Abstract
Background:
Ventral tegmental area (VTA) GABA neurons have been heavily implicated in alcohol reinforcement and reward. In animals that self-administer alcohol, VTA GABA neurons exhibit increased excitability that may contribute to alcohol’s rewarding effects. The present study investigated the effects of acute and chronic ethanol exposure on glutamate (GLU) synaptic transmission to VTA GABA neurons.
Methods:
Whole cell recordings of evoked, spontaneous and miniature excitatory post-synaptic currents (EPSCs) were performed on identified GABA neurons in the VTA of GAD67-GFP+ transgenic mice. Three ethanol exposure paradigms were used: acute ethanol superfusion; a single ethanol injection; and chronic vapor exposure.
Results:
Acute ethanol superfusion increased the frequency of spontaneous EPSCs but inhibited mini EPSC frequency and amplitude. During withdrawal from a single injection of ethanol, the frequency of spontaneous EPSCs was lower than saline controls. There was no difference in AMPA/NMDA ratio between neurons following withdrawal from a single exposure to ethanol. However, following withdrawal from chronic ethanol, spontaneous and mini EPSCs had a greater frequency than air controls. There was no difference in AMPA/NMDA ratio following chronic ethanol.
Conclusions:
These results suggest that presynaptic mechanisms involving local circuit GLU neurons, and not GLU receptors, contribute to adaptations in VTA GABA neuron excitability that accrue to ethanol exposure, which may contribute to the rewarding properties of alcohol via their regulation of mesolimbic dopamine transmission.
Keywords: Ethanol, VTA, GABA, glutamate
Introduction
Alcohol is a widely used substance, with abuse having far-reaching consequences on individuals and on society as a whole (Moss, 2013). The mesolimbic dopamine (DA) system originating in the midbrain ventral tegmental area (VTA) and projecting to the nucleus accumbens (NAc) has been heavily implicated in the reinforcing aspects of alcohol use (Engel and Jerlhag, 2014). In general, alcohol is thought to influence activity of DA neurons in the VTA(Gessa et al., 1985b, Gessa et al., 1985a, Brodie, 2002, Mrejeru et al., 2015), resulting in changes in striatal and accumbal DA release (Gonzales et al., 2004). One particular target of interest in this system is VTA GABA neurons, which inhibit local DA neurons (Tepper et al., 1998, Nugent and Kauer, 2008, Allison et al., 2011, Brown et al., 2012). Our lab has repeatedly shown that VTA GABA neurons are inhibited in vivo by physiologically relevant doses (0.25 – 1.5 g/kg IP) of ethanol, suggesting that alcohol may increase DA activity by inhibiting GABA release onto VTA DA neurons (Steffensen et al., 2009, Steffensen et al., 2011). Furthermore, this apparent disinhibitory effect may not be exclusive to alcohol, as other drugs of abuse, including opioids, cannabinoids and benzodiazepines, also inhibit VTA GABA neuron activity (Johnson and North, 1992a, Szabo et al., 2002, Tan et al., 2010).
This circuit effect also appears to play an important role in reinforcing properties of alcohol, since rats that self-administer alcohol exhibit large increases in VTA GABA firing during withdrawal and during alcohol seeking behavior (Steffensen et al., 2009, Gallegos et al., 1999). This may obtain via direct effects on VTA GABA neurons, an increase in excitatory GLU receptor (GLUR)-mediated synaptic transmission, a decrease in inhibitory GABA receptor (GABAR)-mediated synaptic transmission, a combination of these, or other mechanisms, such as block of electrical transmission between VTA GABA neurons (Stobbs et al., 2004, Steffensen et al., 2011). Indeed, the reinforcing properties of alcohol are thought to involve its actions on GLU N-methyl-D-aspartate receptors (NMDARs) in the VTA (Luscher and Malenka, 2011). However, whether alcohol exposure changes GLU synaptic transmission to VTA GABA neurons is unknown. Since changes in GLU synaptic transmission may contribute to drug seeking behavior, the present study examined alcohol-induced changes in NMDA and AMPA dependent GLU activity to VTA GABA neurons.
A recent study from our lab (Nelson et al., 2018) has demonstrated that GABA neurons, but not DA neurons, become resistant to the inhibitory effects of the GABAA receptor agonist muscimol following a single exposure to acute ethanol, but not chronic exposure, an effect that is blocked by an injection of the NMDA receptor antagonist, MK-801 prior to ethanol injection. Following chronic ethanol exposure this effect persists for up to 7 days following ethanol exposure (Nelson et al., 2018). These findings suggest that adaptations in GABA transmission to VTA GABA neurons accrue to ethanol exposure and that NMDA GLURs are involved in the short-term adaptation. In the current study, three different ethanol exposure paradigms were used to evaluate the effects of ethanol on GLU activity on VTA GABA neurons: 1) acute ex vivo perfusion; 2) acute withdrawal after a single in vivo exposure; and 3) acute withdrawal after chronic in vivo vapor chamber exposure. We hypothesized that excitatory GLU transmission to VTA GABA neurons would show adaptations with acute, but not chronic, ethanol.
Methods
Animal Subjects
Male glutamate decarboxylase-67 (GAD-67)-GFP knock-in on a CD-1 (white albino) mice (Tamamaki et al., 2003) were bred and cared for in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals. Animals were treated in strict accordance with the Brigham Young University Animal Research Committee (IACUC) guidelines and approved protocols, which follow current NIH guidelines. Once weaned at post-natal day 21, all mice were housed in maximum groups of four and given ad libitum access to solid food and water and placed on a reverse light/dark cycle with lights ON from 10 PM to 10 AM. Mice used in injection experiments were lightly and briefly anesthetized with isoflurane (5%), and injected IP with a sterile needle, however, a group of mice were also injected IP without isoflurane anesthesia in order control for any potential drug interactions. Animals returned to their home cages 30 minutes following the injection. Mice ranged in age from PD 18–60. Vapor chamber exposed mice ranged from PD 50–60 whereas all other experiments were performed in mice under PD 36.
Chronic Intermittent Ethanol Exposure
Animals were exposed to chronic intermittent ethanol (CIE) in a modified alcohol vapor chamber system with feedback control of alcohol levels, as reported previously (Nelson et al., 2018). Mice were exposed to a level of ethanol previously determined to produce 200 mg% BALs for 16 hours (1000–0200 hours) beginning in their dark cycle (i.e., reverse cycle light). The duration of exposure was 2 hrs the first day of the first week and doubled each day until 16 hrs was reached and then held constant at 16 hrs for 2 more weeks, as described previously (Nelson et al., 2018). During the first week, intoxication was monitored and increased progressively in the alcohol vapor-exposed mice with mild ataxia on Day 1, moderate ataxia on Day 2, severe ataxia on Day 3 and sedation on Days 4, 5. Tolerance to the sedative effects of ethanol accrued the second week and tolerance to the ataxic effect accrued the third. This level of exposure increased alcohol consumption and adaptations in VTA GABA neuron responses (Nelson et al., 2018). This paradigm has been used previously by our lab as a model of alcohol dependence (Nelson et al., 2018).
Preparation of Brain Slices
All brain slice preparations were performed in GAD67-GFP knock-in mice. Brains were extracted via isoflurane anesthesia (5%). Upon extraction, the brain was cemented with cyanoacrylate (SuperGlue or Krazy Glue) onto a cutting stage. The brain was then sectioned in ice-cold cutting solution (in mM: 194 Sucrose, 30 NaCl, 4.5 KCl, 1 MgCl2, 26 NaH2CO3, 1.2 NaH2PO4, 10 Glucose) with a vibratome (Leica VT1200, Buffalo Grove, IL) and perfused with 95% O2 / 5% CO2. Targeting the VTA, horizontal slices (220μM thick) were placed in an incubation chamber containing artificial cerebral spinal fluid (ACSF; in mM: 124 NaCl, 3 KCl, 1.25 NaH2PO4, 26 NaHCO3, 12 glucose, 1.5 MgSO4, 2 CaCl2) perfused with 95% O2 / 5% CO2 for at least 30 min. Brain slices were placed in a recording tissue chamber with artificial cerebral spinal fluid (ACSF) bubbled with 95% O2 / 5% CO2 and flowing continuously at physiological temperatures (35°C).
Characterization of Neuron Types
GABA neurons were studied in GAD67-GFP knock-in mice. VTA GABA neurons were identified by a characteristic glow under fluorescence illumination. To confirm identification, VTA neurons were characterized using a current-clamp high frequency spiking command waveform obtained from a typical VTA GABA neuron [spikes at 200 Hz for 500msec; (Steffensen et al., 2008)]. GABA neurons follow the command waveform with fidelity while DA neurons do not. Neurons that did not fluoresce and/or exhibit a non-cation specific inward rectifying current (Ih) with low input resistance, and did not follow high frequency spiking by the command waveform were assumed to be DA neurons, and were not examined in this study (Allison et al., 2006, Allison et al., 2011, Johnson and North, 1992b, Margolis et al., 2006, Steffensen et al., 2011).
Whole-cell Recordings in vitro
A K-gluconate pipette solution [in mM: 123 K-gluconate, 0.2 EGTA, 10 HEPES, 8 NaCl, 2 Mg-ATP, 2 Na3-GTP, and 4.5 QX-314 (pH 7.3)] was used for excitatory post-synaptic current (EPSC) studies. The sodium channel blocker QX-314 was included in pipettes to eliminate action potentials interfering with data collection in the cell being recorded. Pipettes having tip resistances of 2.5 – 6MΩ, and series resistances typically ranging from 7 to 15 MΩ were used. Voltage clamp recordings were filtered at 2 kHz with an Axon Instruments Multiclamp 700B amplifier and digitized at 5 kHz using an Axon 1440A digitizercontrolled with Axon Instruments pClamp ver10 software on PC computers. EPSCs were recorded in the presence of 100 μM picrotoxin to block GABA and glycine mediated synaptic currents. Minis were recorded in the presence of 500 μM lidocaine to block action-potential dependent release. For measurement of AMPA/NMDA ratio, evoked EPSCs (eEPSCs) were recorded at +40 mV to remove the magnesium block from the NMDA receptor. The NMDA receptor antagonist APV (50 μM) was given partway through the experiment to block NMDA currents, and the NMDA component of the total current was calculated by subtraction. The maximum positive peak current amplitude was measured for AMPA and NMDA receptors for data analysis whenever it occurred, which for the latter usually occurred within 15 msec. Peak amplitude of NMDA currents was selected for data analysis (rather than decay kinetics), since it is a common practice (see Ungless et al, 2001) and seems to vary less between GABA cells. To evoke EPSCs, we used a concentric bipolar platinum/iridium electrode from MicroProbes for Life Science (Gaithersburg, MD).
Drug Preparation and Administration
Drugs used in vitro were made fresh in distilled water, added to ACSF, and superfused on brain slices and included: APV (50 μM; Abcam), picrotoxin (100 μM; Sigma-Aldrich), lidocaine (500 μM; Sigma-Aldrich). Ethanol (5 – 60 mM) was added to ACSF after baseline recordings. Drugs used in injections were added to sterile 0.9% saline and injected IP (16% ethanol v/v).
Statistical Analyses
Results are presented as raw mean values and percent control ± SEM. Results between groups were compared using a two-tailed unpaired t test or ANOVA. Experiments relying on variance in time or current were analyzed using mixed models ANOVA with post hoc t-test at individual points. Statistical significance required ≥ 95% level of confidence (p ≤ 0.05). Analysis software included Minianalysis (Synaptosoft. Decatir. GA), Clampfit (Axon Instruments), Microsoft Excel, and Igor Pro (Wavemetrics, Oswego, OR). Significance levels are are indicated on graphs with asterisks *,** and corresponded to significance levels p<0.05, and 0.01, respectively. Figures were constructed with Igor Pro software.
Results
Effects of Acute Superfused Ethanol on VTA GABA Neuron Spontaneous and Mini EPSCs
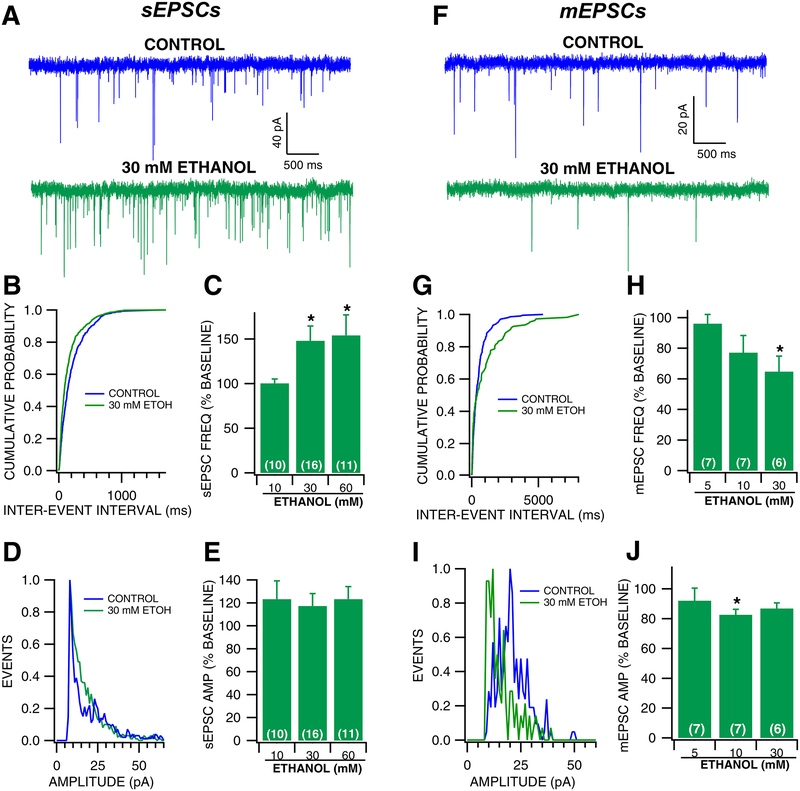
In order to understand ethanol’s effects on GLU activity on VTA GABA neurons, ethanol was superfused during the recordings of whole cell spontaneous EPSCs (sEPSCs) and mini EPSCs (mEPSCs) to VTA GABA neurons (Fig. 1). Ethanol exhibited significant dose-dependent effects on sEPSC frequency [Ethanol 10–60 mM, Fig. 1B–C; two-way mixed-measures ANOVA (Ethanol × concentration); Ethanol: F(1,33) = 2.782, p =0.105; concentration: F(2,33) = 1.296, p = 0.287; interaction: F(2,33) = 4.033, p = 0.0271] with a 47% increase in sEPSC frequency at 30 mM ethanol. However, ethanol had no apparent effect on sEPSC amplitude [Ethanol 10–60 mM, Fig. 1D–E; two-way mixed-measures ANOVA (Ethanol × concentration); Ethanol: F(1,33) = 10.65, p =0.0028; concentration: F(2,33) = 4.251, p = 0.0240; interaction: F(2,33) = 0.376, p = 0.690], suggesting that ethanol alters the activity of presynaptic GLU neurons. Additionally, ethanol had no effect on the kinetics of sEPSCs as measured by half width (F(3,44) = 1, p =0.404). In order to determine if ethanol alters activity-independent sEPSCs, mEPSCs were recorded in the presence of the sodium channel blocker lidocaine (500 μM), which effectively blocks action potentials in VTA GABA neurons (Steffensen et al., 2008). Lidocaine also has the added advantage of blocking TTX-resistant sodium channels (Tan et al., 2014). Lidocaine reduces GABA sEPSC frequency by 55% (Control: 3.436 ± 0.838; Lidocaine: 1.545 ± 0.393; t(6) =2.702, p = 0.0177), and had no apparent effect on sEPSC amplitude (Control: 20.344 ± 3.681; Lidocaine: 18.207 ± 3.179; t(6) =1.534, p = 0.088). Contrary to sEPSCs, ethanol significantly decreased mEPSC frequency [Ethanol 5–30 mM, Fig. 1G–H; two-way mixed-measures ANOVA (Ethanol × concentration); Ethanol: F(1,17) = 7.539, p =0.0138; concentration: F(2,17) = 1.479, p = 0.256; interaction: F(2,17) = 1.516, p = 0.248] with a 35% decrease at 30 mM, and mEPSC amplitude [Ethanol 10–60 mM, Fig. 1I–J; two-way mixed-measures ANOVA (Ethanol × concentration); Ethanol: F(1,16) = 7.037, p =0.0174; concentration: F(2,16) = 0.373, p = 0.695; interaction: F(2,16) = 0.0481, p = 0.953] with an 18% decrease at 10 mM. Ethanol also had no effect on mEPSC half-width (F(3,36) = 0.10, p =0.962). Thus, acute ethanol increases action potential-dependent, but not action potential-independent GLU activity to VTA GABA neurons.
Figure 1: Effects of acute superfused ethanol on VTA GABA neuron spontaneous and mini EPSCs.
(A) Representative 5 sec traces of sEPSCs recorded from a VTA GABA neuron before and during 30 mM ethanol superfusion. (B) Representative cumulative probability plot for sEPSC inter-event intervals before and during 30 mM ethanol superfusion. (C) sEPSC frequency was significantly increased from baseline during superfusion of 30 mM and 60 mM ethanol. (D) Representative histogram plot of sEPSC amplitudes before and during 30 mM ethanol superfusion. (E) sEPSC amplitude was increased following ethanol superfusion at all doses (10 mM, 30 mM, 60 mM). (F) Representative 5 sec traces of mEPSCs recorded from a VTA GABA neuron before and during 30 mM ethanol superfusion. (G) Representative cumulative probability plot for mEPSC inter-event intervals before and during 30 mM ethanol superfusion. (H) mEPSC frequency was decreased following ethanol superfusion. (I) Representative histogram plot of mEPSC amplitudes before and during 30 mM ethanol superfusion. (J) mEPSC amplitude was decreased following ethanol superfusion. Asterisk * represents significance level p<0.05.
Effects of Withdrawal from a Single in vivo Ethanol Injection on VTA GABA Neuron EPSCs
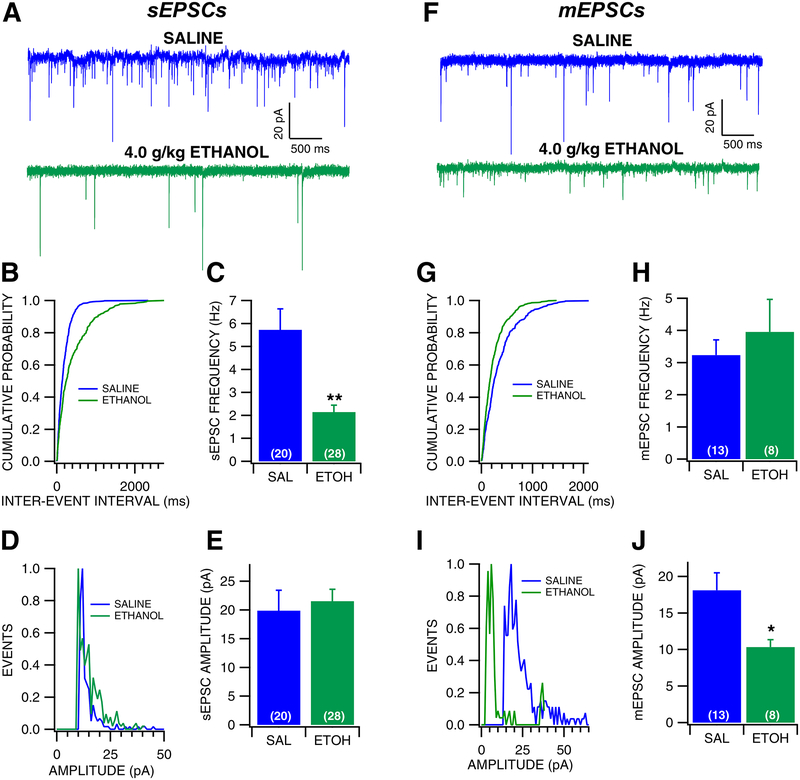
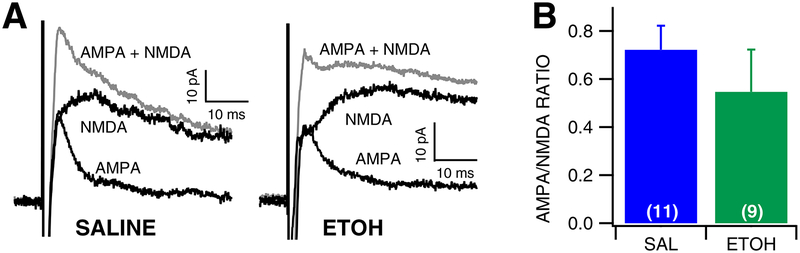
The effects of ethanol withdrawal on GLU transmission to VTA GABA neurons were evaluated in the slice preparation 24 hr after a single in vivo ethanol injection (4.0 g/kg, IP; Fig. 2). Intraperitoneal injections were performed under brief, light isoflurane anesthesia to reduce the stress of the saline/ethanol injection, and sEPSC responses were recorded in VTA GABA neurons. These same experiments were also performed in non-isoflurane exposed mice. Withdrawal from an ethanol injection resulted in decreased sEPSC frequency in both isoflurane (frequency: F (1, 21) = 11.174, p = 0.0032; amplitude: F (1, 21) = 0.0885, p = 0.769), as well as non-isoflurane exposed mice (frequency: F (1, 25) = 12.550, p = 0.0017; amplitude: F (1, 25) = 0.981, p = 0.332). Thus, groups were collapsed and considered together (Fig. 2A–E). Ethanol-exposed mice exhibited a 42% lower baseline frequency of sEPSCs than saline-treated mice, which was statistically significant (Fig. 2B–C, F(1, 47) = 17.915, p < 0.0001). However, there was no apparent effect of ethanol on sEPSC amplitude (Fig. 2D–E, F(1,47) = 0.1823, p = 0.671). Additionally, there was no apparent effect of ethanol on sEPSC kinetics as measured by half-width (Saline: 1.997 ± 0.166 msec; Ethanol: 2.501 ± 0.216 msec; F(1,41) = 4.085, p = 0.0748), suggesting that there was no change in GLU receptor subunit composition as result of ethanol exposure. Mini EPSCs were then recorded in VTA GABA neurons following acute ethanol exposure and withdrawal. Ethanol had no apparent effect on mEPSC frequency (Fig. 2G–H, F(1, 20) = 0.527, p = 0.477), but resulted in significantly lower mEPSC amplitudes (Fig. 2I–J, F (1, 20) = 6.028, p = 0.0239), suggesting that the change in sEPSC frequency was due to ethanol effects related to action-potential dependent GLU release, but the change in mEPSC amplitude was suggestive of postsynaptic effects of ethanol. However, there was no apparent effect of ethanol on mEPSC kinetics as measured by half-width (Saline: 2.369 ± 0.282 ms; Ethanol: 2.988 ± 0.486 ms; F(1,20) = 1.411, p = 0.249). As an additional measure of plasticity, AMPA/NMDA ratio experiments in VTA GABA neurons were performed 24 hours following acute ethanol (4.0 g/kg, i.p.; Fig. 3). There was no difference in AMPA/NMDA ratio following acute ethanol exposure (Fig. 3B, F(1, 18) = 1.2184, p = 0.2842), therefore ethanol does not appear to change relative expression of AMPA receptors and that changes observed with sEPSCs and mEPSCs were likely due to changes in GLU release.
Figure 2: Effects of withdrawal from a single in vivo ethanol injection on VTA GABA neuron EPSCs.
(A) Representative traces of sEPSCs recorded from a VTA GABA neuron following single in vivo injection of saline or ethanol (4.0 g/kg, IP). (B) Representative cumulative probability plot for sEPSC inter-event intervals following saline vs ethanol exposure. (C) sEPSC frequency was significantly decreased in mice injected with ethanol compared to mice injected with saline. (D) Representative histogram plot of sEPSC following saline vs ethanol exposure. (E) There was no difference in sEPSC amplitude between mice injected with saline vs ethanol. (F) Representative traces of mEPSCs recorded from a VTA GABA neuron following an injection of saline vs ethanol. (G) Representative cumulative probability plot for mEPSC inter-event intervals following saline vs ethanol exposure. (H) There was no difference in mEPSC frequency between mice injected with saline vs ethanol. (I) Representative histogram plot of mEPSC following saline vs ethanol exposure. (J) mEPSC amplitude was decreased during withdrawal from a single injection of ethanol compared to saline. Asterisks *, ** represent significance levels p<0.05 and p<0.01, respectively.
Figure 3: Lack of effects of withdrawal from a single in vivo ethanol injection on VTA GABA neuron AMPA/NMDA ratio.
(A) Representative traces of AMPA and NMDA currents recorded from a VTA GABA neuron following a single injection of saline vs ethanol (4.0 g/kg, IP). (B) There was no significant difference in AMPA/NMDA ratio following saline vs ethanol injection.
Effects of Withdrawal from Chronic Ethanol Exposure on VTA GABA Neuron EPSCs.
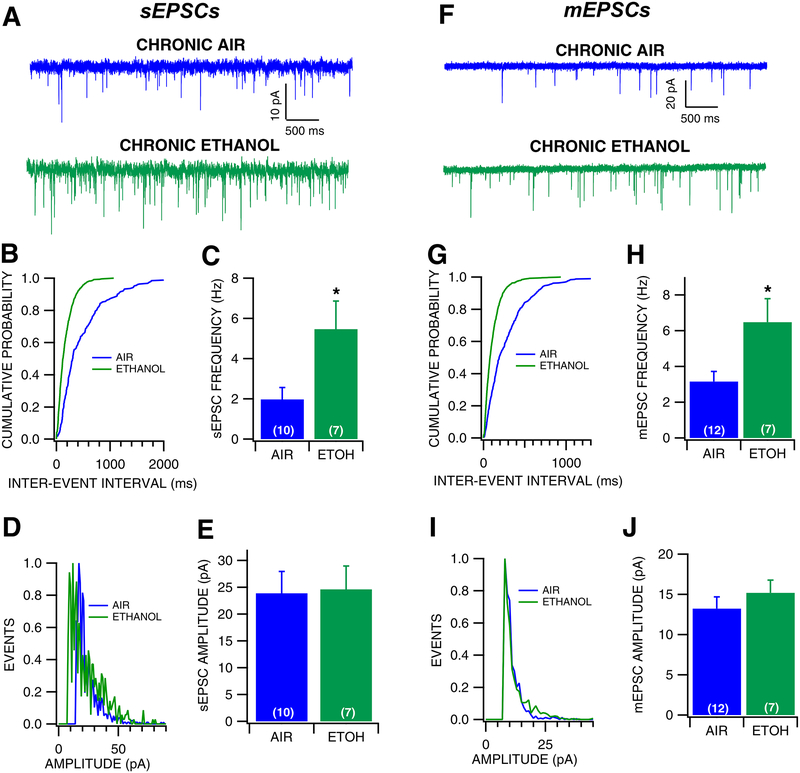
The effects of chronic ethanol and withdrawal on GLU activity on VTA GABA neurons were tested. Mice were exposed to ethanol for a period of 3 weeks through the CIE vapor exposure paradigm, and whole cell recordings were conducted 24 hours following the last ethanol exposure (Fig. 4). CIE significantly increased the frequency of sEPSCs (Fig. 4B–C, F(1, 20) = 7.172, p = 0.0145) by 276%, but had no effect on sEPSC amplitude (Fig. 4D–E, F(1, 20) = 0.0142, p = 0.906) or sEPSC kinetics (Air: 3.016 ± 0.445 msec; Ethanol: 2.632 ± 0.244 msec; F(1, 21) = 0.383, p = 0.543). Similarly, chronic vapor exposure significantly increased the frequency of mEPSCs (Fig. 4G–H, F(1, 17) = 4.593, p = 0.0469) by 168%, but had no effect on mEPSC amplitude (Fig. 4I–J, F(1, 17) = 0.758, p = 0.396), suggesting that during withdrawal from chronic ethanol there is increased probability of GLU release on VTA GABA neurons. Additionally, chronic ethanol vapor exposure significantly altered mEPSC kinetics, as the half width was decreased by 56% (Air: 2.979 ± 0.461 msec; Ethanol: 1.665 ± 0.105 msec; F(1, 18) = 4.557, p = 0.048), suggesting that CIE exposure altered GLU receptor subunit composition. CIE exposure had no effect on AMPA/NMDA ratio in VTA GABA neurons (Fig. 5, F(1, 20) = 0.7598, p = 0.3937). Thus, CIE increases GLU activity to VTA GABA neurons and decrease current decay rates, likely due to modified subunit composition.
Figure 4: Effects of withdrawal from chronic ethanol vapor exposure on VTA GABA neuron EPSCs.
(A) Representative 5 sec traces of sEPSCs recorded from a VTA GABA neuron following chronic ethanol vapor or chronic air exposure. (B) Representative cumulative probability plot for sEPSC inter-event intervals following air vs ethanol exposure. (C) sEPSC frequency was significantly increased following chronic ethanol compared to chronic air exposure. (D) Representative histogram plot of sEPSC following air vs ethanol exposure. (E) There was no difference in sEPSC amplitude between chronic ethanol and chronic air conditions. (F) Representative traces of mEPSCs recorded from a VTA GABA neuron during withdrawal from chronic air vs ethanol vapor. (G) Representative cumulative probability plot for sEPSC inter-event intervals following air vs ethanol exposure. (H) mEPSC frequency was increased during withdrawal from chronic ethanol exposure compared to chronic air exposure. (I) Representative histogram plot of sEPSC following air vs ethanol exposure. (J) There was no difference in mEPSC amplitude during withdrawal from chronic air vs ethanol exposure. Asterisk *represents significance level p<0.05.
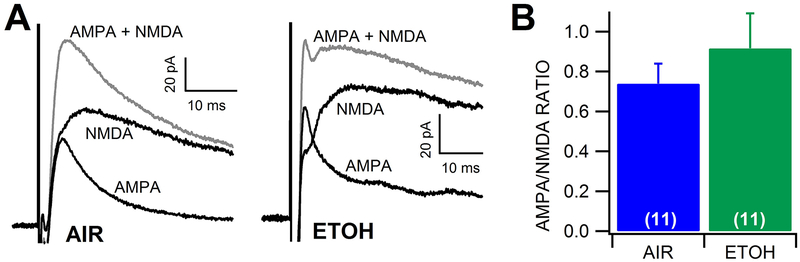
Figure 5: Effects of withdrawal from chronic ethanol vapor exposure on VTA GABA neuron AMPA/NMDA ratios.
(A) Representative traces of AMPA and NMDA currents recorded from a VTA GABA neuron following chronic ethanol vapor vs air exposure. (B) There was no significant difference in AMPA/NMDA ratio between chronic air vs ethanol conditions.
Discussion
The purpose of this study was to investigate ethanol’s effects on GLU transmission to VTA GABA neurons. Acute superfused ethanol had mixed effects on excitatory synaptic events. Spontaneous EPSCs increased, but mini EPSCs decreased with acute ethanol, suggesting that acute ethanol has a variety of targets related to GLU synaptic transmission in the VTA. It significantly enhances sEPSCs, but decreases mEPSCs, suggesting that excitatory effects of ethanol on sEPSCs overshadow inhibitory effects on mEPSCs. While withdrawal from acute ethanol depressed excitation, by decreasing sEPSC frequency, withdrawal from chronic ethanol enhanced excitatory synaptic transmission to VTA GABA neurons, increasing both sEPSC and mEPSC frequency. These results suggest that ethanol is modulating VTA GABA neuron activity in part through its effects on action potential-dependent GLU synaptic transmission and, consistent with our hypothesis, that acute effects of ethanol on GLU transmission would differ from chronic effects. However, contrary to our hypothesis, GLU transmission to VTA GABA neurons was affected by chronic ethanol, albeit differentially.
Acute Ethanol Effects on Excitatory Synaptic Transmission to VTA GABA Neurons
Ethanol superfusion has complicated effects on excitatory synaptic events on VTA GABA neurons. Acute ethanol increases sEPSC frequency at 30 mM and 60 mM, but reduces mEPSC frequency and amplitude suggesting that ethanol has biphasic effects on GLU activity to VTA GABA neurons. Similarly, previous in vivo studies have indicated a transient increase in VTA GABA excitability after ethanol, which is followed by temporary inhibition (Ludlow et al., 2009, Steffensen et al., 2001, Steffensen et al., 2009). This transient increase in firing rate is thought to be due to low dose effects of ethanol on GLU activity, since GLU-dependent stimulus-evoked discharges are enhanced by low doses (<0.1 g/kg), but inhibited by high doses (>1 g/kg) of ethanol (Stobbs et al., 2004, Steffensen et al., 1998, Steffensen et al., 2009). Local GLU neurons comprise only a small subset of VTA neurons (Yamaguchi et al., 2007), but have been implicated in reward (Wang et al., 2015). They synapse onto VTA neurons (Dobi et al., 2010), and project to the NAc (Qi et al., 2016), and are likely responsible for the sEPSCs we recorded here on VTA GABA neurons in the slice preparation. Remote GLU inputs, such as the input from the prefrontal cortex, would likely be severed in the slice preparation, but could elicit mEPSCs via spontaneous release of GLU at terminals. Thus, it is conceivable that adaptations in GLU release could be mediated by these local circuit VTA GLU neurons, although the involvement of remote GLU inputs cannot be discounted.
Ethanol has direct inhibitory effects on a number of ligand gated ion channels including NMDA, AMPA and Kainate GLU receptors (Faingold et al., 1998, Dopico and Lovinger, 2009). For example, ethanol inhibits the NMDA receptor (Weight, 1992, Lovinger et al., 1989) at moderate ethanol concentrations (25 mM) in adult rat hippocampus (Lovinger et al., 1990). Although NMDA receptors are particularly sensitive to ethanol inhibition, AMPA receptors have also garnered attention as a site for ethanol GLU interactions. Ethanol-mediated AMPA receptor inhibition occurs through increases in receptor desensitization (Trussell and Fischbach, 1989, Moykkynen et al., 2003), which may involve interactions with AMPA receptor-regulating proteins (Moykkynen and Korpi, 2012). While the inhibitory effects of ethanol on GLU currents are likely contributing to the marked inhibition observed in in vivo recordings (Gallegos et al., 1999, Stobbs et al., 2004, Steffensen et al., 1998), it is noteworthy that ethanol also increases GABA mediated IPSCs onto VTA GABA neurons via a nicotinic dependent mechanism (Steffensen et al., 2017). Therefore, the inhibitory effects of acute ethanol on VTA GABA firing likely involve multiple mechanism involving GLU, GABA and other systems including gap junction-mediated electrical coupling between VTA GABA neurons (Steffensen et al., 2011, Stobbs et al., 2004). Additionally, alterations in sEPSC and mEPSC frequency could in part be due to ethanol effects on GABA or DA neurons (via GABA(B) or D2 receptors on presynaptic GLU terminals) that cause indirect changes in EPSC frequency.
Withdrawal Effects on Excitatory Synaptic Transmission to VTA GABA Neurons 24 hrs Following a Single Exposure to Ethanol (Non-dependent Condition)
Although bath application of ethanol enhanced GLU release, 24 hrs after a single intoxicating injection of ethanol resulted in a decrease in sEPSC frequency and mEPSCs amplitude. These differences suggest that an ethanol-induced increase in GLU transmission may trigger some feedback inhibitory mechanism to reduce GLU transmission during withdrawal. Interestingly, 24 hr after a single ethanol injection, GABA release onto VTA DA neurons is increased (Wanat et al., 2009, Melis et al., 2002). This previous finding is somewhat complicated by results showing that ethanol prevents GABA LTP onto DA neurons (Guan and Ye, 2010). Additionally, VTA GABA neurons are less sensitive to GABA agonists after an ethanol injection, an effect that is blocked by pretreatment with the GLU receptor antagonist MK-801 (Nelson et al., 2018), demonstrating that ethanol’s acute effects on GLU transmission underlie some of the adaptations observed during acute withdrawal. We did not find any significant effects of withdrawal from a single exposure to ethanol on AMPA/NMDA ratio in VTA GABA neurons, suggesting that receptor plasticity is not operational in VTA GABA neurons, although it has been demonstrated in DA neurons (Ungless et al., 2001). NMDA current kinetics (rise and decay time) differ to a degree from cell to cell (see example traces in Figs 3,5), this high variability is also observed between ethanol and control mice. Therefore, NMDA subtypes may differ between different GABA cells, but ethanol does not appear to change NMDA subtype organization.
Withdrawal Effects on Excitatory Synaptic Transmission Following Withdrawal from Chronic Exposure to Ethanol (Dependent Condition)
We have demonstrated recently that 2–3 weeks of CIE exposure results in tolerance to ethanol effects on GABAR-mediated synaptic transmission to VTA GABA neurons (Nelson et al., 2018) and modifications in VTA GABA neurons (Gallegos et al., 1999), in particular marked increases in baseline firing rates. In this study, CIE resulted in enhanced GLU synaptic transmission. Both sEPSC and mEPSC frequency were greater in CIE-exposed mice compared to chronic air-exposed controls. In other brain regions, CIE has been shown to increase NMDA receptor expression as well as changes in NMDA receptor subunit composition (Qiang et al., 2007, Lack et al., 2007). Presynaptic effects of chronic ethanol have also been observed in other regions, where ethanol exposure resulted in increased GLU release during withdrawal (Lack et al., 2007, Roberto et al., 2006). Increased frequency of GLU release is likely contributing to the increased excitability of VTA GABA neurons following withdrawal from CIE exposure (Gallegos et al., 1999). However, GABA inhibition appears to be reduced as well (Nelson et al., 2018).
Excitatory Plasticity to VTA GABA Neurons Due to Alcohol
Our findings indicate opposing effects on VTA GABA neuron GLU plasticity during withdrawal from acute and chronic ethanol. Withdrawal from a single in vivo ethanol exposure decreases the frequency of sEPSCs, and amplitude of mEPSCs. These results are somewhat inconclusive, and reflect the complexity of circuitry within the VTA and within the mesolimbic DA system that may contribute to these changes. Undoubtedly, GLU plasticity in the VTA is involved in the path to addiction, but no conclusive statement can be made about GLU plasticity in VTA GABA neurons during acute ethanol withdrawal. In contrast, the frequency of excitatory synaptic events (sEPSCs and mEPSCs) during withdrawal from CIE exposure are greater compared to air-exposed controls, suggesting presynaptic modifications occurring due to the chronic exposure and withdrawal. No differences were observed in the amplitude of sEPSCs or mEPSCs, further implicating a presynaptic effect. Further complicating the issue, CIE recordings were performed in mice that were 3–4 weeks older than mice used in acute ethanol studies. Therefore, it’s possible that some differences between acute and CIE effects on GLU transmission are due in part to age differences.
Many drugs of abuse cause increases in AMPA/NMDA ratio in VTA DA neurons (Ungless et al., 2001, Saal et al., 2003). Interestingly, both increases and decreases in AMPA/NMDA ratio have been reported in VTA DA neurons following ethanol exposure (Wanat et al., 2009, Saal et al., 2003). However, VTA GABA neurons are far less understood, but 10X more sensitive to ethanol than DA neurons (Gallegos et al., 1999, Ludlow et al., 2009, Stobbs et al., 2004, Steffensen et al., 2009). Neither of the withdrawal states tested had significant differences on AMPA/NMDA ratio. Although acute effects of ethanol were observed on evoked NMDA EPSCs to VTA GABA neurons, withdrawal from ethanol does not reflect any significant differences. Interestingly, there are different trends between acute and chronic ethanol AMPA/NMDA ratios, however without statistical significance we cannot be sure if those differences are meaningful. We are confident that VTA GABA neurons are a relevant target for ethanol in the brain, and that NMDAR mediated plasticity is involved, but we see no evidence of a change in AMPA/NMDA ratio during withdrawal from acute or chronic ethanol, suggesting that changes in GLUR expression are not a major facet of alcohol adaptations. However, GLU drive to VTA GABA neurons appears functionally relevant in the context of ethanol, perhaps via GLU neurons in the VTA (Dobi et al., 2010, Yamaguchi et al., 2007).
Overall, these results describe robust plasticity in excitatory synapses to VTA GABA neurons that may contribute to the hyperexcitability of these neurons during withdrawal from ethanol (Gallegos et al., 1999), albeit it appears to be a presynaptic mechanism involving local circuit GLU neurons and not the result of GLUR adaptation. The hyperexcitability of VTA GABA neurons appears to be a combination of reduced GABA (Nelson et al., 2018) and enhanced GLU drive to these neurons, which form an important nexus for the regulation of mesolimbic DA transmission and reward signaling. Implications of this study include a molecular mechanism for alcohol’s effect on VTA GABA neurons and a cellular correlate to the hedonic drive to seek alcohol during a period of abstinence.
Acknowledgements:
This work was supported by NIH grants AA020919 and DA035958 to SCS. The authors have no other conflicts of interest to declare.
References
- Allison DW, Ohran AJ, Stobbs SH, Mameli M, Valenzuela CF, Sudweeks SN, Ray AP, Henriksen SJ & Steffensen SC 2006. Connexin-36 gap junctions mediate electrical coupling between ventral tegmental area GABA neurons. Synapse, 60, 20–31. [DOI] [PubMed] [Google Scholar]
- Allison DW, Wilcox RS, Ellefsen KL, Askew CE, Hansen DM, Wilcox JD, Sandoval SS, Eggett DL, Yanagawa Y & Steffensen SC 2011. Mefloquine effects on ventral tegmental area dopamine and GABA neuron inhibition: A physiologic role for connexin-36 gap junctions. Synapse. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodie MS 2002. Increased ethanol excitation of dopaminergic neurons of the ventral tegmental area after chronic ethanol treatment. Alcohol Clin Exp Res, 26, 1024–30. [DOI] [PubMed] [Google Scholar]
- Brown MT, Tan KR, O’connor EC, Nikonenko I, Muller D & Luscher C 2012. Ventral tegmental area GABA projections pause accumbal cholinergic interneurons to enhance associative learning. Nature, 492, 452–6. [DOI] [PubMed] [Google Scholar]
- Dobi A, Margolis EB, Wang HL, Harvey BK & Morales M 2010. Glutamatergic and nonglutamatergic neurons of the ventral tegmental area establish local synaptic contacts with dopaminergic and nondopaminergic neurons. J Neurosci, 30, 218–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dopico AM & Lovinger DM 2009. Acute alcohol action and desensitization of ligand-gated ion channels. Pharmacol Rev, 61, 98–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel JA & Jerlhag E 2014. Alcohol: mechanisms along the mesolimbic dopamine system. Prog Brain Res, 211, 201–33. [DOI] [PubMed] [Google Scholar]
- Faingold CL, N’gouemo P & Riaz A 1998. Ethanol and neurotransmitter interactions--from molecular to integrative effects. Prog Neurobiol, 55, 509–35. [DOI] [PubMed] [Google Scholar]
- Gallegos RA, Criado JR, Lee RS, Henriksen SJ & Steffensen SC 1999. Adaptive responses of GABAergic neurons in the ventral tegmental area to chronic ethanol. J. Pharmacol. Exp. Ther, 291, 1045–1053. [PubMed] [Google Scholar]
- Gessa G, Muntoni F, Boi V & Mereu G 1985a. Effects of Ethanol on Mid-Brain DA and NON-DA Neurons. Society for Neuroscience Abstract, 11, 87.14. [Google Scholar]
- Gessa GL, Muntoni F, Collu M, Vargiu L & Mereu G 1985b. Low doses of ethanol activate dopaminergic neurons of the ventral tegmental area. Brain Res, 348, 201. [DOI] [PubMed] [Google Scholar]
- Gonzales RA, Job MO & Doyon WM 2004. The role of mesolimbic dopamine in the development and maintenance of ethanol reinforcement. Pharmacol Ther, 103, 121–46. [DOI] [PubMed] [Google Scholar]
- Guan YZ & Ye JH 2010. Ethanol blocks long-term potentiation of GABAergic synapses in the ventral tegmental area involving mu-opioid receptors. Neuropsychopharmacology, 35, 1841–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SW & North RA 1992a. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J. Neuroscience, 12, 483–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SW & North RA 1992b. Two types of neurone in the rat ventral tegmental area and their synaptic inputs. J. Physiol. (Lond), 450, 455–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lack AK, Diaz MR, Chappell A, Dubois DW & Mccool BA 2007. Chronic ethanol and withdrawal differentially modulate pre- and postsynaptic function at glutamatergic synapses in rat basolateral amygdala. Journal of Neurophysiology, 98, 3185–3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger DM, White G & Weight FF 1989. Ethanol inhibits NMDA-activated ion current in hippocampal neurons. Science, 243, 1721–4. [DOI] [PubMed] [Google Scholar]
- Lovinger DM, White G & Weight FF 1990. NMDA receptor-mediated synaptic excitation selectively inhibited by ethanol in hippocampal slice from adult rat. J. Neurosci, 10, 1372–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludlow KH, Bradley KD, Allison DW, Taylor SR, Yorgason JT, Hansen DM, Walton CH, Sudweeks SN & Steffensen SC 2009. Acute and chronic ethanol modulate dopamine D2-subtype receptor responses in ventral tegmental area GABA neurons. Alcohol Clin Exp Res, 33, 804–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher C & Malenka RC 2011. Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron, 69, 650–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Lock H, Hjelmstad GO & Fields HL 2006. The ventral tegmental area revisited: is there an electrophysiological marker for dopaminergic neurons? J Physiol, 577, 907–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis M, Camarini R, Ungless MA & Bonci A 2002. Long-lasting potentiation of GABAergic synapses in dopamine neurons after a single in vivo ethanol exposure. J Neurosci, 22, 2074–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss HB 2013. The impact of alcohol on society: a brief overview. Soc Work Public Health, 28, 175–7. [DOI] [PubMed] [Google Scholar]
- Moykkynen T & Korpi ER 2012. Acute effects of ethanol on glutamate receptors. Basic Clin Pharmacol Toxicol, 111, 4–13. [DOI] [PubMed] [Google Scholar]
- Moykkynen T, Korpi ER & Lovinger DM 2003. Ethanol inhibits alpha-amino-3-hydyroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor function in central nervous system neurons by stabilizing desensitization. J Pharmacol Exp Ther, 306, 546–55. [DOI] [PubMed] [Google Scholar]
- Mrejeru A, Marti-Prats L, Avegno EM, Harrison NL & Sulzer D 2015. A subset of ventral tegmental area dopamine neurons responds to acute ethanol. Neuroscience, 290, 649–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson AC, Williams SB, Pistorius SS, Park HJ, Woodward TJ, Payne AJ, Obray JD, Shin SI, Mabey JK & Steffensen SC 2018. Ventral Tegmental Area GABA Neurons Are Resistant to GABA(A) Receptor-Mediated Inhibition During Ethanol Withdrawal. Front Neurosci, 12, 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent FS & Kauer JA 2008. LTP of GABAergic synapses in the ventral tegmental area and beyond. J Physiol, 586, 1487–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi J, Zhang S, Wang HL, Barker DJ, Miranda-Barrientos J & Morales M 2016. VTA glutamatergic inputs to nucleus accumbens drive aversion by acting on GABAergic interneurons. Nat Neurosci, 19, 725–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiang M, Denny AD & Ticku MK 2007. Chronic intermittent ethanol treatment selectively alters N-methyl-D-aspartate receptor subunit surface expression in cultured cortical neurons. Mol Pharmacol, 72, 95–102. [DOI] [PubMed] [Google Scholar]
- Roberto M, Treistman SN, Pietrzykowski AZ, Weiner J, Galindo R, Mameli M, Valenzuela F, Zhu PJ, Lovinger D, Zhang TA, Hendricson AH, Morrisett R & Siggins GR 2006. Actions of acute and chronic ethanol on presynaptic terminals. Alcohol Clin Exp Res, 30, 222–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saal D, Dong Y, Bonci A & Malenka RC 2003. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron, 37, 577–82. [DOI] [PubMed] [Google Scholar]
- Steffensen SC, Bradley KD, Hansen DM, Wilcox JD, Wilcox RS, Allison DW, Merrill CB & Edwards JG 2011. The role of connexin-36 gap junctions in alcohol intoxication and consumption. Synapse, 65, 695–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffensen SC, Lee RS, Stobbs SH & Henriksen SJ 2001. Responses of ventral tegmental area GABA neurons to brain stimulation reward. Brain Res, 906, 190–7. [DOI] [PubMed] [Google Scholar]
- Steffensen SC, Shin SI, Nelson AC, Pistorius SS, Williams SB, Woodward TJ, Park HJ, Friend L, Gao M, Gao F, Taylor DH, Foster Olive M, Edwards JG, Sudweeks SN, Buhlman LM, Michael Mcintosh J & Wu J 2017. alpha6 subunit-containing nicotinic receptors mediate low-dose ethanol effects on ventral tegmental area neurons and ethanol reward. Addict Biol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffensen SC, Svingos AL, Pickel VM & Henriksen SJ 1998. Electrophysiological characterization of GABAergic neurons in the ventral tegmental area. J Neurosci, 18, 8003–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffensen SC, Taylor SR, Horton ML, Barber EN, Lyle LT, Stobbs SH & Allison DW 2008. Cocaine disinhibits dopamine neurons in the ventral tegmental area via use-dependent blockade of GABA neuron voltage-sensitive sodium channels. Eur J Neurosci, 28, 2028–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffensen SC, Walton CH, Hansen DM, Yorgason JT, Gallegos RA & Criado JR 2009. Contingent and non-contingent effects of low-dose ethanol on GABA neuron activity in the ventral tegmental area. Pharmacol Biochem Behav, 92, 68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stobbs SH, Ohran AJ, Lassen MB, Allison DW, Brown JE & Steffensen SC 2004. Ethanol suppression of ventral tegmental area GABA neuron electrical transmission involves N-methyl-D-aspartate receptors. J Pharmacol Exp Ther, 311, 282–9. [DOI] [PubMed] [Google Scholar]
- Szabo B, Siemes S & Wallmichrath I 2002. Inhibition of GABAergic neurotransmission in the ventral tegmental area by cannabinoids. Eur J Neurosci, 15, 2057–61. [DOI] [PubMed] [Google Scholar]
- Tamamaki N, Yanagawa Y, Tomioka R, Miyazaki J, Obata K & Kaneko T 2003. Green fluorescent protein expression and colocalization with calretinin, parvalbumin, and somatostatin in the GAD67-GFP knock-in mouse. J Comp Neurol, 467, 60–79. [DOI] [PubMed] [Google Scholar]
- Tan KR, Brown M, Labouebe G, Yvon C, Creton C, Fritschy JM, Rudolph U & Luscher C 2010. Neural bases for addictive properties of benzodiazepines. Nature, 463, 769–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan ZY, Piekarz AD, Priest BT, Knopp KL, Krajewski JL, Mcdermott JS, Nisenbaum ES & Cummins TR 2014. Tetrodotoxin-resistant sodium channels in sensory neurons generate slow resurgent currents that are enhanced by inflammatory mediators. J Neurosci, 34, 7190–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper JM, Paladini CA & Celada P 1998. GABAergic control of the firing pattern of substantia nigra dopaminergic neurons. Adv Pharmacol, 42, 694–9. [DOI] [PubMed] [Google Scholar]
- Trussell LO & Fischbach GD 1989. Glutamate receptor desensitization and its role in synaptic transmission. Neuron, 3, 209–18. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Whistler JL, Malenka RC & Bonci A 2001. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature, 411, 583–7. [DOI] [PubMed] [Google Scholar]
- Wanat MJ, Sparta DR, Hopf FW, Bowers MS, Melis M & Bonci A 2009. Strain specific synaptic modifications on ventral tegmental area dopamine neurons after ethanol exposure. Biol Psychiatry, 65, 646–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HL, Qi J, Zhang S, Wang H & Morales M 2015. Rewarding Effects of Optical Stimulation of Ventral Tegmental Area Glutamatergic Neurons. J Neurosci, 35, 15948–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weight F 1992. Cellular and molecular physiology of alcohol actions in the nervous system. International Review of Neurobiology, 33, 289–348. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Sheen W & Morales M 2007. Glutamatergic neurons are present in the rat ventral tegmental area. Eur J Neurosci, 25, 106–18. [DOI] [PMC free article] [PubMed] [Google Scholar]