Abstract
Morphogenesis describes the developmental processes that reorganize groups of cells into functional tissues and organs. The spatiotemporal patterning of individual cell behaviors is influenced by how cells perceive and respond to mechanical forces, and determines final tissue architecture. Here, we review recent work examining the physical mechanisms of tissue morphogenesis in vertebrate and invertebrate models, discuss how epithelial cells employ contractility to induce global changes that lead to tissue folding, and describe how tissue form itself is a regulator of cell behavior. We then highlight novel tools to recapitulate these processes in engineered tissues.
Keywords: morphodynamics, dynamic reciprocity, epithelial folding, mechanical stress
Introduction
Morphogenesis determines the unique shape and correct positioning of tissues and organs in the body. Just as all cells come from cells (“omnis cellula e cellula”) [1], all tissues come from cells that contain essentially the same genetic information. Many of the signaling pathways that control organ morphogenesis are conserved across species [2], and common changes in cell adhesion, cell shape, and cell migration drive context-dependent outcomes on a tissue scale. Nonetheless, every tissue exhibits a distinct architecture and function, which indicates that cells integrate information from signaling networks and mechanical cues in a context-dependent manner to determine the physical output of gene expression [3,4].
The spatiotemporal control of morphogenetic processes accommodates and is driven by surface area and volume constraints to give rise to various tissue architectures from arborized networks of blood vessels, neurons, and bronchial tubes to vilified epithelial sheets. In order to meet mass-transport requirements, most animals employ a network of interconnected epithelial tubes with barrier and secretory functions [5]. For instance, the human vascular network enables about five liters of blood to be delivered to tissues each minute [6], while the arborized structure of the lungs maximizes the surface area for gas exchange at the alveolar tips to enable the oxygenation of blood. How groups of epithelial cells form polarized sheets that buckle and bend in response to mechanical and biochemical cues, and thus acquire various shapes and functions, remains mostly a mystery. It is well appreciated, however, that the generation and maintenance of proper tissue architecture is required for homeostasis whereas its loss is a prerequisite for disease [3].
Studies of model organisms and cultured tissues have provided key insights into how mechanical forces generated at the cellular level are integrated with biochemical cues to convert gene expression patterns into sophisticated tissue structures in a context-dependent manner. Most of our understanding of morphogenetic processes emanates from well-defined invertebrate models because of widely available genetic and molecular tools. A well-studied example is the formation of the ventral furrow during Drosophila gastrulation, during which the tension generated by actomyosin contractility across the apical surface of a sheet leads to apical constriction and localized tissue folding [7–9]. This requires dynamic changes in actomyosin contractility at the molecular level to be transmitted across larger length scales through junctional domains between cells in the tissue sheet [10].
Development is choreographed such that tissue structure can be tuned in response to microenvironmental factors. The interactions between the cells that constitute a tissue and their surrounding extracellular matrix (ECM) can guide cellular behavior and changes in tissue morphology. According to the principle of dynamic reciprocity, cells communicate with the ECM through the transport of growth factors or through direct contact with membrane-associated components, and these interactions evolve over time [11]. This crosstalk has been examined extensively in the context of the mammary gland, which can undergo cycles of development, differentiation, and apoptosis in order to accommodate the temporary need to produce and deliver milk [12]. The regulation of ECM remodeling in morphogenesis has revealed that the loss of proper tissue architecture underlies malignant transformation, while reconstitution of normal tissue architecture through the restoration of healthy cell-ECM communication overrides genetic abnormalities [3,4,13–15].
Disruption of the force-generation and transmission machinery leads to aberrant tissue morphologies that underlie many congenital diseases such as defects of neural tube closure, pulmonary hypoplasia, and abnormal alveolar structures [16]. Morphogenesis of diseased tissues relies on the same signaling pathways that guide healthy development. In a way, acquired diseases such as cancers are errors of development, as Virchow asserted, since “tumors appear by the same law which regulated embryonic development” [1].
Here, we discuss how a group of undifferentiated cells employ cytoskeletal contractility, proliferation, apoptosis, and interactions with their surrounding microenvironment to generate complex and reproducible epithelial tissue architectures. We review recent work on how long-range transmission of mechanical forces molds sheets of cells into their final form, and how its dysregulation leads to the disruption of healthy tissue architecture.
Cellular contractility generates tissue bends and folds
Many morphogenetic events that remodel epithelial sheets result from dynamic cell shape changes. A well-known example is apical constriction, in which the apical surface of a cell shrinks due to the purse-string effect produced by actomyosin contractility [17]. This local change in cell geometry impacts global tissue morphology when contractile forces are transmitted across a sheet through cell-cell junctions, and its role has been implicated in cell ingression, cell extrusion, delamination, and wound healing [17,18].
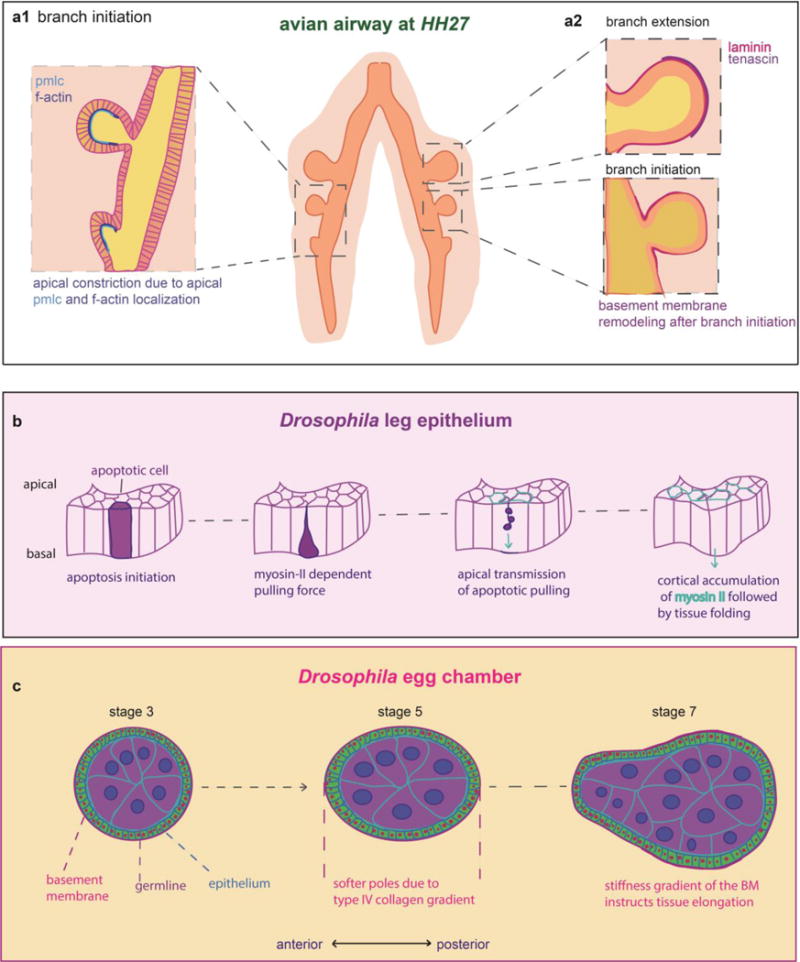
Actomyosin contractility has been shown to underlie the initiation of epithelial buds during branching morphogenesis of the chicken lung. Localization of phosphorylated myosin light chain (pMLC) and filamentous actin (f-actin) to the apical surface of the epithelium was demonstrated to induce cellular shape changes as a result of apical constriction that precede domain branching and induce branch initiation (Figure 1a). Inhibition of actomyosin contractility prevented both apical constriction and domain branching, whereas inhibiting proliferation had no effect on branch initiation [19].
Figure 1. Tissue folding arises in response to cellular contractility and physical constraints imposed by the tissue microenvironment.
A. Domain branching in the chicken lung is preceded by apical constriction [18]. B. In Drosophila, apoptotic cells pull the leg epithelium in the apicobasal direction to drive folding [24] C. The Drosophila egg chamber elongation is driven by stiffness gradients present in the BM [29]. D. Branching in the chicken lung is initiated by FAK and the extension of branches is accompanied by BM remodeling (Spurlin et al., 2018).
Ventral furrow formation in Drosophila is driven by dynamic pulsatile actomyosin contractions [7], and the coordination of these pulses leads to collective apical constriction [20], which drives individual cell shape changes. The transmission of contractile forces relies on the coupling of cell-cell junctions to actomyosin networks [21]; recently, the use of optogenetic tools to manipulate cytoskeletal contractility with spatial specificity demonstrated for the first time that depleting actin from the cortex arrested invagination of the ventral furrow [22]. Guglielmi et al. used light to modulate the levels of plasma membrane phophoinositides, or phophotidylinositol 4,5 biphosphates, which regulate cortical actin polymerization, achieving spatiotemporal control over cellular contractility. These experiments demonstrated that apical constriction is necessary to both initiate and sustain invagination [22]. Since this optogenetic approach provides spatial and temporal control over apical constriction, it could be used in other developmental systems to assess the extent of force transmission required to induce tissue folding.
Actomyosin contractility has an important role in providing the mechanical forces necessary to drive cytokinesis during cell division [23], and causes local tissue deformation by inducing cell- shape changes in apoptotic cells [24]. Recently, it was found that actomyosin contractility drives epithelial folding in the Drosophila leg by creating an apico-basally directed force in apoptotic cells. Following the initiation of apoptosis, it was observed that a cable-like myosin II structure in apoptotic cells deforms the apical surface of the epithelium through myosin II-dependent pulling (Figure 1b). This force then propagates throughout the fold domain via adherens junctions, and finally, the distribution of apoptotic events within the fold domain leads to a global redistribution of myosin II to induce epithelial folding [25].
Cellular contractility drives the initiation of unique tissue patterns, and it is in turn modulated by predefined spatial constraints. During ventral furrow formation in Drosophila, mechanical constraints imposed by the ellipsoid shape of the embryo lead to anisotropic tension along its long axis, causing the actomyosin meshwork to be aligned along the anterior-posterior direction, and leads to ventral furrow formation [26]. These findings point to the reciprocal nature of mechanosensing, since actomyosin contractility can drive tissue folding, but results as a consequence of mechanical constraints imposed by the microenvironment.
Reciprocal interactions between cells and their surrounding microenvironment determine final tissue architecture
Crosstalk between cells and their surrounding microenvironment dictates the varying patterns of cell shape changes, proliferation, apoptosis, and rearrangement of cells within an epithelial sheet. The basement membrane (BM), a specialized type of ECM comprised mainly of laminin, collagen IV, and several large glycoproteins, separates the epithelium from its surrounding mesenchyme [27]. During branching morphogenesis of organs such as the lung, salivary gland, and mammary gland, the epithelium expands rapidly while still being enveloped within a BM [19,28,29].
Recent work has shown that the mechanical properties of the BM dictate organ shape. A stiffness gradient present within the BM was found to determine the aspect ratio of the Drosophila egg chamber (Figure 1c), causing the initially spherical structure to elongate into a football-like shape [30]. It was determined that type IV collagen stiffens the BM, which in turn sculpts the egg chamber. The stiffening behavior of type IV collagen could have implications for branching morphogenesis of vertebrate tissues, including the salivary and mammary glands, since collagen IV is abundant in these BMs as well.
In murine salivary gland morphogenesis, the BM surrounding an emerging branch becomes perforated around the expanding tip, and also translocates towards the stalk to support and sculpt the extending branch. It has been suggested that the perforation of the BM is made possible by myosin-II-dependent pulling as well as protease activity [27]. Similarly, in the embryonic chicken lung, thinning of the BM accompanies branch extension, and BM remodeling persists throughout branch development. Specifically, the distribution of BM proteins tenascin C and laminin changes during branch initiation, suggesting a role for the BM in shaping the developing branch (Spurlin et al., 2018) (Figure 1d). These findings suggest that the BM is not a static scaffold, and that communication between the epithelium and the mesenchyme patterns morphogenesis of these organs [31].
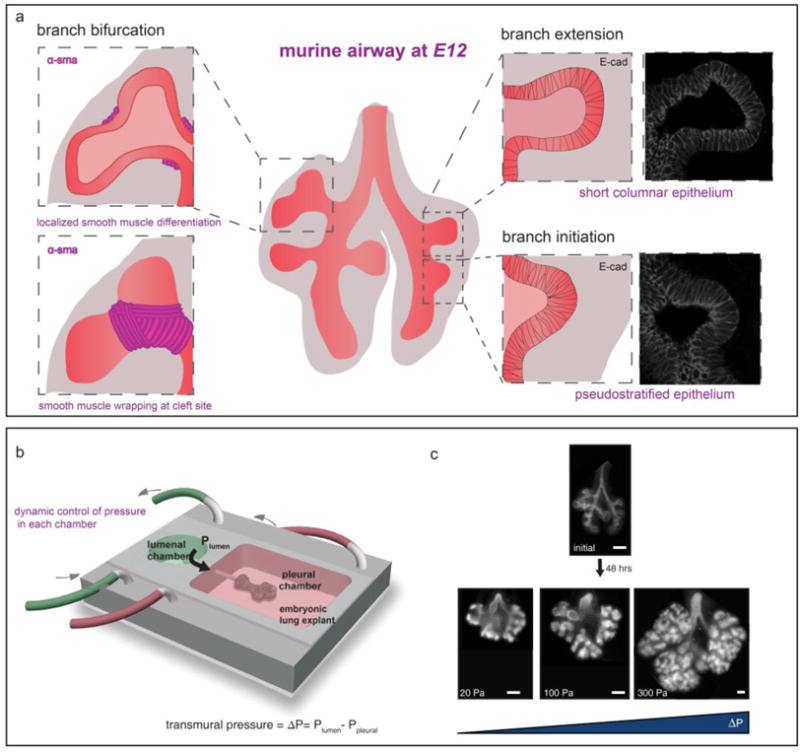
Morphogenesis of the looping structure of the murine gut also requires crosstalk between the growing epithelium and the surrounding mesenchyme. In this case, the developing smooth muscle functions as a stiff sheath in the mesenchyme that compresses the expanding epithelial tube, causing it to buckle inwards to give rise to the ridges that later form intestinal villi [32]. Local smooth muscle differentiation that impacts the mechanical properties of the mesenchyme surrounding the murine airway epithelium also guides its branching morphogenesis. The developing lung emerges from the ventral surface of the foregut endoderm, and is initially a simple epithelial tube surrounded by mesenchyme [33]. New branches emerge sequentially through domain branching followed by orthogonal and planar bifurcations at branch tips [34,35]. The mesenchyme surrounding the airway epithelium sculpts bifurcations of the extending branch through the localized differentiation of alpha-smooth muscle actin (αSMA)-expressing airway smooth muscle cells (Figure 2) [36]. A similar mechanism could underlie domain branching of the mouse lung, which is known to be pseudostratified during branch initiation (Figure 2b). These findings suggest that morphogenesis of the mouse airway and intestinal epithelia are both controlled by their surrounding mechanical microenvironments.
Figure 2. Branching morphogenesis is regulated by mechanical forces imposed by its surrounding microenvironment.
A. Airway branching in the mouse lung is accompanied by localized smooth muscle differentiation at bifurcating tips [34] and stratification of the epithelium during domain branching B. The branching rate of the murine airway epithelium can be modulated using a microfluidic transmural pressure device [37]
In addition to ECM and smooth muscle, epithelial morphogenesis can be instructed by mechanical signals from fluid pressure. Transmural pressure, which is the difference between the pressure inside and outside of an epithelial tube, was recently shown to control the rate of airway epithelial branching in the mouse lung [37]. Microfluidic chest cavities were used to culture embryonic lungs under a range of transmural pressures that represented those observed during normal development and disease. In lungs cultured under low transmural pressure, few new branches formed, whereas under higher pressure, the lungs developed the stereotyped branching pattern that forms in vivo, demonstrating that the rate of epithelial morphogenesis depends on pressure across the fetal lung (Figure 2c). Transmural pressure was also found to govern contraction of airway smooth muscle [37], which suggests that increasing the frequency of smooth muscle contractions could revert the progression of congenital diseases such as airway hypoplasia, a condition in which fetal lungs are under-branched. The mechanical microenvironment facilitates crosstalk between developing or already-patterned epithelia and their surrounding tissues, and is therefore crucial for driving morphogenesis or maintaining homeostasis.
Measuring forces in a physiological context
Although many of the cellular structures that generate and transmit force are known, and the role of the mechanical microenvironment in tissue morphogenesis is widely recognized, it has only recently become possible to measure the mechanical forces exerted by cells on their native microenvironments. Early investigations of cellular mechanics relied primarily on reductionist approaches carried out in culture such as laser ablation. This technique was initially used to sever small portions of the actomyosin network in order to provide insight into the force-generation machinery [38], and to demonstrate that local modifications in actomyosin contractility induce small changes in cell-cell and cell-ECM adhesions that lead to changes in cell shape, which can in turn induce tissue-scale outcomes [21]. Since then, laser ablation has been adapted to in vivo model systems, such as the Drosophila embryo, in order to provide a qualitative sense of how contractile forces are transmitted across developing tissues [39,40].
Even though laser ablation is a useful method that qualitatively reveals cellular tension in different tissue contexts, it does not provide quantitative information about cellular forces that might be at play during tissue development or disease progression. Recently, Campàs and colleagues developed ferrofluid oil droplets –or microrheometers- that can be injected into live tissues to measure the mechanical properties of the tissue surrounding the droplet, allowing one to infer the cellular forces within native tissues based on the deformation of the oil droplet (which has known shape and viscoelastic properties) [41,42]. This technique has been further developed to actively deform the droplet with the help of a magnetic field, and measure the mechanical response of the surrounding tissue. During tailbud elongation in the zebrafish embryo, which is used as a model system for vertebrate body axis elongation, the viscosity and stiffness of the tissue varied along the anterior-posterior axis, with the elongating posterior region displaying lower tissue stiffness and increased fluidity, suggesting that the spatial variations in viscoelastic properties could be instrumental in tissue patterning. Moreover, the use of ferrofluid oil droplets in tissues that have lost their normal architecture (e.g. tumors) could shed light on the mechanical changes that take place in the microenvironment during disease progression.
These methods, although disruptive, have contributed to our understanding of the cellular structures that sense and transmit mechanical information. Currently, non-invasive methods such as Förster resonance energy transfer (FRET)-based sensors, which do not deform the cell or tissue, are being used to study invertebrate development [43,44] and are in the process of being adapted for the study of force transmission in living vertebrate embryos. These sensors employ two fluorophores that are linked by a spring-like peptide that can be compressed or stretched reversibly by intra- or extracellular forces depending on where the force sensor is anchored. Besides providing insight into the role of mechanical forces in embryonic development, these techniques may also promote the engineering of biomaterials that effectively mimic various in vivo mechanical microenvironments, and offer new avenues to investigate the role of mechanics in disease progression.
Towards building organs from scratch
Epithelial folding is a highly complex yet reproducible process in vivo. The ability to recapitulate epithelial folding and organ development in culture would greatly accelerate understanding of the underlying mechanisms and screening of therapeutics. Current efforts in 3D tissue culture models are directed towards recapitulating the complexity of mammalian organogenesis by engineering stem cells, constructing biomimetic materials, and directing tissue architecture in novel culture systems [45]. Over the past century, these efforts have evolved from culturing tissue fragments [46–48], and generating organ-like structures in suspension from dissociated cells [49], to recognizing the role of the ECM in orchestrating tissue morphogenesis [50–52]. 3D collagen or laminin-rich cultures [53,54], micropatterning approaches [3], and more recently, advances in stem cell engineering have paved the way for organoid models of epithelial tissues [55].
Programming of stem cells requires profound understanding of how single cells can be ordered to assemble into epithelial structures, as in the establishment of the long-term culture of multipotent Lgr5+ stem cells that gave rise to “mini guts” [56]. Advances in stem cell engineering have paved the way for organoid models of epithelial tissues. Recently, the directed differentiation of human pluripotent stem cells into progenitors of the ureteric epithelium or the metanephric mesenchyme that give rise to collecting ducts and nephrons, respectively, has led to human kidney organoid cultures that contain renal tubules [57]. These organoid models have several advantages, including accessibility through high-resolution imaging, temporal control, and genetic manipulation, and are promising models of human disease [58,59]. However, organoids are not perfect in the sense that they can only recapitulate certain stages of development and are often difficult to reproduce [60]. In order to induce the development of more refined structures, the characteristics of a microenvironment that can support the differentiation and self-organization of stem cells into organoids need to be determined.
The spatiotemporal distribution of microenvironmental signals determines how these cues will be received and interpreted by cells. For example, intestinal stem cell survival, proliferation, and self-organization can be modulated by synthetic hydrogel networks with tunable ECM stiffness [61], a mechanical property that can now be controlled with spatial precision by modulating the crosslinking of polyethylene glycol (PEG) hydrogels [62]. Such biomimetic scaffolds were shown to allow for intestinal stem cell survival and organoid formation [61]. However, inducing tissue folding in vitro to achieve the complexity of mammalian organogenesis still poses a significant challenge. Recently, inspired by the local strain differences that arise between the folding epithelium and the underlying tissue in many morphogenetic processes, Hughes et al. devised an approach to pattern fibroblasts on ECM-based gels, and observed that these cells, by pulling on the surrounding ECM fibers, created local strains at the epithelial-mesenchymal interface which led to epithelial folding at precise locations similar to the patterning of the mouse gut [63]. The endeavors to mimic cell-cell and cell-ECM interactions in native cellular microenvironments and to instruct self-organization of epithelial sheets in vitro could guide future efforts towards fabrication of tissues that have physiological function.
Conclusions
Cells interact with their surrounding microenvironment in a reciprocal manner, and these interactions are often inhomogeneous, anisotropic, and transient. The spatiotemporal regulation of how mechanical and biochemical signals are perceived and transmitted by cells sculpts epithelial sheets into tissues and organs with unique bends, folds, and curves to accommodate their function. These complex interactions can be partially recapitulated using 3D models, which are becoming more sophisticated with the advent of organoids and engineered hydrogels.
Acknowledgments
Work from the authors’ group was supported by grants from the NIH (GM083997, HL110335, HL118532, HL120142, and CA187692), the NSF (CMMI-1435853), the David & Lucile Packard Foundation, the Alfred P. Sloan Foundation, the Camille & Henry Dreyfus Foundation, and the Burroughs Wellcome Fund. A.A.A. was supported in part by a pre-doctoral fellowship from the New Jersey Commission on Cancer Research. C.M.N. holds a Faculty Scholars Award from the HHMI.
Abbreviations
- αSMA
alpha-smooth muscle actin
- BM
basement membrane
- ECM
extracellular matrix
- f-actin
filamentous actin
- FRET
Förster resonance energy transfer
- PEG
polyethylene glycol
- pMLC
phosphorylated myosin light-chain
- ROCK
Rho-associated protein kinase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Virchow RLK. Cellular Pathology. 1858 [Google Scholar]
- 2.Davies JA. Do different branching epithelia use a conserved developmental mechanism? BioEssays. 2002;24:937–948. doi: 10.1002/bies.10161. [DOI] [PubMed] [Google Scholar]
- 3.Nelson CM, Bissell MJ. Of Extracellular Matrix, Scaffolds, and Signaling: Tissue Architecture Regulates Development, Homeostasis, and Cancer. Annual Review of Cell and Developmental Biology. 2006;22:287–309. doi: 10.1146/annurev.cellbio.22.010305.104315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu R, Boudreau A, Bissell MJ. Tissue architecture and function: dynamic reciprocity via extra- and intra-cellular matrices. Cancer and Metastasis Reviews. 2009;28:167–176. doi: 10.1007/s10555-008-9178-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nelson CM. On Buckling Morphogenesis. Journal of Biomechanical Engineering. 2016;138:021005. doi: 10.1115/1.4032128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shier D, Butler J, Lewis R. Hole’s human anatomy & physiology. McGraw-Hill; 2007. [Google Scholar]
- 7.Martin AC, Kaschube M, Wieschaus EF. Pulsed contractions of an actin–myosin network drive apical constriction. Nature. 2009;457:495–499. doi: 10.1038/nature07522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weng M, Wieschaus E. Myosin-dependent remodeling of adherens junctions protects junctions from Snail-dependent disassembly. The Journal of Cell Biology. 2016;212:219–229. doi: 10.1083/jcb.201508056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He B, Doubrovinski K, Polyakov O, Wieschaus E. Apical constriction drives tissue-scale hydrodynamic flow to mediate cell elongation. Nature. 2014;508:392–396. doi: 10.1038/nature13070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siedlik MJ, Nelson CM. Regulation of tissue morphodynamics: an important role for actomyosin contractility. Current Opinion in Genetics & Development. 2015;32:80–85. doi: 10.1016/j.gde.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bissell MJ, Hall HG, Parry G. How does the extracellular matrix direct gene expression? Journal of Theoretical Biology. 1982;99:31–68. doi: 10.1016/0022-5193(82)90388-5. [DOI] [PubMed] [Google Scholar]
- 12.Gjorevski N, Nelson CM. Integrated morphodynamic signalling of the mammary gland. 2011:12. doi: 10.1038/nrm3168. [DOI] [PubMed] [Google Scholar]
- 13.Bissell MJ, Rizki A, Mian IS. Tissue architecture: the ultimate regulator of breast epithelial function. Current Opinion in Cell Biology. 2003;15:753–762. doi: 10.1016/j.ceb.2003.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vidi P-A, Bissell MJ, Lelièvre SA. Three-Dimensional Culture of Human Breast Epithelial Cells: The How and the Why. In: Randell SH, Fulcher ML, editors. Epithelial Cell Culture Protocols. Humana Press; 2012. pp. 193–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weaver VM, Petersen OW, Wang F, Larabell CA, Briand P, Damsky C, Bissell MJ. Reversion of the Malignant Phenotype of Human Breast Cells in Three-Dimensional Culture and In Vivo by Integrin Blocking Antibodies. The Journal of Cell Biology. 1997;137:231–245. doi: 10.1083/jcb.137.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gleghorn JP, Nelson CM. Sculpting Organs: Mechanical Regulation of Tissue Development. 2012;14:129–154. doi: 10.1146/annurev-bioeng-071811-150043. [DOI] [PubMed] [Google Scholar]
- 17.Martin AC, Goldstein B. Apical constriction: themes and variations on a cellular mechanism driving morphogenesis. Development. 2014;141:1987–1998. doi: 10.1242/dev.102228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heisenberg C-P, Bellaïche Y. Forces in Tissue Morphogenesis and Patterning. Cell. 2013;153:948–962. doi: 10.1016/j.cell.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 19.Kim HY, Varner VD, Nelson CM. Apical constriction initiates new bud formation during monopodial branching of the embryonic chicken lung. Development. 2013;140:3146–3155. doi: 10.1242/dev.093682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xie S, Martin AC. Intracellular signalling and intercellular coupling coordinate heterogeneous contractile events to facilitate tissue folding. Nature Communications. 2015;6:7161. doi: 10.1038/ncomms8161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin AC, Gelbart M, Fernandez-Gonzalez R, Kaschube M, Wieschaus EF. Integration of contractile forces during tissue invagination. The Journal of Cell Biology. 2010;188:735–749. doi: 10.1083/jcb.200910099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guglielmi G, Barry Joseph D, Huber W, De Renzis S. An Optogenetic Method to Modulate Cell Contractility during Tissue Morphogenesis. Developmental Cell. 2015;35:646–660. doi: 10.1016/j.devcel.2015.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murrell M, Oakes PW, Lenz M, Gardel ML. Forcing cells into shape: the mechanics of actomyosin contractility. Nature Reviews Molecular Cell Biology. 2015;16:486–498. doi: 10.1038/nrm4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Toyama Y, Peralta XG, Wells AR, Kiehart DP, Edwards GS. Apoptotic Force and Tissue Dynamics During Drosophila Embryogenesis. Science. 2008;321:1683–1686. doi: 10.1126/science.1157052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25*.Monier B, Gettings M, Gay G, Mangeat T, Schott S, Guarner A, Suzanne M. Apico-basal forces exerted by apoptotic cells drive epithelium folding. Nature. 2015;518:245–248. doi: 10.1038/nature14152. Authors demonstrate that the apoptotic cells in the Drosophila leg epithelium have actively contribute to its folding through a dynamic apico-basal myosin-II cable. Apical localization of myosin-II in apoptotic cells is shown to exert transient forces across the apical surface of the epithelial sheet and drive tissue folding. [DOI] [PubMed] [Google Scholar]
- 26*.Chanet S, Miller CJ, Vaishnav ED, Ermentrout B, Davidson LA, Martin AC. Actomyosin meshwork mechanosensing enables tissue shape to orient cell force. Nature Communications. 2017;8:15014. doi: 10.1038/ncomms15014. This study demonstrates that the ellipsoid shape of the Drosophila embryo imposes a mechanical constraint that promotes anisotropic tension throughout the embryo to ensure successful invagination during ventral furrow formation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harunaga JS, Doyle AD, Yamada KM. Local and global dynamics of the basement membrane during branching morphogenesis require protease activity and actomyosin contractility. Developmental Biology. 2014;394:197–205. doi: 10.1016/j.ydbio.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Daley WP, Yamada KM. ECM-modulated cellular dynamics as a driving force for tissue morphogenesis. Current Opinion in Genetics & Development. 2013;23:408–414. doi: 10.1016/j.gde.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fata JE, Werb Z, Bissell MJ. Regulation of mammary gland branching morphogenesis by the extracellular matrix and its remodeling enzymes. Breast Cancer Research. 2003;6 doi: 10.1186/bcr634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30*.Crest J, Diz-Muñoz A, Chen D-Y, Fletcher DA, Bilder D. Organ sculpting by patterned extracellular matrix stiffness. eLife. 2017:6. doi: 10.7554/eLife.24958. Using atomic force microscopy to measure the stiffness of the egg chamber, the authors found that the stiffness gradient in the basement membrane forms prior to egg chamber elongation, and that the gradient matches the distribution of type IV collagen around the embryo. This study demonstrates that the Drosophila egg chamber elongation is dictated in part by a stiffness gradient imposed by the basement membrane. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Isabella Adam J, Horne-Badovinac S. Rab10-Mediated Secretion Synergizes with Tissue Movement to Build a Polarized Basement Membrane Architecture for Organ Morphogenesis. Developmental Cell. 2016;38:47–60. doi: 10.1016/j.devcel.2016.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shyer AE, Tallinen T, Nerurkar NL, Wei Z, Gil ES, Kaplan DL, Tabin CJ, Mahadevan L. Villification: How the Gut Gets Its Villi. Science. 2013;342:212–218. doi: 10.1126/science.1238842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Warburton D, Schwarz M, Tefft D, Flores-Delgado G, Anderson KD, Cardoso WV. The molecular basis of lung morphogenesis. Mechanisms of Development. 2000;92:55–81. doi: 10.1016/s0925-4773(99)00325-1. [DOI] [PubMed] [Google Scholar]
- 34.Metzger RJ, Klein OD, Martin GR, Krasnow MA. The branching programme of mouse lung development. Nature. 2008;453:745–750. doi: 10.1038/nature07005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spurlin JW, Nelson CM. Building branched tissue structures: from single cell guidance to coordinated construction. Philosophical Transactions of the Royal Society B: Biological Sciences. 2017;372:20150527. doi: 10.1098/rstb.2015.0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36**.Kim Hye Y, Pang M-F, Varner Victor D, Kojima L, Miller E, Radisky Derek C, Nelson Celeste M. Localized Smooth Muscle Differentiation Is Essential for Epithelial Bifurcation during Branching Morphogenesis of the Mammalian Lung. Developmental Cell. 2015;34:719–726. doi: 10.1016/j.devcel.2015.08.012. This paper reveals that αSMA-expressing cells in the embryonic mouse lung are physical mediators of terminal bifurcation in the mouse lung. It is shown that stereotyped patterning of airway smooth muscle drives terminal bifurcations during branching morphogenesis, and that αSMA-expressing cells localize near the epithelium where terminal bifurcations will occur, then wrap around the cleft site to sculpt the extending bifurcations. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37**.Nelson CM, Gleghorn JP, Pang M-F, Jaslove JM, Goodwin K, Varner VD, Miller E, Radisky DC, Stone HA. Microfluidic chest cavities reveal that transmural pressure controls the rate of lung development. Development. 2017;144:4328–4335. doi: 10.1242/dev.154823. Authors use microfluidic chest cavities to culture embryonic mouse lungs and demonstrate that the mechanical properties of the tissue microenvironment control the rate of branching. Embryonic lungs are cultured under various pressure conditions, and it is shown that transmural pressure regulates the rate of branching, as well as the frequency of smooth muscle contractions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paluch E, Heisenberg C-P. Biology and Physics of Cell Shape Changes in Development. Current Biology. 2009;19:R790–R799. doi: 10.1016/j.cub.2009.07.029. [DOI] [PubMed] [Google Scholar]
- 39.Fernandez-Gonzalez R, Simoes SdM, Röper J-C, Eaton S, Zallen JA. Myosin II Dynamics Are Regulated by Tension in Intercalating Cells. Developmental Cell. 2009;17:736–743. doi: 10.1016/j.devcel.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goodwin K, Ellis Stephanie J, Lostchuck E, Zulueta-Coarasa T, Fernandez-Gonzalez R, Tanentzapf G. Basal Cell-Extracellular Matrix Adhesion Regulates Force Transmission during Tissue Morphogenesis. Developmental Cell. 2016;39:611–625. doi: 10.1016/j.devcel.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 41**.Campàs O, Mammoto T, Hasso S, Sperling RA, O’Connell D, Bischof AG, Maas R, Weitz DA, Mahadevan L, Ingber DE. Quantifying cell-generated mechanical forces within living embryonic tissues. Nature Methods. 2013;11:183–189. doi: 10.1038/nmeth.2761. The authors develop ferromagnetic oil droplets that be injected into live tissues and used to apply controlled forces to it. This technique provides the means to measure how the mechanical properties of embryonic tissues vary spatiotemporally during development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Serwane F, Mongera A, Rowghanian P, Kealhofer DA, Lucio AA, Hockenbery ZM, Campàs O. In vivo quantification of spatially varying mechanical properties in developing tissues. Nature Methods. 2016;14:181–186. doi: 10.1038/nmeth.4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamashita S, Tsuboi T, Ishinabe N, Kitaguchi T, Michiue T. Wide and high resolution tension measurement using FRET in embryo. Scientific Reports. 2016;6 doi: 10.1038/srep28535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lagendijk AK, Gomez GA, Baek S, Hesselson D, Hughes WE, Paterson S, Conway DE, Belting H-G, Affolter M, Smith KA, et al. Live imaging molecular changes in junctional tension upon VE-cadherin in zebrafish. Nature Communications. 2017;8 doi: 10.1038/s41467-017-01325-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khademhosseini A, Langer R. A decade of progress in tissue engineering. Nature Protocols. 2016;11:1775–1781. doi: 10.1038/nprot.2016.123. [DOI] [PubMed] [Google Scholar]
- 46.Harrison RG. Observations on the living developing nerve fiber. Exp Biol Med. 1906;4:140–143. [Google Scholar]
- 47.Strangeways TSP, Fell HB. Experimental Studies on the Differentiation of Embryonic Tissues Growing in vivo and in vitro.–II. The Development of the Isolated Early Embryonic Eye of the Fowl when Cultivated in vitro. Proc R Soc B Biol Sci. 1926;100:273–283. [Google Scholar]
- 48.Fell HB, Robison R. The growth, development and phosphatase activity of embryonic avian femora and limb-buds cultivated in vitro. Biochem J. 1929;23:767–784. 765. doi: 10.1042/bj0230767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moscona A, Moscona H. The dissociation and aggregation of cells from organ rudiments of the early chick embryo. J Anat. 1952;86:287–301. [PMC free article] [PubMed] [Google Scholar]
- 50.Orkin RW, Gehron P, McGoodwin EB, Martin GR, Valentine T, Swarm R. A murine tumor producing a matrix of basement membrane. J Exp Med. 1977;145:204–220. doi: 10.1084/jem.145.1.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee EY, Lee WH, Kaetzel CS, Parry G, Bissell MJ. Interaction of mouse mammary epithelial cells with collagen substrata: regulation of casein gene expression and secretion. Proc Natl Acad Sci U S A. 1985;82:1419–1423. doi: 10.1073/pnas.82.5.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bissell DM, Arenson DM, Maher JJ, Roll FJ. Support of cultured hepatocytes by a laminin-rich gel. Evidence for a functionally significant subendothelial matrix in normal rat liver. J Clin Invest. 1987;79:801–812. doi: 10.1172/JCI112887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Simian M, Hirai Y, Navre M, Werb Z, Lochter A, Bissell MJ. The interplay of matrix metalloproteinases, morphogens and growth factors is necessary for branching of mammary epithelial cells. Development. 2001;128:3117–3131. doi: 10.1242/dev.128.16.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fata JE, Mori H, Ewald AJ, Zhang H, Yao E, Werb Z, Bissell MJ. The MAPK(ERK-1,2) pathway integrates distinct and antagonistic signals from TGFalpha and FGF7 in morphogenesis of mouse mammary epithelium. Dev Biol. 2007;306:193–207. doi: 10.1016/j.ydbio.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lancaster MA, Knoblich JA. Organogenesis in a dish: modeling development and disease using organoid technologies. Science. 2014;345:1247125. doi: 10.1126/science.1247125. [DOI] [PubMed] [Google Scholar]
- 56.Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 57.Takasato M, Er PX, Chiu HS, Maier B, Baillie GJ, Ferguson C, Parton RG, Wolvetang EJ, Roost MS, Lopes SMCdS, et al. Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature. 2016;536:238–238. doi: 10.1038/nature17982. [DOI] [PubMed] [Google Scholar]
- 58.Shamir ER, Ewald AJ. Three-dimensional organotypic culture: experimental models of mammalian biology and disease. Nature Reviews Molecular Cell Biology. 2014;15:647–664. doi: 10.1038/nrm3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fatehullah A, Tan SH, Barker N. Organoids as an in vitro model of human development and disease. Nature Cell Biology. 2016;18:246–254. doi: 10.1038/ncb3312. [DOI] [PubMed] [Google Scholar]
- 60.Huch M, Knoblich JA, Lutolf MP, Martinez-Arias A. The hope and the hype of organoid research. Development. 2017;144:938–941. doi: 10.1242/dev.150201. [DOI] [PubMed] [Google Scholar]
- 61.Gjorevski N, Sachs N, Manfrin A, Giger S, Bragina ME, Ordóñez-Morán P, Clevers H, Lutolf MP. Designer matrices for intestinal stem cell and organoid culture. Nature. 2016;539:560–564. doi: 10.1038/nature20168. [DOI] [PubMed] [Google Scholar]
- 62.Rosales AM, Anseth KS. The design of reversible hydrogels to capture extracellular matrix dynamics. Nature Reviews Materials. 2016;1:15012. doi: 10.1038/natrevmats.2015.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63**.Hughes AJ, Miyazaki H, Coyle MC, Zhang J, Laurie MT, Chu D, Vavrušová Z, Schneider RA, Klein OD, Gartner ZJ. Engineered Tissue Folding by Mechanical Compaction of the Mesenchyme. Developmental Cell. 2018;44:165–178 e166. doi: 10.1016/j.devcel.2017.12.004. Inspired by the in vivo strain mismatch between different tissues within developing organs, Hughes et al demonstrate that controlled patterning of mesenchymal cells presages epithelial folding sites. [DOI] [PMC free article] [PubMed] [Google Scholar]


