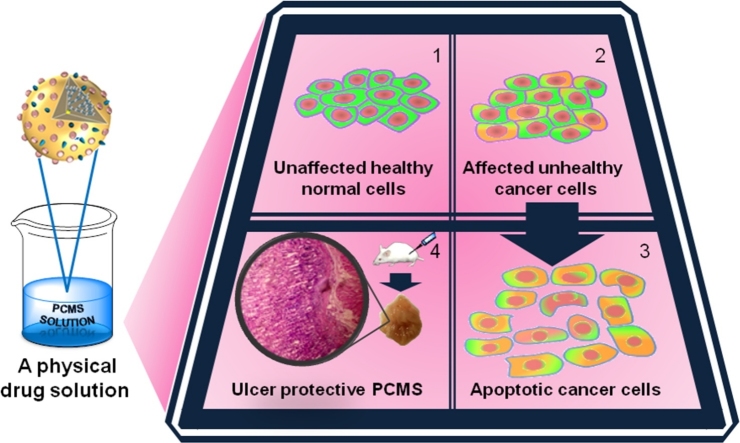
Graphical abstract
Abbreviations: PAMAM, poly(amido)amine; P, PAMAM; PC, PAMAM-controller; PCM, PAMAM controller-motor; PCMS, PAMAM-controller-motor-sensor; AGS, human caucasian gastric adenocarcinoma; G, generation; ROS, radical oxygen species; CNDP, critical nanoscale design parameters; CEES, combined excitation emission spectroscopy
Keywords: Nonchemical drug, Dendrimer toxicity, Gastric ulcer, Matrix metalloproteinase, Inflammation
Highlights
-
•
PCMS is a new physical drug that shows selective activity towards gastric cancer.
-
•
PCMS treated tissues have very comparable ulcer index with the control group.
-
•
In ethanol on mice ulcer model PCMS down regulates both pro- & active- MMP9.
-
•
In a particular narrow dose window PCMS can act as a potential cancer suppressor.
-
•
Physical drug interact with the target molecules via precise resonance vibrations.
Abstract
PC, PCM, PCS, and PCMS are our designed & synthesized ∼8 nm PAMAM dendrimer (P) -based organic supramolecular systems, for example, PCMS has 32 molecular motors (M), 4 pH sensors (S) and 2 multi-level molecular electronic switches (C). We have reported earlier following a preliminary in-vitro test that the synthesized PCMS can selectively target cancer cell nucleotides if triggered wirelessly by an electromagnetic pulse. Here to further verify its drug potential, we have studied the preliminary efficacy, toxicity, and pharmacokinetics of P derivatives (PC, PCM, PCMS) in-vivo and in-vitro. We used ethanol-induced gastric inflammation model and cultured human gastric epithelial cells AGS to examine to the toxicity of PAMAM dendrimers cell permeability and toxicity, in (a) the cultured human gastric epithelium cells (AGS), and in (b) the gastric ulcer mice model. Here we report that the toxicity of PAMAM dendrimer (>G3.5) P can be reduced by adding C, M and S. Gastric ulcer is the primary stage of the manifestation of acute inflammation, even gastric epithelial cancer. Ethanol causes ulceration (ulcer index 30), thus upregulates both pro and active MMP-9. A 50 μl PCMS dose prior to ethanol administration reduces ulceration by ∼80% and downregulates MMP-9 and prevents oxidative damages of gastric tissue by ECM remodeling. Alcohol's inflammation of mouse stomach causes up-regulation of both pro and active MMP-9, resulting in oxidative damages of gastric tissue by ECM remodeling. PCMS in particular dose window reverses & alters ECM remodeling, thus, neutralizing alcohol-induced inflammation & generation of ROS.
1. Introduction
Molecular biology literatures are rich in ab initio structural analysis of different organic and inorganic drugs. However, the mapping of simultaneously operating toxic pathways requires analyzing many body systems. Technologically, underpinning an accurate mechanism for simultaneously operating pathways are always difficult. Proteins and highly complex biomolecules undergo multiple successive structural changes prior to executing their functions [1,2]. A drug agent [3,4] becomes toxic if it causes ions or pH imbalance, harmful dipolar interactions and active chemical site of biomolecules is blocked such that it eventually disrupts the cellular functions. Many potentially bioactive molecules are preliminary conceived as drug, but are not translated from bench to bedside in the post animal-trial scenario [5,6] as they show a cascade of toxic effects beyond the range of tested applications. It causes a huge waste of resources over the years in the drug development process. Therefore, since our synthesized PCMS has showed selective activity in targeting the cancer cell nucleotides [7], then the further study of toxicity and pharmacokinetics became a reason for this study. Many synthetic routes are adopted to resolve the toxicity issues [[8], [9], [10], [11]]. However, toxicity studies are mostly confined within the domain of the target disease, e.g. drug delivery [12], which has repeatedly found vulnerable to the generic medicinal applications. Toxicity of a chemically modified molecule [[13], [14], [15], [16], [17], [18]] turns unpredictable under the variable biological, medical and genetic conditions. The origin of toxicity is poorly understood due to the multitude of variables factors work together in a real-time environment [19,20]. However, it encourages us for developing new technologies like drug delivery, biotechnology, bacteria filtration etc. [[21], [22], [23], [24]].
For years, we are developing PAMAM dendrimers (>G3.5) based chemically inert and purely physical analogue [7,[25], [26], [27], [28]] to the existing chemical drugs, —wirelessly operated by an electromagnetic antenna like remote controller located outside the body. The purely organic device is designed in such a way that it would not release any chemical in the body but physically interact with the target biomolecule in the infected parts with the vibrations in a molecularly precise manner. The organic supra-structure [[25], [26], [27], [28]], thus developed is, approx. 8 nm in size and if an antenna triggers its multiple vibrational modes remotely [[28], [29], [30], [31]], it can generate various kinds of oscillations in the proximity areas of the target molecule. Though earlier we have identified the target potential of our PCMS on the cancer cells, yet the application of such nano-platform is robust and in addition, its design could be manipulated for any target disease by changing composition of molecules of particular properties. Here, as argued above, our interest is in documenting the possible side effects of the remotely controlled PCMS, beyond the target disease and the target organs.
PAMAM dendrimer structure is already proven useful for the drug delivery as it has an amide chain backbone and has a high concentration of dielectric charge like other natural bio-molecules (e.g. polypeptides and proteins) [32,33]. It is structurally close to a globular shaped protein and can form stable complexes with drugs, oligonucleotides, plasmid DNA, and antibodies. It can target any organ, especially brain, lung, heart and kidney where it accumulates temporarily [34]. However, if the peripheral terminal groups if kept free, they affect the cell membrane by triggering apoptosis [35]. PAMAM dendrimers are tunable for both therapeutic applications and diagnostics, however, it is crucial to note the drug usability and cytotoxicity of unmodified pure PAMAM dendrimers before the cytotoxicity test of terminal group neutralized PAMAM dendrimers [36,37]. Dendrimers (>G3.5) cross the neuron cell membranes via endocytosis; however, the surface functionalization of a dendrimer changes its diffusion ability significantly. In the neurons, if one uses a sub-micromolar dose (<100 nM dose is safe), it does not even cause an apoptosis [38]. Amine-terminated PAMAM G2.0, G3.0 and G4.0 limits the non-enzymatic modifications of biomacromolecules, G3.0 works best as the number of surface groups available determines the degree of bonding with the target biomolecules. However, the toxicity of PAMAM G3.0 > G4.0; cationic amine surface of PAMAM G4.0 binds with glucose by stable bonding, so, it has the ability to scavenge the glucose excess to cut the protein glycation and glycoxidation (treating severe hyperglycemia). Thus PAMAM may be used as a therapeutic agent in a poorly controlled diabetes mellitus [39], multiple co-factors are cited in defense. The primary cytotoxicity determining factors of pure PAMAM dendrimers are: (i) the time gap between the repeated administrations and the supplementation duration (prolonged dose accumulates unprotected PAMAMs fraction beyond the threshold tolerance [40],); (ii) the choice of solvent (e.g. PAMAM dendrimer has a long-term stability in methanol); (iii) the route of dendrimer administration and the extrapolation of the in-vitro results in its in-vivo performance; (iv) the surface density of cations to the dendrimer volume ratio (tenability between toxicity and effectiveness of PAMAM); (v) selection of a suitable tissue [41]; (vi) mutually exclusive biodegradability and non-immunogenicity; (vii) cellular uptake & transport, bio-distribution, elimination of target, accumulation, and excretion, all five interconnected features of a drug performance, which depend on the engineering of the dendrimer’s branch-dynamics (Critical Nanoscale Design Parameters, CNDP; [42];). (viii) Temporary accumulation of Dendrimers in liver, pancreas, heart, and kidneys, although the irreversible damage is nominal [43]. (ix) Intracellular signalling pathway (e.g. Akt/mTOR pathway is inhibited by PAMAM via modulation of ROS generation and the Erk1/2 pathway is activated to trigger autophagy in neurons) needs to be protected from PAMAM toxicity for a futuristic neuro-disease treatment [[44], [45], [46]]. (x) PAMAM reaches the brain via systemic circulation or via olfactory nerve route and modulates the gene expression of a brain-derived neurotrophic factor signaling pathway [6].
We edited the several surface groups of PAMAM by attaching a variable number of molecular rotors [[25], [26], [27], [28], [29], [30], [31]] to balance the adoptability and toxicity as well as to support the vibrational tuning. The resultant dynamics of a rotor attached PAMAM changes dramatically the high number of dipole-dipole interactions with the neighboring rotors & the proteins. These rotors displace the end charge amine -NH2 and −COOH terminals which tends to bind with the proteins. These groups are known to damage the cell membrane and cell organelles by unwanted bonding instead of phagocytosis [47] We reported earlier in detail how motors arrest the unpredictable behavior of PAMAM; [7,[25], [26], [27]]. An ordered dynamic behavior is bearable for the cell to work but an uncontrolled dynamics may trigger unwanted molecular processes in a cell [48]. Compatibility and viability with the in-vivo system by escaping the protective immune response can be optimized as we vary the design of the synthetic derivative. From its internalization to the cell invasion through the membrane and a safe secretion from the cell was monitored via CEES spectroscopy of excretion. The functional groups arranged in a particular manner on PAMAM may create a hostile environment for the cells. One of the best examples is our PCMS system, where we added only four pH sensor molecules on the PAMAM surface in addition to the 32 molecular rotors; the proton-sensing ability dramatically improves the derivative’s cellular acceptance. We reported [7] and patented [[28], [29], [30]] PCMS as a wireless, remotely controlled nanobot for regulating the target molecule's dynamics. As we encapsulated a multi-level switch C [[49], [50], [51]], Nile red doped inside PAMAM generated a cyclic triangular oscillation or clocking like vibration between the three components (multilevel switch C, pH sensor, S and the motor, M) inside PCMS [[25], [26], [27]]. We have already described [7] how these three components resonant oscillations enable them to mimicking the vibrations of the desired target (mutated DNA, deformed proteins & their accumulation etc.). Resonant oscillations associated with mechanical vibration destroys the material's weak structural bonds [7] or arrests the molecules key dynamics leading to fatal cellular action in a molecularly precise manner [48]. It is worthy to mention that we did not add a delivery agent to PCMS [52] surface. Here, we have tested multiple modified variants of PAMAM on the human gastric cells in-vitro, mice in-vivo, addressing all the three toxicity challenges mentioned above, i.e. the charge density regulation, controlling unwanted dynamics and composition of right functional groups.
2. Material and methods
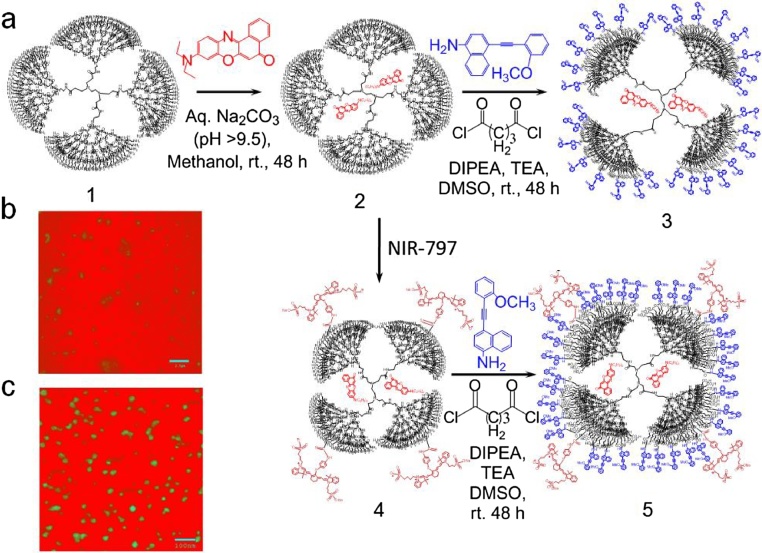
5th generation PAMAM dendrimer [P] was purchased from Aldrich chemicals and we carried out basic characterizations to confirm the structure and its purity. Nile red [C] was bought from Tokai chemicals. Nile red encapsulated PAMAM dendrimer (sample-1, P + C PC) and two other synthetically modified dendrimer derivatives (sample -2 & -3) were used for invitro and in-vivo studies. Sample-2 was prepared by chemically connecting 32 rotor molecules [M] on the surface of the compound PC to result P + C+M = PCM derivative. A chosen pH sensor molecule (NIR-797) [S] is connected to the surface to produce Sample 3 [P + C+M + S=PCMS]. The molecular rotor M was designed, synthesized and characterized in our laboratory. For in-vitro cell culture study, we used human gastric cells as it exhibits much visible change in morphology. Details of the synthesis of organic structures are described in our earlier reports [[25], [26], [27]]. PCMS destroys the cancer cells and Aβ plaques only after a wireless ac/dc signal of a specific frequency is applied. It is a purely physical drug that activates the cyclic energy transfer inside PCMS wirelessly. As far as the toxicity is concerned, the most optimized structure of desired activity among P, PC, PM, PCM, PCS, and PCMS (see notes in the parenthesis above to decode the names) is identified as PCMS. In fact, we started our toxicity studies from P, and subsequently added various alternate functional groups improving two factors, the capacity to trigger the molecule from distance and cytotoxicity. However, on the basis of most important observations regarding the toxicity analysis, we are providing a brief report of three samples only, PC (sample 1), PCM (sample 2) and PCMS (sample 3) (Fig. 1).
Fig. 1.
(a) Schematic presentation of synthetic scheme of PAMAM dendrimer derivatives, from P (1) to PC (2), PCM (3) & PCMS (5) preparation; (b, c) TEM images of PCM &PCMS samples respectively. PCM (scale 2.5 nm) showing particles are not well resolved, yet it can be predicted to be composed of mostly inconsistent spherical aggregates. PCMS (scale 100 nm) image showing well resolved round particles (ranging from ∼8–28 nm).
2.1. Cell culture and viability studies
Human gastric adenocarcinoma cell line AGS were grown in a RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS) and 1% antibiotic solution containing penicillin, streptomycin and an antimycotic agent. This cell line was grown in a humidified incubator containing 5% CO2 and 95% air at 37 °C [53]. For experiments, cells were seeded (2 × 106 cells/well) into 6 well plates and cultured for 24 h. 2.5% ethanol (∼25 mM) was added to cells for 6 h as damaging agent. Protection studies were done with the PCMS (sample 3) 12 h prior to the addition of ethanol. Assays vehicle controls were included which did not affect any of the parameters measured.
To determine cell viability in presence of ethanol, the colorimetric MTT assay was used. AGS cells (1 × 104 cells/well) were cultured in a 96-well plate at 37 °C [54]. On confluency, a set of wells were treated with 2.5% alcohol media only, and in another 5% alcohol was given. For both the cases, 3 time points were chosen, 15, 30 and 60 min. The experiment was repeated for 3 times. A dose vs. time curve was prepared for the standardization of the doses of ethanol [Fig. 6].
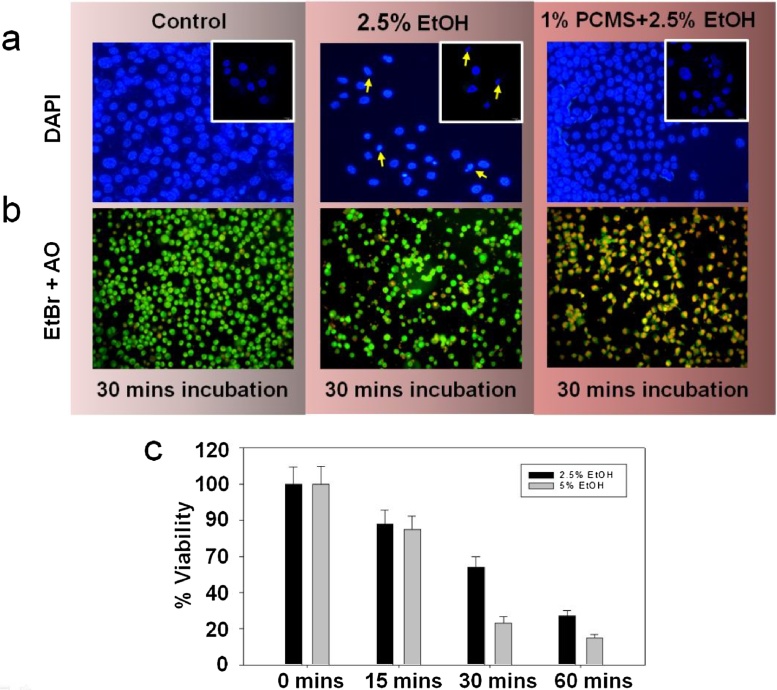
Fig. 6.
DAPI and EtBr-acridine orange staining of AGS cells. AGS cells were grown and treated as described and stained with (a) DAPI (top row panels) and (b) EtBr-acridine orange cocktail (bottom row panels), which revealed the nuclear morphology as well as the apoptotic state depending on the membrane integrity. Inset pictures in the panel-a show the 60X magnified form. (c) It was seen that viability of cells did not decrease significantly on 5 min. exposure for both the concentrations. When the exposure time was further increased for more 15 min., the viability decreased by 50% in case of 2.5% ethanol treated cells whereas viability decreased to ∼25% in 5% ethanol treated cells. We chose the damaging concentration of ethanol as 2.5% and time as 30 min. Beyond that, the cells were excessively damaged.
2.2. DAPI and acridine orange/EtBr staining on AGS cells
Acridine orange/EtBr (AO/EB) cocktail and DAPI staining experiments on AGS cells were performed in order to study the apoptotic behavior and the morphological changes of cells after exposure to either PCMS (sample 3) alone or in combination with 2.5% ethanol. AO/EB staining combines the differential uptake of fluorescent DNA binding dyes AO and EB, and the morphologic aspect of chromatin condensation in the stained nucleus, which allows distinguishing between viable, apoptotic and necrotic cells. Viable cells possess uniform bright green nuclei. Early apoptotic cells show bright green areas of condensed or fragmented chromatin in the nucleus and necrotic cells show uniform bright orange nuclei. After the exposure time, 100 μg/ml of AO/EB and 10 mM of DAPI, DNA-specific fluorescent dyes, were added to the cell monolayer in each separate well and the plates were incubated for 30 °C mins in dark at room temperature [55,56] The stained cells were then observed under an Olympus fluorescence microscope.
2.3. Scratch assay
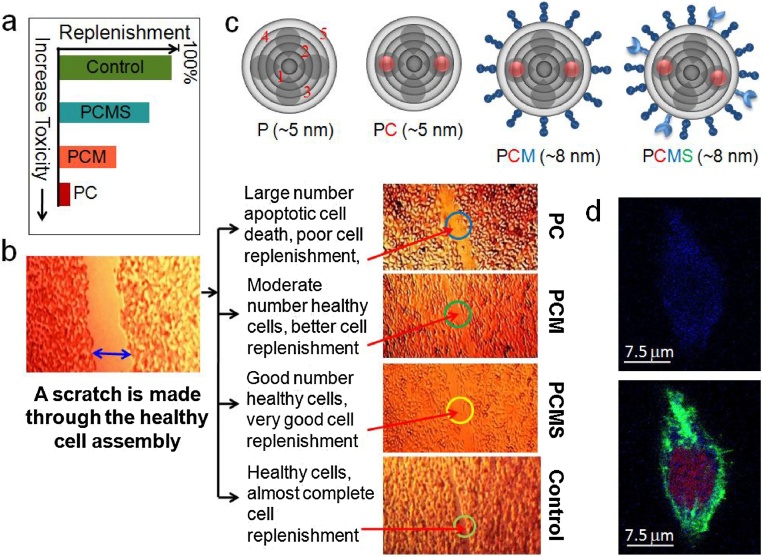
AGS cells were cultured as described above in separate culture wells and after the cells became 80% confluent on the surface, 2 nM of each sample-1, sample-2 and sample-3 were applied separately into three separate sets of the wells and incubated. Then a mechanical scratch was made manually, across the substrate, which looks like a channel through each of the confluent wells [57]. A separate fourth well was kept for control. After this, they were further incubated for 6 h (Fig. 2).
Fig. 2.
(a) An graphical presentation of in vitro gastric cell replenishment profile after treatment with different 5th generation PAMAM dendrimer derivatives. (b) Study of gastric cell replenishment after a 6 h incubation with different 5th generation PAMAM dendrimer derivatives and efficacy of replenishment is compared with the control; (c) A graphical presentation of P (where 1,2,…numbers represent generation of dendrimer branching & dark shadows represent structural cavities), PC (red spheres - controller molecules), PCM (deep-blue dumbbells - molecular rotors) and PCMS (light-blue crab legs - sensor molecule) and their effective diameters; (d) 3D cell images under confocal fluorescence microscopy, blue dots inside cell indicate the evasiveness of PCMS.
2.4. Animal experiment
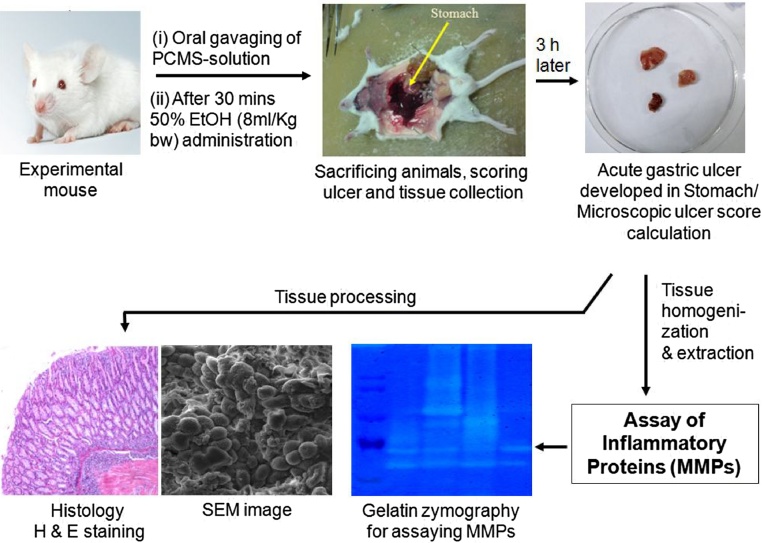
Male Balb/c mice, each weighing approximately 20–25 g m were acclimatized to conditions in animal house (21 ± 2 °C, 60 ± 10% relative humidity, 12 h/12 h light/dark cycle) for 7 days. All the animals except the control group were fasted overnight before experiment with free access to water. The animals were anesthetized with ketamine (12 mg/kg b. wt.) followed by cervical dislocation for killing [52]. The mice were randomly divided into seven groups and each group having four animals were as follows: i) control; ii) ethanol-induced stomach damage (50% EtOH, 6 ml/kg body wt); iii) only PCMS (1.3 mM) 50 μl for 30 min; iv) PCMS (1.3 mM) 10 μl + ethanol (same dose as before); v) PCMS (1.3 mM) 25 μl + ethanol; vi) PCMS (1.3 mM) 50 μl + ethanol, and vii) PCMS (1.3 mM) 100ul + ethanol. Mice were orally fed with 50% ethanol at 6 ml/kg body weight to induce acute ulcer while the control group received only sterile PBS. The PCMS solution was administered prior to ethanol as ulcer-protecting agents. Animals were sacrificed after 3 h, stomachs were isolated and ulcer index were scored. All experimental procedures and protocols used in this study were reviewed and approved by the animal ethics committee of CSIR-Indian Institute of Chemical Biology, and were conducted according to the guidelines.
2.5. Histological analysis
For the histology studies, stomachs from all groups of mice were fixed in formalin and embedded in paraffin. The sections (5 μm) were cut by microtome, stained with hematoxylin and eosin, and assessed under an Olympus microscope (Olympus Optical Co., Hamburg, Germany). Images were captured using Camedia software (E-20 P 5.0 Megapixel) at original magnification 10 × 10 X and processed under Adobe Photoshop version 7.0 [58,59].
2.6. Scanning electron microscopy (SEM)
A small part of stomach tissue was excised from mice of all the groups. Those were fixed in 2.5% glutaraldehyde buffered in 0.1 M phosphate, washed in phosphate buffer thrice and osmicated in 2% osmium tetraoxide. The specimens were dried in a critical point drying apparatus (Quorum Technologies) by liquid CO2, mounted on aluminium stubs and vacuum coated with gold palladium (Polaron SC 7620). Coated specimens were then viewed in a SEM (TESCAN VEGA II L50) operated at 10 kV [59]. The entire specimen was scanned on a monitor at X 2.5K and 5K magnification.
2.7. Tissue extraction
The whole stomachs (including fundic, body and pyloric parts) were washed with normal saline. Stomachs, except connective tissue layer were suspended in 10 mM phosphate-buffered saline (PBS) containing EDTA free protease inhibitor cocktail, minced and centrifuged at 12,000 g for 15 min at 4 °C. The supernatants were collected as PBS extracts. The pellet was then extracted in lysis buffer containing triton-X-100 and centrifuged at 12,000 g for 15 min to obtain TX extracts. All extracts were preserved at −80 °C freezer [[58], [59], [60]].
2.8. Gelatin zymography
For the assay of MMP-2 and MMP-9 activities, tissue extracts were electrophoresed in 8% SDS polyacrylamide gel containing 1 mg/ml gelatin under non-reducing conditions. The gels were washed in 2.5% Triton-X-100 and incubated in calcium assay buffer (40 mM Tris−HCl, pH 7.4, 0.2 M NaCl, 10 mM CaCl2) for 18 h at 37 °C and stained with 0.1% coomassie blue followed by destaining. The zones of gelatinolytic activity came as negative staining. Quantification of zymographic bands was performed using densitometry linked to proper software (Image J) [[58], [59], [60]].
3. Result & discussions
In the recent advancements of multidisciplinary science, PAMAM dendrimer structures are one of the widely used supramolecular drugs. With the change in structural symmetry, its’ different branches reorganize and redistribute the potential energy across its branches, therefore, the property is widely tuneable and new properties may be induced. It has the ability to cross the membrane barrier of a cell and to alter the normal cellular physiology. In order to be a drug, a molecule should be nontoxic, free from adverse effects, and biocompatible. Here, we showed a generic protocol for minimizing the inherent toxicity of PAMAM dendrimer and improving its biocompatibility by improvising the characteristics of a generic and effective drug for the inflammatory diseases. Therefore, we can organize multiple molecular systems in a programmed manner to eliminate toxicity and encode drug activity.
Since our target is to map toxicity of a drug in the domain of its application, we begin our toxicity study with the in-vitro tests on AGS cells. A groove is made by a scratch through the confluent cultured AGS cells after treatment with PCMS and other samples and incubated for 6 h. Replenishment in the scratch area indicated that the cells were relatively healthy with PCMS, having a normal potential of cell division (Fig. 2). Treatment with an external agent to the cells would hamper its normal physiology by interfering with the cell division as well as the cell cycle by imparting cytotoxicity. Our data suggested that unlike PC and PCM PCMS didn’t hamper much the normal rate of cell division of AGS cells, which signifies that PCMS was less-toxic reagent; otherwise, the cells would become unhealthy and apoptotic. In the three different experimental wells of cells (Fig. 2), a treatment with the different forms of modified dendrimer demonstrated an increased rate of cell division from the PC to PCM to PCMS using equal concentration of the molecules. Thus, the decreased order of toxicity is observed due to the functional modification of the PAMAM structure. Surprisingly the addition of pH sensor molecules on the surface of a PAMAM dramatically changes the toxicity scenario. An extensive review of the previous toxicity studies on P as described in the beginning, could be revisited here, and we could suggest that P is the most toxic material and PCMS is the least (P > PC > PCM > PCS > PCMS).
DAPI and acridine orange/EtBr (AO/EB) cocktail staining experiments were performed in order to study the apoptotic behavior and the morphological changes of the cells after administration of 1% PCMS in the serum free media. When the cells were treated with 2.5% ethanol for 30 min. only, the cells showed a loss of adherence, became distorted and apoptotic, which is reflected as a yellow mark in the AO/EtBr staining. DAPI staining by confocal microscopy also revealed a condensed and shrunk nucleus with the fragmented chromatin. If the cells were treated with 1% PCMS for 2 h (data not shown) prior to the ethanol addition, the damage was more pronounced. AO/EtBr staining demonstrated more yellow to orange colored cells with a lesser number of cells in many fields in Fig. 6. More condensed nucleus with significant morphological derangement in the DAPI staining signifies the toxicity of PCMS on isolated cells.
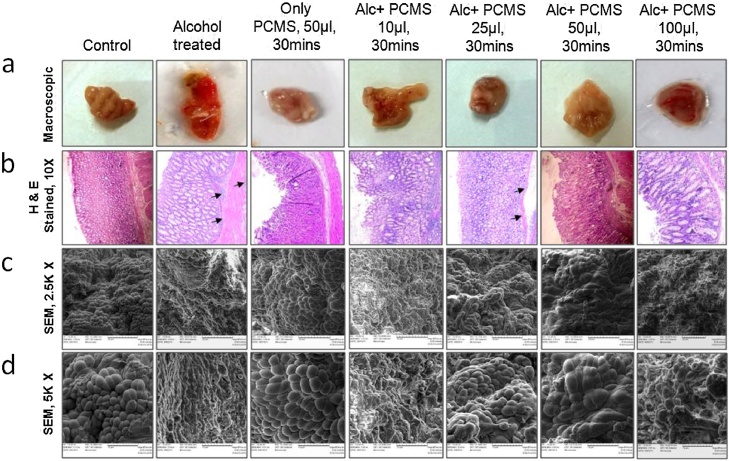
Instead of all, we select only three, PC, PCM and PCMS to study their toxicity on the experimental animal model of gastric inflammation along which is outlined in Fig. 3. However, the result of the animals that were administered with PCMS in different doses is discussed here with full details. After the PCMS is administered in different doses as described in the material and methods section, the animals were then sacrificed. The tissues were collected and processed. The micrographs stated that the surface morphology of the gastric tissues, the thickness of epithelial layer and the integrity of glandular layer of the tissues were perturbed on treatment with ethanol. The toxicity of the cells is rescued by a particular dose window of PCMS as revealed from histology, scanning electron micrographs, both in low and high magnifications as depicted in Fig. 4. Ethanol produced an ulcer index of 30, however, the control group showed a very few patches of ulceration with a minimum ulcer index of 5. When the animals were treated with PCMS (sample 3) only for 30 min, the ulcer index was quite comparable to the control group. However, when they were treated overnight with the PCMS, it became very toxic and the index increased to 35. The animal group that was treated with 10 μl PCMS for 30 min prior to the ethanol administration, developed ulcers as they had prominent patches in the inner lining of their stomach. It signifies that this dose didn’t show much ulceroprotective potential. Another group of animals were treated with 25 μl PCMS as preventive drug prior to ethanol administration and it showed better protection, the ulcer index reduced by 33%. The group which received 50 μl PCMS prior to ethanol administration demonstrated a protection by almost 80%. Interestingly, when the dose of PCMS was increased beyond this limit and administered prior to ethanol, it caused severe ulcer. This observation again proved that the selection of proper dose of a drug is very crucial step before proceeding for the further studies with it.
Fig. 3.
A schematic presentation of the toxicology study experiment of the of 5th generation PAMAM dendrimer derivative PCMS while pretreated to ethanol treated mouse. The alcohol induced ulcer progression of was investigated through SEM imaging, gelatin zymography and tissue histology studies.
Fig. 4.
Macroscopic appearances, histology and scanning electron micrographs of mice gastric tissues after ulcer induction by ethanol and protection by PCMS (sample 3). Gastric ulcers were induced in mice by oral administration of 50% ethanol and PCMS solution was administered orally prior to ethanol treatment in different doses as explained. The control mice received sterile PBS only. After 3 h, mice were sacrificed and the stomachs were processed for histology and SEM studies. Each column in the panel attribute to the experimental condition as designated in the overhead title. (a) Macroscopic pictures of the stomach of mice of all the groups after dissection. (b) Transverse sections of stomach tissues of mice were stained with hematoxylin and eosin and observed at 10X magnification. Histological appearances of gastric epithelium, pits and glands in (i) control, (ii) ethanol treated, (iii) only PCMS treated for 30 min (iv) PCMS 10 μl pretreated ethanol treated, (v) PCMS 25 μl pretreated ethanol treated, (vi) PCMS 50 μl pretreated ethanol treated and (vii) PCMS 100 μl pretreated ethanol treated tissues. Gastric mucosal epithelium and glandular region (black arrows) were demonstrated. (c, d) Scanning electron micrographs of the above mentioned groups were shown. Tissues were processed and photographed as described in “materials and methods” section. Oval or circular epithelial cells were seen with regular arrangement in control and only PCMS treated tissues, while loss of epithelial cells integrity was palpable in ulcerated and PCMS 100 μl treated tissues which was almost restored to control level in PCMS 25 μl and 50 μl treated tissues. Again the tissue architecture is lost in presence of ethanol when the dose is increased beyond 50 μl.
The ulcer scores were seen prominently in the macroscopic pictures in the uppermost panel of Fig. 4. In the second panel of histology the sections stained with hematoxylin and eosin, showed the different layers of the stomach tissues in mice. It showed an exfoliation of the epithelial cells, disruption of the mucosal layer, gastric pits and an infiltration of the inflammatory cells in the ethanol treated group as compared to control. Only PCMS treated tissues showed very comparable morphology with the control group with intact layers of tissues. Tissues from the animals treated with 10 μl PCMS followed by alcohol treatment showed disrupted epithelial layers in stomach tissues. There were significant marks of inflammation as the neutrophil infiltrations were there. This observation signifies that the particular dose of PCMS is not potent as anti-ulcer agent. A dose of 25 μl PCMS and then alcohol treated tissues displayed a marked protection against the ethanol-induced damage. Moreover, an inflammation in the sub mucosal region was also reduced by the pre-treatment of this dose of PCMS compared to the ethanol-treated groups. When the animals were pre-treated with 50 μl PCMS prior to the ethanol treatment, the protection it provided was very significant. There was very little neutrophil infiltration in the submucosal and in the glandular region. On the contrary, when the preventive dose was increased beyond 50 μl, then, due to an excessive acid secretion, the tissue showed more disruption in the structure with erosions in many places.
Scanning electron micrographs showed changes in the surface morphology of the gastric epithelial tissues upon an ethanol treatment. Control tissue exhibited >90% intact epithelial layer as compared to the ulcerated tissues where the tissue architecture and the orientation of the cells were significantly damaged. 50 μl PCMS (sample 3) followed by ethanol treated group showed >75% live epithelial cells with slight erosions, which corroborates the fact obtained from the macroscopic and histological studies. The magnified SEM pictures showed the ultrastructural changes in the tissues very vividly. Excessive damage was observed in higher doses (100 μl) of PCMS while no protection was observed by lower doses (10 μl) of PCMS treatment prior to ethanol administration.
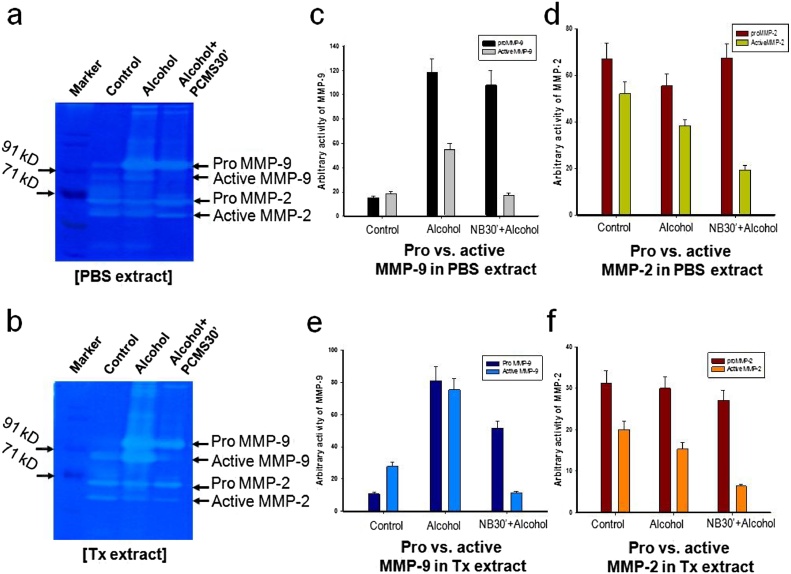
Literatures suggest that an acute dose of ethanol induces gastric inflammation along with the generation of ROS. It causes an up-regulation of pro MMP-9, which, in turn, produces some oxidative damages to the cellular macromolecules. MMPs are capable of degrading the ECM proteins and are highly involved in playing a role in the ECM remodeling gastric tissues. The regulation of MMPs activity, in turn, alters the ECM remodeling which could be modulated by various drugs during a disease management. The up- and down regulation of the MMPs at the gene and the protein levels are thought to be major underlying mechanisms for tissue damage and regeneration during the ulceration as well as healing. To assess the activities of the gelatinases, i.e. MMP-2 and MMP-9, gelatin zymography was done for PBS and TX extracts of the gastric tissues of different groups of mice (Fig. 5a and b) which showed that the optimum dose of 50 μl of PCMS prevented the ethanol-induced gastric ulcer and associated with attenuation of pro MMP-9 activity. Ethanol administration resulted in an almost 6 fold up regulation of synthesized pro MMP-9 activity compared to control (Fig. 5e). PCMS pretreatment reduced the pro MMP-9 by 50% compared to the ethanol-treated group. While comparing stomach of ethanol vs. PCMS + ethanol group, the level of active MMP-9 significantly reversed by PCMS treatment (Fig. 5c, e). There were no significant changes in pro-MMP-2 activities in the presence of alcohol and prior treatment with PCMS (Fig. 5d, f). Previous studies from our laboratory suggested that the pro MMP-9 synthesis and the secretion increase in a dose dependent manner in the gastric tissue of experimental animal treated with alcohol [30]. The present study supports that the activity of pro MMP-9 was higher in an ethanol treated tissue extracts as compared to the control group and PCMS reduced MMP-9 (pro and active) activity significantly in a very narrow dose window. In summary, alcohol produces an inflammation in the inner lining of the mouse stomach, which causes an up-regulation of synthesized and secreted pro MMP-9. In addition, PCMS, in a particular dose window, prevented this change of both pro and active MMP-9 while preventing the inflammation. Histology pictures demonstrated inflammatory cell infiltration in the tissue spaces, thus mediated an extracellular tissue remodeling during ulceration and PCMS reverse the altered ECM architecture protection of gastric inflammation.
Fig. 5.
Role of PCMS (sample 3) on MMP-9, MMP-2 activities during prevention of gastric ulcer. Acute gastric ulcers were induced in rats as described in Fig. 4. Equal amount of (a) PBS extracts and (b) Tx extracts (70 μg proteins in each lane) were electrophoresed in 8% non reducing SDS polyacrylamide gel containing 1 mg/ml of gelatin and MMP activity was estimated. (c) Histographic representation of the comparative analysis of secreted proMMP-9/ activeMMP-9 activities (d) secreted proMMP-2/ vs. activeMMP-2 activities (e) synthesized proMMP-9/activeMMP-9 (f) synthesized proMMP-2/activeMMP-2 activities during acute ulceration and its prevention with 50 μl of PCMS for 30 min only. Pretreatment with 50 μl of PCMS for 30 min significantly reduced the activity of proMMP-9 near to control, both secreted 33 and synthesized forms; whereas, it didn’t show any significant impact on the MMP-2 activities while preventing gastric ulcer.
In summary, our study of PCMS on mice revealed that the molecule showed an anti-ulcerogenic activity against the ethanol-induced gastric ulcer in a very narrow dose window. Beyond that dose, it became even more toxic than the tissue damage caused by ethanol. The treatment duration is also playing a crucial role for showing its antiulcer potential. When the animals were treated with PCMS only for overnight without ethanol, the animals developed high ulcers with significant ulcer scores. As predicted, when it was administered with the same dose for a very small span of time, i.e. 30 min only, it didn’t exhibit its toxicity much; instead, it displayed its antiulcer property. The in-vitro data on human gastric cells also corroborated the fact obtained from the in-vivo studies. In view of this, further long-term animal studies and clinical trials are needed to evaluate and re-validate these findings in the patients suffering from an ethanol induced gastropathy.
Though P alone is claimed as a drug and we have found that the ranking of toxicity is P > PC > PCM > PCS > PCMS, even then, PCMS exhibits toxicity if the dose is not chosen carefully. Earlier we reported the potential of PCMS in treating cancer and Alzheimer’s, and now we demonstrate its potential in treating gastric ulcer, this venture would continue. However, we speculate that one prime reason for PCMS toxicity could possibly be due to the impurity in the synthetic solution. Absolute purity is the ultimate target and we are improving the quality of the solution to reach 99.999% from the current 98%. Once the molecule is purified extensively by removing unwanted toxic materials introduced in the solution, the dose window would be large and thus it could be regulated meticulously for patient-to-patient variations. PCMS may temporarily store in kidney, heart, lung and brain, thus, it is imperative to further study its positive and negative roles as an anti-inflammatory drug against the known diseases of those organs.
Anti-inflammatory agents are either encapsulated in the dendritic cavity or weakly bonded to the dendritic surface. In a sharp contrast, by harnessing the enhanced permeability and retention (EPR) effect of the dendritic nano-platform we have made the first step towards realizing a physical drug that does not chemically interact with any biomaterial. The existing chemical drugs are effective but adopt several unknown interaction pathways to affect various systems. It is impossible to map all possible pathways; where, in a purely physical drug, chemical interactions are completely switched off, even the physical interactions need an extremely precise composition of wireless physical signals. The pH sensor in our PCMS (we added it since cancer cells are acidic, PCMSs are attracted in-vivo) could be replaced by other molecular sensors, to target antibodies, peptides, vitamins, and hormones. The resonance based detection and functional modulation are atomically precise and selective. Even a paradigm shift towards generic programmed drug could be achieved by modifying the molecular rotor and introducing a new dynamics. While treating cancer and Alzheimer's, when we administer a PCMS, do they exclusively perform their job, or engage in several other unwanted activities, this work is the first documentation of such side effects. The efforts would continue until we map all possible side effects of our PCMS until we chemically modify and develop the final design that resolves both the diseases, increasing the longevity of humans.
Conflict of interest
The authors declare that there are no conflict of interest.
Acknowledgements
Authors acknowledge NIMS Sengen-site, JSPS Grants in Aid for Young Scientists (A) for 2009-2011, Grant number 21681015 (Govt of Japan); SERB, Govt. of India for the Grant no. ECR/2016/001534 (2017-2020); CSIR-IICB for providing infrastructure facility and support for toxicology test, and acknowledge the Asian office of Aerospace R&D (AOARD) a part of United States Air Force (USAF) for the Grant no. FA2386-16-1-0003 (2016–2019).
Contributor Information
Subrata Ghosh, Email: subrata@neist.res.in.
Snehasikta Swarnakar, Email: sikta@iicb.res.in.
Anirban Bandyopadhyay, Email: anirban.bandyopadhyay@nims.go.jp.
References
- 1.Gorman C.B., Smith J.C. Effect of repeat unit flexibility on dendrimer conformation as studied by atomistic molecular dynamics simulations. Polymer. 2000;41 [Google Scholar]
- 2.Tomalia D.A., Hall M., Hedstrand D.M. Starburst dendrimers. III. The importance of branch junction symmetry in the development of topological shell molecules. J. Am. Chem. Soc. 1987;109 [Google Scholar]
- 3.Lee C.C., MacKay J.A., Fréchet J.M.J., Szoka F.C. Designing dendrimers for biological applications. Nat. Biotechnol. 2005;23 doi: 10.1038/nbt1171. [DOI] [PubMed] [Google Scholar]
- 4.Duncan R. The dawning era of polymer therapeutics. Nat. Rev. Drug Discov. 2003;2 doi: 10.1038/nrd1088. [DOI] [PubMed] [Google Scholar]
- 5.Jain K., Kesharwani P., Gupta U., Jain N.K. Dendrimer toxicity: let’s meet the challenge. Int. J. Pharm. 2010;394 doi: 10.1016/j.ijpharm.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 6.Win-Shwe T.T., Sone H., Kurokawa Y., Zeng Y., Zeng Q., Nitta H., Hirano S. Effect of PAMAM dendrimer in the mouse brain after a single intranasal instillation. Toxicol. Lett. 2014;228 doi: 10.1016/j.toxlet.2014.04.020. [DOI] [PubMed] [Google Scholar]
- 7.Ghosh S., Chatterjee S., Roy A., Ray K., Swarnakar S., Fujita D., Bandyopadhyay A. Resonant oscillation language of a futuristic nano-machine-module: eliminating cancer cells & Alzheimer Aβ-plaques. Curr. Topic. Med. Chem. 2015;15 doi: 10.2174/1568026615666150225101155. [DOI] [PubMed] [Google Scholar]
- 8.Kannaiyan D., Imae T. pH-Dependent encapsulation of pyrene in PPI-core: PAMAM-shell dendrimers. Langmuir. 2009;25 doi: 10.1021/la8039847. [DOI] [PubMed] [Google Scholar]
- 9.Nagatani H., Sakamoto T., Torikai T., Sagara T. Encapsulation of Anilinonaphthalenesulfonates in carboxylate-terminated PAMAM dendrimer at the polarized water/1,2-dichloroethane interface. Langmuir. 2010;26 doi: 10.1021/la1032477. [DOI] [PubMed] [Google Scholar]
- 10.Maingi V., Kumar M.V.S., Maiti P.K. PAMAM dendrimer-drug interactions: effect of pH on the binding and release pattern. J. Phys. Chem. B. 2012;116 doi: 10.1021/jp211515g. [DOI] [PubMed] [Google Scholar]
- 11.Crooks R.M., Zhao M., Sun L., Chechik V., Yeung L.K. Dendrimer-encapsulated metal nanoparticles: synthesis, characterization, and applications to catalysis. Acc. Chem. Res. 2001;34 doi: 10.1021/ar000110a. [DOI] [PubMed] [Google Scholar]
- 12.Yavuz B., Pehlivan S.B., Ünlü N. Dendrimeric systems and their applications in ocular drug delivery. Sci. World J. 2013;2013 doi: 10.1155/2013/732340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roberts J.C., Bhalgat M.K., Zera R.T. Preliminary biological evaluation of polyamidoamine (PAMAM) Starburst dendrimers. J. Biomed. Mater. Res. 1996;30 doi: 10.1002/(SICI)1097-4636(199601)30:1<53::AID-JBM8>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 14.Heiden T.C., Dengler E., Kao W.J., Heideman W., Peterson R.E. Developmental toxicity of low generation PAMAM dendrimers in zebrafish. Toxicol. Appl. Pharm. 2007;225 doi: 10.1016/j.taap.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Albertazzi L., Gherardini L., Brondi M., Sulis S.S., Bifone A., Pizzorusso T., Ratto G.M., Bardi G. In vivo distribution and toxicity of PAMAM dendrimers in the central nervous system depend on their surface chemistry. Mol. Pharm. 2013;10 doi: 10.1021/mp300391v. [DOI] [PubMed] [Google Scholar]
- 16.Mukherjee S.P., Davoren M., Byrne H.J. In vitro mammalian cytotoxicological study of PAMAM dendrimers –towards quantitative structure activity relationships. Toxicol. In Vitro. 2013;24 doi: 10.1016/j.tiv.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 17.Mukherjee S.P., Lyng F.M., Garcia A., Davoren M., Byrne H.J. Mechanistic studies of in vitro cytotoxicity of poly(amidoamine) dendrimers in mammalian cells. Toxicol. Appl. Pharmacol. 2013;248 doi: 10.1016/j.taap.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 18.Mukherjee S.P., Byrne H.J. Polyamidoamine dendrimer nanoparticle cytotoxicity, oxidative stress, caspase activation and inflammatory response: experimental observation and numerical simulation. Nanomed. Nanotechnol. Biol. Med. 2013;9 doi: 10.1016/j.nano.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 19.Tsatsakis A.M., Kouretas D., Tzatzarakis M.N., Stivaktakis P., Tsarouhas K., Golokhvast K.S., Rakitskii V.N., Tutelyan V., Hernandez A.F., Rezaee R., Chung G., Fenga C., Engin A.B., Neagu M., Arsene A.L., Docea A.O., Gofita E., Calina D., Taitzoglou I., Liesivuori J., Hayes A.W., Gutnikov S., Tsitsimpikou C. Simulating real-life exposures to uncover possible risks to human health: a proposed consensus for a novel methodological approach. Hum. Exp. Toxicol. 2017;36 doi: 10.1177/0960327116681652. [DOI] [PubMed] [Google Scholar]
- 20.Hasanzadeh D., Mahdavi M., Dehghan G., Charoudeh H.N. Farnesiferol C induces cell cycle arrest and apoptosis mediated by oxidative stress in MCF-7 cell line. Toxicol. Rep. 2017;4 doi: 10.1016/j.toxrep.2017.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Medina S.H., El-Sayed M.E.H. Dendrimers as carriers for delivery of chemotherapeutic agents. Chem. Rev. 2009;109 doi: 10.1021/cr900174j. [DOI] [PubMed] [Google Scholar]
- 22.Cheng Y. John Wiley & Sons, Inc.; New Jersey: 2012. Dendrimer Based Drug Delivery System From Theory to Practice. [Google Scholar]
- 23.Christensen J.B., Heegaard P.M.H., Boas U. RSC Publishing; 2006. Dendrimers in Medicine and Biotechnology: New Molecular Tools. [Google Scholar]
- 24.Chen C.Z., Cooper S.L. Interaction between dendrimer biocides and bacterial membranes. Biomaterials. 2002;23 doi: 10.1016/s0142-9612(02)00036-4. [DOI] [PubMed] [Google Scholar]
- 25.Ghosh S., Dutta M., Sahu S., Fujita D., Bandyopadhyay A. Nano molecular-platform: a protocol to write energy transmission program inside a molecule for bio-inspired supramolecular engineering. Adv. Funct. Mater. 2014;24 [Google Scholar]
- 26.Ghosh S., Fujita D., Bandyopadhyay A. An organic jelly made fractal logic gate with an infinite truth table. Sci. Rep. 2015;5 doi: 10.1038/srep11265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghosh S., Dutta M., Ray K., Fujita D., Bandyopadhyay A. Simultaneous one pot synthesis of two fractal structures by swapping of two fractal reaction kinetic states. Phys. Chem. Chem. Phys. 2016;18 doi: 10.1039/c6cp00447d. [DOI] [PubMed] [Google Scholar]
- 28.Sahu S., Ghosh S., Fujita D., Bandyopadhyay A. Live visualizations of single isolated tubulin protein self-assembly via tunneling current: effect of electromagnetic pumping during spontaneous growth of microtubule. Sci. Rep. 2014;4 doi: 10.1038/srep07303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.S. Ghosh, D. Fujita, A. Bandyopadhyay, A molecular chip that generates electrical power from free thermal noise, Japan Patent JP2014-075198, April 1, 2014.
- 30.S. Ghosh, D. Fujita, A. Bandyopadhyay, Thermal noise driven molecular rotor, Japan Patent JP2014-091141, April 25, 2014.
- 31.S. Ghosh D. Fujita, A. Bandyopadhyay, A supramolecular architecture creation by successive phase transitions and radiations, Japan Patent JP2014-219958, April 25, 2014.
- 32.Simonson T., Perahia D., Brünger A.T. Dielectric properties of proteins: microscopic and macroscopic theory. J. Chim. Phys. 1991;88 doi: 10.1016/S0006-3495(91)82282-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thompson D.B., Cronican J.J., Liu D.R. Engineering and identifying supercharged proteins for macromolecule delivery into mammalian cells. Methods Enzymol. 2012;503 doi: 10.1016/B978-0-12-396962-0.00012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McNerny D.Q., Leroueil P.R., Baker J.R. Understanding specific and nonspecific toxicities: a requirement for the development of dendrimer-based pharmaceuticals. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2010;2 doi: 10.1002/wnan.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Savić R.J., Azzam T., Eisenberg A., Nedev H., Rosenberg L., Maysinger D. Block-copolymer micelles as carriers of cell signaling modulators for the inhibition of JNK in human islets of Langerhans. Biomater. 2009;30 doi: 10.1016/j.biomaterials.2009.03.028. [DOI] [PubMed] [Google Scholar]
- 36.Albertazzi L., Gherardini L., Brondi M., Sato S.S., Bifone A., Pizzorusso T., Ratto G.M., Bardi G. In vivo distribution and toxicity of PAMAM dendrimers in the central nervous system depend on their surface chemistry. Mol. Pharm. 2013;10 doi: 10.1021/mp300391v. [DOI] [PubMed] [Google Scholar]
- 37.Mendes P.M. Cellular nanotechnology: making biological interface smarter. Chem. Soc. Rev. 2013;42 doi: 10.1039/c3cs60198f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Albertazz L., Gherardini L., Brondi M., Sato S.S., Bifone A., Bardi T.G.M.G. In vivo distribution of toxicity of PAMAM dendrimers in the central nervous system depend on their surface chemistry. Mol. Pharm. 2013;10 doi: 10.1021/mp300391v. [DOI] [PubMed] [Google Scholar]
- 39.Labieniec-Watala M., Karolczak K., Siewiera K., Watala C. The Janus face of PAMAM dendrimers used to potentially cure nonenzymatic modifications of biomacromolecules in metabolic disorders—a critical review of the pros and cons. Molecules. 2013;18 doi: 10.3390/molecules181113769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feliu N., Pelaz B., Zhang Q., del Pino P., Andreas Nyström A., Parak W.J. Nanoparticle dosage-a nontrivial task of utmost importance for quantitative nanosafety research. Nanomed. Nanobiotechnol. 2016;8 doi: 10.1002/wnan.1378. [DOI] [PubMed] [Google Scholar]
- 41.Jedrych M., Borowska K., Galus R., Jodłowska-Jędrych B. The evaluation of the biomedical effectiveness of poly(amido)amine dendrimers generation 4.0 as a drug and as drug carriers: a systematic review and meta-analysis. Int. J. Pharm. 2014;462 doi: 10.1016/j.ijpharm.2013.12.033. [DOI] [PubMed] [Google Scholar]
- 42.Kannan R.M., Nance E., Kannan S., Tomalia D.A. Emerging concepts in dendrimer-based nanomedicine: from design principles to clinical applications. J. Int. Med. 2014;276 doi: 10.1111/joim.12280. [DOI] [PubMed] [Google Scholar]
- 43.Shcharbin D., Janaszewska A., Klajnert-Maculewicz B., Ziemba B., Dzmitruk V., Halets I., Loznikova S., Shcharbina N., Milowska K., Ionov M., Shakhbazau A., Bryszewska M. How to study dendrimers and dendriplexes III. Biodistribution, pharmacokinetics and toxicity in vivo. J. Control. Release. 2014;181 doi: 10.1016/j.jconrel.2014.02.021. [DOI] [PubMed] [Google Scholar]
- 44.Wang S., Li Y., Fan J., Wang Z., Zeng X., Sun Y., Song P., Ju D. The role of autophagy in the neurotoxicity of cationic PAMAM dendrimers. Biomaterials. 2014;35 doi: 10.1016/j.biomaterials.2014.05.029. [DOI] [PubMed] [Google Scholar]
- 45.Li Y., Zeng X., Wang S., Sun Y., Wang Z., Fan J., Song P., Ju D. Inhibition of autophagy protects against PAMAM dendrimers-induced hepatotoxicity. Nanotoxicology. 2015;9 doi: 10.3109/17435390.2014.930533. [DOI] [PubMed] [Google Scholar]
- 46.Nikitovic D., Corsini E., Kouretas D., Tsatsakis A., Tzanakakis G. ROS-major mediators of extracellular matrix remodeling during tumor progression. Food Chem. Toxicol. 2013;61 doi: 10.1016/j.fct.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 47.Watala M.L., Watala C. PAMAM dendrimers: destined for success or doomed to fail Plain and modified PAMAM dendrimers in the context of biomedical applications. J. Pharm. Sci. 2015;104 doi: 10.1002/jps.24222. [DOI] [PubMed] [Google Scholar]
- 48.Kholodenko B.N. Cell signaling dynamics in time and space. Nat. Rev. Mol. Cell Biol. 2006;7 doi: 10.1038/nrm1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bandyopadhyay A., Miki K. Fabrication of a memory chip by a complete self-assembly process using state-of-the-art multilevel cell (MLC) technology. Adv. Funct. Mater. 2008;18 [Google Scholar]
- 50.Sahu S., Ghosh S., Hirata K., Fujita D., Bandyopadhyay A. Multi-level memory-switching properties of a single brain microtubule. Appl. Phys. Lett. 2013;102 doi: 10.1016/j.bios.2013.02.050. [DOI] [PubMed] [Google Scholar]
- 51.Bandyopadhyay A., Sahu S.S., Fujita D., Wakayama Y. A new approach to extract multiple distinct conformers and co-existing distinct electronic properties of a single molecule by point-contact method. Phys. Chem. Chem. Phys. 2010;12 doi: 10.1039/b913691f. [DOI] [PubMed] [Google Scholar]
- 52.Matai I., Gopinath P. Hydrophobic myristic acid modified PAMAM dendrimers augment the delivery of tamoxifen to breast cancer cells. RSC Adv. 2016;6 [Google Scholar]
- 53.Robinson K., Kenefeck R., Pidgeon E.L., Shakib S., Patel S., Polson R.J., Zaitoun A.M., Atherton J.C. Helicobacter pylori induced peptic ulcer disease is associated with inadequate regulatory T cell responses. Gut. 2008;57 doi: 10.1136/gut.2007.137539. [DOI] [PubMed] [Google Scholar]
- 54.Twentyman P.R., Luscombe M.A. Study of some variables in a tetrazolium dye (MTT) based assay for cell growth and chemosensitivity. Br. J. Cancer. 1997;56 doi: 10.1038/bjc.1987.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Park H.S., Kim G.Y., Nam T.J., Kim N.D., Choi Y.H. Antiproliferative activity of fucoidan was associated with the induction of apoptosis and autophagy in AGS human gastric cancer cells. J. Food Sci. 2011;76 doi: 10.1111/j.1750-3841.2011.02099.x. [DOI] [PubMed] [Google Scholar]
- 56.Jones N.L., Day A.S., Jennings H.A., Sherman P.M. Helicobacter pylori induces gastric epithelial cell apoptosis in association with increased Fas receptor expression. Infect. Immun. 1999;67 doi: 10.1128/iai.67.8.4237-4242.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liang C.C., Park A.Y., Guan J.L. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2007;2 doi: 10.1038/nprot.2007.30. [DOI] [PubMed] [Google Scholar]
- 58.Paul S., Sharma A.V., Mahapatra P.D., Bhattacharya P., Reiter R.J., Swarnakar S. Role of melatonin in regulating matrix metalloproteinase-9 via tissue inhibitors of metalloproteinase-1 during protection against endometriosis. J. Pineal Res. 2008;44 doi: 10.1111/j.1600-079X.2007.00547.x. [DOI] [PubMed] [Google Scholar]
- 59.Singh L.P., Kundu P., Ganguly K., Mishra A., Swarnakar S. Novel role of famotidine in downregulation of matrix metalloproteinase-9 during protection of ethanol-induced acute gastric ulcer. Free Radic. Biol. Med. 2007;43 doi: 10.1016/j.freeradbiomed.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 60.Chakraborty S., Stalin S., Das N., Choudhury S.T., Ghosh S., Swarnakar S. The use of nano-quercetin to arrest mitochondrial damage and MMP-9 upregulation during prevention of gastric inflammation induced by ethanol in rat. Biomaterials. 2012;33 doi: 10.1016/j.biomaterials.2011.12.037. [DOI] [PubMed] [Google Scholar]