Abstract
This study examines altered resting state functional connectivity (rsFC) of the cognitive control network (CCN) in fibromyalgia patients as compared to healthy controls, as well as how effective interventions, such as Tai Chi, can modulate the altered rsFC of the CCN. Patients with fibromyalgia and matched healthy subjects were recruited in this study. Fibromyalgia patients were scanned 12 weeks before and after intervention. The bilateral dorsolateral prefrontal cortex (DLPFC) was used as a seed to explore the rsFC of the CCN. Data analysis was conducted with 21 patients and 20 healthy subjects. Compared to healthy subjects, fibromyalgia patients exhibited increased rsFC between the DLPFC and the bilateral rostral anterior cingulate cortex (rACC) and medial prefrontal cortex (MPFC) at baseline. The rsFC between the CCN and rACC/MPFC further increased after Tai Chi intervention, and this increase was accompanied by clinical improvements. This rsFC change was also significantly associated with corresponding changes in the Overall Impact domain of the Revised Fibromyalgia Impact Questionnaire (FIQR). Further analysis showed that the rACC/MPFC rsFC with both the PAG and hippocampus significantly decreased following Tai Chi intervention. Our study suggests that fibromyalgia is associated with altered CCN rsFC and that effective treatment may elicit clinical improvements by further increasing this altered rsFC. Elucidating this mechanism of enhancing the allostasis process may deepen our understanding of the mechanisms underlying mind-body intervention non-pharmacological treatment of fibromyalgia and facilitate the development of new pain management methods.
Keywords: Resting state functional connectivity, fibromyalgia, cognitive control network, dorsal lateral prefrontal cortex, Tai Chi, anterior cingulate cortex, mind-body intervention
Introduction
Fibromyalgia (FM) is a multidimensional complex disorder characterized by chronic widespread musculoskeletal pain, and physical and psychological limitations (Sumpton & Moulin, 2014). The neuropathology of fibromyalgia remains unclear, but previous studies have suggested that the central nervous system may play an important role in the development and maintenance of fibromyalgia (Jensen, Loitoile, et al., 2012; K. B. Jensen et al., 2013; Tracey & Bushnell, 2009).
Recently, functional magnetic resonance imaging (fMRI) has been used to investigate the pathophysiology (K. Jensen et al., 2013; Jensen, Loitoile, et al., 2012; Loggia et al., 2014; Truini et al., 2016) and treatment of fibromyalgia (Cummiford et al., 2016; Flodin et al., 2015). These studies suggest that patients with fibromyalgia exhibit significant changes in brain function and structure, further endorsing the role of brain in the neuropathology of fibromyalgia. For instance, studies show that fibromyalgia patients exhibit reductions in cortical thickness, brain volume (K. B. Jensen et al., 2013; Kuchinad et al., 2007; Lutz et al., 2008; Robinson, Craggs, Price, Perlstein, & Staud, 2011), and measures of functional connectivity (K. B. Jensen et al., 2013) in the rostral anterior cingulate cortex (rACC) and medial prefrontal cortex (MPFC) compared to healthy controls, demonstrating the important role of rACC/MPFC in the development of FM.
Although these studies significantly enhance our understanding of fibromyalgia, the physiological implications of these brain functional and morphometry changes remain unclear. One possible explanation is that some of these changes may reflect an adaptation process of the brain to fibromyalgia, specifically through allostasis, the process of achieving homeostasis through physiological or behavioral changes. Based on the theory of allostasis, the human body, including the brain, adapts to the altered conditions caused by a disorder, such as persistent pain (Borsook, Maleki, Becerra, & McEwen, 2012). This can be carried out by means of alteration in the hypothalamus – pituitary – adrenal (HPA) axis hormones, the autonomic nervous system, as well as through brain functional and structure changes (McEwen & Wingfield 2003). For example, to adapt and cope with consistent pain, fibromyalgia patients may employ an involuntary distraction strategy through a cognitive control mechanism. In support of this hypothesis, studies have demonstrated that attention can significantly modulate pain perception (Legrain et al., 2009; Lobanov, Quevedo, Hadsel, Kraft, & Coghill, 2013; Torta, Legrain, Mouraux, & Valentini, 2017); that is, focusing on pain may enhance pain responses, while distraction from pain can significantly reduce one’s pain experience (Valet et al., 2004). Further studies suggest that the descending pain modulation system, including the periaqueductal gray (PAG), cingulate cortex, and dorsal lateral prefrontal cortex, may play an important role in the attention regulation of pain (H. Fields, 2004; H. L. Fields, Basbaum, & Heinricher, 2005; Kong, Tu, Zyloney, & Su, 2010).
Cognitive control refers to the set of brain processes necessary for goal-directed thought and action (Cole & Schneider, 2007), which plays a crucial role in the top-down modulation of attention–memory interactions (Cole & Schneider, 2007; Rosen, Stern, Michalka, Devaney, & Somers, 2015; Sheline, Price, Yan, & Mintun, 2010), decision-making, and conflict resolution (Sheline et al., 2010). A key factor in cognitive control processing is attention, a mechanism by which sensory input is selected to enter into awareness and which can regulate one’s pain experience (Bushnell, Ceko, & Low, 2013; Torta et al., 2017; C. Villemure & Bushnell, 2002; C. Villemure & Schweinhardt, 2010; Wiech, 2016; Zeidan et al., 2015; Zeidan, Grant, Brown, McHaffie, & Coghill, 2012). Thus, cognitive control may represent an interesting aspect of allostasis in FM patients.
The brain network most closely related to cognitive executive functioning is the cognitive control network (CCN), which includes the frontal gyrus, parietal gyrus, and anterior cingulate cortex (Petersen & Posner, 2012; Zanto & Gazzaley, 2013). A large body of evidence indicates that the dorsolateral prefrontal cortex (DLPFC) is a key region of the CCN, playing an important role in cognitive control processes (Cieslik et al., 2013; Legrain et al., 2009; Miller & Cohen, 2001; Sheline et al., 2010; Tracey & Mantyh, 2007). Previous studies have also suggested that using the bilateral DLPFC as a seed can be a valuable tool for exploring the function of the CCN using resting state fMRI (Cieslik et al., 2013; J. W. Hwang et al., 2015; Legrain et al., 2009; Miller & Cohen, 2001; Sheline et al., 2010; Tao, Chen, Egorova, et al., 2017; Tracey & Mantyh, 2007).
In this study, in addition to investigating the difference in the CCN between fibromyalgia patients and healthy controls, we also attempted to explore how effective treatment (Tai Chi) can modulate the rsFC in fibromyalgia patients. We believe investigating rsFC changes after effective treatment, as well as the association between these rsFC changes and accompanying clinical symptom reduction, will help us understand the significance of brain functional changes observed in FM patients when compared to healthy controls. We chose Tai Chi intervention because pharmacological treatment of FM only achieves limited success with a significant risk of adverse effects that cannot be tolerated by many patients (Goldenberg, Burckhardt, & Crofford, 2004; Lautenschlager, 2000). Previous randomized controlled trials have shown that Tai Chi, a multicomponent intervention that incorporates physical, psychosocial, emotional, spiritual, and behavioral elements, is a potentially useful therapy for patients with fibromyalgia (C. Wang et al., 2010). Meta-analyses indicate that Tai Chi can enhance cognitive function in older adults, particularly in the realm of executive functioning (Wayne et al., 2014). Imaging studies have also shown that Tai Chi can significantly modulate the function and structure of brain regions associated with cognitive control (Tao, Chen, Egorova, et al., 2017; G. X. Wei, Dong, Yang, Luo, & Zuo, 2014; G. X. Wei, Gong, Yang, & Zuo, 2017; G.X. Wei et al., 2013), further endorsing the role of the CCN in Tai Chi intervention.
In this study, we first compared the resting state functional connectivity (rsFC) of the DLFPC between fibromyalgia patients and healthy subjects to characterize the brain pathophysiology of fibromyalgia, and then investigated how the rsFC of the DLPFC are modulated when symptoms are relieved after longitudinal intervention. We hypothesized that fibromyalgia patients would show increased DLPFC – ACC/MPFC rsFC as a distraction/adaptation mechanism. Mind-body interventions may enhance this coping process by increasing DLPFC- ACC/MPFC connectivity, further triggering the descending pain modulation system to relieve clinical symptoms in fibromyalgia patients. This investigation, which combines both healthy controls and an intervention group, allows us to not only illustrate rsFC changes that are sensitive to symptom improvement or treatment, but also highlights the physiological implications of these changes.
Materials and Methods
Participants
From May 2015 to September 2015, we recruited 24 patients with fibromyalgia (≥21 years old) and 24 healthy controls matched for age, gender, and body mass index (BMI) to participate in the study. After describing the details of the study to interested and eligible subjects, written informed consent was obtained in accordance with the procedures established by Tufts Medical Center/Tufts University Human Institutional Review Board and the Medical Ethics Committee of Massachusetts General Hospital. The study rheumatologist (WFH) performed clinical examinations and confirmed that participants met the eligibility criteria. All FM patients participated in 12 weeks of Tai Chi training (NCT02407665).
Inclusion criteria were: (1) age 21 years or older, (2) fulfillment of the American College of Rheumatology (ACR) 1990 classification criteria and the ACR 2010 diagnostic criteria for fibromyalgia, (3) willingness to complete a 12-week, twice-a-week Tai Chi exercise program, (4) willingness to undergo an fMRI scan at baseline and follow-up visits, and (5) fluent in English. Exclusion criteria were: (1) diagnosed with medical conditions that are known to contribute to fibromyalgia symptomatology, such as thyroid disease, inflammatory arthritis, systemic lupus erythematosus, rheumatoid arthritis, myositis, vasculitis, or Sjogren’s syndrome, (2) inability to pass the Physical Activity Readiness Questionnaire (PAR-Q), (3) score of less than 24 on the Mini-Mental State Examination; (4) plans to relocate from the region during the trial period; (5) verbal confirmation of pregnancy or planned pregnancy during the study period, (6) enrollment in any other clinical trial in the last 30 days, (7) presence of any contraindications to fMRI scanning, including but not limited to: cardiac pacemaker, metal implants, fear of enclosed spaces, pregnancy, and weight > 300 lbs., (8) prior experience with Tai Chi training, or similar types of complementary and alternative medicine (e.g. Qi Gong or yoga) in the past year, and (8) serious medical conditions limiting ability to participate in the Tai Chi training, including dementia, neurological disease, cancer, cardiovascular disease, pulmonary disease, metabolic disease, renal disease, liver disease, or other serious medical conditions, as determined by the study physicians.
Healthy controls were recruited and matched to enrolled fibromyalgia participants. Inclusion criteria were (1) age matched within 5 years (must be ≥ 21 years old), (2) gender and race matched, (3) BMI within 5 kg/m2 (must be < 300 lbs.), and (4) willingness to attend a single evaluation. Exclusion criteria were (1) prior experience with Tai Chi training or similar types of complementary and alternative medicine, (2) chronic or acute pain (e.g. fibromyalgia, osteoarthritis), and (3) presence of any contraindications to fMRI scanning, including but not limited to: cardiac pacemaker, metal implants, fear of closed spaces, pregnancy, and weight > 300 lbs.
Intervention
All participants in the Tai Chi group attended a 60-minute practice session twice a week for 12 weeks at Tufts Medical Center. The instructor (RR), who has extensive experience conducting Tai Chi training programs, followed a standardized Tai Chi protocol developed for patients with fibromyalgia (C. Wang et al., 2010). Participants were also provided with printed materials on fibromyalgia and the Tai Chi mind-body program, including Tai Chi principles, practicing techniques, and safety precautions specifically for participants with fibromyalgia. Every session included the following components: (1) warm-up, (2) Tai Chi movement, (3) breathing techniques, and (4) relaxation. Each component of the program was derived from the classical Yang style Tai Chi 108 posture (1983) condensed for the 12-week intervention program.
All subjects were encouraged to maintain their usual physical activities and to perform no new additional strength training other than their Tai Chi exercises. Subjects were also allowed to continue taking regular medications and maintain routine visits to their physicians throughout the course of the study. Participants were also instructed to practice at least 30 minutes a day at home and to complete daily logs indicating the amount of time that they practiced Tai Chi exercises. Data on class attendance and home practice was recorded and verified using standard case report forms.
Outcome measurements
The primary outcome for the study was the resting state functional connectivity (rsFC) of the bilateral DLPFC (a key region of the cognitive control network). The secondary outcomes were: 1) Revised Fibromyalgia Impact Questionnaire (FIQR) including the three domains, i.e., Function, Overall Impact, and Symptom Severity, and 2) Beck Depression Inventory (BDI-II). All outcome measurements were collected at baseline and after 12 weeks of the intervention for the fibromyalgia cohort and at baseline for the healthy subjects.
MRI data acquisition
fMRI scans were performed at Massachusetts General Hospital at the Martinos Center for Biomedical Imaging. Each fibromyalgia subject participated in an identical fMRI scan before and after 12 weeks of Tai Chi exercise, whereas healthy controls were only scanned at baseline. The fMRI brain imaging acquisition was conducted on a 3.0 Tesla Siemens (Skyra syngo) scanner with a 32-channel head coil. Magnetization-prepared rapid gradient echo (MPRAGE) T1-weighted images were collected (flip angle= 7 degree, voxel size 1.0 × 1.0 × 1.0 mm3). The BOLD resting state functional images were obtained with echo-planar imaging (TR = 3000 ms, TE = 30 ms, flip angle = 85 degrees, slice thickness=2.6 mm, acquisition matrix = 64×64, voxel size = 2.6 × 2.6 × 2.6 mm3, 44 axial slices, scan time 8 min 21 sec). All patients were required to keep their eyes open during the resting state fMRI scan.
Statistical analysis
Clinical data analysis
Statistical analysis was performed using SPSS 19.0 Software (SPSS Inc., Chicago, IL, USA). A threshold of p < 0.05 (2-tailed) was applied. One-way ANOVA and Chi square tests were conducted to compare baseline characteristics of the participants between groups. There were no significant differences in age and gender between the fibromyalgia and matched healthy control groups.
Seed based functional connectivity analysis
Functional BOLD data were preprocessed using SPM 12 (Statistical Parametric Mapping. Welcome Department of Cognitive Neurology, London, UK; implemented by MATLAB R3012b, Math Works, Inc., Natick, MA, USA). During the preprocessing, images were realigned, segmented, and co-registered to each subject’s high-resolution T1 scan, which was used to normalize to the standard Montreal Neurological Institute (MNI) template. Images were also smoothed using an 8 mm full-width at half-maximum (FWHM) Gaussian kernel and filtered with a frequency window of 0.008–0.09 Hz. In addition to these steps, we employed segmentation of gray matter, white matter, and cerebrospinal fluid (CSF) areas for the removal of temporal confounding factors (white matter and CSF) (Whitfield-Gabrieli & Nieto-Castanon, 2012). Data were then submitted to motion correction using the artifact detection toolbox (http://www.nitrc.org/projects/artifact_detect/). For each subject, we treated images as outliers if the composite movement from a preceding image exceeded 0.5 mm or if the global mean intensity was greater than 3 standard deviations from the mean image intensity for the entire resting scan. Outliers were included as regressors in the first-level general linear model along with other six regular motion parameters (Redcay et al., 2013).
Resting state functional connectivity analysis was conducted using the CONN toolbox v15.g (Whitfield-Gabrieli & Nieto-Castanon, 2012) (http://www.nitrc.org/projects/conn). We used an a priori DLPFC seed (peak coordinate: ± 36, 27, 29, with 5 mm radius), which has been used in previous studies (J. Hwang et al., 2015; Sheline et al., 2010; Tao, Chen, Egorova, et al., 2017). Functional connectivity measures were computed between the seed and every other voxel in the brain. First-level correlation maps were produced by extracting the residual BOLD time course from the DLPFC and by computing Pearson’s correlation coefficients between that time course and the time courses of all other voxels in the brain. Correlation coefficients were transformed into Fisher’s ‘Z’ scores to increases normality and allow for improved second-level general linear model analyses.
The baseline DLPFC rsFC of fibromyalgia patients and healthy control subjects were compared using a two-sample t-test. The Tai Chi practice effect (post-practice minus pre-practice) on fibromyalgia patients was compared using a paired t-test. Additionally, we also compared the DLPFC rsFC of fibromyalgia patients after practicing Tai Chi to healthy controls using a two-sample t-test. Age, gender, and BDI scores were included as covariates. A threshold of voxel-wise p < 0.005 (uncorrected) and cluster-level p < 0.05 (family-wise error correction) were applied for data analyses. Given the important role of the periaqueductal grey in pain modulation, we defined the PAG as a region of interest and used small volume correction to correct the p value at a level of p < 0.05. Similar to previous studies (Eippert et al., 2009; Kong et al., 2013b), correction was based on peak coordinates (x, y, z: 1, −25, −12) with a 4 mm radius obtained from a previous PAG meta-analysis (Linnman, Moulton, Barmettler, Becerra, & Borsook, 2012).
Results
The study was completed with 21 fibromyalgia patients and 20 healthy controls. One fibromyalgia patient was excluded from the rsFC analysis due to excessive head movement during scanning. Additionally, one healthy control was excluded due to brain atrophy.
The mean age of fibromyalgia subjects was 53.10 ± 11.58 (mean ± SD) and 52.90 ± 11.12 for control subjects (Table 1). There were no significant differences in age, gender, race, and BMI between the fibromyalgia and healthy control groups. FIQR scores demonstrated moderate to severe fibromyalgia in the majority of the fibromyalgia subjects with an average score of 45.1 ± 18.6. BDI-II scores revealed moderate depression with an average score of 19.71 ± 11.12 in the fibromyalgia group, and a two-sample t-test showed significant differences between the fibromyalgia and healthy control groups in BDI-II scores (2.75 ± 3.77) (p < 0.0001). Paired t-tests showed significant pre- and post-Tai Chi differences in general FIQR scores (mean ± SD, Pre: 45.1 ± 18.6, post: 35.8 ± 21.4, p = 0.003), as well as in the three FIQR domains: Function (pre: 12.1 ± 6.3, post: 8.5 ± 6.4, p = 0.001), Overall Impact (pre: 8.8 ± 6.4, post: 7.0 ± 5.7 p= 0.05), and Symptoms (pre: 25.1 ± 8.4, post: 20.8 ± 12, p = 0.017). Analysis of BDI-II scores showed a significant difference between pre- and post-treatment scores in the fibromyalgia patients (pre: 19.71 ±11.12, post: 9.95 ± 8.55, p = 0.0027).
Table 1.
Baseline Characteristics and Health Outcome Variables of Study Participants, all values are mean (SD) unless otherwise noted.
| Variable | Tai Chi (n=21) | Healthy Controls (n=20) |
|---|---|---|
| Female, no. (%) | 20 (95.24) | 19 (95.00) |
| Age, yr. | 53.10 (11.58) | 52.9 (11.12) |
| Race, no. (%) | ||
| White | 13 (61.90) | 12 (60.00) |
| Black | 6 (28.57) | 6 (30.00) |
| Asian | 2 (9.52) | 2 (10.00) |
| Body Mass Index, kg/m2 | 29.37 (6.96) | 27.28 (3.58) |
| Beck Depression Inventory-II | 19.71 (11.12) | 2.75 (3.77) |
Functional connectivity results
rsFC analysis indicated that fibromyalgia patients showed significantly greater rsFC between the DLPFC and both the bilateral rostral anterior cingulate cortex (rACC) and medial prefrontal cortex (MPFC) compared to healthy controls at baseline. There were no significant DLPFC rsFC decreases in fibromyalgia patients compared to healthy subjects at baseline. The comparison between healthy controls and fibromyalgia patients after intervention showed significant CCN rsFC increases at the bilateral rACC MPFC and temporal pole, and rsFC decreases at the left superior parietal lobule and postcentral gyrus in fibromyalgia patients (Table 2).
Table 2.
Regions with significantly different connectivity between the DLPFC and other brain regions in fibromyalgia patients (FM) and healthy controls before and after treatment.
| Conditions | Brain Regions | mm3 | Cluster centroid (MNI) | |||
|---|---|---|---|---|---|---|
| x | y | z | Z | |||
| FM (pre) > HC | Bilateral rACC/MPFC | 540 | 18 | 48 | 6 | 3.91 |
| HC > FM (pre) | NA | |||||
| FM (post) > FM (pre) | Bilateral rACC/MPFC | 312 | −10 | 58 | −4 | 3.89 |
| Bilateral middle frontal gyrus/superior frontal gyrus | 1380 | −16 | 28 | 38 | 5.61 | |
| Left middle frontal gyrus | 768 | −36 | 26 | 28 | 4.68 | |
| Left middle frontal/medial frontal gyrus | 255 | −20 | 42 | 18 | 4.59 | |
| Right operculum | 246 | 46 | 24 | −2 | 4.22 | |
| Left angular/supramarginal gyrus | 235 | −54 | −58 | 22 | 4.27 | |
| Right precentral gyrus | 211 | 46 | −8 | 40 | 4.71 | |
| Left temporal lobe | 202 | −50 | 12 | −30 | 3.96 | |
| Bilateral middle occipital gyrus | 1561 | 34 | −72 | 22 | 5.57 | |
| Bilateral precuneus | 804 | 28 | −72 | −4 | 5.18 | |
| Right cerebellum | 378 | 40 | −48 | −40 | 4.92 | |
| Left cerebellum | 308 | −22 | −60 | −54 | 4.73 | |
| Left cerebellum/fusiform gyrus | 209 | −44 | −46 | −28 | 4.63 | |
| FM (pre) > FM (post) | Brainstem | 253 | 8 | −24 | −56 | 4.52 |
| FM(post) > HC | Bilateral rACC/MPFC | 2650 | 18 | 42 | 26 | 4.76 |
| Left temporal pole | 251 | −52 | 16 | −14 | 3.96 | |
| Right temporal pole | 190 | 42 | 24 | −22 | 4.05 | |
| HC>FM(post) | Left superior parietal lobule/postcenral gyrus | 449 | −42 | −44 | 56 | 4.22 |
Note: FM(pre)=Fibromyalgia pre-Tai Chi intervention, FM(post)=Fibromyalgia post-Tai Chi intervention, HC=Healthy control, DLPFC=dorsolateral prefrontal cortex, rACC=rostral anterior cingulate cortex, MPFC= medial prefrontal cortex.
A direct comparison between the DLPFC before and after intervention in FM patients indicate a significant rsFC increase with the bilateral rACC/MPFC, frontal gyrus, precuneus, occipital gyrus, cerebellum; left temporal lobe, angular/supramarginal gyrus; and right operculum and precentral gyrus, and a significant rsFC decrease at the brain stem after Tai Chi.
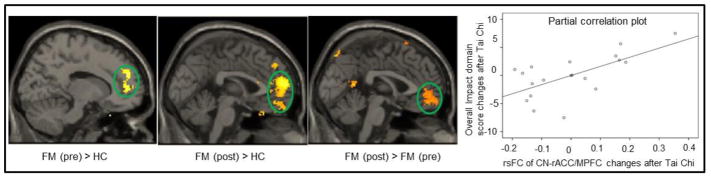
To explore the association between the CCN rsFC changes after intervention and the corresponding Overall Impact domain score changes (a domain reflecting the cognitive aspect of the FIQR), we extracted the Fisher z value of the rACC/MPFC cluster and applied a regression analysis, including age, gender, and BDI-II score as covariates. We found a significant positive association between CCN and rACC/MPFC rsFC changes and corresponding Overall Impact domain score changes (p = 0.01) (Figure 1).
Figure 1.
Comparison of dorsal lateral prefrontal cortex (DLPFC) connectivity maps in fibromyalgia patients and healthy controls. The partial correlation scatter indicates a regression analysis between the Overall Impact domain score and DLPFC-rACC/MPFC rsFC changes.
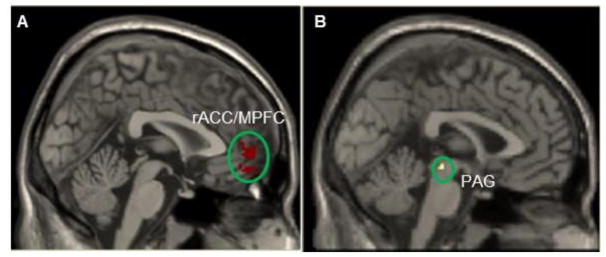
Given the importance of the rACC/MPFC in pain modulation and pathophysiology of chronic pain (H. Fields, 2004; Li et al., 2016; Yu et al., 2014), we extracted the overlapping areas of the rACC/MPFC (contrasts of fibromyalgia post intervention > healthy and fibromyalgia post intervention > fibromyalgia pre-intervention) as an ROI. We defined the ROI using the overlapping area so that we could target the area reflecting both the neuropathology of fibromyalgia and the area that would be modulated by Tai Chi intervention. We then compared the rsFC of the rACC/MPFC before and after Tai Chi using the same method as the ROI of the DLPFC and found that after Tai Chi practice, FM patients showed significant rsFC decreases between the rACC/MPFC and the left hippocampus, parahippocampus, fusiform, cerebellum, right PAG, and caudate, and bilateral inferior temporal gyrus (Table 3, Figure 2). There were also significant rsFC increases between the rACC/MPFC and bilateral MPFC, precuneus, middle frontal gyrus, middle cingulate cortex, supplementary motor cortex, operculum; left middle frontal gyrus, postcentral gyrus/supramarginal gyrus; and right precentral gyrus (Table 3).
Table 3.
Regions with significantly different connectivity between the rACC/MPFC and other brain regions in FM and healthy controls before and after treatment.
| Conditions | Brain Regions | mm3 | Cluster centroid (MNI) | |||
|---|---|---|---|---|---|---|
| x | y | z | Z | |||
| FM (post) > FM (pre) | Right middle frontal gyrus/precentral gyrus | 811 | 42 | 12 | 38 | 6.10 |
| Left postcentral gyrus/supramarginal gyrus/operculum | 1113 | −44 | −24 | 40 | 5.54 | |
| Right middle frontal gyrus | 227 | 40 | 54 | 2 | 4.39 | |
| Bilateral middle cingulate cortex/MPFC/supplementary motor cortex | 361 | −4 | 26 | 36 | 4.07 | |
| Left middle frontal gyrus | 230 | −36 | 40 | 46 | 4.41 | |
| Left middle frontal gyrus | 804 | −38 | 48 | 8 | 5.65 | |
| Bilateral precuneus | 1238 | −6 | −68 | 46 | 5.03 | |
| Right operculum | 239 | 66 | −16 | 16 | 3.76 | |
| FM (pre) > FM (post) | Left inferior temporal gyrus/fusiform gyrus/hippocampus/parahippocampus | 212 | −48 | −18 | −44 | 4.40 |
| Right PAG (small volume correction) | 6 | 2 | −22 | −8 | 2.88 | |
| Right caudate | 264 | 22 | −4 | 36 | 5.58 | |
| Right inferior temporal gyrus | 498 | 52 | −24 | − 38 | 5.02 | |
| Left cerebellum gyrus | 419 | −20 | −62 | − 24 | 5.28 | |
Note: FM (pre) = Fibromyalgia pre-Tai Chi intervention, FM (post)= Fibromyalgia post-Tai Chi intervention, HC=Healthy control, DLPFC= dorsolateral prefrontal cortex, rACC= rostral anterior cingulate cortex, MPFC= medial prefrontal cortex.
Figure 2.
A representative result of resting state functional connectivity using the rostral anterior cingulate cortex (rACC)/medial prefrontal cortex (MPFC) as a seed (ROI). A: seed region used in rsFC analysis. B: periaqueduct grey (PAG) showed significant rsFC decreases with rACC/MPFC after Tai Chi exercise.
Discussion
In this study, we found that compared to matched healthy controls, the CCN of fibromyalgia patients showed increased rsFC with the bilateral rACC/MPFC prior to Tai Chi practice. After intervention, fibromyalgia patients demonstrated further increased rsFC between the CCN and the bilateral rACC and MPFC, which was significantly associated with a decrease in Overall Impact domain scores on the FIQR. Further rsFC analyses using the rACC/MPFC as a seed found that, after Tai Chi, the rsFC between the rACC/MPFC and both the PAG and hippocampus was significantly decreased.
Our findings are consistent with a previous study demonstrating the effects of Tai Chi (C. Wang et al., 2010) and exercise (Flodin et al., 2015) in fibromyalgia patients. In addition, our results are consistent with previous brain imaging studies, which demonstrated a significant modulation effect of Tai Chi on brain function and structure in healthy human subjects (Fong, Chi, Li, & Chang, 2014; Tao, Chen, Liu, et al., 2017; Tao, Chen, Egorova, et al., 2017; Tao et al., 2016; Tao, Liu, et al., 2017; G. X. Wei et al., 2014; G. X. Wei et al., 2017; G.X. Wei et al., 2013) and of exercise on brain responses to heat pain (Ellingson, Stegner, Schwabacher, Koltyn, & Cook, 2016). Our results are also similar with other studies that explore resting state functional connectivity of pain-related brain regions (Flodin et al., 2015) in patients with fibromyalgia.
The DLPFC is a brain region in the dorsal pathway (stream) that is associated with reactions to various stimuli, including pain. Lorenz and colleagues (Lorenz, Minoshima, & Casey, 2003) suggest that the DLPFC exerts active control on pain perception by modulating cortico-subcortical and cortico-cortical pathways. Napadow et al. (Napadow et al., 2010) found a negative association between the right frontoparietal network and both the PAG and hippocampus (greater right frontoparietal network connectivity in relation to lower pain levels) in fibromyalgia patients with greater spontaneous pain. In one of our previous studies, we found significant fMRI signal increases in the bilateral DLPFC and MPFC/ACC during both anticipation and the application of pain within a placebo analgesia conditioning paradigm (Kong et al., 2013a). We also found that the baseline rsFC between the right frontoparietal network and the rACC could significantly predict placebo analgesia.
Both DLPFC and ACC/MPFC play an important role in executive control processing (Petersen & Posner, 2012) and non-pharmacological therapeutic modalities such as mind-body intervention (Tang, Holzel, & Posner, 2015) and acupuncture (Chen et al., 2015; Chen, Spaeth, Retzepi, Ott, & Kong, 2014; X. Wang et al., 2016; Z. Wang et al., 2017), as well as placebo effects (Gollub et al., 2018; Kong et al., 2006; Petrovic, Kalso, Petersson, & Ingvar, 2002; Wager et al., 2004; Zubieta et al., 2005). Studies also suggest that the rACC/MPFC is a key region in the descending pain modulatory system (Eippert & Tracey, 2014; H. Fields, 2004; Kong et al., 2013a; Kucyi, Salomons, & Davis, 2013; Tracey et al., 2002; Yu et al., 2014), forming a core network with the PAG and rostral ventral medulla (RVM) for pain modulation (Kong et al., 2010). Patients with fibromyalgia have been shown to exhibit reduced connectivity during repeated pressure pain stimuli between the rACC and the amygdala, hippocampus, and brainstem (PAG) compared to healthy controls (Jensen, Loitoile, et al., 2012). Thus, we postulate that enhanced rsFC between the DLPFC and the rACC/MPFC in fibromyalgia patients at baseline may indicate that the brain employs a self-regulation mechanism to cope with repeated pain experiences.
Consistent with the aforementioned studies and our hypothesis, we found that after 12 weeks of Tai Chi intervention, the rsFC between the CCN and the rACC/MPFC rsFC was further enhanced, and this increase was associated with decreases in the Overall Impact domain score of the FIQR, suggesting that Tai Chi practice can further amplify this self-regulation process (Tang et al., 2015). Our finding is consistent with a previous study (Jensen, Kosek, et al., 2012) investigating the effects of cognitive behavioral therapy on fibromyalgia patients. Investigators found that rather than reducing pain responses in patients with fibromyalgia, cognitive behavioral therapy increased access to executive regions for the reappraisal of pain. These results are further supported by another brain imaging study (Li et al., 2017) on the effect of acupuncture treatment in patients with migraines, in which the investigators discovered decreased rsFC between the right frontoparietal network and bilateral precuneus in migraine patients when compared to healthy controls. After acupuncture treatment, the rsFC between the frontoparietal network and precuneus was further reduced, and this reduction was associated with headache intensity relief.
Taken together, we believe our results suggest the existence of allostasis in patients with fibromyalgia. Based on the allostasis theory proposed by McEwen and colleagues (McEwen & Stellar, 1993), physiological systems within the body fluctuate to meet demands from external forces to maintain homeostasis. In this context, allostasis represents the ability to protect the body through the altered activity of mediators that normally promote adaptation (Borsook et al., 2012). Most importantly, it seems that different treatment modalities, such as behavioral therapy, mind-body interventions, and acupuncture, can further enhance allostasis to promote self-regulation and adaptation, and this may represent a common mechanism underlying non-pharmacological treatments. Elucidating this mechanism might shed light on the development of new pain management methods.
To further explore the role of the rACC/MPFC in the development of FM and the associated clinical improvements, we compared the rsFC changes of the rACC/MPFC before and after intervention. We found that after 12 weeks of Tai Chi practice, the rsFC between the rACC/MPFC and both the hippocampus and PAG were significantly reduced. Recently, investigators have conceptualized chronic pain as a type of long-term learning (Apkarian, Hashmi, & Baliki, 2011), characterized by an accumulation of nociceptive memory (Yi & Zhang, 2011) and inability to extinguish negative emotional associations and anxiety (Mutso et al., 2014; Ploghaus et al., 2001; Vachon-Presseau et al., 2013; Vachon-Presseau et al., 2016). A recently proposed model of aversive prediction error in pain learning has suggested that the PAG is the primary hub in the pain learning neural network (Roy et al., 2014), relying on the rACC/MPFC and the hippocampus. We (Kong et al., 2018) found that the rsFC of the nucleus accumbens (NAc) showed significant increase with both rACC/MPFC and dorsolateral prefrontal cortex in the boosted acupuncture group using an expectancy manipulation (Kong et al., 2006) as compared to standard acupuncture group without such a manipulation. In another study, we found that acupuncture significantly increased the rsFC between the hippocampus and PAG (Egorova, Gollub, & Kong, 2015) and improved pain intensity in knee osteoarthritis patients, providing further support for the role of the hippocampus in pain modulation.
We also found that after the intervention, fibromyalgia patients showed increased rsFC between the rACC/MPFC and the middle/inferior prefrontal gyrus, middle cingulate, and postcentral gyrus/operculum. It is well known that these regions are involved in the cognitive, affective, and sensory aspects of pain, respectively (Tracey & Mantyh, 2007). The increased rsFC between the rACC/MPFC and these regions may reflect the enhanced modulation effects and therapeutic benefits of Tai Chi.
Although our results may provide some novel insights into the mechanisms underlying the effectiveness of Tai Chi, the lack of a control condition has significantly limited our ability to identify the precise mechanism behind Tai Chi’s therapeutic effects. We would like to emphasize here that the aim of this study was to investigate the rsFC alterations of the CCN in FM patients and how these rsFC changes following effective treatment were associated with symptom relief. These findings have the potential to shed light on our understanding of the pathophysiology and development of FM. We acknowledge that pharmacological medication (all participants were allowed to keep their regular medication during experiment) and other unknown factors may have also contributed to the clinical improvements observed. Nevertheless, this paradigm has been used in previous studies (Rodriguez-Raecke, Niemeier, Ihle, Ruether, & May, 2009). Additional controls should be applied in future studies to elucidate the specific effect of Tai Chi.
In summary, we found that fibromyalgia patients showed increased resting state functional connectivity between the cognitive control network and the rACC/MPFC, which may reflect an adaptive brain response to fibromyalgia. Effective interventions such as Tai Chi can further enhance the cognitive control network and rACC/MPFC rsFC and relieve the clinical symptoms in fibromyalgia patients. Finally, we found the rACC/MPFC rsFC with both the PAG and hippocampus were significantly reduced after the intervention. Our findings suggest the existence of allostasis in patients with fibromyalgia. A novel effective pain management intervention such as Tai Chi may further enhance allostasis to promote self-regulation and adaptation mechanisms and ultimately, provide significant therapeutic benefit to FM patients.
Acknowledgments
This study was supported by the National Center for Complementary and Integrative Health of the National Institutes of Health (R01 AT005521, R01 AT006367 and K24 AT007323, R01AT006364, R01 AT008563, R21AT008707, R61 AT009310, and P01 AT006663 from NIH/NCCIH), the National Center for Research Resources, National Institutes of Health (UL1 RR025752) and the National Center for Advancing Translational Sciences, National Institutes of Health (UL1TR000073 and UL1TR001064).
Dr. Chenchen Wang is supported by R01 AT005521, R01AT006367 and K24AT007323, the National Center for Research Resources, National Institutes of Health (UL1 RR025752) and the National Center for Advancing Translational Sciences, National Institutes of Health (UL1TR000073 and UL1TR001064). Dr. Jian Kong is supported by R01AT006364, R01AT008563, R61 AT009310, R21AT008707, and P01 AT006663 from NIH/NCCIH. We also thank Courtney Lang for her help in manuscript revision and proof reading.
Footnotes
Contribution of the authors
Experimental design: Chenchen Wang, Jian Kong, Ramel Rones, William F Harvey
Data collection: Jian Kong, Emily Wolcott, Jing Tao, Kristen Jorgenson, William F Harvey
Data analysis: Zengjian Wang, Jian Kong
Manuscript preparation: Jian Kong, Zengjian Wang, Chenchen Wang, Kristen Jorgenson, William F Harvey
Conflict of interest
Jian Kong holds equity of a start-up Inc (MNT). All authors declare that they have no competing financial interests.
References
- Apkarian AV, Hashmi JA, Baliki MN. Pain and the brain: specificity and plasticity of the brain in clinical chronic pain. Pain. 2011;152(3 Suppl):S49–64. doi: 10.1016/j.pain.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Board CSE. Simplified “Taijiquan”. Beijing: China Publications Center; 1983. [Google Scholar]
- Borsook D, Maleki N, Becerra L, McEwen B. Understanding migraine through the lens of maladaptive stress responses: a model disease of allostatic load. Neuron. 2012;73(2):219–234. doi: 10.1016/j.neuron.2012.01.001. S0896-6273(12)00025-6 [pii] [DOI] [PubMed] [Google Scholar]
- Bushnell MC, Ceko M, Low LA. Cognitive and emotional control of pain and its disruption in chronic pain. Nat Rev Neurosci. 2013;14(7):502–511. doi: 10.1038/nrn3516. nrn3516 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Spaeth RB, Freeman SG, Scarborough DM, Hashmi JA, Wey HY, … Kong J. The modulation effect of longitudinal acupuncture on resting state functional connectivity in knee osteoarthritis patients. Mol Pain. 2015;11(1):67. doi: 10.1186/s12990-015-0071-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Spaeth RB, Retzepi K, Ott D, Kong J. Acupuncture modulates cortical thickness and functional connectivity in knee osteoarthritis patients. Sci Rep. 2014;4:6482. doi: 10.1038/srep06482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cieslik EC, Zilles K, Caspers S, Roski C, Kellermann TS, Jakobs O, … Eickhoff SB. Is there “one” DLPFC in cognitive action control? Evidence for heterogeneity from co-activation-based parcellation. Cereb Cortex. 2013;23(11):2677–2689. doi: 10.1093/cercor/bhs256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MW, Schneider W. The cognitive control network: Integrated cortical regions with dissociable functions. Neuroimage. 2007;37(1):343–360. doi: 10.1016/j.neuroimage.2007.03.071. [DOI] [PubMed] [Google Scholar]
- Cummiford CM, Nascimento TD, Foerster BR, Clauw DJ, Zubieta JK, Harris RE, DaSilva AF. Changes in resting state functional connectivity after repetitive transcranial direct current stimulation applied to motor cortex in fibromyalgia patients. Arthritis Res Ther. 2016;18:40. doi: 10.1186/s13075-016-0934-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egorova N, Gollub RL, Kong J. Repeated verum but not placebo acupuncture normalizes connectivity in brain regions dysregulated in chronic pain. Neuroimage Clin. 2015;9:430–435. doi: 10.1016/j.nicl.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eippert F, Bingel U, Schoell ED, Yacubian J, Klinger R, Lorenz J, Buchel C. Activation of the opioidergic descending pain control system underlies placebo analgesia. Neuron. 2009;63(4):533–543. doi: 10.1016/j.neuron.2009.07.014. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19709634. [DOI] [PubMed] [Google Scholar]
- Eippert F, Tracey I. Pain and the PAG: learning from painful mistakes. Nat Neurosci. 2014;17(11):1438–1439. doi: 10.1038/nn.3844. [DOI] [PubMed] [Google Scholar]
- Ellingson LD, Stegner AJ, Schwabacher IJ, Koltyn KF, Cook DB. Exercise Strengthens Central Nervous System Modulation of Pain in Fibromyalgia. Brain Sci. 2016;6(1) doi: 10.3390/brainsci6010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields H. State-dependent opioid control of pain. Nat Rev Neurosci. 2004;5(7):565–575. doi: 10.1038/nrn1431. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15208698. [DOI] [PubMed] [Google Scholar]
- Fields HL, Basbaum AI, Heinricher MM. Central nervous system mechanisms of pain modulation. In: McMahon SB, Koltzenburg M, editors. Wall and Melzack’s Textbook of Pain. London: Elsevier - Churchill Livingstone; 2005. pp. 125–142. [Google Scholar]
- Flodin P, Martinsen S, Mannerkorpi K, Lofgren M, Bileviciute-Ljungar I, Kosek E, Fransson P. Normalization of aberrant resting state functional connectivity in fibromyalgia patients following a three month physical exercise therapy. Neuroimage Clin. 2015;9:134–139. doi: 10.1016/j.nicl.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong DY, Chi LK, Li F, Chang YK. The benefits of endurance exercise and Tai Chi Chuan for the task-switching aspect of executive function in older adults: an ERP study. Front Aging Neurosci. 2014;6:295. doi: 10.3389/fnagi.2014.00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg DL, Burckhardt C, Crofford L. Management of fibromyalgia syndrome. Jama. 2004;292(19):2388–2395. doi: 10.1001/jama.292.19.2388. [DOI] [PubMed] [Google Scholar]
- Gollub RL, Kirsch I, Maleki N, Wasan AD, Edwards RR, Tu Y, … Kong J. A Functional Neuroimaging Study of Expectancy Effects on Pain Response in Patients with Knee Osteoarthritis. J Pain. 2018 doi: 10.1016/j.jpain.2017.12.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang J, Egorova N, Yang XQ, Zhang WY, Chen J, Yang XY, … Kong J. Subthreshold depression is associated with impaired resting state functional connectivity of the cognitive control network. Translational Psychiatry. 2015;5:e683. doi: 10.1038/tp.2015.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang JW, Egorova N, Yang XQ, Zhang WY, Chen J, Yang XY, … Kong J. Subthreshold depression is associated with impaired resting-state functional connectivity of the cognitive control network. Transl Psychiatry. 2015;5:e683. doi: 10.1038/tp.2015.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen K, Srinivasan P, Spaeth R, Tan Y, Kosek E, Petzke F, … Kong J. Overlapping structural and functional brain changes in patients with long-term exposure to fibromyalgia. Arthritis & Rheumatism. 2013;65(12):3293–3303. doi: 10.1002/art.38170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen KB, Kosek E, Wicksell R, Kemani M, Olsson G, Merle JV, … Ingvar M. Cognitive Behavioral Therapy increases pain-evoked activation of the prefrontal cortex in patients with fibromyalgia. Pain. 2012;153(7):1495–1503. doi: 10.1016/j.pain.2012.04.010. [DOI] [PubMed] [Google Scholar]
- Jensen KB, Loitoile R, Kosek E, Petzke F, Carville S, Fransson P, … Kong J. Patients With Fibromyalgia Display Less Functional Connectivity In The Brain’s Pain Inhibitory Network. Mol Pain. 2012;8(1):32. doi: 10.1186/1744-8069-8-32. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=22537768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen KB, Srinivasan P, Spaeth R, Tan Y, Kosek E, Petzke F, … Kong J. Overlapping structural and functional brain changes in patients with long-term exposure to fibromyalgia pain. Arthritis Rheum. 2013;65(12):3293–3303. doi: 10.1002/art.38170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, Gollub RL, Rosman IS, Webb JM, Vangel MG, Kirsch I, Kaptchuk TJ. Brain activity associated with expectancy-enhanced placebo analgesia as measured by functional magnetic resonance imaging. J Neurosci. 2006;26(2):381–388. doi: 10.1523/JNEUROSCI.3556-05.2006. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16407533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, Jensen K, Loiotile R, Cheetham A, Wey HY, Tan T, … Gollub R. Functional connectivity of frontoparietal network predicts cognitive modulation of pain. Pain. 2013a;154(3):459–467. doi: 10.1016/j.pain.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, Jensen K, Loiotile R, Cheetham A, Wey HY, Tan Y, … Gollub RL. Functional connectivity of the frontoparietal network predicts cognitive modulation of pain. Pain. 2013b;154(3):459–467. doi: 10.1016/j.pain.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, Tu PC, Zyloney C, Su TP. Intrinsic functional connectivity of the periaqueductal gray, a resting fMRI study. Behav Brain Res. 2010;211(2):215–219. doi: 10.1016/j.bbr.2010.03.042. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=20347878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, Wang ZJ, Leiser J, Minicucci D, Edwards R, Kirsch I, … Gollub L. Enhancing treatment of osteoarthritis knee pain by boosting expectancy: A functional neuroimaging study. NeuroImage: Clinical. 2018;18:325–334. doi: 10.1016/j.nicl.2018.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchinad A, Schweinhardt P, Seminowicz DA, Wood PB, Chizh BA, Bushnell MC. Accelerated Brain Gray Matter Loss in Fibromyalgia Patients: Premature Aging of the Brain? J Neurosci. 2007;27(15):4004–4007. doi: 10.1523/jneurosci.0098-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucyi A, Salomons TV, Davis KD. Mind wandering away from pain dynamically engages antinociceptive and default mode brain networks. Proc Natl Acad Sci U S A. 2013;110(46):18692–18697. doi: 10.1073/pnas.1312902110. 1312902110 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lautenschlager J. Present state of medication therapy in fibromyalgia syndrome. Scand J Rheumatol Suppl. 2000;113:32–36. doi: 10.1080/030097400446616. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11028829. [DOI] [PubMed] [Google Scholar]
- Legrain V, Damme SV, Eccleston C, Davis KD, Seminowicz DA, Crombez G. A neurocognitive model of attention to pain: behavioral and neuroimaging evidence. Pain. 2009;144(3):230–232. doi: 10.1016/j.pain.2009.03.020. [DOI] [PubMed] [Google Scholar]
- Li Z, Lan L, Zeng F, Makris N, Hwang J, Guo T, … Kong J. The altered right frontoparietal network functional connectivity in migraine and the modulation effect of treatment. Cephalalgia. 2017;37(2):161–176. doi: 10.1177/0333102416641665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Liu M, Lan L, Zeng F, Makris N, Liang Y, … Kong J. Altered periaqueductal gray resting state functional connectivity in migraine and the modulation effect of treatment. Sci Rep. 2016;6:20298. doi: 10.1038/srep20298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnman C, Moulton EA, Barmettler G, Becerra L, Borsook D. Neuroimaging of the periaqueductal gray: state of the field. Neuroimage. 2012;60(1):505–522. doi: 10.1016/j.neuroimage.2011.11.095. S1053-8119(11)01400-5 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobanov OV, Quevedo AS, Hadsel MS, Kraft RA, Coghill RC. Frontoparietal mechanisms supporting attention to location and intensity of painful stimuli. Pain. 2013;154(9):1758–1768. doi: 10.1016/j.pain.2013.05.030. S0304-3959(13)00269-8 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loggia ML, Berna C, Kim J, Cahalan CM, Gollub RL, Wasan AD, … Napadow V. Disrupted brain circuitry for pain-related reward/punishment in fibromyalgia. Arthritis Rheumatol. 2014;66(1):203–212. doi: 10.1002/art.38191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz J, Minoshima S, Casey KL. Keeping pain out of mind: the role of the dorsolateral prefrontal cortex in pain modulation. Brain. 2003;126:1079–1091. doi: 10.1093/brain/awg102. [DOI] [PubMed] [Google Scholar]
- Lutz J, Jager L, de Quervain D, Krauseneck T, Padberg F, Wichnalek M, … Schelling G. White and gray matter abnormalities in the brain of patients with fibromyalgia: a diffusion-tensor and volumetric imaging study. Arthritis Rheum. 2008;58(12):3960–3969. doi: 10.1002/art.24070. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Stellar E. Stress and the individual. Mechanisms leading to disease. Arch Intern Med. 1993;153(18):2093–2101. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8379800. [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11283309. [DOI] [PubMed] [Google Scholar]
- Mutso AA, Petre B, Huang L, Baliki MN, Torbey S, Herrmann KM, … Apkarian AV. Reorganization of hippocampal functional connectivity with transition to chronic back pain. J Neurophysiol. 2014;111(5):1065–1076. doi: 10.1152/jn.00611.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napadow V, LaCount L, Park K, As-Sanie S, Clauw DJ, Harris RE. Intrinsic brain connectivity in fibromyalgia is associated with chronic pain intensity. Arthritis Rheum. 2010;62(8):2545–2555. doi: 10.1002/art.27497. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=20506181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen SE, Posner MI. The attention system of the human brain: 20 years after. Annu Rev Neurosci. 2012;35:73–89. doi: 10.1146/annurev-neuro-062111-150525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovic P, Kalso E, Petersson KM, Ingvar M. Placebo and opioid analgesia-- imaging a shared neuronal network. Science. 2002;295(5560):1737–1740. doi: 10.1126/science.1067176. [DOI] [PubMed] [Google Scholar]
- Ploghaus A, Narain C, Beckmann CF, Clare S, Bantick S, Wise R, … Tracey I. Exacerbation of pain by anxiety is associated with activity in a hippocampal network. J Neurosci. 2001;21(24):9896–9903. doi: 10.1523/JNEUROSCI.21-24-09896.2001. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11739597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redcay E, Moran JM, Mavros PL, Tager-Flusberg H, Gabrieli JD, Whitfield-Gabrieli S. Intrinsic functional network organization in high-functioning adolescents with autism spectrum disorder. Front Hum Neurosci. 2013;7:573. doi: 10.3389/fnhum.2013.00573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson ME, Craggs JG, Price DD, Perlstein WM, Staud R. Gray matter volumes of pain-related brain areas are decreased in fibromyalgia syndrome. J Pain. 2011;12(4):436–443. doi: 10.1016/j.jpain.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Raecke R, Niemeier A, Ihle K, Ruether W, May A. Brain gray matter decrease in chronic pain is the consequence and not the cause of pain. J Neurosci. 2009;29(44):13746–13750. doi: 10.1523/JNEUROSCI.3687-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen ML, Stern CE, Michalka SW, Devaney KJ, Somers DC. Cognitive Control Network Contributions to Memory-Guided Visual Attention. Cereb Cortex. 2015 doi: 10.1093/cercor/bhv028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy M, Shohamy D, Daw N, Jepma M, Wimmer GE, Wager TD. Representation of aversive prediction errors in the human periaqueductal gray. Nat Neurosci. 2014;17(11):1607–1612. doi: 10.1038/nn.3832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Price JL, Yan Z, Mintun MA. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proc Natl Acad Sci U S A. 2010;107(24):11020–11025. doi: 10.1073/pnas.1000446107. 1000446107 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumpton JE, Moulin DE. Fibromyalgia. Handb Clin Neurol. 2014;119:513–527. doi: 10.1016/B978-0-7020-4086-3.00033-3. [DOI] [PubMed] [Google Scholar]
- Tang YY, Holzel BK, Posner MI. The neuroscience of mindfulness meditation. Nat Rev Neurosci. 2015;16(4):213–225. doi: 10.1038/nrn3916. [DOI] [PubMed] [Google Scholar]
- Tao J, Chen X, Liu J, Egorova N, Xue X, Liu W, … Kong J. Tai Chi Chuan and Baduanjin Mind-Body Training Changes Resting-State Low-Frequency Fluctuations in the Frontal Lobe of Older Adults: A Resting-State fMRI Study. Front Hum Neurosci. 2017;11:514. doi: 10.3389/fnhum.2017.00514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao J, Chen XL, Egorova N, Liu J, Xue XH, Huang J, … Kong J. Tai Chi Chuan and Baduanjin practice modulates functional connectivity of the cognitive control network in older adults. Sci Rep. 2017;7:41581. doi: 10.1038/srep41581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao J, Liu J, Egorova N, Chen X, Sun S, Xue X, … Kong J. Increased Hippocampus-Medial Prefrontal Cortex Resting-State Functional Connectivity and Memory Function after Tai Chi Chuan Practice in Elder Adults. Front Aging Neurosci. 2016;8:25. doi: 10.3389/fnagi.2016.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao J, Liu J, Liu W, Huang J, Xue X, Chen X, … Kong J. Tai Chi Chuan and Baduanjin Increase Grey Matter Volume in Older Adults: A Brain Imaging Study. J Alzheimers Dis. 2017 doi: 10.3233/JAD-170477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torta DM, Legrain V, Mouraux A, Valentini E. Attention to pain! A neurocognitive perspective on attentional modulation of pain in neuroimaging studies. Cortex. 2017;89:120–134. doi: 10.1016/j.cortex.2017.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey I, Bushnell MC. How neuroimaging studies have challenged us to rethink: is chronic pain a disease? J Pain. 2009;10(11):1113–1120. doi: 10.1016/j.jpain.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Tracey I, Mantyh PW. The cerebral signature for pain perception and its modulation. Neuron. 2007;55(3):377–391. doi: 10.1016/j.neuron.2007.07.012. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17678852. [DOI] [PubMed] [Google Scholar]
- Tracey I, Ploghaus A, Gati JS, Clare S, Smith S, Menon RS, Matthews PM. Imaging attentional modulation of pain in the periaqueductal gray in humans. J Neurosci. 2002;22(7):2748–2752. doi: 10.1523/JNEUROSCI.22-07-02748.2002. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11923440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truini A, Tinelli E, Gerardi MC, Calistri V, Iannuccelli C, La Cesa S, … Di Franco M. Abnormal resting state functional connectivity of the periaqueductal grey in patients with fibromyalgia. Clin Exp Rheumatol. 2016;34(2 Suppl 96):S129–133. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/27157397. [PubMed] [Google Scholar]
- Vachon-Presseau E, Roy M, Martel MO, Caron E, Marin MF, Chen J, … Rainville P. The stress model of chronic pain: evidence from basal cortisol and hippocampal structure and function in humans. Brain. 2013;136(Pt 3):815–827. doi: 10.1093/brain/aws371. [DOI] [PubMed] [Google Scholar]
- Vachon-Presseau E, Tetreault P, Petre B, Huang L, Berger SE, Torbey S, … Apkarian AV. Corticolimbic anatomical characteristics predetermine risk for chronic pain. Brain. 2016 doi: 10.1093/brain/aww100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valet M, Sprenger T, Boecker H, Willoch F, Rummeny E, Conrad B, … Tolle TR. Distraction modulates connectivity of the cingulo-frontal cortex and the midbrain during pain--an fMRI analysis. Pain. 2004;109(3):399–408. doi: 10.1016/j.pain.2004.02.033. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15157701. [DOI] [PubMed] [Google Scholar]
- Villemure C, Bushnell MC. Cognitive modulation of pain: how do attention and emotion influence pain processing? Pain. 2002;95:195–199. doi: 10.1016/S0304-3959(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Villemure C, Schweinhardt P. Supraspinal pain processing: distinct roles of emotion and attention. Neuroscientist. 2010;16(3):276–284. doi: 10.1177/1073858409359200. [DOI] [PubMed] [Google Scholar]
- Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, … Cohen JD. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science. 2004;303:1162–1167. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- Wang C, Schmid CH, Rones R, Kalish R, Yinh J, Goldenberg DL, … McAlindon T. A randomized trial of tai chi for fibromyalgia. N Engl J Med. 2010;363(8):743–754. doi: 10.1056/NEJMoa0912611. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=20818876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Wang Z, Liu J, Chen J, Liu X, Nie G, … Kong J. Repeated acupuncture treatments modulate amygdala resting state functional connectivity of depressive patients. Neuroimage Clin. 2016;12:746–752. doi: 10.1016/j.nicl.2016.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Wang X, Liu J, Chen J, Liu X, Nie G, … Kong J. Acupuncture treatment modulates the corticostriatal reward circuitry in major depressive disorder. J Psychiatr Res. 2017;84:18–26. doi: 10.1016/j.jpsychires.2016.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayne PM, Walsh JN, Taylor-Piliae RE, Wells RE, Papp KV, Donovan NJ, Yeh GY. Effect of tai chi on cognitive performance in older adults: systematic review and meta-analysis. J Am Geriatr Soc. 2014;62(1):25–39. doi: 10.1111/jgs.12611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei GX, Dong HM, Yang Z, Luo J, Zuo XN. Tai Chi Chuan optimizes the functional organization of the intrinsic human brain architecture in older adults. Front Aging Neurosci. 2014;6:74. doi: 10.3389/fnagi.2014.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei GX, Gong ZQ, Yang Z, Zuo XN. Mind-Body Practice Changes Fractional Amplitude of Low Frequency Fluctuations in Intrinsic Control Networks. Front Psychol. 2017;8:1049. doi: 10.3389/fpsyg.2017.01049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei GX, Xu T, Fan FM, Dong HM, Jiang LL, Li H, … Zuo XN. Can Taichi reshape the brain? A brain morphometry study. PLoS One. 2013;8(4):e61038. doi: 10.1371/journal.pone.0061038. doi:61010.61371/journal.pone.0061038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2(3):125–141. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- Wiech K. Deconstructing the sensation of pain: The influence of cognitive processes on pain perception. Science. 2016;354(6312):584–587. doi: 10.1126/science.aaf8934. [DOI] [PubMed] [Google Scholar]
- Yi M, Zhang H. Nociceptive memory in the brain: cortical mechanisms of chronic pain. J Neurosci. 2011;31(38):13343–13345. doi: 10.1523/JNEUROSCI.3279-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu R, Gollub R, Spaetha R, Napadowa V, Wasana A, Kong J. Disrupted functional connectivity of the periaqueductal gray in chronic low back pain. NeuroImage: Clinical. 2014;6:100–108. doi: 10.1016/j.nicl.2014.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanto TP, Gazzaley A. Fronto-parietal network: flexible hub of cognitive control. Trends Cogn Sci. 2013;17(12):602–603. doi: 10.1016/j.tics.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidan F, Emerson NM, Farris SR, Ray JN, Jung Y, McHaffie JG, Coghill RC. Mindfulness Meditation-Based Pain Relief Employs Different Neural Mechanisms Than Placebo and Sham Mindfulness Meditation-Induced Analgesia. J Neurosci. 2015;35(46):15307–15325. doi: 10.1523/JNEUROSCI.2542-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidan F, Grant JA, Brown CA, McHaffie JG, Coghill RC. Mindfulness meditation-related pain relief: evidence for unique brain mechanisms in the regulation of pain. Neurosci Lett. 2012;520(2):165–173. doi: 10.1016/j.neulet.2012.03.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubieta JK, Bueller JA, Jackson LR, Scott DJ, Xu Y, Koeppe RA, … Stohler CS. Placebo effects mediated by endogenous opioid activity on mu-opioid receptors. J Neurosci. 2005;25(34):7754–7762. doi: 10.1523/JNEUROSCI.0439-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]