Summary
Actin has been linked to processes spanning the whole gene expression cascade, from regulating specific transcription factors, such as myocardin-related transcription factor, to chromatin remodeling and RNA polymerase function. However, whether actin controls the transcription of only specific genes or has a global role in gene expression has remained elusive. Our genome-wide analysis reveals, for the first time, that actin interacts with essentially all transcribed genes in Drosophila ovaries. Actin co-occupies the majority of gene promoters together with Pol II, and on highly expressed genes, these two proteins also associate with gene bodies. Mechanistically, actin is required for Pol II recruitment to gene bodies, and manipulation of nuclear transport factors for actin leads to the decreased expression of eggshell genes. Collectively, these results uncover a global role for actin in transcription and demonstrate the in vivo importance of balanced nucleocytoplasmic shuttling of actin in the transcriptional control of a developmental process.
Subject Areas: Molecular Mechanism of Gene Regulation, Developmental Biology, Model Organism
Graphical Abstract

Highlights
-
•
Genome-wide analysis shows actin on all transcribed genes
-
•
Actin binds with RNA polymerase II near transcription start sites of most genes
-
•
On highly expressed genes, actin is also found on the gene bodies
-
•
Nuclear transport of actin is required for transcription during fly development
Molecular Mechanism of Gene Regulation; Developmental Biology; Model Organism
Introduction
In addition to its essential roles as part of the cytoskeleton, actin regulates gene expression in the nucleus. Actin is a component of many chromatin remodeling complexes (reviewed by Kapoor and Shen, 2013) and is linked to transcription by all three RNA polymerases (Hofmann et al., 2004, Hu et al., 2004, Philimonenko et al., 2004). Actin seems to have a positive role in general transcription, since the reduced availability of nuclear actin, due to inhibition of the active nuclear import of actin (Dopie et al., 2012); activation of a mechanosensory complex consisting of emerin, non-muscle myosin II, and actin (Le et al., 2016); or polymerizing nuclear actin into stable filaments (Serebryannyy et al., 2016), attenuates transcription. Nevertheless, the exact mechanism and the in vivo -relevance of this process have remained unclear. Actin also negatively regulates the transcription of specific genes. For example, actin regulates both the nuclear localization and activity of myocardin-related transcription factor A (MRTF-A; also known as MAL/MKL1), which is a cofactor of the essential transcription factor SRF (Miralles et al., 2003, Vartiainen et al., 2007). Actin monomer binding prevents MRTF-A from activating SRF in the nucleus. This regulation has been postulated to take place at the level of target genes (Vartiainen et al., 2007), but how the opposing effects of actin on transcription are resolved on chromatin is not obvious. Moreover, the genome-wide binding pattern of actin in the context of RNA polymerase II (Pol II)-mediated transcription has remained elusive, and previous studies of actin-chromatin interactions are based on few selected genes (Hofmann et al., 2004, Obrdlik et al., 2008). Importantly, actin itself is one of the target genes for SRF (Salvany et al., 2014), generating a feedback loop, where actin levels are controlled by the actin dynamics cycle. Here we show that chromatin binding of actin is not dependent on Mrtf transcription factors and that, at the genome-wide level, actin interacts with essentially all the transcribed genes in Drosophila ovaries, with a pattern depending on the expression level of the gene. Finally, we demonstrate the functional relevance of nuclear actin for gene transcription in vivo.
Results and Discussion
Actin Is Involved in Transcription of Act5C Independently of Mrtf
To clarify the role of actin in general versus gene-specific transcriptional regulation, we examined actin-chromatin interactions in Drosophila ovaries, where Mrtf has been shown to regulate Act5C transcription (Salvany et al., 2014). We performed chromatin immunoprecipitation followed by deep sequencing (ChIP-seq) of Mrtf-GFP, actin, and Pol II phosphorylated at serine 5 (Pol II S5P) in ovaries of wild-type (w1118) and Mrtf mutant (mal-dΔ7) flies, where Mrtf expression is abolished (Somogyi and Rorth, 2004), as well as in flies ubiquitously expressing the GFP-tagged version of Mrtf (tub mal-d3xGFP) (Salvany et al., 2014) (Figure 1A). Deletion and overexpression of Mrtf displayed decreased and increased expression of Act5c, respectively (Figure 1B), and Mrtf bound to promoter and upstream region of the Act5C gene (Figures 1A, 1C, and 1D), in agreement with previous studies (Salvany et al., 2014). Pol II S5P bound to the transcription start sites (TSSs) of Act5C in all three fly strains (Figures 1A, 1C, and 1D). Interestingly, the binding pattern of actin was different from that of Mrtf, and a substantial actin signal was found on the gene body of the Act5C gene (Figures 1A and 1E). Importantly, actin signal was not reduced in mal-dΔ7 flies (Figure 1F), indicating that actin binding to the Act5C gene is not dependent on Mrtf. The functional significance of actin binding to its own gene remains to be investigated.
Figure 1.
Actin Binding to the Act5C Gene Is Not Dependent on Mrtf
(A) ChIP-seq analysis of Mrtf-GFP, actin, and Pol II S5P at the Act5C gene region on chromosome X. Fly strains and antibodies used are indicated on the left, and signal intensity as number of reads is shown above each track; actin and the control antibody IgG are shown on the same scale.
(B) mRNA levels of Act5C in the indicated fly strain measured by qPCR. Rpl32 was used as internal control; data is normalized to mal-dΔ7/+ (heterozygous Mrtf deletion) and is the mean from two independent measurements with standard deviation.
(C and D) Binding profile of Pol II S5P (purple) and Mrtf-GFP (green) on Act5C gene in ovaries from mal-dΔ7 (C) and tub mal-d3xGFP (D) fly strains. Read counts are normalized to inputs.
(E) Binding profile of actin (black) and Mrtf-GFP (green) on the Act5C gene in ovaries from tub mal-d3xGFP fly strain. Read counts are normalized to inputs.
(F) Binding profile of actin on the Act5C gene in ovaries from tub mal-d3xGFP (black) and in mal-dΔ7(light brown) flies.
Actin Interacts with Transcribed Genes with a Pattern Depending on Their Expression Level
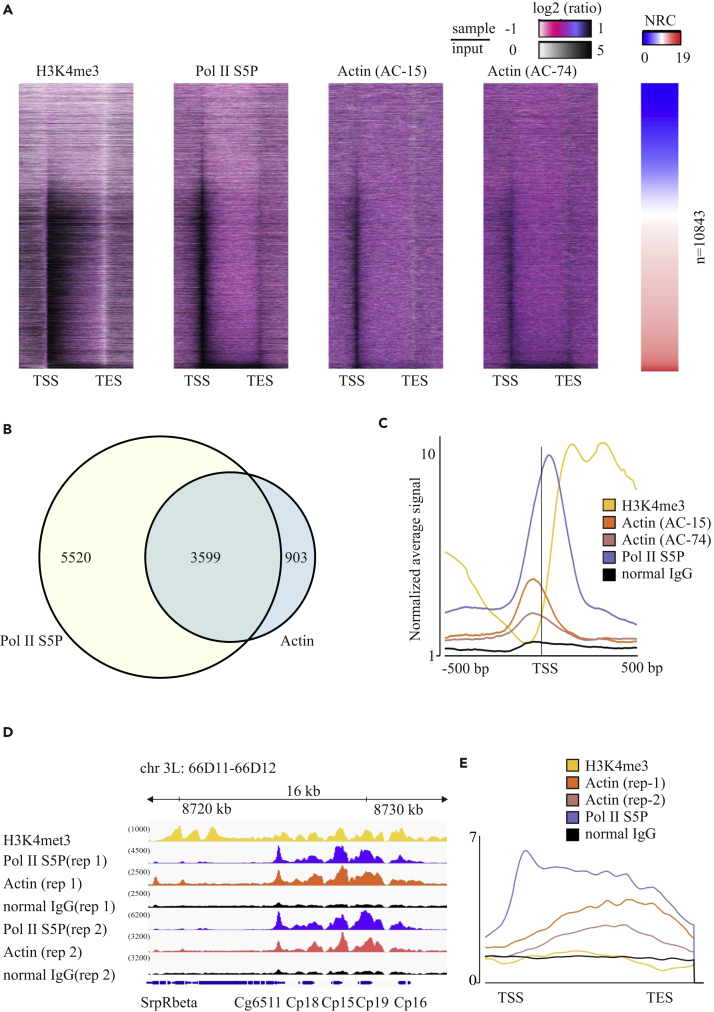
To obtain a genome-wide view of actin-chromatin interactions, further ChIP-seq analysis of the w1118 fly strain revealed actin on the promoters of essentially all transcribed genes together with Pol II S5P (Figure 2A). Peak calling confirmed the substantial overlap between actin and Pol II S5P binding sites (Figure 2B). However, detailed analysis showed that actin binds promoters slightly before the TSS and Pol II S5P enrichment (Figure 2C), indicating that actin could be involved in transcriptional initiation, perhaps via pre-initiation complex formation, as suggested before (Hofmann et al., 2004).
Figure 2.
Actin Colocalizes with Pol II at TSS and Gene Bodies of Transcribed Genes
(A) Heatmap of the ratio between the sample (histone H3K4met3, Pol II S5P, and actin with two antibodies, AC-74 and AC-15) and input ChIP-seq signals across gene regions, standardized and segmented into 200 bins. Transcription start sites (TSS) and transcription end sites (TES) are indicated. Genes are sorted according to normalized read count (NRC) of RNA sequencing data from w1118 fly ovaries (right panel).
(B) Venn diagram showing overlap of actin (AC-74) and Pol II S5P peaks from ChIP-seq.
(C) Average signal of read counts normalized to the input from −500 bp to +500 bp from the TSS of gene loci (n = 10,843).
(D and E) (D) Binding profile of actin and Pol II on chorion genes at 66D locus of chromosome 3L. Antibodies used in ChIP-seq are indicated on the left, and signal intensity as number of reads is shown in parentheses above each track. Results from two experiment replicates (rep) are shown. (E) ChIP-seq with the indicated antibodies with average signal of read counts normalized to input shown across the gene body of known eggshell-protein-encoding genes (Tootle et al., 2011).
Similarly to the Act5C gene (Figure 1), actin was also found, together with Pol II S5P, on the gene bodies of certain genes (Figure 2A, genes at the bottom have highest expression). These included, for example, the highly transcribed chorion genes (Figure 2D) involved in eggshell formation. On these genes actin is enriched more toward the transcription end site than the TSS (Figure 2E). Notably, both actin antibodies produced a very similar binding pattern on chromatin (Figures 2A, 2B, 2D, and 2E). This genome-wide analysis shows that actin interacts with most transcribed genes in Drosophila ovaries and that depending on the expression level of the gene, actin can be found both on the promoters and gene bodies. This genome-wide data can thus consolidate previous ChIP studies of actin that have reported variable binding to different genomic sites depending on the specific gene analyzed (Hu et al., 2004, Obrdlik et al., 2008, Philimonenko et al., 2004, Ye et al., 2008). Whether the binding pattern of actin reflects its dual roles in transcription, both during transcription initiation and elongation, or whether the recruitment to gene bodies represents a specific requirement for actin upon high transcriptional activity, awaits further studies. An obvious candidate for recruiting actin to the genes is Pol II, which based on our ChIP-seq studies co-occupies most actin-binding sites (Figure 2), although not with exactly the same pattern. Other candidates include the different chromatin remodeling complexes containing actin (Kapoor and Shen, 2013), as well as the elongation factor P-TEFb (Qi et al., 2011).
Active Transport of Nuclear Actin Is Required for Eggshell Gene Transcription
To study if active maintenance of nuclear actin levels is required for transcription in Drosophila ovaries similarly as in mammalian cells (Dopie et al., 2012), we generated a mutant of the nuclear actin import receptor, RanBP9 (Drosophila ortholog of Importin-9) (Figure 3A; see also Transparent Methods). Similarly to Importin-9 knockdown in mammalian cells (Dopie et al., 2012), loss of RanBP9 in Drosophila resulted in decreased nuclear actin levels (Figures 3B and 3C), although the total actin levels were not significantly altered (Figure 3D). On the same genetic background, the RanBP9Δ1 mutants were viable, but females laid fewer eggs than control flies (Figure 3E), and these eggs failed to develop.
Figure 3.
Generation of RanBP9 Mutant Fly with Decreased Nuclear Actin
(A) Schematic of the RanBP9 locus. The region of deletion (light yellow) generated by imprecise excision of P{GSV6}GS13460.
(B) Confocal microscopic images of nurse cell nuclei of ovarian egg chambers stained with actin antibodies and DAPI. Scale bar, 10 μm.
(C) Quantitation of nucleus-to-cytoplasm ratio of actin-staining intensities in nurse cells. Data are from three independent experiments with N = 32 (wt/def) and N = 29 (RanBP9Δ1/def). Mann-Whitney test, p < 0.05. Boxes represent 25%–75%, and the error bars range within 1.5 IQR. The line in the middle is median, and the open square is mean.
(D) Western blots from the whole fly lysates probed with anti-actin antibody. Quantitation of actin amount (below the blots) is from three independent experiments with wt/def normalized to 1 and ± representing SD. No significance by student's t test.
(E) Numbers of eggs laid by the indicated flies. N = 289 (wt/def) and N = 214 (RanBP9Δ1/def) from six independent experiments. Student's t test, p < 0.001. Data shown as in (C). Black diamonds are outliers.
In contrast to our previous results from mammalian cells, RNA sequencing analysis of the RanBP9Δ1 mutant ovaries did not reveal dramatic transcriptional downregulation upon inhibiting active nuclear import of actin (Figure 4A and Table S1). We note that in mammalian cells Importin-9 depletion led to a greater reduction in nuclear actin levels (Dopie et al., 2012) than the RanBP9Δ1 deletion reported here (Figure 3C). Whether the fly utilizes additional nuclear import mechanisms for actin or whether the underlying biological complexity creates differential sensitivity to nuclear actin levels remains to be determined. Nevertheless, several genes encoding for chorion proteins showed reduced expression in the RanBP9Δ1 compared with control (marked in red in Figure 4A), and RT-qPCR confirmed the significant downregulation for a subset of them (Figure 4B). Importantly, the same transcripts also showed reduced expression when RanBP9 expression was silenced by RNAi specifically in the follicle cells (Figure 4C), which are the cells that express the chorion genes to deposit the eggshell over the oocyte. Since RanBP9 could also have other import cargoes apart from actin, and rescue with an NLS-actin construct (Dopie et al., 2012) was not possible due to technical reasons in this experimental system, we used overexpression of Exportin 6, the nuclear export receptor for actin (Stuven et al., 2003), as an alternative method to manipulate nuclear actin in follicle cells. Also, this led to a reduction in chorion gene expression (Figure 4C), further supporting the notion that balanced nuclear transport of actin is required for appropriate transcription of eggshell genes. Mechanistically, the RanBP9Δ1 deletion led to decreased binding of both actin and Pol II (Figure 4D) to the chorion genes. Finally, the eggs laid by the RanBP9Δ1 females displayed morphologically abnormal (Figure 4E) and short (Figure 4F) dorsal appendages, which are specialized structures of the eggshell used by the embryo for breathing. Deregulated chorion gene expression thus has phenotypic consequences and could explain why the eggs laid by the RanBP9Δ1 females do not develop.
Figure 4.
RanBP9 Mutants Display Decreased Expression of Chorion Protein Genes and Defective Eggshell Formation
(A) MA plot of RNA-sequencing data. The transcripts of known eggshell proteins are indicated in red.
(B) Relative expression of four chorion protein transcripts in wt/def and RanBP9Δ1/def fly ovaries from five independent experiments. Data are normalized to wt/def. Statistics with student's t test. Error bars represent ± SD.
(C) Relative expression of four chorion protein transcripts in the indicated fly strains from two independent experiments. Data are normalized to c204>RNAi-GFP and error bars represent ± SD. *p < 0.05 with student's t test.
(D) ChIP-seq with actin (right) and Pol II ser5 (left) with average signal of read counts normalized to input shown across the gene body of chorion protein genes.
(E) Scanning electron micrographs of fly eggs with dorsal appendages. Representative images of control (wt/def) and RanBP9Δ1/def eggs are shown. Magnification 450×. Scale bar, 200 μm.
(F) Relative lengths of dorsal appendages from eggs of indicated fly strains. Data are normalized to wt/def. N = 91 (wt/def) and N = 120 (RanBP9Δ1/def) from three independent experiments. Student's t test, p < 0.001. Data shown as in Figure 3C.
Taken together, these results enforce the importance of actin for transcription by showing in a genome-wide format that actin interacts with virtually all genes transcribed by Pol II and that its balanced nuclear transport is required for transcription in vivo. Further studies are required to elucidate the molecular machineries that recruit actin both to the promoters and gene bodies.
Limitations of the Study
Although this study shows the genome-wide binding pattern of actin on chromatin, which has not been available before, the transcription complexes containing and functionally interacting with actin remain unclear and will be an important avenue for further studies. This study takes advantage of the nucleocytoplasmic shuttling mechanism of actin to decrease the amounts of actin in the nucleus. Although targeting both import and export pathways of actin alleviates some specificity issues, development of more precise tools to manipulate actin specifically in the nucleus would benefit the whole research field.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We thank Paula Maanselkä for technical assistance and Dr. Joachim Urban for supplying UASp-Exp6 fly strain. Imaging was carried out at the Light Microscopy Unit (LMU) of the Institute of Biotechnology, SEM sample preparation as well as imaging at Electron Microscopy Unit of the Institute of Biotechnology, and next-generation sequencing at Biomedicum Functional Genomics Unit (FuGU), all of the University of Helsinki. This work was supported by the Academy of Finland, ERC, Sigrid Juselius and Jane & Aatos Erkko Foundation grants to M.K.V.
Author Contributions
Conceptualization, M.K.V. and M.S.; Methodology, M.K.V., M.S., V.H., and M.P.; Investigation, M.S., H.M.M., B.P., J.D., M.H., and R.C.M.; Writing – Original Draft, M.S, H.M.M., and M.K.V.; Writing – Review & Editing, M.S. and M.K.V.; Supervision, M.K.V. and V.H; Project Administration, M.K.V.; Funding Acquisition, M.K.V.
Declaration of Interests
None.
Published: November 30, 2018
Footnotes
Supplemental Information includes Transparent Methods and one table and can be found with this article online at https://doi.org/10.1016/j.isci.2018.10.010.
Supplemental Information
Data from RNA-seq analysis.
References
- Dopie J., Skarp K.P., Kaisa Rajakyla E., Tanhuanpaa K., Vartiainen M.K. Active maintenance of nuclear actin by importin 9 supports transcription. Proc. Natl. Acad. Sci. U S A. 2012;109:E544–E552. doi: 10.1073/pnas.1118880109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann W.A., Stojiljkovic L., Fuchsova B., Vargas G.M., Mavrommatis E., Philimonenko V., Kysela K., Goodrich J.A., Lessard J.L., Hope T.J. Actin is part of pre-initiation complexes and is necessary for transcription by RNA polymerase II. Nat. Cell Biol. 2004;6:1094–1101. doi: 10.1038/ncb1182. [DOI] [PubMed] [Google Scholar]
- Hu P., Wu S., Hernandez N. A role for beta-actin in RNA polymerase III transcription. Genes Dev. 2004;18:3010–3015. doi: 10.1101/gad.1250804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor P., Shen X. Mechanisms of nuclear actin in chromatin-remodeling complexes. Trends Cell Biol. 2013;24:238–246. doi: 10.1016/j.tcb.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le H.Q., Ghatak S., Yeung C.Y., Tellkamp F., Gunschmann C., Dieterich C., Yeroslaviz A., Habermann B., Pombo A., Niessen C.M. Mechanical regulation of transcription controls Polycomb-mediated gene silencing during lineage commitment. Nat. Cell Biol. 2016;18:864–875. doi: 10.1038/ncb3387. [DOI] [PubMed] [Google Scholar]
- Miralles F., Posern G., Zaromytidou A.I., Treisman R. Actin dynamics control SRF activity by regulation of its coactivator MAL. Cell. 2003;113:329–342. doi: 10.1016/s0092-8674(03)00278-2. [DOI] [PubMed] [Google Scholar]
- Obrdlik A., Kukalev A., Louvet E., Farrants A.K., Caputo L., Percipalle P. The histone acetyltransferase PCAF associates with actin and hnRNP U for RNA polymerase II transcription. Mol. Cell. Biol. 2008;28:6342–6357. doi: 10.1128/MCB.00766-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philimonenko V.V., Zhao J., Iben S., Dingova H., Kysela K., Kahle M., Zentgraf H., Hofmann W.A., de Lanerolle P., Hozak P. Nuclear actin and myosin I are required for RNA polymerase I transcription. Nat. Cell Biol. 2004;6:1165–1172. doi: 10.1038/ncb1190. [DOI] [PubMed] [Google Scholar]
- Qi T., Tang W., Wang L., Zhai L., Guo L., Zeng X. G-actin participates in RNA polymerase II-dependent transcription elongation by recruiting positive transcription elongation factor b (P-TEFb) J. Biol. Chem. 2011;286:15171–15181. doi: 10.1074/jbc.M110.184374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvany L., Muller J., Guccione E., Rorth P. The core and conserved role of MAL is homeostatic regulation of actin levels. Genes Dev. 2014;28:1048–1053. doi: 10.1101/gad.237743.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serebryannyy L.A., Parilla M., Annibale P., Cruz C.M., Laster K., Gratton E., Kudryashov D., Kosak S.T., Gottardi C.J., de Lanerolle P. Persistent nuclear actin filaments inhibit transcription by RNA polymerase II. J. Cell Sci. 2016;129:3412–3425. doi: 10.1242/jcs.195867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somogyi K., Rorth P. Evidence for tension-based regulation of Drosophila MAL and SRF during invasive cell migration. Dev. Cell. 2004;7:85–93. doi: 10.1016/j.devcel.2004.05.020. [DOI] [PubMed] [Google Scholar]
- Stuven T., Hartmann E., Gorlich D. Exportin 6: a novel nuclear export receptor that is specific for profilin.actin complexes. EMBO J. 2003;22:5928–5940. doi: 10.1093/emboj/cdg565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tootle T.L., Williams D., Hubb A., Frederick R., Spradling A. Drosophila eggshell production: identification of new genes and coordination by Pxt. PLoS One. 2011;6:e19943. doi: 10.1371/journal.pone.0019943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vartiainen M.K., Guettler S., Larijani B., Treisman R. Nuclear actin regulates dynamic subcellular localization and activity of the SRF cofactor MAL. Science. 2007;316:1749–1752. doi: 10.1126/science.1141084. [DOI] [PubMed] [Google Scholar]
- Ye J., Zhao J., Hoffmann-Rohrer U., Grummt I. Nuclear myosin I acts in concert with polymeric actin to drive RNA polymerase I transcription. Genes Dev. 2008;22:322–330. doi: 10.1101/gad.455908. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data from RNA-seq analysis.