Abstract
To explore the role of ribosomal protein S15A (RPS15A) in breast cancer. The Oncomine database was used to compare the expression of RPS15A in human breast cancer tissues and normal tissues. RPS15A in breast cancer cell line ZR-75-30 and BT474 was specifically knocked down using lentivirus-mediated short hairpin RNAs (shRNAs). RPS15A knockdown efficiency was validated by quantitative polymerase chain reaction and western blot analysis. Subsequently, the functional effects of RPS15A on proliferation of breast cancer cells were investigated by MTT, colony formation and flow cytometry assays. Functional analysis indicated that RPS15A knockdown could inhibit cell proliferation, induced cell cycle arrest and apoptosis. Mechanism analysis revealed RPS15A mediated apoptosis via activating of caspase-3 and PARP cleavage, upregulating of Bad and BAX and downregulating of Bcl-2. Our preliminary study highlighted the importance of RPS15A in breast cancer growth. The inhibition of RPS15A may be a promising therapeutic target for breast cancer treatment.
Electronic supplementary material
The online version of this article (10.1007/s10616-018-0221-9) contains supplementary material, which is available to authorized users.
Keywords: Breast cancer, PRS15A, Cell proliferation, Apoptosis
Introduction
Breast cancer is one of the major causes of cancer death among women worldwide with high incidence and death rates (Zhou et al. 2014). Currently, despite of several advanced therapeutics, such as surgery, hormone therapy and biological therapy (Ottolini et al. 2013), the morbidity and mortality of breast cancer are still high (Karagianni et al. 2014). Recently, seeking novel molecular targets has been one of the most popular issues in improving the efficacy and reducing side effects in the treatment of breast cancer (Zhang et al. 2015).
To our best knowledge, cancer occurrence can be driven by mutations in cancer suppressed genes and oncogenes and presented as uncontrolled protein synthesis in the procedure of cell proliferation. Ribosomes, consisting of a large 60S subunit and a small 40S subunit, have been demonstrated to be responsible for catalyzing protein synthesis. Moreover, ribosome biogenesis plays an important role in the initiation and progression of cancer (Ruggero and Pandolfi 2003). Ribosomal protein S15A (RPS15A), as a highly conserved housekeeping gene, encodes a component of 40S ribosomes subunits. At early stages of translation, RPS15A has been shown to promote capped mRNA to bind small ribosomal subunit and modulate ribosome assembly (Jimenez et al. 2004; Shen et al. 2005). At present, accumulated evidences have indicated that RPS15A is abundantly expressed in plants (Bonham-Smith and Moloney 1994), yeast (Lavoie et al. 1994), and zebrafish (Amsterdam et al. 2004). Moreover, it has been reported that PRS15A is upregulated in various human cancers, including colorectal cancer, prostate cancer and astrocytoma (Kavak et al. 2010). Notably, downregulation of RPS15A has been demonstrated to suppress cell proliferation and arrest cell cycle in hepatic cancer (Maiyu et al. 2014), lung cancer (Zhao et al. 2015) and osteosarcoma cells (Zhang et al. 2014b) in vitro. Oncogene c-Myc plays a crucial role in the development of breast cancer (Zhang et al. 2014a) and can upregulate RPS15A expression levels (Zeller et al. 2003). Based on these evidences, we hypothesized RPS15A may be a novel molecular target for the treatment of breast cancer.
To investigate whether RPS15A could regulate cell growth in the breast cancer cells, the expression of RPS15A was analyzed using related Oncomine data. In addition, the expression of RPS15A was specifically knocked down in human breast cancer cell lines ZR-75-30 and BT474 to explore the biological effects of RPS15A on breast cancer cells and the underlying potential mechanisms.
Materials and methods
Analysis of Oncomine data
Richardson and Curtis in Oncomine database (http://www.oncomine.com) were used to analyze the expression of RPS15A in human breast cancer. The comparison between RPS15A gene expression in breast cancer tissues and normal breast tissues was conducted following the standard procedures as previously described (Rhodes et al. 2007).
Knockdown of RPS15A with specific short hairpin RNAs (shRNAs)
Two target sequence sites for RPS15A (NM_001019) siRNAs (S1: 5′-GTGCAACTCAAAGACCTGGAA′ and S2: 5′-GCATGGTTACATTGGCGAATT′) plus one scrambled sequence (5′-TTCTCCGAACGTGTCACGT-3′) were synthesized and cloned into the NheI/PacI–linearized pFH-L vector (Shanghai Hollybio, Shanghai, China) carrying a green fluorescent protein (GFP) tag. DNA sequencing was performed to confirm lentiviral based shRNA expressing vectors. Generated plasmids were termed as shRPS15A (S1), shRPS15A (S2) and shCon.
Cell culture
Human breast cancer cell lines ZR-75-30 and BT474, as well as embryonic kidney cell 293T (HEK293) were obtained from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). ZR-75-30 and BT474 were cultured in RPMI-1640 (GE Healthcare, Chicago, IL, USA) with 10% fetal bovine serum (FBS; Biowest, Riverside, MO, USA) in atmosphere of 5% CO2 at 37 °C. HEK293 cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM; Hyclone, Logan, UT, USA) with 10% FBS at a temperature of 37 °C in the atmosphere of 5% CO2.
Construction of lentiviral particles and cell transfection
Lentiviral particles were generated by transfecting shRPS15A (S1), shRPS15A (S2) and shCon into 293T cells with packing plasmids pVSVG-I and pCMV△R8.92 (Shanghai Hollybio) according to the manufacturer’s instructions. Subsequently, ZR-75-30 and BT474 cells were seeded into six-well plates and transfected with lentiviral particles, including shRPS15A (S1), shRPS15A (S2) and shCon, respectively. The infection efficiency was measured through counting GFP positive cells under a fluorescence microscopy after 96 h infection.
Quantitative polymerase chain reaction (qPCR) analysis
Total RNA was extracted from breast cancer cells after 5 days infection using Trizol reagent (Invitrogen, Carlsbad, CA, USA) and the first strand of complementary DNA (cDNA) was synthesized according to the manufacturer’s instructions (Promega, Madison, WI, USA). PCR primers were RPS15A-forward (5′-TGACGTGCAACTCAAAGACC-3′), RPS15A-reverse (5′-CCAGAGTCCATGAGGCATTT-3′), actin-forward (5′-GTGGACATCCGCAAAGAC-3′) and actin-reverse (5′-AAAGGGTGTAACGCAACTA-3′). The qPCR was performed to assess the RPS15A mRNA expression on BioRad (Hercules, CA, USA) Connet Real-Time PCR platform using 20 µl reaction mixtures containing 10 µl 2 × SYBR premix ex taq (TAKARA, Beijing, China), 0.5 µl primers (2.5 µM), 5 µl cDNA and 4.5 ddH2O following the amplified procedure: initial denaturation at 95 °C for 1 min and 40 cycles containing 5 s denaturation at 95 °C and 20 s annealing extension at 60 °C. The 2−ΔΔCt method was used to calculate relative quantification of mRNA expression (Livak and Schmittgen 2001).
Western blot analysis
Cells were washed with PBS and then collected after 6 days of infection. Then the cells were lysed using 2X SDS Sample Buffer (10 mM EDTA, 100 mM Tris–Hcl (pH 6.8), 4% SDS and 10% glycine) for 30 min. After the centrifugation at 13,000×g and collection of the supernatants, bicinchoninic acid (BCA; Beyotime, Shanghai, China) protein assay kit was used to determine the protein concentration. 30 µg proteins were separated on 10% polyacrylamide-sodium dodecyl sulfate (SDS-PAGE). The gel was transferred onto polyvinylidene fluoride (PVDF) membranes and the membranes were blocked with 5% nonfat milk. Then the membranes were probed with diluted primary antibodies overnight at 4 °C and incubated with horseradish peroxidase-conjugated secondary antibodies (1:5000, Santa Cruz Biotechnology, Santa Cruz, CA, USA, SC-2054) for 2 h at room temperature. ECL kit (Pierce, Waltham, MA, USA) was used to develop the immunoblots according to the manufacturer’s instruction. The primary antibodies were rabbit anti-RPS15A (1:1000, Ab175054, Abcam, Cambridge, MA, USA), rabbit anti-caspase-3 (1:1000, 19677-1-AP, Proteintech, Rosemont, IL, USA), rabbit anti-cleavage caspase-3 (1:500, #9661, Cell signaling, Beverly, MA, USA), rabbit anti-PARP (1:1000, #9542, Cell signaling) and rabbit anti-GAPDH (1:500000, 10494-1-AP, Proteintech).
MTT analysis for cell proliferation
Cells from three groups (shRPS15A (S1), shRPS15A (S2) and shCon) were seeded in 96 well plates at a density of 2000 cells per well 90 h after transduction. Then 20 µl 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT; 5 mg/ml) was added in each well at different time points after infection (1, 2, 3, 4 and 5 days). After incubation for 4 h at 37 °C, 100 µl acidic isopropanol (10% SDS, 5% isopropanol and 0.01 mol/L HCl) was added to each well. In each assay, the absorbance at 595 nm was detected using a spectrophotometer.
Colony formation assay
Cells from shRPS15A (S1) and shCon groups were seeded in six well plates at a density of 400 cells per well. 120 h after transduction, the cells were cultured to develop to natural monolayer colonies for 5 days. Crystal purple staining method was used to visualize the colonies according to previous description (Yu 2014). Then the colonies containing more than 50 cells were photographed and the number of them were recorded under a fluorescence microscopy.
Cell cycle analysis
Cells from three groups (shRPS15A (S1), shRPS15A (S2) and shCon) were cultured at a density of 6.0 × 104 cells per dish for 96 h 4-day after transduction. Subsequently, cells were collected at 80% confluence and washed with ice-cold PBS, and then stained with propidium iodide (PI) according to the cell cycle kit instructions (Brea, CA, USA). The PI uptake was analyzed using the FC500 flow cytometry systems (Beckman Coulter, Brea, CA, USA).
Flow cytometric apoptosis assay
Cells from the shRPS15A (S1) and shCon groups were collected and washed with PBS 7 day after transduction. Subsequently, cells (6.0 × 104 cells per dish) were stained using the Annexin V-APC/7-AAD detection kit according to the manufacturer’s protocol (eBioscience, San Diego, CA, USA). The percentage of apoptotic cells, including early and late apoptotic cells was determined using a FACS Calibur flow cytometer (BD, Franklin Lakes, NJ, USA).
Statistical analysis
Data were analyzed using the SPSS 19.0 software package and presented as mean ± standard deviation (SD) of three independent experiments. T test was used to conduct statistical comparisons and a two-sided P values < 0.05 were considered significant.
Results
Oncomine data
To investigate the relationship between RPS15A expression and tumor progression, we used Oncomine microarray data to analyze RPS15A gene expression in human breast cancer. In Richardson Breast, RPS15A expression in ductal breast carcinoma tissue was significant increased compared with normal breast tissues (Figure 1 in supplemental figures, P < 0.01). In Curtis Breast, we found RPS15A in 4 datasets has higher expression, including Breast phyllodes tumor, Benign breast Neoplasm, Tubular Breast Carcinoma and Invasive Ductal and invasive Lobular Breast carcinomas (Figure 1 in supplemental figures, P < 0.01). Both results indicated that RPS15A was upregulated in Ductal Breast carcinoma. Hence, in the further study, we selected ductal carcinoma (IDC) cell lines as study objective.
Effective knockdown of RPS15A in breast cancer cells
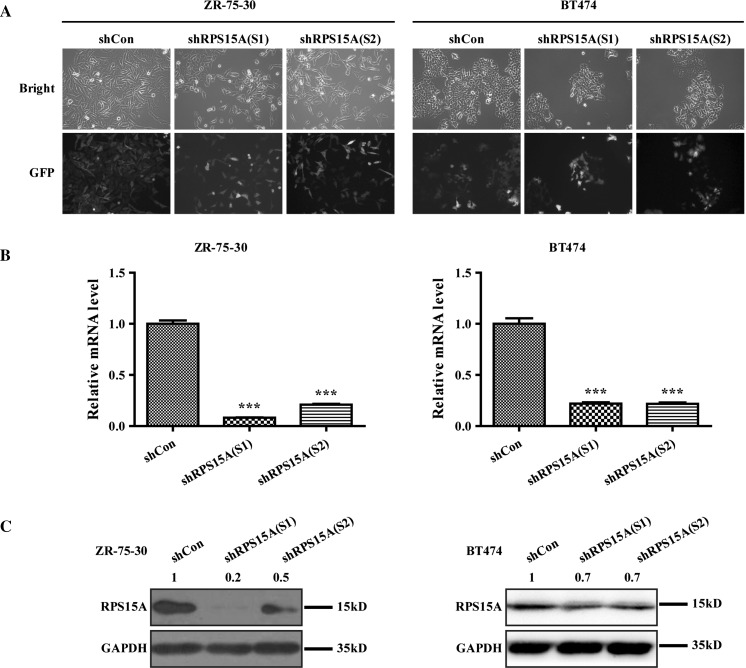
To further explore the functional effect of RPS15A in breast cancer, 2 lentiviral shRNA were successfully constructed to silence RPS15A gene expression in the indicated cells. After transduction, we observed the GFP fluorescence light under a fluorescence microscope and found that virus infection rate was more than 80% (Fig. 1a). Further detection showed that the mRNA expression level of RPS15A was significantly reduced in BT474 cells infected with shRPS15A (S1) or shRPS15A (S2) compared to shCon group (Fig. 1b, P < 0.001). In line with the results of qPCR, western blot analysis revealed that the protein expression levels of RPS15A in BT474 cells were also significantly decreased by shRPS15A (Fig. 1c).
Fig. 1.
Knockdown efficiency of RPS15A by lentivirus transduction of the breast cancer cells, ZR-75-30 and BT474. a Representative microscopic images of breast cancer cells were shown. Transduction efficiency was evidences by the expression of the visible green fluorescence protein (GFP). b RT-PCR was used to analyze the RPS15A mRNA level in three groups of ZR-75-30 and BT474 cells after 5 days infection. c RPS15A Protein levels were detected by western blot analysis in ZR-75-30 and BT474 cells 4 days after transduction. ***P < 0.001; Scale bars, 10 μm
Knockdown of RPS15A suppressed proliferation of breast cancer cells
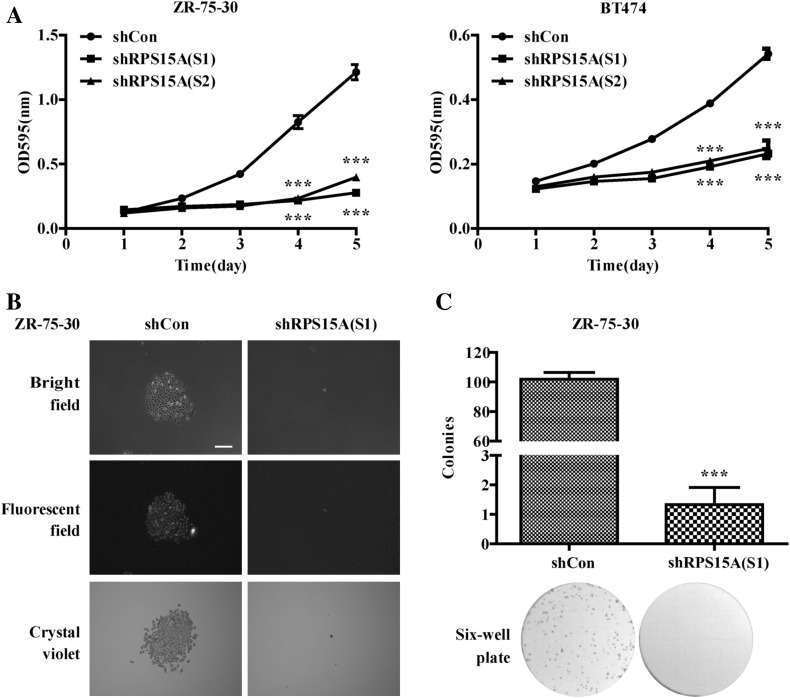
To assess the effect of RPS15A on regulating breast cancer cell proliferation, MTT and colony formation assay were carried out. As shown in Fig. 2a, MTT analysis showed that growth curves of the shRPS15A (S1) and shRPS15A (S2) groups were obviously lower than that of the shCon group, especially at the 4th and 5th day. Statistical analysis demonstrated that shRNA-mediated RPS15A silencing caused a significant decrease in cell proliferation (P < 0.001). Colony formation assay showed that RPS15A knockdown led to remarkably decreased colony number and size (Fig. 2b, c, P < 0.001).
Fig. 2.
RPS15A knockdown inhibited cell proliferation and colony formation in ZR-75-30 and BT474 cells. a Cell proliferation was significantly inhibited after RPS15A knockdown, which was examined by the MTT assay. b Representative photographs of colonies showed a significant inhibition of colony formation in the shRPS15A (S1) condition. c Statistical analysis of the number of colonies (>= 50 single cells) stained with crystal violet. ***P < 0.001; Scale bars, 25 μm
Knockdown of RPS15A arrested cell cycle and promoted cell apoptosis in breast cancer cells
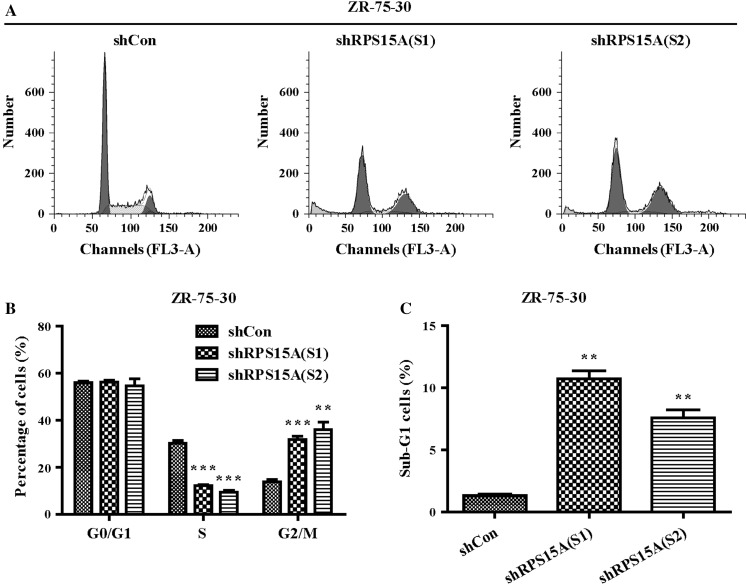
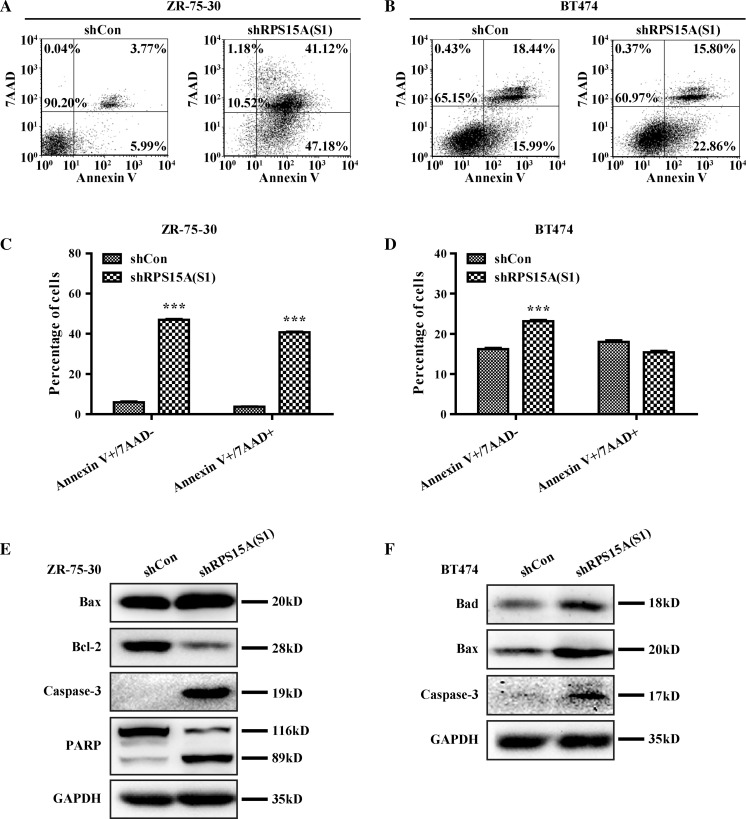
To examine the mechanisms underlying the inhibition effect of knocked down RPS15A on breast cancer cell proliferation, we detected cell cycle distribution and apoptosis percentage. Cell cycle analysis (Fig. 3a, b) showed that the percentage of cells in S phase was significantly decreased in shRPS15A (S1) and shRPS15A (S2) groups compared to that in shCon groups (P < 0.001), whereas there was an apparent increase in cells in the G2/M phase in breast cancer cells transduced with shRPS15A (S2) (P < 0.001). Moreover, the percentage of cells in sub-G1 phase was obviously increased in shRPS15A (S1) and shRPS15A (S2) groups, suggesting that knockdown of RPS15A may promote early stage apoptosis (Fig. 3c). As shown in Fig. 4a–d, the early apoptotic cells (Annexin V+ and 7-AAD−) and late apoptotic cells (Annexin V+ and 7-AAD+) were significantly increased in breast cancer cells transduced with shRPS15A (S1). These results indicated that knockdown of RPS15A arrested breast cell cells at G2/M phase and induced early stage apoptosis.
Fig. 3.
Knockdown of RPS15A induces cell-cycle arrest. a Cell cycle distribution of ZR-75-30 cells was analyzed by flow cytometry. b, c Statistical analysis of the percentage of cells in the different cell cycle phases **P < 0.01; ***P < 0.001, Scale bars, 10 μm
Fig. 4.
Knockdown of RPS15A promotes apoptosis in ZR-75-30 cells. a Representative images showing Annexin V/7-ADD staining results in ZR-75-30 cells. b Representative images showing Annexin V/7-ADD staining results in BT474 cells. c, d Quantification of a and b. e, f Expression of downstream proteins related to apoptosis in ZR-75-30 and BT474 cells by western blot analysis. ***P < 0.001; Scale bars, 10 μm
To further investigate the mechanism underlying the pro-apoptotic effect of RPS15A knockdown, western blot was used to detect the expression of apoptotic related proteins, including Caspase-3, PARP, BAX, Bcl-2 and Bad. As presented in Fig. 4e, f, knockdown of RPS15A elevated the expression of cleaved caspase-3, BAX, Bad, and downregulated the expression of Bcl-2, especially the total PARP (molecular weight = 116KD) was decreased and cleaved PARP (molecular weight = 89KD) was increased after RPS15A knockdown. These evidences demonstrated that knockdown of RPS15A could promote breast cancer cell apoptosis.
Discussion
Breast cancer is the leading cause of cancer death among women worldwide. RPS15A encodes a component of 40S ribosomes subunits and has been elucidated in many kinds of cancer cells. Therefore, it is urgently needed to identify RPS15A as a potential target for breast cancer.
To investigate its biological role in breast cancer, Oncomine data analysis was performed to analyze RPS15A expression and results showed that it was highly expressed in ductal carcinoma compared with normal breast tissue. In support of our findings, gene expression profiling showed that RPS15A may support the progression of breast cancer (Hannemann et al. 2006). Moreover, RPS15A was successfully knocked down in breast cancer cells using RNAi technology. Functional analyses indicated that knockdown of RPS15A significantly inhibited breast cancer cell proliferation and colony formation ability. Previous studies have indicated that RPS15A silencing could inhibit cell proliferation and impair colony formation in hepatic cancer HepG2 and Bel7404 cells (Maiyu et al. 2014), lung cancer A549 cells (Zhao et al. 2015) and osteosarcoma U2OS cells (Zhang et al. 2014b). In addition, we analyzed the relationship between RPS15A and c-Myc in breast cancer using GEPIA database. The results showed a significant positive correlation between them (p = 4.3e−05 in supplemental Fig. 2) (Tang et al. 2017). We deduced that RPS15A may promote breast cancer cell proliferation through mediating c-Myc, which needs further study.
To further elucidate the possible mechanisms that RPS15A might contribute to breast cancer cell proliferation, cell cycle and apoptosis analyses were conducted. Our results showed that cell cycle was arrested in the G0/G1 phase, and the observation of an obvious apoptotic effect in RPS15A silenced breast cancer cells. RPS15A has been reported to be a responsive gene of transforming growth factor-β1 (TGF-β1), which plays a vital role in cell proliferation and differentiation (Akiyama et al. 2000). Consistently, knockdown of RPS15A could drastically arrest cell cycle and promote cell apoptosis in several cancer cells (Maiyu et al. 2014; Zhang et al. 2014b; Zhao et al. 2015). From these points, we can infer that RPS15A can promote breast cancer cell proliferation via arresting cell cycle and affecting cell apoptosis progression.
Further analysis showed RPS15A knockdown resulted in activation of Caspase-3, PARP cleavage, Bad, BAX and downregulation of Bcl-2, Bad, and BAX. Bcl-2 is a member of the Bcl-2 family, which are major regulators of apoptosis. About 20 Bcl-2 family proteins have been identified, which are divided into two classes according to the function in apoptosis: pro-apoptosis and anti-apoptosis. Bad and BAX are pro-apoptosis proteins, and Bcl-2 is an anti-apoptosis protein (Besbes et al. 2015; Chipuk et al. 2010). In the process of apoptosis, Bad can inhibit Bcl-2 activity, but activate BAX. BAX and BAK induce mitochondrial outer membrane permeabilization (MOMP) which allows soluble proteins (Cytochrome c) to diffuse into the cytoplasm, Cytochrome c together with Apaf-1 and dATP forms the apoptosomes promoting the activation of initiator Caspase-9. Caspase-9 subsequently activates effector Caspases, including caspase-3, -6 and -7, which then induce apoptosis by cleaving proteins which are essential for cell survival, such as PARP and DFF45 (Kelly and Strasser 2011; Strasser et al. 2011). The cleavage of Caspase-3 and PARP is the marker of apoptosis, and Caspase-3 catalyzes the cleavage of PARP (Heeres and Hergenrother 2007; Mazumder et al. 2008). Our results clearly suggest that cell death is primarily caused by apoptosis, and cell apoptosis was induced in breast cancer via activation of Caspase-3, PARP cleavage, upregulation of pro-apoptotic factors and downregulation of anti-apoptotic factors. Here we propose that RPS15A, as a ribosomal protein, may play a prominent role in promoting breast cancer cell proliferation.
Conclusions
These data suggest that RPS15A is overexpressed in breast cancer and may promote breast cancer cell growth. Moreover, RPS15A plays a key role in regulating proliferation and apoptosis of breast cancer cells and could be a potential therapeutic target for developing novel treatments against breast cancer. Our preliminary data unravel a new mechanism of RPS15A in gene therapy. However, further analysis in vitro and in vivo will be needed to elucidate the exact molecular mechanism underlying RPS15A-mediated cell apoptotic regulation in breast cancer.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by grants from the Project from Natural Science Foundation of Zhejiang Province (LY18H160033).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no competing interests.
References
- Akiyama N, Matsuo Y, Sai H, Noda M, Kizakakondoh S. Identification of a series of transforming growth factor Β-responsive genes by retrovirus-mediated gene trap screening. Mol Cell Biol. 2000;20:3266–3273. doi: 10.1128/MCB.20.9.3266-3273.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amsterdam A, Sadler KC, Lai K, Farrington S, Bronson RT, Lees JA, Hopkins N. Many ribosomal protein genes are cancer genes in zebrafish. PLoS Biol. 2004;2:0690–0698. doi: 10.1371/journal.pbio.0020139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besbes S, Mirshahi M, Pocard M, Billard C. New dimension in therapeutic targeting of BCL-2 family proteins. Oncotarget. 2015;6:12862–12871. doi: 10.18632/oncotarget.3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonham-Smith PC, Moloney MM. Nucleotide and protein sequences of a cytoplasmic ribosomal protein S15a gene from Arabidopsis thaliana. Plant Physiol. 1994;106:401–402. doi: 10.1104/pp.106.1.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chipuk JE, Moldoveanu T, Llambi F, Parsons MJ, Green DR. The BCL-2 family reunion. Mol Cell. 2010;37:299–310. doi: 10.1016/j.molcel.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannemann J, Velds A, Halfwerk JB, Kreike B, Peterse JL, Vijver MJVD. Classification of ductal carcinoma in situ by gene expression profiling. Breast Cancer Res. 2006;8:R61. doi: 10.1186/bcr1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeres JT, Hergenrother PJ. Poly(ADP-ribose) makes a date with death. Curr Opin Chem Biol. 2007;11:644–653. doi: 10.1016/j.cbpa.2007.08.038. [DOI] [PubMed] [Google Scholar]
- Jimenez L, Becerra A, Landa A. Cloning, expression and partial characterization of a gene encoding the S15a ribosomal protein of Taenia solium. Parasitol Res. 2004;92:414–420. doi: 10.1007/s00436-003-1021-4. [DOI] [PubMed] [Google Scholar]
- Karagianni M, Kaitelidou D, Kalokairinou A, Mantas J. Breast cancer in social media: a literature review. Stud Health Technol Inform. 2014;202:321. [PubMed] [Google Scholar]
- Kavak E, Unlu M, Nister M, Koman A. Meta-analysis of cancer gene expression signatures reveals new cancer genes, sage tags and tumor associated regions of co-regulation. Nucl Acids Res. 2010;38:7008–7021(7014). doi: 10.1093/nar/gkq574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly GL, Strasser A. The essential role of evasion from cell death in cancer. Adv Cancer Res. 2011;111:39–96. doi: 10.1016/B978-0-12-385524-4.00002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie C, Tam R, Clark M, Lee H, Sonenberg N, Lasko P. Suppression of a temperature-sensitive cdc33 mutation of yeast by a multicopy plasmid expressing a Drosophila ribosomal protein. J Biol Chem. 1994;269:14625–14630. [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Maiyu X, et al. Down-regulation of ribosomal protein S15A mRNA with a short hairpin RNA inhibits human hepatic cancer cell growth in vitro. Gene. 2014;536:84–89. doi: 10.1016/j.gene.2013.11.075. [DOI] [PubMed] [Google Scholar]
- Mazumder S, Plesca D, Almasan A. Caspase-3 activation is a critical determinant of genotoxic stress-induced apoptosis. Methods Mol Biol. 2008;414:13–21. doi: 10.1007/978-1-59745-339-4_2. [DOI] [PubMed] [Google Scholar]
- Ottolini D, Calì T, Negro A, Brini M. The Parkinson disease-related protein DJ-1 counteracts mitochondrial impairment induced by the tumour suppressor protein p53 by enhancing endoplasmic reticulum-mitochondria tethering. Hum Mol Genet. 2013;22:2152–2168. doi: 10.1093/hmg/ddt068. [DOI] [PubMed] [Google Scholar]
- Rhodes D, et al. Oncomine 3.0: genes, pathways, and networks in a collection of 18,000 cancer gene expression profiles. Neoplasia. 2007;9:166–180. doi: 10.1593/neo.07112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggero D, Pandolfi PP. Does the ribosome translate cancer? Nat Rev Cancer. 2003;3:179–192. doi: 10.1038/nrc1015. [DOI] [PubMed] [Google Scholar]
- Shen XC, Valencia CA, Szostak JW, Dong B, Liu R. Scanning the human proteome for calmodulin-binding proteins. Proc Natl Acad Sci USA. 2005;102:5969–5974. doi: 10.1073/pnas.0407928102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser A, Cory S, Adams JM. Deciphering the rules of programmed cell death to improve therapy of cancer and other diseases. EMBO J. 2011;30:3667–3683. doi: 10.1038/emboj.2011.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Z, Li C, Kang B, Gao G, Li C, Zhang Z. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucl Acids Res. 2017;45:W98–W102. doi: 10.1093/nar/gkx247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X. Lentivirus-mediated knockdown of eukaryotic translation initiation factor 3 subunit D inhibits proliferation of HCT116 colon cancer cells. Biosci Rep. 2014;34:e00161–e00161. doi: 10.1042/BSR20140078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeller KI, Jegga AG, Aronow BJ, O’Donnell KA, Chi VD. An integrated database of genes responsive to the Myc oncogenic transcription factor: identification of direct genomic targets. Genome Biol. 2003;4:54–56. doi: 10.1186/gb-2003-4-10-r69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, et al. HSPC111 governs breast cancer growth by regulating ribosomal biogenesis. Mol Cancer Res. 2014;12:583–594. doi: 10.1158/1541-7786.MCR-13-0168. [DOI] [PubMed] [Google Scholar]
- Zhang C, Zhang T, Song E, Swa H, Chen X, Zheng L. Ribosomal protein S15A augments human osteosarcoma cell proliferation in vitro. Cancer Biother Radiopharm. 2014;29:451–456. doi: 10.1089/cbr.2014.1698. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zhang GQ, He C, Tao L, Liu F. Role of DJ-1 siRNA in reverse sensitivity of breast cancer cells to chemotherapy and its possible mechanism. Int J Clin Exp Pathol. 2015;8:6944–6951. [PMC free article] [PubMed] [Google Scholar]
- Zhao X, et al. Decreased expression of RPS15A suppresses proliferation of lung cancer cells. Tumor Biol. 2015;36:6733–6740. doi: 10.1007/s13277-015-3371-9. [DOI] [PubMed] [Google Scholar]
- Zhou W, Shi G, Zhang Q, Wu Q, Li B, Zhang Z. MicroRNA-20b promotes cell growth of breast cancer cells partly via targeting phosphatase and tensin homologue (PTEN) Cell Biosci. 2014;4:62. doi: 10.1186/2045-3701-4-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.