Abstract
Aluminum phosphide (AlP), an inexpensive solid fumigant, is frequently used for grain conservation despite its alleged high toxicity. Increased utilization of AlP for agricultural and non-agricultural purposes during the last four decades has resulted in increment of AlP-attributed poisoning numbers. Moreover, due to its limitless accessibility in developing countries, AlP has been increasingly used for suicide. Moisture-exposed AlP undergoes a chemical reaction producing phosphine gas, which in turn inhibits cytochrome oxidase and impedes cellular oxygen consumption. Lethality remains elevated reaching rates of >50% and no effective antidote is available. Nevertheless, experimental and clinical studies suggested that magnesium sulfate, melatonin, N-acetylcysteine, glutathione, sodium selenite, vitamin C and E, triiodothyronine, liothyronine, vasopressin, milrinone, Laurus nobilis L., 6-aminonicotinamide, boric acid, acetyl-L-carnitine and coconut oil, may serve as antidotes by reducing the deleterious oxidative properties of AlP. This article reviews the afore-mentioned chemicals suggested to specifically treat AlP poisoning and discusses their protective mechanisms and main outcomes.
Keywords: Aluminum phosphide, Phosphine, Antidote, Protection, Intoxication
1. Introduction
Aluminum phosphide (AlP) has been extensively used on account of its ideal properties like leaving little residue on food grains and exterminating insects with no impact on seed viability [1]. However, its widespread use has contributed to a marked increase in the related suicidal [2] and accidental poisonings [3] with high-risk mortality [4]. Due to unlimited and uncontrolled accessibility, AlP poisoning is one of the most common causes of poisoning in the developing countries such as India [[5], [6], [7], [8]] and Iran [[9], [10], [11], [12], [13], [14], [15]]. AlP-poisoned cases have been also reported from developed countries [16].
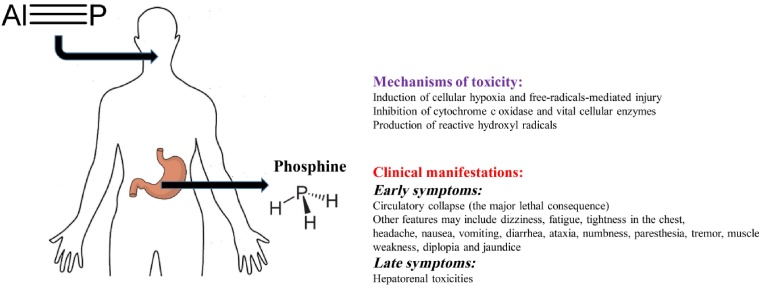
Following AlP ingestion, reaction with hydrochloric acid in the stomach produces a lethal gas called “phosphine” (PH3) (Fig. 1). Interestingly, prompt liberation of this gas following exposure to atmospheric moisture, has also made AlP a potential chemical terrorism agent [17]. Phosphine induces cellular hypoxia by affecting the mitochondria [18], inhibits cytochrome c oxidase [19] and leads to formation of highly reactive hydroxyl radicals [20]. The signs and symptoms of AlP intoxication are nonspecific and appear instantaneously [21]. AlP-related fatality is attributed to cardiac failure caused by inhibition of cytochrome c oxidase, decrement of adenosine triphosphate (ATP) production and cardiomyocyte impairment [22]. Oxidative stress has been shown to play a major role in AlP toxicity [23]. Nevertheless, AlP-induced inhibition of cytochrome c oxidase as the underlying cause of AlP toxicity, has raised controversies [24]. No definitive antidote has been proven clinically efficient [25]. AlP toxicity is mainly treated by supportive approaches [26] including intra-aortic balloon pump [27] and extracorporeal membrane oxygenation (ECMO), a recent promising technique that provides temporary cardiorespiratory support [[28], [29], [30]]. Here, we discuss the pros and cons of different agents suggested as potential antidotes for AlP.
Fig. 1.
Pathophysiology of aluminum phosphide (AlP) intoxication. After ingestion, AlP reacts with stomach acid and releases phosphine (PH3) gas. PH3 reaches the heart through the systemic circulation and causes myocardial cell death and arrhythmias.
2. Mechanisms of AlP-related toxicity
Subsequent to ingestion and upon contacting water/acid in the gastrointestinal (GI) tract, AlP produces PH3 which is then absorbed. Absorption through the skin and eyes has not been frequently reported, but may take place. Since phosphine is a small molecule, it is distributed all over the body. Intact AlP is excreted in the urine. Hypophosphite is the major urine metabolite of PH3 while exhalation is the removing pathway for pure PH3 [31].
By inhibiting the production of cell enzymes and proteins, PH3 causes toxicities similar to that induced by metal phosphides [19]. Non-competitive blockade of cytochrome c oxidase leads to the inhibition of oxidative phosphorylation and cellular respiration and consequently, overproduction of peroxide radicals. Cell membrane dysfunction also happens due to catalase inhibition and glutathione reduction [32]. PH3 inhibits cytochrome c oxidase in the cardiac cell as it negatively affects mitochondria and myocardial proteins. These effects are mediated through impairment of cellular permeability to major ions and alterations in the cardiac cell wall potential [24]. Increases in Heinz bodies (i.e. denatured hemoglobin) and hemichrome formation are also observed following PH3 reaction with hemoglobin, consequently reducing heme capacity [33]. As evidenced by post-mortem histopathological studies, organs such as the heart, lung, kidney, and liver with higher oxygen demands, are more sensitive to PH3-induced damage involving oxygen free-radicals production [34]. In a 70-kg individual, 500 mg AlP has been reported as the lethal dose. Moreover, 400–600 mg/L phosphine level in the air may lead to death after 30 min [31].
3. Approach used in this review
Scientific databases such as PubMed, Scopus and Web of Science were searched for the following terms: ‘aluminum phosphide’, ‘phosphine’, ‘antidote’ and ‘protective role’, from January 1980 up to May 2018. We reviewed the abstracts of relevant articles, looking particularly for those examining special antidotes against AlP. Duplicate articles or articles that did not match our subject, were excluded. Experimental (including in vitro and in vivo) and human studies were presented separately.
4. Magnesium sulfate
4.1. Human studies
Contradictory results have been reported concerning the utilization of magnesium sulfate as therapeutic agent in AlP-intoxicated patients. Based on some studies, AlP intoxication did not induce hypomagnesaemia and magnesium sulfate therapy did not improve the patient conditions [35,36]. By contrast, other investigations showed that magnesium level decreased in AlP poisoning, thus supporting the hypothesis that improving magnesium level can be regarded as a step towards treatment of AlP poisoning [37]. Consistent with this hypothesis, it was suggested that hypomagnesaemia might be the major cause of high mortality in AlP-intoxicated patients and its rectification is lifesaving [38].
The anti-peroxidant effects of magnesium were evaluated in fifty AlP-poisoned patients [39]. After demonstrating that AlP-induced oxidative stress in the early phase of poisoning was responsible for increase in lipid peroxidation and reduction in glutathione (GSH) levels, the authors reported significant improvement in patients receiving magnesium. They explained that AlP-induced oxidative stress was able to induce transient fall in magnesium and magnesium-dependent GSH, leading to increased susceptibility towards oxygen free radical-induced damages and resulting in elevated levels of lipid peroxidation.
5. Melatonin
Melatonin may specifically ameliorate AlP-induced cardiotoxicity. Primarily, its amphiphilic structure allows its penetration into the intracellular compartments such as mitochondria. As it scavenges reactive oxygen species (ROS), melatonin is also known as robust antioxidant. Additionally, melatonin not only extenuates the suppression of respiratory chain complexes but also intensifies ATP production. It prevents apoptosis by restraining the mitochondrial permeability transition pore and disturbing caspase activation after inhibiting cytochrome c release [40]. Therefore, melatonin administration was suggested as a potentially beneficial approach against AlP-induced cardiotoxicity.
6. In vitro and in vivo studies
Several in vitro investigations suggested that melatonin can overcome AlP-induced oxidative damage; however, further researches are still warranted to evaluate the feasibility of using melatonin as antidote to treat AlP-poisoned patients. Interestingly, PH3 was shown to increase lipid peroxidation (as reflected by the levels of malondialdehyde (MDA) and 4-hydroxyalkenals (4-HDA)) in a concentration (0.25–2 mM)- and time (30–150 min)-dependent manner, with the maximum level of 2.9‐fold increment achieved at 90 min at the concentration of 1 mM PH3, in brain homogenates [32]. Similarly, PH3 (4 mg/kg, intraperitoneally (IP)) induced brain DNA oxidation [as reflected by the levels of 8-hydroxyguanosine (8-OH-dG)] in vitro and in the frontal cortex and lowered brain glutathione peroxidase activity in vivo. Melatonin (0.1–2 mM) dose-dependently restored all PH3-induced consequences in the brain, probably through its free-radical scavenging ability
6.1. Animal studies
PH3 (4 mg/kg, IP) was shown to significantly decrease GSH, GSH peroxidase and catalase, 30 min after its administration to male Wistar rats [32]. By contrast, PH3 raised the levels of lipid peroxidation, DNA oxidation and superoxide dismutase (SOD) activity, in the kidney and heart. All these changes were reversed by melatonin 10 mg/kg, administered IP 30 min before injecting PH3) in cardiac tissues, with the exception of SOD activity.
To estimate melatonin-attributed effects on 16.7 mg/kg AlP-induced decline in heart rate and blood pressure, several doses of melatonin (20, 30, 40 and 50 mg/kg; IP) were administered [41]. Significant improvements were observed in the activities of mitochondrial complexes, oxidative stress biomarkers and ADP/ATP ratio following 40 and 50 mg/kg melatonin administration.
In another Wistar rat study, PH3 was shown to significantly decrease GSH concentration and elevate lipid peroxidation in the brain, lungs and liver 30 min after the administration of PH3 (2 mg/kg, IP) [42]. The preventive effects of melatonin (10 mg/kg), vitamin C (30 mg/kg) and β-carotene (6 mg/kg) injected IP 30 min before PH3 administration were thereafter examined. Only melatonin was able to block PH3-induced changes, while vitamin C and β-carotene were not effective.
7. Coconut oil
7.1. Human studies
In a 28-year-old man, coconut oil administered six hours after the ingestion of a lethal amount of AlP (12 g) was shown to reduce the expected amount of absorbed AlP [43]. Thereafter, coconut oil was suggested as a possibly lifesaving and useful antidote for AlP poisoning. In another report, thirty-three AlP-intoxicated patients admitted to the intensive care unit received extensive gastric lavage using a mixture of coconut oil and sodium bicarbonate [36]. The mean length of hospital stay was 5.84 ± 1.86 days and the survival rate was 42%. Based on these findings, the authors claimed that coconut oil was effective in increasing the survival rate. Seven AlP-poisoned patients with severe hemodynamic conditions treated with supportive measures also received gastric lavage with diluted potassium permanganate, coconut oil and sodium bicarbonate [26]. Since 4 out of the 7 patients survived, the authors similarly proposed that coconut oil may be regarded as an efficient therapy as long as no specific antidote exists. Nevertheless, the absence of a control group (i.e. intoxicated patients who were not treated with coconut oil), could be regarded as a limitation and should be considered when interpreting the findings.
8. N-acetylcysteine (NAC)
8.1. Animal studies
The effects of NAC on acute and chronic toxicities of AlP (10, 20, 40 mg/kg, IP) were studied in the heart, lung, kidney and liver of white male mice [44]. Besides delaying the latency to death, NAC (50–100 mg/kg, IP) prevented hepatic necrosis. Interestingly, significant delay in latency to death was also observed following the administration of vitamin C (500–1000 mg/kg, IP) in this study.
The protective effects of NAC and N omega-Nitro-L-arginine methyl ester (L-NAME) against AlP (12.5 mg/kg) administered by intragastric route were investigated in the rat [45]. It was recorded that AlP deteriorated the hemodynamic profile and biochemical parameters leading to the rat death with a mean survival time of 90 ± 10 min. After NAC infusion (6.25 mg/kg/min, intravenous (IV) for 30 min), the parameters of AlP-poisoned rats were significantly improved, while treatment with L-NAME (1 mg/kg/min, IV for 60 min) neither improved the survival time nor the biochemical parameters although it significantly raised blood pressure. Co-administration of NAC and L-NAME to AlP-poisoned animals worsened the survival time as compared to untreated AlP-poisoned rats. Based on these findings, the authors concluded that NAC, in contrast to L-NAME, reduces AlP-induced myocardial oxidative damage and increases survival time. In both mouse and rat models, NAC could successfully increase the survival time.
8.2. Human studies
Studies are controversial [46]. Several case reports like the one reporting a 20-year-old female intoxicated with both AlP and zinc phosphide, who survived after intensive treatment with NAC, digoxin and insulin [47], have been used to claim the beneficial effects of NAC in AlP-poisoned patients.
In a 2013 prospective, randomized, controlled open-label trial recruiting thirty-seven AlP-intoxicated patients (22 treated with NAC and 15 serving as controls), NAC significantly reduced the plasma MDA level (139 ± 28.2 μmol/L in the NAC-treated patients vs. 149.6 ± 35.2 μmol/L in the controls; p = 0.03), duration of hospitalization (2.7 ± 1.8 vs. 8.5 ± 8.2 days; p = 0.02), mechanical ventilation (45.4 vs. 73.3%; p = 0.04) and mortality rate (36 vs. 60% with odds ratio of 2.6 (95%-confidence interval, 0.7–10.1), confirming NAC-attributed benefits in AlP poisoning [48]. Though statistically significant differences were found in MDA levels following treatment with NAC, authors did not discuss the clinical significance of this alteration.
A second study, carried out in an emergency medical unit in India, investigated the antioxidant effects of NAC on mortality in 50 severely AlP-poisoned patients over a period of one year [49]. NAC was given IV in 5% dextrose, with a loading dose of 150 mg/kg over one hour, followed by 50 mg/kg over four hours and 100 mg/kg over 16 h. The mortality rate was 87.5% in the treatment group versus 88.5% in the placebo group. Survivors in the treatment group received 19 g NAC and non-survivors received only 12.15 g NAC. In this study, NAC did not improve the outcome of severe AlP poisoning.
In a case-control study, addition of NAC (300 mg/kg, infused IV for 20 h) to the routine treatment was shown to be beneficial on AlP-attributed cardiac alterations (creatine kinase MB, creatine phosphokinase, heart rate, and mean systolic blood pressure) in 46 patients (23 patients in the NAC group and 23 controls) [50]. NAC prevented sharp heart rate fluctuations. Creatine phosphokinase levels were significantly different 24 h after admission in both groups (one group received intravenous NAC plus conventional treatment while the other group did not receive NAC) in comparison to those measured before treatment (p < 0.001). In a cohort study, oxidative stress was evaluated in patients who received NAC along with supportive treatment [51]. The baseline catalase (p = 0.008) and SOD levels (p < 0.01) were significantly higher among survivors (32.6% of the patients) than non-survivors (67.4% of the patients), suggesting NAC ability to recuperate survival by ameliorating oxidative stress in AlP-poisoned patients.
9. Sodium selenite
9.1. Animal study
Pretreatment with sodium selenite in male albino mice intoxicated by AlP (10, 20 and 40 mg/kg, IP) was shown to reduce pulmonary and liver complications (as evidenced by edema and fatty changes), though it had no effects on mortality latency occurring 35 ± 15 min after AlP administration [44].
10. Vitamin E
10.1. Human studies
Vitamin E has recently been mentioned as a useful treatment for AlP poisoned patients. A 21-year-old male who ingested a 3-g AlP tablet, was successfully treated with a combination of routine approaches and administration of antioxidants such as vitamin C (1000 mg every 12 h via slow IV infusion), vitamin E (400 Units, IM) and NAC (140 mg/kg oral as a loading dose followed by 70 mg/kg oral every 4 h, for up to 17 doses) [52]. Administered at 400 mg BD by intramuscular (IM) route besides other supportive treatments to AlP-poisoned subjects, vitamin E was able to decrease the requirement (30 vs. 62%) and duration of mechanical ventilation and reduce the mortality rate (15% vs. 50%), as compared to controls [53].
11. Triiodothyronine
11.1. Animal study
The cardio-protective effect of triiodothyronine (T3) (1, 2 and 3 μg/kg, IP 30 min after 12 mg/kg AlP administration) was observed in rats [54]. In this study, T3 3 μg/kg significantly improved cardiovascular features (including decrement of heart rate, blood pressure and abnormal QRS complexes, QTc and ST height on the ECG) as well as oxidative stress indices. T3 ameliorated mitochondrial function and ATP levels in the cardiac cells while decreased apoptosis by diminishing caspase 3 and 9 activities and enhancing cell viability. The experimental findings highly suggested beneficial effects of T3 against AlP-induced cardiotoxicity in rats.
12. Liothyronine
12.1. Human study
After gastric lavage, liothyronine (50 μg) was administered to twelve AlP-intoxicated patients via a nasogastric tube [55]. Oral liothyronine significantly ameliorated systolic blood pressure, arterial pH, and total thiol molecules while reducing lipid peroxidation, elevating catalase activity, and maintaining total antioxidant capacity. Altogether, liothyronine was suggested to be a promising adjuvant therapy in AlP-poisoned patients.
13. Vasopressin and milrinone
13.1. Animal study
The protective effects of vasopressin (2.0 IU/kg, IP) and milrinone (0.25 mg/kg) on cardiovascular function, oxidative stress and apoptosis, were investigated in rats poisoned with AlP (12.5 mg/kg, by gavage) [54]. Vasopressin and milrinone not only had short-term cardio-protective effects but also reconstructed mitochondrial function, improved ATP level and reduced the oxidative damage in long-term which protected cardiomyocytes against apoptosis.
14. Laurus nobilis L
14.1. In vitro study
The defensive effects of the leaf extract of Laurus nobilis L. (LNE), a plant with antioxidant and antibacterial properties, against AlP-induced genotoxicity and oxidative damages were investigated in cultured human blood cells [56]. Co-application of LNE (25, 50, 100 and 200 mg/l) and AlP (58 mg/l) decreased the total oxidative levels but increased the total antioxidant capacity (TAC) as compared to the controls. The preventive role of LNE in moderating AlP-induced DNA-damage was pointed out, as sister chromatid exchange and chromosome aberration were also decreased in AlP-exposed rats treated with LNE in comparison to the controls.
14.2. Animal study
The protective effects of LNE (200 mg/kg for 14 successive days, IP) against AlP toxicity were investigated in the rat [57]. LNE was shown to suppress the genetic damage and oxidative stress caused by AlP. The protective effect of LNE was attributed to its antioxidant and free-radical scavenging features.
15. 6-aminonicotinamide (6-AN)
15.1. In vitro study
AlP-poisoned patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency were reported to survive surprisingly [58,59]. Therefore, to investigate the probable effects of G6PD deficiency in protecting the patients against AlP toxicity, isolated hepatocytes were treated with 6-aminonicotinamide (6-AN; 3 μg/ml for 2 h) as an inhibitor of the NADP+-dependent 6-phosphogluconate dehydrogenase [60]. All analyzed parameters such as cell viability, ROS formation, mitochondria membrane potential (MMP), lysosomal integrity, reduced GSH content, and lipid peroxidation significantly decreased in hepatocytes pretreated with 6-AN for 10 min compared to the controls, providing evidence on the protective role of G6PD deficiency which could be induced by 6-AN.
15.2. Boric acid
Boric acid, a non-toxic Lewis acid, may act as a “trapping agent” for phosphine which is a Lewis base, leading to phosphine excretion in the urine. Thus, boric acid appears as a potential antidote for AlP poisoning [61].
15.3. In vitro Study
One-gram AlP tablets were added to 200 ml of various solvents including distilled water, activated charcoal diluted in distilled water and saturated boric acid solution [62]. Each solution was used at both normal and acidified pH and separately examined. The volume of released gas and the rate of gas evolution throughout reactions of AlP tablets were compared among the various groups. Although boric acid did not significantly decrease the amount of released gas, it significantly reduced the grade of gas evolution. A gaseous adduct was created in the reaction between AlP and boric acid. These findings showed possible benefits of boric acid in treatment of PH3 poisoning.
16. Acetyl-L-carnitine
16.1. Animal study
Effects of acetyl-L-carnitine (ALCAR; 100, 200, and 300 mg/kg, IP) on AlP-induced toxicity were investigated in a rodent model with regard to mitochondrial respiratory chain activity, ATP production, oxidative stress and cellular apoptosis/necrosis [63]. ALCAR significantly ameliorated oxidative stress (i.e. reduced elevated ROS and plasma iron levels) and elevated the activity of cytochrome oxidase which in turn amplified ATP production. Moreover, flow cytometric assays used to report apoptotic/necrotic cells percentages and evaluate caspase-3 and caspase-9 activities, showed that ALCAR prevented AlP-induced apoptosis in cardiomyocytes.
A detailed summary of the novel modalities investigated for treatment of AlP poisoning, is given in Table 1.
Table 1.
Antidotes studied for treatment of AlP intoxication.
| Treatment | Experimental model | Dose/route of AlP | Dose/route | Main findings |
|---|---|---|---|---|
| Magnesium sulfate | Human study | – | – | Improved oxidative stress status |
| Melatonin | In vitro (Rat brain homogenate) | 0.25-2 mM | 0.1-2mM | Antioxidant activity Increased ATP production Prevention of apoptosis |
| Animal study (Rats) | 4 mg/kg IP | 10 mg/kg IP | ||
| Animal study (Rats) | 16.7 mg/kg | 40-50 mg/kg IP | ||
| Animal study (Rats) | 2 mg/kg | 10 mg/kg IP | ||
| Coconut oil | Human study | 12 g | Oral | Increased survival rate |
| N-acetyl cysteine | Animal study (Mice) | 10-20-40 mg/kg IP | 50-100 mg/kg IP | Delaying the latency of death Prevention of hepatic necrosis |
| Animal study (Rats) | 12.5 mg/kg | 6.25 mg/kg/min Infusion for 30 min | Improvement of hemodynamic profile and biochemical parameters | |
| Human study | – | 140 mg/kg infusion (loading dose) Followed by 70 mg/kg/IV infusion every 4 h |
Reduction of the duration of hospitalization and mechanical ventilation Decrement of mortality rate |
|
| Case report | – | 300 mg/kg infusion for 20 h | Improvement of cardiac alteration | |
| Sodium selenite | Animal study (Mice) | 10-20-40 mg/kg IP | 3 mg/kg | Reduction of pulmonary and liver complications |
| Vitamin E | Case report | 3 g | 400 units IM | Decrement of mechanical ventilation duration Reduction of the mortality |
| Triiodothyronine | Animal study (Rats) | 12 mg/kg | 3 μg/kg | Improvement of cardiovascular complications Decrement of oxidative stress Increment of ATP levels Decrement of apoptosis rate |
| Liothyronine | Human study | 50 μg oral | Amelioration of cardiac complications and oxidative stress | |
| Vasopressin | Animal study (Rats) | 12.5 mg/kg gavage | 2 IU/kg IP | Cardio protective effects Increment of ATP Decrement of oxidative damage and apoptosis |
| Milrinone | Animal study (Rats) | 12.5 mg/kg gavage | 0.25 mg/kg | |
| Laurus nobilis | In vitro (Cultured human blood cells) | 58 mg/l | 25, 50, 100 and 200 mg/l | Decrement of oxidative stress Decrement of DNA damage |
| Animal study (Rats) | – | 200 mg/kg for 14 days, IP | Suppression of genetic damage Decrement of oxidative stress |
|
| 6-aminonicothinamide | In vitro (Isolated rat hepatocyte) | – | 3 μg/ml for 2 h | Decrement of ROS formation and lipid peroxidation Increment of cell viability |
| Boric acid | In vitro (Distilled water, activated charcoal, and Saturated boric acid solution) | 1 g/200 ml | Saturated boric acid solution | Decrement of the grade of gas evolution |
| Acetyl-l-carnitine | Animal study (Rats) | – | 100, 200, 300 mg/kg, IP | Increment of cytochrome oxidase and ATP production Decrement of oxidative stress Decrement of apoptosis |
IV: intravenous, IM: intramuscular and IP: intraperitoneal.
17. Discussion
Several experimental and clinical investigations have suggested some interest to various substances, able to counteract AlP-related toxicity and thus improve the prognosis of AlP-poisoned patients. However, physicians should be cautious. The level of evidence is still very low. Recommendations for utilization of all these antidotes to treat AlP-poisoned patients require further assessments.
Magnesium sulfate did not ameliorate the mortality rate in AlP-intoxicated patients [35]. The only obvious outcome following administration of magnesium sulfate was the modification of oxidative stress parameters [39]. Similar to magnesium sulfate, sodium selenite did not affect the mortality latency, but seemed able to attenuate pulmonary and hepatic complications [44]. Melatonin showed interesting antioxidant activities and improved mitochondrial complex activities, and ADP/ATP ratio [40]. Since the effects of melatonin on mortality rate have not been mentioned, more thorough assessments in this regard should be done. Coconut oil covers the stomach and decreases AlP absorption rate and may be used as a potent protective agent against AlP toxicity. Several case series suggested that coconut oil is able to improve AlP poisoning outcome [36,43]. As an adjutant therapy, NAC may combat AlP-induced cardiotoxicity, prevent liver necrosis, and ameliorate hemodynamic conditions and biochemical parameters. Even at high doses, NAC was well-tolerated without causing side effects and actually reduced AlP-induced mortality rate [50]. Vitamin E decreased the fatality rate [53] and exerted more marked effects when co-administered with NAC [52]. T3 [54], vasopressin [54] and ALCAR [63] were suggested to improve AlP-related alterations in cardiovascular function, ATP levels and apoptosis. Based on in vitro studies, 6-aminonicotinamide showed protective activity in hepatocytes [60]. Finally, although not fully investigated, boric acid theoretically seems to be a potential antidote by trapping PH3 [61,62].
18. Conclusion
Oxidative stress and ROS production, as well as inflammatory signaling, mediate the mechanisms of AlP-related toxicity in the poisoned patient. Therefore, using an antioxidant may theoretically be beneficial to limit the toxicity as lifesaving antidote. Various antioxidants have been investigated with considerable efforts; but discrepancies exist among the findings. More in-depth researches are still required to assess the current candidates and find out the most efficacious one able to reduce AlP-induced mortality.
Funding
This work was supported by the Vice Chancellor of Research, Mashhad University of Medical Sciences, Mashhad, Iran.
Conflict of interest
The authors report no conflicts of interest.
Acknowledgement
Authors are thankful to the Vice Chancellor of Research, Mashhad University of Medical Sciences, Mashhad, Iran.
References
- 1.Sankhla M.S., Kushwah R.S., Sharma K., Kumar R. Aluminium phosphide: a fatal poisoning. Interdiscip. Toxicol. 2017;8(2):65–67. doi: 10.1515/intox-2015-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anger F., Paysant F., Brousse F., Normand I.L., Develay P., Galliard Y. Fatal aluminum phosphide poisoning. J. Anal. Toxicol. 2000;24(2):90–92. doi: 10.1093/jat/24.2.90. [DOI] [PubMed] [Google Scholar]
- 3.Shadnia S., Mehrpour O., Abdollahi M. Unintentional poisoning by phosphine released from aluminum phosphide. Hum. Exp. Toxicol. 2008;27(1):87–89. doi: 10.1177/0960327107086241. [DOI] [PubMed] [Google Scholar]
- 4.Hassanian-Moghaddam H. 2014. Phosphides and Phosphine: Mechanisms for Toxicity and Range of the Problem. Clin. Toxicol.: INFORMA HEALTHCARE 52 VANDERBILT AVE; pp. 397–398. NEW YORK, NY 10017 USA. [Google Scholar]
- 5.Kapoor A., Sinha U., Singh A., Mehrotra R. An epidemiological study of aluminium phosphide poisoning at Allahabad. Indian Internet J. Forensic Med. Toxicol. 2006;4(1) [Google Scholar]
- 6.Chaudhary S., Momin S., Vora D.H., Modi P., Chauhan V., Chotaliya D. An epidemiological study of fatal aluminium phosphide poisoning at Rajkot. IOSR J. Pharm. 2013;3(1):17–23. [Google Scholar]
- 7.Saraswat P., Gupta B., Malhotra V., Goyal V. Prevalence of fatalities due to aluminium phosphide poisoning in Southern Rajasthan (an epidemiological study) J. Forensic Med. Toxicol. 1985;11:1–7. [Google Scholar]
- 8.Chugh S., Ram S., Arora B., Malhotra K. Incidence & outcome of aluminium phosphide poisoning in a hospital study. Indian J. Med. Res. 1991;94:232–235. [PubMed] [Google Scholar]
- 9.Kordrostami R., Akhgari M., Ameri M., Ghadipasha M., Aghakhani K. Forensic toxicology analysis of self-poisoning suicidal deaths in Tehran, Iran; Trends between 2011-2015. DARU J. Pharm. Sci. 2017;25(1):15. doi: 10.1186/s40199-017-0181-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shadnia S., Sasanian G., Allami P., Hosseini A., Ranjbar A., Amini-Shirazi N. A retrospective 7-years study of aluminum phosphide poisoning in Tehran: opportunities for prevention. Hum. Exp. Toxicol. 2009;28(4):209–213. doi: 10.1177/0960327108097194. [DOI] [PubMed] [Google Scholar]
- 11.Moghadamnia A., Abdollahi M. An epidemiological study of poisoning in northern Islamic Republic of Iran. East Mediterr. Health J. 2002;8(1):88–94. [PubMed] [Google Scholar]
- 12.Mehrpour O., Singh S. Rice tablet poisoning: a major concern in Iranian population. Hum. Exp. Toxicol. 2010;29(8):701–702. doi: 10.1177/0960327109359643. [DOI] [PubMed] [Google Scholar]
- 13.Hosseinian A., Pakravan N., Rafiei A., Feyzbakhsh S. Aluminum phosphide poisoning known as rice tablet: A common toxicity in North Iran. Indian J. Med. Sci. 2011;65(4):143–150. [PubMed] [Google Scholar]
- 14.Hassanian-Moghaddam H., Pajoumand A. Two years epidemiological survey of aluminum phosphide poisoning in Tehran. Iran. J. Toxicol. 2007;1(1):35–39. [Google Scholar]
- 15.Nosrati A., Karami M., Esmaeilnia M. Aluminum phosphide poisoning: A case series in north Iran. Asia Pac. J. Med. Toxicol. 2013;2(3):111–113. [Google Scholar]
- 16.Wilson R., Lovejoy M., Jr, Jaeger R.J., Landrigan P.L. Acute phosphine poisoning aboard a grain freighter. Epidemiol. Clin. Pathol. Find. JAMA. 1980;244(2):148–150. [PubMed] [Google Scholar]
- 17.Bogle R., Theron P., Brooks P., Dargan P., Redhead J. Aluminium phosphide poisoning. Emerg. Med. J. 2006;23(1) doi: 10.1136/emj.2004.015941. e03-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nath N.S., Bhattacharya I., Tuck A.G., Schlipalius D.I., Ebert P.R. Mechanisms of phosphine toxicity. J. Toxicol. 2011;2011:1–9. doi: 10.1155/2011/494168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh S., Bhalla A., Verma S.K., Kaur A., Gill K. Cytochrome-c oxidase inhibition in 26 aluminum phosphide poisoned patients. Clin. Toxicol. 2006;44(2):155–158. doi: 10.1080/15563650500514467. [DOI] [PubMed] [Google Scholar]
- 20.Gurjar M., Baronia A.K., Azim A., Sharma K. Managing aluminum phosphide poisonings. J. Emerg. Trauma. Shock. 2011;4(3):378–384. doi: 10.4103/0974-2700.83868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bumbrah G.S., Krishan K., Kanchan T., Sharma M., Sodhi G.S. Phosphide poisoning: A review of literature. Forensic Sci. Int. 2012;214(1):1–6. doi: 10.1016/j.forsciint.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 22.Mehrpour O., Jafarzadeh M., Abdollahi M. A systematic review of aluminium phosphide poisoning. Arch. Ind. Hygiene Toxicol. 2012;63(1):61–73. doi: 10.2478/10004-1254-63-2012-2182. [DOI] [PubMed] [Google Scholar]
- 23.Kariman H., Heydari K., Fakhri M., Shahrami A., Dolatabadi A.A., Mohammadi H.A. Aluminium phosphide poisoning and oxidative stress: serum biomarker assessment. J. Med. Toxicol. 2012;8(3):281–284. doi: 10.1007/s13181-012-0219-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anand R., Binukumar B., Gill K.D. Aluminum phosphide poisoning: an unsolved riddle. J. Appl. Toxicol. 2011;31(6):499–505. doi: 10.1002/jat.1692. [DOI] [PubMed] [Google Scholar]
- 25.Moghadamnia A.A. An update on toxicology of aluminum phosphide. DARU J. Pharm. Sci. 2012;20(1):25. doi: 10.1186/2008-2231-20-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agrawal V.K., Bansal A., Singh R.K., Kumawat B.L., Mahajan P. Aluminum phosphide poisoning: possible role of supportive measures in the absence of specific antidote. Indian J. Crit. Care Med. 2015;19(2):109. doi: 10.4103/0972-5229.151019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bagheri-Moghaddam A., Abbaspour H., Tajoddini S., Mohammadzadeh V., Moinipour A., Dadpour B. Using intra-aortic balloon pump for management of cardiogenic shock following aluminum phosphide poisoning; Report of 3 cases. Emergency. 2017;6(1):3. [PMC free article] [PubMed] [Google Scholar]
- 28.Hassanian‐Moghaddam H., Zamani N., Rahimi M., Hajesmaeili M., Taherkhani M., Sadeghi R. Successful treatment of aluminium phosphide poisoning by extracorporeal membrane oxygenation. Basic Clin. Pharmacol. Toxicol. 2016;118(3):243–246. doi: 10.1111/bcpt.12481. [DOI] [PubMed] [Google Scholar]
- 29.De Lange D., Sikma M., Meulenbelt J. Extracorporeal membrane oxygenation in the treatment of poisoned patients. Clin. Toxicol. 2013;51(5):385–393. doi: 10.3109/15563650.2013.800876. [DOI] [PubMed] [Google Scholar]
- 30.Mohan B., Singh B., Gupta V., Ralhan S., Gupta D., Puri S. Outcome of patients supported by extracorporeal membrane oxygenation for aluminum phosphide poisoning: an observational study. Indian Heart J. 2016;68(3):295–301. doi: 10.1016/j.ihj.2016.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hashemi-Domeneh B., Zamani N., Hassanian-Moghaddam H., Rahimi M., Shadnia S., Erfantalab P. A review of aluminium phosphide poisoning and a flowchart to treat it. Arh. Hig. Rada Toksikol. 2016;67(3):183–193. doi: 10.1515/aiht-2016-67-2784. [DOI] [PubMed] [Google Scholar]
- 32.Hsu C.H., Chi B.C., Casida J.E. Melatonin reduces phosphine-induced lipid and DNA oxidation in vitro and in vivo in rat brain. J. Pineal Res. 2002;32(1):53–58. doi: 10.1034/j.1600-079x.2002.10809.x. [DOI] [PubMed] [Google Scholar]
- 33.Shadnia S., Soltaninejad K., Hassan ian-Moghadam H., Sadeghi A., Rahimzadeh H., Zamani N. Methemoglobinemia in aluminum phosphide poisoning. Hum. Exp. Toxicol. 2011;30(3):250–253. doi: 10.1177/0960327110384287. [DOI] [PubMed] [Google Scholar]
- 34.Moazezi Z., Abedi S.H. A successful management of aluminum phosphide intoxication. Casp. J. Intern. Med. 2011;2(3):286–288. [PMC free article] [PubMed] [Google Scholar]
- 35.Siwach S.B., Singh P., Ahlawat S., Dua A., Sharma D. Serum & tissue magnesium content in patients of aluminium phosphide poisoning and critical evaluation of high dose magnesium sulphate therapy in reducing mortality. J. Assoc. Phys. India. 1994;42(2):107–110. [PubMed] [Google Scholar]
- 36.Bajwa S.J., Bajwa S.K., Kaur J., Singh K., Panda A. Management of celphos poisoning with a novel intervention: A ray of hope in the darkest of clouds. Anesth. Essays Res. 2010;4(1):20–24. doi: 10.4103/0259-1162.69301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Siwach S.B., Dua A., Sharma R., Sharma D., Mehla R.K. Tissue magnesium content and histopathological changes in non-survivors of aluminium phosphide poisoning. J. Assoc. Phys. India. 1995;43(10):676–678. [PubMed] [Google Scholar]
- 38.Chugh S.N., Kumar P., Aggarwal H.K., Sharma A., Mahajan S.K., Malhotra K.C. Efficacy of magnesium sulphate in aluminium phosphide poisoning--comparison of two different dose schedules. J. Assoc. Phys. India. 1994;42(5):373–375. [PubMed] [Google Scholar]
- 39.Chugh S.N., Kolley T., Kakkar R., Chugh K., Sharma A. A critical evaluation of anti-peroxidant effect of intravenous magnesium in acute aluminium phosphide poisoning. Magnes. Res. 1997;10(3):225–230. [PubMed] [Google Scholar]
- 40.Asghari M.H., Abdollahi M., Oliveira M.R., Nabavi S.M. A review of the protective role of melatonin during phosphine‐induced cardiotoxicity: focus on mitochondrial dysfunction, oxidative stress and apoptosis. J. Pharm. Pharmacol. 2017;69(3):236–243. doi: 10.1111/jphp.12682. [DOI] [PubMed] [Google Scholar]
- 41.Asghari M.H., Moloudizargari M., Baeeri M., Baghaei A., Rahimifard M., Solgi R. On the mechanisms of melatonin in protection of aluminum phosphide cardiotoxicity. Arch. Toxicol. 2017;91(9):3109–3120. doi: 10.1007/s00204-017-1998-6. [DOI] [PubMed] [Google Scholar]
- 42.Hsu C.-H., Han B.-C., Liu M.-Y., Yeh C.-Y., Casida J.E. Phosphine-induced oxidative damage in rats: attenuation by melatonin. Free Radic. Biol. Med. 2000;28(4):636–642. doi: 10.1016/s0891-5849(99)00277-4. [DOI] [PubMed] [Google Scholar]
- 43.Shadnia S., Rahimi M., Pajoumand A., Rasouli M.-H., Abdollahi M. Successful treatment of acute aluminium phosphide poisoning: possible benefit of coconut oil. Hum. Exp. Toxicol. 2005;24(4):215–218. doi: 10.1191/0960327105ht513oa. [DOI] [PubMed] [Google Scholar]
- 44.Moghadamnia A.A., Rahmani F.A., Javadian S., Dibavand N. Aluminium phosphide poisoning in mice and the procedure for its managements. JBUMS. 2000;2(4):25–33. [Google Scholar]
- 45.Azad A., Lall S., Mittra S. Effect of N-acetylcysteine and L-NAME on aluminium phosphide induced cardiovascular toxicity in rats. Acta Pharmacol. Sin. 2001;22(4):298–304. [PubMed] [Google Scholar]
- 46.Chaudhry D., Rai A.S. N-acetyl cysteine in aluminum phosphide poisoning: myth or hope. Indian J. Crit. Care Med. 2014;18(10):646–647. doi: 10.4103/0972-5229.142172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Manoochehri A., Ahangar R.M., Bigvand P., Nakhaee S., Mehrpour O. A case of successful treatment of heart failure due to simultaneous poisoning with aluminum phosphide and zinc phosphide. Iran. Red Crescent Med. J. 2018 (In Press) [Google Scholar]
- 48.Tehrani H., Halvaie Z., Shadnia S., Soltaninejad K., Abdollahi M. Protective effects of N-acetylcysteine on aluminum phosphide-induced oxidative stress in acute human poisoning. Clin. Toxicol. 2013;51(1):23–28. doi: 10.3109/15563650.2012.743029. [DOI] [PubMed] [Google Scholar]
- 49.Bhalla A., Jyothinath P., Singh S. Antioxidant therapy in patients with severe aluminum phosphide poisoning: A pilot study. Indian J. Crit. Care Med. 2017;21(12):836. doi: 10.4103/0972-5229.220744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Taghaddosinejad F., Farzaneh E., Ghazanfari-Nasrabad M., Eizadi-Mood N., Hajihosseini M., Mehrpour O. The effect of N-acetyl cysteine (NAC) on aluminum phosphide poisoning inducing cardiovascular toxicity: a case–control study. SpringerPlus. 2016;5(1):1948. doi: 10.1186/s40064-016-3630-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Agarwal A., Robo R., Jain N., Gutch M., Consil S., Kumar S. Oxidative stress determined through the levels of antioxidant enzymes and the effect of N-acetylcysteine in aluminum phosphide poisoning. Indian J. Crit. Care Med. 2014;18(10):666. doi: 10.4103/0972-5229.142176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oghabian Z., Mehrpour O. Treatment of aluminium phosphide poisoning with a combination of intravenous glucagon, digoxin and antioxidant agents. Sultan Qaboos Univ. Med. J. 2016;16(3):e352. doi: 10.18295/squmj.2016.16.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Halvaei Z., Tehrani H., Soltaninejad K., Abdollahi M., Shadnia S. Vitamin E as a novel therapy in the treatment of acute aluminum phosphide poisoning. Turk. J. Med. Sci. 2017;47(3):795–800. doi: 10.3906/sag-1512-6. [DOI] [PubMed] [Google Scholar]
- 54.Abdolghaffari A.H., Baghaei A., Solgi R., Gooshe M., Baeeri M., Navaei-Nigjeh M. Molecular and biochemical evidences on the protective effects of triiodothyronine against phosphine-induced cardiac and mitochondrial toxicity. Life Sci. 2015;139:30–39. doi: 10.1016/j.lfs.2015.07.026. [DOI] [PubMed] [Google Scholar]
- 55.Goharbari M.H., Taghaddosinejad F., Arefi M., Sharifzadeh M., Mojtahedzadeh M., Nikfar S. Therapeutic effects of oral liothyronine on aluminum phosphide poisoning as an adjuvant therapy: a clinical trial. Hum. Exp. Toxicol. 2017;37(2):107–117. doi: 10.1177/0960327117694074. [DOI] [PubMed] [Google Scholar]
- 56.Türkez H., Toğar B. Aluminium phosphide-induced genetic and oxidative damages in vitro: attenuation by Laurus nobilis L. leaf extract. Indian J. Pharmacol. 2013;45(1):71. doi: 10.4103/0253-7613.106439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Turkez H., Togar B. Aluminum phosphide-induced genetic and oxidative damages in rats: attenuation by Laurus nobilis leaf extract. Toxicol. Ind. Health. 2013;29(7):579–583. doi: 10.1177/0748233711433942. [DOI] [PubMed] [Google Scholar]
- 58.Humayun M., Haider I., Badshah A., Subhan F. Protective role of G6PD deficiency in aluminium phosphide poisoning. J. Coll. Phys. Surg. Pak. 2015;25:S66–8. https://doi.org/04.2015/JCPSP.S66S68. [PubMed] [Google Scholar]
- 59.Zamani N., Mehrpour O. Protective role of G6PD deficiency in poisoning by aluminum phosphide; are there possible new treatments? Eur. Rev. Med. Pharmacol. Sci. 2013;17(7):994–995. [PubMed] [Google Scholar]
- 60.Salimi A., Paeezi M., Yousefsani B.S., Shadnia S., Hassanian-Moghaddam H., Zamani N. Inhibition of glucose-6-phosphate dehydrogenase protects hepatocytes from aluminum phosphide-induced toxicity. Pestic. Biochem. Physiol. 2017;143:141–146. doi: 10.1016/j.pestbp.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 61.Soltani M., Shetab-Boushehri S.F., Mohammadi H., Shetab-Boushehri S.V. Proposing boric acid as an antidote for aluminium phosphide poisoning by investigation of the chemical reaction between boric acid and phosphine. J. Med. Hypotheses Ideas. 2013;7(1):21–24. [Google Scholar]
- 62.Soltani M., Shetab-Boushehri S.F., Shetab-Boushehri S.V. Chemical reaction between boric acid and phosphine indicates boric acid as an antidote for aluminium phosphide poisoning. Sultan Qaboos Univ. Med. J. 2016;16(3) doi: 10.18295/squmj.2016.16.03.007. e303-e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Baghaei A., Solgi R., Jafari A., Abdolghaffari A.H., Golaghaei A., Asghari M.H. Molecular and biochemical evidence on the protection of cardiomyocytes from phosphine-induced oxidative stress, mitochondrial dysfunction and apoptosis by acetyl-l-carnitine. Environ. Toxicol. Pharmacol. 2016;42:30–37. doi: 10.1016/j.etap.2015.12.019. [DOI] [PubMed] [Google Scholar]