Abstract
Dyslipidemia is associated with glomerular injury. However, the effect of statins on chronic kidney disease (CKD) progression remains controversial. We aimed to investigate the efficacy of statins for renal protection in patients with CKD. The retrospective cohort study comprised 3441 patients diagnosed with CKD in multiple medical centers. We divided the patients into two cohorts based on statin prescription, and compared proportions and risks of CKD progression events between the two groups. CKD progression event was defined as an average annual decline of eGFR >5 mL/min/1.73 m2 or advancement to the dialysis stage. The result revealed that among all incident patients with CKD, 28.7% and 30.3% of the users and nonusers demonstrated CKD progression, respectively. The crude odds ratio (OR) of CKD progression was 0.93 [95% confidence interval (CI) 0.78–1.10]. After adjustment for baseline characteristics, the adjusted OR was 0.80 (95% CI 0.63–1.01). The sensitivity analysis results showed consistent OR for CKD progression, stratification by age, sex, Charlson score, and statins use within 1 year before index date. The effect of statins was significant in patients with CKD stage 3B-5 (OR 0.68, 95% CI 0.48–0.95), but not statistically significant in those with CKD stage 1–3A (OR 0.97, 95% CI 0.68–1.38). The effect of statins was significant in patients with proteinuria ≥1000 mg/day (OR 0.63, 95% CI 0.43–0.92), but not statistically significant in those with proteinuria <1000 mg/day (OR 1.02, 95% CI 0.74–1.41).
Introduction
Chronic kidney disease (CKD) is a global health concern1. According to systemic review data and a meta-analysis of observational studies until September 2014, the global prevalence of CKD was approximately 13.4%, and the prevalence of stage 3–5 CKD was 10.6%1. An estimated 5.4 million people will be receiving dialysis due to end-stage renal disease (ESRD) in 2030, and globally, the number of patients receiving dialysis is increasing the fastest in Asia2. According to the 2017 United States Renal Data System (USRDS) report, the highest prevalence and incidence of ESRD among all countries investigated was noted in Taiwan3. Among patients with ESRD in Taiwan, median estimated glomerular filtration rate (eGFR) at initial dialysis was approximately 4.7 mL/min/1.73 m2 4.
Cardiovascular disease (CVD), which may occur even at the earliest stages of CKD without manifestations of vascular disease, is the leading cause of morbidity and mortality among patients with CKD5. CKD is associated with increased CVD risk, severity of which increases as kidney function deteriorates6. Statins are the mainstay of primary and secondary prevention of CVD in the general population7. The Kidney Disease Improving Global Outcomes (KDIGO) lipid management guidelines suggest statin initiation for primary prevention in all patients with CKD above the age of 50 years, and all adult CKD patients with diabetes who are not receiving dialysis8. In Taiwan, in adults with eGFR <60 mL/min/1.73 m2 and without chronic dialysis, statins therapy is recommended only if low-density lipoprotein cholesterol levels are ≥100 mg/dL9.
Patients with CKD are more likely to exhibit elevated triglyceride and low high-density lipoprotein cholesterol levels, which were the risk factors for CVD10. On the basis of the experimental evidence, dyslipidemia is associated with tubulointerstitial and glomerular injuries, which may result in glomerulosclerosis11,12. Statins inhibit HMG-CoA reductase activity and thus play a beneficial role in dyslipidemia treatment13. Many studies have investigated the renoprotective effects of statins. The ALERT trial and Scandinavian Simvastatin Survival Study have demonstrated that statins slowed CKD progression14,15; however, other studies such as the SHARP study, ALLHAT study, and ASUCA trial have reported that statins exhibit little to no effects on CKD progression16–18. Recent systematic reviews and meta-analyses have indicated that statins do not reduce risk of kidney failure events in adults not receiving dialysis, where kidney failure events are defined as 25–50% decrease in eGFR, doubling of serum creatinine level, or advancement to ESRD stage during follow-up period; however, according to the same reports, statins may reduce proteinuria and eGFR decline19. Therefore, we evaluated the renoprotection efficacy of statins in patients with CKD.
Methods
National Health Insurance Database and Multicenter CKD Cohort
The National Health Insurance Database (NHID) is a research database developed by the Ministry of Health and Welfare20, including National Health Insurance claims data more than 99% of 23 million Taiwan residents. The Ministry of Health and Welfare annually releases the Longitudinal Health Insurance Database for research purposes, which contains the insured population’s registration files and medical claims data, including data on basic demographic characteristics, inpatient and ambulatory care, diagnostic codes, medical expenditure, operations, prescriptions, examinations, and procedures. From January 1, 2008, to Dec 31, 2013, a multicenter project was conducted to survey risk factors for CKD in the Taiwan population. This project was supported by the Bureau of Health Promotion, Ministry of Health and Welfare, Taiwan. The present CKD retrospective observational cohort study is based on the data of 7956 patients from the multicenter project. We used national identification numbers to link laboratory data from study hospitals to the NHID. This research project was approved by the Ethics Committee of Taipei Medical University-Shuang Ho Hospital (TMU-JIRB 20124036), Tri-Service General Hospital (TSGHIRB 100-05-197), Cardinal Tien Hospital (TMU-JIRB 201204035), Changhua Christian Hospital (CCHIRB 20405), Kaohsiung Medical University Chung-Ho Memorial Hospital (KMUHIRB 20120019), Kaohsiung Chang Gung Memorial Hospital (101-1096B), National Cheng Kung University Hospital (A-ER-101-117) and China Medical University Hospital (DMR101-IRB2-273(CR-1)). After a complete explanation of the study, written informed consent was obtained from all participants. All clinical and biological samples were collected after patient consent. All the study methods were in accordance with the guidelines approved by the joint institutional review board and aforementioned governmental regulations.
Study Population
The initial study cohort comprised 7956 patients diagnosed with CKD between November 1, 2008, and June 30, 2013, in multiple medical centers (Fig. 1). We calculated eGFRs by using the Chronic Kidney Disease Epidemiology Collaboration equation, as recommended by KDIGO guidelines. The inclusion criteria were as follows: kidney damage evident as structural or functional abnormalities or eGFR of <60 mL/min/1.73 min2 for more than 3 months, with this eGFR being used to determine CKD stage21. The exclusion criteria were as follows: age <20 years, follow-up duration <1 year, absence of major variables [e.g., age; sex; baseline CKD stage; and Charlson comorbidity scores, including these for diabetes mellitus (DM), coronary artery disease, and stroke], and cancer patients. The multiple imputation method was used to account for missing data on smoking, alcohol use, and betel nut use22. We input missing data on the basis of patients’ sex and CKD stage. After patients with loss to follow-up, with missing data, or with cancer were removed from the database, the final study cohort comprised 3441 CKD patients.
Figure 1.
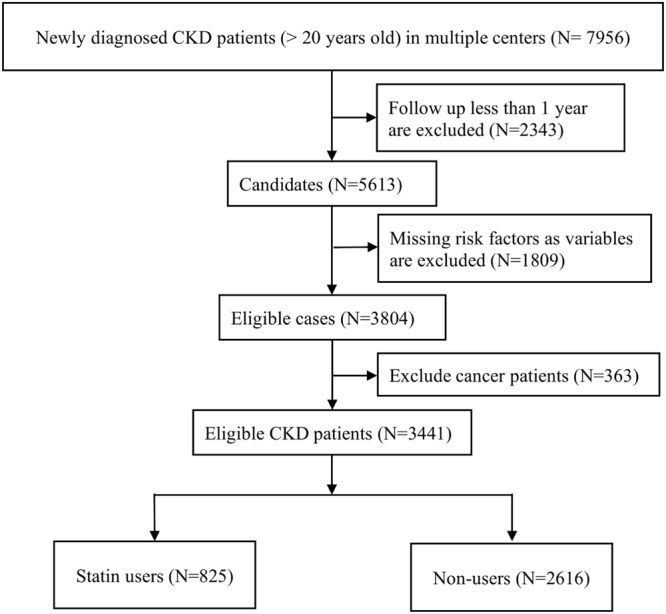
Flowchart demonstrates the selection criteria and process of eligible CKD patients.
Study Design
The patients were divided into two cohorts based on statin prescription: statin users (n = 825, those receiving a ≥90-day statin prescription within 180 days of the index date) and nonusers (n = 2616, those not receiving statins or receiving <90-day statin prescription within 180 days of the index date). Patients were individually tracked from the index date to December, 2015, to determine CKD progression. The primary outcome was CKD progression, defined as an average annual decline of eGFR >5 mL/min/1.73 m2 23 or advancement to the dialysis stage.
Statistical Analysis
We compared the demographic data between statin users and nonusers by using Pearson’s chi-squared test and t test. We calculated proportions of CKD progression events; then, a logistic regression model was used to calculate crude odds ratios (ORs) between statin users and nonusers. After additional adjustments for the potential confounding factors including age, sex, comorbidities, smoking, alcohol use, betel nut use, body mass index (BMI), baseline eGFR, baseline urine protein-to-creatinine ratio (UPCR), and statin use within 1 year before the index date, adjusted ORs (aORs) were calculated. We also performed sensitivity analysis after stratification according to age (<65 and ≥65 years), sex, Charlson comorbidity index score (≤3 and >3), baseline CKD stage (1–3 A and 3B–5), proteinuria (<1000 mg/day and ≥1000 mg/day) and stain use within 1 year before the index time. All analyses were performed the SAS system for Windows (version 9.3.1; SAS Institute Inc., Cary, NC, USA). P < 0.05 was considered significant.
Results
Demographic Characteristics
From November 1, 2008, to June 30, 2013, across multiple medical centers, a total of 7956 patients with CKD, aged 20–85 years, fulfilling the inclusion criteria were identified. Among them, those who had ever received dialysis or kidney transplant were excluded. After the additional exclusion of patients with a follow-up of <1 year (n = 2343), with incomplete or missing data (n = 1809), and with cancer (n = 363), 3441 patients with CKD were enrolled (Fig. 1). Detailed demographic information for the cohort is provided in Table 1. The mean age of statin users and nonusers were 62.99 ± 12.82 and 62.05 ± 14.62 years, respectively; 52.73% and 57.72% of statin users and nonusers were men, respectively. Among all patients, 1717 and 1724 exhibited early (1–3A)- and late (3B–5)-stage CKD, respectively. Compared with the nonusers, a higher proportion of the users had DM (53.45% vs 39.60%), hypertension (84.61% vs 76.26%), coronary artery disease (3.39% vs 2.60%), stroke (18.18% vs 16.59%), BMI of >25 (57.09 vs 44.38%), and more statin use within 1 year before the index date (86.79% vs 14.33%). The mean eGFR of the users and nonusers was 52.58 ± 34.21 and 51.25 ± 36.37 mL/min/1.73 m2, respectively. Furthermore, the users had a significantly higher baseline cholesterol, fasting glucose and HbA1c levels than did the nonusers (p = 0.0023; p = 0.0016; p = 0.003, respectively). Baseline levels of serum albumin, electrolytes, uric acid, hemoglobin, hematocrit, and proteinuria did not differ significantly between the groups. The following levels of cholesterol of the users and nonusers was 177.6 ± 43.15 and 177.14 ± 41.06 mg/dl, respectively (p = 0.8049).
Table 1.
Baseline Characteristics of Patients with CKD Stage 1–5.
| Characteristic | Statin Users | Statin Nonusers | P-value |
|---|---|---|---|
| Number of patients | 825 | 2616 | |
| Age, mean (SD), years | 62.99 ± 12.82 | 62.05 ± 14.62 | 0.0755 |
| Age group, years | <0.0001 | ||
| 20–44 | 74 (8.97) | 340 (13.00) | |
| 45–64 | 355 (43.03) | 1057 (40.41) | |
| 65–74 | 252 (30.55) | 658 (25.15) | |
| ≥75 | 144 (17.45) | 561 (21.44) | |
| Male | 435 (52.73) | 1510 (57.72) | 0.0116 |
| Waist, cm | 89.18 ± 11.56 | 86.47 ± 11.57 | <0.0001 |
| Body mass index, kg/m2 | <0.0001 | ||
| <18.5 | 11 (1.33) | 87 (3.33) | |
| 18.5–24.9 | 343 (41.58) | 1368 (52.29) | |
| 25–29.9 | 338 (40.97) | 870 (33.26) | |
| ≥30 | 133 (16.12) | 291 (11.12) | |
| Smoking | 203 (24.61) | 659 (25.19) | 0.7352 |
| Alcohol | 83 (10.06) | 268 (10.24) | 0.8790 |
| Betel nut | 44 (5.33) | 155 (5.93) | 0.5255 |
| Statin drug used within 1 year before the index date | 716 (86.79) | 375 (14.33) | <0.0001 |
| Baseline CKD stage | 0.3652 | ||
| 1–3A | 423 (51.27) | 1294 (49.46) | |
| 3B–5 | 402 (48.73) | 1322 (50.54) | |
| Comorbidities before the index date | |||
| Diabetes Mellitus | 441 (53.45) | 1036 (39.60) | <0.0001 |
| Coronary artery disease | 28 (3.39) | 68 (2.60) | 0.2269 |
| Stroke | 150 (18.18) | 434 (16.59) | 0.2883 |
| Hypertension | 698 (84.61) | 1995 (76.26) | <0.0001 |
| Charlson comorbidity index | 0.0034 | ||
| ≤3 | 443 (53.70) | 1567 (59.90) | |
| 4–5 | 222 (26.91) | 645 (24.66) | |
| >5 | 160 (19.39) | 404 (15.44) | |
| Charlson comorbidity index, mean (SD) | 3.63 ± 2.14 | 3.3 ± 2.17 | 0.0002 |
| Baseline examination data | |||
| Fasting glucose, mg/dL | 120.44 ± 44.07 | 114.32 ± 43.06 | 0.0016 |
| HbA1c, % | 7.16 ± 1.56 | 6.87 ± 2.10 | 0.0030 |
| Total cholesterol, mg/dL | 188.27 ± 48.45 | 182.18 ± 44.01 | 0.0023 |
| Triglyceride, mg/dL | 146.71 ± 85.41 | 139.28 ± 105.12 | 0.0516 |
| Albumin, g/dL | 4.05 ± 0.90 | 4.38 ± 11.53 | 0.2829 |
| Serum Na, mmol/L | 141.99 ± 55.3 | 139.34 ± 5.77 | 0.3012 |
| Serum K, mmol/L | 4.75 ± 5.94 | 4.52 ± 3.34 | 0.3700 |
| Serum Ca, mg/dL | 9.04 ± 0.63 | 8.98 ± 2.07 | 0.2995 |
| Serum P, mg/dL | 3.99 ± 1.68 | 4.01 ± 1.02 | 0.8228 |
| Uric acid, mg/dL | 7.01 ± 2.66 | 6.97 ± 2.49 | 0.6780 |
| Hb, mg/dL | 12.22 ± 2.27 | 12.10 ± 2.54 | 0.2592 |
| Hct, mg/dL | 36.79 ± 18.55 | 35.85 ± 6.69 | 0.1864 |
| UPCR, mg/g | 5682.23 ± 124358.24 | 1063.28 ± 2758.89 | 0.2864 |
| eGFR, mL/min/1.73 m2 | 52.58 ± 34.21 | 51.25 ± 36.37 | 0.3383 |
| Follow-up examination data | |||
| Fasting glucose, mg/dL | 118.24 ± 39.48 | 114.75 ± 40.31 | 0.0604 |
| HbA1c, % | 6.83 ± 1.35 | 6.65 ± 1.38 | 0.0219 |
| Total cholesterol, mg/dL | 177.60 ± 43.15 | 177.14 ± 41.06 | 0.8049 |
| Triglyceride, mg/dL | 143.19 ± 92.53 | 138.64 ± 123.98 | 0.3159 |
| Albumin, g/dL | 4.03 ± 0.50 | 4.72 ± 23.90 | 0.2826 |
| Serum Na, mmol/L | 138.33 ± 9.21 | 138.48 ± 6.98 | 0.7455 |
| Serum K, mmol/L | 4.40 ± 0.64 | 4.43 ± 1.34 | 0.4016 |
| Serum Ca, mg/dL | 9.06 ± 0.86 | 9.03 ± 1.14 | 0.5658 |
| Serum P, mg/dL | 4.20 ± 1.22 | 4.13 ± 1.24 | 0.2760 |
| Uric acid, mg/dL | 6.95 ± 4.21 | 6.80 ± 3.44 | 0.4404 |
| Hb, mg/dL | 12.01 ± 2.48 | 12.51 ± 20.62 | 0.3069 |
| Hct, mg/dL | 35.70 ± 6.48 | 36.15 ± 20.90 | 0.4200 |
| UPCR, mg/g | 1315.04 ± 2029.19 | 1225.66 ± 2219.41 | 0.4392 |
| eGFR, mL/min/1.73 m2 | 49.07 ± 33.47 | 52.00 ± 41.07 | 0.0487 |
SD, standard deviation; CKD, chronic kidney disease; UPCR, urine protein and creatinine ratio; eGFR, estimated glomerular filtration rate.
Long-Term Risks of CKD Progression Events
Table 2 presents proportions and risk of CKD progression events. CKD progression occurred in 237 (28.73%) users and 793 (30.31%) nonusers. The crude OR and aOR (after adjustment for confounding factors) for CKD progression were 0.93(95% confidence interval [CI], 0.78–1.10), and 0.80 (95% CI, 0.63–1.10), respectively. Stratified by CKD stage, the statin effects in reduction of CKD progression was significant in CKD stage 3B-5 (aOR 0.68, 95% CI 0.48–0.95), but not statistically significant in those with CKD stage 1–3A (aOR 0.97, 95% CI 0.68–1.38).
Table 2.
Proportion and risk of CKD progression in patients with CKD stage 1–5, according to stain use.
| No. of events | No. of patients | Proportion (%) | Unadjusted odds ratio | Adjusted odds ratiob | |
|---|---|---|---|---|---|
| Total | |||||
| Statin Users | 237 | 825 | 28.73 | 0.93 (0.78, 1.10) | 0.80 (0.63, 1.01) |
| Statin Nonusers | 793 | 2616 | 30.31 | 1.00 | 1.00 |
| Stage 1–3a | |||||
| Statin Users | 121 | 423 | 28.61 | 1.08 (0.85, 1.38) | 0.97 (0.68, 1.39) |
| Statin Nonusers | 350 | 1294 | 27.05 | 1.00 | 1.00 |
| Stage 3b-5 | |||||
| Statin Users | 116 | 402 | 28.86 | 0.81 (0.63, 1.03) | 0.68 a (0.48, 0.95) |
| Statin Nonusers | 443 | 1322 | 33.51 | 1.00 | 1.00 |
Note: Values in parentheses are 95% confidence interval.
aP < 0.001.
bAdjustments were made for age, sex, diabetes mellitus, coronary artery disease, stroke, cancer, Charlson comorbidity index, Statin drug used within 1 year before the index date, body mass index, smoking, alcohol, betel nut, urine protein and creatinine ratio, and baseline estimated glomerular filtration rate.
Figure 2 presents the sensitivity analysis results. Consistent aORs were noted for CKD progression, stratification by age, sex, Charlson score, and statins use within 1 year before index date. The effect of statins was significant in patients with proteinuria ≥1000 mg/day (aOR 0.63, 95% CI 0.43–0.92), but not statistically significant in those with proteinuria <1000 mg/day (aOR 1.02, 95% CI 0.74–1.41).
Figure 2.
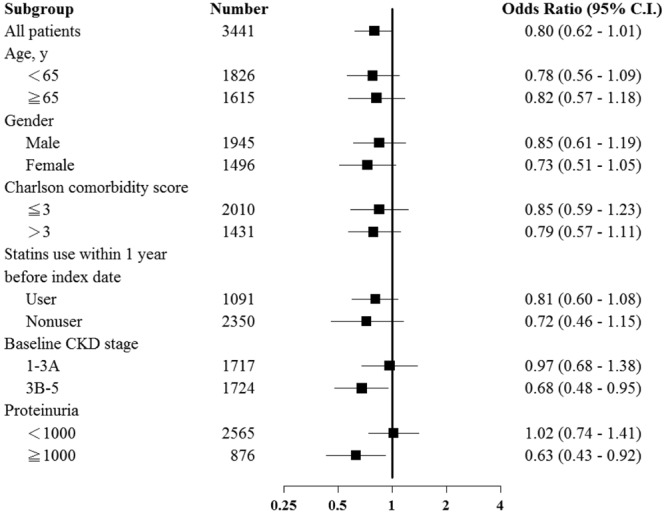
Subgroup analysis of risk of CKD among population with statin users and non-users.
Discussion
Our study demonstrated several critical findings with potential implications in therapies for CKD. In CKD stage 3B-5, statin use was significantly associated with a decrease in the number of CKD progression events, defined as an annual eGFR decline of ≥5 or advancement to the dialysis stage. The renoprotective effect of statins had the trend but not statistically significant in all CKD patients after adjustment for demographic and clinical characteristics including age, sex, comorbidities, alcohol use, betel nut use, smoking, BMI, baseline eGFR, baseline UPCR, and statin use within 1 year before the index date (crude OR 0.93, 95% CI 0.78–1.10; aOR 0.80, 95% CI 0.62–1.01). In the subgroup of patients with CKD stage 3B-5 (aOR 0.68, 95% CI 0.48–0.95), and those with proteinuria ≥1000 mg/day (aOR 0.63, 95% CI 0.43–0.92), statins exhibited renoprotective effects with statistically significant.
Because of their main mechanism of inhibiting HMG-CoA reductase activity, statins are widely used to treat hyperlipidemia. In addition, statins have other therapeutic benefits, such as anti-inflammatory and antioxidant properties, apoptosis induction, vascular smooth muscle proliferation inhibition, platelet activation and aggregation reduction, and increase in atherosclerotic plaque stability24. Many of these effects are potentially arise from small G-protein disruption24. Because of its resultant biological and genetic stress, the activation of G-protein signaling is pivotal in renal pathologies25.
The effects of statins on CKD progression remain subject to debate. A 2016 meta-analysis19 examined the data of 57 randomized control trials (RCTs), with 143,888 participants and 8,498 kidney failure events and suggested that statin therapy results in mild decreases in proteinuria levels and an eGFR decline of 0.41 mL/min/1.73 m2 per year. Su et al.19 defined kidney failure events as 25–50% decrease in eGFR, doubling of serum creatinine level, or advancement to ESRD stage during the follow-up period. Statin use did not prevent or mitigate these kidney failure events, consistent with the results of a systemic review conducted by Zhong et al.26; in total, 23 RCTs with 39,419 participants were analyzed, and the authors concluded that statins significantly reduced microalbuminuria, proteinuria, and clinical deaths, but did not slow the clinical progression of non-end-stage CKD significantly.
The 2012 KDIGO guidelines27,28 suggest that dialysis should be initiated when the eGFR is approximately 5–9 mL/min/1.73 m2. The 2014 Canadian Society of Nephrology clinical practice guidelines suggest that chronic dialysis should be initiated when eGFR decreases to 6 mL/min/1.73 m2, even if no clinical indicators are exhibited. Although data have shown high prevalence and incidence of ESRD in the Taiwan population3, patients in Taiwan begin dialysis with poor clinical conditions (mean hematocrit 24.2%, mean serum albumin 3.2 g/dL) with low residual renal function (eGFR 4.7 mL/min/1.73 m2)4. Hwang et al.4 attributed the delay in dialysis to patient awareness and attitudes toward dialysis treatment; in Taiwan, to initiate dialysis, pre-ESRD patients tend to wait for the presentation of uremic symptoms and other clinical signs, rather than following the eGFR criteria. The effects of dialysis initiation timing remains controversial. Although dialysis initiation timing is relatively late in Taiwan, mortality due to CKD is low4.
Our study enrolled patients from multiple medical centers in Taiwan, and national identification numbers were used to link patients with corresponding data in the NHID20. In contrast to definitions used in previous studies, we identified CKD progression events as either an annual average eGFR decline >5 mL/min/1.73 m2 or advancement to the dialysis stage. Because observational studies are prone to bias and being confounded, sensitivity analyses were performed, the results of which suggested that statin use benefited in those with CKD stage 3B-5 and proteinuria ≥1000 mg/day. A possible explanation for this finding is that patients may require a higher dosage of statin therapy. Shepherd et al.29 reported that although some eGFR improvement occurred after low-dosage (10 mg/day) atorvastatin treatment, high-dosage atorvastatin led to a more significant eGFR improvement. However, a 2014 meta-analysis30 involving 6 RCTs compared high-intensity statin therapy (atorvastatin 80 mg or rosuvastatin 20 or 40 mg) with moderate-to-mild statin treatment or placebo and noted that the effect of the high-intensity and moderate-to-mild therapies on eGFR improvement was not substantially different; regarding the safety of statin use, the prevalence of adverse events was low, and the pooled results showed no significant differences in adverse event prevalence among patients receiving high-intensity or nonintensive statin therapy or placebo30. Therefore, the effect of high-intensity statin on renal function is difficult to determine conclusively, and more evidence from high-quality studies is required.
This study has some limitations. First, this was a retrospective cohort study with a short follow-up period (1–6 years). Second, our criterion for defining CKD progression (annual eGFR decline >5 mL/min/1.73 m2) was broader than that of similar studies. It is worth noting that the beneficial effects of statin can be seen in patients with more than one gram of urine protein, because urinary protein itself is an indicator that can lead to deterioration of kidney function.
Conclusion
This national cohort study on CKD found that statins effectively delay CKD progression in CKD stage 3B-5 patients, particularly among those with proteinuria ≥1000 mg/day, for whom the benefits of treatment are clear. Thus, statin therapy may have a net clinical benefit for preventing CKD progression, particularly considering the high burden of dialysis. The protective effect of CKD on kidneys may differ according to statin dosage, and additional evidence is required to confirm these benefits. Our results suggest that statin usage in CKD with stage 3B-5 and those with proteinuria ≥1000 mg/day is critical, particularly for targeting CKD progression outcomes.
Acknowledgements
The study was supported by grant from the Shuang Ho Hospital, Taipei Medical University (grant no. 104TMU-SHH-13).
Author Contributions
Yuh-Feng Lin had full access to all of the data in the study and was responsible for the integrity of the data and the accuracy of the data analysis. Research idea and study design: Mei-Yi Wu, Ping-Jen Hu, Chung-Shun Wong, Yuh-Feng Lin. Data acquisition: Mei-Yi Wu, Tzu-Ting Chen, Yun-Chun Wu, Sui-Lung Su, Kuo-Cheng Lu, Jin-Shuen Chen, Fung-Chang Sung, Chien-Te Lee, Yu Yang, Shang-Jyh Hwang, Ming-Cheng Wang, Yung-Ho Hsu, Hung-Yi Chiou. Data analysis/interpretation: Mei-Yi Wu, Ping-Jen Hu, Chung-Shun Wong, Tsu-Chen Lin, Tsu-Chen Lin. Statistical analysis: Tzu-Ting Chen, Yun-Chun Wu. Supervision or mentorship: Chung-Shun Wong, Yuh-Feng Lin.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ping-Jen Hu and Mei-Yi Wu contributed equally.
Contributor Information
Chung-Shun Wong, Email: johnson7617@gmail.com.
Yuh-Feng Lin, Email: linyf@shh.org.tw.
References
- 1.Hill NR, et al. Global Prevalence of Chronic Kidney Disease - A Systematic Review and Meta-Analysis. PloS one. 2016;11:e0158765. doi: 10.1371/journal.pone.0158765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liyanage T, et al. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet. 2015;385:1975–1982. doi: 10.1016/S0140-6736(14)61601-9. [DOI] [PubMed] [Google Scholar]
- 3.System, U. S. R. D. 2017 USRDS annual data report: Epidemiology of kidney disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, https://www.usrds.org/adr.aspx 2017.
- 4.Hwang SJ, Yang WC, Lin MY, Mau LW, Chen HC. Impact of the clinical conditions at dialysis initiation on mortality in incident haemodialysis patients: a national cohort study in Taiwan. Nephrol. Dial. Transplant. 2010;25:2616–2624. doi: 10.1093/ndt/gfq308. [DOI] [PubMed] [Google Scholar]
- 5.Di Angelantonio E, et al. Chronic kidney disease and risk of major cardiovascular disease and non-vascular mortality: prospective population based cohort study. BMJ. 2010;341:c4986. doi: 10.1136/bmj.c4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am. J. Kidney Dis. 1998;32:S112–119. doi: 10.1053/ajkd.1998.v32.pm9820470. [DOI] [PubMed] [Google Scholar]
- 7.Law MR, Wald NJ, Rudnicka AR. Quantifying effect of statins on low density lipoprotein cholesterol, ischaemic heart disease, and stroke: systematic review and meta-analysis. BMJ. 2003;326:1423. doi: 10.1136/bmj.326.7404.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tonelli M, Wanner C. Lipid management in chronic kidney disease: synopsis of the Kidney Disease: Improving Global Outcomes 2013 clinical practice guideline. Ann. Intern. Med. 2014;160:182. doi: 10.7326/m13-2453. [DOI] [PubMed] [Google Scholar]
- 9.Li YH, et al. 2017 Taiwan lipid guidelines for high risk patients. J. Formos. Med. Assoc. 2017;116:217–248. doi: 10.1016/j.jfma.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 10.Parikh NI, et al. Cardiovascular disease risk factors in chronic kidney disease: overall burden and rates of treatment and control. Arch. Intern. Med. 2006;166:1884–1891. doi: 10.1001/archinte.166.17.1884. [DOI] [PubMed] [Google Scholar]
- 11.Satirapoj B, et al. Oxidized low-density lipoprotein antigen transport induces autoimmunity in the renal tubulointerstitium. Am. J. Nephrol. 2012;35:520–530. doi: 10.1159/000338484. [DOI] [PubMed] [Google Scholar]
- 12.Vaziri ND, Navab M, Fogelman AM. HDL metabolism and activity in chronic kidney disease. Nat. Rev. Nephrol. 2010;6:287–296. doi: 10.1038/nrneph.2010.36. [DOI] [PubMed] [Google Scholar]
- 13.Stancu C, Sima A. Statins: mechanism of action and effects. J. Cell. Mol. Med. 2001;5:378–387. doi: 10.1111/j.1582-4934.2001.tb00172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fellstrom B, et al. Effect of fluvastatin on renal end points in the Assessment of Lescol in Renal Transplant (ALERT) trial. Kidney Int. 2004;66:1549–1555. doi: 10.1111/j.1523-1755.2004.00919.x. [DOI] [PubMed] [Google Scholar]
- 15.Huskey J, et al. Effect of simvastatin on kidney function loss in patients with coronary heart disease: findings from the Scandinavian Simvastatin Survival Study (4S) Atherosclerosis. 2009;205:202–206. doi: 10.1016/j.atherosclerosis.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 16.Rahman M, et al. Progression of kidney disease in moderately hypercholesterolemic, hypertensive patients randomized to pravastatin versus usual care: a report from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) Am. J. Kidney Dis. 2008;52:412–424. doi: 10.1053/j.ajkd.2008.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baigent C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377:2181–2192. doi: 10.1016/S0140-6736(11)60739-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kimura G, et al. Effects of atorvastatin on renal function in patients with dyslipidemia and chronic kidney disease: assessment of clinical usefulness in CKD patients with atorvastatin (ASUCA) trial. Clin. Exp. Nephrol. 2017;21:417–424. doi: 10.1007/s10157-016-1304-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Su X, et al. Effect of Statins on Kidney Disease Outcomes: A Systematic Review and Meta-analysis. Am. J. Kidney Dis. 2016;67:881–892. doi: 10.1053/j.ajkd.2016.01.016. [DOI] [PubMed] [Google Scholar]
- 20.National Health Insurance Database, Ministry of Health and Welfare, Taiwan. https://dep.mohw.gov.tw/DOS/np-2497-113.html.
- 21.Stevens PE, Levin A. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann. Intern. Med. 2013;158:825–830. doi: 10.7326/0003-4819-158-11-201306040-00007. [DOI] [PubMed] [Google Scholar]
- 22.Yuan Y. Multiple Imputation Using SAS Software. J. Stat. Softw. 2011;45:14450. doi: 10.18637/jss.v045.i06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inker LA, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am. J. Kidney Dis. 2014;63:713–735. doi: 10.1053/j.ajkd.2014.01.416. [DOI] [PubMed] [Google Scholar]
- 24.Baradaran A, Rafieian-Kopaei HZ, An M. update on renoprotective and nephrotoxicity of statins. Ann. Res. Antioxid. 2016;1:e16. [Google Scholar]
- 25.Park F. Activators of G protein signaling in the kidney. J. Pharmacol. Exp. Ther. 2015;353:235–245. doi: 10.1124/jpet.115.222695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Z, Wu P, Zhang J, Wang S, Zhang G. The effect of statins on microalbuminuria, proteinuria, progression of kidney function, and all-cause mortality in patients with non-end stage chronic kidney disease: A meta-analysis. Pharmacol. Res. 2016;105:74–83. doi: 10.1016/j.phrs.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 27.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney inter., Suppl3, 5–14 (2013).
- 28.Nesrallah GE, et al. Canadian Society of Nephrology 2014 clinical practice guideline for timing the initiation of chronic dialysis. CMAJ. 2014;186:112–117. doi: 10.1503/cmaj.130363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shepherd J, et al. Effect of intensive lipid lowering with atorvastatin on renal function in patients with coronary heart disease: the Treating to New Targets (TNT) study. Clin. J. Am. Soc. Nephrol. 2007;2:1131–1139. doi: 10.2215/CJN.04371206. [DOI] [PubMed] [Google Scholar]
- 30.Yan YL, et al. High-intensity statin therapy in patients with chronic kidney disease: a systematic review and meta-analysis. BMJ open. 2015;5:e006886. doi: 10.1136/bmjopen-2014-006886. [DOI] [PMC free article] [PubMed] [Google Scholar]


