Abstract
Globally, approximately one in three of all adults suffer from multiple chronic conditions (MCCs). This review provides a comprehensive overview of the resulting epidemiological, economic and patient burden.
There is no agreed taxonomy for MCCs, with several terms used interchangeably and no agreed definition, resulting in up to three-fold variation in prevalence rates: from 16% to 58% in UK studies, 26% in US studies and 9.4% in Urban South Asians.
Certain conditions cluster together more frequently than expected, with associations of up to three-fold, e.g. depression associated with stroke and with Alzheimer's disease, and communicable conditions such as TB and HIV/AIDS associated with diabetes and CVD, respectively. Clusters are important as they may be highly amenable to large improvements in health and cost outcomes through relatively simple shifts in healthcare delivery.
Healthcare expenditures greatly increase, sometimes exponentially, with each additional chronic condition with greater specialist physician access, emergency department presentations and hospital admissions. The patient burden includes a deterioration of quality of life, out of pocket expenses, medication adherence, inability to work, symptom control and a high toll on carers. This high burden from MCCs is further projected to increase.
Recommendations for interventions include reaching consensus on the taxonomy of MCC, greater emphasis on MCCs research, primary prevention to achieve compression of morbidity, a shift of health systems and policies towards a multiple-condition framework, changes in healthcare payment mechanisms to facilitate this change and shifts in health and epidemiological databases to include MCCs.
Keywords: Multiple chronic conditions, Multimorbidity, Chronic disease, Noncommunicable diseases, Communicable diseases, Health care costs, Health policy, Review
1. Introduction
1.1. Investment in noncommunicable disease
Three in five global deaths are attributed to four major non-communicable diseases (NCDs) – cardiovascular disease, cancer, chronic lung diseases and diabetes (Wang et al., 2016). The increasing burden of NCDs, which fall disproportionately on low-income countries (LICs), has made prevention and management of NCDs a global priority. In 2011, the United Nations convened a High-Level Meeting on NCDs, calling for whole-of-society, whole-of-government and multi stakeholder action to prevent and control NCDs (United Nations, 2011). The 66th annual World Health Assembly endorsed the World Health Organization (WHO) Action Plan for the prevention and control of NCDs 2013–2020 (World Health Organization, 2013). A report by the National Academy of Medicine focuses on strategies to better serve high need patients including those with more than one chronic condition (National Academy of Medicine, 2017).
One in three adults lives with more than one chronic condition, or multiple chronic conditions (MCC) and accrue a disproportionate health and cost burden (Marengoni et al., 2011). This figure is closer to three out of four in older adults living in developed countries and is predicted to rise dramatically (Buttorff et al., 2017), with the proportion of patients with four+ diseases almost doubling between 2015 and 2035 in the UK (Kingston et al., 2018). Yet the area of MCCs remains grossly understudied.
The purpose of this review is to provide an overview of the available evidence base on the health, economic and patient burden from MCC.
2. Methods
2.1. Data sources and availability
Data used for the report include searches conducted in the academic literature and ‘snowballing’ to identify other referenced articles and reports. A review of English language literature through May 15, 2017 was performed using electronic databases (MEDLINE, PubMed). Search terms used included “multiple chronic conditions”, “multimorbidity”, “polychronicity”, “comorbidities”, “chronic conditions”, “chronic diseases”, “chronic disease clusters”. Additional articles were identified by searching each article's reference section. Other data repositories were scoured for primary data such as the WHO (Global Health Observatory Data Repository, n.d.) and the Global Burden of Diseases, Injuries, and Risk Factors (GBD) study developed by the Institute of Health Metrics and Evaluation (IHME) (Institute for Health Metrics and Evaluation, n.d.).
Due to the breadth of information on the subject of MCC, this review was written as a narrative review to gather methodologically sound data across diverse geographic regions, income-levels, ages, and chronic diseases in an effort to identify the evidence base and gaps.
2.2. Definitions of MCC
The lack of a single definition for what constitutes MCC has resulted in considerable heterogeneity in estimates. This report presents estimates where available, but it is important to consider these are highly dependent on the number of chronic conditions included in the definition, as well how chronic conditions are defined. The simplest definition of MCC is the presence of two or more chronic diseases, but what constitutes a chronic disease is also variable across the literature (Lefèvre et al., 2014). For example, some studies define chronic conditions by their respective organ system (e.g., chronic lung disease), whereas others differentiate within organ systems (e.g., COPD and interstitial lung disease) (Diederichs et al., 2010).
Various indices have been used to assess the number and severity of chronic diseases. Perhaps the most well-known of these is the Charlson Comorbidities Index and its adaptations, originally established to predict mortality in hospital patients (Yurkovich et al., 2015). Other indices have been derived from medical data, medication groups, diagnoses groups (Starfield et al., 2005), or organ systems (e.g., Chronic Disease Score) (Ionescu-Ittu et al., 2007). However, the Charlson Comorbidity Index and other available measures are not widely or consistently used in reporting MCC (McPhail, 2016).
3. Observations
3.1. Epidemiology of chronic conditions
The top conditions contributing to mortality and morbidity combined using disability adjusted life years (DALYs) in high-income countries (HIC) include ischemic heart disease (IHD), stroke, lung cancer, depression, diabetes and, back and neck pain (GBD, 2015). In low-income countries (LIC) and middle-income countries (MIC), the top diseases similarly include IHD, stroke, diabetes and depression, but also communicable diseases such as diarrhea, HIV and malaria, and road traffic injuries.
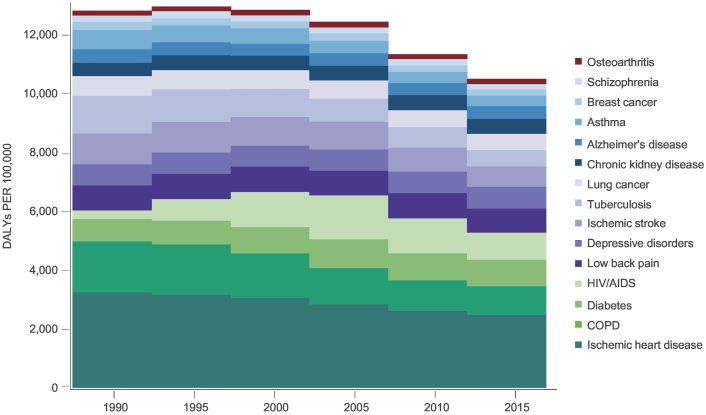
Fig. 1 illustrates the global burden of chronic disease as measured by DALYs from 1990 to 2015. The shift over the last 25 years highlights the reduction in DALYs due to IHD, resulting from an increase in the prevalence of IHD but a reduction in mortality.
Fig. 1.
Change Over Time for Age-standardized DALYs (rate per 100,000) for Leading Chronic Conditions (1990–2015) (GBD, 2015).
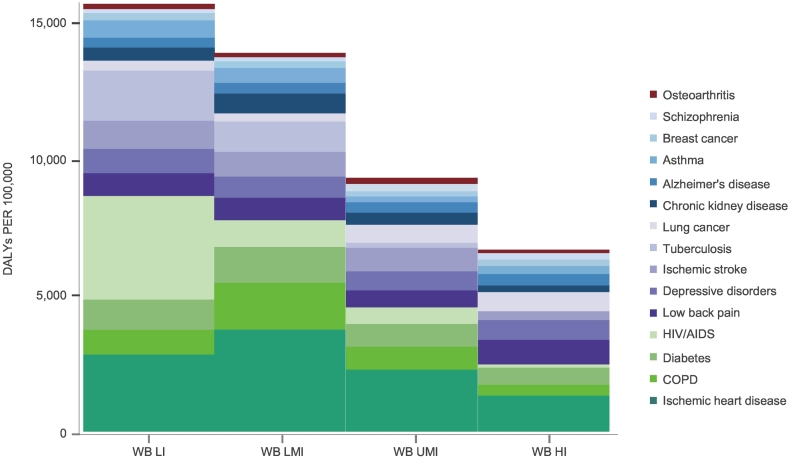
Fig. 2 demonstrates the burden of chronic disease by country-level income group in 2015. The combined burden of chronic diseases is greatest in LIC and largely attributed to the burden of HIV/AIDS. While the socioeconomic status (SES) of a country's population can explain the variation in HIV/AIDS and IHD, this is not the case for other conditions such as low back pain, depression, and arthritis, which are relatively homogenous between countries with differing SES.
Fig. 2.
Age-standardized DALYs (rate per 100,000) for leading chronic diseases (1995–2015) for World Bank Low Income (WB LI), Low Middle Income (WB LMI), Upper Middle Income (WB UMI), and High Income Countries (WB HI) (GBD, 2015).
While NCDs predominate, excessive DALY rates are seen for IHD and chronic obstructive pulmonary disease (COPD) in LMICs. Communicable diseases such as tuberculosis (TB) remain high in LICs and despite the advances made against HIV/AIDS over the last 15 years; it remains the largest contributor to DALYs in LICs. Other chronic conditions and symptoms such as depression and low back pain also highly contribute to DALYs in countries of all income brackets.
3.1.1. Prevalence of multiple chronic conditions (MCC)
Prevalence estimates for MCC are highly heterogeneous with methodological differences, including the number of chronic conditions included in the count, leading to estimates that vary up to three-fold (Fortin et al., 2012). Most US-based studies use a list of 20 chronic diseases classified by the Department of Health and Human Services (Centers for Medicare and Medicaid Services, 2015), while some reviews include 40 diseases and up to 140 conditions (Salisbury et al., 2011; Barnett et al., 2012). UK prevalence estimates for MCC range from 16% (17 chronic conditions considered) to 58% (114 chronic conditions considered) (Salisbury et al., 2011). When including 10 physical chronic conditions, approximately 25.5% of the United States population were reported to have MCC, and the prevalence increases to 50% of adults 45 to 65 years, and up to 81% of adults older than 65 years (Ward et al., 2014). For adults over 50 years, rates of MCC vary from 45% in China to 71% in Russia (Garin et al., 2015).
3.1.2. Future projections of MCC
As populations age, the time people live with disability and chronic disease is increasing such that MCC prevalence rates are closer to three quarters of older adults in developed countries (Divo et al., 2014). A simulation model of UK primary care patients predicts a dramatic rise such that patients with four or more diseases will almost double between 2015 and 2035 (Kingston et al., 2018). Furthermore, two-thirds of those with four or more diseases are predicted to have poor mental (dementia, depression, cognitive impairment no dementia) (Kingston et al., 2018). The majority of gains in life expectancy (3.6 years in men, 2.9 years in women) will be spent with four or more diseases (2.4 out of 3.6 years or 65.9% in men; 2.5 out of 2.9 years or 85.2% in women), due to increased prevalence of, rather than longer survival with, MCC (Kingston et al., 2018).
3.2. Global prevalence of MCC by chronic disease type
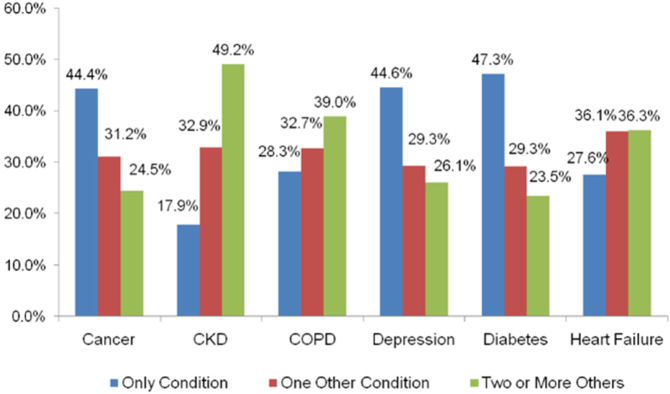
Fig. 3 shows that the highest proportion of MCC is observed with chronic kidney disease (CKD) (82.1% have a secondary condition, in particular heart failure and diabetes) (Schneider et al., 2009). For diabetes, depression, and cancer, individuals were more likely to only have the primary condition.
Fig. 3.
Proportion (%) of Medicare beneficiaries with MCC by selected chronic condition (2005) (Schneider et al., 2009).
3.3. MCC and demographics
In the US, women aged 18–64 are more likely than men to have MCC (Buttorff et al., 2017), to have two diseases (14.5% vs. 13.0%) and three diseases (12.6% vs. 10.7%), but this may be attributed to a greater tendency for female health-seeking behavior (Ward et al., 2014). For those under age 45, there is considerable heterogeneity as to the primary chronic condition, but this diminishes for those aged over 45. More than half of those with cancer, COPD, or arthritis who are under age 45 had MCC (Buttorff et al., 2017).
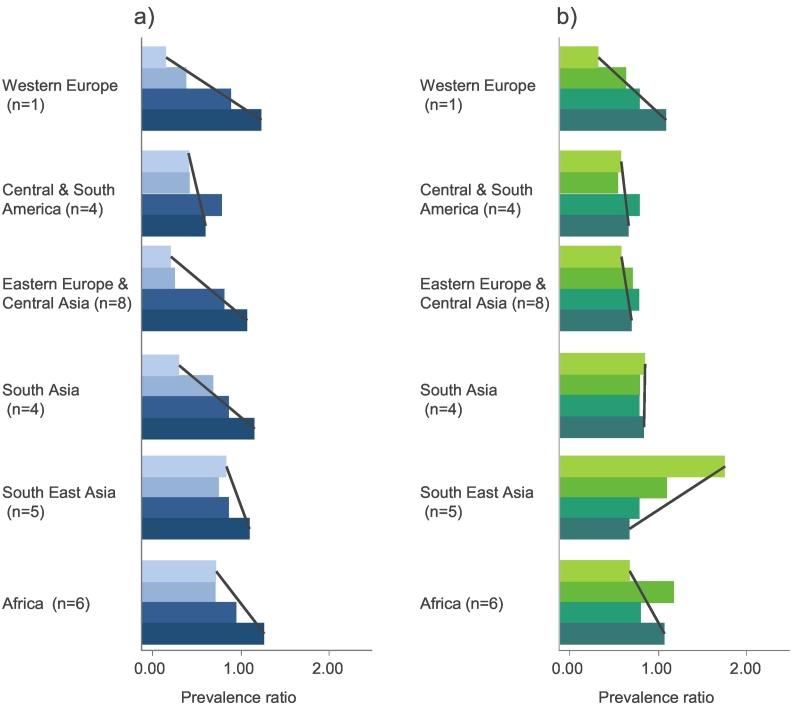
Globally, the relationship between SES and MCC is dependent on both geography and age. Fig. 4 reports prevalence ratios of MCC across SES groups and age from the World Health Survey (Afshar et al., 2015). Among adults under 55 years, there is a strong negative relationship between SES and MCC in most regions, which is most pronounced in Western Europe and, Eastern Europe and Central Asia. This relationship does not hold for adults over 55 years, with no or only weak relationships seen in all regions, other than South East Asia where a positive relationship between SES and MCC is seen (Afshar et al., 2015). This is consistent with other studies in India that reported greater chronic disease (obesity, CVD and MCC) in higher SES groups (Subramanian et al., 2013; Arokiasamy & Uttamacharya, 2015). This geographical and age pattern may reflect the distribution of key risk factors for chronic diseases such as unhealthy diet, physical inactivity, tobacco use and alcohol consumption among SES groups, which are higher in wealthier populations in developing countries and lower income groups in developed countries.
Fig. 4.
a) Socioeconomic gradient of MCC prevalence (2003) by regions for age category 1 (<55). b) Socioeconomic gradient of MCC by regions for age category 2 (≥ 55) (2012) taken from Afshar et al., (2015)
Note: Lightest shade: first category (higher education), Darkest shade: final category (less than primary school education). MCC prevalence ratios are based on prevalence of MCC in the third category, set at 1.
3.3.1. Chronic disease clusters
There is a paucity of published comprehensive research on clusters of chronic conditions and their impact on patients, health systems and healthcare costs. One systematic review examining clusters included 39 studies and >70,000,000 patients in 12 countries (Violan et al., 2014). Only three of the included studies used all chronic health conditions, remaining studies selected a variable number of conditions, which ranged between 5 and 335. The review provides a useful summary of MCC clusters, but the authors stated limitations due to heterogeneity in study design, sampling design (primary care sample vs. general population), age (as MCC is highly associated with age), and the definition used for MCC (Violan et al., 2014).
Table 1 provides a summary of available evidence for chronic disease clusters for the leading global chronic diseases (by DALYs) and their relationship with other chronic diseases. The most strongly associated clusters include Alzheimer's disease and stroke (relative risk of 5.5) (Tatemichi et al., 1994), depressive disorders and stroke (relative risk 3.2) (Huang et al., 2010), CVD and stroke alongside depression (odds ratio of 1.43) (Van der Kooy et al., 2007), alongside long-term communicable diseases in developing countries such as TB and Diabetes (relative risk of 3.11) (Baker et al., 2012; Jeon & Murray, 2008), and HIV/AIDS and CVD (relative risk of 1.6–2.0) (Islam et al., 2012). Other conditions that cluster together strongly include TB and COPD, CVD and asthma, depressive disorders and low back pain, depressive disorders and Alzheimer's disease, diabetes and depressive disorders, breast cancer and CVD, diabetes and osteoarthritis, and COPD and depressive disorders.
Table 1.
Clustering and strength of association between common chronic conditions.
| Primary condition | Secondary condition | Risk |
|---|---|---|
| COPD | Depressive disorders (Schneider et al., 2010) | OR = 1.4 |
| TB (Lee et al., 2013) | HR = 2.5 | |
| Diabetes | CVD (Selvin et al., 2004) | RR = 1.2 |
| COPD (Ehrlich et al., 2009) | HR = 1.2 | |
| Depressive disorders (Ali et al., 2006) | OR = 1.6 | |
| Ischemic stroke (Rodriguez et al., 2002) | RR = 1.9, RR = 3.1 | |
| TB (Jeon & Murray, 2008) | RR = 3.1 | |
| Asthma (Ehrlich et al., 2009) | HR = 1.1 | |
| Osteoarthritis (Louati et al., 2015) | OR = 1.5 | |
| HIV/AIDS | CVD (Islam et al., 2012) | On ART: RR = 2.0, Not on ART: 1.6 |
| Depressive disorders | CVD (Van der Kooy et al., 2007) | RR = 1.5 |
| IHD (Van der Kooy et al., 2007) | RR = 1.5 | |
| Diabetes (Nouwen et al., 2010) | RR = 1.2 | |
| Low back pain (Carroll et al., 2004) | HR = 3.9 | |
| Ischemic stroke (Van der Kooy et al., 2007) | RR = 1.4 | |
| Alzheimer's disease (Diniz et al., 2013) | RR = 1.9 | |
| Ischemic stroke | Depressive disorders (Huang et al., 2010) | RR = 3.2 |
| Alzheimer's disease (Tatemichi et al., 1994) | RR = 5.5 | |
| CKD | CVD (Weiner et al., 2004) | RR = 1.2 |
| Asthma | CVD (Australian Bureau of Statistics, 2012) | RR = 2.1 |
| Breast cancer | CVD (Hooning et al., 2007) | IR = 1.3 |
| Osteoarthritis | Diabetes (Louati et al., 2015) | OR = 1.4 |
| Alzheimer's disease (Huang et al., 2015) | HR = 1.3 |
OR = odds ratio; RR = relative risk; IR = incidence rate; ART = antiretroviral therapy; IHD = Ischemic Heart Disease; TB = Tuberculosis; COPD = chronic obstructive pulmonary disease; CVD = cardiovascular diseases (includes ischemic heart disease and ischemic stroke), CKD = chronic kidney disease.
Existing studies have concentrated on disease combinations or chronic disease risk factors, with limited consideration of the potential impact of clustering of certain conditions. Clustering can occur by virtue of high prevalence rates, shared risk factors or due to causation of one condition by another. An alternate categorization of clusters is by concordance (shared risk factors or disease pathways) and discordance (seemingly unrelated conditions). It is important to distinguish between these categories in particular for the prediction and prevention of subsequent chronic conditions. The treatment and management of clusters of conditions may also be impacted upon by whether they are concordant vs. discordant. For example, medications for one condition (e.g., TB) might exacerbate another chronic condition (e.g., diabetes) or increase risks associated with the disease, particularly if the conditions are discordant (Magnan et al., 2015).
By tackling clusters rather than individual diseases, interventions and systems can tackle difficulties faced by patients including medication design, approaches to screening and detection, and care guidelines. Clinical guidelines must consider not only the quantity of conditions, but the quality, to determine chronic condition interrelatedness and how this may impact treatment options, diagnostic processes and management. Moreover, healthcare costs for MCC patients increase exponentially and are expected to be greater than the additive effect of treating patients with each individual chronic condition. Current estimates of healthcare costs for chronic disease are likely to underestimate the true costs for such patients if they ignore clusters.
3.3.2. Tuberculosis and diabetes
A systematic review of 13 observational studies demonstrated DM is associated with more than a three-fold increased risk of developing TB (Baker et al., 2012; Jeon & Murray, 2008). Subgroup analyses revealed that this relationship was significantly stronger in non-North American countries. The mechanism of the increased risk is unclear as is whether the higher risk is due to reactivation of dormant TB or the acquiring of new infections. Some cross-sectional studies have shown a positive correlation between the presence of latent TB and diabetes (Hensel et al., 2016; Magee et al., 2015). Whether the latent TB is more likely to reactivate has not yet been reported. Furthermore, TB patients who have diabetes are less responsive to anti-TB medication (Baker et al., 2012).
The association between TB and diabetes is bi-directional; that is, patients with TB are also at higher risk of developing glycemic dysfunction and diabetes. The biological mechanism for this remains unclear and it may be the anti-TB medication, rather than the TB itself, that causes glycemic dysfunction.
3.3.3. TB and COPD
A systematic review of studies evaluating TB and COPD suggests the two chronic diseases occur together more frequently than by chance alone. COPD patients have a 3-fold higher risk of developing TB (Sarkar et al., 2017) and COPD is an independent risk factor for developing TB (hazard ratio = 2.47) (Lee et al., 2013). This could be due to their common risk factors of smoking, low SES, biomass fuel exposure and vitamin D deficiency (Sarkar et al., 2017).
3.3.4. Depression and chronic diseases
One study that examined the clustering of depression with other chronic diseases in a sample of adults aged 50–74 years (Pruchno et al., 2016) reported that as the number of chronic diseases increase, so do depressive symptoms. The prevalence of depressive symptoms was 10.5% with zero conditions, 14.4% with one condition, 20.8% with two conditions, 30.1% with three conditions, 37.3% with four conditions and 58.3% with five conditions (Pruchno et al., 2016). Research from the World Health Survey demonstrated that the prevalence of depression in respondents with chronic diseases was higher than in those without chronic diseases (Moussavi et al., 2007). Respondents with depression had the lowest health scores among chronic disease conditions. Furthermore, the decrement in health score from the combination of diabetes and depression was significantly greater than the sum of the two conditions separately (Moussavi et al., 2007).
3.3.5. HIV/AIDS and CVD
While the introduction of antiretroviral therapy (ART) has reduced the risk of HIV-related mortality worldwide, it has increased the risk of CVD among HIV patients. A meta-analysis of studies examining the relationship reported a substantially increased pooled relative risk (RR) of CVD of 1.61 (95% CI: 1.43–1.81) for HIV patients compared to HIV-uninfected people (Islam et al., 2012). HIV patients on ART treatment have an increased risk of CVD compared to both individuals with HIV who are not being treated (RR = 1.52; 95% CI: 1.35–1.70) and HIV-uninfected people (RR = 2.0; 95% CI: 1.70–2.37). The CVD risk also depends on the duration of ART; CVD risk may be higher after initiating ART, which may be mediated by an increase in dyslipidemia, a reduction in insulin sensitivity and increased body fat redistribution (Hemkens & Bucher, 2014).
3.3.6. Diabetes and stroke
While the increased risk of stroke among diabetics is well reported, the magnitude of risk varies by study population. One study comparing risk of stroke in diabetes patients between two cohort studies of different populations found that Japanese American men in the Honolulu Heart Program had a relative risk of stroke of 1.9 (95%CI: 1.5–2.4) whereas American men in the Framingham study had a higher relative risk of stroke of 3.1 (95% CI: 1.6–5.8) (Rodriguez et al., 2002). This difference in risk could not be explained by differing risk factor profiles alone.
3.4. The financial burden of MCC
MCC is associated with substantial increases in healthcare costs and resource utilization (McPhail et al., 2015) attributable to elevated use of primary care and specialist physician services, greater medication use, emergency department presentations and hospital admissions (both frequency of admissions and bed days) (McPhail et al., 2015).
Older age, undesirable lifestyle factors, and low SES have been consistently associated with the development of MCC (McPhail et al., 2015). Three important and interrelated challenges for contemporary healthcare policy include (Barnett et al., 2012):
-
•
The aging nature of population demographics,
-
•
Development of chronic diseases at younger ages,
-
•
And socioeconomic inequalities in the distribution of MCC and its effects.
The scarcity of robust economic evaluations in the field represents a considerable challenge for resource allocation decision-making intended to reduce the burden of MCC. Although the literature is sparse, one systematic review and several published studies are summarized below.
3.4.1.1. Determining costs from multiple chronic conditions
The cost drivers of excess utilization, patterns of usage, physician access, medication use, bed utilization, out of pocket healthcare costs and cost effectiveness of interventions for MCC are summarized below and in Table 2.
Table 2.
Summary of studies relating to cost and healthcare utilization for patients with MCC. Adapted from Lehnert (2011) (Lehnert et al., 2011).
| Study & country | Description & year | Impact |
|---|---|---|
| Healthcare costs | ||
| Fishman et al. (1997) United States (Fishman et al., 1997) |
Cross-sectional study with diagnostic and procedural data (1992) from Group Health Cooperative (GHC) of Pudget Sound (Western Washington State, U.S.) |
Each additional CC resulted in an expected increase in annual Healthcare costs (HCCs) of between 80% - 300%, depending on age, sex, and CC profile. |
| Hoffman et al. (1996) United States (Hoffman et al., 1996) |
Cross-sectional study with data from the 1987 National Medical Expenditure Survey (household component) | In comparison with elders with acute conditions only ($2713), those with one CC had annual HCCs about 1.8 times ($4887), and those with two or more CCs had costs about 3.6 times as high ($9881). |
| Crystal et al. (2000) United States (Crystal et al., 2000) |
Cross-sectional study with 1995 Medicare Current Beneficiary Survey data (use and cost files) |
The number of CCs was significantly and positively associated with total HCCs, annual OPE, and OPE as percentage of income (persons without CCs spent 13.8% of their income, those with five or more CCs 25.5%). |
| Hwang et al. (2001) United States (Hwang et al., 2001) |
Cross-sectional study with 1996 Medicare Expenditure Panel Survey data (household Component |
OPE increased with each additional CC and was about twice as high for elders with two CCs compared with those without CCs. This association was found for OPE for prescription drugs, home health, office visits, hospital use, and medical equipment but not for OPE for dental services and vision aids. |
| Physician usage | ||
| Hessel et al. (2000) Germany (Hessel et al., 2000) |
Cross-sectional study with data from a household survey by the University of Leipzig, Germany, March/April 1996 | The number of medical conditions was significantly and positively associated with the annual number of physician visits and number of medications taken on a daily basis (CCs were strongest predictor in each of the multiple regression analyses). |
| Bed utilization | ||
| Chan et al. (2002) Australia (Chan et al., 2002) |
Cross-sectional study with data from a household survey in the Randwick Municipality of Sydney (Australia), March 1998 to June 1999 | Multiple (three or more) CCs were a strong and significant predictor of emergency department admissions. |
| Ionescu-Ittu et al. (2007) Canada (Ionescu-Ittu et al., 2007) |
Cross-sectional study with random sample drawn from provincial administrative databases in Quebec, Canada, for 2000–2001 | Comorbidity was a significant independent predictor of emergency department use. In a multivariate analysis, comorbidity had a comparatively weak effect on emergency department use: One additional score on CCI increased the rate of emergency department use by 7%, one score on the CDS by 4%. |
| Landi et al. (2004) Italy (Landi et al., 2004) |
Observational cohort study with administrative data from six Italian home health care agencies (longitudinal data, 1997–2002) | Elders with any HA (at baseline) had significantly more CCs (3.9) than those without HA (3.2). In a multivariate analysis, elderly persons with five or more CCs were more than twice as likely to incur an HA, compared with those without CCs (during 1-year follow-up). |
| Librero et al. (1999) Spain (Librero et al., 1999) |
Cross-sectional study with administrative (hospital discharge) data from Valencia Health Service, Spain, 1993–1994 | Results from logistic regression with age comorbidity interaction: Patients aged 65 to 79 in the highest morbidity group (5+) had significantly lower chances of being hospitalized (OR 0.51) than those without CCs, whereas patients with moderate morbidity burden (1 to 2) had significantly higher chances (OR 1.24). |
| Condelius et al. (2008) Sweden (Condelius et al., 2008) |
Cross-sectional study with administrative registry data (2001) from four municipalities | In multivariate analyses, the number of CCs was significantly associated with acute and total number of admissions, and (less strongly) with planned HAs. |
| Condelius et al. (2008) Sweden (Condelius et al., 2008) |
Cross-sectional study with administrative registry data (2001) from four municipalities in southern Sweden | Elders with three or more HAs had significantly more CCs (3.45) than those with one (1.64) or two stays (2.61). |
| Chu and Pei (1999) Hong Kong (Chu & Pei, 1999) |
Prospective case–control study with emergency admissions (using administrative data) at Queen Mary Hospital of Hong Kong, 1996 | Compared with controls, readmission cases had significantly more CCs (3.1 vs.2.6). Number of CCs was a significant risk factor for early unplanned readmission in a multivariate analysis (OR 1.30). |
| Medication | ||
| Fahlman et al. (2006), United States (Fahlman et al., 2006) |
Retrospective review (crosssectional) of retail and mail order prescription claims data from Medicare + Choice (collected between January 1998 and December 2000), United States | Beneficiaries with higher numbers of comorbidities had significantly greater numbers of prescriptions (8 prescriptions for each additional comorbidity) and higher annual prescription drug expenditures and higher OPE. |
3.5. Cost
Most studies to date have asserted a positive association between MCC and healthcare utilization outcomes (including physician visits, hospitalizations, use of medications) and healthcare cost outcomes (including medication, out of pocket, total healthcare expenditures) (Lehnert et al., 2011; Paez et al., 2009). In fact, several studies have reported a near exponential relationship, in which expenditures approximately doubled with each additional CC (Schneider et al., 2009; Wolff et al., 2002). This finding suggests costs for MCC are not simply additive but there is an interaction resulting in costs increasing exponentially, and this should be taken into account in reporting of costs from chronic disease.
3.6. Patterns of usage
MCC has been associated with higher levels of health resource utilization, including medications, primary care and outpatient specialist services, as well as emergency department presentations and hospitalizations (McPhail et al., 2016). For adults 65–69 years old in the US in 1999, data show the odds of incurring a hospital admission for an adverse event increase with the number of chronic conditions (Wolff et al., 2002). Both for ambulatory care sensitive conditions (OR: 1 = 7.49, 2 = 18.10, 3 = 36.43, ≥4 = 98.52) and preventable complications (1 = 6.02, 2 = 13.60, 3 = 29.17, ≥4 = 91.35) (Wolff et al., 2002). The greater use of non-emergency care for preventable conditions suggests some of the access utilization is avoidable.
There is considerable variation in the magnitude of increases in healthcare utilization (HCU) reported between studies, health systems and data sources from which study findings were derived. In terms of HCU, all evidence points to more complex in- and outpatient-care scenarios, with disproportionately higher use of services by specialists (Wolff et al., 2002), seeing a multitude of physicians (Anderson, 2010) and confronting them with more problems at each encounter (Beasley et al., 2004). In addition, MCC patients use significantly more prescription medications and have higher prescription drug expenditures (Mueller et al., 1997; Sambamoorthi et al., 2003.
Age and living arrangements (e.g. living alone) are positively associated with hospital use (Landi et al., 2004; Rapoport et al., 2004; Shelton et al., 2000; Librero et al., 1999; Condelius et al., 2008), and female gender and supplementary insurance are associated with an increased use of prescription medications (Sambamoorthi et al., 2003; Lawson et al., 2013), independent of the number of chronic conditions (CCs).
3.7. Physician access
Older adults with MCC utilize between two and five times more physician appointments than peers without chronic diseases (Xakellis, 2005; Paez et al., 2009; Schneider et al., 2009). A Canadian study reported 51% greater use of physician services for each additional chronic disease (Rapoport et al., 2004). People with MCC are also more likely to see a specialist physician for a CC that would fall within the scope of primary care service (Starfield et al., 2005).
3.8. Medication use
Several studies have found patients with three or more CCs had prescription medication costs that were 6.6 times greater than peers without CCs, and 2.1 times greater than peers with one or two comorbidities (Moxey et al., 2002). Amongst US Medicare beneficiaries, patients with five or more CCs used an additional eight prescriptions for each additional comorbidity during their last year of life (Fahlman et al., 2006).
3.9. Bed utilization
Greater emergency department presentations and hospital admissions are also reported. One US study found older patients with three or more CCs utilized 25 times more hospital bed-days during 14.6 times more hospital admissions than peers without any CCs (Schneider et al., 2009).
3.10. Out of pocket healthcare costs
Individual patients are also impacted by the elevated costs of MCC if they are responsible for healthcare usage costs (Smith et al., 2012). For example, the out-of-pocket expenses (OOPE) are twice as high for older adults with MCC than those without MCC and the elderly and low-income families are disproportionately affected (Rogowski et al., 1997).
3.11. Cost effectiveness of interventions for MCC
The largest study, a Cochrane systematic review, examined the effect of primary care and community interventions for MCC patients and reported that cost savings were plausible for interventions related to pharmaceutical use and risk factor prevention but the cost-effectiveness of interventions was not reported (Smith et al., 2012). The authors postulated cost savings were plausible based on favorable intervention effects related to pharmaceutical use and reductions in chronic disease risk factors, but this cost effectiveness was not specifically reported. The paucity of cost-effectiveness data to inform allocation decisions related to MCC remains a concern.
3.12. Geographical variation in healthcare cost
The impact of MCC on healthcare costs and resources will likely differ across health systems, geographical regions, disease combinations, and socioeconomic and demographic factors (Lawson et al., 2013; Hopman et al., 2015; Rapoport et al., 2004). Despite this, most studies to date are from developed countries.
3.13. Clusters of diseases
Existing studies have concentrated on disease combinations or chronic disease risk factors, with limited consideration of the potential impact of intervening in concordant versus discordant clustering of disease combinations (Damery et al., 2015; Hsieh et al., 2015; Katon et al., 2012; Panagioti et al., 2014; Candrilli et al., 2015; Tonelli et al., 2015).
3.13.1. Impact of MCC on patients and families
The impact of having MCC on the patient and caregivers remains underexamined but important considerations include their ability to work, remain productive lead independent lives, and further financial constraints due to out-of-pocket healthcare costs. Patients report compounded effects such as adhering to medication and self-care (Hajat & Kishore, 2018).
Research has indicated that MCC is associated with poorer physical function and functional decline, with on average 50% risk of functional decline with each additional condition (Kadam et al., 2007; Marengoni et al., 2009).
3.13.2. Unmet needs & challenges
Despite the increasing burden of MCCs across the world, intervention funding and political action are non-commensurate with major disparities between the burden of disease and the funding allocated (Dieleman et al., 2014), particularly in LICs and MICs. For example, in 2010, HIV/AIDS accounted for 3.7% of the burden of disease in LICs and MICs, whereas NCDs accounted for 49.8% of the burden. The development assistance allocated for health was just 2.3% for NCDs and 45.9% for HIV/AIDS (Dieleman et al., 2014).
Future projections suggest much of the life-expectancy gains will be spent with disability due to chronic conditions, such that the compression of morbidity, that is delaying the onset of chronic disease as far as possible, becomes increasingly pertinent (Fries et al., 1984). As a large proportion of chronic conditions contributing to MCC are amenable to prevention through lifestyle behavior change, compression of morbidity can only be achieved through early intervention through primary prevention of chronic conditions.
Unfortunately, traditional health systems and major disease programs rarely address the chronic diseases that occur together, instead taking a single-disease framework. For example, reports indicate physicians underestimate the presence of depression in cancer patients because oncology visits are focused only on physiologic treatment and symptom management (Passik et al., 1998). The shift from a single-disease focus to a broad consideration of other diseases was the result of a successful, multidisciplinary application of behavioral and social science that must be applied to all areas of health and medicine (Holland, 2002).
The literature on cost effectiveness of interventions that tackle more than one chronic condition is sparse and studies that exist highlight methodological problems with designing such studies (Smith et al., 2012). Regarding primary prevention, long-term or lifetime modeling of potential attainment of health and cost benefits are required to demonstrate tangible health benefits and reductions in health service utilization for MCC interventions (Drummond & McGuire, 2001; Weinstein et al., 2003).
Unfortunately, long-term modeling may also come with untenable levels of uncertainty such as determining how long lifestyle behavior change interventions will last (Lefèvre et al., 2014). Studies examining secondary prevention would require many years of ongoing intervention (and follow-up) among large samples before benefits can be directly observed such as the outcomes of myocardial infarction or stroke (Li et al., 2008; Lindström et al., 2013; Diabetes Prevention Program Research Group, 2015).
4. Opportunities for action and intervention
The opportunity to reduce the burden of MCC lies with healthcare providers, the pharmaceutical industry, policy makers, the digital health industry, and the broader public health community. There have been some promising advances in tackling MCC, particularly in the field of high technology solutions. The emerging solutions and models target issues of MCC burden, functional health, quality of life and health care costs. However, many other opportunities exist, including measures for prevention, health systems and professionals, and smarter and tailored development of medication and patient support systems.
Public health prevention of chronic conditions may be the most impactful in terms of cost and health outcomes. Distinguishing between modifiable and non-modifiable risk factors is critical for developing effective interventions that prevent the onset of disease. Beyond prevention, healthcare systems should develop models of care and systems that facilitate cross-condition management. For example, using symptom-based care guidelines in resource-poor settings can empower non-physician prescribing and be an effective strategy for simultaneously managing communicable and NCDs (Fairall et al., 2005). Further, patients with few concordant chronic conditions should be targeted as potential patients at risk of suboptimal care, since these patients are often earlier in their disease progression. Empowering and educating physicians can improve patient health, given the impact physician-patient relationships, including time, rapport, communication and trust, can have on patients care and personal health (Hajat & Yach, 2015).
Efforts to increase adherence to medication among those with chronic diseases could improve health and reduce healthcare costs (Berg et al., 1992). For example, fixed-dose combination medicine, which combines multiple medications into a single pill, can simplify treatment regimens and increase adherence (Bangalore et al., 2007). Other technology providing adherence data to patients and their caregivers can improve adherence (Frias et al., 2017). Technological innovations provide the tools to support on-demand physician care in areas with physician shortages low resources (Eccles, 2012). Artificial intelligence provides opportunities to maximize care for patients with MCC by predicting drug receptivity, adherence and interactions, while using data repositories to provide personalized care and targeted disease management (Mukherjee, 2017).
5. Conclusions
Existing data suggest that between16–57% of adults in developed countries suffer from more than one chronic condition. Developing countries now need to deal with the double burden of long-term communicable conditions alongside NCDs, with clustering and causality between common conditions. From the relatively sparse evidence-base, MCC has been shown to be associated with substantially greater increases in healthcare costs and different patterns of resource utilization. The increasing proportion of older adults in the population, increasing proportion of younger adults with MCC who will live to advanced ages, together with the predicted increase in prevalence of those living with MCC, all have worrying implications for policy and healthcare funding. Compression of morbidity through prevention of chronic disease would be the most impactful approach and requires lifetime lifestyle behavior change.
There are substantial gaps in the knowledge base, such as taxonomy, availability and consistency of data, and economic evaluations of interventions. Furthermore, the major sources of data don't directly report on or tackle MCC and the evidence increasingly provides strong justification for a shift in this approach. The concerns of patients with MCC, such as the presence of chronic pain and the inability to remain in work, are not yet widely recognized or reported.
Prominent findings include the sharp rise in healthcare costs with each additional chronic condition, in addition to clustering of chronic conditions, which further increase and complicate the health and cost burden from MCC. Both of these attributes should be taken into account for health system design in moving away from a single-disease framework towards a patient-centered model that deals with several chronic conditions. An innovative approach to existing health system payment models would help to facilitate this shift.
Clinical practice guidelines, which primarily focus on single diseases, fail to consider how MCC should be managed, particularly among older adults. Guiding principles for older adults with MCC proposed by the American Geriatrics Society offer new directions for clinicians to provide more appropriate care (Boyd et al., 2012). Incorporating patient preferences into medical decision-making, framing medical decisions in the context of risks, burdens, benefits, and prognosis, considering treatment complexity and feasibility and prioritizing treatments with high benefit and little harm can enhance quality of life and promote patient-centered health outcomes among patients with MCC.
Interventions for MCC are lacking. A few initiatives promise to be impactful, including measures to increase medication adherence (such as fixed dose combination medication) and multi-condition management (such as patient-based guidelines). There is a need for healthcare providers to rethink and test new models of healthcare provision to prepare for future escalating costs of managing MCC in aging populations.
Acknowledgments
CH and ES designed the study, conducted the research and wrote the manuscript. The authors would like to thank Dr Derek Yach for his valuable oversight of this work. This work was supported by funding from Teva Pharmaceuticals Ltd., but the content of this review was developed independently of Teva.
References
- Afshar S., Roderick P.J., Kowal P., Dimitrov B.D., Hill A.G. Multimorbidity and the inequalities of global ageing: a cross-sectional study of 28 countries using the World Health Surveys. BMC Public Health. 2015;15(1):776. doi: 10.1186/s12889-015-2008-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali S., Stone M.A., Peters J.L., Davies M.J., Khunti K. The prevalence of co-morbid depression in adults with type 2 diabetes: a systematic review and meta-analysis. Diabet. Med. 2006;23(11):1165–1173. doi: 10.1111/j.1464-5491.2006.01943.x. [DOI] [PubMed] [Google Scholar]
- Anderson G. Chronic care: making the case for ongoing care. 2010. http://www.rwjf.org/files/research/50968chronic.care.chartbook.pdf Available:
- Arokiasamy P., Uttamacharya Jain K. Multi-morbidity, functional limitations, and self-rated health among older adults in India: cross-sectional analysis of LASI pilot survey, 2010. SAGE Open. 2015;5(1) [Google Scholar]
- Australian Bureau of Statistics Australian Health Survey 2011–2012. 2012. http://www.abs.gov.au/australianhealthsurvey Available:
- Baker M.A., Lin H.H., Chang H.Y., Murray M.B. The risk of tuberculosis disease among persons with diabetes mellitus: a prospective cohort study. Clin. Infect. Dis. 2012;54(6):818–825. doi: 10.1093/cid/cir939. [DOI] [PubMed] [Google Scholar]
- Bangalore S., Kamalakkannan G., Parkar S., Messerli F.H. Fixed-dose combinations improve medication compliance: a meta-analysis. Am. J. Med. 2007;120(8):713–719. doi: 10.1016/j.amjmed.2006.08.033. [DOI] [PubMed] [Google Scholar]
- Barnett K., Mercer S.W., Norbury M., Watt G., Wyke S., Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet. 2012;380(9836):37–43. doi: 10.1016/S0140-6736(12)60240-2. 7. [DOI] [PubMed] [Google Scholar]
- Beasley J.W., Hankey T.H., Erickson R. How many problems do family physicians manage at each encounter? A WReN study. Ann. Fam. Med. 2004;2(5):405–410. doi: 10.1370/afm.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg J.S., Dischler J., Wagner D.J., Raia J.J., Palmer-Shevlin N. Medication compliance: a healthcare problem. Ann. Pharmacother. 1992;27(9 Suppl):S1–S24. [PubMed] [Google Scholar]
- Boyd C.M., McNabney M.K., Brandt N. American Geriatrics Society Expert Panel on the Care of Older Adults with Multimorbidity. Guiding principles for the care of older adults with multimorbidity: an approach for clinicians. J. Am. Geriatr. Soc. 2012;60:E1–25. doi: 10.1111/j.1532-5415.2012.04188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttorff C., Ruder T., Bauman M. RAND Corporation; Santa Monica, CA: 2017. Multiple Chronic Conditions in the United States.https://www.rand.org/pubs/tools/TL221.html [Google Scholar]
- Candrilli S.D., Meyers J.L., Boye K., Bae J.P. Health care resource utilization and costs during episodes of care for type 2 diabetes mellitus related comorbidities. J. Diabetes Complicat. 2015;29(4):529–533. doi: 10.1016/j.jdiacomp.2014.12.009. [DOI] [PubMed] [Google Scholar]
- Carroll L.J., Cassidy J.D., Côté P. Depression as a risk factor for onset of an episode of troublesome neck and low back pain. Pain. 2004;107(1–2):134–139. doi: 10.1016/j.pain.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Centers for Medicare and Medicaid Services Multiple Chronic Conditions. 2015. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-reports/Chronic-Conditions/MCC_Main.html Available:
- Chan D.K., Chong R., Basilikas J., Mathie M., Hung W.T. Survey of major chronic iIlnesses and hospital admissions via the emergency department in a randomized older population in Randwick, Australia. Emerg Med. 2002;14(4):387–392. doi: 10.1046/j.1442-2026.2002.00343.x. [DOI] [PubMed] [Google Scholar]
- Chu L.W., Pei C.K. Risk factors for early emergency hospital readmission in elderly medical patients. Gerontology. 1999;45(4):220–226. doi: 10.1159/000022091. [DOI] [PubMed] [Google Scholar]
- Condelius A., Edberg A.K., Jakobsson U., Hallberg I.R. Hospital admissions among people 65+ related to multimorbidity, municipal and outpatient care. Arch. Gerontol. Geriatr. 2008;46(1):41–55. doi: 10.1016/j.archger.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Crystal S., Johnson R., Harman J., Sambamoorthi U., Kumar R. Out-of-pocket health care costs among older Americans. J. Gerontol. Soc. Sci. 2000;55(1):S5I–62. doi: 10.1093/geronb/55.1.s51. [DOI] [PubMed] [Google Scholar]
- Damery S., Flanagan S., Combes G. The effectiveness of interventions to achieve co-ordinated multidisciplinary care and reduce hospital use for people with chronic diseases: study protocol for a systematic review of reviews. Syst. Rev. 2015;4:64. doi: 10.1186/s13643-015-0055-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diabetes Prevention Program Research Group Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: the Diabetes Prevention Program Outcomes Study. Lancet Diabetes Endocrinol. 2015;3(11):866–875. doi: 10.1016/S2213-8587(15)00291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diederichs C., Berger K., Bartels D.B. The measurement of multiple chronic diseases—a systematic review on existing multimorbidity indices. J. Gerontol. A Biol. Sci. Med. Sci. 2010;66(3):301–311. doi: 10.1093/gerona/glq208. [DOI] [PubMed] [Google Scholar]
- Dieleman J.L., Graves C.M., Templin T. Global health development assistance remained steady in 2013 but did not align with recipients' disease burden. Health Aff. 2014;33(5):878–886. doi: 10.1377/hlthaff.2013.1432. [DOI] [PubMed] [Google Scholar]
- Diniz B.S., Butters M.A., Albert S.M., Dew M.A., Reynolds C.F. Late-life depression and risk of vascular dementia and Alzheimer's disease: systematic review and meta-analysis of community-based cohort studies. Br. J. Psychiatry J. Ment. Sci. 2013;202(5):329–335. doi: 10.1192/bjp.bp.112.118307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divo M.J., Martinez C.H., Mannino D.M. Ageing and the epidemiology of multimorbidity. Eur. Respir. J. 2014;44(4):1055–1068. doi: 10.1183/09031936.00059814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond M.F., McGuire A. Oxford University Press; New York, NY: 2001. Economic Evaluation in Health Care: Merging Theory with Practice. [Google Scholar]
- Eccles N. Telemedicine in developing countries: challenges and successes. Harvard Coll. Glob. Health Rev. 2012 https://www.hcs.harvard.edu/hghr/print/spring-2011/telemedicine-developing/ Available: (February 1) [Google Scholar]
- Ehrlich S.F., Quesenberry C.P., Van Den Eeden S.K., Shan J., Ferrara A. Patients diagnosed with diabetes mellitus are at increased risk for asthma, chronic obstructive pulmonary disease, pulmonary fibrosis, and pneumonia but not lung cancer. Diabetes Care. 2009;33(1):55–60. doi: 10.2337/dc09-0880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahlman C., Lynn J., Doberman D., Gabel J., Finch M. Prescription drug spending for Medicare+ choice beneficiaries in the last year of life. J. Palliat. Med. 2006;9(4):884–893. doi: 10.1089/jpm.2006.9.884. [DOI] [PubMed] [Google Scholar]
- Fairall L.R., Zwarenstein M., Bateman E.D. Effect of educational outreach to nurses on tuberculosis case detection and primary care of respiratory illness: pragmatic cluster randomised controlled trial. BMJ. 2005;331(7519):750–754. doi: 10.1136/bmj.331.7519.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman P., Von Korff M., Lozano P., Hecht J. Chronic care costs in managed care. Health Aff. 1997;3:239–247. doi: 10.1377/hlthaff.16.3.239. [DOI] [PubMed] [Google Scholar]
- Fortin M., Stewart M., Poitras M.E., Almirall J. Maddocks H. a systematic review of prevalence studies on multimorbidity: toward a more uniform methodology. Ann. Fam. Med. 2012;10(2):142–151. doi: 10.1370/afm.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frias J., Virdi N., Raja P., Kim Y., Savage G., Osterberg L. Effectiveness of digital medicines to improve clinical outcomes in patients with uncontrolled hypertension and type 2 diabetes: prospective, open-label, cluster-randomized pilot clinical trial. J. Med. Internet Res. 2017;19(7) doi: 10.2196/jmir.7833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries J.F., Nesse R.M., Schneider E.L., Brody J.A. Aging, natural death, and the compression of morbidity. N. Engl. J. Med. 1984;310(10):659–660. [PubMed] [Google Scholar]
- Garin N., Koyanagi A., Chatterji S. Global multimorbidity patterns: a cross-sectional, population-based, multi-country study. J. Gerontol. A Biol. Sci. Med. Sci. 2015;71(2):205–214. doi: 10.1093/gerona/glv128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GBD GBD Compare. 2015. https://vizhub.healthdata.org/gbd-compare/ Accessed:
- Global Health Observatory Data Repository. http://apps.who.int/gho/data/node.home Available: [DOI] [PubMed]
- Hajat C., Kishore S.P. The case for a global focus on multiple chronic conditions. BMJ Global Health. 2018;3(3) doi: 10.1136/bmjgh-2018-000874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajat C., Yach D. Physician, exercise thyself. Bhekisisa. 2015 http://bhekisisa.org/article/2015-08-06-physician-exercise-thyself Available: [Google Scholar]
- Hemkens L.G., Bucher H.C. HIV infection and cardiovascular disease. Eur. Heart J. 2014;35(21):1373–1381. doi: 10.1093/eurheartj/eht528. [DOI] [PubMed] [Google Scholar]
- Hensel R.L., Kempker R.R., Tapia J., Oladele A., Blumberg H.M., Magee M.J. Increased risk of latent tuberculous infection among persons with pre-diabetes and diabetes mellitus. Int. J. Tuberc. Lung Dis. 2016;20(1):71–78. doi: 10.5588/ijtld.15.0457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessel A., Gunzelmann T., Geyer M., Brähler E. Inanspruchnahme medizinischer Leistungen und Medikamenteneinnahme bei über 60jährigen in Deutschland–gesundheitliche, sozialstrukturelle, sozio-demographische und subjektive Faktoren. Z. Gerontol. Geriatr. 2000;33(4):289–299. doi: 10.1007/s003910070049. [DOI] [PubMed] [Google Scholar]
- Hoffman C., Rice D., Sung H.Y. Persons with chronic conditions: their prevalence and costs. JAMA. 1996;276(18):1473–1479. [PubMed] [Google Scholar]
- Holland J.C. History of psycho-oncology: overcoming attitudinal and conceptual barriers. Psychosom. Med. 2002;64(2):206–221. doi: 10.1097/00006842-200203000-00004. [DOI] [PubMed] [Google Scholar]
- Hooning M.J., Botma A., Aleman B.M. Long-term risk of cardiovascular disease in 10-year survivors of breast cancer. J. Natl. Cancer Inst. 2007;99(5):365–375. doi: 10.1093/jnci/djk064. [DOI] [PubMed] [Google Scholar]
- Hopman P., Heins M.J., Rijken M., Schellevis F.G. Health care utilization of patients with multiple chronic diseases in The Netherlands: differences and underlying factors. Eur. J. Intern. Med. 2015;26(3):190–196. doi: 10.1016/j.ejim.2015.02.006. [DOI] [PubMed] [Google Scholar]
- Hsieh H.M., Gu S.M., Shin S.J., Kao H.Y., Lin Y.C., Chiu H.C. Costeffectiveness of a diabetes pay-for-performance program in diabetes patients with multiple chronic conditions. PLoS One. 2015;10(7) doi: 10.1371/journal.pone.0133163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C.Q., Dong B.R., Lu Z.C., Yue J.R., Liu Q.X. Chronic diseases and risk for depression in old age: a meta-analysis of published literature. Ageing Res. Rev. 2010;9(2):131–141. doi: 10.1016/j.arr.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Huang S.W., Wang W.T., Chou L.C., Liao C.D., Liou T.H., Lin H.W. Osteoarthritis increases the risk of dementia: a nationwide cohort study in Taiwan. Sci. Rep. 2015;5 doi: 10.1038/srep10145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang W., Weller W., Ireys H., Anderson G. Out-of-pocket medical spending for care of chronic conditions. Health Aff. 2001;6:267–278. doi: 10.1377/hlthaff.20.6.267. [DOI] [PubMed] [Google Scholar]
- Institute for Health Metrics and Evaluation Global Burden of Disease. http://www.healthdata.org/gbd/data Available:
- Ionescu-Ittu R., McCusker J., Ciampi A. Continuity of primary care and emergency department utilization among elderly people. Can. Med. Assoc. J. 2007;177(11):1362–1368. doi: 10.1503/cmaj.061615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam F.M., Wu J., Jansson J., Wilson D.P. Relative risk of cardiovascular disease among people living with HIV: a systematic review and meta-analysis. HIV Med. 2012;13(8):453–468. doi: 10.1111/j.1468-1293.2012.00996.x. [DOI] [PubMed] [Google Scholar]
- Jeon C.Y., Murray M.B. Diabetes mellitus increases the risk of active tuberculosis: a systematic review of 13 observational studies. PLoS Med. 2008;5(7):e152. doi: 10.1371/journal.pmed.0050152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadam U.T., Croft P.R., North Staffordshire GP Consortium Group Clinical multimorbidity and physical function in older adults: a record and health status linkage study in general practice. Fam. Pract. 2007;24(5):412–419. doi: 10.1093/fampra/cmm049. [DOI] [PubMed] [Google Scholar]
- Katon W., Russo J., Lin E.H. Cost-effectiveness of a multicondition collaborative care intervention: a randomized controlled trial. Arch. Gen. Psychiatry. 2012;69(5):506–514. doi: 10.1001/archgenpsychiatry.2011.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingston A., Robinson L., Booth H., Knapp M., Jagger C. Projections of multi-morbidity in the older population in England to 2035: estimates from the population ageing and care simulation (PACSim) model. Age Ageing. 2018;47(3):374–380. doi: 10.1093/ageing/afx201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landi F., Onder G., Cesari M. Comorbidity and social factors predicted hospitalization in frail elderly patients1. J. Clin. Epidemiol. 2004;57(8):832–836. doi: 10.1016/j.jclinepi.2004.01.013. [DOI] [PubMed] [Google Scholar]
- Lawson K.D., Mercer S.W., Wyke S. Double trouble: the impact of multimorbidity and deprivation on preference-weighted health related quality of life a cross sectional analysis of the Scottish Health Survey. Int. J. Equity Health. 2013;12(1):67. doi: 10.1186/1475-9276-12-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C.H., Lee M.C., Shu C.C. Risk factors for pulmonary tuberculosis in patients with chronic obstructive airway disease in Taiwan: a nationwide cohort study. BMC Infect. Dis. 2013;13(1):194. doi: 10.1186/1471-2334-13-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefèvre T., d'Ivernois J.F., De Andrade V., Crozet C., Lombrail P., Gagnayre R. What do we mean by multimorbidity? An analysis of the literature on multimorbidity measures, associated factors, and impact on health services organization. Rev. Epidemiol. Sante Publique. 2014;62(5):305–314. doi: 10.1016/j.respe.2014.09.002. [DOI] [PubMed] [Google Scholar]
- Lehnert T., Heider D., Leicht H. Health care utilization and costs of elderly persons with multiple chronic conditions. Med. Care Res. Rev. 2011;68(4):387–420. doi: 10.1177/1077558711399580. [DOI] [PubMed] [Google Scholar]
- Li G., Zhang P., Wang J. The long-term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: a 20-year follow-up study. Lancet. 2008;371(9626):1783–1789. doi: 10.1016/S0140-6736(08)60766-7. [DOI] [PubMed] [Google Scholar]
- Librero J., Peiró S., Ordiñana R. Chronic comorbidity and outcomes of hospital care: length of stay, mortality, and readmission at 30 and 365 days. J. Clin. Epidemiol. 1999;52(3):171–179. doi: 10.1016/s0895-4356(98)00160-7. [DOI] [PubMed] [Google Scholar]
- Lindström J., Peltonen M., Eriksson J.G. Improved lifestyle and decreased diabetes risk over 13 years: long-term follow-up of the randomised Finnish diabetes prevention study (DPS) Diabetologia. 2013;56(2):284–293. doi: 10.1007/s00125-012-2752-5. [DOI] [PubMed] [Google Scholar]
- Louati K., Vidal C., Berenbaum F., Sellam J. Association between diabetes mellitus and osteoarthritis: systematic literature review and meta-analysis. RMD Open. 2015;1(1) doi: 10.1136/rmdopen-2015-000077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee M.J., Kempker R.R., Kipiani M. Diabetes mellitus is associated with cavities, smear grade, and multidrug-resistant tuberculosis in Georgia. Int. J. Tuberc. Lung Dis. 2015;19(6):685–692. doi: 10.5588/ijtld.14.0811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnan E.M., Palta M., Johnson H.M., Bartels C.M., Schumacher J.R., Smith M.A. The impact of a patient's concordant and discordant chronic conditions on diabetes care quality measures. J. Diabetes Complicat. 2015;29(2):288–294. doi: 10.1016/j.jdiacomp.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marengoni A., Von Strauss E., Rizzuto D., Winblad B., Fratiglioni L. The impact of chronic multimorbidity and disability on functional decline and survival in elderly persons. A community-based, longitudinal study. J. Intern. Med. 2009;265(2):288–295. doi: 10.1111/j.1365-2796.2008.02017.x. [DOI] [PubMed] [Google Scholar]
- Marengoni A., Angleman S., Melis R. Aging with multimorbidity: a systematic review of the literature. Ageing Res. Rev. 2011;10:430–439. doi: 10.1016/j.arr.2011.03.003. [DOI] [PubMed] [Google Scholar]
- McPhail S.M. Multimorbidity in chronic disease: impact on health care resources and costs. Risk Manag. Healthcare Policy. 2016;9:143. doi: 10.2147/RMHP.S97248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussavi S., Chatterji S., Verdes E., Tandon A., Patel V., Ustun B. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet. 2007;370(9590):851–858. doi: 10.1016/S0140-6736(07)61415-9. [DOI] [PubMed] [Google Scholar]
- Moxey E.D., O’Connor, Novielli K.D., Teutsch S., Nash DB. Prescription drug use in the elderly: a descriptive analysis. Health Care Financ. Rev. 2002;24(4):127–141. [PMC free article] [PubMed] [Google Scholar]
- Mueller C., Schur C., O'connell J. Prescription drug spending: the impact of age and chronic disease status. Am. J. Public Health. 1997;87(10):1626–1629. doi: 10.2105/ajph.87.10.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S. Prepare for the digital health revolution. Fortune. 2017;175(6):37–45. http://fortune.com/2017/04/20/digital-health-revolution/ Available: [Google Scholar]
- National Academy of Medicine Effective Care For High-Need Patients. 2017. https://nam.edu/wpcontent/uploads/2017/06/Effective-Care-for-High-Need-Patients.pdf Available:
- Nouwen A., Winkley K., Twisk J. Type 2 diabetes mellitus as a risk factor for the onset of depression: a systematic review and meta-analysis. Diabetologia. 2010;12:2480–2486. doi: 10.1007/s00125-010-1874-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paez K.A., Zhao L., Hwang W. Rising out-of-pocket spending for chronic conditions: a ten-year trend. Health Aff. 2009;28(1):15–25. doi: 10.1377/hlthaff.28.1.15. [DOI] [PubMed] [Google Scholar]
- Panagioti M., Richardson G., Murray E. Queen's Printer and Controller of HMSO; Southampton, UK: 2014. Reducing Care Utilisation through Self-management Interventions (RECURSIVE): A Systematic Review and Meta-analysis. [PubMed] [Google Scholar]
- Passik S.D., Dugan W., McDonald M.V., Rosenfeld B., Theobald D.E., Edgerton S. Oncologists' recognition of depression in their patients with cancer. J. Clin. Oncol. 1998;16(4):1594–1600. doi: 10.1200/JCO.1998.16.4.1594. [DOI] [PubMed] [Google Scholar]
- Pruchno R.A., Wilson-Genderson M., Heid A.R. Multiple chronic condition combinations and depression in community-dwelling older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2016;71(7):910–915. doi: 10.1093/gerona/glw025. [DOI] [PubMed] [Google Scholar]
- Rapoport J., Jacobs P., Bell N.R., Klarenbach S. Refining the measurement of the economic burden of chronic diseases in Canada. Age. 2004;20(39):1–643. [PubMed] [Google Scholar]
- Rodriguez B.L., D'agostino R., Abbott R.D. Risk of hospitalized stroke in men enrolled in the Honolulu Heart Program and the Framingham Study: a comparison of incidence and risk factor effects. Stroke. 2002;33(1):230. doi: 10.1161/hs0102.101081. (-6.0-236) [DOI] [PubMed] [Google Scholar]
- Rogowski J., Lillard L.A., Kington R. The financial burden of prescription drug use among elderly persons. Gerontologist. 1997;37(4):475–482. doi: 10.1093/geront/37.4.475. [DOI] [PubMed] [Google Scholar]
- Salisbury C., Johnson L., Purdy S., Valderas J.M., Montgomery A.A. Epidemiology and impact of multimorbidity in primary care: a retrospective cohort study. Br. J. Gen. Pract. 2011;61(582):e12–e21. doi: 10.3399/bjgp11X548929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambamoorthi U., Shea D., Crystal S. Total and out-of-pocket expenditures for prescription drugs among older persons. The Gerontologist. 2003;43(3):345–359. doi: 10.1093/geront/43.3.345. [DOI] [PubMed] [Google Scholar]
- Sarkar M., Gowda S., Madabhavi I., Dogra K. Tuberculosis associated chronic obstructive pulmonary disease. Clin. Respir. J. 2017;3:285–295. doi: 10.1111/crj.12621. [DOI] [PubMed] [Google Scholar]
- Schneider K.M., O'Donnell B.E., Dean D. Prevalence of multiple chronic conditions in the United States' Medicare population. Health Qual. Life Outcomes. 2009;7(1):82. doi: 10.1186/1477-7525-7-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider C., Jick S.S., Bothner U., Meier C.R. COPD and the risk of depression. CHEST Journal. 2010;137(2):341–347. doi: 10.1378/chest.09-0614. [DOI] [PubMed] [Google Scholar]
- Selvin E., Marinopoulos S., Berkenblit G. Meta-analysis: glycosylated hemoglobin and cardiovascular disease in diabetes mellitus. Ann. Intern. Med. 2004;141(6):421–431. doi: 10.7326/0003-4819-141-6-200409210-00007. [DOI] [PubMed] [Google Scholar]
- Shelton P., Sager M.A., Schraeder C. The community assessment risk screen (CARS): identifying elderly persons at risk for hospitalization or emergency department visit. Am. J. Manag. Care. 2000;6(8):925–933. [PubMed] [Google Scholar]
- Smith S.M., Soubhi H., Fortin M., Hudon C., O'Dowd T. Managing patients with multimorbidity: systematic review of interventions in primary care and community settings. BMJ. 2012;345 doi: 10.1136/bmj.e5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starfield B., Lemke K.W., Herbert R., Pavlovich W.D., Anderson G. Comorbidity and the use of primary care and specialist care in the elderly. Ann. Fam. Med. 2005;3(3):215–222. doi: 10.1370/afm.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian S.V., Corsi D.J., Subramanyam M.A., Davey Smith G. Jumping the gun: the problematic discourse on socioeconomic status and cardiovascular health in India. Int. J. Epidemiol. 2013;42(5):1410–1426. doi: 10.1093/ije/dyt017. [DOI] [PubMed] [Google Scholar]
- Tatemichi T.K., Paik M., Bagiella E. Risk of dementia after stroke in a hospitalized cohort: results of a longitudinal study. Neurology. 1994;44(10):1885. doi: 10.1212/wnl.44.10.1885. [DOI] [PubMed] [Google Scholar]
- Tonelli M., Wiebe N., Guthrie B. Comorbidity as a driver of adverse outcomes in people with chronic kidney disease. Kidney Int. 2015;88(4):859–866. doi: 10.1038/ki.2015.228. [DOI] [PubMed] [Google Scholar]
- United Nations General Assembly, Sixty-Sixth Session. High-Level Meeting of the General Assembly on the Prevention and Control of Non-communicable Diseases. 2011. http://www.who.int/nmh/events/un_ncd_summit2011/3rd_plenary_meeting.pdf Accessed:
- Van der Kooy K., Van Hout H., Marwijk H., Marten H., Stehouwer C., Beekman A. Depression and the risk for cardiovascular diseases: systematic review and meta analysis. Int. J. Geriatr. Psychiatry. 2007;22(7):613–626. doi: 10.1002/gps.1723. [DOI] [PubMed] [Google Scholar]
- Violan C., Foguet-Boreu Q., Flores-Mateo G. Prevalence, determinants and patterns of multimorbidity in primary care: a systematic review of observational studies. PLoS One. 2014;9(7) doi: 10.1371/journal.pone.0102149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Naghavi M., Allen C. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1459–1544. doi: 10.1016/S0140-6736(16)31012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward B.W., Schiller J.S., Goodman R.A. Peer reviewed: multiple chronic conditions among us adults: a 2012 update. Prev. Chronic Dis. 2014;11 doi: 10.5888/pcd11.130389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner D.E., Tighiouart H., Amin M.G. Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: a pooled analysis of community-based studies. J. Am. Soc. Nephrol. 2004;15(5):1307–1315. doi: 10.1097/01.asn.0000123691.46138.e2. [DOI] [PubMed] [Google Scholar]
- Weinstein M.C., O'brien B., Hornberger J. Principles of good practice for decision analytic modeling in health-care evaluation: report of the ISPOR Task Force on Good Research Practices—modeling studies. Value Health. 2003;6(1):9–17. doi: 10.1046/j.1524-4733.2003.00234.x. [DOI] [PubMed] [Google Scholar]
- Wolff J.L., Starfield B., Anderson G. Prevalence, expenditures, and complications of multiple chronic conditions in the elderly. Arch. Intern. Med. 2002;162(20):2269–2276. doi: 10.1001/archinte.162.20.2269. [DOI] [PubMed] [Google Scholar]
- World Health Organization . 2013. Global Action Plan for the Prevention and Control of Noncommunicable Diseases 2013–2020. [Google Scholar]
- Xakellis G.C. Are patients who use a generalist physician healthier than those who seek specialty care directly? Fam. Med. 2005;37(10):719–726. [PubMed] [Google Scholar]
- Yurkovich M., Avina-Zubieta J.A., Thomas J., Gorenchtein M., Lacaille D. A systematic review identifies valid comorbidity indices derived from administrative health data. J. Clin. Epidemiol. 2015;68(1):3–14. doi: 10.1016/j.jclinepi.2014.09.010. [DOI] [PubMed] [Google Scholar]