Abstract
The interleukin-21 (IL-21) protein was found to be expressed at an elevated level in clinical samples of colorectal cancer patients without or with a parasitic infection that were collected from Sudan in our previous study. The IL-21 gene in HT29 and HCT116 cells was then correlated to cell proliferation and cell migration, as well as the cellular mechanisms associated with gene expressions in our present study. Our results demonstrated that silencing the IL-21 gene in HCT116 cells increased the cytotoxic level and fibroblast growth factor-4 (FGF4) mRNA expression in the cancer cells. Moreover, specific gene silencing reduced the migration of cancer cells compared to non-silenced cancer cells. These events were not observed in IL-21-silenced HT29 cells. Neutralizing FGF4 in conditioned medium of IL-21-silenced HCT116 cells further increased the cytotoxic level and restored the migratory activity of HCT116 cells in the culture compared to silencing the IL-21 gene alone in the cancer cells. Our results indicate the importance of both silencing the IL-21 gene and co-expression of the FGF4 protein in HCT116 cells, which pave the way for the discovery of important factors to be used as biomarkers for the design of drugs or cost-effective supplements to effectively treat the patients having infectious disease and HCT116 cells of colorectal cancer simultaneously in the future.
Keywords: Infectious disease, Colorectal cancer, IL-21, FGF4, Cell proliferation, Cell migration
Introduction
Infectious disease is apparently related to inflammation, where inflammatory cells produce potential genotoxic mediators, such as reactive oxygen, nitrogen species and pro-inflammatory cytokines that may cause genomic instability, dysregulation of oncogenes and oncosuppressor genes during infection (Herrera et al. 2005; Trakatelli et al. 2005). The mutation of these molecular disturbances leads to the progression of dysplasia and carcinoma. Another factor that might play a significant role in colorectal carcinogenesis in infected patients is the existence of concomitant enterobacterial infections in the host. In both clinical and experimental studies, various strains of Enterobacteriaceae have been described in association with tropical infection disease, such as schistosomes, which grant a survival advantage to bacteria by inducing immune suppression (Chieffi 1992). Some of these organisms can stimulate colorectal carcinogenesis through multiple pathways, such as the production of reactive oxygen intermediates, dysregulation of the T cell response and modification of the host epithelial carbohydrate expression (Hope et al. 2005).
There is a significantly higher rate of synchronous tumours in patients with Schistosoma spp. colorectal cancer than in patients with spontaneous colorectal cancer in an endemic population (Madbouly et al. 2007). This phenomenon can be due to the infection that is caused by chronic Schistosomal inflammation throughout the colon and rectum, an event that is similar to that described in the case of the colitis-associated cancer patient. Chronic intestinal inflammation, on the other hand, is a well-known risk factor for colorectal cancer progression (Jess et al. 2012). The chronic inflammatory response provoked by schistosome antigens provides the proliferative motivation that is necessary to promote cancer growth from potentially malignant foci by other carcinogens (Ming-Chai et al. 1980). The increase in the epithelial cell proliferation post-infection probably contributes to carcinogenesis, but it is insufficient to cause cancers alone. Our present study found a high level of interleukin-21 (IL-21) protein in the serum of colorectal cancer patients infected with S. mansoni. Hence, the involvement of this protein in cell proliferation and other mechanisms of colorectal carcinogenesis are studied.
IL-21 is a cytokine produced by CD4 T-cells and natural killer T (NKT) cells (Parrish-Novak et al. 2000; Coquet et al. 2007). It targets a broad range of immune cells within both the lymphoid and myeloid lineages (Spolski and Leonard 2008). This cytokine also increases anti-tumour activity in various mouse models (Leonard and Spolski 2005), suggesting that IL-21 bridges the innate and adaptive immune responses (Collins et al. 2003). IL-21 influences cell differentiation, cell fate, cell proliferation and the survival of diverse immune cell subsets (Kesselring et al. 2012). It also plays a prominent role in tumour growth and immune surveillance of colitis-associated tumourigenesis. In addition, this cytokine controls the balance between T-helper type 17 (Th17) and T-helper type 1 (Th1) cell subsets, and is necessary for the homeostasis of a tumour-supportive microenvironment characterized by extensive infiltration of Th17 cells. Secretion of this Th17 cell-associated cytokine leads to the induction of chemokines, matrix metalloproteinases and antimicrobial peptides in the surrounding tissue, leading to inflammation, and the recruitment of neutrophils and macrophages that may lead to cancer development.
Our study aimed to investigate the role of IL-21 in colorectal cancer cells by performing specific gene silencing using small interfering RNA (siRNA) in colorectal cancer cells. Cell proliferation and other cellular activities post IL-21 gene silencing of the cancer cells were then investigated. Although our contribution may be small, it is believed that by studying IL-21 in-depth, the molecular targets associated with the parasitic infection and inflammation can be better understood for the improved design of drugs or cost-effective supplements. Japan and the coastal plain of the People’s Republic of China have been successfully freed from schistosomiasis over the last two decades (Olveda et al. 2014). However, countries in South East Asia, such as the Philippines have not experienced similar success in eliminating this dreaded disease because of health education and research, efforts towards environmental modification and improvement remain lacking. Moreover, the countries are burdened with the flood, which tends to contaminate river water and other sources of water with the infective-stage cercariae (Leonardo et al. 2016). Therefore, more studies should be conducted to understand better this neglected tropical disease that is molecularly associated with colorectal cancer in an endemic population to enable the improved design of drugs or supplements that are affordable in the region.
Materials and methods
Silencing IL-21 gene in colorectal cancer cells by siRNA
A mouse specific lyophilized IL-21 siRNA reagent system (Santa Cruz Biotechnology, Dallas, TX, USA) was used to silence the IL-21 gene in HT29 and HCT116 cells. Each cell line was seeded in 6-well culture plates at a density of 2 × 105 cells per well in 2 ml of antibiotic-free DMEM supplemented with 10% FBS. The culture was incubated at 37 °C in a humidified atmosphere of 5% (v/v) CO2 until the cells reached 80% confluence. To carry out the transfection, Solution A was prepared by diluting 4 μL of fluorescein-conjugated IL-21 siRNA duplex with 100 μL of siRNA transfection medium, whereas Solution B was prepared by diluting 6 μL of siRNA transfection reagent (as optimized in our previous study) with 100 μL of siRNA transfection medium. Solution A was then added directly to Solution B and was mixed gently by pipetting. The mixture was incubated at room temperature for 15–45 min. Each cell line was then washed with 2 ml of siRNA transfection medium. The mixture of Solution A and Solution B was then added to 800 μL of siRNA transfection medium. The mixture was mixed gently and overlaid onto the washed cells. The transfection reaction was incubated at 37 °C for 72 h in a CO2 incubator. The efficacy of specific gene silencing was examined using a fluorescence microscope, whereas the IL-21 protein level in the silenced cells was further confirmed by Western blotting. The IL-21-silenced cells were then subjected to fibroblast growth factor-4 (FGF4) analysis by real-time PCR.
Analysis of FGF4 mRNA expression in IL-21-silenced colorectal cancer cells by real-time PCR
The old growth medium of the culture of IL-21-silenced colorectal cancer cells, as prepared above, were discarded and the cells were subjected to RNA extraction using the RNeasy Mini Kit (QIAGEN, Valencia, CA, USA). The concentration of extracted total RNA was determined using a NanoPhotometer, whereas the integrity of extracted RNA was checked using 1% (w/v) agarose gel electrophoresis. The good quality of extracted total RNA was reverse transcribed to cDNA using the RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, Basingstoke, England, UK) and the product of reverse transcription (cDNA) was kept at − 20 °C until it was used for the analysis of gene expression by real-time PCR. The specific primers were designed for quantitative amplification of the internal control gene and FGF4 using Primer Express 2.0 software (Applied Biosystems, Foster, CA, USA). Forward β-actin: 5′-CATTGCCGACAGGATGCA-3′, Reverse β-actin: 5′-CCGATCCACACGGAGTACTTG-3′, Forward FGF4: 5′-CAACTACAACGCCTACGAGTCCTA-3′ and Reverse FGF4: 5′-CCTTCTTGGTCTTCCCATTCTTG-3′. Real-time PCR was performed using the Power SYBR® Green PCR Master Mix (Applied Biosystems, Foster, CA, USA) and a 7500 Fast Real-Time PCR System (Applied Biosystems, Foster, CA, USA). For this, a volume of 12.5 μL of 2 × SYBR Green PCR Master Mix, 20 pmol of each forward and reverse primer and 5 μL of cDNA (25 ng) were mixed as a reaction. A sufficient volume of deionized water was added to the mixture to bring the volume of the reaction to 25 μL. The thermocycler program was carried out as follows: 50 °C for 3 min and 95 °C for 5 min as the short hot-start, followed by 35–40 cycles of 95 °C for 10 s for denaturation, annealing and extension steps at 60 °C for 30 s. Finally, the melting curve of each amplicon was generated with 1 °C temperature increments from 72 to 95 °C. All the reactions were performed in triplicate and included 3 non-template reactions as negative controls. Data analysis was carried out according to the manufacturer’s instructions. The experiment was repeated at least twice. The mRNA expression level of the target gene was normalized to that of β-actin in the test sample. Thereafter, the fold change of the target gene mRNA expression level was calculated using the 2−ΔΔCt method (Livak and Schmittgen 2001).
Cytotoxic effect of IL-21-silenced HCT116 cells in growth medium without or with FGF4 neutralizing antibody by colourimetric LDH cytotoxicity assay
Quantitative cytotoxicity was performed in IL-21-silenced colorectal cancer cells using the Lactate Dehydrogenase (LDH) Cytotoxicity Assay Kit (BioVision, Milpitas, CA, USA), based on the measurement of LDH activity released from damaged cells in the culture supernatant. HT29 and HCT116 cells were first transfected with IL-21 siRNA, as described above. Thereafter, the transfected cells were harvested and the cell suspension was adjusted to a suitable concentration with 10% growth medium and seeded into a 24-well plate. The cultures were incubated in growth medium without or with FGF4 neutralizing antibody at 37 °C in a humidified atmosphere of 5% (v/v) CO2. A volume of 100 μL of culture medium was withdrawn at 24, 48 and 72 h of incubation for LDH activity measurement, according to the manufacturer’s instructions. The culture medium was transferred to a flat-bottomed 96-well plate and 100 μL of the mixture of lactate and tetrazolium salt was added to each well in triplicate. The plate was incubated at room temperature in the dark for 30 min. The absorbance reading of all the reactions was measured at 490 nm and the reference wavelength of 620 nm was used for the measurement using an ELISA microplate reader. The percentage cytotoxicity was calculated as follows:
Cytotoxicity (%) = [(Test sample − Low control)/(High control − Low control)] × 100
Low control denotes the absorbance of the medium from a culture without the addition of any substance.
High control denotes the absorbance of the medium from a culture in which the cells were treated with 1% (v/v) Triton X-100 to release 100% of the available LDH.
Cell migration of IL-21-silenced HCT116 cells in growth medium without or with FGF4-neutralizing antibody by wound healing assay
The scratch-wound cell migration assay was conducted to determine the migration of IL-21-silenced HCT116 cells based on the repopulation of wounded culture. Non-silenced HCT116 cells were used as the control for this part of the study. In the process, the cancer cells were seeded in a 24-well plate at a density of 1 × 105 cells per well. The cultures were incubated at 37 °C in a humidified atmosphere of 5% (v/v) CO2 until the cells reached 80% confluence, such as after 24 h. The cultures were then subjected to the silencing of IL-21 for 72 h. The cultures were maintained in growth medium without or with the FGF4 neutralizing antibody. A scratch on the culture (wound) was made using a yellow pipette tip. The cell debris was removed and fresh medium in the culture was replaced. The wound of each culture was photographed after 0, 24, 48 and 72 h. Image J software (National Institutes of Health, Bethesda, MD, USA) was used to measure the wound closure of each culture at each time point by averaging 3 individual measurements. Each measurement was performed in triplicate and was repeated in at least 2 independent experiments.
Analysis of FGF4-related gene mRNA expression in IL-21-silenced HCT116 cells by real-time PCR
The same cDNA used for FGF4 mRNA expression analysis was used for the analysis of gene expression related to FGF4 by real-time PCR. The specific primer design and the real-time PCR were performed as above. All primers used for the real-time PCR are listed in Table 1. The same internal control was used for the analysis. All the reactions were performed in triplicate and included three negative controls as above. Data analysis was performed according to the manufacturer’s instructions. The experiment was repeated at least twice. The expression level of each target gene was normalized to that of the β-actin in the test sample. Thereafter, the fold change of the target gene expression level was calculated using the 2−ΔΔCt method, as described above.
Table 1.
The list of primers used for the analysis by real-time PCR in this study
| Gene | Primer sequence |
|---|---|
| Ki67 | Forward 5′-AACTATCCTCGTCTGTCCCAACAC-3′ |
| Reverse 5′-CGGCCATTGGAAAGACAGAT-3′ | |
| PCNA | Forward 5′-AGAAGGTGTTGGAGGCACTCA-3′ |
| Reverse 5′-GGTTTACACCGCTGGAGGTAA-3′ | |
| Caspase-3 | Forward 5′-GTGCTATTCTGAGGCGGTTGT-3′ |
| Reverse 5′-CACGGATACACAGCCACAGGTAA-3′ | |
| Caspase-9 | Forward 5′-GTGAGGCTGTCCTGTACATTGTG-3′ |
| Reverse 5′-GAAGACGCGTTACTGGCATTG-3′ | |
| TGFα | Forward 5′- CAGACCTTCCTACTTGGCCTGTAA-3′ |
| Reverse 5′- GACGGAGTTCTTGACAGAGTTTTG-3′ | |
| CCL5 | Forward 5′-AGCCTCTCCCACAGGTACCAT-3′ |
| Reverse 5′-GGCAGTAGCAATGAGGATGACA-3′ | |
| EGF | Forward 5′-TGTGGTTCTCAGATTGGGCTATG-3′ |
| Reverse 5′-GATGAGGGCTTCAGCATGCT-3′ | |
| BNIP3 | Forward 5′-AACTGGAGTCTGACTTGGTTCGTT-3′ |
| Reverse 5′-CCAGGATCTAACAGCTCTTCAGTGA-3′ | |
| IL-17 | Forward 5′-AGAGATATCCCTCTGTGATC-3′ |
| Reverse 5′-TACCCCAAAGTTATCTCAGG-3′ | |
| IL-13 | Forward 5′-CCACGGTCATTGCTCTCACTTGCC-3′ |
| Reverse 5′-CCTTGTGCGGGCAGAATCCGCTCA-3′ | |
| IL-6 | Forward 5′-CCAGTACCCCCAGGAGAAGATT-3′ |
| Reverse 5′-CCGTCGAGGATGTACCGAAT-3′ |
Statistical analysis
GraphPad Prism 6.0 Software (GraphPad Software Inc., USA) was used for statistical analyses. Data were analysed using paired and unpaired Student’s t tests. One-way analysis of variance (ANOVA) was performed to estimate the statistical significance of the data. A P value less than 0.05 was reported as statistically significant.
Results
IL-21 gene-silenced colorectal cancer cells
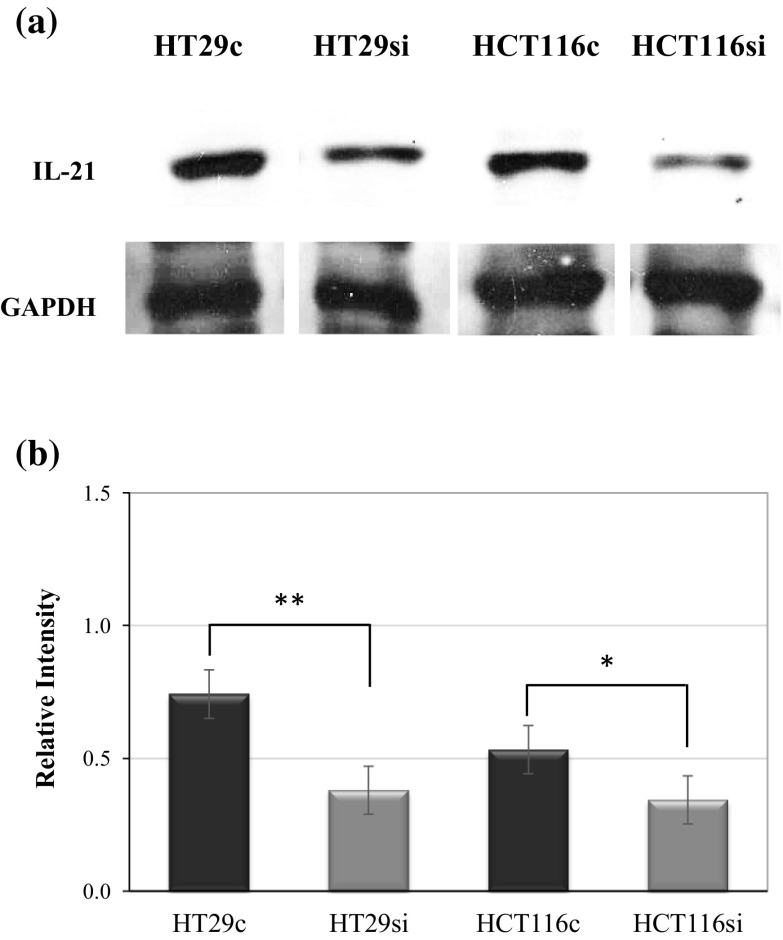
Figure 1a presents a high efficacy of specific gene silencing at protein level, where the level of IL-21 protein expression was significantly decreased in both IL-21-silenced HT29 and HCT116 cells. Only faint protein bands were detected in the specific gene-silenced colorectal cancer cells compared with the non-silenced colorectal cancer cells. In statistical analysis, the level of IL-21 protein in IL-21-silenced HT29 cells was decreased from 0.74 units to 0.38 units (P < 0.01), whereas the level of IL-21 protein in IL-21-silenced HCT116 cells was decreased from 0.53 to 0.34 units (P < 0.05) (Fig. 1b).
Fig. 1.
Efficacy of IL-21 silencing in colorectal cancer cells by Western blotting. a The protein band of IL-21 in IL-21-silenced colorectal cancer cells relative to the control. GAPDH was used as the endogenous control. b The intensity of the IL-21 protein band in HT29 control (HT29c), IL-21-silenced HT29 cells (HT29si), HCT116 control (HCT116c) and IL-21-silenced HCT116 cells (HCT116si). The data are expressed as the mean ± SD (n = 3); *P < 0.05 and **P < 0.01
FGF4 mRNA expression in IL-21 gene-silenced HT29 and HCT116 cells
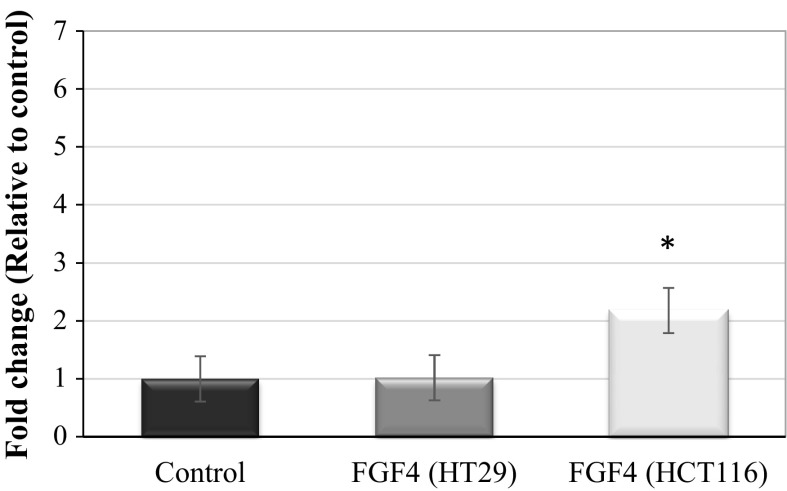
Figure 2 shows the FGF4 mRNA expression after IL-21 silencing in HT29 and HCT116 cells. The analysis showed no significant differences in the mRNA expression of FGF4 in IL-21-silenced HT29 cells, but up-regulation of FGF4 mRNA expression in IL-21-silenced HCT116 cells, compared with that of the control. The mRNA expression of FGF4 was observed to be significantly up-regulated to a 2.18-fold change (P < 0.05) in IL-21-silenced HCT116 cells compared with that of the control. Therefore, only IL-21-silenced HCT116 cells were studied in subsequent experiments.
Fig. 2.
mRNA expression of FGF4 in HT29 and HCT116 cells post IL-21 silencing. The targeted gene expression was normalized to the expression of β-actin. Three replications were performed in at least two independent experiments. The data are represented as the mean ± SD (n = 3); *P < 0.05
Cytotoxic effect of IL-21-silenced HCT116 cells in growth medium without or with FGF4- neutralizing antibody
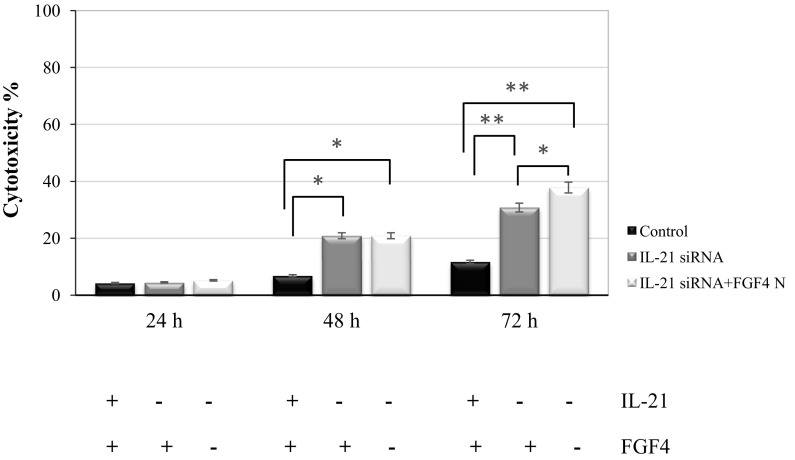
The role of IL-21 in the cytotoxic effect or proliferation of HCT116 cells was determined in the growth medium without or with the FGF4-neutralizing antibody. The LDH assay revealed an increase in the cytotoxic effect post-silencing the IL-21 gene in HCT116 cells for 48 and 72 h (Fig. 3). The increase in the cytotoxic effect of IL-21-silenced HCT116 cells in growth medium without or with the FGF4-neutralizing antibody at 48 h of cultivation was significant (20.9%, P < 0.01) compared with that of the non-silenced HCT116 cells (6.8%). The percentage cytotoxicity of the IL-21-silenced HCT116 cells was increased further when the silenced cancer cells were incubated in the growth medium without or with the FGF4-neutralizing antibody for 72 h, whereby the percentage cytotoxicity of IL-21-silenced HCT116 cells incubated without or with FGF4-neutralizing antibody was 30.8% (P < 0.001) and 38.5% (P < 0.01), respectively, compared with that of the control (11.6%). Comparison of the percentage cytotoxicity in the IL-21-silenced HCT116 cells in the growth medium without and with the FGF4-neutralizing antibody was also significant (P < 0.05) at 72 h of cultivation. Therefore, silencing IL-21 induces cytotoxicity and was more influenced by FGF4 neutralizing antibody in the cells, though the percentage cytotoxicity for all observations was below 40%.
Fig. 3.
Percentage (%) cytotoxicity in IL-21-silenced HCT116 cells cultivated in growth medium without or with FGF4-neutralizing antibody for 24, 48 and 72 h. Three replications were carried out in at least 2 independent experiments. The data are represented as the mean ± SD (n = 3); *P < 0.05 and **P < 0.01
Migration of IL-21-silenced HCT116 cells in growth medium without or with FGF4-neutralizing antibody
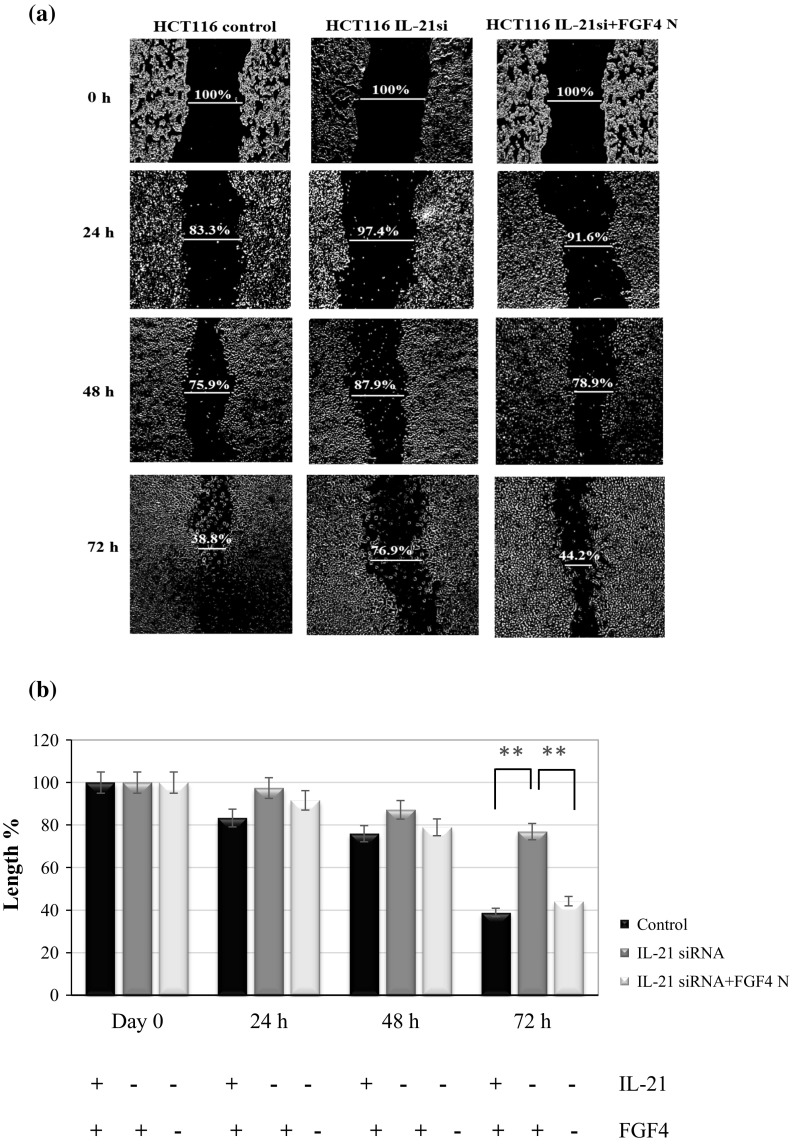
The assay revealed that the wound, which was 100% of the length of scratch, was not fully covered in IL-21-silenced HCT116 cells at the end of the assay compared with that of the non-silenced HCT116 cells. The wound of non-silenced HCT116 cells and IL-21-silenced HCT116 cells remained 38.8 and 76.9%, respectively, at 72 h following the assay (Fig. 4a). However, the wound of the IL-21-silenced HCT116 cells was covered and left only 44.2% at 72 h following the process when the specific gene-silenced colorectal cancer cells were cultivated in the growth medium that contained FGF4-neutralizing antibody. In brief, a relatively slower invasion rate was observed in IL-21-silenced HCT116 cells than in non-silenced HCT116 cells (P < 0.01) (Fig. 4b), whereby FGF4 was expressed in the IL-21-silenced HCT116 cells as detected by real-time PCR. However, HCT116 cells returned active when IL-21-silenced HCT116 cells were incubated in the growth medium that contained the FGF4-neutralizing antibody (P < 0.01), indicating that neutralizing FGF4 in the conditioned medium restored the migratory activity of the IL-21-silenced HCT116 cells. Therefore, reduced expression of IL-21 and co-expression of FGF4 are required to reduce the migration of HCT116 cells.
Fig. 4.
Migration of IL-21-silenced HCT116 cells. a Migration capacity of non-silenced HCT116 cells (control), IL-21-silenced HCT116 cells in growth medium only (HCT116 siRNA) and IL-21-silenced HCT116 cells in growth medium which contained the FGF4-neutralizing antibody (HCT116 siRNA + FGF4 N). All the cells were incubated for 24, 48 and 72 h. Cell migration was estimated by measuring the wound region of the culture. b Graph of the statistical analysis of the wound region of the culture. The data are expressed as the mean ± SD (n = 3); **P < 0.01
FGF4-related gene mRNA expressions in IL-21-silenced HCT116 cells maintained in growth medium without or with FGF4-neutralizing antibody
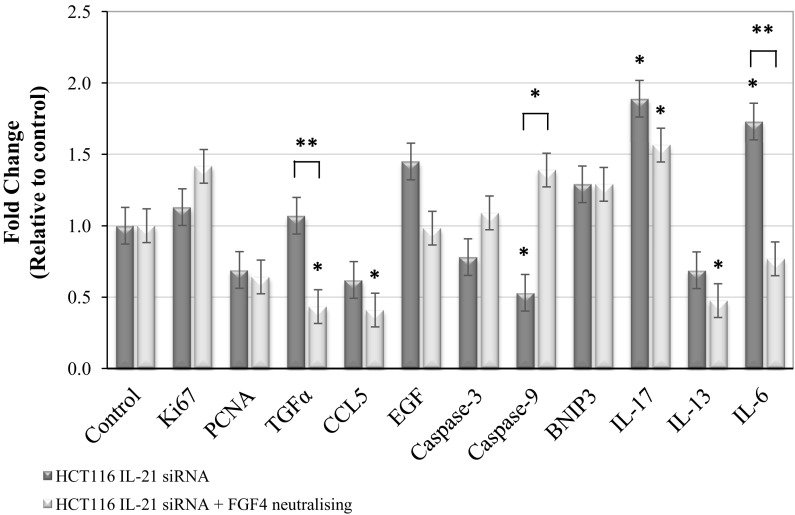
Figure 5 shows the FGF4 and IL-21-associated mRNA expressions that could be correlated with cellular mechanisms in HCT116 cells quantitatively. When only IL-21 was silenced in the cells, the effects on the reduction of cell proliferation and cell migration were observed. The mRNA expression of HCT116 cells after silencing IL-21 showed significant down-regulation of caspase-9 (0.53-fold, P < 0.05) compared with that of the control cells, indicating that no apoptosis was induced following the specific gene silencing. The analysis also showed significant up-regulation of IL-6 (1.73-fold, P < 0.05) and IL-17 (1.89-fold, P < 0.05) in the IL-21-silenced HCT116 cells. Therefore, IL-6 and IL-17 may play important roles in the reduction of cell proliferation and cell migration of IL-21-silenced HCT116 cells. As for the notion that FGF4 protein was neutralized in the conditioned medium of IL-21-silenced HCT116 cells, which showed a continual reduction of cell proliferation but regained cell migration, the significant down-regulation of mRNA expressions of TGFα (0.43-fold, P < 0.05), CCL5 (0.41-fold, P < 0.05) and IL-13 (0.48-fold, P < 0.05) were observed, which may indicate the continually cellular mechanisms in the cancer cells following the event. The induction of IL-17 (1.57-fold, P < 0.05) mRNA expression was also found in the IL-21-silenced HCT116 cells maintained in the growth medium with the FGF4-neutralizing antibody. Therefore, IL-17 may be the target gene for continual reduction of cell proliferation in IL-21-silenced HCT116 cells. Further analysis showed significant down-regulation of TGFα (P < 0.01) and IL-6 (P < 0.01) in IL-21-silenced HCT116 cells maintained in the growth medium with the FGF4-neutralizing antibody, indicating an alternative modification, such as regained cell migration in the cells, compared with those of the IL-21-silenced HCT116 cells maintained in the growth medium only. Indeed, extending this event may induce apoptosis in the cells as the induction of the caspase-9 mRNA expression (P < 0.05) was observed.
Fig. 5.
mRNA gene expression in IL-21-silenced HCT116 cells. The cell proliferation (Ki67, PCNA, TGFα, CCL5 and EGF), apoptosis (Caspase-3, Caspase-9 and BNIP3) and other related gene expressions (IL-17, IL-13 and IL-6) at the mRNA level in IL-21-silenced HCT116 cells were determined by real-time PCR. The gene expression level in control was set as 1.0-fold, < 1.0-fold indicated down-regulation and > 1.0-fold indicated up-regulation. The data represented mean ± SD (n = 3); *P < 0.05 and **P < 0.01
Discussion
Our study demonstrated that the IL-21 gene was silenced in HT29 and HCT116 cells, whereby the specific gene protein expression was reduced. However, only FGF4 mRNA expression was observed to be significantly up-regulated in the IL-21-silenced HCT116 cells. The percentages cytotoxicity in IL-21-silenced HCT116 cells were observed to increase significantly and continued to be up-regulated when IL-21-silenced HCT116 cells were maintained in the growth medium with an FGF4-neutralizing antibody. Silencing the IL-21 gene for 72 h revealed the inhibition of cell migration in HCT116 cells. However, cell migration was restored when the FGF4 in the conditioned medium was neutralized. Real-time PCR analysis revealed that TGFα, CCL5, IL-6, IL-13 and IL-17 were a group of genes that may play important roles in FGF4 and IL-21-associated proliferation and migration of HCT116 cells.
This study clarified the potential role of IL-21 that IL-21 may play in the patients who were diagnosed with the progression of colorectal cancer and schistosomiasis. The initial study conducted by a team at the Tropical Medicine Research Institute (TMRI) in Sudan showed up-regulation of the IL-21 protein level in colorectal cancer patients with schistosomiasis. This phenomenon increased the potential role of IL-21 in the patients who were diagnosed with colorectal carcinogenesis during chronic inflammation by infection. IL-21 was recently characterized as a Th2 cytokine that inhibited the differentiation of naïve T helper cells into IFN-γ-producing Th1 cells (Wurster et al. 2002) and played an indispensable role in the pathogenesis of the disease in infections (Wynn 2004; Pearce and MacDonald 2002). IL-21 also plays a pathogenic role in many chronic inflammatory processes that are associated with an enhanced risk of cancer (Monteleone et al. 2009). This relationship indicates the role of IL-21 in the development of colorectal cancer in patients with parasitic infection. The study by Mantovani et al. (2008) revealed that chronic inflammation is a major driving force for the initiation and progression of tumours in many tissues. There is an increased risk of colon cancer, which is related to the duration, extent and severity of inflammatory disease (Rutter et al. 2004; Gupta et al. 2007).
A study of IL-21 in colorectal cancer and parasitic infections (Schistosoma mansoni and Nippostrongylus brasiliensis) indicated that IL-21R deficiency had a profound effect on the progression of schistosomiasis (John et al. 2006). The egg-induced inflammatory response was decreased significantly in mice without IL-21R as compared to that of the wild-type mice. A marked reduction in secondary granuloma formation and a faster resolution of primary granulomas in the lung were also observed in IL-21R deficient mice. This phenomenon demonstrated the importance of IL-21R in granulomatous inflammation. Another study by Kesselring et al. (2012) clearly showed that IL-21 had a prominent function in tumour growth and the immune surveillance of colitis-associated tumourigenesis. Consequently, our study is consistent with previous reports that indicate patients diagnosed with colorectal cancer and schistosomiasis produce a higher level of IL-21 than that in schistosomiasis patients only or in patients who are solely diagnosed with colorectal cancer only, clarifying the role of IL-21 in the development of colorectal cancer in schistosomiasis patients.
Evidence has indicated that the overexpression of IL-21 in tumour cells suppresses tumour growth through enhanced antitumour immunity (Skak et al. 2008). However, like many other cytokines, IL-21 has opposed functions on the growth of tumours, depending on the tissue context and local immune activation. Intestinal cytokine networks are critical mediators of tissue homeostasis, inflammation and tumourigenesis. In colorectal cancer, established cytokines, such as IL-21, promote several key hallmarks of cancer, including resistance to apoptosis, irregular growth, proliferation, induction of genetic instability, angiogenesis, invasiveness and metastasis (West et al. 2015).
In this study, the role of IL-21 in HCT116 cells that may be associated with schistosomiasis were investigated by silencing the IL-21 gene using a specific IL-21 siRNA. Silencing the IL-21 gene reduced the proliferation and inhibited the migration of HCT116 cells. Thus, IL-21 may be an attractive target for preventing and treating colorectal cancer that is associated with parasitic infection. IL-21 has been used as a molecular target for the development of strategies to treat human cancers. IL-21 processes anti-tumour activity in several different preclinical tumour models, including plasmid gene delivery, tumour cell secretion and in study models that are enhanced by foreign antigens, vaccines and adoptively transferred tumour-specific lymphocytes (Davis et al. 2009). By contrast, studies in mice showed that IL-21 has a prominent function in tumour growth, whereby it induces tumour cell proliferation, limiting the tumour immune surveillance of colitis-associated colorectal cancer and resulting in extensive tumour growth (Kesselring et al. 2012). This phenomenon is supported by an in vitro study of the role of IL-21 in the development of colorectal cancer progression and proliferation.
The data collected from the LDH cytotoxicity assay of our study indicated that IL-21 silencing displayed an inhibitory property on the growth of HCT116 cells. This result is similar to a recent finding in colitis-associated colon cancer that indicated the absence of IL-21 induced apoptosis of tumour cells, activated the tumour immune surveillance and led to limited tumour growth (Kesselring et al. 2012). The cytotoxic effect detected in the IL-21-silenced HCT116 cells in our study was more influenced when the IL-21-silenced HCT116 cells were maintained in the growth medium that contained the FGF4-neutralizing antibody, though the cytotoxic level observed to be less than 40%. This phenomenon indicates that both silencing the IL-21 gene in HCT116 cells and neutralizing the FGF4 in HCT116 cell conditioned medium increases the cytotoxic level of HCT116 cells, compared to silencing the IL-21 gene alone in HCT116 cells, though both modifications remained insufficient to induce apoptosis in the cancer cells.
Despite the reduction in proliferation, our results also showed that IL-21 was highly involved in the migration of HCT116 cells. Silencing IL-21 by siRNA caused HCT116 cells to lose their response to cell migration. Measurement of the rate of cell migration revealed that the wound was never covered by the process of healing after 72 h compared with the non-silenced colorectal cancer cells. This research finding supports the hypothesis proposed by Wang et al. (2015) in breast cancer cells that silencing IL-21 in MDA-MB-231 cells persuades an anti-invasive activity in the cells. IL-21 can bind to other receptors that induce the invasive and healing processes (Habib et al. 2002; Zeng et al. 2005). Although the inhibition of cell migration detected in our study was observed in the IL-21-silenced HCT116 cells, the activity of cell migration is restored when the FGF4-neutralizing antibody is added to the conditioned medium of IL-21-silenced HCT116 cells. Therefore, IL-21 gene silencing and FGF4 protein expression in conditioned media are required for the inhibition of cell migration in HCT116 cells. Exclusion of the FGF4 protein expression is the factor that must be considered to design new drugs or supplements for infectious colorectal cancer treatment, as it restores the migratory activity of the cancer cells. Indeed, the effect of FGF4 protein inhibition is more effective when the strategy is replaced with inhibitor.
FGF4 is a member of the FGF superfamily of proteins that is involved in various stages of embryonic development (Kosaka et al. 2009). FGF4 is well known, in turn, to have a direct or indirect association with the induction of apoptosis (Hirai et al. 2004; Pradhan et al. 2004). The role of FGF4 in inducing apoptosis in colorectal cancer has not been reported previously. However, a similar study can be found in the studies of other cancers. For example, many studies in multiple myeloma (MM) have shown that FGFR3 inhibition by antagonistic antibodies or small-molecule inhibitors causes reduced proliferation and increased apoptosis of FGFR3-positive MM cell lines (Trudel et al. 2004; Grand et al. 2004a, b; Trudel, et al. 2005, 2006). Our results showed significant up-regulation of the FGF4 mRNA expression in HCT116 cells after the IL-21 gene was silenced, this gene plays an important role in the proliferation and migration of cancer cells only as indicated in the study because no apoptosis was induced in IL-21-silenced HCT116 cells. The reduction in the proliferation and inhibition of migration in IL-21-silenced HCT116 cells was likely also regulated by the mRNA expression of the group of genes identified in IL-21-silenced HCT116 cells from this study.
The mRNA expression of IL-17, which was shown to be significantly up-regulated in IL-21-silenced HCT116 cells, may also be correlated with the reduction of cell proliferation, whereby the reduction was more prominently observed with continuous up-regulation of IL-17 mRNA expression when the cells were maintained in growth medium containing FGF4-neutralizing antibody. IL-17 is a pleiotropic proinflammatory cytokine that promotes cancer-elicited inflammation and prevents cancer cells from immune surveillance (Dang et al. 2013). Our result demonstrated that silencing IL-21 in HCT116 cells induced a high level of IL-17 in the cells compared with that of the control cells. This result is opposed to the previous result in colitis-associated colorectal cancer (Kesselring et al. 2012). The study reported that the absence or low level of IL-21 induced the progression of colorectal cancer in an adoptive transfer model while promoting an immune response characterized by a high level of IL-17. This result may also be due to the alternative functions of IL-17 and IL-21 in cancer cells. Furthermore, IL-17 is considered to be an advocate for the development of colorectal cancer, although this statement is controversial (Dang et al. 2013). For the detection of colorectal cancer patients who are with schistosome infection at an early stage, in which the disease can be treated more effectively, further studies are warranted to explore the correlation of IL-21 with the identified group of genes and proliferation in HCT116 cells, e.g. the mRNA expressions of TGFα, CCL5, IL-13 and IL-6 may also be correlated with the further reduction of proliferation in IL-21-silenced HCT116 cells in the medium with a neutralizing FGF4 antibody. Perhaps, the efficacy and sustainability of specific gene silencing at mRNA level should also be investigated before other cellular mechanisms are explored.
In conclusion, compelling epidemiological data, immuno-pathological data and molecular features of infectious colorectal cancer describe a possible role for chronic infection in promoting the carcinogenesis of colorectal cancer. The similarities to colitis-induced carcinoma point to inflammation as a key factor in the carcinogenic process in the present study. We also indicate that both silencing the IL-21 gene in the HCT116 cells and neutralizing the FGF4 in conditioned medium of the cancer cells increases the cytotoxic level and restores the migratory activity of HCT116 cells compared to silencing the IL-21 gene alone. Therefore, these factors may be considered for future drug and supplement design to treat schistosomiasis patients with colorectal cancer effectively.
Acknowledgements
The authors would like to thank USM-TWAS Postgraduate Fellowship and Tropical Medicine Research Institute (TMRI), Sudan for providing support to the first author for the study. This study was also supported by Fundamental Research Grant Scheme (FRGS) Fasa 2/2013 (Grant No. 203/CIPPM/6711336) for the study of FGF4, Fundamental Research Grant Scheme (FRGS) Fasa 1/2017 (Grant No. 203/CIPPM/6711599) for the study of IL-21 and Sumitomo Foundation for Japan-related projects (REG. No. 158401-49).
References
- Chieffi PP. Interrelationship between schistosomiasis and concomitant diseases. Mem Inst Oswaldo Cruz. 1992;87:291–296. doi: 10.1590/S0074-02761992000800045. [DOI] [PubMed] [Google Scholar]
- Collins M, Whitters MJ, Young DA. IL-21 and IL-21 receptor: a new cytokine pathway modulates innate and adaptive immunity. Immunol Res. 2003;28:131–140. doi: 10.1385/IR:28:2:131. [DOI] [PubMed] [Google Scholar]
- Coquet JM, Kyparissoudis K, Pellicci DG, Besra G, Berzins SP, Smyth MJ, Godfrey DI. IL-21 is produced by NKT cells and modulates NKT cell activation and cytokine production. J Immunol. 2007;178:2827–2834. doi: 10.4049/jimmunol.178.5.2827. [DOI] [PubMed] [Google Scholar]
- Dang W, Pin W, Qi H, Yang L, Jun Y, Jian H. Interleukin-17: a promoter in colorectal cancer progression. Clin Dev Immunol. 2013;2013:436307. doi: 10.1155/2013/436307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis ID, Brady B, Kefford RF, Millward M, Cebon J, Skrumsager BK, Mouritzen U, Hansen LT, Skak K, Lundsgaard D, Frederiksen KS, Kristjansen PE, McArthur G. Clinical and biological efficacy of recombinant human interleukin-21 in patients with stage IV malignant melanoma without prior treatment: a phase IIa trial. Clin Cancer Res. 2009;15:2123–2129. doi: 10.1158/1078-0432.CCR-08-2663. [DOI] [PubMed] [Google Scholar]
- Grand EK, Chase AJ, Heath C, Rahemtulla A, Cross NC. Targeting FGFR3 in multiple myeloma: inhibition of t(4;14)-positive cells by SU5402 and PD173074. Leukemia. 2004;18:962–966. doi: 10.1038/sj.leu.2403347. [DOI] [PubMed] [Google Scholar]
- Grand EK, Grand FH, Chase AJ, Ross FM, Corcoran MM, Oscier DG, Cross NC. Identification of a novel gene, FGFR1OP2, fused to FGFR1 in 8p11 myeloproliferative syndrome. Genes Chromosomes Cancer. 2004;40:78–83. doi: 10.1002/gcc.20023. [DOI] [PubMed] [Google Scholar]
- Gupta RB, Harpaz N, Itzkowitz S, Hossain S, Matula S, Kornbluth A, Bodian C, Ullman T. Histologic inflammation is a risk factor for progression to colorectal neoplasia in ulcerative colitis: a cohort study. Gastroenterology. 2007;133:1099–1105. doi: 10.1053/j.gastro.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habib T, Senadheera S, Weinberg K, Kaushansky K. The common γ chain (γc) is a required signaling component of the IL21 receptor and supports IL21-induced cell proliferation via JAK3. Biochemistry. 2002;41:8725–8731. doi: 10.1021/bi0202023. [DOI] [PubMed] [Google Scholar]
- Herrera LA, Benitez-Bribiesca L, Mohar A, Ostrosky-Wegman P. Role of infectious diseases in human carcinogenesis. Environ Mol Mutagen. 2005;45:284–303. doi: 10.1002/em.20122. [DOI] [PubMed] [Google Scholar]
- Hirai K, Sasaki H, Yamamoto H, Sakamoto H, Kubota Y, Kakizoe T, Terada M, Ochiya T. HST-1/FGF-4 protects male germ cells from apoptosis under heat-stress condition. Exp Cell Res. 2004;294:77–85. doi: 10.1016/j.yexcr.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Hope ME, Hold GL, Kain R, El-Omar EM. Sporadic colorectal cancer: role of the commensal microbiota. FEMS Microbiol Lett. 2005;244:1–7. doi: 10.1016/j.femsle.2005.01.029. [DOI] [PubMed] [Google Scholar]
- Jess T, Simonsen J, Jørgensen KT, Pedersen BV, Nielsen NM, Frisch M. Decreasing risk of colorectal cancer in patients with inflammatory bowel disease over 30 years. Gastroenterology. 2012;143:375–381. doi: 10.1053/j.gastro.2012.04.016. [DOI] [PubMed] [Google Scholar]
- John P, Mallika K, Thirumalai RR, Robert WT, Joseph FU, Allen WC, Deborah AY, Mary C, Michael JG, Thomas AW. The IL21 receptor augments Th2 effector function and alternative macrophage activation. J Clin Invest. 2006;116:2044–2055. doi: 10.1172/JCI27727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesselring R, Jauch D, Fichtner-Feigl S. Interleukin 21 impairs tumor immunosurveillance of colitis-associated colorectal cancer. OncoImmunology. 2012;1:537–538. doi: 10.4161/onci.19407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaka N, Sakamoto H, Terada M, Ochiya T. Pleiotropic function of FGF-4: its role in development and stem cells. Dev Dyn. 2009;238:265–276. doi: 10.1002/dvdy.21699. [DOI] [PubMed] [Google Scholar]
- Leonard WJ, Spolski R. Interleukin-21: a modulator of lymphoid proliferation, apoptosis and differentiation. Nat Rev Immunol. 2005;5:688–698. doi: 10.1038/nri1688. [DOI] [PubMed] [Google Scholar]
- Leonardo L, Chigusa Y, Kikuchi M, Kato-Hayashi N, Kawazu S-I, Angeles JM, Fontanilla IK, Tabios IK, Moendeg K, Goto Y, Fornillos RJ, Tamayo PG, Chua JC. Schistosomiasis in the Philippines: challenges and some successes in control. Southeast Asian J Trop Med Public Health. 2016;47:651–666. [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔCt) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Madbouly KM, Senagore AJ, Mukerjee A, Hussien AM, Shehata MA, Navine P, Delaney CP, Fazio VW. Colorectal cancer in a population with endemic Schistosoma mansoni: is this an at-risk population? Int J Colorectal Dis. 2007;22:175–181. doi: 10.1007/s00384-006-0144-3. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- Ming-Chai C, Chi-Yuan C, Pei-Yu C, Jen-Chun H. Evolution of colorectal cancer in schistosomiasis: transitional mucosal changes adjacent to large intestinal carcinoma in colectomy specimens. Cancer. 1980;46:1661–1675. doi: 10.1002/1097-0142. [DOI] [PubMed] [Google Scholar]
- Monteleone I, Pallone F, Monteleone G. Interleukin-23 and Th17 cells in the control of gut inflammation. Mediators Inflamm. 2009;2029:297645. doi: 10.1155/2009/297645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olveda DU, Li Y, Olveda RM, Lam AK, McManus DP, Chau TNP, Harn DA, Williams GM, Gray DJ, Ross AGP. Bilharzia in the Philippines: past, present, and future. Int J Infect Dis. 2014;18:52–56. doi: 10.1016/j.ijid.2013.09.011. [DOI] [PubMed] [Google Scholar]
- Parrish-Novak J, Dillon SR, Nelson A, Hammond A, Sprecher C, Gross JA, Johnston J, Madden K, Xu W, West J, Schrader S, Burkhead S, Heipel M, Brandt C, Kuijper JL, Kramer J, Conklin D, Presnell SR, Berry J, Shiota F, Bort S, Hambly K, Mudri S, Clegg C, Moore M, Grant FJ, Lofton-Day C, Gilbert T, Rayond F, Ching A, Yao L, Smith D, Webster P, Whitmore T, Maurer M, Kaushansky K, Holly RD, Foster D. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature. 2000;408:57–63. doi: 10.1038/35040504. [DOI] [PubMed] [Google Scholar]
- Pearce EJ, MacDonald AS. The immunobiology of schistosomiasis. Nat Rev Immunol. 2002;2:499–511. doi: 10.1038/nri843. [DOI] [PubMed] [Google Scholar]
- Pradhan A, Mijovic A, Mills K, Cumber P, Westwood N, Mufti GJ, Rassool FV. Differentially expressed genes in adult familial myelodysplastic syndromes. Leukemia. 2004;18:449–459. doi: 10.1038/sj.leu.2403265. [DOI] [PubMed] [Google Scholar]
- Rutter M, Saunders B, Wilkinson K, Rumbles S, Schofield G, Kamm M, Williams C, Price A, Talbot I, Forbes A. Severity of inflammation is a risk factor for colorectal neoplasia in ulcerative colitis. Gastroenterology. 2004;126:451–459. doi: 10.1053/j.gastro.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Skak K, Kragh M, Hausman D, Smyth MJ, Sivakumar PV. Interleukin 21: combination strategies for cancer therapy. Nat Rev Drug Discov. 2008;7:231–240. doi: 10.1038/nrd2482. [DOI] [PubMed] [Google Scholar]
- Spolski R, Leonard WJ. Interleukin-21: basic biology and implications for cancer and autoimmunity. Annu Rev Immunol. 2008;26:57–79. doi: 10.1146/annurev.immunol.26.021607.090316. [DOI] [PubMed] [Google Scholar]
- Trakatelli C, Frydas S, Hatzistilianou M, Papadopoulos E, Simeonidou I, Founta A, Paludi D, Petrarca C, Castellani ML, Papaioannou N, Salini V, Conti P, Kempuraj D, Vecchiet J. Chemokines as markers for parasite induced inflammation and tumors. Int J Biol Markers. 2005;20:197–203. doi: 10.1177/172460080502000401. [DOI] [PubMed] [Google Scholar]
- Trudel S, Ely S, Farooqi Y, Affer M, Robbiani DF, Chesi M, Bergsage PL. Inhibition of fibroblast growth factor receptor 3 induces differentiation and apoptosis in t(4;14) myeloma. Blood. 2004;103:3521–3528. doi: 10.1182/blood-2003-10-3650. [DOI] [PubMed] [Google Scholar]
- Trudel S, Li ZH, Wei E, Wiesmann M, Chang H, Chen C, Reece D, Heise C, Stewart AK. CHIR-258, a novel, multitargeted tyrosine kinase inhibitor for the potential treatment of t(4;14) multiple myeloma. Blood. 2005;105:2941–2948. doi: 10.1182/blood-2004-10-3913. [DOI] [PubMed] [Google Scholar]
- Trudel S, Stewart AK, Rom E, Wei E, Li ZH, Kotzer S, Chumakov I, Singer Y, Chang H, Liang SB, Yayon A. The inhibitory anti-FGFR3 antibody, PRO-001, is cytotoxic to t(4;14) multiple myeloma cells. Blood. 2006;107:4039–4046. doi: 10.1182/blood-2005-10-4179. [DOI] [PubMed] [Google Scholar]
- Wang L, Cui Y, Ruge F, Jiang WG. Interleukin 21 and its receptor play a role in proliferation, migration and invasion of breast cancer cells. Cancer Genomics Proteomics. 2015;12:211–221. [PubMed] [Google Scholar]
- West RN, McCuaig S, Franchini F, Powrie F. Emerging cytokine networks in colorectal cancer. Nat Rev Immunol. 2015;15:615–629. doi: 10.1038/nri3896. [DOI] [PubMed] [Google Scholar]
- Wurster AL, Rodgers VL, Satoskar AR, Whitters MJ, Young DA, Collins M, Grusby MJ. Interleukin 21 is a T helper (Th) cell 2 cytokine that specifically inhibits the differentiation of naive Th cells into interferon γ-producing Th1 cells. J Exp Med. 2002;196:969–977. doi: 10.1084/jem.20020620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TA. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat Rev Immunol. 2004;4:583–594. doi: 10.1038/nri1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng R, Spolski R, Finkelstein SE, Oh S, Kovanen PE, Hinrichs CS, Pise-Masison CA, Radonovich MF, Brady JN, Restifo NP. Synergy of IL21 and IL-15 in regulating CD8 T-cellexpansion and function. J Exp Med. 2005;201:139–148. doi: 10.1084/jem.20041057. [DOI] [PMC free article] [PubMed] [Google Scholar]