Abstract
Abstract
Fibroblast growth factor 21 (FGF21) potentially regulates glucose and lipid metabolism in energy homeostasis. We investigated dynamic changes in goat adipocytes treated with 75 nM FGF21 for 24, 36 and 48 h. Compared to controls, FGF21-treated adipocytes displayed smaller lipid droplets and altered levels of the mRNA transcripts encoding several lipolysis genes. The genes with elevated mRNA levels included: ATGL, HSL, CPT-1, and UCP1, and this was observed mainly at 24 and 36 h (P < 0.05). Some gene expression was attenuated including lipogenesis genes, such as SREBP1, PPARγ, C/EBPα, and ACC. This attenuation was observed mainly at 24 h (P < 0.05). Among the genes that were significantly induced or inhibited, ATGL, PGC1α, and C/EBPα were observed a significant effect at 48 h (P < 0.05). In addition, FGF21 treatment greatly increased number of mitochondria and the expression of genes implicated in mitochondrial biogenesis, such as PGC1α, NRF1, and TFAM. These results suggest that FGF21 treatment induced lipolysis more effectively than it suppressed lipogenesis in goat adipocytes, and that mitochondrial biogenesis plays an important role in these cells.
Graphical abstract
Keywords: FGF21, Adipocyte, Lipolysis, Lipogenesis, Mitochondria
Introduction
Adipose tissue is the major storage site of triglycerides (TG), an important fuel source providing free fatty acids (FFA) and gluconeogenic carbons in the form of glycerol. With escalating obesity and diabetes in current populations the adipose tissue in dietary sources is a concern (Lasa et al. 2012). Compared to other livestock such as pigs, cattle, and sheep, goats deposit less subcutaneous and intramuscular fat (Webb et al. 2005). Hence, goat meat appeals to clients as a source of lean meat, especially in developed countries with a high incidence of cardiovascular disease. Therefore, insights into the molecular machinery of goat lipid metabolism may contribute to our understanding of fat-related diseases and meat quality of domestic animals.
Fibroblast growth factor 21 (FGF21) specifically regulates glucose and lipid metabolism (Coskun et al. 2008; Emanuelli et al. 2014), and therefore plays critical role in energy homeostasis in mammals (Kharitonenkov et al. 2005). FGF21 is predominantly enriched in energy regulating organs like skeletal muscle and adipose tissue (Kharitonenkov and Larsen 2011; Murata et al. 2011). Increasing FGF21 expression elevates lipolysis in adipose tissue and plasma levels of FFAs during fasting (Hotta et al. 2009). Mild obesity and adipocyte hypertrophy were discovered in KO-FGF21 mice (Badman et al. 2009; Hotta et al. 2009). Many metabolic disorders in rodents and nonhuman primates have been cured through recombinant FGF21 treatment (Xu et al. 2009). Additionally, FGF21 might be closely related to mitochondria function (Luo and McKeehan 2013), since skeletal muscle and brown adipose tissue are rich in mitochondria (Fisher et al. 2012; Ji et al. 2015).
Although studies on FGF21 have been carried out in model mammals such as human and mice, the biological function of the FGF21 gene in domestic animals is still limited. Here, we systematically illustrate the effects of FGF21 on the size of lipid droplets, gene expression in lipid metabolism (lipolysis and lipogenesis), and number of mitochondria within goat adipocytes. These results provide useful data on lipid metabolism in large-size mammals.
Materials and methods
Isolation of preadipocytes from fat tissue
Three healthy male Nanjiang Brown kids (3d old) were provided by the Nanjiang Brown Goat Breeding Center (Nanjiang, Sichuan, China). Subcutaneous adipose tissue was collected from beneath back skin and rinsed with phosphate buffered saline (PBS medium supplemented with 50 U/mL penicillin and streptomycin; Hyclone, Logan, UT, USA). Tissue was then sliced into approximately 1-mm3 sections under sterile conditions and digested with type I collagenase for about 60–90 min at 37 °C with constant agitation. The digested tissue was filtered through a 200 μm nylon mesh screen to separate cells from undigested tissue fragments and debris and then centrifuged at 1400 ×g for 5 min. The cells were centrifuged 1400 ×g for 5 min, counted using a hemocytometer, resuspended in DMEM-F12 medium (Hyclone) supplemented with 10% (v/v) fetal bovine serum (Gibco, Mulgrave, VIC, Australia).
Preadipocyte culturing
In order to normalize the growth of goat preadipocytes, we first studied the differentiation of goat adipocytes. Preadipocytes were cultured under humidified air containing 5% CO2 at 37 °C. The medium was changed every second day. The cells became confluent after 48 h (day 2), differentiation into adipocytes was initiated as follows: medium was supplemented with 0.5 mM 3-isobuty-methylxanthine (MIX; Sigma St. Louis, MO, USA), 1 μM dexamethasone (Sigma), and 10 μg/mL insulin (Sigma) for 2 days. Then the culture medium was replaced with DMEM-F12 supplemented with 10% fetal bovine serum and 10 µg/mL insulin for another 2 days. Thereafter, the cells were cultured in DMEM-F12 medium containing only 10% FBS.
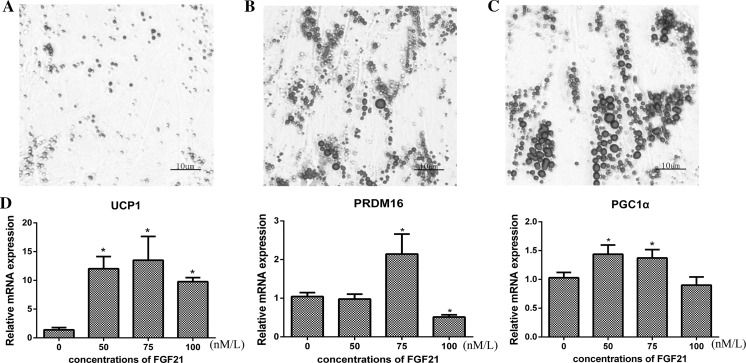
On day 4, the adipocytes harbored a few obvious lipid droplets (Fig. 1a), and on day 8 a large number and larger sized lipid droplets arose (Fig. 1b). On day 12 approximately 90% of the adipocytes differentiated (Fig. 1c). These results imply that after about 12 days of culture, the preadipocytes matured into adipocytes.
Fig. 1.
Morphologic characteristics of goat preadipocytes: Goat preadipocytes were stained with oil red O solution, visualized by light microscopy, and photographed. Preadipocytes were stained with oil red O after differentiation on day 4 a and day 8 b. c Preadipocytes differentiated into adipocytes contained lipid droplets in the cytoplasm. Adipocytes were stained with oil red O. d The gene expression of UCP1, PRDM16 and PGC1α were detected by qRT- PCR (SEM, n = 3, * p < 0.05). (Color figure online)
FGF21 treatment and cell harvesting
To achieve optimal experimental conditions, we based our protocol on previous studies involving preadipocyte culture as well as research on FGF21 that was reported in human cells (Fisher et al. 2012). We added recombinant human FGF21 protein (PROSPEC, Rehovot, Israel) to mature adipocytes (day 12) with final concentrations of 0 nM, 50 nM, 75 nM and100 nM and then harvested cells 24 h later. The mRNA levels of PGC1α, PRDM16, and UCP1 increased in adipocytes treated with FGF21of 75 nM (Fig. 1d).
Therefore, in further studies, goat adipocytes cultured 12 days were treated with FGF21 at final concentration of 75 nM. The adipocytes were harvested to extract RNA and DNA or analyzed histochemically, at 24, 36 and 48 h.
RNA and DNA extraction and cDNA synthesis
Total RNA (TRIzol reagent, Invitrogen, Carlsbad CA, USA) and genomic DNA (TIANGEN, Beijing. China) were extracted using the manufacturer’s protocol. Their concentration and quality were further assessed using the NanoDrop spectrophotometer (Bio-Rad, Benicia, CA, USA). cDNA was synthesized from 1 ug of total RNA using the PrimeScript RT reagent Kit with oligo-dT primers (Takara, Dalian, China).
Lipid droplet staining
Cytoplasmic lipid droplets were stained using oil red O (Sigma). Generally, adipocytes were fixed with 4% formalin in phosphate buffer for 1 h at room temperature and stained for 30 min with a filtered 60% (w/v) oil red O solution in distilled water. Cells were washed with distilled water and then visualized and photographed using light microscopy.
The cytoplasmic lipid droplets were analyzed using a phase-contrast microscope and Image-Pro Plus software from Media Cybernetics (Carlsbad, CA, USA). Stained adipocytes were scanned using × 40 objective magnifications. A field at objective magnification was chosen for analysis.
Fluorescent mitochondrial visualization
MitoTracker Green FM (Invitrogen, Carlsbad CA, USA) was used to evaluate mitochondrial number in adipocytes. The cells were incubated with 150 nM MitoTracker at 37 °C for 30 min and washed 3 times with prewarmed PBS. Mitochondria were quantified using fluorescence microscopy and Image-Pro Plus software from Media Cybernetics (Carlsbad, CA, USA).
Cloning of goat FGF21 CDS
The primers for goat FGF21 were designed according to its predicted cDNA sequence (GenBank accession no.XM_005692688.1) (Table 1) and synthesized by the Tsing Ke Biotechnology Company (Chengdu, China). PCR was performed using 12.5 μL 2 × Taq PCR master mix (TIANGEN, Beijing, China), 1 μL each of forward and reverse primers (final 10 μM), 2 μL cDNA and 8.5 μL sterile water. Conditions for PCR were 5 min at 95 °C, followed by 35 cycles of denaturation at 94 °C for 30 s, 67 °C (annealing temperature) for 30 s, and 72 °C for 1 min, and final extension at 72 °C for 7 min. The PCR products were purified, cloned (pMD 19-T vector, Takara, Dalian, China), and then sequenced by Tsing Ke Biotechnology Company (Chengdu, China).
Table 1.
Specific primers used in this study
| Gene | Sequences 5′ to 3′ |
|---|---|
| FGF21 | Forwarda: ATGGGCTGGGACGAGGCCAAGTTCAAG Reverse: TCAAGAAGTGTAGCTGGGGCTTCGG |
| FGF21 | Forward: ACTGTGGGTCCCTGTGCTG Reverse: GGGCTGGAGTCTGGAATGG |
| ATGL | Forward: GGAGCTTATCCAGGCCAATG Reverse: TGCGGGCAGATGTCACTCT |
| HSL | Forward: GCCAACACCCAGGAAGAAA Reverse: GAATGCCCGCAAAGACG |
| ACC | Forward: AAGGTTATGTGAAGGATGTGGATGA Reverse: CAATAATCTTCTGATGCCTGCGTT |
| FAS | Forward: GGGCTCCACCACCGTGTTCCA Reverse: GCTCTGCTGGGCCTGCAGCTG |
| SREBP1 | Forward: CTGCTGACCGACATAGAAGACAT Reverse: GTAGGGCGGGTCAAACAGG |
| C/EBPα | Forward: CCGTGGACAAGAACAGCAA Reverse: GGCGGTCATTGTCACTGG |
| PPARγ | Forward: GTGTCACTCCTGAACGAAAT Reverse: GGAAATGCTGGAGAAGTCAA |
| PGC1α | Forward: AAGCCAACCAAGATAACC Reverse: TACAACTCAGACTGCTCGGG |
| FIS1 | Forward: ACAGAGCCGCAGAACAAC Reverse: CACAGCAAGTCCGATGAGT |
| OPA1 | Forward: AGGGTTGTTGTGGTTGG Reverse: CCGCATTCGGAGTTCTA |
| Mfn1 | Forward: CGGGCACAGATGTCACTA Reverse: GGCTTGGAAAGTCGCTCA |
| Mfn2 | Forward: CGTGGTCCTCAAGGTTTACA Reverse: TCCATTTGACTCCGCACA |
| NRF1 | Forward: TCCAAACCCAACCCTGTC Reverse: GGGATAAATGCCCGAAGC |
| TFAM | Forward: TGGCACATCACAGGTAAA Reverse: GTTCCTCCCAGGATTTCA |
| UCP1 | Forward: ATCTCCACGGTCCCAAAC Reverse: ACAACAGCGGACACGAAG |
| GCG | Forward: GCTGCTGCTTTGTCTGC Reverse: GCTTCGGGTTCTGGATT |
| COX2 | Forward: GTAGAAACGGTCTGAACT Reverse: TTTACTGTGAGGGATGG |
| HSP-90 | Forward: GCCCGAGATAGAAGACGTTG Reverse: AGTCGTTGGTCAGGCTCTTG |
| ALAS | Forward: ATGTGGCCCACGAGTTTGG Reverse: CTTGTGCTGGCGATGTACC |
| HMBS | Forward: CTTGCCAGAGAAGAGTGTGG Reverse: CAGCCGTGTGTTGAGGTTTC |
aThe primer used for real-time PCR of FGF21 cloning and other primers used for qRT-PCR
Quantifying the mRNA levels of target genes
The qRT-PCR was performed with a total volume of 10 μL containing 5 μL 2 × SYBR Premix ExTaqII, 0.4 μL each primer (10 μM) and 0.8 μL diluted cDNA. The PCR conditions were as follows: 95 °C for 30 s; 40 cycles of 94 °C for 15 s, primer specific Tm for 30 s, and 72 °C for 30 s. HSP-90, ALAS and HMBS were used as internal control genes (Najafpanah et al. 2013). Primer sequences were listed in Table 1.
Investigating mitochondrial DNA copy number
The copy number of mitochondrial DNA (mtDNA) per adipocyte was assessed using real-time qPCR. The amount of COX2 and GCG, single copy gene in mitochondrial and nuclear DNA, respectively, was analyzed. PCR conditions were similar to qPCR described previously except using DNA as template. The ratio of COX2 to GCG was used to quantify mtDNA molecules in each adipocyte.
Statistical analyses
The expression levels of target genes were calculated using 2−ΔΔCt methods (Livak and Schmittgen 2001). Values between treatment and control group at each time point were compared via unpaired t test in IBM SPSS version 22. Data are presented as mean ± SE. P < 0.05 was considered significant.
Results
Characteristics of goat FGF21
The goat FGF21 CDS sequenced here was the same as the predicted nucleotide sequence of FGF21 in the NCBI database (XM_005692688.1). It contained an open reading frame (ORF) of 630 base pairs and encoded 209 amino acids. The putative goat FGF21 protein shared 99.04, 66.53, 75.24, 91.39 and 85.71% identity with that of cow (XP_010813507.1), sheep (XP_011950505.2), mouse (AAH49592.1), pig (ACS34770.1), and human (AAH18404.1), respectively.
In addition, goat and human FGF domains (126 residues from Gln43 to Pro168) were highly conserved, retaining 86.51% identity. They had almost the same receptor interaction binding sites (a conserved 17 residue segment) and similar heparin binding sites (glycine box) (Fig. 2). Therefore, it is reasonable to use human FGF21 in this study.
Fig. 2.
Alignment of goat and human FGF21 amino acid sequences. The domain structure was analyzed by the online software SMART. Gray blocks represent the protein domain of FGF21, red and blue blocks describe the receptor interaction binding sites and heparin binding sites of goat and human, respectively. (Color figure online)
FGF21 decreased the size of lipid droplets in goat adipocytes
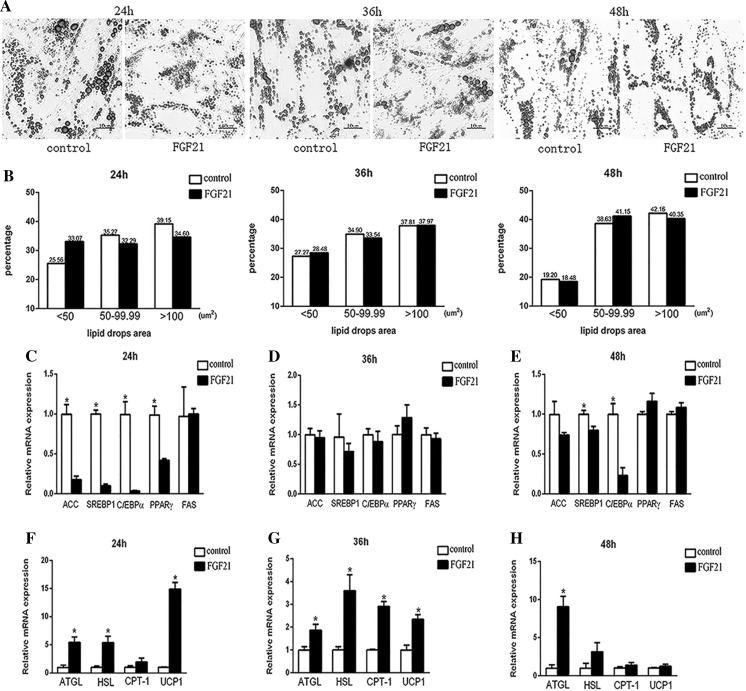
Generally, adipocytes displayed significantly smaller lipid droplets following FGF21 treatment (Fig. 3a). 24 h after FGF21 treatment, small lipid droplets (< 50 um2) increased by 8% (FGF21 treatment, 33.07%, n = 309; control, 25.57%, n = 257; P = 0.026), while medium (50–99.9 um2) and large (> 100 um2) droplets decreased by 3% (FGF21 treatment, 32.29%, n = 316; control, 35.27%, n = 275; P = 0.044) and 5% (FGF21 treatment, 34.63%, n = 503; control, 39.16%, n = 453; P = 0.074), respectively. At 36 h the percentage of large lipid droplets increased slightly, accompanied by a slight decrease of small droplets. Furthermore, the percentage of both medium and large size lipid droplets increased whereas small droplets declined dramatically at 48 h after FGF21 treatment (Fig. 3b).
Fig. 3.
Effects of FGF21 on lipid droplet variation and gene expression pattern of adipogenesis and lipolysis in goat adipocytes. a Completely differentiated goat adipocytes were treated with FGF21. Untreated cells were stained with oil red O solution, visualized by a light microscope, and photographed after 24 h, 36 h and 48 h. b The sizes of lipid droplets were measured and quantified from the image of cells stained with oil red O. The staining of lipid droplets was analyzed using the Image-Pro Plus system. Stained adipocytes were scanned using × 400 objective magnification. Relative mRNA expression levels of SREBP1, PPARγ, C/EBPα, ACC and FAS in goat adipocytes treated with FGF21 and control shown at 24 h c, 36 h d and 48 h e. The mRNA levels of genes involved in lipolysis as determined by qRT-PCR at 24 h (f), 36 h g and 48 h h (SEM, n = 3, * p < 0.05). (Color figure online)
FGF21 inhibited lipogenesis and simultaneously stimulated lipolysis in goat adipocytes
Lipid amount demonstrates the dynamic equilibrium of fat deposit and expenditure. Obviously, FGF21 treatment dramatically suppressed the expression of SREBP1, PPARγ, C/EBPα and ACC at 24 h (Fig. 3c; P < 0.05). While at 36 and 48 h, mRNA levels of ACC, SREBP1 and C/EBPα still decreased with FGF21 treatment (Figs. 2e, 3d; P > 0.05), PPARγ mRNA levels increased slightly (P > 0.05). Nevertheless, FAS expression was unaffected by FGF21 treatment throughout the experiment (P > 0.05).
Meanwhile, FGF21 treatment increased the levels of ATGL, HSL, CPT-1 and UCP1 mRNAs by 2–15 fold at 24 h (Fig. 3f; P < 0.05) and by 2–3.5 fold at 36 h (Fig. 3g; P < 0.05), but at 48 h only ATGL mRNA levels were significantly elevated (Fig. 3h; P < 0.05). The expression levels of UCP1, the most known lipolysis gene induced by FGF21, were decreased temporally.
Comparatively, FGF21 affected lipolysis genes more efficiently than lipogenesis genes since at 36 h the expression of the four genes involving in lipolysis still differed significantly (P < 0.05). Intriguingly, at 48 h ATGL mRNA was still consistently elevated, but accompanied by significantly downregulated expression of SREBP1 and C/EBPα.
FGF21 induced mitochondria number and function in goat adipocyte
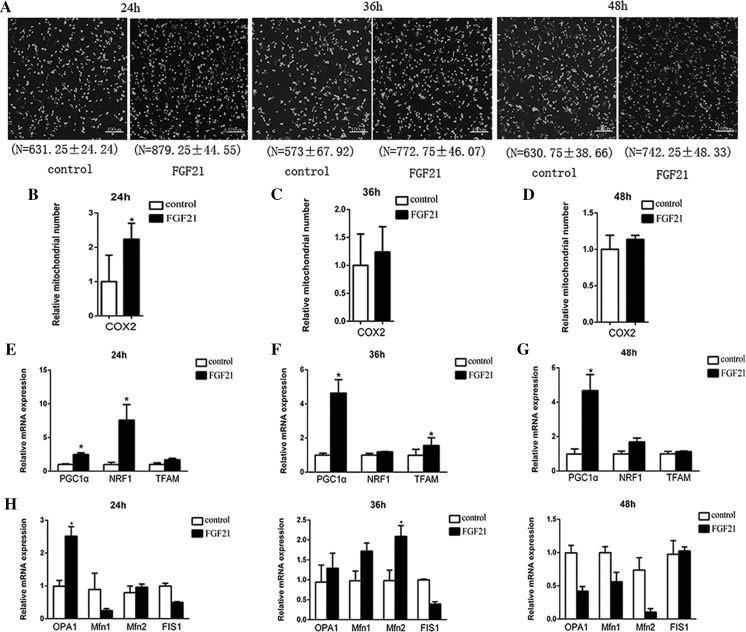
Generally, mitochondrial number increased throughout the investigation in cells treated with FGF21 (Fig. 4a), and simultaneously mtDNA copy number increased especially at 24 h (Fig. 4b–d). Correspondingly, expression of PGC1α, NRF1, and TFAM, which are involved in mitochondria biogenesis, were upregulated in adipocytes. Notably, PGC1α exhibited significantly higher mRNA levels in treated adipocytes throughout the investigation (P < 0.05), whereas expression levels of NRF1 and TFAM only significantly increased at 24 h and 36 h (P < 0.05; Fig. 4e–g). As for genes involved in mitochondrial fusion (OPA1, Mfn1 and Mfn2) and fission (FIS1), FGF21 treatment increased mRNA levels of OPA1 and Mfn2 at 24 and 36 h, respectively (P < 0.05), but expression of FIS1 was almost unaffected (P > 0.05; Fig. 4h). These results confirm that FGF21 mainly enhances mitochondrial biogenesis.
Fig. 4.
Mitochondrial DNA copy number and mitochondrial-biogenesis-related gene expression in goat adipocytes treated with 75 nM FGF21. a Mitochondrial DNA copy number. The goat adipocytes treated with FGF21 and control cells were stained with Mito Tracker green at 24, 36 and 48 h, and then imaged using a fluorescence microscope (× 40 magnification, n = 3). The cellular mtDNA content was assessed by qRT-PCR analysis with primers designed to target COX2 and GCG as a reference gene at 24 h b, 36 h c and 48 h d (n = 3). The ratio of COX2 to GCG (Batista et al. 2014) reflects the number of mitochondria per adipocyte. The mRNA levels of PGC1α, NRF1, and TFAM were analyzed using qRT-PCR and normalized to Hsp-90, ALAS and HMBS levels at 24 h e, 36 h f and 48 h g (n = 3, *P < 0.05). h: Treatment with FGF21, relative mRNA expression levels of OPA1, Mfn1, Mfn2 and FIS1 at 24h, 36h, and 48h
Discussion
FGF21 has been described as a hormone with a potential role in lipolysis in adipocytes. It has been shown that FGF21 transgenic mice cause lipolysis of mature adipocytes (Inagaki et al. 2007), and FGF21 protein stimulates lipolysis in cultured 3T3-L1 adipocytes (Chen et al. 2011). Our work found that FGF21 decreased the size of lipid droplets in goat adipocytes even at 48 h after treatment. This corresponded to elevated mRNA levels for ATGL, HSL, CPT-1 and UCP1 genes, which are critical in lipolysis (Dong et al. 2013; Walther and Farese 2012). These results confirm the potential role of FGF21 in adipocyte lipolysis.
Among the lipolysis genes investigated, ATGL maintained higher expression levels throughout the investigation. ATGL is the rate-limiting enzyme for lipolysis, specific for initiation of triacylglycerol hydrolysis (Ong et al. 2013), and highly expressed in adipose tissue (Wang et al. 2011). ATGL-Ad-infected 3T3-L1 cells and MEF (mouse embryonic fibroblasts) adipocytes have smaller lipid droplets (Miyoshi et al. 2008), which is consistent with the lipid droplet morphology observed in FGF21-treated goat adipocytes in our study. In addition, ATGL-mediated lipolysis controls the expression of UCP1 by generating ligands or precursor ligands for nuclear receptors (Hondares et al. 2011). ATGL and HSL coordinately catabolize stored TG (Wang et al. 2013), as well. These data indicate that an increase in ATGL plays key role in lipolysis induced by FGF21.
Lipolysis genes were mostly affected by FGF21 treatment, and this behavior also closely relates to mitochondrial function. For example, ATGL-mediated lipolysis controls mitochondrial oxidative phosphorylation (Dimova et al. 2012); CPT-1 accelerates the long-chain acetyl-CoA entry into mitochondria (Morris et al. 2012), which contain the primary sites for the tricarboxylic acid cycle and beta oxidation of fatty acids (Alaynick 2008). We found that FGF21 enhanced mitochondrial number, mtDNA copy number, and mRNA levels of mitochondrial biogenesis genes including PGC1α, TFAM and NRF1. Specifically, PGC1α was induced throughout all three stages investigated.
PGC1α is a key factor of mitochondrial biogenesis. As a positive regulator of mitochondrial biogenesis and oxidative metabolism (Handschin and Spiegelman 2006), PGC1α expression increased in brown adipocytes and muscle after cold exposure, hyperphagia, or adrenaline (Harms and Seale 2013; Sparks et al. 2005). A previous study demonstrated that FGF21can increase PGC1α expression in human adipocytes (Chau et al. 2010). Our study also demonstrated that PGC1α was upregulated in the presence of FGF21.We also observed that FGF21 enhanced the expression of NRF1 and TFAM. NRF1 is an intermediate transcription factor, which stimulates the synthesis of TFAM which is a final effector activating the duplication of mtDNA (Vina et al. 2009). Interestingly, overexpression of PGC1α protein can stimulate genes including NRF1 and TFAM (Doerks et al. 2002). Additionally, UCP1 is located in downstream of PGC1α (Fedorenko et al. 2012), and UCP1 possesses mitochondrial oxidative capacity without concomitant synthesis of ATP (Flachs et al. 2013). The expression of UCP1 mRNA indicates that at early stages of FGF21 stimulation (especially 24 h), lipid heat expenditure is critical in lipolysis. Furthermore, FGF21 treatment only partly elevated expression of genes associated with mitochondrial fusion (OPA1 and Mfn2). These results imply that FGF21 elevates mitochondrial function mainly via mitochondrial biogenesis (including mtDNA copy number), in which PGC1α may play key roles.
Simultaneously, expression of adipogenesis-related genes including PPARγ, SREBP1, C/EBPα and ACC were downregulated dramatically by FGF21 treatment at 24 h. Adipogenesis is a process in which preadipocytes become adipocytes and begin lipid biosynthesis through conversion of acetyl-CoA into fatty acids and cholesterol (Shimano et al. 1999; Siersbaek et al. 2012). ACC is a key rate-limiting enzyme in de novo fatty acid synthesis (Postic and Girard 2008), while PPARγ directly affects the accumulation of intracellular triglycerides (Grimaldi et al. 2010; Walczak and Tontonoz 2002) and cells lacking PPARγ are unable to generate lipids even in the presence of an induction cocktail (Rosen et al. 1999). The dramatically downregulated ACC and PPARγ mRNA levels in FGF21-treated adipocytes at 24 h suggest that de novo lipid synthesis may be restricted. This effect could be the main co-contributor to the obvious small lipid droplets in adipocytes with lipolysis genes.
Intriguingly, expression of C/EBPα and SREBP1 dramatically decreased again at 48 h, after almost equal levels between control and treated adipocytes at 36 h. C/EBPα promotes transcription of genes specifically expressed in adipocytes (Karadeniz et al. 2011). C/EBPα and PPARγ cross-regulate the transcriptional pathway of adipogenesis (Wu et al. 1999). SREBP1 deficiency in 3T3-L1 cells strongly inhibits adipocyte differentiation and suppresses the expression of PPARγ, C/EBPα and FAS (Kim and Spiegelman 1996). Therefore, it is likely that C/EBPα and SREBP1 are the main factors in a substantial network of suppressed lipogenesis factors induced by FGF21 treatment at later stages, but further studies are required to address this issue.
In conclusion, FGF21 decreased lipid droplet size in goat adipocytes as long as 48 h post-treatment and elevated mitochondrial function mainly via mitochondrial biogenesis (including mtDNA copy number). We also found that ATGL and PGC1α may play key roles in this process. Although FGF21 treatment induced lipolysis more effectively, suppression of lipogenesis, including de novo lipid synthesis, co-contributed to the smaller lipid droplets in adipocytes at 24 h, and at later stages (48 h) C/EBPα and SREBP1, the main lipogenesis factors, are suppressed by FGF21 treatment. Interestingly, ATGL-mediated lipolysis plays a critical role in generating ligands or precursor ligands for nuclear receptors, such as, PPARα (Hondares et al. 2011). This suggests that lipolysis and lipogenesis are possibly coupled in a substantial network within FGF21 adipocytes, but further studies are required to address this issue.
Abbreviations
- FGF21
Fibroblast growth factor
- ATGL
Adipose triglyceride lipase
- HSL
Hormone sensitive lipase
- CPT-1
Carnitine palmitoyltransferase 1
- ACC
Acetyl-coa carboxylase
- FAS
Fatty acid synthase
- SREBP1
Sterol regulatory element binding protein 1
- C/EBPα
CCAAT/enhancer binding protein
- PPARγ
Peroxisome proliferator-activated receptor γ
- PGC1α
Peroxisome proliferator activated receptor-γ co-activator 1-α
- NRF1
Nuclear respiratory factors 1
- TFAM
Mitochondria transcription factor A
- UCP1
Uncoupling protein 1
- GCG
Glucagon gene
- COX2
Cytochrome c oxidase 2
- HSP-90
Heat shock protein 90
- ALAS
Aminolevulinate synthase
- HMBS
Hydroxyl methyl bilane synthase
- FIS1
Fission protein 1
- OPA1
Optic atrophy 1
- Mfn1
Mitochondria fusion protein 1
- Mfn2
Mitochondria fusion protein 2
Funding
This work was supported by Sichuan province science and technology support program (2014NZ0077, 2015NZ0112).
Conflict of interest
All the authors stated no conflict of interest.
Ethical approval
This work was approved by Institutional Animal Care and Use Ethics Committee of Sichuan Agriculture University and carried out in accordance with the “Guidelines for Experimental Animals” of the Ministry of Science and Technology (Beijing, PR China).
References
- Alaynick WA. Nuclear receptors, mitochondria and lipid metabolism. Mitochondrion. 2008;8:329–337. doi: 10.1016/j.mito.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badman MK, Koester A, Flier JS, Kharitonenkov A, Maratos-Flier E. Fibroblast growth factor 21-deficient mice demonstrate impaired adaptation to ketosis. Endocrinology. 2009;150:4931–4940. doi: 10.1210/en.2009-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista RITP, et al. Methodological strategies for transgene copy number quantification in goats (Capra hircus) using real-time. PCR Biotechnol Progr. 2014;30:1390–1400. doi: 10.1002/btpr.1946. [DOI] [PubMed] [Google Scholar]
- Chau MDL, Gao J, Yang Q, Wu Z, Gromada J. Fibroblast growth factor 21 regulates energy metabolism by activating the AMPK–SIRT1–PGC-1α pathway. Proc Natl Acad Sci USA. 2010;107:12553–12558. doi: 10.1073/pnas.1006962107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, et al. Growth hormone induces hepatic production of fibroblast growth factor 21 through a mechanism dependent on lipolysis in adipocytes. J Biol Chem. 2011;286:34559–34566. doi: 10.1074/jbc.M111.285965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coskun T, et al. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology. 2008;149:6018–6027. doi: 10.1210/en.2008-0816. [DOI] [PubMed] [Google Scholar]
- Dimova NV, et al. APC/C-mediated multiple monoubiquitylation provides an alternative degradation signal for cyclin B1. Nat Cell Biol. 2012;14:168–176. doi: 10.1038/ncb2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerks T, Copley RR, Schultz J, Ponting CP, Bork P. Systematic identification of novel protein domain families associated with nuclear functions. Genome Res. 2002;12:47–56. doi: 10.1101/gr.203201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong M, et al. Cold exposure promotes atherosclerotic plaque growth and instability via UCP1-dependent lipolysis. Cell Metab. 2013;18:118–129. doi: 10.1016/j.cmet.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuelli B, et al. Interplay between FGF21 and insulin action in the liver regulates metabolism. J Clin Invest. 2014;124:515–527. doi: 10.1172/JCI67353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko A, Lishko PV, Kirichok Y. Mechanism of fatty-acid-dependent UCP1 uncoupling in brown fat mitochondria. Cell. 2012;151:400–413. doi: 10.1016/j.cell.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher FM, et al. FGF21 regulates PGC-1alpha and browning of white adipose tissues in adaptive thermogenesis. Gene Dev. 2012;26:271–281. doi: 10.1101/gad.177857.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flachs P, Rossmeisl M, Kuda O, Kopecky J. Stimulation of mitochondrial oxidative capacity in white fat independent of UCP1: a key to lean phenotype. BBA Mol Cell Biol L. 2013;1831:986–1003. doi: 10.1016/j.bbalip.2013.02.003. [DOI] [PubMed] [Google Scholar]
- Grimaldi B, et al. PER2 controls lipid metabolism by direct regulation of PPARgamma. Cell Metab. 2010;12:509–520. doi: 10.1016/j.cmet.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handschin C, Spiegelman BM. Peroxisome proliferator-activated receptor gamma coactivator 1 coactivators, energy homeostasis, and metabolism. Endocr Rev. 2006;27:728–735. doi: 10.1210/er.2006-0037. [DOI] [PubMed] [Google Scholar]
- Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nat Med. 2013;19:1252–1263. doi: 10.1038/nm.3361. [DOI] [PubMed] [Google Scholar]
- Hondares E, et al. Peroxisome proliferator-activated receptor (PPAR) induces ppar coactivator 1 (PGC-1) gene expression and contributes to thermogenic activation of brown fat: involvement of PRDM16. J Biol Chem. 2011;286:43112–43122. doi: 10.1074/jbc.M111.252775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotta Y, et al. Fibroblast growth factor 21 regulates lipolysis in white adipose tissue but is not required for ketogenesis and triglyceride clearance in liver. Endocrinology. 2009;150:4625–4633. doi: 10.1210/en.2009-0119. [DOI] [PubMed] [Google Scholar]
- Inagaki T, et al. Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell Metab. 2007;5:415–425. doi: 10.1016/j.cmet.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Ji K, et al. Skeletal muscle increases FGF21 expression in mitochondrial disorders to compensate for energy metabolic insufficiency by activating the mTOR-YY1-PGC1alpha pathway. Free Radic Bio Med. 2015;84:161–170. doi: 10.1016/j.freeradbiomed.2015.03.020. [DOI] [PubMed] [Google Scholar]
- Karadeniz F, Karagozlu MZ, Pyun S-Y, Kim S-K. Sulfation of chitosan oligomers enhances their anti-adipogenic effect in 3T3-L1 adipocytes. Carbohydr Polym. 2011;86:666–671. doi: 10.1016/j.carbpol.2011.05.005. [DOI] [Google Scholar]
- Kharitonenkov A, Larsen P. FGF21 reloaded: challenges of a rapidly growing field. Trends Endocrin Met. 2011;22:81–86. doi: 10.1016/j.tem.2010.11.003. [DOI] [PubMed] [Google Scholar]
- Kharitonenkov A, et al. FGF-21 as a novel metabolic regulator. J Clin Invest. 2005;115:1627–1635. doi: 10.1172/JCI23606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JB, Spiegelman BM. ADD1/SREBP1 promotes adipocyte differentiation and gene expression linked to fatty acid metabolism. Gene Dev. 1996;10:1096–1107. doi: 10.1101/gad.10.9.1096. [DOI] [PubMed] [Google Scholar]
- Lasa A, Schweiger M, Kotzbeck P, Churruca I, Simon E, Zechner R, Portillo MP. Resveratrol regulates lipolysis via adipose triglyceride lipase. J Nutr Biochem. 2012;23:379–384. doi: 10.1016/j.jnutbio.2010.12.014. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2 − ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Luo Y, McKeehan WL. Stressed Liver and Muscle Call on Adipocytes with FGF21. Front Endocrinol. 2013 doi: 10.3389/fendo.2013.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi H, Perfield JW, 2nd, Obin MS, Greenberg AS. Adipose triglyceride lipase regulates basal lipolysis and lipid droplet size in adipocytes. J Cell Biochem. 2008;105:1430–1436. doi: 10.1002/jcb.21964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris EM, Meers GM, Booth FW, Fritsche KL, Hardin CD, Thyfault JP, Ibdah JA. PGC-1alpha overexpression results in increased hepatic fatty acid oxidation with reduced triacylglycerol accumulation and secretion. Am J Physiol Gastr L. 2012;303:G979–G992. doi: 10.1152/ajpgi.00169.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata Y, Konishi M, Itoh N. FGF21 as an Endocrine regulator in lipid metabolism: from molecular evolution to physiology and pathophysiology. Int J Sport Nutr Exe. 2011;2011:981315. doi: 10.1155/2011/981315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najafpanah MJ, Sadeghi M, Bakhtiarizadeh MR. Reference genes selection for quantitative real-time PCR using rankaggreg method in different tissues of capra hircus. PLoS ONE. 2013 doi: 10.1371/journal.pone.0083041.t001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong KT, Mashek MT, Bu SY, Mashek DG. Hepatic ATGL knockdown uncouples glucose intolerance from liver TAG accumulation. Faseb J. 2013;27:313–321. doi: 10.1096/fj.12-213454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postic C, Girard J. Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: lessons from genetically engineered mice. J Clin Invest. 2008;118:829–838. doi: 10.1172/JCI34275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen ED, et al. PPARγ is required for the differentiation of adipose tissue in vivo and in vitro. Mol Cell. 1999;4:611–617. doi: 10.1016/S1097-2765(00)80211-7. [DOI] [PubMed] [Google Scholar]
- Shimano H, et al. Sterol Regulatory element-binding protein-1 as a key transcription factor for nutritional induction of lipogenic enzyme genes. J Biol Chem. 1999;274:35832–35839. doi: 10.1074/jbc.274.50.35832. [DOI] [PubMed] [Google Scholar]
- Siersbaek R, Nielsen R, Mandrup S. Transcriptional networks and chromatin remodeling controlling adipogenesis. Trends Endocrin Met. 2012;23:56–64. doi: 10.1016/j.tem.2011.10.001. [DOI] [PubMed] [Google Scholar]
- Sparks LM, Xie H, Koza RA, Mynatt R, Hulver MW, Bray GA, Smith SR. A high-fat diet coordinately downregulates genes required for mitochondrial oxidative phosphorylation in skeletal muscle. Diabetes. 2005;54:1926–1933. doi: 10.2337/diabetes.54.7.1926. [DOI] [PubMed] [Google Scholar]
- Vina J, Gomez-Cabrera MC, Borras C, Froio T, Sanchis-Gomar F, Martinez-Bello VE, Pallardo FV. Mitochondrial biogenesis in exercise and in ageing. Adv Drug Deliver Rev. 2009;61:1369–1374. doi: 10.1016/j.addr.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Walczak R, Tontonoz P. PPARadigms and PPARadoxes: expanding roles for PPARγ in the control of lipid metabolism. J Lipid Res. 2002;43:177–186. [PubMed] [Google Scholar]
- Walther TC, Farese RV., Jr Lipid droplets and cellular lipid metabolism. Annu Rev Biochem. 2012;81:687–714. doi: 10.1146/annurev-biochem-061009-102430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, et al. Unique regulation of adipose triglyceride lipase (ATGL) by perilipin 5, a lipid droplet-associated protein. J Biol Chem. 2011;286:15707–15715. doi: 10.1074/jbc.A110.207779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang PP, She MH, He PP, Chen WJ, Laudon M, Xu XX, Yin WD. Piromelatine decreases triglyceride accumulation in insulin resistant 3T3-L1 adipocytes: role of ATGL and HSL. Biochimie. 2013;95:1650–1654. doi: 10.1016/j.biochi.2013.05.005. [DOI] [PubMed] [Google Scholar]
- Webb EC, Casey NH, Simela L. Goat meat quality. Small Ruminant Res. 2005;60:153–166. doi: 10.1016/j.smallrumres.2005.06.009. [DOI] [Google Scholar]
- Wu Z, et al. Cross-regulation of C/EBPα and PPARγ controls the transcriptional pathway of adipogenesis and insulin sensitivity. Mol Cell. 1999;3:151–158. doi: 10.1016/S1097-2765(00)80306-8. [DOI] [PubMed] [Google Scholar]
- Xu J, et al. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes. 2009;58:250–259. doi: 10.2337/db08-0392. [DOI] [PMC free article] [PubMed] [Google Scholar]