Abstract
Oil extraction from the kernels of Argania spinosa (L.) Skeels (Sapotaceae), an endemic tree of Morocco, produces argan press-cake (APC) used as a shampoo and to treat sprains, scabies, and for healing wounds. We have previously reported that argan oil has antimelanogenesis effect. Here, we determined if the by-product, APC, has melanogenesis regulatory effect using B16 murine melanoma cells. The effect of APC ethanol extract on cell proliferation and melanin content of B16 cells were measured, and to elucidate the mechanism involved, the expression level of melanogenic enzymes tyrosinase (TYR), dopachrome tautomerase (DCT), and tyrosinase-related protein 1 (TRP1) were determined and mRNA expression level of microphthalmia- associated transcription factor (Mitf) and Tyr genes were quantified. APC ethanol extract showed a significant melanin biosynthesis inhibitory effect on B16 cells in a time-dependent manner without cytotoxicity, which could be due to the decreased expression of TYR, TRP1, and DCT in a time-dependent manner. APC extract down regulated Mitf and Tyr. Decreased TRP1 and DCT levels could be due to post-translational modifications. These results suggest that APC extract may be used as a new source of natural whitening products and may be introduced as an important pharmacological agent for the treatment of hyperpigmentation disorders.
Keywords: Argania spinosa, Argan press-cake, Melanogenesis, Mitf, Tyr
Introduction
The color of the skin, hair and eyes of mammals is derived from the production and distribution of pigmented biopolymers known as melanins. Melanins are synthesized within specialized and unique organelles called melanosomes that are produced in melanocytes and in retinal pigment epithelial cells (Slominski et al. 2004). This pigment protects the skin and eyes from the harmful effects of UV irradiation (Fitzpatrick et al. 1979). Melanin synthesis begins with the hydroxylation of amino acid l-tyrosine to L-3-(3,4-dihydroxyphenyl)-alanine (DOPA) and of DOPA to DOPA-quinone. These two reactions are catalyzed by, the rate-limiting enzyme of melanogenesis, TYR. The conjugation of DOPA-quinone with cysteine or glutathione yields 5-S-cysteinyl-DOPA and glutathionyl-DOPA, which are progressively converted into red/yellow pigment pheomelanin. DOPA-quinone in the absence of thiol compounds may spontaneously oxidize to DOPAchrome. DCT, called also tyrosinase-related protein 2 (TRP2), isomerizes DOPAchrome to 5,6-dihydroxyindole-2-carboxylic acid (DHICA). TRP1 has been shown to oxidize DHICA to indole-5,6-quinone-2-carboxylic acid, indolic precursors of the black/brown pigment eumelanin (Hearing 1999). The production of these melanogenic enzymes is controlled by the microphthalmia-associated transcription factor (MITF), at mRNA level (Bertolotto et al. 1998). Pigmented melanosomes are then transported towards the cell membrane at the dendrite tips of melanocytes and then transferred to neighboring keratinocytes by a still not well characterized mechanism (Marks and Seabra 2001). Once in keratinocytes, melanosomes are positioned over the sun-exposed side of nuclei to form supranuclear melanin caps that protects DNA from the harmful UV-radiation (Byers et al. 2003; Park et al. 2009).
However, the overproduction and accumulation of melanin causes aesthetic problems and various related pigment disorders like melasma, freckles or lentigines. Although a number of hypopigmenting products have been developed, relatively few of them are used mainly due to safety concerns. For example, hydroquinone and kojic acid were among the most used depigmenting agents before serious side effects such as allergy, contact dermatitis, eczema and cytotoxicity arise (Serra-Baldrich et al. 1998; Raynaud et al. 2001). Therefore, the development of safe and effective whitening agents is one of the challenges for cosmetic industry.
Argania spinosa (L.) Skeels (Sapotaceae) is an endemic tree of Morocco. It presents great ecological and socioeconomic roles in this area (Morton and Voss 1987). The fruit of A. spinosa has an oleaginous kernel from which well-known oil is used in folk medicine and cosmetics. We have previously reported that argan oil inhibits melanogenesis in B16 melanoma cells (Villareal et al. 2013). The others parts of argan tree have also been widely used in traditional remedies in Morocco. APC, a by-product of argan oil extraction, is used as a shampoo. It is also advocated to treat sprains, scabies, and wounds (Moukal 2004). Despite its efficacy in curing various skin diseases, the effect of APC on skin pigmentation has not yet been determined. Here, the effect of APC extract on melanin biosynthesis and the mechanism involved was determined.
Materials and methods
Chemicals and reagents
Dulbecco’s modified eagle’s medium (DMEM), fetal bovine serum (FBS), 4 mmol/l l-glutamine, 3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide or MTT, trizma base, trypsin (ethylene-diamine-tetra-acetic acid [EDTA]), acetonitrile and protease inhibitor cocktail, were purchased from Sigma–Aldrich (USA). Penicillin/streptomycin was purchased from Lonza, Walkersville Inc., (MD, USA). Dithiothreitol (DTT) and tetramethylethylenediamine (TEMED) were purchased from Amersham Bioscience (Sweden). Ammonium persulfate (APS) and protein rainbow marker were purchased from GE Healthcare (United Kingdom). Sodium dodecyl sulfate (SDS) was purchased from GE Healthcare (Sweden). Lysis buffer (RIPA buffer) was obtained from Promega. All other chemicals were purchased from Wako (Japan).
Extraction of APC
The fruits of Argania spinosa were harvested at complete maturity in July 2011 from Sidi ifni region (southwest of Morocco). They were dried in the dark at 25 °C. Argan fruits were manually peeled, then their nut’s shells were cracked and kernels were collected to extract argan oil by mechanical press using a Komet DD 85 G press (IBG Monforts Oekotec GmbH & Co. KG, Mönchengladbach, Germany). Ten grams (10 g) of APC was extracted with 100 ml ethanol 70% for 2 weeks at room temperature. The extract was then centrifuged (1000 g, 15 min) and the supernatant was filter-sterilized using a 0.45 µm pore size filter (Millipore, Billerica, MA, USA) and stored at −80 °C until use.
APC oil content
APC (10 g) was extracted with 100 ml of hexane at room temperature for 24 h with agitation. The solvent was then evaporated to dryness under vacuum. The residue was weighed to get the oil content of APC.
Cells and cell culture
B16 murine melanoma cells were purchased from Riken Cell Bank (Tsukuba, Japan) and maintained as a monolayer culture in DMEM supplemented with 10% FBS, 4 mmol/l l-glutamine, 50 units/ml penicillin, and 50 µg/ml streptomycin; and incubated in a 37 °C humidified atmosphere with 5% CO2. Photographs of the cells were taken using Leica DFC290 HD camera (Beckman Coulter, CA, USA).
MTT assay
Cell viability was assessed using the MTT assay by following previously reported method (Villareal et al. 2013). In brief, B16 cells were seeded (3 × 103 cells/well) onto 96-well plates and cultured as described above for 24 h. The medium was then replaced with medium containing APC extract at various concentrations (0 or control, 0.01, 0.02, 0.1, 0.2, 1.0, and 2.0 mg/ml). After incubation for 48 h, MTT (5 mg/ml) was added at 10 µl per well; the plates were covered with aluminum foil and incubated at 37 °C for further 18 h. SDS (10%) was then added at 100 µl per well, followed by overnight incubation at 37 °C to completely dissolve the formazan crystals. The absorbance was measured at 570 nm using a microplate reader (Powerscan HT, Dainippon Pharmaceuticals USA Corporation, Marlborough, MA, USA). Blanks, containing only medium, MTT, and SDS were used to correct the absorbance. The absorbance of the control cells were set as 100% cell proliferation.
Measurement of melanin content
The melanin content of B16 cells was measured using a modified version of a previously reported method (Kawano et al. 2007). B16 melanoma cells were seeded onto 100-mm dishes at a density of 5 × 105 cells per Petri dish and cultivated using the method described above. After overnight incubation, the medium was replaced with medium containing arbutin (100 µmol/l) or APC extract (25, 50, 100 or 150 µg/ml) and incubated for 48 or 72 h. The medium was then removed, and the cells were washed twice with phosphate-buffered saline (PBS) and harvested by trypsinization. The harvested cells were pelleted and the cell membrane was dissolved by sonication after addition of 0.1% Triton X-100. The synthesized melanin was then purified and precipitated in 10% trichloroacetate and dissolved in 8 mol/l NaOH followed by incubation for 2 h at 80 °C. The absorbance of the solution was measured at 410 nm and the melanin content was calculated using a standard curve for synthetic melanin. The melanin content in control was considered as 100% and results were expressed as a percentage of the control. The cell viability and total cell count were assessed using the ViaCount Program of Guava PCA (GE Healthcare, UK Ltd.) following the manufacturer’s instructions.
Western blot
B16 melanoma cells at a density of 5 × 105 cells per dish were seeded onto 100-mm Petri dishes and cultivated using the method described above. After overnight incubation, the medium was replaced by medium with or without APC extract (50 µg/ml). The plates were then incubated for 48 or 72 h. The medium was then removed, the cells were washed twice with cold PBS and total protein was extracted using RIPA buffer according to the manufacturer’s instructions. Ten micrograms (10 µg) of extracted protein sample were resolved in 10% sodium dodecyl sulphate (SDS)-polyacrylamide gel electrophoresis, transferred to polyvinylidene difluoride (PVDF) membrane, and detected by primary antibodies (anti-TYR, anti-TRP1, anti- DCT, or anti-GAPDH antibodies) from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). The signal was detected using LiCor Odyssey Infrared Imaging System after reaction with IRDye-labeled secondary anti-Mouse IgG or anti-Goat IgG antibodies (LI-COR, Lincoln, NE, USA).
Total RNA extraction
B16 cells were seeded onto 100-mm Petri dish at a density of 3 × 106 cells per dish and cultivated as described above. After overnight incubation, the cells were treated with fresh medium without (control) or with APC extract (50 µg/ml). After 12 or 24 h, the medium was removed and the cells were washed twice with cold PBS. The total RNA was then extracted using ISOGEN kit (Nippon Gene, Tokyo, Japan) and quantified using a Nanodrop 2000 spectrophotometer (Nanodrop Technologies, Wilmington, DE, USA) following the manufacturer’s instructions. The extracted RNA (1 µg) was used for reverse transcription polymerase chain reaction (RT-PCR) using SuperScript III Reverse Transcriptase Kit (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s instructions.
Quantitative real-time PCR analysis
Gene expression was determined using TaqMan Gene expression Assays (Applied Biosystem, Carlsbad, CA, USA). Real-time PCR (rt-PCR) was performed using the 7500 Fast Real-time PCR System (Applied Biosystems) under the following conditions: 2 min at 50 °C and 10 min at 95 °C, followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min. Gapdh was used as the internal control. The Assay ID of the primers used for rt-PCR are the following: Mitf–Mm00434954_m1, Tyr–Mm00495818_m1 and Gapdh–Mm99999915_g1. Data were analyzed using 7500 Fast System SDS Software 1.3.1 (Applied Biosystems).
Statistical analysis
The results are expressed as the mean ± standard deviation (SD) of three independent experiments. The data were statistically analyzed using one-way analysis of variance with a Fisher’s Least Significant Difference (LSD) post hoc test. P values less than 0.05 were considered to be statistically significant.
Results
APC oil content
APC was analyzed for the presence of residue of oil at the end of the mechanical process of argan kernels oil extraction. The determination of APC oil content revealed that the oil residue was negligible (0.6% w/w).
APC extract has no cytotoxic effect on B16 cells
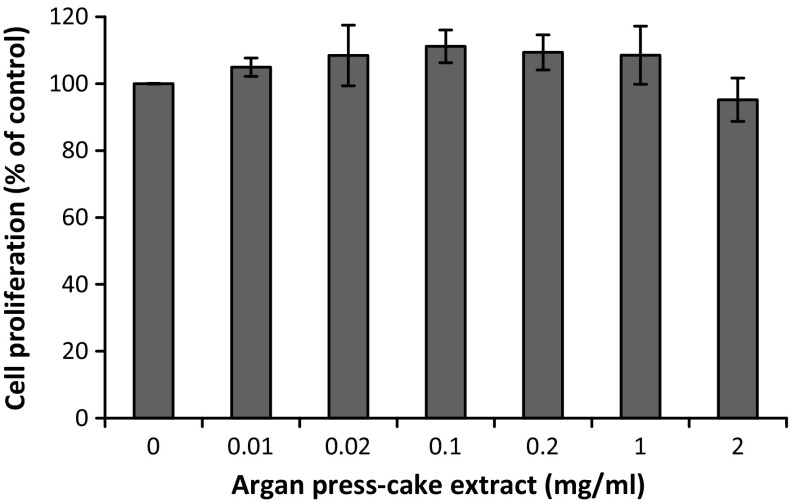
To evaluate the effect of APC extract on B16 cell proliferation, MTT assay was performed. As shown in Fig. 1, APC extract was not cytotoxic on B16 melanoma cells at all tested concentrations (0.01–2.00 mg/ml) after 48 h of incubation.
Fig. 1.
Effect of APC extract on B16 melanoma cells proliferation. After treatment of B16 cells with APC extract at various concentrations for 48 h, cell proliferation was determined by MTT assay as described in “Materials and Methods” section. Data are presented as the mean ± SD of three independent experiments. Statistical significance *P < 0.05 was defined as control versus sample
APC extract has an inhibitory effect on melanogenesis on B16 melanoma cells
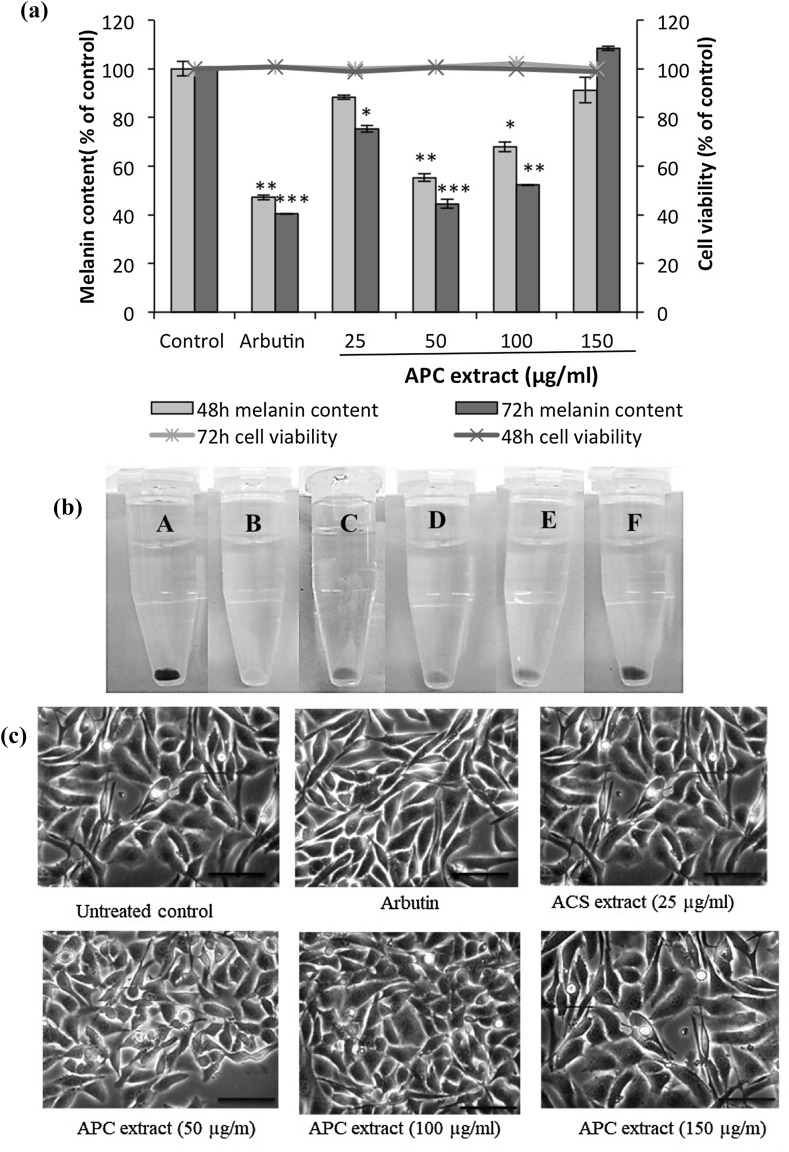
Treatment with APC extract caused a significant decrease in the melanin content of B16 cells in time- and dose-dependent manners (Fig. 2a). At a dose of 50 µg/ml, APC extract showed the highest melanogenesis inhibitory effect compared to other tested APC extract’s concentrations (25, 100 or 150 µg/ml). Melanin content in B16 cells treated during 72 h with 50 µg/ml of APC extract was reduced by 55.5%; while it decreased by 47.8% in B16 cells treated with 100 µg/ml and by 24.5% in B16 cells treated with 25 µg/ml of APC extract. Either at 48 h or at 72 h, melanogenesis was not inhibited by APC extract at the dose of 150 μg/ml.
Fig. 2.
Effect of APC extract on the melanin content of B16 murine melanoma cells, 48 and 72 h after treatment. (a) B16 cells were seeded at a density of 5 × 105 cells per 100-mm dish. After overnight incubation, the cells were treated without (control) or with 25, 50, 100 and 150 µg/ml of APC extract for 48 and 72 h. Cells treated with 100 µmol/l arbutin served as a positive control. The synthesized melanin and total number of cells were then measured as described in “Materials and Methods” section. The melanin content was expressed as melanin content per cell and then as percentage of control (% of control). The bar graph indicates the melanin content (left-hand y-axis) while the line graph indicates cell viability (right-hand y-axis). The results are presented as the mean ± SD of three independent experiments. Statistical significance *P < 0.05 was defined as control versus sample. (b) The extracted melanin from B16 cells treated without (A) or with 100 µmol/l of arbutin (B), or with APC extract at 25 µg/ml (C), APC extract at 50 µg/ml (D), APC extract at 100 µg/ml (E), APC at 150 µg/ml (F). (c) Images of B16 cells treated with APC extract or arbutin (400× magnification)
In comparison with arbutin, a well-known melanogenesis inhibitor used as a positive control, cells treated with APC extract at 50 µg/ml had almost the same melanin content as cells treated with arbutin at 100 µmol/l. The color of the extracted melanin from treated B16 cells clearly showed that the melanin content was markedly decreased by APC extract treatment (Fig. 2b) . Moreover, APC extract did not have an effect on the morphology of B16 cells (Fig. 2c).
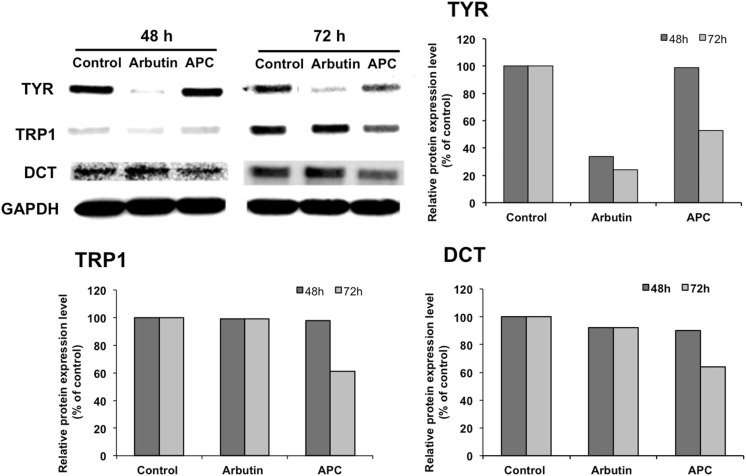
APC extract modulated the melanogenic enzymes expression in B16
As previously reported (Gaggioli et al. 2003), melanogenesis is mediated by three major enzymes: TYR, TRP1, and DCT. The expression level of these enzymes in APC extract-treated cells (50 µg/ml for 48 or 72 h) was determined via western blot analysis. The results showed that APC extract decreases the expression level of those three enzymes in a time-dependent manner (Fig. 3a). The analysis of densitometric values of proteins expressions showed that TYR, TRP1 and DCT expressions levels decreased significantly after 72 h by 47, 49, and 36%, respectively (Fig. 3b). The positive control arbutin, as expected, significantly decreased the expression level of TYR by 66 and 76% after 48 and 72 h of treatment, respectively. However, no significant changes were observed on TRP-1 and DCT expressions levels.
Fig. 3.
Effect of APC extract on melanogenic enzymes TYR, TRP1, and DCT expression level, determined by western blot analysis (a) and their densitometric values (b). B16 melanoma cells were seeded in 100-mm dish at a density of 5 × 105 cells per dish. After overnight incubation, cells were treated without (Control) or with 50 μg/ml of APC extract and incubated for further 48 and 72 h. Total proteins were then extracted and resolved by SDS-PAGE. The resolved proteins were then blotted onto a PVDF membrane and detected by anti-TYR, anti-TRP1, anti-DCT and GAPDH antibodies (as a loading control). The signal was detected using Odyssey Fc Imaging System. Values are mean ± SD derived from three independent experiments. Statistical significance *P < 0.05 was defined as control versus sample
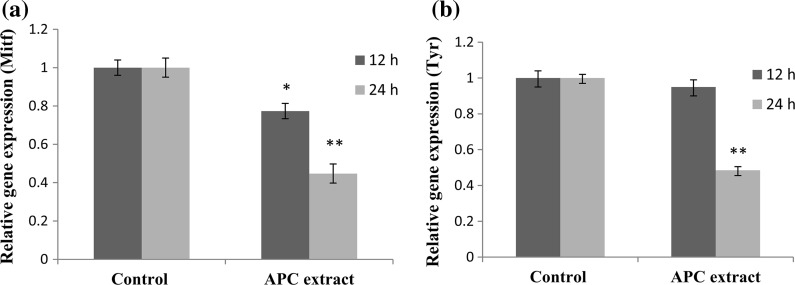
APC extract regulated the expression of Mitf and Tyr
The relative changes in the expression of Mitf and Tyr genes were evaluated by real-time PCR. Mitf, the master melanogenic transcription factor, was significantly decreased by 20 and 48% after treatment with APC extract for 12 and 24 h, respectively (Fig. 4a). Tyr was significantly down-regulated by 51.5% compared to the control (Fig. 4b).
Fig. 4.
Effect of APC extract on the expression of (a) microphthalmia-associated transcription factor (Mitf) and (b) tyrosinase (Tyr) genes in B16 cells. Cells were seeded onto 100-mm dish at a density of 3 × 106 cells per dish. After overnight incubation, the cells were treated without or with APC extract (50 µg/ml) for 12 or 24 h. Real-time PCR was then used to determine the expression level of Mitf and Tyr genes. Data are expressed as the mean ± SD of three independent experiments. Statistical significance *P < 0.05 and **P < 0.01 were defined as control versus sample
Discussion
In the recent years, there has been a growing demand for cosmetic skin-lightening products (Kim et al. 2015). In Western countries, these products are usually used for the prevention or treatment of irregular hyperpigmentation conditions like melasma, freckles and sun spots. While in Asia and Africa, skin-whitening formulations are also popularly used to make the skin color lighter. Although a number of whitening agents have been developed, those from natural sources are preferred and will predominate in the cosmetics market driven by consumers’ growing interest in safer and more natural ingredients. To our knowledge, this is the first study to report that APC, a by-product of argan oil extraction, has an inhibitory effect on melanogenesis in B16 melanoma cells.
APC extract did not inhibit the proliferation of B16 melanoma cells at any concentrations tested (up to 2 mg/ml) suggesting that APC extract had no cytotoxic effects on B16 melanoma cells (Fig. 1). The quantification of melanin content revealed that APC extract has a bi-phasic inhibitory effect on melanogenesis: low doses of APC extract (25, 50 µg/ml) showed a positive dose-dependent inhibitory effect (decreased melanin biosynthesis with the highest effect at 50 µg/ml) while at higher doses (100, 150 µg/ml), the inhibitory effect faded out and the melanin content was higher (Fig. 2). A similar bi-phasic effect has been observed when HTC cells, a hepatocyte cell line derived, were treated with APC extract to evaluate their responses to insulin. APC extract exhibited an insulin-sensitizing action at low doses and almost no effect at high doses (Samane et al. 2006). One hypothetical explanation for this concentration-dependent effect, in our case, is either the antagonism or the synergy that may occur between the different compounds present in APC extract that enhances the melanogenesis inhibitory effect at the low concentrations, and cancels this effect at the higher concentration.
We have previously reported that 48 h of treatment with argan oil (1/1000 v/v) inhibited melanogenesis in B16 cells by about 30% (Villareal et al. 2013). Interestingly, at this point in time, APC extract had a more effective anti-melanogenesis effect (44.7%) compared to argan oil. This APC extract inhibitory activity cannot be attributed to the presence of oil residue, since the latter is negligible in APC (0.6%). APC extract contains mainly saponins (arganine A-F, mi-saponin A) as previously reported (Charrouf et al. 1992; Henry et al. 2013). APC saponins are bidesmosidic and their aglycone belongs to the oleanane family which has been reported having inhibitory effects on melanogenesis (Fujimoto et al. 2012; Nakamura et al. 2012). Although APC saponins appear to be the main component that regulates melanogenesis, the other components of APC extract like phenolic acid derivatives and flavonoids (Rojas et al. 2005; El Monfalouti et al. 2012) may also play an important role. Therefore, the utilization of a fractionation approach may help the identification of the active principles of APC extract in inhibiting melanogenesis.
Melanin synthesis is controlled by the melanogenic enzymes, TYR, DCT and TRP1, mutations in which cause hypopigmented or diluted color of skin. APC extract treatment significantly decreased the expression level of TYR, TRP1 and DCT by 47, 49 and 36%, respectively, after 72 h of treatment, while arbutin significantly decreased only the expression level of TYR by 76% (Fig. 3). In order to elucidate the molecular mechanism that controls the decrease of melanogenic enzymes expression level, we used real-time PCR to evaluate the relative changes in the expression of Mitf and Tyr genes. Mitf and Tyr expression were significantly decreased by APC extract treatment (Fig. 4). Mitf which is a key regulator of melanogenesis, regulates the expression of a numerous genes involved in melanocyte differentiation and pigment formation (Vachtenheim et al. 2010; Yajima et al. 2011). In addition, it is well known that Mitf controls the production of the melanogenic enzymes at mRNA level (Bertolotto et al. 1998) and our results have shown that the early events (12 and 24 h) following APC extract treatment involves down regulation of Mitf expression. This fact suggests then that APC extract inhibits melanogenesis in B16 melanoma cells through the down-regulation of Mitf expression that leads to the decrease of the transcription of the tyrosinase gene leading to a depletion of this enzyme. However, given that the expressions of the Trp1 and Dct mRNA were not altered; the observed lower expression levels of TRP1 and DCT are possibly due to post-translational modifications.
In addition to its action at mRNA level, our results suggest that APC extract may also act through other (non transcriptional) mechanisms of melanogenesis regulation. This hint is shown up by the decrease of melanin content of B16 melanoma cells at 48 h, while the expressions of TYR, TRP1 and DCT were not altered at this point in time. So, considering the diversity of the components in APC extract (saponins, phenolic acid derivatives, flavonoids), we presume that this latter (APC extract) may exert its early inhibitory effect by targeting other key points of melanogenesis process such as (1) through the inhibition of the catalytic activity of melanogenic enzymes, (2) directly by destroying existing melanin, (3) by scavenging the intermediate products of melanin synthesis, and/or (4) through post-translational modifications that conduct to inactive proteins. More analysis should be performed to make clear the mechanisms involved in the down-regulation of melanogenesis caused by treatment with APC extract.
Conclusion
We demonstrated in this study that APC extract significantly decreases melanin synthesis in B16 melanoma cells without cytotoxicity. We also found out that APC extract had, at an earlier time, a higher anti-melanogenesis effect compared to argan oil. Our results suggest that APC extract inhibits melanogenesis through the down-regulation of Mitf causing a decrease in the expression level of melanogenic enzymes, TYR, DCT and TRP1. Decreased TRP1 and DCT levels could be due to post-translational modifications. Recently, the utilization of single skin whitening agent is extended to the use of complex mixture, especially plants extract. To the best of our knowledge, this is the first report demonstrating the anti-melanogenesis activity of APC extract, providing a scientific basis for its cosmetic application. However, the exact mechanism by which the anti-melanogenesis effect occurs, as well as the active principle of APC extract remain to be elucidated.
Acknowledgements
Authors are grateful to the following organizations for their support: JST-JICA Japan’s SATREPS project; Japan Student Services Organization (JASSO); and Centre National pour la Recherche Scientifique et Technique (CNRST) Morocco.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Contributor Information
Chemseddoha Gadhi, Email: dgadhi@uca.ac.ma.
Hiroko Isoda, Email: isoda.hiroko.ga@u.tsukuba.ac.jp.
References
- Bertolotto C, Abbe P, Hemesath TJ, Bille K, Ortonne J-P, Ballotti R. Microphthalmia gene product as a signal transducer in cAMP-induced differentiation of melanocytes. J Cell Biol. 1998;142:827–835. doi: 10.1083/jcb.142.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers HR, Maheshwary S, Amodeo DM, Dykstra SG. Role of cytoplasmicdynein in perinuclear aggregation of phagocytosed melanosomes and supranuclear melanin cap formation in human keratinocytes. J Investig Dermatol. 2003;121:813–820. doi: 10.1046/j.1523-1747.2003.12481.x. [DOI] [PubMed] [Google Scholar]
- Charrouf Z, Wieruszeski JM, Fkih-Tetouani S, Leroy Y, Charrouf M, Fournet B. Triterpenoid saponins from Argania spinosa. Phytochemistry. 1992;31:2079–2086. doi: 10.1016/0031-9422(92)80367-N. [DOI] [PubMed] [Google Scholar]
- El Monfalouti H, Charrouf Z, Belviso S, Ghirardello D, Scursatone B, Guillaume G, Denhez C, Zeppa G. Analysis and antioxidant capacity of the phenolic compounds from argan fruit (Argania spinosa (L.) Skeels) Eur J Lipid Sci Technol. 2012;114:446–452. doi: 10.1002/ejlt.201100209. [DOI] [Google Scholar]
- Fitzpatrick TB, Szabo G, Seiji M, Quevedo WC. Biology of the melanin pigmentary system. In: Fitzpatrick TB, Eisen A, Wolff K, Freedberg I, Austen K, editors. Dermatology in general medicine. New York: McGraw-Hill; 1979. pp. 131–145. [Google Scholar]
- Fujimoto F, Nakamura S, Nakashima S, Matsumoto T, Uno K, Ohta T, Miura T, Matsuda H, Yoshikawa M. Medicinal flowers. XXXV. Nor-Oleanane-type and acylatedoleanane-type triterpene saponins from the flower buds of Chinese Camellia japonica and their inhibitory effects on melanogenesis. Chem Pharm Bull. 2012;60:1188–1194. doi: 10.1248/cpb.c12-00473. [DOI] [PubMed] [Google Scholar]
- Gaggioli C, Busca R, Abbe P, Ortonne JP, Ballotti R. Microphthalmia associated transcription factor (MITF) is required but is not sufficient to induce the expression of melanogenic genes. Pigment Cell Res. 2003;16:374–382. doi: 10.1034/j.1600-0749.2003.00057.x. [DOI] [PubMed] [Google Scholar]
- Hearing VJ. Biochemical control of melanogenesis and melanosomal organization. J Investig Dermatol. 1999;4:24–28. doi: 10.1038/sj.jidsp.5640176. [DOI] [PubMed] [Google Scholar]
- Henry M, Kowalczyk M, Maldini M, Piacente S, Stochma A, Oleszek W. Saponin inventory from Argania spinosa kernel cakes by liquid chromatography and mass spectrometry. Phytochem Anal. 2013;24:616–622. doi: 10.1002/pca.2440. [DOI] [PubMed] [Google Scholar]
- Kawano M, Matsuyama K, Miyamae Y, Shinmoto H, Kchouk ME, Morio T, Shigemori H, Isoda H. Antimelanogenesis effect of Tunisian herb Thymelaea hirsuta extract on B16 murine melanoma cells. Exp Dermatol. 2007;16:977–984. doi: 10.1111/j.1600-0625.2007.00618.x. [DOI] [PubMed] [Google Scholar]
- Kim JS, Seo YC, No RH, Lee HY. Improved cosmetic activity by optimizing the Lithospermum erythrorhizon extraction process. Cytotechnology. 2015;67:51–65. doi: 10.1007/s10616-013-9657-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks MS, Seabra MC. The melanosome: membrane dynamics in black and white. Nat Rev Mol Cell Biol. 2001;2:738–748. doi: 10.1038/35096009. [DOI] [PubMed] [Google Scholar]
- Morton JF, Voss GL. The argan tree (Argania spinosa, sapotaceae) a desert source of edible oil. Econ Bot. 1987;41:221–233. doi: 10.1007/BF02858970. [DOI] [Google Scholar]
- Moukal A. L’arganier, Argania spinosa L. Skeels, usage thérapeutique, cosmétique et alimentaire. Phytothérapie. 2004;5:135–141. doi: 10.1007/s10298-004-0041-2. [DOI] [Google Scholar]
- Nakamura S, Moriura T, Park S, Fujimoto K, Matsumoto T, Ohta T, Matsuda H, Yoshikawa M. Melanogenesis inhibitory and fibroblast proliferation accelerating effects of noroleanane- and oleanane-type triterpene oligoglycosides from the flower buds of Camellia japonica. J Nat Prod. 2012;75:1425–1430. doi: 10.1021/np3001078. [DOI] [PubMed] [Google Scholar]
- Park HY, Kosmadaki M, Yaar M, Gilchrest BA. Cellular mechanisms regulating human melanogenesis. Cell Mol Life Sci. 2009;66:1493–1506. doi: 10.1007/s00018-009-8703-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raynaud E, Cellier C, Perret JL. Depigmentation for cosmetic purposes: prevalence and side-effects in a female population in Senegal. Ann Dermatol Venereol. 2001;128:720–724. [PubMed] [Google Scholar]
- Rojas LB, Quideau SP, Pardon P, Charrouf Z. Colorimetric evaluation of phenolic content and GC-MS characterization of phenolic composition of alimentary and cosmetic argan oil and press cake. J Agric Food Chem. 2005;53:9122–9127. doi: 10.1021/jf051082j. [DOI] [PubMed] [Google Scholar]
- Samane S, Noël J, Charrouf Z, Amarouch H, Haddad PS. Insulin-sensitizing and anti-proliferative effects of Argania spinosa seed extracts. Evid Based Complement Altern Med. 2006;3:317–327. doi: 10.1093/ecam/nel015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra-Baldrich E, Tribó MJ, Camarasa JG. Allergic contact dermatitis from kojic acid. Contact Dermat. 1998;39:86–87. doi: 10.1111/j.1600-0536.1998.tb05843.x. [DOI] [PubMed] [Google Scholar]
- Slominski A, Tobin DJ, Shibahara S, Wortsman J. Melanin pigmentation in mammalian skin and its hormonal regulation. Phys Rev. 2004;84:1155–1228. doi: 10.1152/physrev.00044.2003. [DOI] [PubMed] [Google Scholar]
- Vachtenheim J, Ondrusova L, Borovansky J. SWI/SNF chromatin remodeling complex is critical for the expression of microphthalmia-associated transcription factor in melanoma cells. Biochem Biophys Res Commun. 2010;392:454–459. doi: 10.1016/j.bbrc.2010.01.048. [DOI] [PubMed] [Google Scholar]
- Villareal MO, Kume S, Bourhim T, Bakhtaoui FZ, Kashiwagi K, Han J, Gadhi C, Isoda H. Activation of MITF by argan oil leads to the inhibition of the tyrosinase and dopachrome tautomerase expressions in B16 murine melanoma cells. Evid Based Complement Altern Med. 2013;2013:340107. doi: 10.1155/2013/340107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajima I, Kumasaka MY, Thang ND. Molecular network associated with MITF in skin melanoma development and progression. J Skin Cancer. 2011;2011:730170. doi: 10.1155/2011/730170. [DOI] [PMC free article] [PubMed] [Google Scholar]