | 441 (54) | 372 (46) | 0.55 | 61(16) | 311 (84) | 0.34 |
| Disease characteristics | ||||||
| Diagnosis | 0.80 | 0.72 | ||||
| Hematologic malignancy, n (%) | 165 (54) | 141 (46) | 25 (18) | 116 (82) | ||
| Solid tumor, n (%) | 277 (52) | 256 (48) | 36 (14) | 220 (86) | ||
| Brain tumor, n (%) | 53 (65) | 28 (35) | 6 (21) | 22 (78) | ||
| Months from diagnosis to enrollment, median (IQR) | 25 (15-50.6) | 30.6 (20-50.6) | 0.14 | 26.3 (17.9-54.1) | 30.7 (20.5-50.4) | 0.96 |
| Disease status active/progressive | 323 (55) | 267 (45) | 0.63 | 48 (18) | 219 (82) | 0.12 |
| Months since last disease progression, median (IQR) | 6.1 (3.7-9.5) | 5.6 (3.2-9.4) | 0.38 | 5.4 (3.2-9.4) | 6.1 (3.4-8.7) | 0.28 |
| Treatment (past 10 days) | ||||||
| Cancer-directed treatment (any) | 256 (53) | 231 (47) | 0.12 | 39 (17) | 192 (83) | 0.42 |
| Type of cancer treatment | ||||||
| Chemotherapy | 245 (53) | 214 (47) | 0.23 | 38 (18) | 176 (82) | 0.24 |
| Procedure | 36 (44) | 47 (56) | 0.07 | 13 (28) | 34 (72) | 0.05 |
| Surgery | 3 (27) | 8 (73) | 0.21 | 1 (12) | 7 (88) | 0.21 |
| Radiation therapy SCT conditioning |
14 (36) | 25 (64) | 0.13 | 0 | 25 (100) | 0.13 |
| Opioid therapy | 56 (40) | 85 (60) | 0.02 | 6 (7) | 79 (93) | 0.07 |
| Hemoglobin (g/dl), mean (SD) | 10.9 (1.7) | 10.7 (1.9) | <0.01 | 10.7 (2.4) | 10.7 (1.9) | 0.94 |
| Physical Symptoms:High Distress** | ||||||
| Anorexia | 67 (29) | 168 (71) | <.001 | 17 (10) | 151 (90) | <0.01 |
| Nausea | 63(28) | 160 (72) | <0.001 | 6 (4) | 154 (96) | <0.001 |
| Cough | 74 (46) | 86 (54) | 0.06 | 4 (5) | 82 (95) | <0.01 |
| Diarrhea | 72 (37) | 123 (63) | <0.001 | 15 (12) | 108 (88) | 0.49 |
| Pain | 155 (43) | 205 (57) | <0.01 | 14 (7) | 191 (93) | <0.001 |
| Difficulty sleeping | 62 (31) | 140 (69) | <0.001 | 15 (11) | 125 (89) | 0.27 |
| Psychological Symptoms:High Distress** | ||||||
| Sadness | 60 (38) | 100 (62) | <0.001 | 10 (10) | 90 (90) | 0.1 |
| Irritability | 104 (42) | 141 (58) | <0.001 | 20 (14) | 121 (86) | 0.52 |
| Worry | 63 (39) | 99 (61) | <0.01 | 5 (5) | 94 (95) | <0.01 |
Adjusted for intervention arm and respondent clustering (patient level clustering)
Symptom distress scores: High distress defined as a score ≥44 for PQ-MSAS 7-12, and ≥33 for PQ-MSAS-13-18 and PQ-MSAS-proxy-full.
Abbreviations: IQR, inter-quartile range; SD, standard deviation
TABLE 3.
Factors associated with reports of fatigue and fatigue distress (multivariate models)
| OR | 95%CI | p* | |
|---|---|---|---|
| Fatigue | |||
| Age | 1.06 | 1.0-1.13 | 0.04 |
| Hemoglobin | 0.79 | 0.69-0.91 | 0.001 |
| High distress symptoms** | |||
| Anorexia | 3.37 | 1.96-5.79 | <0.001 |
| Nausea | 3.29 | 1.97-5.5 | <0.001 |
| Difficulty sleeping | 2.93 | 1.70-5.03 | <0.001 |
| Sadness | 1.96 | 1.06-3.64 | 0.03 |
| Irritability | 1.96 | 1.10-3.50 | 0.02 |
| Fatigue Distress** | |||
| High distress symptoms* | |||
| Nausea | 5.01 | 1.99-12.57 | <0.001 |
| Cough | 4.25 | 1.41-12.81 | .01 |
| Pain | 2.3 | 1.13-4.70 | .02 |
| Worry | 2.52 | 0.89-7.11 | .08 |
Adjusted for intervention arm and respondent clustering (patient level clustering)
Symptom distress scores: High distress defined as a score ≥44 (PQ-MSAS 7-12), and ≥33 (PQ-MSAS-13-18, PQ-MSAS-proxy-full).
Abbreviations: OR, odds ratio; 95% CI, 95% confidence interval
Factors Associated with Fatigue Distress
In the subgroup of surveys in which fatigue was noted, female sex was associated with fatigue distress. Other variables, including diagnosis, disease status, receipt of cancer treatment and Hb were not (Table 2). Older child age, receipt of opioids and undergoing a procedure were marginally associated with fatigue distress. Both distressing physical (nausea, cough, pain) and psychological (worry) symptoms were associated with fatigue distress. In multivariate analyses only distressing nausea, cough, and pain were associated with fatigue distress. (Table 3)
Discussion
This sizeable, multicenter cohort of children with advanced cancer reported fatigue nearly fifty percent of the time. Moreover, a substantial proportion of their fatigue reports revealed high fatigue frequency, severity or bother, and the majority had fatigue distress. These data support the conclusion that, when present, fatigue may present a significant burden to children with advanced cancer.
Fatigue was associated with older age, lower Hb and distress from multiple symptoms. Prior studies of children with earlier stage cancer and adults with advanced cancer have revealed associations between fatigue and a range of similar factors, including patient factors (e.g. age),5,33 clinical factors (e.g. anemia)34 and symptoms.7,32,35 Our findings, in conjunction with those of others, underscore the complex and multifactorial nature of fatigue.
The factors we observed to be associated with fatigue in this advanced cancer setting differ from those associated with earlier-stage fatigue. For example, we found that neither anemia nor recent receipt of any cancer treatment in the prior 10 days was associated with fatigue. This stands in contrast with findings from other studies of children/adolescents with earlier-stage cancer were more likely to report fatigue if they were anemic, on treatment or had recently received treatment.11,14,17,29,33,34,36,37 This difference may be due to the fact that for children with advanced cancer, symptom distress figures more prominently than other factors such as cancer-directed therapy or anemia.
We found that fatigue is experienced as distressing when in association with other uncontrolled physical symptoms such as nausea, cough, pain and possibly worry. The mechanism by which a child with a high burden of other symptoms is more likely to experience fatigue distress is not well understood but has been previously described.35 Suffering from a different symptom may reduce a child’s threshold for experiencing fatigue distress (and vice versa). Treatment of other symptoms (i.e. polypharmacy) may also lead to fatigue. Clinical experience suggests that suffering from uncontrolled symptoms is exhausting; these data provide evidence for this important observation.
Our findings have important implications for the care of children with advanced cancer. First, fatigue may present a high degree of burden for some children, and interventions targeting their fatigue are warranted. Second, relief of fatigue distress may be within grasp, as treatments to relieve many other distressing symptoms contributing to fatigue (e.g. nausea) exist. An important corollary of this is that fatigue need not be simply accepted by clinicians or patients. We need communication about a child’s fatigue and the strategies we may have in hand to relieve it.
Third, while a differential diagnosis for fatigue and identification and treatment of non-symptom factors is often emphasized, optimal fatigue treatment should be primarily focused on concomitant uncontrolled symptoms. The co-occurrence of fatigue with other bothersome symptoms may explain why single interventions aimed at fatigue, such as methylphenidate, have had variable success in relieving fatigue.38–41 This may also explain why intensive, multimodal treatment of multiple concurrent symptoms is known to reduce fatigue, fatigue distress and interference with functioning for adults with advanced cancer42,43 as do global approaches such as coaching interventions promoting self-care.44
This study has several strengths. First, the vast majority of symptom reports were self-reported by the child. Second, the multicenter design and sizeable sample (relative to other studies in this population) increases the generalizability of findings. Third, the MSAS permitted a deeper examination of the symptom, fatigue’s various dimensions and the burden experienced by the child, which are not one and the same.35,45 Finally, we evaluated fatigue and fatigue distress in a comprehensive manner, evaluating an array of child, clinical and symptom factors together, as a child would experience them, to better understand the nature of pediatric advanced cancer fatigue.
Our findings must be interpreted in light of the study’s limitations. First, limited diversity of the study population may have precluded detection of associations between fatigue or fatigue distress and race or ethnicity. Second, fatigue assessments were not systematically tied to chemotherapy cycles, limiting analysis of fatigue variation throughout the treatment course. Third, fatigue outcomes were at times (albeit infrequently) based on parent proxy report. Parents are, however, generally the proxy of choice, and their use prevented otherwise non-random loss of data. Because parent reports of child outcomes can be influenced by the parent’s state,45,46 future efforts involving parent proxy reporting of child symptoms would be strengthened by concomitant assessments of parents. In addition, analyses were focused on factors hypothesized a priori to be associated with fatigue; other factors not included might still impact fatigue. Finally, future efforts in this vein might also employ an instrument dedicated to fatigue assessment, thereby providing an even deeper understanding of this complex symptom.
Children with advanced cancer experience high burden from fatigue. Importantly, fatigue and associated distress are primarily related to other symptoms, which may be amenable to treatment. Thus, future interventions to mitigate suffering from fatigue should focus on overall symptom control. Such a strategy may well strengthen our ability to mitigate fatigue and improve the overall wellbeing of children with advanced cancer.
Acknowledgments
We are indebted to the children and families for their participation in the study and the contributions of Sarah Aldridge, CPNP-AC, CPHON, Lindsay Teittinen, ARNP, Janis Rice, MPH, Karen Carroll, BS, and Karina Bloom, BS, Gabriela Andrade, MD and Chris Wong, MD (enrollment, data collection); Bridget Neville, MPH (data management and coding); and Kun Chen, PhD (data analysis). We are also grateful for the DFCI Clinical Research Informatics team and Pediatric Palliative Care Research Network for their contributions.
Research support: NIH/NCI 1K07 CA096746-01, Charles H. Hood Foundation Child Health Research Award, and American Cancer Society Pilot and Exploratory Project Award in Palliative Care of Cancer Patients and Their Families (Wolfe, PI); NIH/NHLBI 1K23 HL107452 (Ullrich, PI)
Footnotes
Author Contributions
| Conceptualization | Data Curation | Formal Analysis | Funding Acquisition | Investigation | Methodology | Project Administration | Resources | Software | Supervision | Validation | Visualization | Writing- original draft | Writing- reviewing and editing | |
| Ullrich | ||||||||||||||
| Dussel | ||||||||||||||
| Orellana | ||||||||||||||
| Kang | ||||||||||||||
| Rosenberg | ||||||||||||||
| Feudtner | ||||||||||||||
| Wolfe |
Trial Registration: clinicaltrials.gov NCT01838564
Conflicts of interest: none
References
- 1.Jalmsell L, Kreicbergs U, Onelov E, Steineck G, Henter JI. Symptoms Affecting Children with Malignancies During the Last Month of Life: A Nationwide Follow-Up. Pediatrics. 2006;117(4):1314–1320. doi: 10.1542/peds.2005-1479. [DOI] [PubMed] [Google Scholar]
- 2.Wolfe J, Grier HE, Klar N, et al. Symptoms and Suffering at the End of Life in Children with Cancer. N Engl J Med. 2000;342(5):326–333. doi: 10.1056/NEJM200002033420506. [DOI] [PubMed] [Google Scholar]
- 3.Theunissen JM, Hoogerbrugge PM, van Achterberg T, Prins JB, Vernooij-Dassen MJ, van den Ende CH. Symptoms in the Palliative Phase of Children with Cancer. Pediatr Blood Cancer. 2007;49(2):160–165. doi: 10.1002/pbc.21042. [DOI] [PubMed] [Google Scholar]
- 4.Pritchard M, Burghen E, Srivastava DK, et al. Cancer-Related Symptoms Most Concerning to Parents During the Last Week and Last Day of Their Child’s Life. Pediatrics. 2008;121(5):e1301–1309. doi: 10.1542/peds.2007-2681. [DOI] [PubMed] [Google Scholar]
- 5.Van Cleve L, Munoz CE, Savedra M, et al. Symptoms in Children with Advanced Cancer: Child and Nurse Reports. Cancer Nurs. 2012;35(2):115–125. doi: 10.1097/NCC.0b013e31821aedba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.von Lutzau P, Otto M, Hechler T, Metzing S, Wolfe J, Zernikow B. Children Dying from Cancer: Parents’ Perspectives on Symptoms, Quality of Life, Characteristics of Death, and End-of-Life Decisions. J Palliat Care. 2012;28(4):274–281. [PubMed] [Google Scholar]
- 7.Ullrich CK, Dussel V, Hilden JM, et al. Fatigue in Children with Cancer at the End of Life. J Pain Symptom Manage. 2010;40(4):483–494. doi: 10.1016/j.jpainsymman.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 8.van der Geest IM, Darlington AS, Streng IC, Michiels EM, Pieters R, van den Heuvel-Eibrink MM. Parents’ Experiences of Pediatric Palliative Care and the Impact on Long-Term Parental Grief. J Pain Symptom Manage. 2014;47(6):1043–1053. doi: 10.1016/j.jpainsymman.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 9.Gibson F, Mulhall AB, Richardson A, Edwards JL, Ream E, Sepion BJ. A Phenomenologic Study of Fatigue in Adolescents Receiving Treatment for Cancer. Oncol Nurs Forum. 2005;32(3):651–660. doi: 10.1188/05.ONF.651-660. [DOI] [PubMed] [Google Scholar]
- 10.Ream E, Gibson F, Edwards J, Seption B, Mulhall A, Richardson A. Experience of Fatigue in Adolescents Living with Cancer. Cancer Nurs. 2006;29(4):317–326. doi: 10.1097/00002820-200607000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Erickson JM, Beck SL, Christian B, et al. Patterns of Fatigue in Adolescents Receiving Chemotherapy. Oncol Nurs Forum. 2010;37(4):444–455. doi: 10.1188/10.ONF.444-455. [DOI] [PubMed] [Google Scholar]
- 12.Hicks J, Bartholomew J, Ward-Smith P, Hutto CJ. Quality of Life among Childhood Leukemia Patients. J Pediatr Oncol Nurs. 2003;20(4):192–200. doi: 10.1177/1043454203253969. [DOI] [PubMed] [Google Scholar]
- 13.Hinds PS, Hockenberry-Eaton M, Gilger E, et al. Comparing Patient, Parent, and Staff Descriptions of Fatigue in Pediatric Oncology Patients. Cancer Nurs. 1999;22(4):277–288. doi: 10.1097/00002820-199908000-00004. quiz 288-279. [DOI] [PubMed] [Google Scholar]
- 14.Enskar K, von Essen L. Prevalence of Aspects of Distress, Coping, Support and Care among Adolescents and Young Adults Undergoing and Being Off Cancer Treatment. Eur J Oncol Nurs. 2007;11(5):400–408. doi: 10.1016/j.ejon.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 15.Rosenberg AR, Orellana L, Ullrich C, et al. Quality of Life in Children with Advanced Cancer: A Report from the Pediquest Study. J Pain Symptom Manage. 2016 doi: 10.1016/j.jpainsymman.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moody K, Meyer M, Mancuso CA, Charlson M, Robbins L. Exploring Concerns of Children with Cancer. Support Care Cancer. 2006;14(9):960–966. doi: 10.1007/s00520-006-0024-y. [DOI] [PubMed] [Google Scholar]
- 17.Walker AJ, Gedaly-Duff V, Miaskowski C, Nail L. Differences in Symptom Occurrence, Frequency, Intensity, and Distress in Adolescents Prior to and One Week after the Administration of Chemotherapy. J Pediatr Oncol Nurs. 2010;27(5):259–265. doi: 10.1177/1043454210365150. [DOI] [PubMed] [Google Scholar]
- 18.Hedstrom M, Ljungman G, von Essen L. Perceptions of Distress among Adolescents Recently Diagnosed with Cancer. J Pediatr Hematol Oncol. 2005;27(1):15–22. doi: 10.1097/01.mph.0000151803.72219.ec. [DOI] [PubMed] [Google Scholar]
- 19.Wolfe J, Hammel JF, Edwards KE, et al. Easing of Suffering in Children with Cancer at the End of Life: Is Care Changing? J Clin Oncol. 2008;26(10):1717–1723. doi: 10.1200/JCO.2007.14.0277. [DOI] [PubMed] [Google Scholar]
- 20.Hendricks-Ferguson V. Physical Symptoms of Children Receiving Pediatric Hospice Care at Home During the Last Week of Life. Oncol Nurs Forum. 2008;35(6):E108–115. doi: 10.1188/08.onf.e108-e115. [DOI] [PubMed] [Google Scholar]
- 21.Wolfe J, Orellana L, Cook EF, et al. Improving the Care of Children with Advanced Cancer by Using an Electronic Patient-Reported Feedback Intervention: Results from the Pediquest Randomized Controlled Trial. J Clin Oncol. 2014;32(11):1119–1126. doi: 10.1200/JCO.2013.51.5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wolfe J, Orellana L, Ullrich C, et al. Symptoms and Distress in Children with Advanced Cancer: Prospective Patient-Reported Outcomes from the Pediquest Study. J Clin Oncol. 2015;33(17):1928–1935. doi: 10.1200/JCO.2014.59.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dussel V, Orellana L, Soto N, et al. Feasibility of Conducting a Palliative Care Randomized Controlled Trial in Children with Advanced Cancer: Assessment of the Pediquest Study. J Pain Symptom Manage. 2015;49(6):1059–1069. doi: 10.1016/j.jpainsymman.2014.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Collins JJ, Byrnes ME, Dunkel IJ, et al. The Measurement of Symptoms in Children with Cancer. J Pain Symptom Manage. 2000;19(5):363–377. doi: 10.1016/s0885-3924(00)00127-5. [DOI] [PubMed] [Google Scholar]
- 25.Collins JJ, Devine TD, Dick GS, et al. The Measurement of Symptoms in Young Children with Cancer: The Validation of the Memorial Symptom Assessment Scale in Children Aged 7-12. J Pain Symptom Manage. 2002;23(1):10–16. doi: 10.1016/s0885-3924(01)00375-x. [DOI] [PubMed] [Google Scholar]
- 26.Drake R, Frost J, Collins JJ. The Symptoms of Dying Children. J Pain Symptom Manage. 2003;26(1):594–603. doi: 10.1016/s0885-3924(03)00202-1. [DOI] [PubMed] [Google Scholar]
- 27.Tomlinson D, Hinds PS, Bartels U, Hendershot E, Sung L. Parent Reports of Quality of Life for Pediatric Patients with Cancer with No Realistic Chance of Cure. J Clin Oncol. 2011;29(6):639–645. doi: 10.1200/JCO.2010.31.4047. [DOI] [PubMed] [Google Scholar]
- 28.Hinds PS, Nuss SL, Ruccione KS, et al. Promis Pediatric Measures in Pediatric Oncology: Valid and Clinically Feasible Indicators of Patient-Reported Outcomes. Pediatr Blood Cancer. 2013;60(3):402–408. doi: 10.1002/pbc.24233. [DOI] [PubMed] [Google Scholar]
- 29.Perdikaris P, Merkouris A, Patiraki E, Tsoumakas K, Vasilatou-Kosmidis E, Matziou V. Evaluating Cancer Related Fatigue During Treatment According to Children’s, Adolescents’ and Parents’ Perspectives in a Sample of Greek Young Patients. Eur J Oncol Nurs. 2009;13(5):399–408. doi: 10.1016/j.ejon.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 30.Spathis A, Booth S, Grove S, Hatcher H, Kuhn I, Barclay S. Teenage and Young Adult Cancer-Related Fatigue Is Prevalent, Distressing, and Neglected: It Is Time to Intervene. A Systematic Literature Review and Narrative Synthesis. J Adolesc Young Adult Oncol. 2015;4(1):3–17. doi: 10.1089/jayao.2014.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Atay S, Conk Z, Bahar Z. Identifying Symptom Clusters in Paediatric Cancer Patients Using the Memorial Symptom Assessment Scale. Eur J Cancer Care (Engl) 2012;21(4):460–468. doi: 10.1111/j.1365-2354.2012.01324.x. [DOI] [PubMed] [Google Scholar]
- 32.Smith AW, Bellizzi KM, Keegan TH, et al. Health-Related Quality of Life of Adolescent and Young Adult Patients with Cancer in the United States: The Adolescent and Young Adult Health Outcomes and Patient Experience Study. J Clin Oncol. 2013;31(17):2136–2145. doi: 10.1200/JCO.2012.47.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hinds PS, Hockenberry M, Tong X, et al. Validity and Reliability of a New Instrument to Measure Cancer-Related Fatigue in Adolescents. J Pain Symptom Manage. 2007;34(6):607–618. doi: 10.1016/j.jpainsymman.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller E, Jacob E, Hockenberry MJ. Nausea, Pain, Fatigue, and Multiple Symptoms in Hospitalized Children with Cancer. Oncol Nurs Forum. 2011;38(5):E382–393. doi: 10.1188/11.ONF.E382-E393. [DOI] [PubMed] [Google Scholar]
- 35.Perdikaris P, Merkouris A, Patiraki E, Papadatou D, Vasilatou-Kosmidis H, Matziou V. Changes in Children’s Fatigue During the Course of Treatment for Paediatric Cancer. Int Nurs Rev. 2008;55(4):412–419. doi: 10.1111/j.1466-7657.2008.00644.x. [DOI] [PubMed] [Google Scholar]
- 36.Erickson JM, Beck SL, Christian BR, et al. Fatigue, Sleep-Wake Disturbances, and Quality of Life in Adolescents Receiving Chemotherapy. J Pediatr Hematol Oncol. 2011;33(1):e17–25. doi: 10.1097/MPH.0b013e3181f46a46. [DOI] [PubMed] [Google Scholar]
- 37.Bruera E, Yennurajalingam S, Palmer JL, et al. Methylphenidate and/or a Nursing Telephone Intervention for Fatigue in Patients with Advanced Cancer: A Randomized, Placebo-Controlled, Phase Ii Trial. J Clin Oncol. 2013;31(19):2421–2427. doi: 10.1200/JCO.2012.45.3696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bruera E, Valero V, Driver L, et al. Patient-Controlled Methylphenidate for Cancer Fatigue: A Double-Blind, Randomized, Placebo-Controlled Trial. J Clin Oncol. 2006;24(13):2073–2078. doi: 10.1200/JCO.2005.02.8506. [DOI] [PubMed] [Google Scholar]
- 39.Escalante CP, Meyers C, Reuben JM, et al. A Randomized, Double-Blind, 2-Period, Placebo-Controlled Crossover Trial of a Sustained-Release Methylphenidate in the Treatment of Fatigue in Cancer Patients. Cancer J. 2014;20(1):8–14. doi: 10.1097/PPO.0000000000000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roth AJ, Nelson C, Rosenfeld B, et al. Methylphenidate for Fatigue in Ambulatory Men with Prostate Cancer. Cancer. 2010;116(21):5102–5110. doi: 10.1002/cncr.25424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Raaf PJ, de Klerk C, Timman R, Busschbach JJ, Oldenmenger WH, van der Rijt CC. Systematic Monitoring and Treatment of Physical Symptoms to Alleviate Fatigue in Patients with Advanced Cancer: A Randomized Controlled Trial. J Clin Oncol. 2013;31(6):716–723. doi: 10.1200/JCO.2012.44.4216. [DOI] [PubMed] [Google Scholar]
- 42.Given B, Given CW, McCorkle R, et al. Pain and Fatigue Management: Results of a Nursing Randomized Clinical Trial. Oncol Nurs Forum. 2002;29(6):949–956. doi: 10.1188/02.ONF.949-956. [DOI] [PubMed] [Google Scholar]
- 43.Ream E, Richardson A, Alexander-Dann C. Supportive Intervention for Fatigue in Patients Undergoing Chemotherapy: A Randomized Controlled Trial. J Pain Symptom Manage. 2006;31(2):148–161. doi: 10.1016/j.jpainsymman.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 44.Poder U, Ljungman G, von Essen L. Parents’ Perceptions of Their Children’s Cancer-Related Symptoms During Treatment: A Prospective, Longitudinal Study. J Pain Symptom Manage. 2010;40(5):661–670. doi: 10.1016/j.jpainsymman.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 45.Parsons SK, Shih MC, Duhamel KN, et al. Maternal Perspectives on Children’s Health-Related Quality of Life During the First Year after Pediatric Hematopoietic Stem Cell Transplant. J Pediatr Psychol. 2006;31(10):1100–1115. doi: 10.1093/jpepsy/jsj078. [DOI] [PubMed] [Google Scholar]


