Abstract
In attempt to improve long-term disease control outcomes for high-risk prostate cancer, numerous clinical trials have tested the addition of chemotherapy (CTX)—either adjuvant or neoadjuvant—to definitive local therapy, either radical prostatectomy (RP) or radiation therapy (RT).
Neoadjuvant trials generally confirm safety, feasibility, and pre-RP PSA reduction, but rates of pathologic complete response are rare, and no indications for neoadjuvant CTX have been firmly established. Adjuvant regimens have included CTX alone or in combination with androgen deprivation therapy (ADT).
Here we provide a review of the relevant literature, and also quantify utilization of CTX in the definitive management of localized high-risk prostate cancer by querying the National Cancer Data Base (NCDB). Between 2004 and 2013, 177 patients (of 29,659 total) treated with definitive RT, and 995 (of 367,570 total) treated with RP had CTX incorporated into their treatment regimens. Low numbers of RT + CTX patients precluded further analysis of this population, but we investigated the impact of CTX on overall survival (OS) for patients treated with RP +/− CTX. Disease-free survival or biochemical-recurrence-free survival are not available through the NCDB. Propensity-score matching (PSM) was conducted as patients treated with CTX were a higher-risk group. For non-matched groups, OS at 5-years was 89.6% for the CTX group versus 95.6%, for the no-CTX group (p < 0.01). The difference in OS between CTX and no-CTX groups did not persist after PSM, with 5-year OS 89.6% versus 90.9%, respectively (HR 0.99; P = 0.88).
In summary, CTX was not shown to improve OS in this retrospective study. Multimodal regimens—such as RP followed by ADT, RT, and CTX; or RT in conjunction with ADT followed by CTX—have shown promise, but long-term follow-up of randomized data is required.
Keywords: chemotherapy, adjuvant, neoadjuvant, high-risk prostate cancer, radiation therapy, prostatectomy
INTRODUCTION
In attempt to improve disease control outcomes for high-risk prostate cancer, numerous clinical trials have tested the addition of chemotherapy (CTX)—either adjuvant or neoadjuvant—to definitive local therapy, either radical prostatectomy (RP) or radiation therapy (RT).
Neoadjuvant regimens supplemented to local therapy have included estramustine and etoposide,1 docetaxel alone,2–6 or docetaxel in combination with mitoxantrone,7–10 estramustine,11,12 capecitabine,13 nab-paclitaxel,14 gefitinib,15 bevacizumab,16 and/or androgen deprivation therapy (ADT).17–23 These neoadjuvant trials generally confirm feasibility, safety, and PSA reduction prior to RP, but pathologic complete response is rare and no indications for neoadjuvant CTX have been firmly established.7,17
Adjuvant regimens following RP have included CTX alone24–28 or in combination with ADT and RT.29–32 The recently published results of NRG Oncology/RTOG Study 0621—a phase 2 trial of adjuvant RT, ADT, and docetaxel for high-risk post-RP patients—are encouraging and the 3-year progression-free survival of 73% demonstrates a significant improvement from historical controls, but the authors acknowledge that randomized studies are needed.31 Following RT and ADT, the addition of adjuvant CTX also seems promising as 4-year results from the randomized phase 3 trial RTOG 0521 suggest a 10% improvement in disease-free survival, and a 4% improvement to overall survival (OS).33
Incorporation of CTX into definitive treatment regimens remains outside the routine standard of care, especially since the relative trials are generally small in number of patients included, limited in terms of long-term follow-up, mostly single arm, and heterogeneous in terms of inclusion criteria and treatment paradigms. Due to the rise of active surveillance, prostate cancer incidence seems likely to move away from low-risk patients and towards high-risk patients. Additionally, with increasing public awareness of active surveillance, some patients may be reluctant for treatment intensification, even in warranted scenarios. RT-based regimens now incorporate use of more effective dose-escalated modalities in addition to long-term ADT. With these changes to management of high-risk prostate cancer, the question of the exact scenarios for which CTX has been tested and may provide a supplemental benefit gains importance.
NEOADJUVANT CHEMOTHERAPY
In the early 1990s several reports emerged describing the effectiveness of CTX for metastatic castration-resistant prostate cancer, which was at that time a relatively novel concept.34–36 Subsequently, interest arose in moving CTX to the definitive setting. Table 1 demonstrates a comparison of the numerous trials that will be discussed below in terms of regimens, eligibility criteria, and outcomes.
Table 1.
Trial comparisons.
| Study | Regimen | Inclusion criteria; Any of the below |
Local therapy |
Number of patients who completed CTX and underwent local therapy |
Median follow- up time (months) |
Percent with recurrence* |
|---|---|---|---|---|---|---|
| Neoadjuvant CTX | ||||||
|
| ||||||
| Clark et al, 2001; Cleveland Clinic1 | Estramustine + etoposide × 3 cycles (28 day cycles) | T2b-T3; GS ≥8; PSA ≥15 | RP | 18 | 14 | 12 |
|
| ||||||
| Hussain et al, 2003; Karmanos and Michigan12 | Docetaxel + estramustine q21days for 3–6 cycles | T2b-T3; GS ≥8; PSA ≥15 | RP or RT | 28 | 130 | 64 |
|
| ||||||
| Ryan et al, 2004; Memorial Sloan Kettering39 | Vinblastine (6 weeks on, 2 weeks off) + estramustine × 2 neoadjuvant cycles, followed by concurrent vinblastine and estramustine with RT | GS ≥8 and PSA ≥10; GS ≥7 and PSA ≥20; T3 and PSA ≥20; T4; N1 | RT | 23 | 60 | 65 |
|
| ||||||
| Febbo et al, 2005; Dana Farber3 | Weekly docetaxel × 6 months | T3; GS ≥8; PSA ≥20 | RP | 19 | 26.5 | 63.2 |
|
| ||||||
| Vuky et al, 2009; Virginia Mason Medical Center15 | Docetaxel (3 weeks on 1 week off) and daily gefitinib × two months | T2b-T3; GS ≥8; PSA ≥20 | RP | 22 | 28 | 34 |
|
| ||||||
| Ross et al, 2012; Prostate Cancer Clinical Trials Consortium16 | Docetaxel × 6 (q21 days) with bevacizumab (q21 days) given with the first 5 cycles | T3; GS ≥8; PSA ≥20; PSA velocity >2 ng/mL/y | RP | 37 | N/A | 49 |
|
| ||||||
| Zhao et al, 2015; Cleveland Clinic6 | Weekly docetaxel × 6 weeks | T2b-T3; GS≥8; PSA ≥15 | RP | 28 | 49.5 | 57 |
|
| ||||||
| Bergstrom et al, 2017; Oregon/VA Portland/Washington8 | Docetaxel (weekly) + mitoxantrone (3 out of 4 weeks) × 4 months | T2c-T3a; GS ≥4+3; PSA ≥15 | RP | 54 | 120 | 63 |
|
| ||||||
| Neoadjuvant CTX + ADT | ||||||
|
| ||||||
| Pettaway et al, 2000; MD Anderson20 | LHRH agonist and antiandrogen + alternating cycles (× 12 weeks) of ketoconazole + doxorubicin or vinblastine + estramustine | T3; GS 7 with PSA ≥10; T1-2 with GS ≥8 | RP | 33 | 13 | 31 |
|
| ||||||
| Konety et al, 2004; Memorial Sloan Kettering18 | LHRH agonist + 4–6 cycles of carboplatin, paclitaxel, and estramustine | ≥T3; GS ≥8; PSA ≥20 | RP | 35 | 29 | 55 |
|
| ||||||
| Prayer-Galetti et al, 2007; Italy21 | LHRH agonist + docetaxel (q21 days) and estramustine × 4 cycles | ≥T3; GS ≥8; PSA ≥15 | RP | 18 | 53 | 58 |
|
| ||||||
| Kelly et al, 2008; CALGB 9981146 | LHRH agonist + paclitaxel weekly, carboplatin monthly, and estramustine × 4 cycles | ≥T3b; GS ≥7 and PSA >20 | RT | 27 | 38 | 70 |
|
| ||||||
| Chi et al, 2008; Canadian multicenter17 | LHRH agonist and antiandrogen + docetaxel (6 weeks with 3 or on 2 weeks off) for 3 cycles | ≥T3; GS ≥8; PSA ≥20; GS 7 with 3 or more positive cores; PSA ≥10 with 3 or more positive cores | RP | 64 | 42.7 | 30 |
|
| ||||||
| Sella et al, 2008; Israel22 | LHRH agonist and antiandrogen + docetaxel (q21days) and estramustine × 4 cycles | ≥T2c; GS ≥8; PSA ≥20 | RP | 22 | 23.6 | 45.4 |
|
| ||||||
| Mellado et al, 2009; Spain45 | LHRH agonist and antiandrogen + docetaxel (3 weeks on, 1 week off) × 3 cycles | T3; T1c-T2 with GS ≥4+3 or PSA >20 | RP | 51 | 35 | 41.2 |
|
| ||||||
| Narita et al, 2012; Akita University, Japan19 | LHRH agonist and antiandrogen + docetaxel (weekly) and estramustine × 6 weeks | ≥T3; GS ≥9; PSA ≥15 | RP | 18 | 18 | 16.7 |
|
| ||||||
| Thalgott et al, 2014; Germany23 | LHRH agonist and antiandrogen | >40% 5-yr biochemical recurrence risk62 | RP | 29 | 48.6 | 55.2 |
|
| ||||||
| Fizazi, et al. 2015; GETUG 1247 | LHRH agonist alone LHRH agonist + docetaxel (q3 weeks) and estramustine × 4 cycles | ≥T3; GS ≥8; vs. PSA >20; N1 | RP or RT | 206 vs. 207 | 105.6 | 54 vs. 43 |
|
| ||||||
| Adjuvant CTX | ||||||
|
| ||||||
| Schmidt et al, 2006; National Prostatic Cancer Project—RP Protocol49 | Cyclophosphamide q3weeks × 2 years vs. estramustine × 2 years vs. observation | T2c – T3b; N1 | RP | 184 | 120 | 56 vs. 46 vs. 46 |
|
| ||||||
| Schmidt et al, 2006; National Prostatic Cancer Project—RT Protocol49 | Cyclophosphamide q3weeks × 2 years vs. estramustine × 2 years vs. observation | T2c – T3b; N1 | RT | 253 | 120 | 77 vs. 49 vs. 63 |
|
| ||||||
| Kibel et al, 2007; Multicenter25 | Docetaxel (3 weeks on, 1 week off) × 6 cycles | >50% 3-yr biochemical recurrence | RP | 76 | 29.2 | 60.5 |
|
| ||||||
| Cetnar et al, 2008; University of Pennsylvania24 | Paclitaxel weekly (3 weeks on, 1 week off) and estramustine × 4 cycles | ≥50% 2-year PSA failure63 | RP | 17 | 24 | 30 |
|
| ||||||
| Ahlgren et al, 2016 SPCG 1253 | Docetaxel q3weeks × 6 cycles vs. survellance | pT2 with positive margin and GS ≥4+3; pT3b and GS >3+4; N1 and GS >3+4 | RP | 459 | 56.8 | 47.9 vs. 38.9 |
|
| ||||||
| Adjuvant CTX + RT + ADT | ||||||
|
| ||||||
| Hussain et al, 2012; University of Maryland55 | LHRH agonist + paclitaxel weekly concurrent with adjuvant RT | pT3N0N+ disease or rising PSA ≥ 0.05 | RP | 30 | 74.9 | 37 |
|
| ||||||
| Hurwitz et al, 2017; RTOG 062131 | LHRH agonist and antiandrogen + docetaxel (q3weeks) × 6 cycles | post-RP PSA nadir > 0.2 ng/mL and GS ≥7; post-RP PSA nadir of <0.2 but ≥pT3 and GS ≥8 | RT | 74 | 52.8 | 35.1 |
Different regimen types and lengths allow only rough comparisons due to the possibility immortal time bias. Biochemical or clinical recurrence.
Neoadjuvant chemotherapy without androgen deprivation therapy
In 1998 at Cleveland Clinic, a trial was initiated that treated high-risk patients with three cycles of estramustine and etoposide prior to RP and bilateral pelvic lymphadenectomy.1 The regimen was delivered to 18 patients, and while there was a higher than expected rate of organ-confined disease on surgical pathology, histologically, there was no evidence for antitumor effect beyond what would have been expected with ADT alone.1 This apparent lack of a markedly improved antitumor effect compared to ADT, in conjunction with a relatively high rate (17%) of thromboembolic adverse events (a known association with the synthetic steroidal estrogen, estramustine), led the authors to determine that while the regimen was feasible, other potentially more efficacious regimens should be considered.1
In 1999 at the Karmanos Cancer Institute and University of Michigan, 21 men with were treated with estramustine and docetaxel for a maximum of six cycles, this time prior to either RP or RT.12 Again, histological activity was demonstrated, but relative activity of CTX compared to ADT remained in question.12 None of the 10 patients who underwent RP had a pathologic complete response.12 To offset the thrombotic effects of estramustine (and after 3 patients developed deep venous thrombosis), low-dose warfarin was eventually instituted, which effectively stopped thromboembolism in the remaining patients.12 PSA was lowered to a degree consistent with the above Cleveland Clinic trial.1 Namely, median PSA nadir was 0.5 ng/mL, with 76% of patients achieving a 90% decline and the remainder achieving a decline between 50 – 90%.12 Comparatively, in the Cleveland clinic trial, half of the patients achieved an undetectable PSA prior to RP (less than 0.2 ng/mL), and the remaining patients had PSAs between 0.2 and 0.7 ng/mL (median 98% reduction).1
Subsequently in 2001, the Cleveland Clinic group began a new Phase II trial of weekly docetaxel alone for six weeks prior to RP.2,6 Again, reductions in serum PSA following the CTX regimen were demonstrated in the majority of the 29 patients (79%); though, only 24.1% of patients had ≥ 50% reduction in PSA,2,6 which was somewhat less than the rates demonstrated in the Karmanos/Michigan study of estramustine and docetaxel.12 Again, the neoadjuvant CTX regimen was feasible and reasonably well tolerated, with no significant increase to RP morbidities.6 None of these patients achieved a pathologic complete response.6 The most recent report of these patients describes 36% alive and recurrence-free.6 Notably, PSA response to neoadjuvant therapy—an endpoint in several of these neoadjuvant chemotherapy trials—was not found to be a predictor of long-term outcomes.6 In a later attempt by this group to see if another agent might more effectively elicit tumor response, 18 patients were treated with nab-paclitaxel prior to RP.14 Again, no pathologic complete responses were generated and only 16% had PSA reductions > 50%.14 It should be noted that there is evidence from the metastatic setting that suggests PSA reductions with CTX may be enhanced by the addition of prednisone, though it is unclear if this benefit might be translated to the neoadjuvant setting.37
In 2001 a study was open through Dana Farber that again tested neoadjuvant docetaxel, but this time docetaxel was given for 6 months instead of 6 weeks.3 Endorectal MRI was also incorporated to monitor tumor response to CTX.3 In the 19 patients, median maximum tumor volume decreased by 1.5 cm3 (48.3%) and median prostate size decreased by 3.6 cm3 (28.9%) after the six months of docetaxel.3 PSA decrease of ≥50% was noted in the majority of patients (58%) and mean PSA reduction was 64%.3 No pathologic complete responses were demonstrated.3
Also in 2001, a multicenter Phase 1/2 study opened in the Northwest; neoadjuvant treatment was with docetaxel and mitoxantrone for 16 weeks prior to RP.7–10 The regimen was safely tolerated, and of the 54 patients included in the most recent report, recurrence-free survival was 29%.8 Lymph node status, PSA density, and increased prostate VEGF expression were found to be predictive of recurrence.8
VEGF expression had been previously hypothesized to be involved in pathogenesis of prostate metastases,38 and in further investigation of VEGF as a driver of prostate cancer progression, the Prostate Cancer Clinical Trials Consortium performed a phase 2 multicenter trial, with patients treated 2006 – 2008, of docetaxel and the VEGF inhibitor bevacizumab for six cycles (21 day cycles).16 Of the 41 patients included in analysis, 22% achieved >50% decline in PSA with neoadjuvant therapy, and 29% achieved >50% decline in tumor volume on endorectal MRI.16 Again, no patients experienced a pathologic complete response.16
Other failures in obtaining pathologic complete response were demonstrated in Friedman et al (3–6 months of docetaxel and capecitabine; 40% achieving ≥50% PSA reduction),13 and Vuky et al (2 months of neoadjuvant docetaxel and gefitinib).15
Collectively, determining the effect of neoadjuvant CTX on biochemical control and pathologic disease characteristics such as surgical margin status was not possible via the above phase II trials. Testosterone monitoring conducted in the above trials generally suggests that CTX on its own does appear to act in a mechanism independent from ADT to lower PSA; the extent to which there may be additive or synergistic effects between CTX and ADT remains unclear.
Finally, with respect to neoadjuvant CTX prior to RT, this has been investigated in single-institutional Phase 2 trial, Ryan et al, which enrolled a very high-risk (relative to many of the above trials) cohort of patients to a protocol of neoadjuvant vinblastine and estramustine followed by the same regimen concurrent with RT (75.6 Gy).39,40 PSA nadirs of 0 were achieved in 70% of the 23 patients, and this seemed to be a strong predictor of outcomes, as not achieving a PSA nadir of 0 was associated with a fivefold increase in risk of developing metastatic disease.39,40
Neoadjuvant chemotherapy with androgen deprivation therapy
In 2006 the Cancer and Leukemia Group B (CALGB) 90203 randomized phase 3 trial was initiated testing RP with or without the addition of neoadjuvant ADT and docetaxel.11 Adjuvant RT is allowed at the discretion of the treating physician. Men eligible for this study must have an estimated ≤60% probability of freedom from disease recurrence at 5 years following RP.11 CALGB 90203 has met its accrual goal, though results will not be available for several years.44
Several phase II trials investigating a similar paradigm have been conducted, and confirmed the safety and feasibility of their various regimens.18,20,21 As expected, PSA values decreased dramatically with the addition of ADT, compared to the above trials of neoadjuvant CTX alone. Interestingly, an Italian study of 19 men included one patient with a pathologic complete response and another six patients (31%) with only small foci of tumor remaining comprising <10% of the prostate volume.21 Disease-free survival was found to be associated with pathologic response to neoadjuvant therapy.21 In a Canadian multicenter study of 64 men, two achieved a pathologic complete response and an additional 25% had ≤5% tumor remaining;17 in a Spanish study of 51 men, three achieved a pathologic complete response;45 and in a Japanese study of 18 men, two achieved a pathologic complete response.19 Conversely, in an Israeli study of 22 men,22 and in a German study of 30 men,23 no pathologic complete responses were demonstrated. It does seem that pathologic complete responses, though rare, are possible with the addition of ADT to neoadjuvant CTX.
Prior to RT, neoadjuvant CTX and ADT has been investigated in a multicenter phase 2 study, Cancer and Leukemia Group B (CALGB) 99811.46 In addition to ADT, patients received neoadjuvant carboplatin, paclitaxel, and estramustine.46 The regimen was found to be tolerable and feasible in the multicenter setting.46
Data from the randomized Phase 3 GETUG 12 trial—testing the addition of adjuvant docetaxel and estramustine versus ADT alone prior to definitive local therapy via RP or RT—continues to mature.47 After staging lymphadenectomy, local therapy was decided upon at multidisciplinary conference; patients with node-negative disease could undergo RP or RT, while patients with node-positive disease could undergo RT or no local therapy.47 Early results show a benefit for CTX in terms of 8-year relapse-free survival (62% vs. 50%) but not yet metastasis-free survival or OS.47
ADJUVANT CHEMOTHERAPY
Adjuvant therapy affords patients with more favorable pathologic characteristics to perhaps be spared treatment regimen intensification via RT or CTX. The obvious cost of moving treatment escalation to the adjuvant setting is that there is no chance of tumor response/downstaging—which can influence surgical outcome, prognosis, and need for adjuvant RT.
Adjuvant chemotherapy without androgen deprivation therapy
Perhaps the earliest attempt to evaluate adjuvant CTX was the National Prostate Cancer Project, that enrolled 1978 – 1985, and randomized patients, via two protocols (post-RP and post-RT, staging pelvic lymphadenectomy required for both arms), to either cyclophosphamide, estramustine, or observation.49 When interpreting the results (Table 1), it should be noted that lymph node involvement was considerably lower in the RP arm (29%) compared to the RT arm (63%).49 The authors ultimately concluded that adjuvant estramustine was of clearest benefit in RT patients with extensive (>20%) pelvic nodal involvement.49
At the University of Pennsylvania between 2001 and 2004, 17 patients with at least 50% probability of 2-year PSA failure were treated with adjuvant paclitaxel and estramustine following RP.24 The actual median risk of PSA failure in this group of men was 70%, and, interestingly, only 30% developed PSA failure (P = 0.001).24
Between 2002 – 2004, patients at >50% recurrence risk after RP accrued on a relatively large phase II multicenter trial (Kibel et al) investigating adjuvant docetaxel for six cycles.25 Using a nomogram comprised of historical controls, the median progression-free survival of 15.7 months was deemed to be better than the predicted 10 months, though 30% of patients experienced Grade ≥3 toxicity.25
Initiated in 2006, Veteran’s Affairs Cooperative Studies Program (CSP) 553 was a randomized trial testing RP with or without the addition of adjuvant docetaxel and prednisone.50,51 VA CSP 553 did not meet its accrual goal of 300 men and was closed early; so far it has been reported in abstract form only, with the underpowered results demonstrating a non-statistically significant trend for benefit in terms of progression free survival for the overall intention to treat population, but a statistically significant benefit for African American patients and ≥T3b tumors, on the pre-specified subgroup analyses.52
In 2016, results of SPCG12 were reported in abstract form; from 2005 – 2010, 459 Scandinavian men were randomized to docetaxel versus surveillance with results showing no benefit for docetaxel in terms of biochemical disease free survival.53 In fact, numerically the docetaxel arm did worse by approximately 10%, though this was not statistically significant, and the authors even suggest that a certain subgroup of patients seems to progress biochemically more rapidly with docetaxel monotherapy.53
Adjuvant chemotherapy with androgen deprivation therapy
Extrapolating from efficacy of ADT in other settings, it seems possible or even likely that the combination of CTX and ADT could be superior to CTX alone. RTOG 99-02 was a phase III randomized trial testing the addition of four cycles of paclitaxel, estramustine, and etoposide to ADT and RT.54 Due to excess thromboembolic toxicity, despite the eventual addition of warfarin, the trial was stopped early of its intended sample size of 1,440, after a total of 397 patients were accrued.54 Toxicities were considerably more common in the experimental arm—gastrointestinal, renal/genitourinary, and especially, hematologic (40% experienced Grade 3–4)—though late toxicities at 2 or 3 years were not different between arms.54
Early results of RTOG 0521—a randomized phase III trial of adjuvant ADT and six cycles of docetaxel following definitive RT in men with any of 1) Gleason ≥9; 2) ≥T2, Gleason 8, PSA <20; or 3) Gleason ≥7, PSA ≥20—have been reported in abstract form and demonstrated a statistically significant benefit for the addition of docetaxel in terms of 4-year disease-free survival (65% vs. 55%) and OS (93% vs. 89%).33 Node positive men and men with PSA >150 were excluded. Longer follow-up is planned and will reveal if the degree of these survival differences magnify over time.
Trimodality therapy—surgery, adjuvant radiation therapy, and chemotherapy
University of Maryland initiated a protocol in 1999 testing the addition of adjuvant ADT and concurrent chemoradiation (paclitaxel) following RP.55 Of the 30 patients enrolled at a median follow-up time of 74.9 months, 37% experienced biochemical progression.55
The recently published results of RTOG 0621 demonstrated favorable 3-year freedom from progression of 73%.31 This regimen was deemed well-tolerated except for Grade 3 and 4 neutropenia, and this treatment paradigm is the most uniform among the adjuvant CTX trials given that it pre-specifies use of ADT and adjuvant RT. Longer-term follow-up is needed before an analysis of OS will be performed, and a follow-up phase 3 study is being planned—NRG-GU002—which will randomize men treated with RP with high-risk post-operative features to receive ADT and adjuvant RT with or without six cycles of adjuvant docetaxel.56
In summary of the above, results from adjuvant CTX trials are encouraging, though will require confirmation in large phase 3 trials.
CHEMOTHERAPY UTILIZATION IN THE UNITED STATES
In attempt to quantify the extent to which CTX has been incorporated into definitive treatment of high-risk prostate cancer thus far in the United States, we sought to utilize the National Cancer Data Base (NCDB) to investigate utilization patterns and compare OS between regimens +/− CTX.
Methods and materials
The NCDB is a joint project of the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society, and is the largest clinical registry in the world, incorporating approximately 70% of new cancer cases from the United States.57 Given no patient identifiers are available through the NCDB, no institutional review board approval was required to conduct this investigation.
Patients that were included had histologically-proven invasive prostate adenocarcinoma and met at least one of the following criteria: American Joint Committee on Cancer (AJCC) stage ≥T2c or greater, Gleason score ≥8, or PSA ≥20. Exclusion criteria consisted of: patients with rare histologies including sarcomas, and neuroendocrine/small-cell cancers, metastatic patients, cases with missing outcomes, and cases with unknown CTX status. Node positive patients were not excluded.
Cases that were treated with CTX more than 8 months following local therapy were excluded in order to maintain a population of non-metastatic patients who received either neoadjuvant or adjuvant CTX only. Eight months was selected as a reasonable cutoff beyond which CTX delivery would be more likely to be for metastatic and not adjuvant treatment—while still allowing an adequate interval for the completion of adjuvant RT following RP, if delivered.
Incorporation of CTX into the treatment regimen remains investigational and a non-standard of care approach, and so low numbers of cases were to be expected. Patients treated with either local therapy—RP or RT were both initially included. It became apparent that the number of patients treated with definitive RT and CTX was too small to pursue further statistical analysis of OS. The number of patients treated with RP and CTX was considerably higher, so we proceeded with analysis of OS. Statistical analysis was conducted using SAS Version 9.4 with software macros designed by the Biostatistics and Bioinformatics Shared Resource of the Winship Cancer Institute at Emory University.58 The significance level was set at 0.05. Descriptive statistics were generated to summarize patient, disease, and treatment characteristics. Patients receiving neoadjuvant and adjuvant CTX were grouped together to preserve sample size. The univariate associations between covariates and study cohorts (CTX vs. no CTX) were assessed using the Chi-square test for categorical covariates and ANOVA for numerical covariates. Univariate analysis (UVA) between each covariate including study cohorts and study outcome were assessed using Cox proportional hazards models and log-rank tests, and logistic regression for binary cohorts (CTX). Start date for OS was established as the date of onset of definitive treatment, which was taken as RP unless neoadjuvant CTX was utilized, in which case the start date was established as the initiation of CTX, because, as described above, clinical trials testing the addition of neoadjuvant CTX have demonstrated high degree of tumor down-staging, and progression during neoadjuvant CTX is very rare. In multivariable analysis (MVA), the Cox proportional hazard model was applied for OS, and the model was built by a backward variable selection method applying an alpha = .20 removal criteria. Kaplan Meier plots were produced to compare the survival curves by cohorts.
Propensity score matching (PSM) method was also implemented to reduce treatment selection bias. A logistic regression model predicting CTX was carried out to estimate the propensity score by covariates that predict OS in multivariable model and known confounders. Patients from the CTX group were matched to the no CTX group at a ratio of 1:5 based on the propensity score using a greedy algorithm.59 After matching, the balance of covariates between the two cohorts was evaluated by the standardized differences and a value of < 0.1 was considered as negligible imbalance.60 The effects were estimated in the matched sample by a Cox model with a robust variance estimator for OS.61
Results
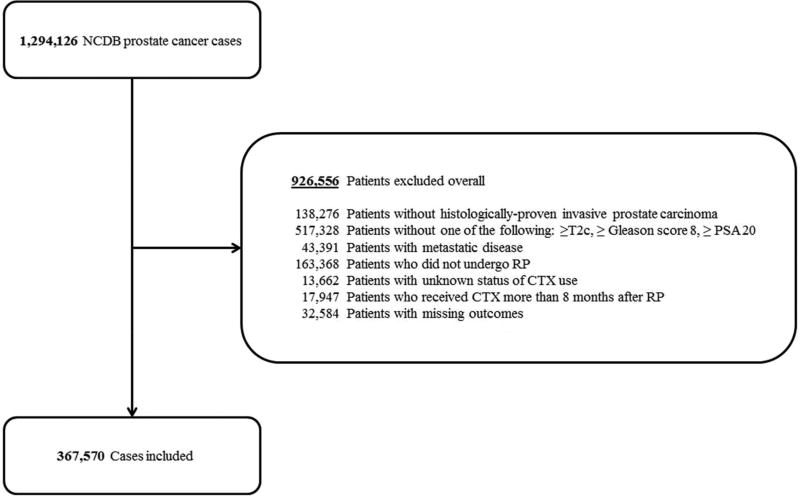
High-risk prostate cancer patients diagnosed between 2004 and 2013 were included in the analysis. During this timeframe, 29,659 patients who met the above criteria were treated with definitive RT, and only 177 of these had CTX incorporated into the treatment regimen; 367,570 patients who met the above criteria underwent RP, and 995 of these patients received CTX. Given the small numbers of patients treated with RT and CTX, only analysis of RP patients treated with CTX was continued. For Consolidated Standards of Reporting Trials (CONSORT) diagram outlining change in RP patient numbers by exclusion criteria, see Figure 1.
Figure 1.
Consolidated Standards of Reporting Trials (CONSORT) diagram.
NCDB = National Cancer Data Base; PSA = Prostate-specific antigen; RP = Radical prostatectomy; CTX = Chemotherapy
For UVA of patient and tumor characteristics by cohort, please see Table 2. All of following were associated (all P < 0.001, unless otherwise specified) with use of CTX: younger age, white race (P = 0.007), academic/research facility type, earlier year of diagnosis, increasing clinical and pathologic T stage, positive clinical and pathologic N stage, positive surgical margins, increasing Gleason score, increasing PSA, positive surgical margins, use of RT, and use of ADT. Distance to facility was not significantly different between CTX and no CTX groups (P = 0.512). The CTX group had a longer median time to RP after diagnosis compared to the no CTX group (111 versus 76.5 days, respectively; P < 0.001). However, when considering median time from diagnosis to start of any definitive treatment regimen (either RP or start date of CTX in the case of neoadjuvant CTX treatment), the CTX group had a shorter median time to onset of therapy compared to the no CTX group (51 versus 65 days, respectively; P < 0.001). The CTX group had a longer median duration to the start of ADT compared to the no CTX group (81 versus 66 days, respectively; P < 0.001).
Table 2.
Patient and Clinical Characteristics and Treatment Details by Cohort
| Variable | No CTX (n = 366,575) | CTX (n = 995) | P* |
|---|---|---|---|
| Age at diagnosis, median, y | 61 | 60 | < 0.001 |
| Race, No. (%) | |||
| White | 304,387 (83.0) | 862 (86.6) | 0.007 |
| Black | 45,367 (12.4) | 92 (9.3) | |
| Others/Unknown | 16,821 (4.6) | 41 (4.1) | |
| Charlson-Deyo Score, No. (%) | |||
| 0 | 307,154 (83.8) | 852 (85.6) | 0.113 |
| 1 | 52,957 (14.4) | 122 (12.3) | |
| 2+ | 6,464 (1.8) | 21 (2.1) | |
| Facility Type, No. (%) | |||
| Non-Academic/Research Program | 210,830 (57.6) | 334 (33.6) | < 0.001 |
| Academic/Research Program | 155,393 (42.4) | 659 (66.3) | |
| Year of Diagnosis, No. (%) | |||
| ≥ 2004 – ≤ 2007 | 129,192 (35.2) | 501 (50.4) | < 0.001 |
| > 2007 – ≤ 2009 | 83,964 (22.9) | 220 (22.1) | |
| > 2009 – ≤ 2011 | 83,900 (22.9) | 162 (16.3) | |
| > 2011 – ≤ 2013 | 69,519 (19.0) | 112 (11.3) | |
| AJCC clinical T stage, No. (%) | |||
| 1 | 213,891 (58.4) | 328 (33.0) | < 0.001 |
| 2 | 89,747 (24.5) | 319 (32.1) | |
| 3 | 10,243 (2.8) | 164 (16.5) | |
| 4 | 358 (0.1) | 15 (1.5) | |
| Unknown | 52,336 (14.3) | 169 (17.0) | |
| AJCC clinical N stage, No. (%) | |||
| 0 | 282,538 (77.1) | 663 (66.6) | < 0.001 |
| 1 | 1,067 (0.3) | 47 (4.7) | |
| Unknown | 82,970 (22.6) | 285 (28.6) | |
| AJCC pathologic T stage, No. (%) | |||
| 1–2 | 26,1170 (71.2) | 242 (24.3) | < 0.001 |
| 3 | 93,604 (25.5) | 630 (63.3) | |
| 4 | 2,113 (0.6) | 38 (3.8) | |
| Unknown | 9,688 (2.6) | 85 (8.5) | |
| AJCC pathologic N stage, No. (%) | |||
| 0 | 263,117 (71.8) | 623 (62.6) | < 0.001 |
| 1 | 9,745 (2.7) | 253 (25.4) | |
| Unknown | 93,713 (25.6) | 119 (12.0) | |
| Gleason Score, No. (%) | |||
| 2–7 | 315,957 (86.2) | 348 (35.0) | < 0.001 |
| 8–10 | 45,214 (12.3) | 585 (58.8) | |
| Unknown | 5,404 (1.5) | 62 (6.2) | |
| PSA, No. (%) | |||
| < 10 | 34,719 (9.5) | 187 (18.8) | < 0.001 |
| ≤ 10- < 20 | 264,260 (72.1) | 530 (53.3) | |
| ≥ 20 | 24,624 (6.7) | 198 (19.9) | |
| Unknown | 42,972 (11.7) | 80 (8.0) | |
| Surgical Margin, No. (%) | |||
| Negative | 275,028 (75.0) | 529 (53.2) | < 0.001 |
| Positive | 87,590 (23.9) | 414 (41.6) | |
| Unknown | 3,957 (1.1) | 52 (5.2) | |
| RT, No. (%) | |||
| No RT | 344,656 (94.0) | 727 (73.1) | < 0.001 |
| Adjuvant RT | 15,781 (4.3) | 196 (19.7) | |
| RT–adjuvant criteria not met | 6,138 (1.7) | 72 (7.2) | |
| ADT, No. (%) | |||
| No | 338,908 (92.4) | 346 (34.8) | < 0.001 |
| Yes | 18,193 (5.0) | 634 (63.7) | |
| Unknown | 9,474 (2.6) | 15 (1.5) |
Abbreviations: CTX, Chemotherapy; AJCC, American Joint Committee on Cancer; PSA, Prostate-specific antigen; RT, Radiation therapy; ADT, Androgen deprivation therapy
Bolded P Values are significant
In the MVA (Table 3), use of CTX was associated with inferior OS with a hazard ratio (HR) of 1.19 (95% confidence interval [CI] 1.01 – 1.39; P = 0.039). As expected, age at diagnosis, race, Charlson-Deyo score, facility type, AJCC clinical T and N stage, AJCC pathologic T and N stage, Gleason score, PSA, and surgical margin status were all significantly predictive for OS.
Table 3.
Multivariable Subgroup Analysis of Overall Survival for the No CTX and CTX Cohorts
| Variable | Hazard Ratio (95% CI) | P* |
|---|---|---|
| Chemotherapy use | ||
| Yes | 1.19 (1.01 – 1.39) | 0.039 |
| No | ― | ― |
| Age at diagnosis, median | 1.06 (1.06 – 1.06) | < 0.001 |
| Race, No. (%) | ||
| White | ― | ― |
| Black | 1.41 (1.35 – 1.47) | < 0.001 |
| Others/Unknown | 0.78 (0.72 – 0.84) | < 0.001 |
| Charlson-Deyo Score, No. (%) | ||
| 0 | ― | ― |
| 1 | 1.59 (1.54 – 1.65) | < 0.001 |
| 2+ | 2.59 (2.41 – 2.77) | < 0.001 |
| Facility Type, No. (%) | ||
| Non-Academic/Research Program | ― | ― |
| Academic/Research Program | 0.87 (0.84 – 0.90) | < 0.001 |
| Year of Diagnosis, No. (%) | ||
| ≥ 2004 – ≤ 2007 | ― | ― |
| > 2007 – ≤ 2009 | 1.05 (1.01 – 1.09) | 0.023 |
| > 2009 – ≤ 2011 | 1.05 (0.99 – 1.10) | 0.082 |
| > 2011 – ≤ 2013 | 1.08 (1.00 – 1.16) | 0.050 |
| AJCC clinical T stage, No. (%) | ||
| 1 | ― | ― |
| 2 | 1.11 (1.08 – 1.15) | < 0.001 |
| 3 | 1.30 (1.22 – 1.39) | < 0.001 |
| 4 | 1.52 (1.19 – 1.93) | < 0.001 |
| Unknown | 1.12 (1.07 – 1.18) | < 0.001 |
| AJCC clinical N stage, No. (%) | ||
| 0 | ― | ― |
| 1 | 1.22 (1.03 – 1.42) | 0.018 |
| Unknown | 0.99 (0.95 – 1.04) | 0.739 |
| AJCC pathologic T stage, No. (%) | ||
| 1–2 | ― | ― |
| 3 | 1.35 (1.31 – 1.40) | < 0.001 |
| 4 | 2.07 (1.86 – 2.31) | < 0.001 |
| Unknown | 1.16 (1.07 – 1.25) | < 0.001 |
| AJCC pathologic N stage, No. (%) | ||
| 0 | ― | ― |
| 1 | 1.55 (1.45 – 1.66) | < 0.001 |
| Unknown | 0.97 (0.93 – 1.01) | 0.101 |
| Gleason Score, No. (%) | ||
| 2–7 | ― | ― |
| 8–10 | 1.65 (1.60 – 1.71) | < 0.001 |
| Unknown | 1.23 (1.12 – 1.35) | < 0.001 |
| PSA, No. (%) | ||
| < 10 | 0.82 (0.78 – 0.85) | < 0.001 |
| ≤ 10– < 20 | ― | ― |
| ≥ 20 | 0.97 (0.92 – 1.03) | 0.381 |
| Unknown | 0.94 (0.89 – 0.99) | 0.028 |
| Surgical Margin, No. (%) | ||
| Negative | ― | ― |
| Positive | 1.15 (1.12 – 1.19) | < 0.001 |
| Unknown | 0.93 (0.82 – 1.06) | 0.277 |
Abbreviations: CTX, Chemotherapy; CI, Confidence interval; AJCC, American Joint Committee on Cancer; PSA, Prostate-specific antigen; ADT, Androgen deprivation therapy
Bolded P Values are significant
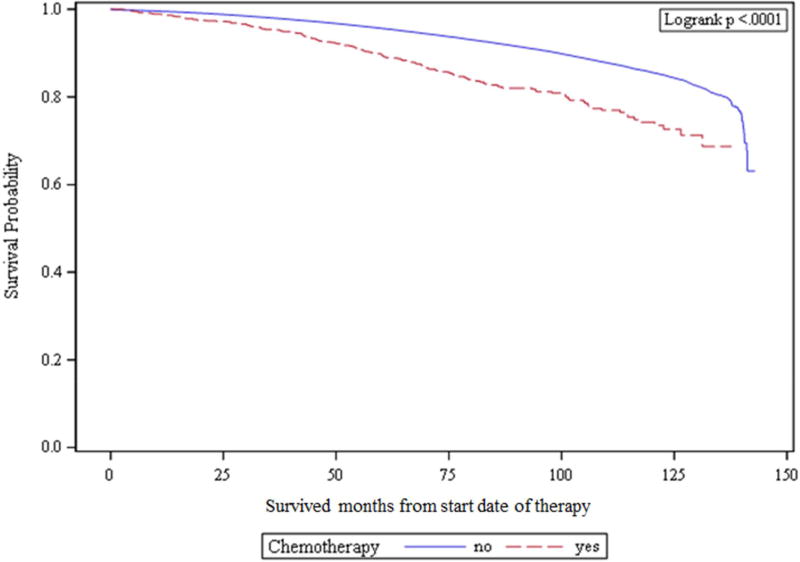
Kaplan-Meier analysis demonstrated 89.6% versus 96.5% 10-year OS for CTX and no CTX groups, respectively (P < 0.01). See Figure 2 for Kaplan Meier curves demonstrating OS for unmatched cohorts.
Figure 2.
Kaplan-Meier curves demonstrating overall survival for unmatched cohorts.
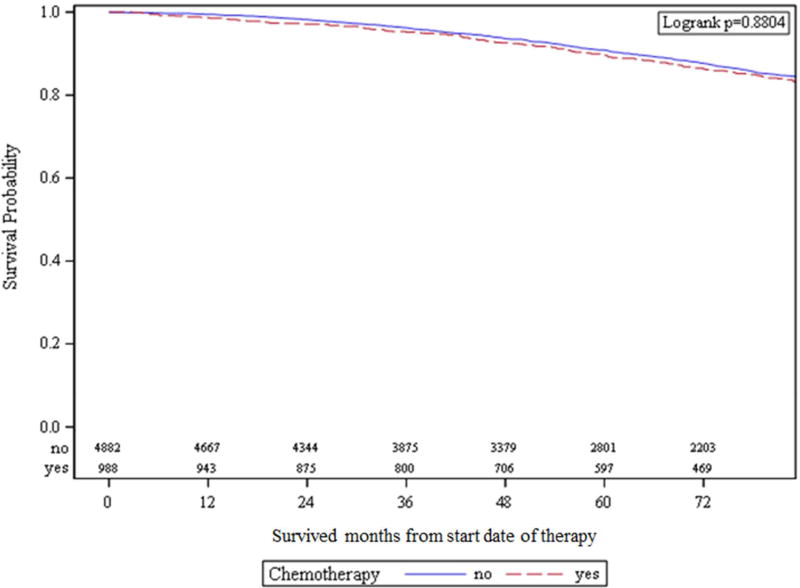
With PSM by ratio of 1:5, a total of 5,870 patients were matched—4882 patients in the no CTX group and 988 patients in the CTX group. Table 4 demonstrates balance check for PSM groups; groups were well-matched. After PSM, 10-year OS was not significantly different between groups—89.6% versus 90.9% for the CTX and the no CTX groups, respectively (Figure 3; HR 0.99; 95% CI 0.82 – 1.19; P = 0.88).
Table 4.
Patient and Clinical Characteristics and Treatment Details for Propensity-Matched Cohorts
| Variable | No CTX | CTX | P | SD |
|---|---|---|---|---|
| Age at diagnosis, median, y (SD) | 59.79 (7.39) | 59.45 (7.46) | 0.332 | 0.034 |
| Race, No. (%) | ||||
| White | 4,204 (86.11) | 857 (86.74) | 0.871 | 0.018 |
| Black | 464 (9.50) | 90 (9.11 | 0.014 | |
| Others/Unknown | 214 (4.38) | 41 (4.15) | 0.012 | |
| Charlson-Deyo Score, No. (%) | ||||
| 0 | 4,143 (84.86) | 845 (85.53) | 0.813 | 0.019 |
| 1 | 639 (13.09) | 122 (12.35) | 0.022 | |
| 2+ | 100 (2.05) | 21 (2.13) | 0.005 | |
| Facility Type, No. (%) | ||||
| Non-Academic/Research Program | 1,752 (35.89) | 334 (33.81) | 0.213 | 0.044 |
| Academic/Research Program | 3,130 (64.11) | 654 (66.19) | 0.044 | |
| Year of Diagnosis, No. (%) | ||||
| ≥ 2004 – ≤ 2007 | 2,415 (49.47) | 494 (50.00) | 0.966 | 0.011 |
| > 2007 – ≤ 2009 | 1,075 (22.02) | 220 (22.27) | 0.006 | |
| > 2009 – ≤ 2011 | 830 (17.00) | 162 (16.40) | 0.016 | |
| > 2011 – ≤ 2013 | 562 (11.51) | 112 (11.34) | 0.006 | |
| AJCC clinical T stage, No. (%) | ||||
| 1 | 1,636 (33.51) | 328 (33.20) | 0.887 | 0.007 |
| 2 | 1,613 (33.04) | 319 (32.29) | 0.016 | |
| 3 | 733 (15.01) | 159 (16.09) | 0.030 | |
| 4 | 63 (1.29) | 15 (1.52) | 0.019 | |
| Unknown | 837 (17.14) | 167 (16.90) | 0.006 | |
| AJCC clinical N stage, No. (%) | ||||
| 0 | 3,316 (67.92) | 659 (66.7) | 0.642 | 0.026 |
| 1 | 201 (4.12) | 46 (4.66) | 0.026 | |
| Unknown | 1,365 (27.96) | 283 (28.64) | 0.015 | |
| AJCC pathologic T stage, No. (%) | ||||
| 1–2 | 1,110 (22.74) | 242 (24.49) | 0.646 | 0.041 |
| 3 | 3,197 (65.49) | 628 (63.56) | 0.040 | |
| 4 | 178 (3.65) | 38 (3.85) | 0.011 | |
| Unknown | 397 (8.13) | 80 (8.1) | 0.001 | |
| AJCC pathologic N stage, No. (%) | ||||
| 0 | 3,114 (63.79) | 621 (62.85) | 0.786 | 0.019 |
| 1 | 1,175 (24.07) | 248 (25.1) | 0.024 | |
| Unknown | 593 (12.15) | 119 (12.04) | 0.003 | |
| Gleason Score, No. (%) | ||||
| 2–7 | 1,659 (33.98) | 347 (35.12) | 0.736 | 0.024 |
| 8–10 | 2,951 (60.45) | 584 (59.11) | 0.027 | |
| Unknown | 272 (5.57) | 57 (5.77) | 0.009 | |
| PSA, No. (%) | ||||
| < 10 | 977 (20.01) | 186 (18.83) | 0.845 | 0.030 |
| ≤ 10– < 20 | 2,556 (52.36) | 529 (53.54) | 0.024 | |
| ≥ 20 | 983 (20.14) | 198 (20.04) | 0.002 | |
| Unknown | 366 (7.50) | 75 (7.59) | 0.004 | |
| Surgical Margin, No. (%) | ||||
| Negative | 2,596 (53.17) | 529 (53.54) | 0.901 | 0.007 |
| Positive | 2,066 (42.32) | 412 (41.70) | 0.013 | |
| Unknown | 220 (4.51) | 47 (4.76) | 0.012 | |
| ADT, No. (%) | ||||
| No | 1,704 (34.90) | 345 (34.92) | 0.963 | 0.000 |
| Yes | 3,098 (63.46) | 628 (63.56) | 0.002 | |
| Unknown | 80 (1.64) | 15 (1.52) | 0.010 | |
| Time from diagnosis to treatment onset (RP or CTX), median, days (SD) | 61.24 (39.52) | 59.75 (49.99) | 0.301 | 0.035 |
Abbreviations: CTX, Chemotherapy; SD, Standardized difference; AJCC, American Joint Committee on Cancer; PSA, Prostate-specific antigen; ADT, Androgen deprivation therapy
Figure 3.
Kaplan-Meier curves demonstrating overall survival for propensity-matched cohorts.
Discussion
Low numbers of high-risk prostate cancer patients have been treated in the United States with regimens including CTX, and these patients are an especially high-risk group within the high-risk criteria. This was to be expected, as incorporation of CTX into a definitive treatment of prostate cancer is still, at this point, non-standard, and so a patient treated with CTX would have been most likely to be either 1) very high risk and judged by his physicians to potentially benefit from addition of CTX to improve outcomes or 2) enrolled on a clinical trial specifically testing addition of CTX. The numbers of patients for whom CTX was incorporated into the treatment regimen was significantly higher than the sum of all patients enrolled on known relevant clinical trials, meaning that at least some patients are being treated with this technique off-trial.
With PSM, the inferior OS for the CTX group dissipated, suggesting that in a group of more closely matched patients, at the very least CTX does not appear to worsen long-term OS. Notably, however, the NCDB is unable to account for potential lasting morbidities caused by CTX—such as neuropathy—which can impact quality of life in long-term survivors of treatment. Even though results from our PSM groups do not show a benefit from CTX in terms of OS, it is possible that a benefit might be seen in terms of freedom from biochemical recurrence, disease-free survival, or distant metastasis-free survival, all of which are not available through the NCDB. Additionally, PSM using the NCDB cannot account for many prognostic factors for RP patients—including pre-treatment PSA velocity, post-RP PSA nadir, and perineural invasion. While summed Gleason score is available, more than half of the patients in the database have unknown values of the primary and secondary Gleason patterns, and so this is another factor that cannot be taken into account via PSM. The NCDB also does not distinguish between adjuvant and early-salvage RT, meaning our adjuvant RT group is likely a mix between these different scenarios, with early-salvage RT representing a more unfavorable population. Identification of specific CTX agents is also not available in the NCDB. Due to evolving CTX paradigms (towards basis around docetaxel), many of the patients included in the NCDB during the timeframe analyzed were likely treated with regimens that would no longer be favored. Finally, the NCDB is subject to the inherent limitations of retrospective data; for example, it is possible that a small portion of our patient population may have been treated without definitive intent due to errors in coding, despite our careful selection criteria. It should be noted that the NCDB database used for this analysis ends at 2014, and the incidence of CTX use may rise in the future with ongoing publications of positive clinical trials.
CONCLUSIONS
Incorporation of CTX into the treatment regimen for high-risk prostate cancer patients treated with RP remains uncommon and investigational, as demonstrated by the available literature, and this analysis of the NCDB. Multimodal regimens such as RP followed by ADT, RT, and CTX, or RT in conjunction with ADT followed by CTX, have shown promise, but will require long-term follow-up of randomized data—treatment with these regimens should still be explicitly reserved for the clinical trial setting. Further studies may elucidate the particular clinical and pathologic scenarios for which a benefit from CTX might be maximized.
Acknowledgments
This study was supported by NIH/NINR K99R00NR014587 and the Oncology Nursing Society Foundation. Research reported in this publication was also supported in part by the Biostatistics and Bioinformatics Shared Resource of Winship Cancer Institute of Emory University and NIH/NCI under award number P30CA138292. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The National Cancer Data Base is a joint program of the Commission on Cancer of the American College of Surgeons and the American Cancer Society.
Abbreviation List
- CTX
Chemotherapy
- RP
Radical prostatectomy
- ADT
Androgen deprivation therapy
- RT
Radiation therapy
- NCDB
National Cancer Data Base
- OS
Overall survival
- CoC
Commission on Cancer
- AJCC
American Joint Committee on Cancer
- PSA
Prostate-specific antigen
- UVA
Univariate analysis
- MVA
Multivariable analysis
- PSM
Propensity score matching
- HR
Hazard ratio
- CI
Confidence interval
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: No conflicts to declare.
References
- 1.Clark PE, Peereboom DM, Dreicer R, Levin HS, Clark SB, Klein EA. Phase II trial of neoadjuvant estramustine and etoposide plus radical prostatectomy for locally advanced prostate cancer. Urology. 2001;57:281–5. doi: 10.1016/s0090-4295(00)00914-6. [DOI] [PubMed] [Google Scholar]
- 2.Dreicer R, Magi-Galluzzi C, Zhou M, et al. Phase II trial of neoadjuvant docetaxel before radical prostatectomy for locally advanced prostate cancer. Urology. 2004;63:1138–42. doi: 10.1016/j.urology.2004.01.040. [DOI] [PubMed] [Google Scholar]
- 3.Febbo PG, Richie JP, George DJ, et al. Neoadjuvant docetaxel before radical prostatectomy in patients with high-risk localized prostate cancer. Clin Cancer Res. 2005;11:5233–40. doi: 10.1158/1078-0432.CCR-05-0299. [DOI] [PubMed] [Google Scholar]
- 4.Magi-Galluzzi C, Zhou M, Reuther AM, Dreicer R, Klein EA. Neoadjuvant docetaxel treatment for locally advanced prostate cancer: a clinicopathologic study. Cancer. 2007;110:1248–54. doi: 10.1002/cncr.22897. [DOI] [PubMed] [Google Scholar]
- 5.Oh WK, George DJ, Kaufman DS, et al. Neoadjuvant docetaxel followed by radical prostatectomy in patients with high-risk localized prostate cancer: a preliminary report. Semin Oncol. 2001;28:40–4. doi: 10.1016/s0093-7754(01)90153-8. [DOI] [PubMed] [Google Scholar]
- 6.Zhao B, Yerram NK, Gao T, Dreicer R, Klein EA. Long-term survival of patients with locally advanced prostate cancer managed with neoadjuvant docetaxel and radical prostatectomy. Urol Oncol. 2015;33:164 e19–23. doi: 10.1016/j.urolonc.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 7.Beer TM, Garzotto M, Lowe BA, et al. Phase I study of weekly mitoxantrone and docetaxel before prostatectomy in patients with high-risk localized prostate cancer. Clin Cancer Res. 2004;10:1306–11. doi: 10.1158/1078-0432.ccr-1021-03. [DOI] [PubMed] [Google Scholar]
- 8.Bergstrom CP, Ruffell B, Ho CM, et al. Docetaxel and mitoxantrone before radical prostatectomy in men with high-risk prostate cancer: 10-year follow-up and immune correlates. Anticancer Drugs. 2017;28:120–6. doi: 10.1097/CAD.0000000000000438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garzotto M, Higano CS, O'Brien C, et al. Phase 1/2 study of preoperative docetaxel and mitoxantrone for high-risk prostate cancer. Cancer. 2010;116:1699–708. doi: 10.1002/cncr.24960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garzotto M, Myrthue A, Higano CS, Beer TM. Neoadjuvant mitoxantrone and docetaxel for high-risk localized prostate cancer. Urol Oncol. 2006;24:254–9. doi: 10.1016/j.urolonc.2005.11.034. [DOI] [PubMed] [Google Scholar]
- 11.Eastham JA, Kelly WK, Grossfeld GD, Small EJ, Cancer, Leukemia Group B Cancer and Leukemia Group B (CALGB) 90203: a randomized phase 3 study of radical prostatectomy alone versus estramustine and docetaxel before radical prostatectomy for patients with high-risk localized disease. Urology. 2003;62(Suppl 1):55–62. doi: 10.1016/j.urology.2003.09.052. [DOI] [PubMed] [Google Scholar]
- 12.Hussain M, Smith DC, El-Rayes BF, et al. Neoadjuvant docetaxel and estramustine chemotherapy in high-risk/locallyadvanced prostate cancer. Urology. 2003;61:774–80. doi: 10.1016/s0090-4295(02)02519-0. [DOI] [PubMed] [Google Scholar]
- 13.Friedman J, Dunn RL, Wood D, et al. Neoadjuvant docetaxel and capecitabine in patients with high risk prostate cancer. J Urol. 2008;179:911–5. doi: 10.1016/j.juro.2007.10.064. discussion 5–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shepard DR, Dreicer R, Garcia J, et al. Phase II trial of neoadjuvant nab-paclitaxel in high risk patients with prostate cancer undergoing radical prostatectomy. J Urol. 2009;181:1672–7. doi: 10.1016/j.juro.2008.11.121. discussion 7. [DOI] [PubMed] [Google Scholar]
- 15.Vuky J, Porter C, Isacson C, et al. Phase II trial of neoadjuvant docetaxel and gefitinib followed by radical prostatectomy in patients with high-risk, locally advanced prostate cancer. Cancer. 2009;115:784–91. doi: 10.1002/cncr.24092. [DOI] [PubMed] [Google Scholar]
- 16.Ross RW, Galsky MD, Febbo P, et al. Phase 2 study of neoadjuvant docetaxel plus bevacizumab in patients with high-risk localized prostate cancer: a Prostate Cancer Clinical Trials Consortium trial. Cancer. 2012;118:4777–84. doi: 10.1002/cncr.27416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chi KN, Chin JL, Winquist E, Klotz L, Saad F, Gleave ME. Multicenter phase II study of combined neoadjuvant docetaxel and hormone therapy before radical prostatectomy for patients with high risk localized prostate cancer. J Urol. 2008;180:565–70. doi: 10.1016/j.juro.2008.04.012. discussion 70. [DOI] [PubMed] [Google Scholar]
- 18.Konety BR, Eastham JA, Reuter VE, et al. Feasibility of radical prostatectomy after neoadjuvant chemohormonal therapy for patients with high risk or locally advanced prostate cancer: results of a phase I/II study. J Urol. 2004;171:709–13. doi: 10.1097/01.ju.0000108122.36893.5a. [DOI] [PubMed] [Google Scholar]
- 19.Narita S, Tsuchiya N, Kumazawa T, et al. Short-term clinicopathological outcome of neoadjuvant chemohormonal therapy comprising complete androgen blockade, followed by treatment with docetaxel and estramustine phosphate before radical prostatectomy in Japanese patients with high-risk localized prostate cancer. World J Surg Oncol. 2012;10:1. doi: 10.1186/1477-7819-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pettaway CA, Pisters LL, Troncoso P, et al. Neoadjuvant chemotherapy and hormonal therapy followed by radical prostatectomy: feasibility and preliminary results. J Clin Oncol. 2000;18:1050–7. doi: 10.1200/JCO.2000.18.5.1050. [DOI] [PubMed] [Google Scholar]
- 21.Prayer-Galetti T, Sacco E, Pagano F, et al. Long-term follow-up of a neoadjuvant chemohormonal taxane-based phase II trial before radical prostatectomy in patients with non-metastatic high-risk prostate cancer. BJU Int. 2007;100:274–80. doi: 10.1111/j.1464-410X.2007.06760.x. [DOI] [PubMed] [Google Scholar]
- 22.Sella A, Zisman A, Kovel S, Yarom N, Leibovici D, Lindner A. Neoadjuvant chemohormonal therapy in poor-prognosis localized prostate cancer. Urology. 2008;71:323–7. doi: 10.1016/j.urology.2007.08.060. [DOI] [PubMed] [Google Scholar]
- 23.Thalgott M, Horn T, Heck MM, et al. Long-term results of a phase II study with neoadjuvant docetaxel chemotherapy and complete androgen blockade in locally advanced and high-risk prostate cancer. J Hematol Oncol. 2014;7:20. doi: 10.1186/1756-8722-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cetnar JP, Malkowicz SB, Palmer SC, Wein AJ, Vaughn DJ. Pilot trial of adjuvant paclitaxel plus estramustine in resected high-risk prostate cancer. Urology. 2008;71:942–6. doi: 10.1016/j.urology.2007.11.117. [DOI] [PubMed] [Google Scholar]
- 25.Kibel AS, Rosenbaum E, Kattan MW, et al. Adjuvant weekly docetaxel for patients with high risk prostate cancer after radical prostatectomy: a multi-institutional pilot study. J Urol. 2007;177:1777–81. doi: 10.1016/j.juro.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 26.Ploussard G, Paule B, Salomon L, et al. Pilot trial of adjuvant paclitaxel plus androgen deprivation for patients with high-risk prostate cancer after radical prostatectomy: results on toxicity, side effects and quality-of-life. Prostate Cancer Prostatic Dis. 2010;13:97–101. doi: 10.1038/pcan.2009.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmidt JD, Gibbons RP, Murphy GP, Bartolucci A. Adjuvant therapy for clinical localized prostate cancer treated with surgery or irradiation. Eur Urol. 1996;29:425–33. doi: 10.1159/000473791. [DOI] [PubMed] [Google Scholar]
- 28.Wang J, Halford S, Rigg A, Roylance R, Lynch M, Waxman J. Adjuvant mitozantrone chemotherapy in advanced prostate cancer. BJU Int. 2000;86:675–80. doi: 10.1046/j.1464-410x.2000.00894.x. [DOI] [PubMed] [Google Scholar]
- 29.Dorff TB, Flaig TW, Tangen CM, et al. Adjuvant androgen deprivation for high-risk prostate cancer after radical prostatectomy: SWOG S9921 study. J Clin Oncol. 2011;29:2040–5. doi: 10.1200/JCO.2010.32.2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guttilla A, Bortolus R, Giannarini G, et al. Multimodal treatment for high-risk prostate cancer with high-dose intensity-modulated radiation therapy preceded or not by radical prostatectomy, concurrent intensified-dose docetaxel and long-term androgen deprivation therapy: results of a prospective phase II trial. Radiat Oncol. 2014;9:24. doi: 10.1186/1748-717X-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hurwitz MD, Harris J, Sartor O, et al. Adjuvant radiation therapy, androgen deprivation, and docetaxel for high-risk prostate cancer postprostatectomy: Results of NRG Oncology/RTOG study 0621. Cancer. 2017 doi: 10.1002/cncr.30620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schweizer MT, Huang P, Kattan MW, et al. Adjuvant leuprolide with or without docetaxel in patients with high-risk prostate cancer after radical prostatectomy (TAX-3501): important lessons for future trials. Cancer. 2013;119:3610–8. doi: 10.1002/cncr.28270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sandler HM, Hu C, Rosenthal SA, et al. A phase III protocol of androgen suppression and 3DCRT/IMRT versus AS and 3DCRT/IMRT followed by chemotherapy with docetaxel and prednisone for localized, high-risk prostate cancer (RTOG 0521). Abstract LBA5002; Presented May 31, 2015 at the ASCO 2015 Annual Meeting. [Google Scholar]
- 34.Hudes GR, Greenberg R, Krigel RL, et al. Phase II study of estramustine and vinblastine, two microtubule inhibitors, in hormone-refractory prostate cancer. J Clin Oncol. 1992;10:1754–61. doi: 10.1200/JCO.1992.10.11.1754. [DOI] [PubMed] [Google Scholar]
- 35.Pienta KJ, Redman B, Hussain M, et al. Phase II evaluation of oral estramustine and oral etoposide in hormone-refractory adenocarcinoma of the prostate. J Clin Oncol. 1994;12:2005–12. doi: 10.1200/JCO.1994.12.10.2005. [DOI] [PubMed] [Google Scholar]
- 36.Sella A, Kilbourn R, Amato R, et al. Phase II study of ketoconazole combined with weekly doxorubicin in patients with androgen-independent prostate cancer. J Clin Oncol. 1994;12:683–8. doi: 10.1200/JCO.1994.12.4.683. [DOI] [PubMed] [Google Scholar]
- 37.Teply BA, Luber B, Denmeade SR, Antonarakis ES. The influence of prednisone on the efficacy of docetaxel in men with metastatic castration-resistant prostate cancer. Prostate Cancer Prostatic Dis. 2016;19:72–8. doi: 10.1038/pcan.2015.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roberts E, Cossigny DA, Quan GM. The role of vascular endothelial growth factor in metastatic prostate cancer to the skeleton. Prostate Cancer. 2013;2013:418340. doi: 10.1155/2013/418340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ryan CJ, Zelefsky MJ, Heller G, et al. Five-year outcomes after neoadjuvant chemotherapy and conformal radiotherapy in patients with high-risk localized prostate cancer. Urology. 2004;64:90–4. doi: 10.1016/j.urology.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 40.Zelefsky MJ, Kelly WK, Scher HI, et al. Results of a phase II study using estramustine phosphate and vinblastine in combination with high-dose three-dimensional conformal radiotherapy for patients with locally advanced prostate cancer. J Clin Oncol. 2000;18:1936–41. doi: 10.1200/JCO.2000.18.9.1936. [DOI] [PubMed] [Google Scholar]
- 41.Powell IJ, Tangen CM, Miller GJ, et al. Neoadjuvant therapy before radical prostatectomy for clinical T3/T4 carcinoma of the prostate: 5-year followup, Phase II Southwest Oncology Group Study 9109. J Urol. 2002;168:2016–9. doi: 10.1016/S0022-5347(05)64285-1. [DOI] [PubMed] [Google Scholar]
- 42.Soloway MS, Hachiya T, Civantos F, Murphy WM, Gomez CC, Ruiz HE. Androgen deprivation prior to radical prostatectomy for T2b and T3 prostate cancer. Urology. 1994;43:52–6. doi: 10.1016/0090-4295(94)90219-4. [DOI] [PubMed] [Google Scholar]
- 43.Sorrentino C, Musiani P, Pompa P, Cipollone G, Di Carlo E. Androgen deprivation boosts prostatic infiltration of cytotoxic and regulatory T lymphocytes and has no effect on disease-free survival in prostate cancer patients. Clin Cancer Res. 2011;17:1571–81. doi: 10.1158/1078-0432.CCR-10-2804. [DOI] [PubMed] [Google Scholar]
- 44.ClinicalTrials.gov. [Accessed Apr 18, 2017];Chemotherapy after prostatectomy (CAP) for high-risk prostate carcinoma (CAP). NCT00132301. https://clinicaltrials.gov/ct2/show/NCT00132301.NCT00132301.
- 45.Mellado B, Font A, Alcaraz A, et al. Phase II trial of short-term neoadjuvant docetaxel and complete androgen blockade in high-risk prostate cancer. Br J Cancer. 2009;101:1248–52. doi: 10.1038/sj.bjc.6605320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kelly WK, Halabi S, Elfiky A, et al. Multicenter phase 2 study of neoadjuvant paclitaxel, estramustine phosphate, and carboplatin plus androgen deprivation before radiation therapy in patients with unfavorable-risk localized prostate cancer: results of Cancer and Leukemia Group B 99811. Cancer. 2008;113:3137–45. doi: 10.1002/cncr.23910. [DOI] [PubMed] [Google Scholar]
- 47.Fizazi K, Faivre L, Lesaunier F, et al. Androgen deprivation therapy plus docetaxel and estramustine versus androgen deprivation therapy alone for high-risk localised prostate cancer (GETUG 12): a phase 3 randomised controlled trial. Lancet Oncol. 2015;16:787–94. doi: 10.1016/S1470-2045(15)00011-X. [DOI] [PubMed] [Google Scholar]
- 48.Ryan CJ, Smith MR, Fizazi K, et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2015;16:152–60. doi: 10.1016/S1470-2045(14)71205-7. [DOI] [PubMed] [Google Scholar]
- 49.Schmidt JD, Gibbons RP, Murphy GP, Bartolucci A. Evaluation of adjuvant estramustine phosphate, cyclophosphamide, and observation only for node-positive patients following radical prostatectomy and definitive irradiation. Investigators of the National Prostate Cancer Project. Prostate. 1996;28:51–7. doi: 10.1002/(SICI)1097-0045(199601)28:1<51::AID-PROS7>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 50.Montgomery B, Lavori P, Garzotto M, et al. Veterans Affairs Cooperative Studies Program study 553: Chemotherapy after prostatectomy, a phase III randomized study of prostatectomy versus prostatectomy with adjuvant docetaxel for patients with high-risk, localized prostate cancer. Urology. 2008;72:474–80. doi: 10.1016/j.urology.2008.02.050. [DOI] [PubMed] [Google Scholar]
- 51.ClinicalTrials.gov. [Accessed Apr 18, 2017];Surgery with or without docetaxel and leuprolide or goserelin in treating patients with high-risk localized prostate cancer. NCT00430183. https://clinicaltrials.gov/ct2/show/NCT00430183.
- 52.Lin DGM, Aronson W, Basler J, Beer T, Brophy M, Kelly K, Lee K, Lu Y, Markle V, McGuire V, Nseyo U, Ringer R, Savage S, Shih MC, Uchio E, Wang Y, Yang C, Montomery B. VA CSP#553: Chemotherapy after prostatecetomy (CAP) for high risk prostate carcinoma: A phase III randomized study. The Journal of Urology. 2016;195:e1071. doi: 10.1016/j.eururo.2019.12.020. [DOI] [PubMed] [Google Scholar]
- 53.Ahlgren GFP, Tammela TLJ, Kellokumpu-Lehtinen P, Borre M, Angelsen A, Iversen JR, Sverrisdottir A, Jonsson E, Sengelov L. A randomized phase III trial between adjuvant docetaxel and surveillance after radical prostatectomy for high risk prostate cancer: Results of SPCG12. Journal of Clinical Oncology. 2016;34:5001. [Google Scholar]
- 54.Rosenthal SA, Bae K, Pienta KJ, et al. Phase III multi-institutional trial of adjuvant chemotherapy with paclitaxel, estramustine, and oral etoposide combined with long-term androgen suppression therapy and radiotherapy versus long-term androgen suppression plus radiotherapy alone for high-risk prostate cancer: preliminary toxicity analysis of RTOG 99-02. Int J Radiat Oncol Biol Phys. 2009;73:672–8. doi: 10.1016/j.ijrobp.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 55.Hussain A, Wu Y, Mirmiran A, et al. Long-term follow-up of a prospective trial of trimodality therapy of weekly paclitaxel, radiation, and androgen deprivation in high-risk prostate cancer with or without prior prostatectomy. Int J Radiat Oncol Biol Phys. 2012;82:167–74. doi: 10.1016/j.ijrobp.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 56.Hurwitz MD. [Accessed Apr 26, 2017];NRG-GU002: Phase II-III trial of adjuvant radiotherapy and androgen deprivation following radical prostatectomy with or without adjuvant docetaxel. https://www.nrgoncology.org/Clinical-Trials/NRG-GU002.
- 57.Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15:683–90. doi: 10.1245/s10434-007-9747-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nickleach D, Liu Y, Shrewsberry A, Ogan K, Kim S, Wang Z. SAS macros to conduct common biostatistical analyses and generate reports. http://analytics.ncsu.edu/sesug/2013/PO-05.pdf.
- 59.Parsons LS. Reducing bias in a propensity score matched-pair sample using greedy matching techniques. SAS SUGL. 2001;26:214–26. [Google Scholar]
- 60.Austin PC, Grootendorst P, Anderson GM. A comparison of the ability of different propensity score models to balance measured variables between treated and untreated subjects: a Monte Carlo study. Stat Med. 2007;26:734–53. doi: 10.1002/sim.2580. [DOI] [PubMed] [Google Scholar]
- 61.Lin DY, Wei LJ. The robust interference for the Cox Proportional Hazards Model. Journal of the American Statistical Association. 1989;84:1074–8. [Google Scholar]
- 62.Kattan MW, Eastham JA, Stapleton AM, Wheeler TM, Scardino PT. A preoperative nomogram for disease recurrence following radical prostatectomy for prostate cancer. J Natl Cancer Inst. 1998;90:766–71. doi: 10.1093/jnci/90.10.766. [DOI] [PubMed] [Google Scholar]
- 63.D'Amico AV, Whittington R, Malkowicz SB, et al. The combination of preoperative prostate specific antigen and postoperative pathological findings to predict prostate specific antigen outcome in clinically localized prostate cancer. J Urol. 1998;160:2096–101. doi: 10.1097/00005392-199812010-00041. [DOI] [PubMed] [Google Scholar]