Abstract
The anti-apoptotic protein Bcl-2 is upregulated in several cancers, including diffuse large B-cell lymphoma (DLBCL) and chronic lymphocytic leukemia (CLL). In a subset of these cancer cells, Bcl-2 blocks Ca2+-mediated apoptosis by suppressing the function of inositol 1,4,5-trisphosphate (IP3) receptors (IP3Rs) located at the endoplasmic reticulum (ER). A peptide tool, called Bcl-2/IP3 receptor disruptor-2 (BIRD-2), was developed to disrupt Bcl-2/IP3R complexes, triggering pro-apoptotic Ca2+ signals and killing Bcl-2-dependent cancer cells. In DLBCL cells, BIRD-2 sensitivity depended on the expression level of IP3R2 channels and constitutive IP3 signaling downstream of the B-cell receptor. However, other cellular pathways probably also contribute to BIRD-2-provoked cell death. Here, we examined whether BIRD-2-induced apoptosis depended on extracellular Ca2+ and more particularly on store-operated Ca2+ entry (SOCE), a Ca2+-influx pathway activated upon ER-store depletion. Excitingly, DPB162-AE, a SOCE inhibitor, suppressed BIRD-2-induced cell death in DLBCL cells. However, DPB162-AE not only inhibits SOCE but also depletes the ER Ca2+ store. Treatment of the cells with YM-58483 and GSK-7975A, two selective SOCE inhibitors, did not protect against BIRD-2-induced apoptosis. Similar data were obtained by knocking down STIM1 using small interfering RNA. Yet, extracellular Ca2+ contributed to BIRD-2 sensitivity in DLBCL, since the extracellular Ca2+ buffer ethylene glycol tetraacetic acid (EGTA) blunted BIRD-2-triggered apoptosis. The protective effects observed with DPB162-AE are likely due to ER Ca2+-store depletion, since a similar protective effect could be obtained using the sarco/endoplasmic reticulum Ca2+-ATPase inhibitor thapsigargin. Thus, both the ER Ca2+-store content and extracellular Ca2+, but not SOCE, are critical factors underlying BIRD-2-provoked cell death.
Introduction
Cell death and survival is regulated by the Bcl-2-protein family, which consists of pro-apoptotic and anti-apoptotic family members1. The anti-apoptotic protein Bcl-2 is upregulated in a large number of cancer cells, including B-cell lymphomas like chronic lymphocytic leukemia (CLL) and diffuse large B-cell lymphoma (DLBCL)2,3. Bcl-2 prevents apoptotic cell death by neutralizing pro-apoptotic family members, including the executioner proteins Bak and Bax and the BH3-only protein Bim, at the mitochondria4,5. BH3-mimetic compounds, like venetoclax, disrupt the binding between Bcl-2 and pro-apoptotic BH3-only proteins, thereby triggering apoptotic cell death in cancer cells that depend on Bcl-2's function at the mitochondria for their survival6,7. Furthermore, the Bcl-2 protein is also located at the endoplasmic reticulum (ER), the main intracellular Ca2+ store8,9. There, Bcl-2 binds with its Bcl-2 homology 4 (BH4) domain to the central, modulatory domain of the inositol 1,4,5-trisphosphate (IP3) receptor (IP3R)10. In this way, Bcl-2 blocks excessive, pro-apoptotic, IP3R-mediated Ca2+ release from the ER, thereby preventing mitochondrial Ca2+ overload and subsequent apoptotic cell death10. Based on the binding site of Bcl-2 on the IP3R, a peptide tool was developed in an attempt to target pro-survival Bcl-2 proteins at the ER in cancer cells11. This cell-permeable peptide, called Bcl-2/IP3R disruptor-2 (BIRD-2), is capable of stripping Bcl-2 from the IP3R, without affecting Bcl-2/Bim complexes. BIRD-2 was shown to kill Bcl-2-dependent cancer cells, like DLBCL and CLL cells, by eliciting spontaneous, pro-apoptotic Ca2+ signals12,13. On the other hand, the survival of normal peripheral mononuclear blood cells was not affected by the peptide tool. Furthermore, follicular lymphoma and small-cell lung cancer cells could be killed by BIRD-2 as well and the peptide even decreased the in vivo tumor growth of human myeloma cells in xenografted mouse models14,15. Interestingly, in DLBCL cells BIRD-2 sensitivity correlated to the expression level of isoform 2 of the IP3R, which is the isoform with the highest sensitivity towards its ligand IP312. DLBCL cells with high IP3R2 levels, like SU-DHL-4 cells, were very sensitive to BIRD-2, whereas cells with low IP3R2 expression levels, such as OCI-LY-1, appeared to be rather resistant to the peptide. On the other hand, OCI-LY-1 cells are very sensitive to BH3-mimetic drugs, like venetoclax16. Recent work from our group showed that there exists an opposite correlation between the susceptibility of DLBCL cells to BIRD-2 and venetoclax16. Additionally, constitutive IP3 signaling also underlies BIRD-2 sensitivity in B-cell cancers17. DLBCL and primary CLL cells could be protected from BIRD-2-triggered apoptosis by blocking constitutive phospholipase C and IP3 signaling.
However, it is not clear whether other cellular factors contribute to BIRD-2-induced cell death in cancer cells. In particular, we found that BIRD-2 provoked spontaneous Ca2+ oscillations in B-cell malignancies13, which eventually result in Ca2+ overload via IP3R-mediated Ca2+ fluxes12. In many cells, Ca2+ oscillations are maintained through the concerted action of Ca2+ release from the ER and Ca2+ influx from the extracellular milieu. Therefore, we assessed whether extracellular Ca2+ and Ca2+ entry mechanisms such as store-operated Ca2+ entry (SOCE) contributed to BIRD-2 cytotoxicity. SOCE is an important Ca2+-influx pathway that is activated upon ER-store depletion18. It is mediated through STIM and Orai proteins19–23. STIM proteins are present in the ER membrane where they serve as luminal Ca2+ sensors, while Orai proteins are located in the plasma membrane and function as Ca2+-influx channels20–22. Upon depletion of the ER Ca2+ store, STIM1 proteins form oligomers that translocate to parts of the ER membrane that are in close contact with the plasma membrane. There, STIM1 activates Orai1 channels to trigger Ca2+ influx23,24. The BIRD-2 peptide provokes IP3-induced Ca2+ release in cancer cells that depend for their survival on the function of Bcl-2 at the ER. Hence, we hypothesized that BIRD-2 treatment results in SOCE activation, subsequently enabling Ca2+ influx that is contributing to intracellular Ca2+ overload and thus cell death. Here, we focused on SU-DHL-4 cells to test our hypothesis, since this DLBCL cell model displays the highest sensitivity towards BIRD-212,16. Our data indicate that despite the fact that extracellular Ca2+ is important for BIRD-2-induced cell death in these cells, SOCE was dispensable as pharmacological SOCE inhibitors YM-58483 and GSK-7975A and Stim1 knockdown failed to protect against BIRD-2-induced apoptosis. Interestingly, DPB162-AE, a SOCE inhibitor that also depletes the ER Ca2+ stores, did protect against BIRD-2-induced cell death. However, this is likely through its action at the ER, since ER Ca2+-store depletion brought about by the sarco/ER Ca2+-ATPase (SERCA) inhibitor thapsigargin (TG) too suppressed BIRD-2 toxicity.
Results
BIRD-2-induced cell death in SU-DHL-4 cells is reduced by DPB162-AE, a SOCE inhibitor that also depletes the ER Ca2+ store
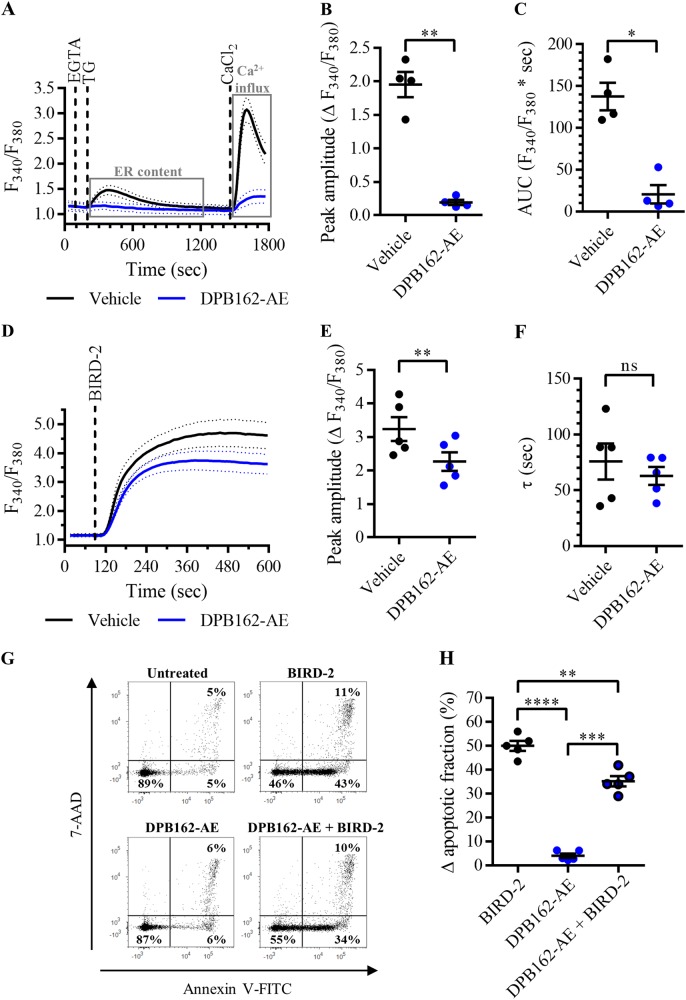
In DLBCL cell lines, BIRD-2 potently induces apoptotic cell death by disrupting Bcl-2/IP3R complexes, resulting in excessive Ca2+ signaling12,16. Since BIRD-2 triggers Ca2+ release from the ER, we wondered whether SOCE occurs upon BIRD-2 application and thus contributes to the cell death-inducing properties of the peptide tool. We explored this hypothesis by focusing on the BIRD-2-sensitive DLBCL cell model, SU-DHL-416, in which we studied the impact of the SOCE inhibitor DPB162-AE on the BIRD-2-induced Ca2+ rise and cell death response. In this cell model, DPB162-AE acts as a potent SOCE inhibitor (Fig. 1a), confirming previously published data25. Pre-treatment of Fura-2 AM-loaded SU-DHL-4 cells with 10 µM DPB162-AE for 30 min reduced Ca2+ influx after store depletion (Fig. 1a). Ca2+ influx was quantified by measuring the peak amplitude. Compared to the control condition, the peak amplitude was significantly reduced in cells pre-treated with DPB162-AE (Fig. 1b). However, in accordance with our previous findings25, DPB162-AE did not only inhibit SOCE, but also depleted the ER Ca2+ store (Fig. 1a). The TG-releasable Ca2+ was quantified by measuring the area under the curve (AUC), which was significantly reduced in cells pre-treated with DPB162-AE (Fig. 1c). Next, it was determined whether DPB162-AE treatment has an effect on the pro-apoptotic, cytosolic Ca2+ rise triggered by BIRD-2 addition. Therefore, SU-DHL-4 cells were loaded with Fura-2 AM and pre-treated for 30 min with 10 µM DPB162-AE. Subsequently, BIRD-2, at a concentration of 10 µM which is the IC50 value of the peptide in SU-DHL-4 cells16, was added while monitoring the cytosolic Ca2+ levels (Fig. 1d). Compared to the control condition, the BIRD-2-provoked Ca2+ rise was reduced in cells pre-treated with DPB162-AE. The cytosolic Ca2+ response was quantified by measuring the peak amplitude and the time constant τ. Compared to cells pre-treated with vehicle, the peak amplitude was significantly reduced in DPB162-AE-pre-treated cells (Fig. 1e). The time constant τ also displayed a tendency to be lower in DPB162-AE-pre-treated cells, although there was no significant difference with the control condition (Fig. 1f). Then, it was investigated whether DPB162-AE also reduces BIRD-2-triggered apoptotic cell death. To do so, SU-DHL-4 cells were pre-treated for 30 min with 10 µM DPB162-AE, followed by treatment with 10 µM BIRD-2. After 2h of peptide treatment, cell death was measured via Annexin V-FITC and 7-aminoactinomycin D (7-AAD) staining of the cells (Fig. 1g). BIRD-2 induced approximately 50% of apoptotic cell death in the SU-DHL-4 cells, whereas treatment with DPB162-AE was only slightly toxic (~5% apoptosis) for the cells (Fig. 1h). Interestingly, DPB162-AE pre-treatment significantly reduced BIRD-2-triggered apoptosis compared to cells treated with BIRD-2 alone. These results indicate that DPB162-AE protects against BIRD-2-provoked cell death, although we do not know whether this is due to its inhibitory effect on SOCE or due to depletion of the ER Ca2+ store.
Fig. 1. DPB162-AE reduces the cytotoxic effects of BIRD-2 in SU-DHL-4 cells.
a Analysis of Ca2+ influx after store depletion in SU-DHL-4 cells using the ratiometric Ca2+ indicator Fura-2 AM. Cells were pre-treated for 30 min with vehicle (black line) or with 10 µM DPB162-AE (blue line). To deplete the ER Ca2+ store, 3 mM EGTA and 1 µM thapsigargin (TG) were added as indicated. After store depletion, Ca2+ influx was triggered by adding 10 mM CaCl2. The curves represent the mean ± SEM of four independent experiments. Quantification of the Ca2+ influx is provided as the peak amplitude (∆ F340/F380) in b, whereas the TG-releasable Ca2+ is quantified as the AUC (F340/F380 × s) in c. d Analysis of the cytosolic Ca2+ response in SU-DHL-4 cells using Fura-2 AM. Cells were pre-treated for 30 min with vehicle (black line) or 10 µM DPB162-AE (blue line). Addition of 10 µM BIRD-2 is indicated by the dotted line. The curves represent the mean ± SEM of five independent experiments. The BIRD-2-provoked cytosolic Ca2+ rise is quantified by measuring the peak amplitude (∆ F340/F380), shown in e, and the time constant τ (s), which is shown in f. g Representative scatter plots from flow cytometry analysis detecting apoptosis in SU-DHL-4 cells stained with Annexin V-FITC and 7-AAD. Cells were pre-treated with or without 10 µM DPB162-AE 30 min prior to application of 10 µM BIRD-2. After 2 h of BIRD-2 treatment, apoptotic cell death was detected by measuring the Annexin V-FITC-positive fraction. h Quantitative analysis of five independent experiments detecting apoptosis in SU-DHL-4 cells treated for 2 h with 10 µM BIRD-2, with or without pre-treatment with 10 µM DPB162-AE. The ∆ apoptotic fraction is plotted, which corresponds to the apoptotic fraction corrected for the percentage of apoptosis detected in untreated cells. In the dot plots, data are represented as the mean ± SEM of at least four independent experiments. Statistically significant differences were determined with a paired two-tailed Student’s t test or a one-way ANOVA, as appropriate (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001). NS not significant
Selective inhibition of SOCE with pharmacological compounds does not protect against BIRD-2-triggered cell death
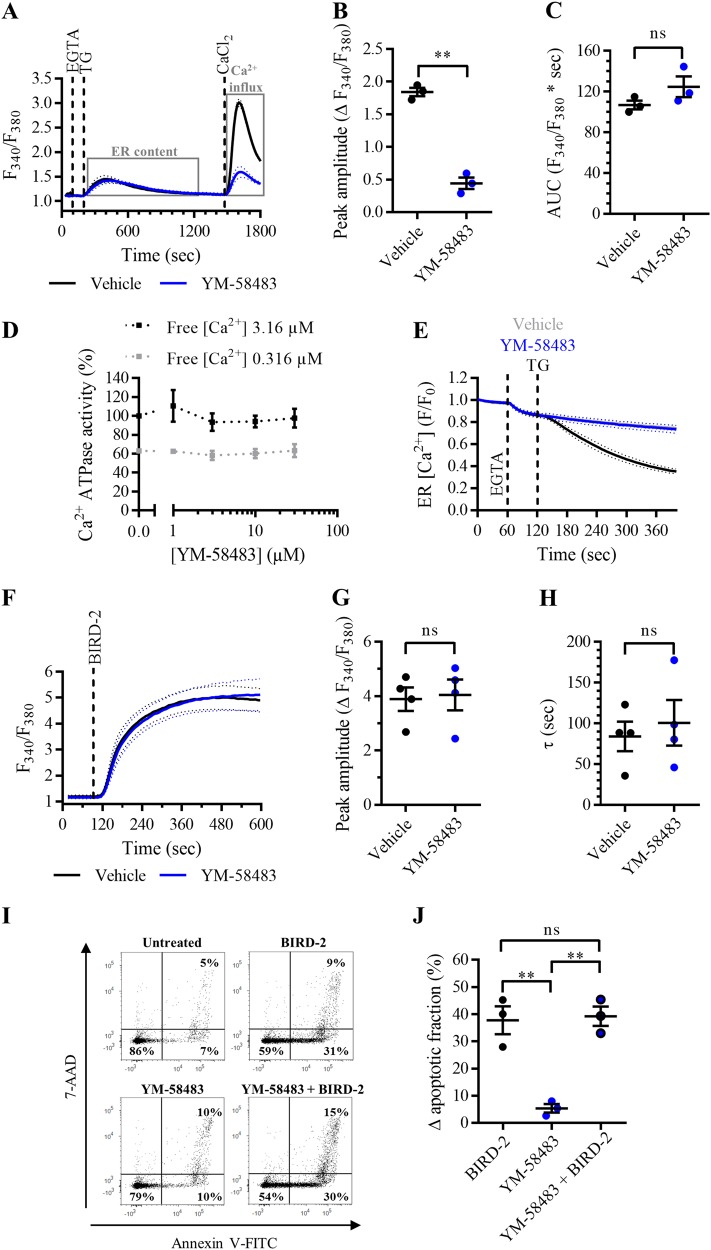
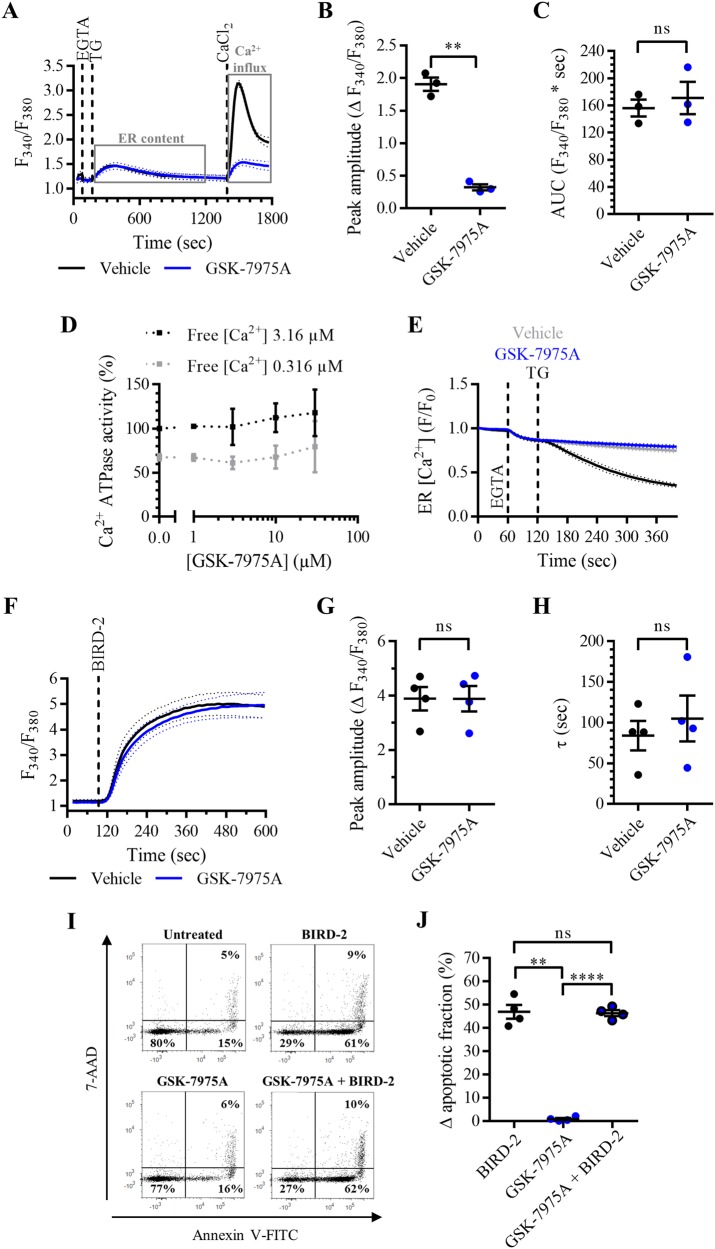
Pharmacological tools that selectively inhibit SOCE were used to test whether DPB162-AE treatment confers protection to BIRD-2 via inhibition of the Ca2+-influx pathway. For this purpose, YM-58483 and GSK-7975A were used. First, it was validated that YM-58483 selectively inhibited SOCE. Compared to vehicle-pre-treated cells, Ca2+ influx after store depletion was reduced in SU-DHL-4 cells pre-treated with 3 µM YM-58483 (Fig. 2a). SOCE was quantified by measuring the peak amplitude, which was significantly reduced in YM-58483-pre-treated cells (Fig. 2b). Moreover, YM-58483 did not alter the ER Ca2+ content, since there was no significant difference in the AUC between YM-58483-pre-treated and vehicle-pre-treated cells (Fig. 2c). To ascertain that YM-58483 has no effect on the ER Ca2+ levels, it was determined whether the compound affects ER Ca2+ uptake by measuring the ATPase activity of SERCA2b, the house-keeping SERCA isoform (Fig. 2d), and whether it affects ER Ca2+ leak by directly measuring ER Ca2+ levels upon YM-58483 application (Fig. 2e). First, SERCA2b ATPase activity was measured in the presence of increasing concentrations of YM-58483 at submaximal (free [Ca2+] 0.316 µM) and maximal (free [Ca2+] 3.16 µM) [Ca2+] in COS microsomes overexpressing SERCA2b (Fig. 2d). Compared to the control condition, YM-58483 does not alter SERCA2b ATPase activity at maximal and submaximal [Ca2+]. Furthermore, it was examined whether the SOCE inhibitor induces an ER Ca2+-leak pathway by measuring the ER Ca2+ levels in HeLa cells transfected with the G-CEPIA1er plasmid26. ER Ca2+ levels were decreased upon application of 1 µM TG, which inhibits the SERCA pump (Fig. 2e). On the other hand, acute addition of 3 µM YM-58483 did not reduce ER Ca2+ levels. These results indicate that YM-58483 has no effect on the ER Ca2+-store content and it can therefore be used as a selective inhibitor of SOCE. Next, it was determined whether inhibiting SOCE with YM-58483 protects against BIRD-2 cytotoxicity in SU-DHL-4 cells. The cytosolic Ca2+ rise provoked by the addition of 10 µM BIRD-2 was measured in SU-DHL-4 cells pre-treated with vehicle or 3 µM YM-58483. Strikingly, the BIRD-2-triggered Ca2+ rise was comparable between cells treated with YM-58483 and the control condition (Fig. 2f). The cytosolic Ca2+ response was quantified by measuring the peak amplitude and the time constant τ. There was neither a significant difference between the peak amplitude (Fig. 2g) nor the time constant τ (Fig. 2h) of YM-58483-pre-treated and vehicle-pre-treated SU-DHL-4 cells. Furthermore, apoptotic cell death triggered by 10 µM BIRD-2 was not reduced in SU-DHL-4 cells that were pre-treated for 30 min with 3 µM YM-58483, compared to cells treated with BIRD-2 alone (Fig. 2i). BIRD-2 treatment as well as YM-58483 + BIRD-2 treatment induced approximately 40% of apoptotic cell death, while treatment of the cells with the SOCE inhibitor alone was only slightly toxic (~5% apoptosis) for the cells (Fig. 2j). These results already strongly suggest that SOCE is not activated by BIRD-2 in the SU-DHL-4 DLBCL cells. Furthermore, these results were confirmed by a second pharmacological SOCE inhibitor, GSK-7975A. Treatment of SU-DHL-4 cells with 3 µM GSK-7975A potently inhibited Ca2+ influx after store depletion (Fig. 3a, b). GSK-7975A is a selective inhibitor of SOCE, as this compound neither reduced the ER Ca2+ content (Fig. 3a–c) nor affected SERCA2b ATPase activity (Fig. 3d) nor altered the ER Ca2+ levels (Fig. 3e). Similarly to YM-58483, GSK-7975A did not reduce the cytosolic Ca2+ rise induced by 10 µM BIRD-2 addition to SU-DHL-4 cells (Fig. 3f). The peak amplitude (Fig. 3g) and the time constant τ (Fig. 3h) were comparable between vehicle-treated and GSK-7975A-treated cells. Finally, apoptotic cell death triggered by 10 µM BIRD-2 was not reduced by inhibiting SOCE with GSK-7975A (Fig. 3i). Of note, GSK-7975A by itself was absolutely not toxic for the SU-DHL-4 cells. Hence, BIRD-2 cytotoxicity was comparable between cells treated with peptide alone or with GSK-7975A in combination with BIRD-2 (Fig. 3j). In summary, these results indicate that BIRD-2 does not depend on Ca2+ influx through SOCE for its pro-apoptotic effects in SU-DHL-4 DLBCL cells.
Fig. 2. SOCE inhibition with YM-58483 does not reduce cell death triggered by BIRD-2 in SU-DHL-4 cells.
a Analysis of Ca2+ influx after store depletion in SU-DHL-4 cells using the ratiometric Ca2+ indicator Fura-2 AM. Cells were pre-treated for 30 min with vehicle (black line) or with 3 µM YM-58483 (blue line). To deplete the ER Ca2+ store, 3 mM EGTA and 1 µM TG were added as indicated. After store depletion, Ca2+ influx was triggered by adding 10 mM CaCl2. The curves represent the mean ± SEM of three independent experiments. Quantification of the Ca2+ influx is provided as the peak amplitude (∆ F340/F380) in b, whereas the TG-releasable Ca2+ is quantified as the AUC (F340/F380 × s) in c. d Dose–response curve of YM-58483 on SERCA2b ATPase activity (%). The Ca2+-dependent ATPase activity was measured at maximal (free [Ca2+] 3.16 µM) and submaximal (free [Ca2+] 0.316 µM) [Ca2+] for different treatments, including vehicle (control) and different [YM-58483]. Data were normalized to the values obtained in the control condition at maximal [Ca2+], which was set at 100%. Data are represented at the mean ± SEM of three independent experiments. e Single-cell analysis of the ER Ca2+ levels in HeLa cells transfected with G-CEPIA1er plasmid. Cells were treated with vehicle (gray curve), 1 µM TG (black curve), or 3 µM YM-58483 (blue curve) 60 s after the addition of 3 mM EGTA. Data are represented as the mean ± SEM of three independent experiments (n > 100 cells/condition). f Analysis of the cytosolic Ca2+ response in SU-DHL-4 cells using Fura-2 AM. Cells were pre-treated for 30 min with vehicle (black line) or 3 µM YM-58483 (blue line). Addition of 10 µM BIRD-2 is indicated by the dotted line. The curves represent the mean ± SEM of four independent experiments. The BIRD-2-provoked cytosolic Ca2+ rise is quantified by measuring the peak amplitude (∆ F340/F380), shown in g, and the time constant τ (s), which is shown in h. i Representative scatter plots from flow cytometry analysis detecting apoptosis in SU-DHL-4 cells stained with Annexin V-FITC and 7-AAD. Cells were pre-treated with or without 3 µM YM-58483 30 min prior to application of 10 µM BIRD-2. After 2 h of BIRD-2 treatment, apoptotic cell death was detected by measuring the Annexin V-FITC-positive fraction. j Quantitative analysis of three independent experiments detecting apoptosis in SU-DHL-4 cells treated for 2 h with 10 µM BIRD-2, with or without pre-treatment with 3 µM YM-58483. The ∆ apoptotic fraction is plotted, which corresponds to the apoptotic fraction corrected for the percentage of apoptosis detected in untreated cells. In the dot plots, data are represented as the mean ± SEM of at least three independent experiments. Statistically significant differences were determined with a paired two-tailed Student’s t test or a one-way ANOVA, as appropriate (**p < 0.01). NS not significant.
Fig. 3. SOCE inhibition with GSK-7975A does not reduce apoptosis induced by BIRD-2 in SU-DHL-4 cells.
a Analysis of Ca2+ influx after store depletion in SU-DHL-4 cells using the ratiometric Ca2+ indicator Fura-2 AM. Cells were pre-treated for 30 min with vehicle (black line) or with 3 µM GSK-7975A (blue line). To deplete the ER Ca2+ store, 3 mM EGTA and 1 µM TG were added as indicated. After store depletion, Ca2+ influx was triggered by adding 10 mM CaCl2. The curves represent the mean ± SEM of three independent experiments. Quantification of the Ca2+ influx is provided as the peak amplitude (∆ F340/F380) in b, whereas the TG-releasable Ca2+ is quantified as the AUC (F340/F380 × s) in c. d Dose–response curve of GSK-7975A on SERCA2b ATPase activity (%). The Ca2+-dependent ATPase activity was measured at maximal (free [Ca2+] 3.16 µM) and submaximal (free [Ca2+] 0.316 µM) [Ca2+] for different treatments, including vehicle (control) and different [GSK-7975A]. Data were normalized to the values obtained in the control condition at maximal [Ca2+], which was set at 100%. Data are represented at the mean ± SEM of three independent experiments. e Single-cell analysis of the ER Ca2+ levels in HeLa cells transfected with G-CEPIA1er plasmid. Cells were treated with vehicle (gray curve), 1 µM TG (black curve), or 3 µM GSK-7975A (blue curve) 60 s after the addition of 3 mM EGTA. Data are represented as the mean ± SEM of three independent experiments (n > 100 cells/condition). f Analysis of the cytosolic Ca2+ response in SU-DHL-4 cells using Fura-2 AM. Cells were pre-treated for 30 min with vehicle (black line) or 3 µM GSK-7975A (blue line). Addition of 10 µM BIRD-2 is indicated by the dotted line. The curves represent the mean ± SEM of four independent experiments. The BIRD-2-provoked cytosolic Ca2+ rise is quantified by measuring the peak amplitude (∆ F340/F380), shown in g, and the time constant τ (s), which is shown in h. i Representative scatter plots from flow cytometry analysis detecting apoptosis in SU-DHL-4 cells stained with Annexin V-FITC and 7-AAD. Cells were pre-treated with or without 3 µM GSK-7975A 30 min prior to application of 10 µM BIRD-2. After 2 h of BIRD-2 treatment, apoptotic cell death was detected by measuring the Annexin V-FITC-positive fraction. j Quantitative analysis of four independent experiments detecting apoptosis in SU-DHL-4 cells treated for 2 h with 10 µM BIRD-2, with or without pre-treatment with 3 µM GSK-7975A. The ∆ apoptotic fraction is plotted, which corresponds to the apoptotic fraction corrected for the percentage of apoptosis detected in untreated cells. In the dot plots, data are represented as the mean ± SEM of at least three independent experiments. Statistically significant differences were determined with a paired two-tailed Student’s t test or a one-way ANOVA, as appropriate (**p < 0.01, ****p < 0.0001). NS not significant.
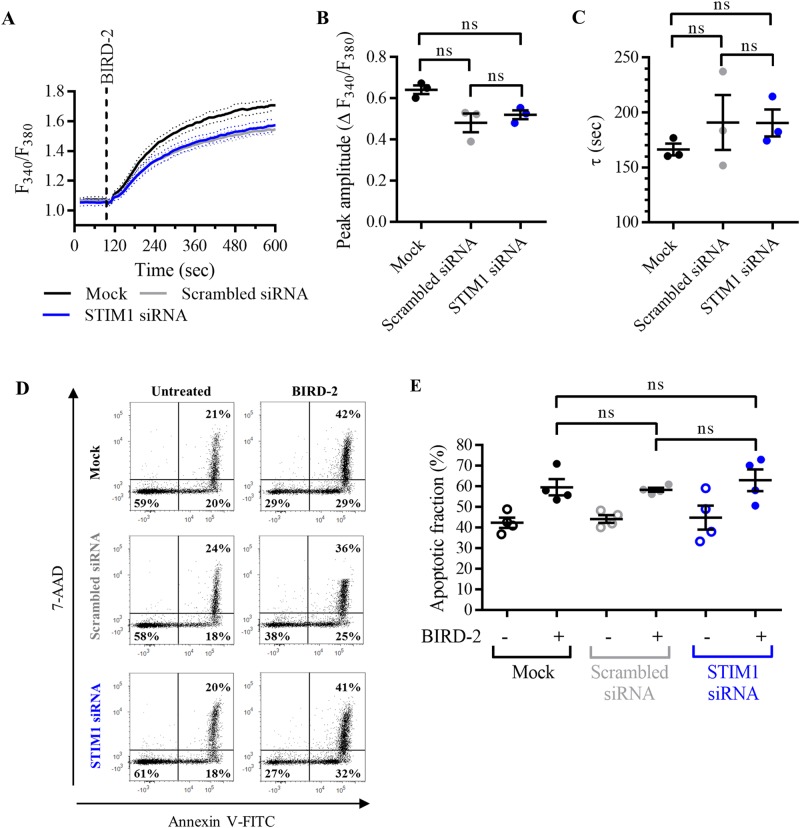
Knockdown of STIM1 with siRNA does not confer protection against BIRD-2-induced apoptosis in SU-DHL-4 cells
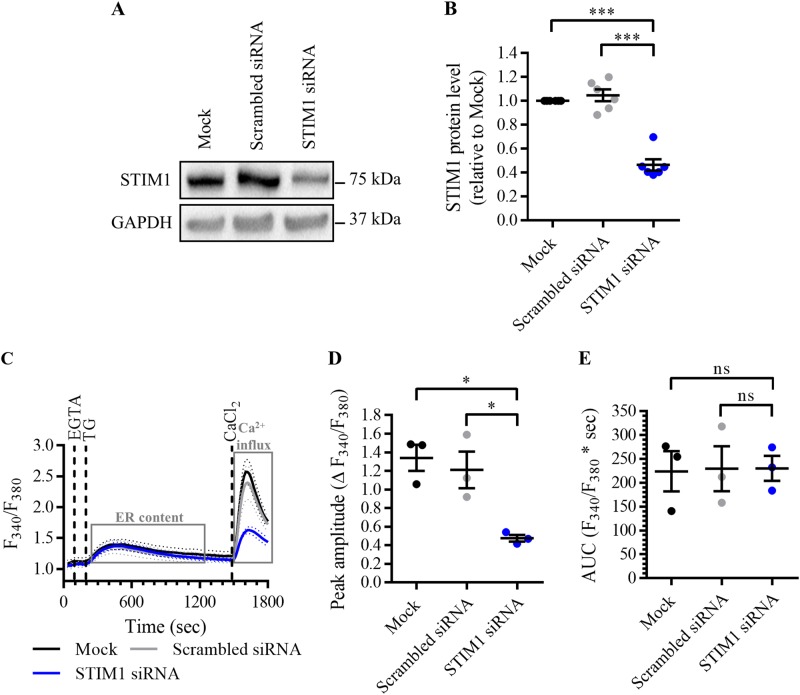
Next, we targeted SOCE using small interfering RNA (siRNA) against STIM1 (Fig. 4a). Mock-transfected SU-DHL-4 cells and cells transfected with scrambled siRNA were used as control conditions. Compared to mock-transfected cells, the expression level of STIM1 was for 60% reduced in cells transfected with STIM1 siRNA, but not affected by scrambled siRNA (Fig. 4b). Next, it was validated that STIM1 knockdown inhibited Ca2+ influx after store depletion (Fig. 4c), as done before. Compared to the control conditions, the peak amplitude, used to quantify SOCE, was significantly reduced in cells transfected with siRNA against STIM1 (Fig. 4d). On the other hand, the AUC, used to quantify the TG-releasable Ca2+, was not reduced by knockdown of STIM1 (Fig. 4e), indicating that STIM1 siRNA selectively blocks SOCE without affecting the ER Ca2+-store content. Next, it was determined whether STIM1 knockdown affects the BIRD-2-induced Ca2+ and cell death response. The cytosolic Ca2+ rise provoked by 10 µM BIRD-2 was comparable between SU-DHL-4 cells transfected with STIM1 siRNA, scrambled siRNA and mock-transfected cells (Fig. 5a), indicated by very similar peak amplitudes (Fig. 5b) and time constants (Fig. 5c). Moreover, apoptotic cell death triggered by 2 h treatment with 10 µM BIRD-2 was comparable for all conditions (Fig. 5d). About 40% of the SU-DHL-4 cell population was already dead after transfection by electroporation (Fig. 5e). Upon treatment with 10 µM BIRD-2, apoptotic cell death was increased to approximately 60% in mock-transfected cells as well as in cells transfected with scrambled and STIM1 siRNA. These data further underpin that SOCE is not involved in BIRD-2-induced apoptosis in SU-DHL-4 cells.
Fig. 4. Knockdown of STIM1 with siRNA inhibits SOCE without depleting the ER Ca2+ store.
a A representative western blot of six independent experiments showing the expression level of STIM1 in mock-transfected SU-DHL-4 cells, or cells transfected with scrambled siRNA or with siRNA against STIM1. The expression level of GAPDH was used as loading control. b Quantification of the STIM1/GAPDH protein levels of six independent experiments relative to the level in mock-transfected SU-DHL-4 cells, which was set at 1. c Analysis of Ca2+ influx after store depletion in transfected SU-DHL-4 cells using the ratiometric Ca2+ indicator Fura-2 AM. To deplete the ER Ca2+ store, 3 mM EGTA and 1 µM TG were added as indicated. After store depletion, Ca2+ influx was triggered by adding 10 mM CaCl2. The curves represent the mean ± SEM of three independent experiments. Quantification of the Ca2+ influx is provided as the peak amplitude (∆ F340/F380) in d, whereas the TG-releasable Ca2+ is quantified as the AUC (F340/F380 × s) in e. In the dot plots, data are represented as the mean ± SEM of at least three independent experiments. Statistically significant differences were determined with a one-way ANOVA (*p < 0.05, ***p < 0.001). NS not significant.
Fig. 5. Knockdown of STIM1 with siRNA does not reduce apoptosis triggered by BIRD-2 in SU-DHL-4 cells.
a Analysis of the cytosolic Ca2+ response in mock-transfected SU-DHL-4 cells, or cells transfected with scrambled siRNA or with siRNA against STIM1, using Fura-2 AM. Addition of 10 µM BIRD-2 is indicated by the dotted line. The curves represent the mean ± SEM of three independent experiments. The BIRD-2-provoked cytosolic Ca2+ rise is quantified by measuring the peak amplitude (∆ F340/F380), shown in b, and the time constant τ (s), which is shown in c. d Representative scatter plots from flow cytometry analysis detecting apoptosis in transfected SU-DHL-4 cells stained with Annexin V-FITC and 7-AAD. Cells were treated for 2 h with 10 µM BIRD-2, after which apoptotic cell death was detected by measuring the Annexin V-FITC-positive fraction. e Quantitative analysis of four independent experiments detecting apoptosis in mock-transfected SU-DHL-4 cells or cells transfected with scrambled siRNA or with STIM1 siRNA. Cells were treated for 2 h with 10 µM BIRD-2. In the scatter plots, data are represented as the mean ± SEM of at least three independent experiments. Statistically significant differences were determined with a one-way ANOVA. NS not significant.
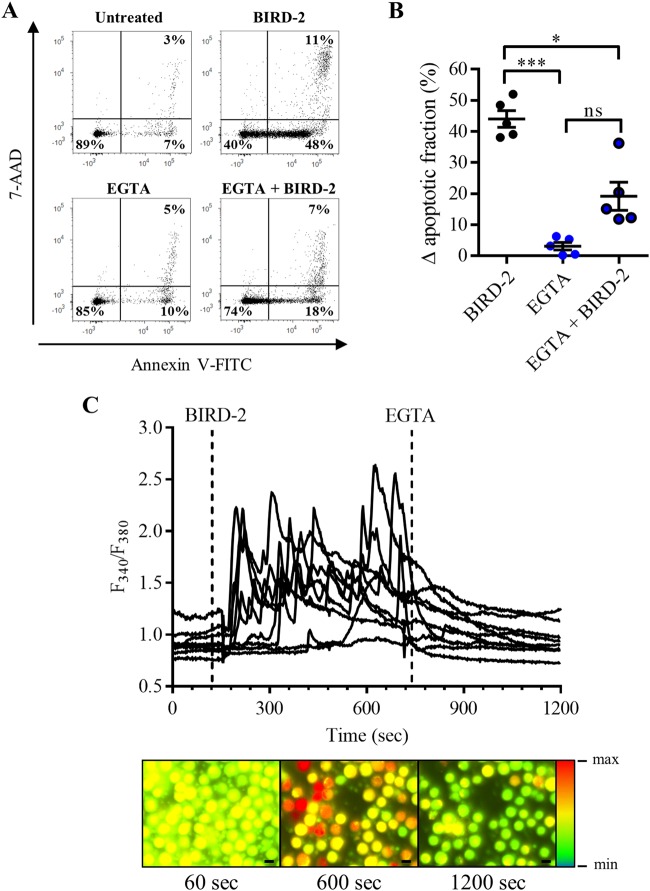
BIRD-2-induced cell death depends on Ca2+ present in the extracellular environment
Next, it was examined whether BIRD-2-induced apoptosis solely depends on intracellular Ca2+ by chelating Ca2+ from the extracellular environment with ethylene glycol tetraacetic acid (EGTA). Therefore, SU-DHL-4 cells were treated with 3 mM EGTA 30 min prior to the addition of 10 µM BIRD-2. After 2 h of BIRD-2 treatment, apoptotic cell death was measured via Annexin V-FITC and 7-AAD staining of the cells (Fig. 6a). BIRD-2 killed approximately 45% of the cell population, whereas treatment with only EGTA was not toxic for the cells (Fig. 6b). Interestingly, BIRD-2-triggered apoptosis was significantly reduced to approximately 20% in SU-DHL-4 cells pre-treated with EGTA, indicating BIRD-2-induced cell death depends on extracellular Ca2+. Next, we determined whether chelating extracellular Ca2+ also affects the cytosolic Ca2+ response provoked by BIRD-2. Therefore, single-cell Ca2+ levels were monitored in Fura-2 AM-loaded cells by fluorescence microscopy. Addition of 10 µM BIRD-2 provoked cytosolic Ca2+ oscillations in the SU-DHL-4 cells (Fig. 6c). However, treating the cells with 3 mM EGTA immediately suppressed these oscillations and caused a rapid decline in the cytosolic Ca2+ levels. These results indicate that, although SOCE is not involved, the cell death-inducing characteristics of BIRD-2 do depend on intracellular Ca2+ oscillations that are driven by Ca2+ influx from the extracellular environment, confirming previous results obtained in human myeloma cell lines14.
Fig. 6. BIRD-2-triggered cell death is reduced by chelating extracellular Ca2+ with EGTA in SU-DHL-4 cells.
a Representative scatter plots from flow cytometry analysis detecting apoptosis in SU-DHL-4 cells stained with Annexin V-FITC and 7-AAD. Cells were pre-treated with or without 3 mM EGTA 30 min prior to application of 10 µM BIRD-2. After 2 h of BIRD-2 treatment, apoptotic cell death was detected by measuring the Annexin V-FITC-positive fraction. b Quantitative analysis detecting apoptosis in SU-DHL-4 cells treated for 2 h with 10 µM BIRD-2, with or without a pre-treatment of 30 min with 3 mM EGTA. The ∆ apoptotic fraction is plotted, which corresponds to the apoptotic fraction corrected for the percentage of apoptosis detected in untreated cells. Data are represented as the mean ± SEM of five independent experiments. Statistically significant differences were determined with a one-way ANOVA (*p < 0.05, ***p < 0.001). c Single-cell cytosolic Ca2+ signals detected in SU-DHL-4 cells using the ratiometric Ca2+ indicator Fura-2 AM. The addition of 10 µM BIRD-2 and 3 mM EGTA are indicated by the dotted lines. Representative pseudo-color images before and after BIRD-2/EGTA addition are shown. The pseudo-color gradient at the right of the panel indicates an increasing fluorescence ratio. NS not significant.
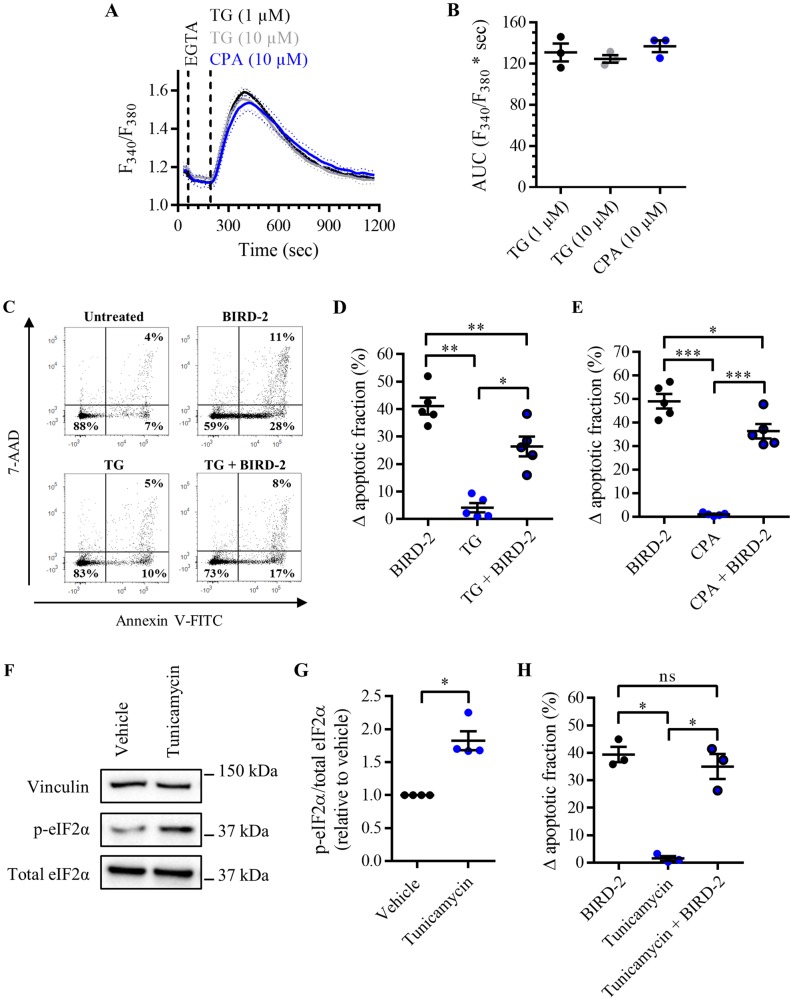
BIRD-2-triggered apoptosis depends on the ER Ca2+-store content
Finally, we determined whether the protection against BIRD-2-induced cell death by DPB162-AE was due to the effects of this compound on the ER Ca2+ content. If so, SERCA inhibitors TG and cyclopiazonic acid (CPA), which cause ER Ca2+-store depletion, should mimic the effects of DPB162-AE and thus suppress BIRD-2-induced cell death. To determine the ability of these compounds to trigger ER Ca2+ depletion, the cytosolic Ca2+ responses were measured in Fura-2 AM-loaded SU-DHL-4 cells upon addition of 1 and 10 µM TG and 10 µM CPA (Fig. 7a). The AUC was comparable in the three conditions, indicating that 1 µM TG and 10 µM CPA are sufficient to completely deplete the ER (Fig. 7b). Subsequently, the effect of ER depletion by TG on BIRD-2-induced apoptosis in SU-DHL-4 cells was determined (Fig. 7c). Therefore, cells were pre-treated for 30 min with 1 µM TG, after which 10 µM BIRD-2 was added. BIRD-2 (10 µM, 2 h) induced approximately 40% of apoptosis, whereas TG by itself only induced toxicity in 5% of the cell population (Fig. 7d). Interestingly, BIRD-2-triggered cell death was significantly reduced to approximately 25% in SU-DHL-4 cells pre-treated with TG. Comparable results were obtained in cells pre-treated with 10 µM CPA instead of TG (Fig. 7e), indicating that the ER Ca2+ content is a critical determinant for BIRD-2-provoked cell death. To exclude that TG and CPA conferred protection against BIRD-2 through ER stress and the subsequent unfolded protein response (UPR), which occur upon SERCA inhibition/ER Ca2+-store depletion, another ER-stress inducer that did not act through ER Ca2+ dysregulation was used. Therefore, we applied tunicamycin, which induces ER stress and activates the UPR by blocking N-linked glycosylation. Treatment for 4 h with 5 µg/ml tunicamycin triggered ER stress, which was observed as an increase in p-eIF2α, whereas total eIF2α levels remained unaffected (Fig. 7f, g). Next, it was determined whether tunicamycin affects BIRD-2-induced apoptosis in SU-DHL-4 cells. Pre-treatment for 4 h with 5 µg/ml tunicamycin did not protect against BIRD-2-provoked cell death (Fig. 7h), suggesting that ER stress/UPR activation was not responsible for the TG-mediated cell death protection against BIRD-2. In summary, these data indicate that the ER Ca2+ content is a critical determinant of BIRD-2-induced cell death in SU-DHL-4 DLBCL cells.
Fig. 7. BIRD-2-triggered apoptosis is reduced by depleting the ER Ca2+ store in SU-DHL-4 cells.
a Analysis of the cytosolic Ca2+ response in SU-DHL-4 cells using Fura-2 AM. After the addition of 3 mM EGTA, 1 or 10 µM TG or 10 µM CPA were added to deplete the ER Ca2+ store. The curves represent the mean ± SEM of three independent experiments. The TG/CPA-releasable Ca2+ is quantified by measuring the AUC (F340/F380 × s), which is shown in b. c Representative scatter plots from flow cytometry analysis detecting apoptosis in SU-DHL-4 cells stained with Annexin V-FITC and 7-AAD. Cells were pre-treated with or without 1 µM TG 30 min prior to application of 10 µM BIRD-2. After 2 h of BIRD-2 treatment, apoptotic cell death was detected by measuring the Annexin V-FITC-positive fraction. d Quantitative analysis of five independent experiments detecting apoptosis in SU-DHL-4 cells treated for 2 h with 10 µM BIRD-2, with or without a pre-treatment of 30 min with 1 µM TG. e Quantitative analysis of five independent experiments detecting apoptosis in SU-DHL-4 cells treated for 2 h with 10 µM BIRD-2, with or without a pre-treatment of 30 min with 10 µM CPA. f A representative western blot of four independent experiments showing the expression levels of total eIF2α and p-eIF2α in SU-DHL-4 cells treated for 4 h with 5µg/ml tunicamycin. The expression level of vinculin was used as a loading control. g Quantification of the p-eIF2α/total eIF2α-protein levels in SU-DHL-4 relative to vehicle-treated cells, which was set at 1. h Quantitative analysis of three independent experiments detecting apoptosis in SU-DHL-4 cells treated for 2 h with 10 µM BIRD-2, with or without a pre-treatment of 4 h with 5µg/ml tunicamycin. In d, e, and h the ∆ apoptotic fraction is plotted, which corresponds to the apoptotic fraction corrected for the percentage of apoptosis detected in untreated cells. In the dot plots, data are represented as the mean ± SEM of at least three independent experiments. Statistically significant differences were determined with a one-way ANOVA (*p < 0.05, **p < 0.01, ***p < 0.001). NS not significant
Discussion
The main finding of this study is that cell death induced by BIRD-2, a peptide tool antagonizing the function of the anti-apoptotic protein Bcl-2 at the ER, is dependent on both the ER Ca2+-store content and extracellular Ca2+, but not on Ca2+ influx through SOCE. BIRD-2 alleviates Bcl-2's inhibitory action on the IP3R by targeting the BH4 domain of Bcl-2. In this way, BIRD-2 triggers Ca2+-mediated apoptotic cell death in cancer cells that depend for their survival on Bcl-2's anti-apoptotic function at the ER, such as the SU-DHL-4 DLBCL cells used in this study. Our results indicate that Ca2+ present in the extracellular environment as well as the ER Ca2+ levels both critically contribute to BIRD-2-induced cell death in DLBCL cancer cells.
First, we investigated whether SOCE, an important Ca2+ influx pathway activated upon ER-store depletion, is involved in BIRD-2-induced cell death, since BIRD-2 triggers apoptotic cell death through excessive Ca2+ release from the ER. Furthermore, the key SOCE players STIM and Orai have been implicated in the control of cell death and survival pathways27,28. The contribution of Ca2+ influx through STIM/Orai signaling to BIRD-2-induced cell death was determined by using pharmacological SOCE inhibitors. One of the compounds used was DPB162-AE, a 2-APB analog developed as a more potent and more selective SOCE inhibitor compared to 2-APB29. Previous work from our lab showed that DPB162-AE is indeed a very potent inhibitor of SOCE, although it also affected the ER Ca2+ content25. At concentrations required for adequate SOCE inhibition, DPB162-AE depleted the ER Ca2+ store by inducing an ER Ca2+-leak pathway. Here, we showed that DPB162-AE treatment reduced the BIRD-2-induced pro-apoptotic Ca2+ signaling and cell death responses in SU-DHL-4 DLBCL cells. However, it is not clear whether this protection was the result of SOCE inhibition, since DPB162-AE also depletes the ER Ca2+ store. Because of this, YM-58483 and GSK-7975A, two SOCE inhibitors that do not alter the ER Ca2+ content, were used to examine the contribution of SOCE to BIRD-2-triggered apoptosis. Both compounds potently inhibited SOCE in SU-DHL-4 cells, without reducing ER Ca2+ levels. Surprisingly, SOCE inhibition with these pharmacological tools did neither reduce BIRD-2-induced cell death nor suppress the BIRD-2-triggered cytosolic Ca2+ response. Furthermore, these results were confirmed by knocking down STIM1 with siRNA, which also blocked SOCE without affecting ER Ca2+ levels. Hence, these data indicate that SOCE does not contribute to BIRD-2-induced cell death.
There are several plausible reasons why SOCE may not be activated upon BIRD-2 application, despite the fact that BIRD-2 provokes Ca2+ oscillations that originate from the ER. First, it might be that BIRD-2 treatment does not completely deplete the ER Ca2+ store, since BIRD-2 does not inhibit SERCA activity. As a consequence, the SERCA pump might partially refill the ER Ca2+ store after treatment with BIRD-2, thus evading complete depletion of the ER Ca2+ store. Second, besides SOCE through STIM/Orai signaling, other Ca2+-influx pathways might be stimulated upon BIRD-2 application. For example, transient receptor potential (TRP) channels, more particularly TRPC channels, have been proposed to be activated by ER store depletion30,31. Third, ectopic Bcl-2 overexpression has been implicated in downregulation of SOCE in both HeLa and LNCaP prostate cancer epithelial cells, which could be attributed to Bcl-2's ability to reduce ER Ca2+ loading32,33. Recent work confirmed that overexpression of wild-type Bcl-2 resulted in decreased SOCE, though accompanied by an increase in steady-state ER Ca2+ levels, protecting against TG-induced cell death34. This Bcl-2 property could be abrogated by the α5-helical Bcl-2 mutant 144WGR146/144AAA146, which partially depleted ER Ca2+ stores by itself and boosted SOCE after TG treatment, resulting in aggravated TG-induced cell death. Yet, it remains unclear whether such SOCE modulation can occur by endogenously elevated Bcl-2 proteins, such as in the B-cell cancer cell model used here. Importantly, in this study we showed that Ca2+ present in the extracellular environment contributes to BIRD-2 toxicity, as chelating extracellular Ca2+ with EGTA conferred protection against BIRD-2-provoked cell death in SU-DHL-4 cells. Moreover, the cytosolic Ca2+ oscillations provoked by BIRD-2 treatment were abruptly suppressed by the addition of the extracellular Ca2+ chelator. Hence, although SOCE is not activated by the peptide tool, our results indicate that BIRD-2 does not solely depend on the intracellular Ca2+ levels for its pro-apoptotic effects. This study revealed a hitherto unappreciated role for extracellular Ca2+ in BIRD-2-induced cell death. Yet, the exact molecular mechanisms by which extracellular Ca2+ enters BIRD-2-sensitive cells and contributes to BIRD-2-induced cell death remain elusive.
Besides extracellular Ca2+, the ER Ca2+ stores also determine apoptotic cell death mediated by BIRD-2 in DLBCL cancer cells, as we previously showed that IP3R inhibition suppresses BIRD-2 sensitivity12. Indeed, treatment of SU-DHL-4 cells with the SERCA inhibitors TG and CPA protected against BIRD-2-induced cell death. As such, we hypothesize that the protective effects of DPB162-AE against BIRD-2-induced cell death are due to the ability of DPB162-AE to lower ER Ca2+ levels and not due to its SOCE-inhibitory properties. Importantly, the observed protection is a direct consequence of ER Ca2+-store depletion, and is not caused by ER stress/UPR activation, since tunicamycin, an ER-stress inducer that does not directly target the Ca2+-signaling machinery, could not reduce BIRD-2-induced apoptosis. Hence, our work implicates that the proper filling of the ER Ca2+ stores is a critical factor underlying the susceptibility of DLBCL cancer cells towards BIRD-2 exposure. This is in line with previous work showing that the ER Ca2+-filling state is a critical determinant in the apoptotic sensitivity of cells towards cell death inducers35,36.
We had previously shown that BIRD-2-induced apoptotic cell death in DLBCL cancer cells depends on multiple cellular determinants. First, DLBCL cells with high expression levels of IP3R2, the isoform with the highest sensitivity to its ligand IP3, are very sensitive to BIRD-2, whereas cells with low IP3R2 expression levels are less susceptible to BIRD-2-induced cell death12. Second, we have recently shown that constitutive IP3 signaling is an additional determinant of the sensitivity of B-cell cancers, including DLBCL and CLL, to BIRD-217. Constitutive IP3 signaling has a pro-survival role in cancer cells, but it can be switched into pro-death signaling by disturbing Bcl-2/IP3R-complex formation with BIRD-2. Finally, here we show that Ca2+-mediated cell death induced by BIRD-2 depends on the Ca2+ levels of the ER as well as on the presence of Ca2+ in the extracellular environment, but not on Ca2+ influx via STIM/Orai signaling. Thus, our data indicate that both extracellular Ca2+ and the ER Ca2+-store content contribute to cell death induced by BIRD-2.
Materials and methods
Cell culture
DLBCL SU-DHL-4 cells were kindly provided by Dr. A. Letai (Dana-Farber Cancer Institute, USA) and cultured in suspension in RPMI-1640 medium (Invitrogen, Merelbeke, Belgium). Human cervical carcinoma HeLa cells were cultured in Dulbecco's modified Eagle's medium medium (Invitrogen, Merelbeke, Belgium). All media were supplemented with 10% heat-inactivated fetal bovine serum, l-glutamine (100× GlutaMAX, Gibco/Invitrogen, Merelbeke, Belgium) and penicillin and streptomycin (100× Pen/Strep, Gibco/Invitrogen, Merelbeke, Belgium). Cells were cultured at 37 °C and 5% CO2. All human cell lines used in this study have been authenticated using autosomal short tandem repeat profiling performed by the University of Arizona Genetics Core and fully matched the DNA fingerprint present in reference databases.
Antibodies and reagents
The following antibodies were used for immunoblotting: anti-GAPDH antibody (Sigma-Aldrich, Munich, Germany, G8795), anti-STIM1 antibody (Abnova, Taipei, Taiwan, PAB12017), anti-total eukaryotic initiation factor 2α (eIF2α) antibody (Thermo Scientific, Waltham, MA, USA, AHO0802), anti-phospho-eIF2α (p-eIF2α) antibody (Thermo Scientific, Waltham, MA, USA, MA5-15133), anti-vinculin antibody (Sigma-Aldrich, Munich, Germany, V9131). Reagents were as follows: EGTA (Acros Organics, Geel, Belgium, 409910250), TG (Alomone labs, Jerusalem, Israel, T-650), CPA (Enzo Life Sciences, Farmingdale, NY, USA, BML-CA415-0010), calcium chloride (CaCl2) (Merck, Darmstadt, Germany, 102382), Fura-2 AM (Life Technologies, Carlsbad, CA, USA, F1221), tunicamycin (Sigma-Aldrich, Munich, Germany, T7765), and YM-58483 (Abcam, Cambridge, UK, ab144413). GSK-7975A was a gift from GlaxoSmithKline (Stevenage, UK). DPB162-AE was kindly provided by Dr. K. Mikoshiba (Brain Science Institute, RIKEN, Tokyo, Japan). BIRD-2 peptide (sequence: RKKRRQRRRGGNVYTEIKCNSLLPLAAIVRV) was purchased from LifeTein (South Plainfield, NJ, USA) with a purity of >85%.
siRNA transfection
Sequences for siRNA were: STIM1 siRNA, 5′-GGAGGAUAAUGGCUCUAUUdTdT-3′ and scrambled siRNA, 5′-AAUUCUCCGAACGUGUCACdTdT-3′. SU-DHL-4 cells were transfected by electroporation using AMAXA Kit V (Lonza, Basel, Switzerland, VCA-1003) program P-005. Briefly, 5 × 106 cells were transfected with 2 µg STIM1 siRNA or scrambled siRNA. After transfection, cells were seeded at 1 × 106 cells/ml and approximately 36 h post-transfection the change in gene expression was measured by western blot analysis.
Cytosolic Ca2+ measurements in intact cell populations
Cytosolic Ca2+ levels were monitored with Fura-2 AM as previously described12,37. Briefly, Fura-2 AM measurements were performed by seeding the SU-DHL-4 cells in 96-well plates (Greiner) with poly-l-lysine coating. The cells were loaded for 30 min with 1.25 µM Fura-2 AM at room temperature in modified Krebs solution (containing 150 mM NaCl, 5.9 mM KCl, 1.2 mM MgCl2, 11.6 mM HEPES (pH 7.3), 11.5 mM glucose and 1.5 mM CaCl2), followed by a de-esterification step of 30 min in the absence of Fura-2 AM. During the de-esterification cells were treated with vehicle, 3 µM YM-58483, 3 µM GSK-7975A, or 10 µM DPB162-AE. Fluorescence was monitored on a FlexStation 3 microplate reader (Molecular Devices, Sunnyvale, CA, USA) by alternately exciting the Ca2+ indicator at 340 and 380 nm and collecting emitted fluorescence at 510 nm. EGTA (final concentration 3 mM), TG (final concentration 1 µM), CaCl2 (final concentration 10 mM), or BIRD-2 (final concentration 10 µM) were added as indicated. All traces are shown as the ratio of emitted fluorescence of Fura-2 (F340/F380).
Single-cell cytosolic Ca2+ imaging
Single-cell cytosolic Ca2+ measurements were performed as previously described38. In brief, SU-DHL-4 cells were loaded with Fura-2 AM as described above. Single-cell imaging was performed using a Zeiss Axio Observer Z1 Inverted Microscope equipped with a 20× air objective and a high-speed digital camera (Axiocam Hsm, Zeiss, Jena, Germany). Data are shown as the ratio of emitted fluorescence of Fura-2 (F340/F380).
Single-cell ER Ca2+ imaging
Single-cell ER Ca2+ measurements were performed as previously described39. ER Ca2+ levels were measured with the genetically encoded Ca2+ indicator G-CEPIA1er, which was kindly provided by Dr. M. Iino (The University of Tokyo, Tokyo, Japan)26. The G-CEPIA1er construct was introduced into HeLa cells utilizing X-tremeGENE HP DNA transfection reagent (Roche, Mannheim, Germany, 06366546001) according to the manufacturer’s protocol. A Zeiss Axio Observer Z1 Inverted Microscope equipped with a 20× air objective and a high-speed digital camera (Axiocam Hsm, Zeiss, Jena, Germany) were used for these measurements. Changes in fluorescence were monitored in the GFP channel (480/520 nm excitation/emission). To chelate extracellular Ca2+, EGTA was added to a final concentration of 3 mM. One minute later, 1 µM TG or different SOCE inhibitors were added. All traces were normalized (F/F0) where F0 is the starting fluorescence of each trace.
ATPase activity measurements
Ca2+-dependent ATPase activity experiments were performed on microsomes of COS cells overexpressing SERCA2b using an endpoint colorimetric assay responding to the released inorganic phosphate, as previously described40. The SOCE inhibitors were pre-incubated with the microsomes for 30 min at 4 °C, followed by 5 min at 37 °C. The Ca2+-dependent ATPase activity was measured at maximal (free [Ca2+] 3.16 µM) and submaximal (free [Ca2+] 0.316 µM) [Ca2+].
Apoptosis assay
SU-DHL-4 cells (5 × 105 cells/ml) were treated for 2 h with 10 µM BIRD-2 with or without a pre-treatment of 30 min with 3 µM YM-58483, 3 µM GSK-7975A, 10 µM DPB162-AE, 3 mM EGTA, 1 µM TG, 10 µM CPA, or 5 µg/ml tunicamycin. Subsequently, cells were pelleted by centrifugation and incubated with Annexin V-FITC (Life Technologies, Carlsbad, CA, USA, V13245) and 7-AAD (Becton Dickinson, Franklin Lakes, NJ, USA, 555815). Cell suspensions were analyzed with an Attune Acoustic Focusing Flow Cytometer (Applied Biosystems). Cell death by apoptosis was scored by quantifying the population of Annexin V-FITC-positive cells using the FlowJo version 10 software. Data are plotted as the ∆ apoptotic fraction (=apoptotic fraction in treated cells − apoptotic fraction in untreated cells).
Western blot analysis
SU-DHL-4 cells were washed with phosphate-buffered saline and incubated at 4 °C with lysis buffer (20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1.5 mM MgCl2, 0.5 mM dithiothreitol, 1% Triton-X-100, a phosphatase inhibitor cocktail tablet (PhosSTOP, Roche, Mannheim, Germany) and 1 tablet complete EDTA-free protease inhibitor (Thermo Scientific, Brussels, Belgium)) for 30 min on a head-over-head rotor. Cell lysates were centrifuged for 5 min at 4000 × g and analyzed by western blotting as previously described41.
Statistical analysis
Results are expressed as the mean ± SEM. The number of independent experiments is always indicated. Significance was determined using a two-tailed paired Student’s t test or a one-way analysis of variance (ANOVA) with a post hoc Tukey’s test, as appropriate. Differences were considered significant at p < 0.05.
Acknowledgements
We thank Marina Crabbé, Anja Florizoone, and Kirsten Welkenhuyzen for their excellent technical assistance. We thank Dr. A. Letai (Dana-Farber Cancer Institute, United States) for providing the SU-DHL-4 cells. We are very grateful to Dr. M. Iino (The University of Tokyo, Japan) for providing the G-CEPIA1er plasmid. We also thank GlaxoSmithKline (Stevenage, United Kingdom) for providing GSK-7975A. This work was supported by grants from the Research Foundation – Flanders (FWO) (G.0C91.14N to GB and JBP, G.0B11.15N to PV and G.0A34.16N to GB), the Research Council – KU Leuven (OT14/101), and Belgian Science Policy/InterUniversity Attraction Pole Program IAP P7/13. G.B., J.B.P. and M.K. are partners within the Scientific Research Community "Ca2+ signaling in health, disease & therapy (CaSign)" supported by the FWO (W0.019.17N). M.B. is holder of a Ph.D. Fellowship from the FWO.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Edited by N. Barlev
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Brunelle JK, Letai A. Control of mitochondrial apoptosis by the Bcl-2 family. J. Cell Sci. 2009;122:437–441. doi: 10.1242/jcs.031682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reed JC. Bcl-2-family proteins and hematologic malignancies: history and future prospects. Blood. 2008;111:3322–3331. doi: 10.1182/blood-2007-09-078162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Placzek WJ, et al. A survey of the anti-apoptotic Bcl-2 subfamily expression in cancer types provides a platform to predict the efficacy of Bcl-2 antagonists in cancer therapy. Cell Death Dis. 2010;1:1–9. doi: 10.1038/cddis.2010.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chipuk JE, Moldoveanu T, Llambi F, Parsons MJ, Green DR. The BCL-2 family reunion. Mol. Cell. 2010;37:299–310. doi: 10.1016/j.molcel.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Letai A. Diagnosing and exploiting cancer’s addiction to blocks in apoptosis. Nat. Rev. Cancer. 2008;8:121–132. doi: 10.1038/nrc2297. [DOI] [PubMed] [Google Scholar]
- 6.Besbes S, Mirshahi M, Pocard M, Billard C. New dimension in therapeutic targeting of BCL-2 family proteins. Oncotarget. 2015;6:12862–12871. doi: 10.18632/oncotarget.3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vervloessem T, et al. Bcl-2 inhibitors as anti-cancer therapeutics: the impact of and on calcium signaling. Cell Calcium. 2018;70:102–116. doi: 10.1016/j.ceca.2017.05.014. [DOI] [PubMed] [Google Scholar]
- 8.Rong Y, Distelhorst CW. Bcl-2 protein family members: versatile regulators of calcium signaling in cell survival and apoptosis. Annu. Rev. Physiol. 2008;70:73–91. doi: 10.1146/annurev.physiol.70.021507.105852. [DOI] [PubMed] [Google Scholar]
- 9.Chen R, et al. Bcl-2 functionally interacts with inositol 1,4,5-trisphosphate receptors to regulate calcium release from the ER in response to inositol 1,4,5-trisphosphate. J. Cell Biol. 2004;166:193–203. doi: 10.1083/jcb.200309146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rong Y, et al. The BH4 domain of Bcl-2 inhibits ER calcium release and apoptosis by binding the regulatory and coupling domain of the IP3 receptor. Proc. Natl. Acad. Sci. USA. 2009;106:14397–14402. doi: 10.1073/pnas.0907555106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rong Y, et al. Targeting Bcl-2-IP3 receptor interaction to reverse Bcl-2’s inhibition of apoptotic calcium signals. Mol. Cell. 2008;31:255–265. doi: 10.1016/j.molcel.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akl H, et al. IP3R2 levels dictate the apoptotic sensitivity of diffuse large B-cell lymphoma cells to an IP3R-derived peptide targeting the BH4 domain of Bcl-2. Cell Death Dis. 2013;4:e632. doi: 10.1038/cddis.2013.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhong F, et al. Induction of Ca2+-driven apoptosis in chronic lymphocytic leukemia cells by peptide-mediated disruption of Bcl-2-IP3 receptor interaction. Blood. 2011;117:2924–2934. doi: 10.1182/blood-2010-09-307405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lavik A, et al. A synthetic peptide targeting the BH4 domain of Bcl-2 induces apoptosis in multiple myeloma and follicular lymphoma cells alone or in combination with agents targeting the BH3-binding pocket of Bcl-2. Oncotarget. 2015;6:27388–27402. doi: 10.18632/oncotarget.4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greenberg EF, et al. Synergistic killing of human small cell lung cancer cells by the Bcl-2-inositol 1,4,5-trisphosphate receptor disruptor BIRD-2 and the BH3-mimetic ABT-263. Cell Death Dis. 2015;6:e2034. doi: 10.1038/cddis.2015.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vervloessem T, et al. Reciprocal sensitivity of diffuse large B-cell lymphoma cells to Bcl-2 inhibitors BIRD-2 versus venetoclax. Oncotarget. 2017;8:111656–111671. doi: 10.18632/oncotarget.22898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bittremieux, M. et al. Constitutive IP3 signaling underlies the sensitivity of B-cell cancers to the Bcl-2/IP3 receptor disruptor BIRD-2. Cell Death Differ. 10.1038/s41418-018-0142-3 (2018). [DOI] [PMC free article] [PubMed]
- 18.Parekh AB, Putney JW., Jr. Store-operated calcium channels. Physiol. Rev. 2005;85:757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- 19.Peinelt C, et al. Amplification of CRAC current by STIM1 and CRACM1 (Orai1) Nat. Cell Biol. 2006;8:771–773. doi: 10.1038/ncb1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roos J, et al. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J. Cell Biol. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang SL, et al. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature. 2005;437:902–905. doi: 10.1038/nature04147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liou J, et al. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr. Biol. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vig M, et al. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science. 2006;312:1220–1223. doi: 10.1126/science.1127883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soboloff J, Rothberg BS, Madesh M, Gill DL. STIM proteins: dynamic calcium signal transducers. Nat. Rev. Mol. Cell. Biol. 2012;13:549–565. doi: 10.1038/nrm3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bittremieux M, et al. DPB162-AE, an inhibitor of store-operated Ca2+ entry, can deplete the endoplasmic reticulum Ca2+ store. Cell Calcium. 2017;62:60–70. doi: 10.1016/j.ceca.2017.01.015. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki J, et al. Imaging intraorganellar Ca2+ at subcellular resolution using CEPIA. Nat. Commun. 2014;5:1–13. doi: 10.1038/ncomms5153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanwar J, Motiani RK. Role of SOCE architects STIM and Orai proteins in cell death. Cell Calcium. 2018;69:19–27. doi: 10.1016/j.ceca.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 28.Lang F, et al. To die or not to die SGK1-sensitive ORAI/STIM in cell survival. Cell Calcium. 2018;74:29–34. doi: 10.1016/j.ceca.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 29.Goto J, et al. Two novel 2-aminoethyl diphenylborinate (2-APB) analogues differentially activate and inhibit store-operated Ca2+ entry via STIM proteins. Cell Calcium. 2010;47:1–10. doi: 10.1016/j.ceca.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salido GM, Sage SO, Rosado JA. TRPC channels and store-operated Ca2+ entry. Biochim. Biophys. Acta. 2009;1793:223–230. doi: 10.1016/j.bbamcr.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 31.Ong HL, Souza LB, de, Cheng KT, Ambudkar IS. Physiological functions and regulation of TRPC channels. Handb. Exp. Pharmacol. 2014;223:1005–1034. doi: 10.1007/978-3-319-05161-1_12. [DOI] [PubMed] [Google Scholar]
- 32.Vanden Abeele F, et al. Bcl-2-dependent modulation of Ca2+ homeostasis and store-operated channels in prostate cancer cells. Cancer Cell. 2002;1:169–179. doi: 10.1016/S1535-6108(02)00034-X. [DOI] [PubMed] [Google Scholar]
- 33.Pinton P, et al. Reduced loading of intracellular Ca2+ stores and downregulation of capacitative Ca2+ influx in Bcl-2-overexpressing cells. J. Cell Biol. 2000;148:857–862. doi: 10.1083/jcb.148.5.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chiu W, et al. Bcl-2 regulates store-operated Ca2+ entry to modulate ER stress-induced apoptosis. Cell Death Discov. 2018;4:37. doi: 10.1038/s41420-018-0039-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pinton P, et al. The Ca2+ concentration of the endoplasmic reticulum is a key determinant of ceramide-induced apoptosis: significance for the molecular mechanism of Bcl-2 action. EMBO J. 2001;20:2690–2701. doi: 10.1093/emboj/20.11.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pinton P, Rizzuto R. Bcl-2 and Ca2+ homeostasis in the endoplasmic reticulum. Cell Death Differ. 2006;13:1409–1418. doi: 10.1038/sj.cdd.4401960. [DOI] [PubMed] [Google Scholar]
- 37.Decuypere JP, et al. Ins(1,4,5)P3 receptor-mediated Ca2+ signaling and autophagy induction are interrelated. Autophagy. 2011;7:1472–1489. doi: 10.4161/auto.7.12.17909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vervliet T, et al. Bcl-2 binds to and inhibits ryanodine receptors. J. Cell Sci. 2014;127:2782–2792. doi: 10.1242/jcs.150011. [DOI] [PubMed] [Google Scholar]
- 39.Vervliet T, et al. Ryanodine receptors are targeted by anti-apoptotic Bcl-XL involving its BH4 domain and Lys87 from its BH3 domain. Sci. Rep. 2015;5:1–10. doi: 10.1038/srep09641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vandecaetsbeek I, Holemans T, Wuytack F, Vangheluwe P. High-throughput measurement of the Ca2+-dependent ATPase activity in COS microsomes. Cold Spring Harb. Protoc. 2014;8:865–875. doi: 10.1101/pdb.prot076885. [DOI] [PubMed] [Google Scholar]
- 41.Monaco G, et al. Selective regulation of IP3-receptor-mediated Ca2+ signaling and apoptosis by the BH4 domain of Bcl-2 versus Bcl-Xl. Cell Death Differ. 2012;19:295–309. doi: 10.1038/cdd.2011.97. [DOI] [PMC free article] [PubMed] [Google Scholar]