Abstract
Neonatal seizures are harmful to the developing brain and are associated with mortality and long-term neurological comorbidities. Hypoxic-ischemic encephalopathy (HIE) seizures represent a significant proportion of such seizures. Phenobarbital (PB) remains the first line anti-seizure drug (ASD) treatment but fails ~50% of the time. Translational models of neonatal seizures are crucial to investigating mechanisms underlying PB-resistance. A model of PB-resistant ischemic seizures in post-natal day 7 (P7) CD-1 mice reported K-Cl cotransporter 2 (KCC2) degradation that has been shown to be due to activation of the TrkB pathway. We investigated PB-efficacy in a pentyleneltetrazole (PTZ) model of neonatal seizures in the same strain and age using identical treatment protocols to gain insights into mechanisms underlying PB-resistance. A single dose of PTZ (80 mg/kg; IP) consistently induced repetitive seizures that did not progress to status epilepticus (SE). PB (25 mg/kg; IP, single dose) significantly suppressed the PTZ-induced seizures. This was associated with significant KCC2 upregulation and stable Na-K-Cl cotransporter 1 (NKCC1) expression at 24h. The TrkB pathway was not activated. PTZ seizure burdens were significantly higher than those reported for ischemic seizures, indicating seizure severity did not dictate the differences in PB-efficacy. Bumetanide (BTN) (0.1–0.2 mg/kg; IP) did not work as an anti-seizure agent, similar to the ischemic model. When investigating mechanisms underlying the emergence of PB-resistance in translational models, the method by which seizures are induced may dictate mechanisms underlying emergence of PB-resistance.
Keywords: Neonatal seizures, Pentyleneltetrazole, Phenobarbital, Bumetanide, KCC2, Rabbit α-KCC2 (RRID:AB_310611), Mouse α-KCC2 (RRID:AB_2721238), Rabbit α-phospho-KCC2-S940 (RRID:AB_2721198), Mouse α-TrkB (RRID:AB_397508), Rabbit α-phospho-TrkB-T816 (RRID:AB_2721199), Mouse α-PLCγ (RRID:AB_2163544), Rabbit α-phospho-PLCγ-T783 (RRID:AB_330855), Rabbit-NKCC1 (RRID:AB_91514), Mouse α-actin (RRID:AB_2637092)
1. Introduction
Neonatal seizures are an early sign of brain injury in newborns (Thibeault-Eyebalin et al., 2009) that can disrupt normal brain development. Phenobarbital (PB) is the first-line anti-seizure drug (ASD) administered clinically but is only efficacious in about 50% of neonatal seizures (Low et al., 2016).
KCC2 and NKCC1 expression and function affect the anti-seizure efficacy of GABAA-agonists (Khirug et al., 2010). The higher [Cl-]i in immature neurons may contribute to the pharmaco-resistant seizures in the immature brain to 1st line-GABAA agonists (Kirmse et al., 2011). KCC2 expression increases exponentially during the perinatal period in both humans and rodents (Kaila et al., 2014). This age-dependent KCC2 upregulation plays an essential role in the GABAergic signaling shift from depolarization to hyperpolarization (Kaila et al., 2014). KCC2 activity is potentiated via serine 940 (S940) phosphorylation and has a crucial role in determining seizure susceptibility (Moore et al., 2017).
Kang et al. (2015a) characterized a permanent unilateral carotid-ligation model of neonatal ischemic-seizures in CD-1 pups to establish a translational model for age-dependent PB-resistant seizures. This model was also used to investigate bumetanide (BTN), an NKCC1 antagonist and short acting diuretic, to rescue PB-resistance as proposed by laboratories whose data initiated two clinical trials for BTN [NCT01434225 (2015); NCT00830531 (2017); Pressler et al., 2015b]. BTN failed as an anti-seizure adjunct in the model. Various pre-clinical models demonstrate that the mechanism of insult and severity of seizure burden can heavily influence ASD efficacy (Dzhala et al., 2008; Cleary et al., 2013; Loscher et al., 2013; Kang et al., 2014).
Rivera et al. (2002) have shown phospholipase C gamma [PLCγ] phosphorylation by TrkB receptor activation results in a KCC2 downregulation. Ischemia can induce BDNF upregulation (Bejot et al., 2011), which binds to TrkB and initiates a signaling cascade that may result in KCC2 downregulation (Dai et al., 2014). Following ischemic insult, KCC2 downregulation was shown in P7 CD-1 mice (Kang et al., 2015a). A single chemoconvulsant-induced seizure episode during P5–7 may result in an upregulation of KCC2 (Khirug et al., 2010) in a non-SE model. This is in contrast to downregulation of KCC2 seen in an adult SE model (Gonzalez et al., 2016). This study characterized PTZ-induced neonatal seizures in CD-1 pups at P7 to determine their response to PB and BTN, to investigate the role of the TrkB pathway and associated KCC2 functional modulation.
2. Methods
i. Study Approval
All procedures were directed in compliance to guidelines by the Committee on the Ethics of Animal Experiments, Johns Hopkins University. Animal Care and Use Committee of Johns Hopkins approved all protocols and animals used in this study. CD-1 mice litters with dams were purchased from Charles River Laboratories Inc. (Wilmington, MA, USA). Litters (n=10 in each litter) were delivered with dams at post-natal 3 or 4 days old (P3 or P4) and were allowed to acclimate until P7 (weight of pups ranged from 4–6g at P7). Food and water were provided ad libitum. Each litter had equal male and female pups.
ii. PTZ administration for insult and sub-dermal EEG electrode implantation
At P7, pups were subject to sub-dermal electrode implantation under isoflurane anesthesia similar to previous studies (Kang et al., 2015a; Kang et al., 2015b). Silver wire electrodes, made for sub-dermal use in humans (IVES EEG; Model # SWE-L25 –MA, IVES EEG solutions, USA), were implanted and fixed in position with adhesive (KrazyGlue) on the scalp of the pups. Three sub-dermal EEG scalp electrodes were used: 1 recording, 1 reference overlying the bilateral parietal cortex, and one ground electrode overlying the rostrum, similar to previous studies (Kang et al., 2015a; Kang et al., 2015b). The pups were then subjected to a single PTZ injection (80 mg/kg, Sigma Aldrich Cat# P6500) intraperitoneally (IP). Pups were tethered by connecting the sub-dermal electrodes to a preamplifier within a recording chamber for 3h continuous video-EEG (vEEG) recording similar to previous studies (Kang et al., 2015a; Kang et al., 2015b). Chamber temperature was maintained with isothermal pads at a constant 36°C.
iii. Experimental Design/ Treatment Groups
PB (25 mg/kg, Sigma Aldrich Cat# P5178; dissolved in phosphate-buffered saline) and BTN (0.1–0.2 mg/kg, Sigma Aldich Cat#: B3023; dissolved in 100% ethyl alcohol) were made fresh on the day of experiment and stored at −20°C until time of use. Following PTZ injection (80 mg/kg; IP), pups [EEG sample size: n=13 males, n=13 females, control pups (n=6) were not subject to EEG; see Suppl. Table 1] were randomly assigned to four different treatment groups 1.) Control (no drug) 2.) PTZ+saline+saline 3.) PTZ+PB+Saline 4.) PTZ+PB+BTN [reported below as: 1.) Control 2.) PTZ 3.) +PB 4.) +PB+BTN groups]. PTZ was injected immediately after electrode implantation. The experimental paradigm is depicted in Figure 1A. PB injection followed 1h post-PTZ and BTN injection followed 1h post-PB. A smaller sample set was subject to 2h experiments with treatment groups for western blotting analysis - 1.) Control 2.) PTZ 3.) PTZ+PB. These pups were not subject to EEG recordings (n=20). In our 24h data set, there were no statistically significant differences in our proteins of interest between both +PB and +PB+BTN groups. For that reason, the +PB+BTN treatment group was not part of the 2h data set.
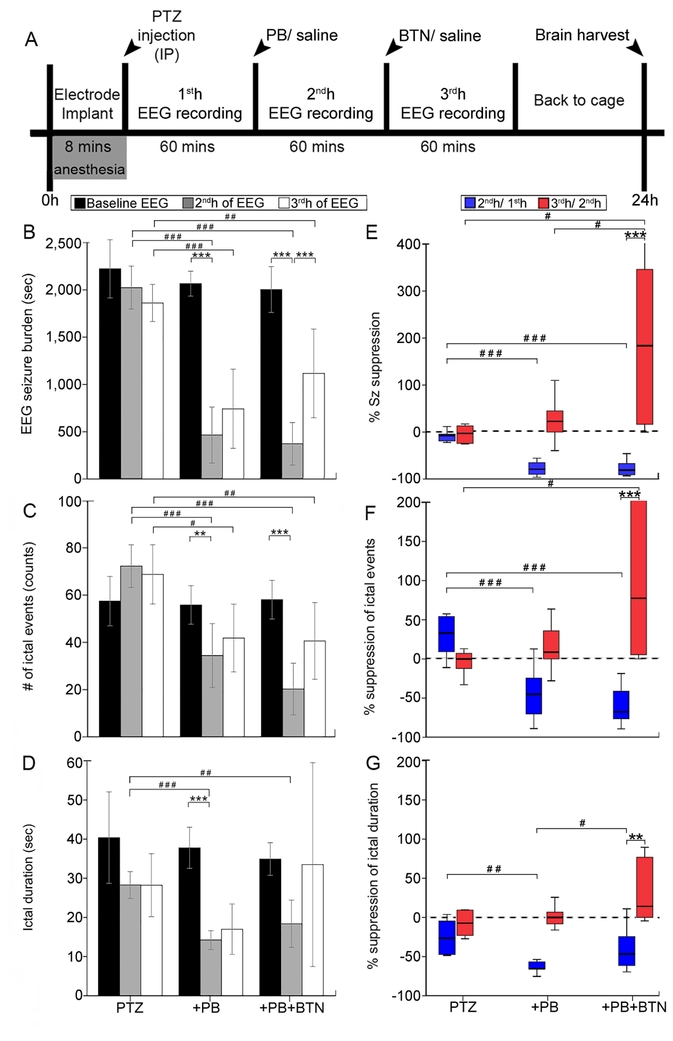
Figure 1:
(A) Experimental paradigm (B – D) EEG seizure burden, number of ictal events (counts), and ictal durations (sec) in PTZ, +PB, +PB+BTN treatment groups. Seizure burden post-PTZ remained stable over the 3h-recording period when no PB treatment was administered. PB (25 mg/kg; IP) was efficacious as an anti-seizure agent (grey bars) to suppress PTZ-induced seizures. BTN, administered as an adjunct to PB, failed to improve PB-efficacy (white bars), and led to a loss of PB-suppressed seizures in treatment group administered. (E – G) Corresponding % suppression of seizure burden, % suppression of ictal events, and % suppression of ictal duration. EEG sample size: n=13 males, n=13 females. Within groups: *P < 0.05, **P < 0.01, ***P ≤ 0.001, two-way ANOVA with Bonferroni post-hoc correlations. Between groups: #P < 0.05, ##P < 0.01, ###P ≤ 0.001, one-way ANOVA with Bonferroni post-hoc correlations.
PTZ was specifically chosen over kainic acid because we wanted to induce repetitive seizures similar to the ischemia model (Kang et al., 2015a) that did not progress into SE in any pup over the 3h-recording period (Velisek et al., 1992). Additionally, kainic acid protocols that induce SE require termination of SE to prevent mortality (Velisek et al., 1992). The single dose of PTZ (80 mg/kg; IP) in this study induced repetitive seizures and allowed for a direct comparison to the characterized ischemia model in the same strain at the same age. This protocol did not result in SE in any pup.
iv. Video-EEG recording and analyses
Sirenia Acquisition Software was used to acquire continuous EEG recording with synchronous video capture (Pinnacle Technology Inc., v 1.6.4.). Data acquisition was done with 400Hz sampling rates, with a pre-amplifier gain of 100. Filters were applied to remove ambient background noise (1Hz to 25Hz) low-pass. Data were scored by visualizing the raw EEG trace in 10-second epochs. Similar to previous studies (Kang et al., 2015a; Kang et al., 2015b), seizures were defined as electrographic ictal events that consisted of high amplitude rhythmic spikes; diffuse peak frequency of (7 – 8Hz) lasting ≥ 6 seconds. Brief epileptiform discharges lasting < 5 seconds were not included for seizure burden calculations in this study. Scored 3h-recordings were quantitated and analyzed for total seizure burden, number of ictal events and ictal durations.
v. Power EEG analysis
Sirenia Sleep software (Pinnacle Technology Inc., v 1.6.4) generated EEG power analysis. EEG spectral power (0.5 – 50 Hz) analysis was run on the acquired EEGs after Fourier’s transformation for each 10-second epoch. Spectral power data were binned as delta (0.5–4.0 Hz), theta (5.5–8.0 Hz), alpha (8.0–13.0 Hz), beta (13.0–30.0 Hz) and gamma (35–50 Hz). EEG power was calculated for entire 3h-recording period (3600 seconds duration each, i.e.; 360 epochs each). EEG power for every 30 epochs, (i.e., 12 bins each hour) was calculated for the 3h-recording period. EEG power analysis was done for recordings with no electrical noise contamination (n=4 for PTZ only group, n=8 for +PB group, n=9 for +PB+BTN group).
vi. Western blot post-PTZ administration
All pups were anesthetized with chloral hydrate (90 mg/mL; IP) 2h and 24h’s post-PTZ before trans-cardiac perfusion. Whole brains were harvested at 2 h and 24h’s post-PTZ administration, separated by L (left) and R (right) cerebral hemispheres and frozen on dry ice. Brains were stored at −80°C until further use. Homogenized brain lysates were suspended in cell lysis buffer with 1% of 100x protease/phosphatase inhibitor cocktail. To quantify total protein concentrations, Bradford Assay was conducted at 570nm wavelength. For gel electrophoresis, samples were diluted for 50ug of protein, at 20ul of loading volume. Samples were run on 4–20% gradient 1.5 mm 15 wells SDS gels (Invitrogen, Grand Island, NY, USA) for 80–90 minutes with 130V and transferred onto polyvinylidene difluoride (PVDF) membranes for 20h wet-transfer at 30V. After the transfer, the PVDF membranes underwent 1h-blocking step in Rockland buffer before an overnight incubation with primary antibodies: rabbit α-KCC2 (used for 24h analysis, 1:1000, Millipore Cat# 07–432, RRID:AB_310611), mouse α-KCC2 (used for 2h analysis when Ab became commercially available to run KCC2 and its phospho on same gel, Aviva Systems Biology, Cat# OASE00240, RRID:AB_2721238), rabbit α-phospho-KCC2-S940 (1:1000, Aviva Systems Biology, Cat# OAPC00188, RRID:AB_2721198), mouse α-TrkB (1:1000, BD Biosciences Cat# 610102, RRID:AB_397508), rabbit α-phospho-TrkB-T816 (1:500, Millipore, Cat# ABN1381, RRID:AB_2721199), mouse α-PLCγ (1:1000, Thermo Fisher Scientific Cat# LF-MA0050, RRID:AB_2163544), rabbit α-phospho-PLCγ -T783 (1:1000, Cell Signaling Technology Cat# 2821S, RRID:AB_330855) [1h-blocking step in 1x TBS in 5% nonfat dry milk for antibodies PLCγ and pPLCγ-T783, not Rockland], rabbit-NKCC1 (1:500, Millipore Cat# AB3560P, RRID:AB_91514) and mouse α-actin (1:10000, LI-COR Biosciences Cat# 926–42213, RRID:AB_2637092). pTrkB-T816 was specifically chosen because it has been shown to promote the phosphorylation of PLCγ via BDNF phosphorylation (He et al., 2010). pKCC2-S940 was specifically chosen because of its known ability to stabilize KCC2 on the cell surface, and thus promoting its extrusion power (Moore et al., 2017). In this study, rabbit α-KCC2 (1:1000, Millipore Cat# 07–432, RRID:AB_310611) and its phospho, rabbit α-phospho-KCC2-S940 (1:1000, Aviva Systems Biology, Cat# OAPC00188, RRID:AB_2721198) were run on separate gels due to same host and close molecular weights. Mouse α-TrkB (1:1000, BD Biosciences Cat# 610102, RRID:AB_397508) and rabbit α-phospho-TrkB-T816 (1:500, Millipore, Cat# ABN1381, RRID:AB_2721199) were run on the same gel (see Suppl. Fig. 1 for entire blot). Mouse α-PLCγ (1:1000, Thermo Fisher Scientific Cat# LF-MA0050, RRID:AB_2163544) and rabbit α-phospho-PLCγ -T783 (1:1000, Cell Signaling Technology Cat# 2821S, RRID:AB_330855) were run on the same gel. On the next day, PVDF membranes were washed with TBS containing 1% tween detergent (TBS-T) and were incubated in chemiluminescent secondaries for 1h (goat α-mouse 700 LT and goat α-rabbit 800 LT, LI-COR Biosciences). Chemiluminescent protein bands were analyzed using Odyssey infrared imaging system 2.1 (LI-COR Biosciences). Optical density of each protein sample was normalized to the actin bands run on each lane. Ratio of phosphorylated protein to total protein was calculated by dividing phosphorylated protein normalized to its actin by total protein normalized to its actin for each sample.
vii. Statistics
All statistical tests were done using SPSS24 (IBM, Armonk, NY U.S.) and Prism7.0 (GraphPad Software, La Jolla, CA, USA). Group averages of total seizure burden, number of ictal events, and ictal duration between each treatment group were compared using one-way measures ANOVA and repeated measures ANOVA with Bonferroni’s post-hoc correlations, similar to previous studies (Kang et al., 2015a; Kang et al., 2015b). Mauchely’s test determined sphericity for EEG data. Two-way ANOVA with Bonferroni post-hoc correlations were conducted within treatment groups and independent t-tests and one-way ANOVA with Bonferroni post-hoc correlations were conducted between treatment groups. All data are reported as means ± 1 S.D. Correlation analyses were performed using nonparametric comparisons (Spearman’s test, two-tail). Differences with p value < alpha at 0.05 (p < 0.05) were considered statistically significant (Between groups: repeated measures ANOVA, one-way ANOVA, independent t-tests; # = p < 0.05 # # = p < 0.01; # # # = p < 0.001 and within groups: two-way ANOVA with Bonferroni post-hoc correlations * = p < 0.05 ** = p < 0.01; *** = p < 0.001 for Figs.1 – 5).
Figure 5:
Western blot quantification of post-PTZ expression of TrkB and p-TrkB (T816), PLCγ, p-PLCγ (T783). (A) Representative western blots for TrkB, p-TrkB (T816) and their respective actins. (B-C) TrkB and p-TrkB (T816) expression remained stable, indicating no activation of TrkB pathway. (D – E) Ratio of p-TrkB (T816)/ TrkB of 24h and 2h post-PTZ, respectively, showed no significant changes. (F) Mean TrkB and p-TrkB (T816) percent of control shows no activation of TrkB pathway. (G) Representative western blots for PLCγ, p-PLCγ (T783) and their respective actins (H-I) PLCγ and p-PLCγ (T783) expression showed no significant differences at 24h. (J-K) Ratio of p-PLCγ (T783) to total PLCγ of 24h and 2h post-PTZ pups showed significant increase in +PB group at 24h. (L) At 2h, mean p-PLCγ (T783) percent of control shows significant increase (50%) in PTZ group compared to control and PLCγ shows significant increase compared to control (50%) in +PB group. At 24h, p-PLCγ (T783) percent of control in +PB group showed a significant reduction compared to controls (45%). There was also an additional significant difference in PLCγ in PTZ only and +PB treatment groups. Sample size: n=32 for 24h data, n=20 for 2h data. Between groups: #P < 0.05, ##P < 0.01, ###P ≤ 0.001, independent t-tests & one-way ANOVA with Bonferroni post-hoc correlations.
3. Results
PB effectively suppresses PTZ-induced seizures.
EEG seizure burden quantification allowed for the testing of PB’s anti-seizure efficacy and BTN’s non-efficacy. A single dose of PTZ (80 mg/kg; IP) consistently induced repetitive seizures in the 1sth (mean seizure burden = 2230 ± 310 seconds in PTZ group, 2152 ± 219 seconds in +PB group, 1966 ± 240 seconds in +PB+BTN group, Fig. 1B) that remained stable over the 3h-recording period (F(4,65)= 14.4, p>0.99 for PTZ only group 1st hr to 2nd h, and 2nd h to 3rd h, two-way ANOVA, Fig. 1B). None of the pups in this study went into SE during the 3h-recording period and was associated with no mortality. A single loading dose of PB (25 mg/kg; IP) significantly decreased seizure burden (F(2,23)= 63.129, p<0.001, one-way ANOVA for both treatment groups, Fig. 1B) when given at 1h post PTZ injection. BTN (0.1–0.2 mg/kg; IP) given 1h following PB, aggravated PB-suppressed seizures (F(4,65)= 14.4, p=0.001, two-way ANOVA, Fig. 1B). PB significantly reduced both the ictal event counts (F(2,23)=27.799 p<0.001, one-way ANOVA for both treatment groups, Fig. 1C) and ictal durations in the 2ndh (F(2,23)=17.441, p<0.001 in +PB group and p=0.002 in +PB+BTN group, one-way ANOVA, Fig. 1D). Repeated measures ANOVAs using a within subjects design for the analysis of PB and BTN were evaluated. Mauchly’s test for sphericity was not significant in regards to seizure burden (p=0.14) or ictal events (p=0.25), but was significant for ictal duration (p=0.001). Repeated measures ANOVA between treatment group comparisons showed significance in seizure burden (F(2,19)=56.623, p<0.001), events (F(2,18)=22.241, p<0.001) and duration (F(2,19)=4.083, p=0.033).
Percent seizure suppression showed no significant change in the 2nd and 3rdh of recording in the PTZ only group (F(2,41)= 8.41, p>0.99, two-way ANOVA, Fig. 1E). Percent seizure suppression by PB was significant in both treatment groups (F(2,23)=48.772, p<0.001 in +PB group and F(2,23)=48.772, p<0.001 in +PB+BTN group, one-way ANOVA, Fig. 1E). BTN resulted in aggravation of PB-suppressed seizures (F(2,41)= 8.41, p<0.001, two-way ANOVA, Fig. 1E) in the 3rd h; however, this aggravation was significantly higher than the PB seizure rebound detected in the +PB group (F(2,18)=5.570, p=0.045, 3rd h +PB+BTN vs. 3rd h +PB, one-way ANOVA, Fig. 1E). Percent suppression of ictal event counts followed similar trends (Fig. 1F). PB significantly suppressed ictal events in both treatment groups (F(2,23)=19.312, p<0.001 in +PB group and F(2,23)=19.312, p<0.001 in +PB+BTN group respectively, one-way ANOVA, Fig. 1F). PB efficaciously decreased ictal durations in the +PB group (F(2,23)=8.105, p=0.002, one-way ANOVA, Fig. 1G), but not in +PB+BTN group (F(2,23)=8.105, p=0.339, one-way ANOVA, Fig. 1G). BTN significantly decreased percent seizure suppression of duration in the 3rd h(F(2,41)= 2.25, p=0.003, two-way ANOVA, Fig. 1G). Therefore, in the PTZ model, PB was an efficacious anti-seizure agent but BTN failed as an adjunct to PB.
Sex as a biological variable
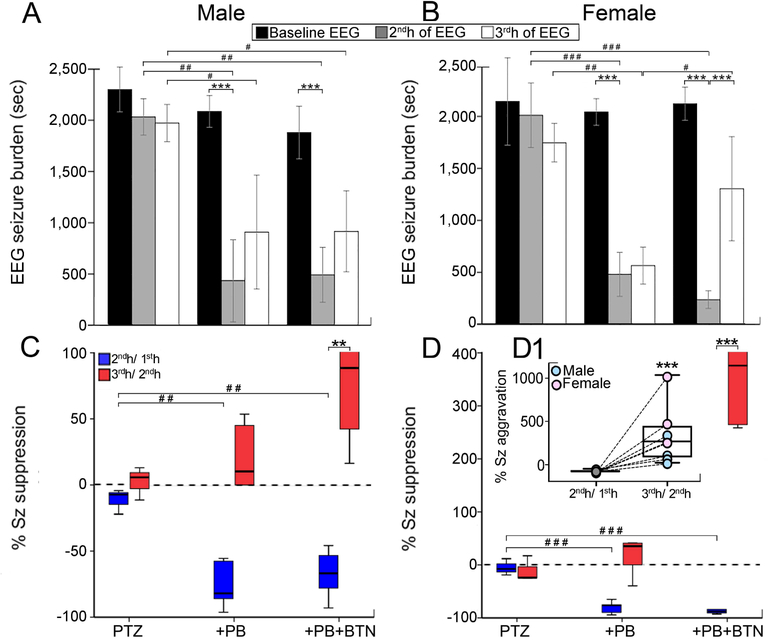
Data were analyzed for sex differences. Both males and females had similar seizure burdens over the 3h-recording period (mean = 6287±576s for males, Fig. 2A, mean = 5967±926s for females, Fig. 2B). Significant decrease in seizure burden post-PB was detected in both sexes (F(2,10)=19.199, p=0.001 for males in both groups, Fig. 2A, F(2,10)=59.400, p<0.001 for females in both groups, Fig. 2B, one-way ANOVA). Females showed significant post BTN aggravation (F(4,28)= 14.4, p<0.001, two-way ANOVA, Fig. 2B). Percent seizure suppression by PB was significant in males (F(2,10)=14.625, p=0.001 in +PB group and F(2,10)=14.625, p=0.003 in +PB+BTN group, one-way ANOVA, Fig. 2C) and females (F(2,10)=50.910, p<0.001 in +PB and +PB+BTN groups, one-way ANOVA, Fig. 2D). The 3rd h in the +PB+BTN group showed significant percent seizure aggravation in males (F(2,17)= 4.26, p=0.003, two-way ANOVA, Fig. 2C) and females [F(2,18)= 8.44, p<0.001, two-way ANOVA, Fig. 2D (see inset, Fig. 2D1)]. Ictal event counts, durations, and their respective percent suppressions between sexes were similar. Overall, PB-efficacy to treat PTZ-induced seizures was similar in both sexes.
Figure 2:
Seizure burden analysis by sex. (A - B) EEG seizure burden. PB was efficacious as an anti-seizure agent in both sexes. (C - D) Corresponding seizure suppression (%) followed similar trends for PB suppression, PB rebound and BTN. (D1) For +PB+BTN treatment group, males and females showed differences in BTN aggravation. EEG sample size: n=13 males, n=13 females. Within groups: *P < 0.05, **P < 0.01, ***P ≤ 0.001, two-way ANOVA with Bonferroni post-hoc correlations. Between groups: #P < 0.05, ##P < 0.01, ###P ≤ 0.001, one-way ANOVA with Bonferroni post-hoc correlations.
Electrographic seizure burden vs. EEG power
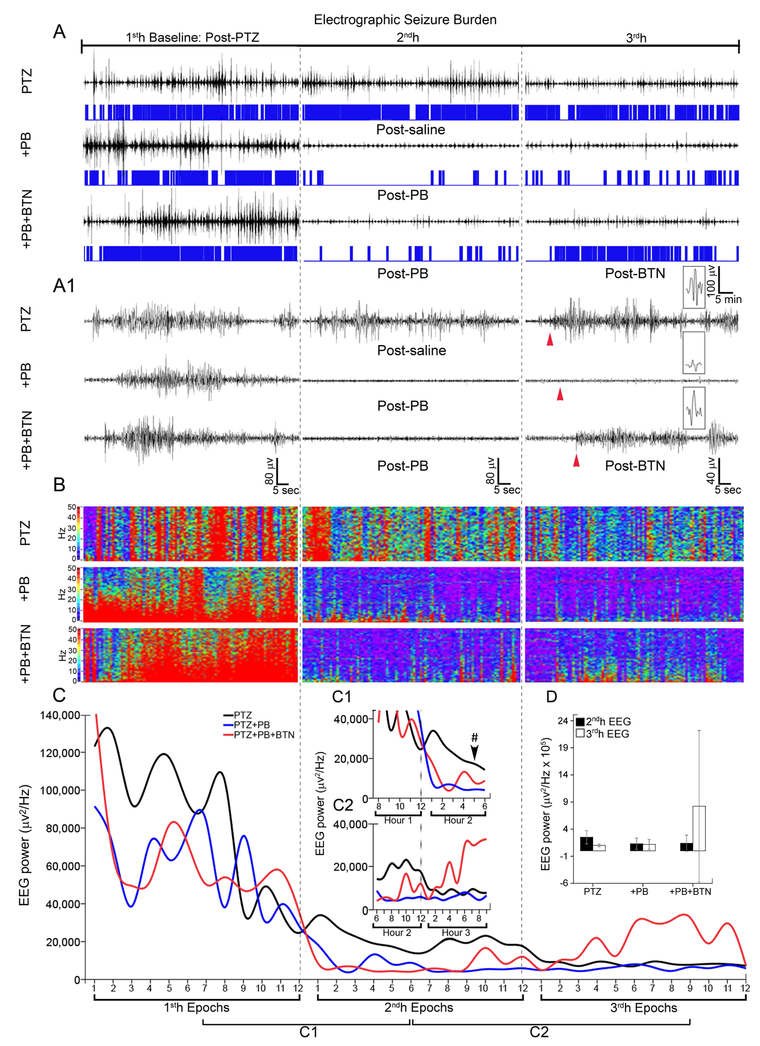
PTZ-induced seizure EEGs were quantitated (representative traces in 3A, 3A1) using manual seizure scoring and were compared to automated spectral power analysis. Pups in the PTZ only group continued to have repetitive seizures (Fig. 3A, 3A, 1) over the 3h-recording period. Seizure amplitude decreased in the PTZ only group over time (Fig. 3A). This phenomenon has been previously reported in a similar animal model of continuous seizures over a 2h-recording period (Zayachkivsky et al., 2015). Human EEGs also show a reduction in overall seizure power with treatment (Pan et al., 2009). BTN related seizure aggravation was associated with an increase in EEG spectral power, which is apparent in the 3rdh EEG trace (Fig. 3A, 3A, 1) as well as in the 3rdh spectral heat map and EEG power analysis. Frequency histograms, that visually display both ictal events and duration over the entirety of the three hours (located under 1h EEG traces, in blue), shows that although EEG amplitude is decreasing without treatment, seizure burden stays high, as shown in Fig. 1B. Heat maps (Fig. 3B) for the same EEG traces shown in 3A/3A1 allow for the clear visualization of PB seizure suppression in the PTZ model as well as the BTN related seizure aggravation in the 3rd h. Spectral power also consistently decreased over the 3h-recording period regardless of treatment group (Fig. 3C) associated with decrease in seizure amplitudes reported for Fig. 3A, A1. Frequency histograms for +PB group and +PB+BTN groups visually show seizure burden trends discussed in Fig. 1B. However, this is unlike the PTZ group that retains a high seizure burden but diminishing EEG power. Regardless of seizures alleviated with effective treatment or not, power decreased over time. This study showed that automated power EEG spectrum analysis may be a useful adjunct, but does not act in lieu of quantitated seizure burden scoring in acute seizure analysis. To evaluate PB and BTN effects upon this declining EEG power trend, epochs around the time of drug delivery were examined closely. PB injection resulted in a significant decrease in EEG power (Fig. 3C1). BTN injection resulted in an increase in EEG power (Fig. 3C2) after epoch 6 in the 3rdh, which was not significant due to variability (Fig. 3D).
Figure 3:
(A – A1) Representative electrographic traces of seizures recorded with scalp electrodes hourly plus frequency histograms (A) and 1 minute traces (A1) for PTZ, +PB, +PB+BTN treatment groups. (B) Representative heat maps for seizure burden show post-PB suppression and post-BTN aggravation, (C) Mean EEG power, epoch duration: 5 minutes showed an reduction of EEG power over 3h-recording period in all treatment groups (C1) EEG power suppression associated with efficacious PB treatment, not detected in PTZ only group (Hr2 Ep5 - #P=0.027, one-way ANOVA with Bonferroni post-hoc correlations). (C2) Post-BTN EEG power increase not detected in PTZ, +PB groups, however this increase was not significant. (D) Total calculated mean EEG power shows non-significant modulation because of large variability, indicating EEG power is not as reliable as EEG seizure burdens. Sample size: n=4 for PTZ group, n=8 for +PB group, n=9 for +PB+BTN group.
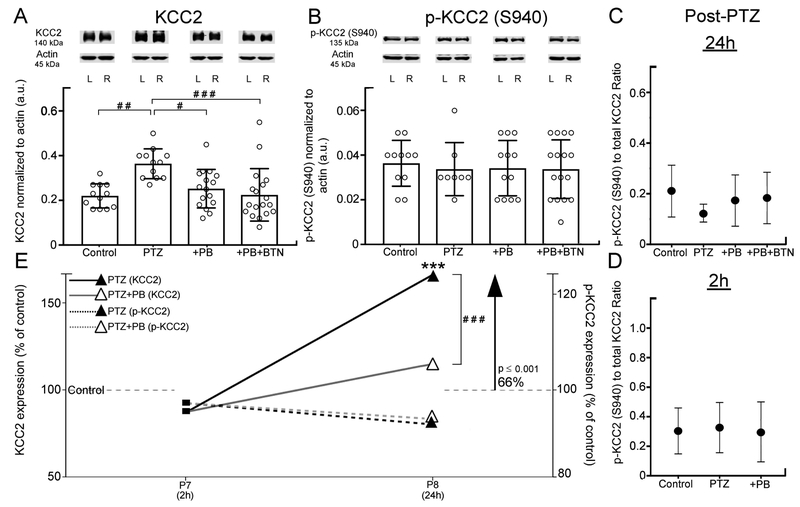
KCC2 and pKCC2-S940 expression in the PTZ model
Cl- cotransporter function is known to underlie seizure susceptibility (Moore et al., 2017). KCC2 is the major neuronal Cl- extruder. To investigate KCC2 and pKCC2-S940 expression, expression profiles at 2 and 24h post-PTZ were quantitated and compared. Significant KCC2 upregulation was detected at 24h in the PTZ only group (F(3,54)= 6.979, p=0.001, one-way ANOVA, Fig. 4A). In contrast, in +PB and +PB+BTN groups that showed significant seizure suppression following PB (Fig. 1B), the KCC2 levels dropped back to control levels (p>0.99 for both treatment groups, one-way ANOVA, Fig. 4A). pKCC2-S940 expression levels remained stable in all treatment groups (Fig. 4B) at 24h. At 2h, no KCC2 or pKCC2 degradation was detected. Ratio of pKCC2-S940 to total KCC2 protein remained stable between all treatment groups at both 24h (Fig. 4C) and 2h (Fig. 4D). Comparison of percent expression of control of KCC2 and pKCC2 in 2 and 24h data sets compared between all treatment groups indicated that total KCC2 expression increased significantly in the PTZ only group but not in other groups where seizures were suppressed by PB (t26 = 5.194, p<0.001, independent t-test, Fig. 4E). Therefore, PTZ-induced seizures resulted in significant KCC2 upregulation at 24h, which was prevented by efficacious PB-seizure suppression in the model.
Figure 4:
Western blot quantifications of post-PTZ expression of KCC2 and p-KCC2 (S940). (A) Representative western blots for KCC2 (B) p-KCC2 (S940) and their respective actins. At 24h, KCC2 upregulation was significant in PTZ only treatment group compared to control, +PB group and +PB+BTN group (bar graph in A). No significant change in p-KCC2 (S940) post-PTZ or post-treatment was detected (bar graph in B). (C) Ratio of p-KCC2 (S940)/ KCC2 24h post-PTZ showed stable ratios. (D) Ratio of p-KCC2 (S940)/ KCC2 2h post-PTZ showed stable ratios. (E) Mean KCC2 and p-KCC2 (S940) percent of control showed a temporal upregulation of KCC2. Sample size: n=32 for 24h data, n=20 for 2h data. Between groups: #P < 0.05, ##P < 0.01, ###P ≤ 0.001, independent t-tests & one-way ANOVA with Bonferroni post-hoc correlations.
PTZ and the TrkB pathway
We quantitated expression levels of TrkB, pTrkB-T816, and its downstream intracellular adaptor protein, PLCγ and pPLCγ-T783 in the same pup brains at 2h and 24h post-PTZ. PTZ only, +PB and +PB+BTN had no effect on TrkB and pTrkB-T816 expression levels compared to naïve control brains both at both 2h and 24h (Fig. 5B and 5C for 24h data, 2h data not shown). pTrkB-T816 / total TrkB ratios also showed no significant changes at 24h and 2h (Fig. 5D and 5E). TrkB and p-TrkB-T816 percent expression of control showed no significant changes (Fig. 5F).
PLCγ and pPLCγ expression remained stable in PTZ only and +PB groups at 2 and 24h (Fig. 5H and 5I for 24h data, 2h data not shown). Ratios of pPLCγ/total PLCγ showed a significant increase in the +PB treatment group (F(3,54)=4.695, p=0.003, one-way ANOVA, Fig. 5J) at 24h, but was stable at 2h (Fig. 5K). At 2h, PLCγ percent expression of control was significantly upregulated in +PB group, but not in the PTZ only group (F(2,25) = 5.157, p=0.02, one-way ANOVA, Fig. 5L). At 2h, p-PLCγ-T783 was significantly upregulated in PTZ only group compared to control (F(2,25) = 3.956, p=0.036, one-way ANOVA, Fig. 5L). At 24h post-PTZ, PLCγ in the +PB group not only returned to control levels, but was significantly downregulated compared to the age-matched PTZ only group (F(2,39) = 67.848, p<0.001, one-way ANOVA, Fig. 5L). Therefore, it is possible that the PLCγ modulation is a result of effectors that phosphorylate and activate pathways independent of TrkB receptor activation.
PTZ vs. Ischemia
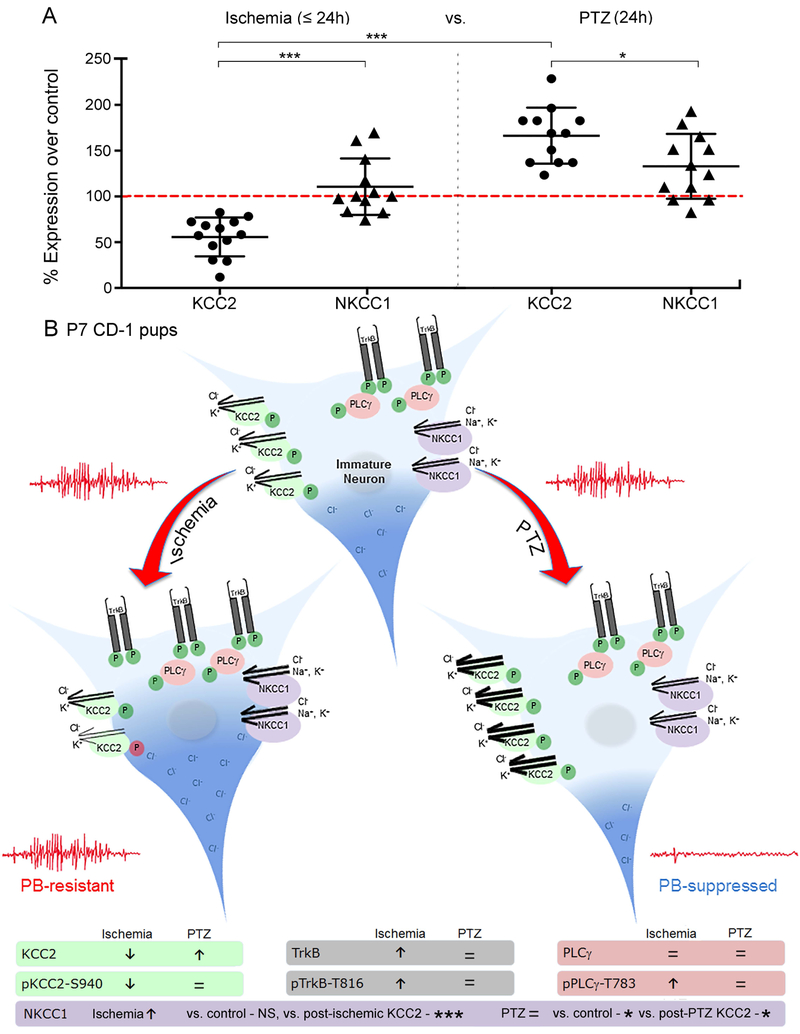
We analyzed the model-specific differences between post-insult expression of KCC2 and NKCC1 between ischemia and PTZ in CD-1 P7 pups. There was a significant difference between post-ischemic and post-PTZ KCC2 levels (F(4,57)=29.82, p<0.001, one-way ANOVA, Fig. 6A) with significant KCC2 degradation following ischemia vs. significant KCC2 upregulation following PTZ; but no significant differences in NKCC1 expression between models was detected. Within each model, the significant differences between KCC2 and NKCC1 expression in both the ischemic model (F(4,57)=29.82, p<0.001, one-way ANOVA, Fig. 6A) and the PTZ model (F(4,57)=29.82, p=0.0303, one-way ANOVA, Fig. 6A) were due to the differences in KCC2 expression.
Figure 6:
Mechanistic differences in ischemia and PTZ model (A) Significant model-specific differences in KCC2 expression, but no model-specific differences in NKCC1 expression. Sample size: n=12 for Ischemia-induced NKCC1 expression, n=6 for PTZ-induced NKCC1 expression. One-way ANOVA with Bonferroni post-hoc correlations: *P < 0.05, **P < 0.01, ***P ≤ 0.001. Additional information: (Kang et al., 2015a) (B) Schematic of model specific changes in KCC2 and TrkB pathway activation.
Correlations between EEG seizure burdens and western blot data for proteins of interest in the PTZ model showed a significant correlation between total seizure burden and NKCC1 (rs=0.71, n=12, p=0.01) in the PTZ only group. This significance was lost in the +PB (rs= −0.01, n=10, p=0.973) and +PB+BTN (rs=0.34, n=12, p=0.281) groups. No other correlations were significant.
4. Discussion
This study quantitated EEG seizure burdens associated with systemic delivery of PTZ in a mouse model of neonatal seizures and reveals critical insights: 1.) A single IP injection induced sustained episodic electrographic seizures over the 3h-recording period without inducing SE. 2.) The EEG seizure burdens were significantly higher than a newly characterized neonatal ischemic seizure model in the same mouse strain at the same age (Kang et al., 2015a). 3.) In spite of the significantly higher seizure burdens compared to ischemia-induced seizures, PTZ-induced seizures were PB-responsive to a loading dose that failed in the ischemia model. 4.) Cl- cotransporters were differentially modulated in the PTZ model compared to the ischemia model in the same strain at the same age. Therefore, the difference in PB-responsiveness detected between the models may reflect an important role of chloride cotransporters, specifically KCC2, in the emergence of refractoriness in neonatal seizures.
Chloride cotransporters in PTZ v. Ischemia-induced seizures
Model-specific insults can modulate chloride cotransporters’ expression and function differentially (Fig. 6, Suppl. Table 2). This study showed a significant upregulation of KCC2 following PTZ-induced seizures in pups; in contrast, the previously characterized ischemia-induced seizures caused a significant downregulation of KCC2. The ischemia-induced TrkB pathway activation and KCC2 degradation in the ischemic seizure model may underlie the emergence of PB-resistance (Kang et al., 2015a). PTZ-induced seizures showed no TrkB pathway activation and a significant upregulation of KCC2 at 24h. These seizures were also significantly PB-responsive. Therefore, pre-clinical models of neonatal seizures that result in differential effects on KCC2 expression and function may underlie opposite anti-seizure effects of GABA agonists like PB.
BTN, a NKCC1 antagonist, results in lower intracellular Cl- levels, and restores GABAergic inhibition in neurons (Dzhala et al., 2008). Drugs that restore low Cl- ions by blocking NKCC1 have been proposed as novel therapies for a wide range of disorders (Loscher et al., 2013). For this reason, BTN has been proposed as a novel anti-seizure agent to rescue PB-resistance in neonatal seizures [NCT01434225 (2015); NCT00830531 (2017); Dzhala et al., 2008]. However, a resulting phase I/II clinical trial, NEMO was terminated prematurely because of PB-inefficacy for HIE seizures and ototoxicity observed in 3 out of 11 babies (Pressler et al., 2015a). In pre-clinical studies, BTN’s anti-seizure responses vary depending on the animal model (Ben-Ari et al., 2016), suggesting that the efficacy of BTN may be dependent on the insult specific alterations Cl- cotransporter function rather than the seizures themselves (Ben-Ari et al., 2016; Gale, 1995). BTN failed as an adjunct therapeutic agent in the PTZ model when given 1h post-PB in P7 CD-1 pups. It also failed in the ischemic model (Kang et al., 2015a). Both models allow for the comparison of post-treatment EEG seizure burdens to the baseline (i.e. untreated) seizure burden in each pup, data, which is not likely to be available in human studies. HIE seizures have natural ebbs and crests in temporal seizure frequency which makes this assessment more complicated when quantitated over shorter periods (≤24h) of EEG recordings (McBride et al., 2000; Nash et al., 2011). Seizures can recur more than 24h apart in neonates exhibiting HIE seizures undergoing hypothermia therapy (Wusthoff et al., 2011; Lynch et al., 2015). Failure of BTN as an efficacious adjunct to PB has been attributed to its poor blood-brain barrier permeability (Puskarjov et al., 2014) and efflux from the brain by drug transporters (Rommerman et al., 2017). Less than 1% of BTN has been shown to reach the brain 1h post-IP injection (Puskarjov et al., 2014), yet its efficacy in few pre-clinical models has been attributed to its neuronal actions on NKCC1 (Cleary et al., 2013; Dzhala et al., 2008; Vlaskamp et al., 2017). The post-BTN aggravation of PB-suppressed seizures in the PTZ model could be due to 1. PB-rebound seizures not responsive to BTN alone (Low et al., 2016) 2. Diuretic effects of BTN [NCT01434225 (2015); NCT00830531 (2017)] 3. BTN’s action on NKCC1 expressing ependymal cells lining cerebral blood vessels (Dzhala et al., 2008; Kahle et al., 2009) 4. Changes to extracellular matrix volumes in the seizing brain (Glykys et al., 2017). Cerebral edema has been proposed to aggravate seizures in HIE where the role of hyperosmolar agents has been investigated with differential results (Vannucci et al., 1990; Haglund et al., 2005).
TrkB pathway
The PTZ model did not result in TrkB receptor activation. The significant modulation of pPLCγ/ total PLCγ ratio at 24h in +PB group was, therefore, independent of TrkB receptor activation. PLCγ is phosphorylated on three tyrosine residues: T771, T783, and T1253. Independent of TrkB pathway, pPLCγ at T783 is essential for lipase activation and is known to be phosphorylated by ERK pathway activation (Margolis et al., 1990; Yang et al., 2014)
Use of chemoconvulsants in models of neonatal seizures
Chemoconvulsants are used in animal models of seizures because they are convenient. However, when used to assess acute seizures using systemic delivery, their extensive cellular and physiological actions, most which are independent of seizure phenomena, make it challenging to separate from direct effects of seizures (Gale, 1995). Therefore, the use of such agents has been recommended for acute focally-evoked seizure models only (Gale, 1995). This study showed that when compared to ischemia, a systemic agent like PTZ has opposite effects on KCC2 function that can explain the associated ASD efficacies. The CD-1 neonatal seizure PTZ model provides critical insights related to translational models used to model neonatal seizures: the mechanism by which one induces seizures may dictate the efficacy of standard and/or novel therapeutic agents being tested.
Supplementary Material
Acknowledgements:
Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number R01HD090884 (SDK). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations:
- (PB)
Phenobarbital
- (PTZ)
Pentyleneltetrazole
- (BTN)
Bumetanide
- (KCC2)
K-Cl cotransporter 2
- (NKCC1)
Na-K-Cl cotransporter 1
- (ASD)
Anti-seizure drug
- (HIE)
hypoxic-ischemic encephalopathy
- (SE)
Status Epilepticus
- (L)
Left
- (R)
Right
Footnotes
Authors have no conflicts of interest to disclose.
Reference List
- 1.Bejot Y, Prigent-Tessier A, Cachia C et al. Time-dependent contribution of non neuronal cells to BDNF production after ischemic stroke in rats. Neurochemistry International 2011;58:102–11. [DOI] [PubMed] [Google Scholar]
- 2.Ben-Ari Y, Damier P, Lemonnier E Failure of the Nemo Trial: Bumetanide Is a Promising Agent to Treat Many Brain Disorders but Not Newborn Seizures. Frontiers in Cellular Neuroscience 2016;10:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cleary RT, Sun H, Huynh T et al. Bumetanide Enhances Phenobarbital Efficacy in a Rat Model of Hypoxic Neonatal Seizures. PLoS One 2013;8:e57148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dai S, Ma Z. BDNF-trkB-KCC2-GABA pathway may be related to chronic stress-induced hyperalgesia at both the spinal and supraspinal level. Medical Hypotheses 2014;83:772–4. [DOI] [PubMed] [Google Scholar]
- 5.Dzhala VI, Talos DM, Sdrulla DA, Brumback AC, Mathews GC, Benke TA, Delpire E, Jensen FE, Staley KJ (2005) NKCC1 transporter facilitates seizures in the developing brain. Nat Med 11:1205–1213. [DOI] [PubMed] [Google Scholar]
- 6.Dzhala VI, Brumback AC, Staley KJ. Bumetanide enhances phenobarbital efficacy in a neonatal seizure model. Ann Neurol 2008;63:222–35. [DOI] [PubMed] [Google Scholar]
- 7.Gale K. Chemoconvulsant seizures: Advantages of focally-evoked seizure models. The Italian Journal of Neurological Sciences 1995;16:17–25. [DOI] [PubMed] [Google Scholar]
- 8.Glykys J, Dzhala V, Egawa K, Kahle KT, Delpire E, Staley K. Chloride Dysregulation, Seizures, and Cerebral Edema: A Relationship with Therapeutic Potential. Trends in Neurosciences 40:276–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gonzalez MI. Regulation of the cell surface expression of chloride transporters during epileptogenesis. Neuroscience Letters 2016;628:213–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haglund MM, Hochman DW. Furosemide and Mannitol Suppression of Epileptic Activity in the Human Brain. Journal of Neurophysiology 2005;94:907–18. [DOI] [PubMed] [Google Scholar]
- 11.He XP, Pan E, Sciarretta C, Minichiello L, McNamara JO. Disruption of TrkB-mediated PLCγ signaling inhibits limbic epileptogenesis. J Neurosci 2010;30:6188–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kahle KT, Simard JM, Staley KJ, Nahed BV, Jones PS, Sun D. Molecular Mechanisms of Ischemic Cerebral Edema: Role of Electroneutral Ion Transport. Physiology 2009;24:257–65. [DOI] [PubMed] [Google Scholar]
- 13.Kaila K, Price TJ, Payne JA, Puskarjov M, Voipio J. Cation-chloride cotransporters in neuronal development, plasticity and disease. Nat Rev Neurosci 2014;15:637–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kadam SD, Markowitz GJ, Kang SK, Kim ST, Johnston MV. Age and gender dependent severity of ischemic neonatal seizures: response to phenobarbital + bumetanide combination therapy in a mouse model. AES annual meeting #3.035. 2011. [Google Scholar]
- 15.Kang SK, Kadam SD. Pre-Clinical Models of Acquired Neonatal Seizures: Differential Effects of Injury on Function of Chloride Co-Transporters. Austin journal of cerebrovascular disease & stroke 2014;1:1026. [PMC free article] [PubMed] [Google Scholar]
- 16.Kang SK, Markowitz GJ, Kim ST, Johnston MV, Kadam SD. Age- and sex-dependent susceptibility to phenobarbital-resistant neonatal seizures: role of chloride co-transporters. Frontiers in Cellular Neuroscience 2015;9:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang SK, Johnston MV, Kadam SD. Acute TrkB-inhibition rescues phenobarbital-resistant seizures in a mouse model of neonatal ischemia. The European journal of neuroscience 2015;42:2792–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khirug S, Ahmad F, Puskarjov M, Afzalov R, Kaila K, Blaesse P. A Single Seizure Episode Leads to Rapid Functional Activation of KCC2 in the Neonatal Rat Hippocampus. The Journal of Neuroscience 2010;30:12028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kirmse K, Witte OW, Holthoff K. GABAergic depolarization during early cortical development and implications for anticonvulsive therapy in neonates. Epilepsia 2011;52:1532–43. [DOI] [PubMed] [Google Scholar]
- 20.Loscher W, Puskarjov M, Kaila K. Cation-chloride cotransporters NKCC1 and KCC2 as potential targets for novel antiepileptic and antiepileptogenic treatments. Neuropharmacology 2013;69:62–74. [DOI] [PubMed] [Google Scholar]
- 21.Low E, Stevenson NJ, Mathieson SR et al. Short-Term Effects of Phenobarbitone on Electrographic Seizures in Neonates. Neonatology 2016;110:40–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lynch NE, Stevenson NJ, Livingstone V et al. The temporal characteristics of seizures in neonatal hypoxic ischemic encephalopathy treated with hypothermia. Seizure 2015;33:60–5. [DOI] [PubMed] [Google Scholar]
- 23.Margolis B, Li N, Koch A et al. The tyrosine phosphorylated carboxyterminus of the EGF receptor is a binding site for GAP and PLC-gamma. The EMBO Journal 1990;9:4375–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McBride MC, Laroia N, Guillet R. Electrographic seizures in neonates correlate with poor neurodevelopmental outcome. Neurology 2000;55:506. [DOI] [PubMed] [Google Scholar]
- 25.Moore YE, Kelley MR, Brandon NJ, Deeb TZ, Moss SJ. Seizing Control of KCC2: A New Therapeutic Target for Epilepsy. Trends in Neurosciences 2017;40:555–71. [DOI] [PubMed] [Google Scholar]
- 26.Nash KB, Bonifacio SL, Glass HC et al. Video-EEG monitoring in newborns with hypoxic-ischemic encephalopathy treated with hypothermia. Neurology 2011;76:556–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.NCT01434225. NEMO1:NEonatal Seizure Using Medication Off-patent (NEMO1). September-14-2015.
- 28.NCT00830531. Pilot Study of Bumetanide for Newborn Seizures. 10-December-2017.
- 29.Pan JW, Zaveri HP, Spencer DD, Hetherington HP, Spencer SS. Intracranial EEG power and metabolism in human epilepsy. Epilepsy research 2009;87:18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pressler RM, Boylan GB, Marlow N et al. Bumetanide for the treatment of seizures in newborn babies with hypoxic ischaemic encephalopathy (NEMO): an open-label, dose finding, and feasibility phase 1/2 trial. The Lancet Neurology 2015;14:469–77. [DOI] [PubMed] [Google Scholar]
- 31.Pressler RM, Boylan GB, Marlow N et al. Bumetanide for neonatal seizures - back from the cotside. Nature Reviews Neurology 2015;11:724. [DOI] [PubMed] [Google Scholar]
- 32.Puskarjov M, Kahle KT, Ruusuvuori E, Kaila K. Pharmacotherapeutic targeting of cation-chloride cotransporters in neonatal seizures. Epilepsia 2014;55:806–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Puskarjov M, Ahmad F, Khirug S, Sivakumaran S, Kaila K, Blaesse P (2015) BDNF is required for seizure-induced but not developmental up-regulation of KCC2 in the neonatal hippocampus. Neuropharmacology 88:103–109. [DOI] [PubMed] [Google Scholar]
- 34.Rivera C, Li H, Thomas-Crusells J et al. BDNF-induced TrkB activation down-regulates the potassium chloride cotransporter KCC2 and impairs neuronal Cl- extrusion. The Journal of Cell Biology 2002;159:747–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rommerman K, Fedrowitz M, Hampel P, Kaczmarek E, Tollner K, Erker T, Sweet DH, Losher W. Multiple blood brain barrier transport mechanisms limitbumetanide accumulation, and therapeutic potential, in the mammalian brain. Neuropharmacology 2017; 117: 182–194 [DOI] [PubMed] [Google Scholar]
- 36.Thibeault-Eybalin MP, Lortie A, Carmant L. Neonatal Seizures: Do They Damage the Brain? Pediatric Neurology 2009;40:175–80. [DOI] [PubMed] [Google Scholar]
- 37.Vannucci RC. Current and Potentially New Management Strategies for Perinatal Hypoxic-Ischemic Encephalopathy. Pediatrics 1990;85:961. [PubMed] [Google Scholar]
- 38.Velisek L, Kubova H, Pohl M, Stankova L, Mare+í P, Schickerova R. Pentylenetetrazol-induced seizures in rats: an ontogenetic study. Naunyn-Schmiedeberg’s Archives of Pharmacology 1992;346:588–91. [DOI] [PubMed] [Google Scholar]
- 39.Vlaskamp C, Poil SS, Jansen F et al. Bumetanide As a Candidate Treatment for Behavioral Problems in Tuberous Sclerosis Complex. Frontiers in Neurology 2017;8:469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wusthoff C, Dlugos DJ, Gutierrez-Colina A et al. Electrographic Seizures During Therapeutic Hypothermia for Neonatal Hypoxic-ischemic Encephalopathy. Journal of child neurology 2011;26:724–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang P, You G, Zhang W et al. Correlation of preoperative seizures with clinicopathological factors and prognosis in anaplastic gliomas: A report of 198 patients from China. Seizure 2014;23:844–51. [DOI] [PubMed] [Google Scholar]
- 42.Zayachkivsky A, Lehmkuhle MJ, Ekstrand JJ, Dudek FE. Ischemic injury suppresses hypoxia-induced electrographic seizures and the background EEG in a rat model of perinatal hypoxic-ischemic encephalopathy. Journal of Neurophysiology 2015;114:2753–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.