Abstract
Background and Aims
Information on cell cycle duration (T) in the root apical meristem (RAM) provides insight into root growth, development and evolution. We have previously proposed a simple method for evaluating T based on the dynamics of root growth (V), the number of cells in the RAM (Nm) and the length of fully elongated cells (l), which we named the rate-of-cell-production (RCP) method. Here, a global analysis was performed to confirm the reliability of this method in a range of angiosperm species and to assess the advantages of this approach.
Methods
We measured V, Nm and l from live or fixed cleared primary roots of seedlings or adventitious roots of bulbs and used this information to estimate the average T values in 73 angiosperm species via the RCP method. The results were then compared with published data obtained using the classical but laborious and time-consuming 3H-thymidine method.
Key Results
In most species examined, the T values obtained by the RCP method were nearly identical to those obtained by the 3H-thymidine method.
Conclusions
The global analysis demonstrated that the relationship between the variables V, Nm and l in roots in the steady state of growth is correctly described by the equation T = (ln2 Nm l)V−1. Thus, the RCP method enables cell cycle duration in the RAM to be rapidly and accurately determined. This method can be performed using live or fixed roots for each individual cell type. The simplicity of the approach suggests that it will be widely used in phenomics, evolutionary ecology and other plant biology studies.
Keywords: Angiosperms, cell cycle, cell cycle duration, cell proliferation, longitudinal zonation pattern, root phenotyping, root apical meristem, root development, root growth
INTRODUCTION
To fulfil their functions, roots must grow continuously throughout the plant’s life cycle. This is possible due to root apical meristem (RAM) activity and subsequent rapid cell elongation. The RAM is the source of all cells from which the root is built. Therefore, cell patterning in the RAM and the dynamics of cell division determine the architecture of the root system and are thus of paramount importance for the plant. Understanding these processes was one of the main interests of the late Peter W. Barlow. He and the senior author of this article proposed two exponential models for cell multiplication in the RAM that are essential for understanding root growth and RAM maintenance (Ivanov, 1974; Barlow, 1976b; Shishkova et al., 2008). Many aspects of root meristem patterning and organization were studied by Barlow, including the quiescent centre of the RAM and its stem cell properties (Barlow, 1973, 1976c, 1987, 1994, 1997, 2015a, b, 2016; Dubrovsky and Barlow, 2015), cell cycle duration within the RAM (Barlow and MacDonald, 1973; Francis and Barlow, 1988; Barlow and Woodiwiss, 1992), the role of plant hormones in cell proliferation in the RAM (Barlow, 1976a, 1992; Barlow and Pilet, 1981, 1984; Barlow et al., 1991; Müller et al., 1993, 1994; Ponce et al., 2005), and the relationship between cell cycle duration (T) and haploid DNA content (Francis et al., 2008).
The root is an exceptionally convenient system for studying cell proliferation due to the relative simplicity of its structure, the clear distinction between the RAM and the elongation zone, and the ease of treating roots with various compounds. Howard and Pelc (1953) were the first to introduce the cell cycle concept as we know it today. The authors proposed a fundamentally new approach for studying cell proliferation based on short-term (pulse) labelling of cells with a radioactive DNA precursor (32P at that time) and subsequent analysis of the labelled cells. The authors used Vicia faba roots, an appropriate system for obtaining numerous labelled cells (see Dubrovsky and Ivanov, 2003). Pulse labelling has since been performed in many studies based on this approach. Quastler and Sherman (1959), who analysed cell population kinetics in the mouse intestinal epithelium, further improved the method used to determine the duration of the cell cycle and its phases. By applying certain assumptions about cell behaviour and examining the relative proportions of labelled (mitotic) and unlabelled (interphase) cells after administering a DNA precursor, tritiated thymidine, the authors developed a graphical method for estimating the duration of the cell cycle and its phases. This approach became popular for analysing plant roots. Short-term incubation of roots in a nutrient solution supplemented with 3H-thymidine and their subsequent fixation at different time intervals after transfer into a solution without 3H-thymidine allows the percentage of labelled cells undergoing mitosis to be estimated. The labelled mitoses values are then plotted against time, and the resulting curves can be used to determine the duration of the cell cycle and its periods. This method, known as the labelled mitoses or 3H-thymidine (hereafter thymidine) method, has several limitations, as analysed by Baskin (2000). One of the disadvantages is the potential effect of radioactivity on the cell cycle itself (Torre and Clowes, 1974). Furthermore, the method is laborious, prompting Francis et al. (2008) to acknowledge the researchers ‘who strove through sleepless nights to obtain the cell cycle times’. Nonetheless, the thymidine method has been widely used in both animal and plant studies, and has yielded much data. Furthermore, some other methods have been developed to determine cell cycle duration, including the colchicine (Van’t Hof et al., 1960; Van’t Hof and Sparrow, 1963) and caffeine (Giménez-Martín et al., 1965) methods. However, these approaches are not always practical. Overall, cell cycle duration has been determined in 170 plant species from 53 genera belonging to 38 families (Grif et al., 2002). However, in recent years, this method has fallen out of favour. During the period of its greatest popularity (1970–1975), 107 papers were published in which this method was implemented, whereas in 1995–2000, only nine such studies were published (Grif et al., 2002). In the past decade, this method has almost never been used.
More recently, a kinematic approach was developed based on analyses of the velocities of cell displacement or cell flux (Sharp et al., 1988; Silk et al., 1989; Beemster and Baskin, 1998; van der Weele et al., 2003; Fiorani and Beemster, 2006; Yang et al., 2017). Among other approaches (e.g. Cools et al., 2010), the DNA precursors bromodeoxyuridine and 5-ethynyl-2′, deoxy-uridine, sometimes in combination with flow cytometry, have occasionally been used instead of thymidine for determination of T in the RAM (Moretti et al., 1992; Lucretti et al., 1999; Hayashi et al., 2013).
Ivanov (1968) proposed a simple method for T estimation based on the organization of the RAM and root growth. This method relies on data including the number of meristematic cells in files, the length of the cells that have completed their growth and root growth rate. The rationale behind this method, the assumptions and a proposed model of the relationship between the rate of root growth and cell production have been described in detail (Ivanov and Dubrovsky, 1997). Because this method is based on the analysis of cell production over time, it is referred to as the rate-of-cell-production (RCP) method. The cell cycle duration values obtained with the RCP and thymidine methods have been shown to match using a few examples (Ivanov and Dubrovsky, 1997). This method has been repeatedly performed using various species: maize, Zea mays (Ivanov, 1994), wheat, Triticum aestivum (Demchenko, 1976), Cactaceae (Dubrovsky et al., 1998) and Arabidopsis thaliana (Dubrovsky et al., 2000; Tapia-López et al., 2008; Garay‐Arroyo et al., 2013; López-Bucio et al., 2014; Napsucialy-Mendivil et al., 2014). However, no global analysis of the RCP method has thus far been performed. Therefore, in the current study, we determined T in 73 angiosperm species using the RCP method and compared the results with published data obtained using the thymidine method. The results of this study confirm that the RCP method is a simple, rapid and accurate approach for determining T in roots.
MATERIAL AND METHODS
Species analysed and growth conditions
The roots of 73 species (Tables 1 and 2) were analysed. For 68 of these species, the primary roots of seedlings were examined and for five species, the adventitious roots obtained from bulbs were examined. Seeds from many of the species were obtained from the Vavilov Research Institute of Plant Industry in Saint Petersburg, Russia (VIR), and from other research centres mentioned in the Acknowledgments. The respective varieties or lines are marked ‘VIR’ in Table 3, along with a number corresponding to their collection. Seeds were germinated in Petri dishes maintained in darkness on filter paper moistened with purified and filtered tap water. The bulbs were grown in dark glass bottles filled with tap water under natural illumination. The temperature during the experiments was between 20 and 25 °C. Root length was measured using a ruler with an accuracy of 1 mm. After germination, root growth increased daily. When the roots started to grow at a constant rate, root tips (1–1.5 cm in length) were excised and fixed in 70 % ethanol. Prior to fixation, thicker roots were cut lengthwise with a razor blade. Immediately before analysis, thin roots were rinsed three times (5 min each) in distilled water, transferred onto a microscope slide in 50 % glycerol, and covered with a coverslip. Thicker roots or longitudinally halved root tips with denser layers of meristematic cells were cleared using the protocol of Malamy and Benfey (1997) and mounted in 50 % glycerol. For the roots of each species, the length of the RAM (Lm) was measured as the length from the distal boundary of the RAM to the point where a sharp increase in cell length began.
Table 1.
Cell cycle duration (T) determined by the rate-of-cell-production (RCP) method, published data obtained by the 3H-thymidine (thym) method and their comparison
| Species | T by RCP method (h) | T by thymidine method (h) | Reference | Difference (h) | Difference (%) |
|---|---|---|---|---|---|
| Aegilops squarrosa auct. (tauschii Coss.) | 11.2 ± 1.0 | 11.4 | Davies and Rees (1975) | −0.2 | −1.8 |
| Aegilops umbellulata Zhuk. | 12.9 ± 1.2 | 10.7 | Kidd et al. (1987) | 2.2 | 17.1 |
| Agoseris heterophylla (Nutt.) Greene | 10.9 ± 1.2 | 8.8 | Price and Bachmann (1976) | 2.1 | 19.3 |
| Agoseris retrorsa (Benth.) Greene | 11.8 ± 1.0 | 9.0 | Price and Bachmann (1976) | 2.8 | 23.7 |
| Allium carinatum L. | 15.1 ± 1.2 | 9.2 | Bösen and Nagl (1978) | 5.9 | 39.1 |
| Allium sativum L. | 22.7 ± 2.8 | 21.6 ± 1.8 | See Table 2 | 1.1 | 4.8 |
| Allium tuberosum Rottler ex Spreng. | 20.7 ± 3.0 | 20.6 | Van’t Hof (1965), Matagne (1968), Bryant (1969) | 0.1 | 0.5 |
| Allium сера L. | 15.5 ± 1.6 | 16.0 ± 1.6 | See Table 2 | −0.5 | −3.2 |
| Allum porrum L. | 23.0 ± 2.5 | 18.0 ± 1.2 | See Table 2 | 5.0 | 21.7 |
| Anacyclus radiatus L. | 11.6 ± 1.5 | 14.0 | Nagl (1974, 1978) | −2.4 | −20.7 |
| Anthemis austriaca L. | 8.0 ± 1.2 | 7.0 | Nagl (1974, 1978) | 1.0 | 12.5 |
| Anthemis cota L. | 8.2 ± 1.0 | 6.0 | Nagl (1974, 1978) | 2.2 | 26.8 |
| Anthemis tinctoria (L.) J. Gay ex Guss. | 12.0 ± 1.2 | 12.0 | Nagl (1974, 1978) | 0.0 | 0.0 |
| Artemisia absinthum L. | 9.3 ± 1.3 | 9.5 | Nagl (1974, 1978) | −0.2 | −2.2 |
| Artemisia annua L. | 10.7 ± 1.0 | 8.0 | Nagl (1974, 1978) | 2.7 | 25.2 |
| Avena pilosa (Roem. & Schult.) Bieb. | 9.2 ± 1.7 | 8.9 | Yang and Dodson (1970) | 0.3 | 3.3 |
| Avena strigosa Schreb | 8.7 ± 1.4 | 9.8 ± 0.3 | Yang and Dodson (1970) | −1.1 | −12.6 |
| Beta vulgaris L. | 12.7 ± 1.4 | 16.0 | Titsu and Popovici (1970) | −3.3 | −26.0 |
| Brassica junceae (L.) Czern. | 12.5 ± 1.2 | 12.0 | Srivastava and Levania (1978) | 0.5 | 4.0 |
| Coriandrum sativum L. | 13.7 ± 3.1 | 13.0 | Olszewska et al. (1990) | 0.7 | 5.1 |
| Crepis capillaris L. | 10.3 ± 1.3 | 11.0 ± 1.0 | See Table 2 | −0.7 | −6.8 |
| Crepis tectorum L. | 12.4 ± 2.6 | 12.0 | Langridge et al. (1970) | 0.4 | 3.2 |
| Cucurbita pepo L. | 12.8 ± 3.0 | 18.0 | Marciniak et al. (1978) | −5.2 | −40.6 |
| Dactylis glomerata L. | 9.6 ± 1.5 | 12.1 ± 1.8 | See Table 2 | −2.5 | −26.0 |
| Daucus carota L. | 8.0 ± 2.2 | 8.0 | Bayliss (1975) | 0.0 | 0.0 |
| Epilobium hirsutum L. | 14.0 ± 1.6 | 7.0 | Thomas (1992) | 7.0 | 50.0 |
| Eragrostis tef (Zuccagni) Troffer | 11.1 ± 2.1 | 9.7 | Kidd et al. (1987) | 1.4 | 12.6 |
| Fagopyrum esculentum Moench. | 7.1 ± 1.0 | 6.0 | Seyhodjaev (1971) | 1.1 | 15.5 |
| Festuca rubra L. | 14.7 ± 1.8 | 16.2 ± 0.2 | See Table 2 | −1.5 | −10.2 |
| Glycine max (L.) Merr. | 13.0 ± 1.2 | 8.6 ± 1.1 | See Table 2 | 4.4 | 33.8 |
| Helianthus annuus L. | 12.2 ± 1.5 | 12.0 ± 1.2 | See Table 2 | 0.2 | 1.6 |
| Hordeum bulbosum L. | 12.7 ± 2.2 | 14.0 | Kidd et al. (1987) | −1.3 | −10.2 |
| Hordeum vulgare L. | 10.5 ± 0.6 | 12.5 ± 0.3 | See Table 2 | −2.0 | −19.0 |
| Hyacinthus orientalis L. | 33.0 ± 3.5 | 24.0 | Evans and Rees (1971) | 9.0 | 27.3 |
| Impatiens balsamina L. | 10.2 ± 1.4 | 9.0 | Van’t Hof (1965) | 1.2 | 11.8 |
| Lactuca sativa L. | 12.0 ± 1.3 | 10.0 | Mazzuka et al. (2000) | 2.0 | 16.7 |
| Lathyrus articulatus L. | 18.3 ± 1.3 | 14.3 | Evans and Rees (1971) | 4.0 | 21.9 |
| Lathyrus latifolius L. | 16.4 ± 0.9 | 24.0 | Olszewska et al. (1990) | −7.6 | −46.3 |
| Lathyrus odoratus L. | 23.3 ± 2.5 | 20.0 | Olszewska et al. (1990) | 3.3 | 14.5 |
| Lathyrus tingitanus L. | 18.5 ± 2.4 | 16.8 | Evans et al. (1972) | 1.7 | 9.2 |
| Lilium longiflorum Thunb. | 51.0 ± 5.9 | 24.0 | Kidd et al. (1987) | 27.0 | 52.9 |
| Linum usitatissimum L. | 13.7 ± 1.2 | 14.0 | Evans et al. (1972) | −0.3 | −2.2 |
| Lolium perenne L. | 9.2 ± 0.9 | 8.1 | Evans et al. (1972) | 1.1 | 12.0 |
| Luzula purpurea Lowe | 21.0 ± 3.6 | 22.0 | Montezuma-de-Carvalho (1962) | −1.0 | −4.8 |
| Lycopersicum esculentum L. ssp. Cultum | 9.7 ± 1.2 | 13.0 | Van’t Hof (1965), Titsu (1967) | −3.3 | −34.0 |
| Melandrium album (Mill.) Garcke | 15.1 ± 1.2 | 15.5 | Choudhury (1969) | −0.4 | −2.6 |
| Nicotiana plumbaginifolia Viv. | 12.8 ± 1.5 | 11.0 | Gupta (1969) | 1.8 | 14.1 |
| Nicotiana tabacum L. | 11.2 ± 1.8 | 9.0 | Gupta (1969) | 2.2 | 19.6 |
| Nigella damascena L. | 14.0 ± 1.7 | 16.5 | Evans et al. (1972) | −2.5 | −17.9 |
| Ornithogalum umbellatum L. | 49.3 ± 8.9 | 14.0 | Tagliasacchi et al. (1983) | 35.3 | 71.6 |
| Oryza sativa L. | 7.9 ± 0.8 | 10.8 | Kidd et al. (1987) | −2.9 | −36.7 |
| Papaver nudicale L. | 12.7 ± 2.2 | 10.0 | Olszewska et al. (1990) | 2.7 | 21.3 |
| Papaver orientale L. | 12.0 ± 1.6 | 16.0 | Olszewska et al. (1990) | −4.0 | −33.3 |
| Papaver somniferum L. | 10.8 ± 1.2 | 12.0 | Olszewska et al. (1990) | −1.2 | −11.1 |
| Pennisetum americanum (L.) Leeke | 11.3 ± 0.8 | 12.4 | Kidd et al. (1987) | −1.1 | −9.7 |
| Phalaris canariensis L. | 14.6 ± 1.0 | 14.5 | Prasad and Gоdward (1965) | 0.1 | 0.7 |
| Pisum sativum L. | 11.7 ± 0.7 | 15.3 ± 1.0 | See Table 2 | −3.6 | −30.8 |
| Pyrrhopappus caroliniana L. | 12.7 ± 3.1 | 12.0 | Price and Bachmann (1976) | 0.7 | 5.5 |
| Rumex thyrsiflorus Fingerh. | 17.8 ± 1.7 | 16.0 | Zuk (1969) | 1.8 | 10.1 |
| Scilla sibirica Andrews | 77.0 ± 10.5 | 67.0 | Baumann (1972) | 10.0 | 13.0 |
| Secale cereale L. | 12.7 ± 1.1 | 14.0 ± 1.3 | See Table 2 | −1.3 | −10.2 |
| Sorghum bicolor (L.) Moench | 17.0 ± 1.1 | 13.9 | Kidd et al. (1987) | 3.1 | 18.2 |
| Triticosecale Wittm. & A.Camus | 13.0 ± 1.1 | 11.7 ± 0.4 | Kaltsikes (1971), Kidd et al. (1987) | 1.3 | 10.0 |
| Triticum aestivum L. | 11.6 ± 0.9 | 14.3 ± 1.2 | See Table 2 | −2.7 | −23.3 |
| Triticum dicoccoides (Körn. ex Asch. & Graebn.) Schweinf. | 11.1 ± 0.8 | 12.7 | Davies and Rees (1975) | −1.6 | −14.4 |
| Triticum monococcum L. | 13.7 ± 1.0 | 12.0 | Davies and Rees (1975) | 1.7 | 12.4 |
| Triticum spelta L. | 19.0 ± 2.9 | 19.7 | Davies and Rees (1975) | −0.7 | −3.7 |
| Triticum timopheevi (Zhuk.) Zhuk. | 14.5 ± 1.2 | 15.0 | Davies and Rees (1975) | −0.5 | −3.4 |
| Triticum turgidum (durum) Desf. | 13.7 ± 1.1 | 12.3 ± 0.9 | Kaltsikes (1971) | 1.4 | 10.2 |
| Tropaeolum majus L. | 25.5 ± 1.7 | 8.0 | Olszewska et al. (1990) | 17.5 | 68.6 |
| Vicia faba L. | 12.8 ± 1.1 | 16.3 ± 1.3 | See Table 2 | −3.5 | −27.3 |
| Vicia sativa L. | 13.4 ± 1.2 | 13.2 ± 0.8 | See Table 2 | 0.2 | 1.5 |
| Zea mays L. | 12.0 ± 0.7 | 12.5 ± 0.2 | See Table 2 | −0.5 | −4.2 |
Dif, difference between the two methods in hours and relative terms. Data are means ± s.e.
Table 2.
Comparison of cell cycle duration values in the root apical meristem obtained by the thymidine method for the same species reported in different studies (means ± s.e.)
Table 3.
Cell cycle duration (T) in the root apical meristem of different varieties of the same species obtained by the RCP method (n = 8, means ± s.e.)
| Species and variety or cultivar | Cell cycle duration, T (h) |
|---|---|
| Pisum sativum L. ‘Premium’ | 17.2 ± 1.9 |
| Pisum sativum L. ‘Miracle’ | 15.6 ± 2.0 |
| Pisum sativum L. ‘Ramensky’ | 10.0 ± 0.7 |
| Pisum sativum L. ‘Pioneer’ | 14.4 ± 1.3; 16.5 ± 1.4 |
| Pisum sativum L. ‘Alpha’ | 10.7 ± 0.7; 12.7 ± 0.7 |
| Pisum sativum L. VIR No. 2227 | 13.8 ± 0.8 |
| Pisum sativum L. VIR No. 6892 | 16.6 ± 1.0 |
| Pisum sativum L. VIR No. 993 | 15.9 ± 0.8 |
| Allium porrum L. VIR No. 2078 | 26.0 ± 2.6 |
| Allium porrum L. VIR No. 2389 | 23.0 ± 2.5 |
| Triticum dicoccoides (Körn.ex Asch. & Graebn.) Schweinf. VIR No. 61842 | 11.1 ± 0.8 |
| Triticum dicoccoides (Körn.ex Asch. & Graebn.) Schweinf. VIR No. 61833 | 9.9 ± 1.0 |
| Hordeum bulbosum L. VIR No. 250 | 12.4 ± 2.2 |
| Hordeum bulbosum L. VIR No. 613 | 10.0 ± 1.1 |
| Daucus carota L. ‘Long red’ | 8.0 ± 2.2 |
| Daucus carota L. VIR No. 4 | 12.0 ± 1.1; 1.0 ± 1.4 |
| Coriandrum sativum L. VIR No. 360 | 13.7 ± 3.1 |
| Coriandrum sativum L. VIR No. 420 | 16.0 ± 2.5 |
| Cucurbita pepo L. ‘Aeronaut’ | 12.8 ± 3.0 |
| Cucurbita pepo L. VIR No. 4800 | 16.7 ± 1.0 |
| Cucurbita pepo L. ‘Gribovsky early’ | 19.5 ± 3.0 |
| Glycine max (L.) Merr. ‘Killer whale’ | 10.1 ± 1.2 |
| Glycine max (L.) Merr. ‘Flyer’ | 18.0 ± 1.6 |
| Zea mays L. ‘Interkras’ | 9.6 ± 0.7; 14.4 ± 1.4 |
| Zea mays VIR No. 1329 | 9.5 ± 0.3 |
| Zea mays VIR No. 6634 | 9.2 ± 0.8 |
| Zea mays VIR No. 6343 | 11.8 ± 1.2 |
| Zea mays VIR No. 18399 | 12.3 ± 0.8 |
| Zea mays L. VIR No. 14a | 5.6 ± 0.4; 7.3 ± 0.5 |
| Zea mays L. VIR No. 15b | 7.2 ± 0.6; 7.1 ± 0.4 |
| Zea mays L. VIR No. 23427 | 10.4 ± 1.0 |
| Zea mays L. VIR No. 23427 | 8.8 ± 0.9 |
| Zea mays L. VIR No. 19019 | 8.5 ± 0.6 |
| Zea mays L. VIR No. 19019 | 8.2 ± 0.6 |
| Zea mays L. VIR No. 18997 | 13.2 ± 1.1 |
| Zea mays L. VIR No. G | 6.9 ± 0.4 |
| Zea mays L. VIR No. Е | 8.5 ± 0.6 |
| Zea mays L. VIR No. Е | 8.5 ± 0.6 |
| Tropaeolum majus L. ‘Golden highlight’ | 24.0 ± 1.6; 27.0 ± 1.7 |
| Tropaeolum majus L. ‘American Queen’ | 22.8 ± 1.2 |
| Tropaeolum majus L. ‘Empress of India’ | 26.9 ± 2.0 |
| Lilium longiflorum L. ‘Eagle’ | 51.0 ± 6.0 |
| Lilium longiflorum L. ‘Nuance’ | 55.0 ± 7.4 |
Principles of the RCP method and practical considerations
The RCP method has been described in detail (Ivanov and Dubrovsky, 1997). Here, we briefly outline the principles of this approach. It is based on the simplest model of cell proliferation in the RAM under the following assumptions: (1) the average cell cycle duration (T) for all meristematic cells is the same; (2) all meristematic cells proliferate; (3) the number of cells in a meristem (or in a cell file within the meristem) is constant; and (4) the flux of cells into and out of the non-proliferating elongation zone is the same (Ivanov and Dubrovsky, 1997). Numerous studies confirm that these assumptions are valid for roots growing at a constant rate (Baskin, 2000; Fiorani and Beemster, 2006; Yang et al., 2017).
In the vast majority of wild-type (non-mutant) plants of various species, the RAM cells of the growing root are in an active proliferation state. The average T is constant along the meristem above the quiescent centre (Balodis and Ivanov, 1970; Barlow and MacDonald, 1973; Clowes, 1976; Baskin, 2000). Detailed analysis indicates that there is no reason to assume that several cell populations exist in the meristem that differ in T (Ivanov, 1974, 1987, 1994; Webster and MacLeod, 1980), although T may fluctuate through consecutive divisions. The values of T for individual cells vary within a range of approximately 15–20 % (Ivanov, 1971).
The decrease in the mitotic index in the basal half of the meristem is caused by a gradual exit of cells from the cell cycle and not by an increase in T (Balodis and Ivanov, 1970). The only exception is developing metaxylem cell files in some monocots, in which mitoses terminate much more closely to the root apex than in other tissues, but these cells comprise only a small fraction of all cells in the meristem. Note that at the boundary of the meristem and the elongation zone, there is the transition domain of the RAM in which cells do not divide, instead growing at almost the same relative rate as in the proliferation domain of the meristem (Verbelen et al., 2006; Baluška et al., 2010; Ivanov and Dubrovsky, 2013). The length of the transition domain varies among species (Ivanov and Dubrovsky, 2013). In some species, for instance A. thaliana, the transition domain may comprise up to 15–27 % of the meristem length (Pacheco-Escobedo et al., 2016), but it is usually shorter in other species (e.g. Z. mays, Allium cepa) (Ivanov and Dubrovsky, 2013; Kirschner et al., 2017). Note that in a root that obeys the above-mentioned assumptions where T is constant along the meristem, the cells located at a level that is about half the length of the RAM, and all of the cells above that level, transit to the elongation zone during a time period equal to one cycle. This observation suggests that cells pass through the transition domain during a short period of time, i.e. shorter than T. This explains why the probability of observing divisions in the transition domain is very low. Thus, if the root grows at a constant rate and the cell number in the RAM does not change over time, the root growth rate (V), length of fully elongated cells (l), number of meristematic cells in a file (Nm) and T are associated via a simple relationship (Ivanov, 1974, 1994; Ivanov and Dubrovsky, 1997):
| (1) |
Under these assumptions, not all cells from the basal half of the meristem have time to divide before exiting the meristem. If the root grows at a non-constant rate and Nm and l change over time, the average values can be used instead, although this may cause errors.
The number of meristematic cells in a file was calculated as the ratio between RAM length (Lm) and the average length of cortical meristematic cells (lm). The latter was determined for eight to ten 50-µm portions per root located along the RAM by counting the number of cells per portion and dividing the length of the portion by the number of cells. Fifty fully elongated cortical cells per root were measured with an ocular micrometer, and the average elongated cell length (l) was used to estimate T. Cell lengths in the middle cortex layers were usually measured. For most species, eight roots were examined per experiment. For some species, independent experiments were performed two to four times (Supplementary Data Table S1). The values of T (h) were calculated using eqn (1). For each variable of eqn (1), the average value and standard error (s.e.) were estimated. The s.e. of T was estimated using the following equation:
| (2) |
where x̅ and y̅ are average variables and Sx̅ and Sy̅ are standard errors of the respective averages, and Z̅ and S̅Z̅ are the x̅-to-y̅ ratio (or x̅ and y̅ product) and the standard error of the ratio (or the product), respectively (Urbach, 1964). T values reported in different laboratories for a species were used to estimate the global average value; s.e. was also estimated using eqn (2). For comparison purposes, only those reported T values determined by the thymidine method which were obtained for plants grown at 23 ± 2 °C were used (listed in Table 1).
RESULTS
Duration of the mitotic cycle estimated by the 3H-thymidine method varies little for roots of the same species
Before comparing the results obtained by the two methods, we analysed the variability of the data for T values reported for the same species obtained by various laboratories. We obtained data reported more than once for the same species from young seedlings of 17 species grown at 22–24 °C (Table S1). For most of these species, T varied within a narrow limit, with a few exceptions. This result is somewhat surprising, since the data encompass research results from several studies performed in different countries and years on the roots of seedlings of different ages under diverse growing conditions.
We encountered several difficulties in comparing these data. T values can vary across different varieties or cultivars of the same species. Some reports do not mention the cultivars or varieties examined. Creber et al. (1993) recorded a variation of T in roots from different populations of Dactylis glomerata, which also differed slightly in terms of haploid DNA content. However, the curves of labelled mitoses in Creber et al. (1993) diverge significantly from the classical curves, which probably indicates significant heterogeneity of T in individual roots or heterogeneity in T within the RAM. Furthermore, it is important to consider that T can change in the same root during growth. Gahan and Hurst (1976) showed that in maize roots, the average T value changed a few times over a 20-d period. Notably, in most of the studies cited (Table 2), the roots of seedlings were sampled several days after germination, when they were growing at a constant and relatively high rate.
To date, an evaluation of the accuracy of T estimated by the thymidine method has not been reported. The T values are frequently provided at an accuracy of up to tenths of an hour, but the method used to determine the percentage of labelled mitoses is clearly less precise. For example, to measure the percentage of mitotic labelled cells, 100 mitoses are often analysed each time root cells are fixed. If the proportion of labelled mitoses equals 50 %, the standard deviation for the analysis of 100 mitoses is at least 5 %. Therefore, the actual accuracy of T value determinations is at least 10 %. Nevertheless, despite these shortcomings, there is a striking similarity among the estimated T values for roots of the same species obtained in different studies performed in different laboratories and years. This finding allowed us to compare T for the same species obtained by the thymidine versus RCP methods.
Estimating cell cycle duration using the RCP method
We determined T in the RAMs of various angiosperm species using the RCP method. Under our growth conditions, the variation of T within a species occurred in a relatively narrow window (Tables 1 and 3). The standard error of T estimated by the RCP method for a single species was approx. 10 % (Table 1). The reproducibility of repeated estimates was on the order of 10–15 % (Table S1). These results are similar to those obtained by the thymidine method (Table 1, Fig. 1).
Fig. 1.
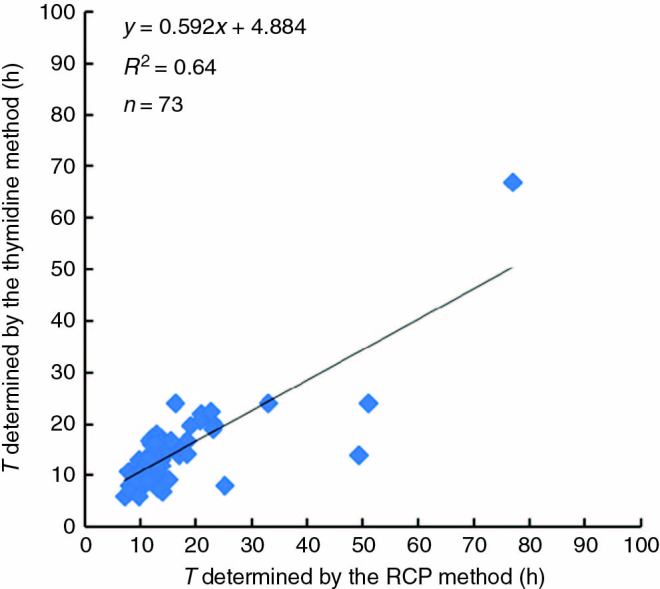
Correlation between cell cycle duration determined using the rate-of-cell-production (RCP) method (obtained in this study) and the 3H-thymidine method (reported in the literature) for the same species. For 73 species, the primary data are presented in Tables 1 and 2; correlation coefficient r = 0.80. If excluding data from the Epilobium, Tropaeolum, Ornithogalum and Lilium species (n = 69), R2 = 0.86 and r = 0.93.
We determined T in the seedling roots of different varieties of several plant species (Table 3). This question has not previously been addressed in the literature. We found that in some species, such as maize, variations between varieties were high, and T determined by the RCP method ranged from a minimum of 5.1 to a maximum of 14.4 h (Table 3). Nonetheless, the average T values determined by the RCP and thymidine methods differed by only 30 % (Table 1). Such differences are of interest, since it is unlikely that the roots of different varieties of plants differ significantly in terms of haploid DNA content, a generally recognized factor that affects T (e.g. Francis et al., 2008). There are several possible explanations for the variation in T. In Dactylis glomerata, a significantly larger T, as determined by the thymidine method (Table 2), was detected in seedlings from various populations with some deviations in haploid DNA content, although these deviations were <10 % (Creber et al., 1993). There might be another explanation for the variability in T among populations (Table 3). T is noticeably higher in roots before a constant growth rate is attained compared to roots growing at a constant rate, as observed for Vicia faba and Pisum sativum roots (data not shown). This difference might not have been accounted for in some studies.
Comparison of the results obtained from the thymidine versus RCP methods
We determined T for 73 angiosperm species using the RCP method and compared the results with published results obtained by the thymidine method. For 69 of the species, the estimates of T obtained by the two methods were nearly identical (Table 1, Fig. 1). The T values obtained differed significantly from previously published data for only a few species: Epilobium hirsutum, Tropaeolum majus, Ornithogalum umbellatum and Lilium longiflorum. Repeated T estimates using the RCP method obtained from different groups of seedlings grown from different seed stocks coincided (Table S1). Hence, these differences were not due to discrepancies in the methods used for these species. Overall, these results indicate that the two methods yield similar results, which confirm the previously observed similarity in T values reported for only a few species (Ivanov and Dubrovsky, 1997). Thus, the RCP method, which is based on a simple exponential model of cell proliferation in the RAM, confirms the results of the more complicated thymidine method.
Root growth rate calculated based on measured l and Nm values and from published T values obtained by the thymidine method agrees with measured values
To validate the model of the link between cell cycle duration, rate of cell production and rate of root growth (Ivanov and Dubrovsky, 1997), we compared the experimentally determined root growth rates (V) with the root growth rates (Vc) calculated based on published data, where T was determined by the thymidine method and from measured Nm and l values. From eqn (1), it follows that
| (3) |
Nm and l were measured in the species listed in Table 1, and T values were obtained from published results (listed in Table 1). In most cases, the Vc values coincided with experimentally measured V values, except for a few species where data from the thymidine and RCP methods greatly diverged (i.e. Epilobium hirsutum, Tropaeolum majus and Ornithogalum umbellatum) (Fig. 2).
Fig. 2.
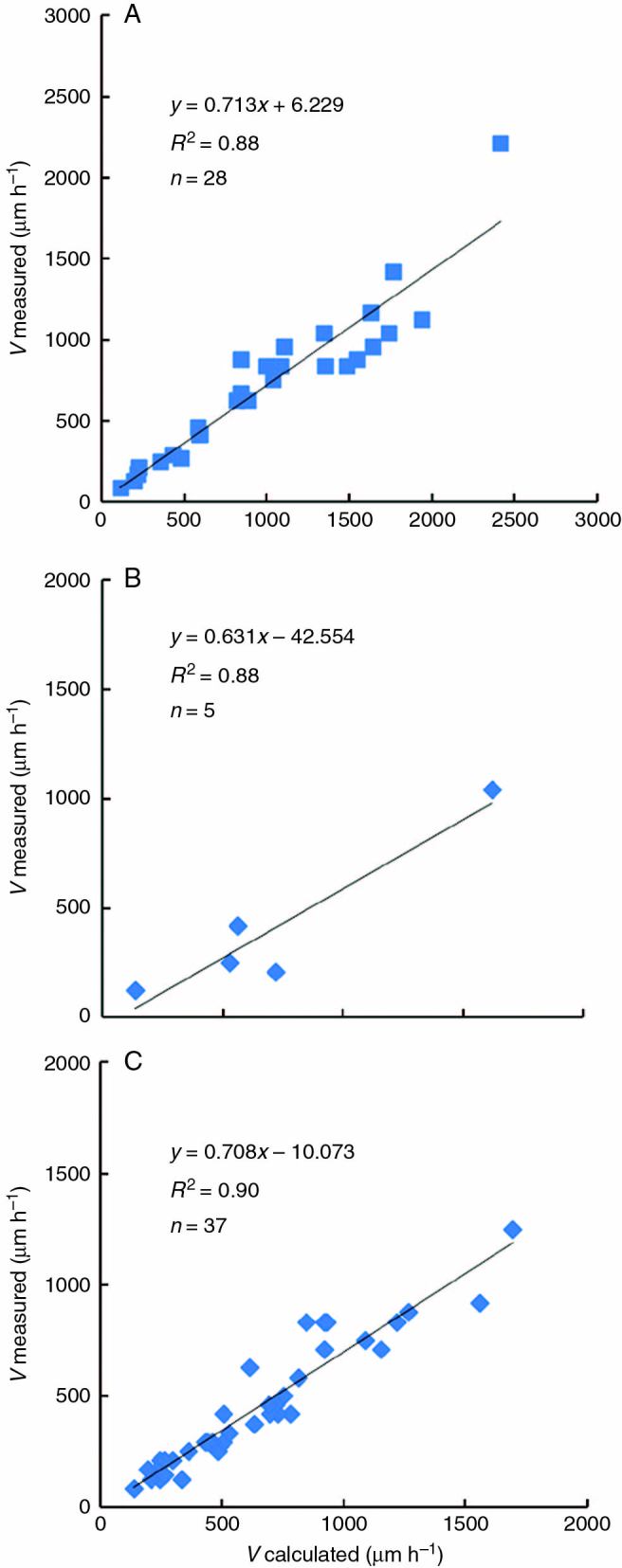
Correlation between the measured rate of root growth and the predicted rate of root growth calculated based on the cell cycle duration reported in the literature (determined by the 3H-thymidine method). The rate of root growth was calculated using eqn (3). (А) Primary roots of monocotyledonous species. (B) Primary roots of dicotyledonous species. (C) Adventitious roots of monocotyledonous species. Correlation coefficients r = 0.94 (A, B) and 0.95 (C).
DISCUSSION
Our results show that T values determined by the RCP method are in good agreement with published data obtained by the thymidine method for 69 out of 73 species; it remains unclear why these values differ in the five species. Overall, this analysis demonstrates that (1) despite their different methodological approaches, the two methods yield nearly the same or similar results; (2) the model used in the RCP approach appears to be correct; and (3) despite the differences in growth conditions used in different laboratories and the different methods of analysis, not much variability was found in the distribution of T measurements within a species. In practical terms, this indicates that the RCP method, which is much simpler and less time-consuming than the thymidine method, can be routinely used for many species. Prior to the current study, the RCP approach was used in only a few laboratories.
The good agreement in the values obtained by the RCP and thymidine methods suggests that the assumptions used for the exponential model of cell proliferation to estimate cell cycle duration are correct. We are aware that any assumption represents a simplification. Nonetheless, the results obtained by the two methods differed by only an average of 0.6 ± 2.7 % for 69 of the 73 species [mean ± s.e., calculated based on data in Table 1: Epilobium hirsutum, Tropaeolum majus, Ornithogalum umbellatum and Lilium longiflorum were excluded (see below)], which is surprisingly accurate for a biological process. Nevertheless, it might appear that the main assumptions of the model (that all cells in the RAM proliferate and that the average T for all meristematic cells is the same) are weak. To explain the basis of these assumptions, we must consider the longitudinal organization of root growth zones and the behaviour of the cells within them. Some authors considered that cells in the basal portion of the RAM have a longer cycle time than in the rest of the RAM (Hejnowicz, 1959) or that not all cells in the basal RAM portion proliferate (Clowes, 1971, 1976), which contradict our assumptions. However, these conclusions were drawn based on labelled mitosis curves and did not consider the exponential age distribution of cells within the RAM (Ivanov, 1974, 1994; Webster and Davidson, 1980). Our analysis showed that the proximal portion of the RAM represents a transition domain where cells still divide (Lavrekha et al., 2017), and the relative cell growth rate is the same as in the rest of the RAM (Ivanov and Dubrovsky, 2013). Also, the duration of the last cell cycle in the RAM is the same as in the proliferation domain (Balodis and Ivanov, 1970), but exit from the cell cycle at the end of the RAM is a heterogeneous process. At a given time point, cell division is less common in this domain than in the other regions, but not because cells divide less frequently or because not all cells proliferate. The lower incidence of cell division is a consequence of cell flux. With increasing distance from the quiescent centre, the cells are displaced more rapidly from the RAM (Ivanov, 1974, 1994; Ivanov and Dubrovsky, 1997, 2013). This analysis shows that during the time equal to one cell cycle, ln2 number of cells in the meristem (~69 %) are displaced from the RAM to the elongation zone. Therefore, during the last cycle, while the cells are displaced from the RAM, not all of them have sufficient time to pass through mitosis. This explains why the mitotic index decreases sharply in the transition domain. On the other hand, cells in the quiescent centre, including initial cells, have much longer cycle times (Barlow, 1976b;Ivanov, 1994), but these cells commonly comprise less than 1 % of all cells in the RAM. Therefore, we assume that the average cycle time is the same for all cells. If the differences in T between individual cells were significant, the cell length within the RAM would also vary significantly, but we know this is not the case (Ivanov, 1971; Baskin, 2000). Analysis of heterogeneity of T in sister cells within the maize RAM showed they do not vary by more than 15–20 % (Ivanov, 1971). Recent time-lapse studies in arabidopsis showed that variation in sister cell T averaged 8.3 % (von Wangenheim et al., 2017b; data extracted from their Video 3, n = 6 sister groups). Another source of heterogeneity is that some cell types (metaxylem in monocots) stop proliferating earlier, but the fraction of these cells is also not significant. Therefore, in the model, it is assumed that all cells have the same average T and that all cells proliferate. It is important to note that a reference thymidine method for determination of T also assumes that all cells proliferate, and only average T for all the meristem is determined. The use of live-cell imaging and high-resolution visualization of vertically grown roots (Maizel et al., 2011; von Wangenheim et al., 2017a, b) will reveal how close the results obtained by the two methods are to reports of T determinations based on time-lapse studies (see also Table S2).
Another important aspect of this work is related to the identification of the proximal meristem boundary. We defined the RAM as an area that includes the proliferation and transition domains, where cells proliferate more actively in the former than in the latter (Ivanov and Dubrovsky, 2013). However, dividing cells are indeed present in the transition domain and recent work on arabidopsis confirms this notion (Lavrekha et al., 2017). When T is determined by the thymidine method, all RAM cells, counting those in the transition domain, are included in the analysis. As the goal of this study was to compare the RCP method with the thymidine method, the number of RAM cells was determined for both domains instead of only the proliferation domain. Clearly, this can be one of the limitations of the RCP method used here. Strictly speaking, as the method applies an exponential model, only the proliferation domain of the RAM should be considered and eqn (1) should take into account the number of cells in the proliferation domain and not the total number of meristematic cells. This corrected approach was successfully used for determination of T in arabidopsis (see below). The main result of the current study is a good overall agreement in T values determined using the RCP and thymidine methods; the difference for all the species was not statistically significant (P > 0.05, Student’s t-test) and T determined by the RCP method was on average only 10 % greater than that determined by the 3H-thymidine method. This suggests that the exponential model can indeed be applied to the entire RAM, at least in practical terms, when determining the average T in the RAM. It is important to underline that the RCP approach permits estimation of average T values and does not reflect possible differences between different cell types, cell locations within the RAM and variations in T between sister cells.
Table 1 includes a comparison of the methods for the 73 species for which determinations by the thymidine method are available. Note that some data available in the literature lack certain details (plant age, temperature, growth conditions) and the results might not be comparable. The T values obtained by the RCP method differed significantly from published data for Epilobium hirsutum. The T value for this species determined by the thymidine method was obtained from a review by Francis et al. (2008), who cite a PhD thesis that is not publicly available. Data for another outlier, Tropaeolum majus, were taken from Olszewska et al. (1990), but no details were provided in their study. Our results obtained by the RCP method for this species were consistent for three different varieties and differed by only 15.6 % (Table 3). Similarly, for adventitious roots of Ornithogalum umbellatum, no data were available for parameters such as bulb age and root growth dynamics (Tagliasacchi et al., 1983). One possible explanation for why all outliers among T values determined by the RCP method were larger than previously reported values is that in these studies, no root growth dynamics were evaluated, and T was determined during the root growth acceleration stage.
It is also interesting to compare the results of T determinations by the RCP method with those of other methods used in the model plant A. thaliana. This species was not included in our current analysis because, although many reports of T determinations are available, the only data obtained by the thymidine method are for young seedlings, and the RCP method cannot be applied to plants of a similar age (Table S2). A single T determination was obtained by the thymidine method for arabidopsis roots of seedlings on the first day after germination (Van’t Hof et al., 1978), when no steady-state growth has been attained (Table S2). Since in the reported studies using the RCP method, T was estimated at later stages, a pertinent comparison with the thymidine method is not possible. Details of T estimation in arabidopsis by the RCP method can be found (Napsucialy-Mendivil et al., 2014; López-Bucio et al., 2014). In most studies, T values obtained by the RCP method use the number of cells in the proliferation domain for eqn (1); however, some studies use the number of cells in the entire RAM (Table S2). Taking into account estimations obtained using other methods, irrespective of ecotype, the average T in roots of arabidopsis seedlings aged 6–12 d after germination (dag) grown at 20–23 °C is 16.6 ± 0.8 h, which is comparable to the average T of 15.0 ± 1.2 h (in both samples n = 8, mean ± s.e., P > 0.05 Student’s t-test, Table S2) estimated by the RCP method for 5–8 dag arabidopsis seedlings grown at the same temperature when the number of cells was determined in the proliferation domain. Interestingly, the T values were very close for the same ecotype (Col-0) at the same temperature (23 °C) and age (5 dag), i.e. 14.3 h in time-lapse studies (Yin et al., 2014; von Wangenheim et al., 2017) and 16.4 h estimated by the RCP method (Table S2). These studies show that, similar to other species, an exponential model of cell proliferation is appropriate for estimating cell cycle duration in the arabidopsis RAM.
In summary, our results indicate that in 96 % of the species examined, T values in the RAM determined by the thymidine and RCP methods were very similar. This indicates that the RCP method is a reliable, straightforward approach that can be used to investigate numerous subjects. Notably, T is a highly stable parameter, as estimations for a single species grown under different conditions and in different countries, years and varieties produced highly similar values. Our data also indicate that T can be considered a species-specific feature. The duration of the cell cycle in the RAM is an important root trait. Analysis of this trait will provide insight into root growth mechanisms and their endogenous and exogenous control and into the general evolutionary ecology and phenomics of roots. However, this trait has not yet been used in large-scale analyses investigating these processes (Walter and Schurr, 2005; Furbank and Tester, 2011; Comas et al., 2012; Maherali, 2017; Valverde-Barrantes et al., 2017). The application of the RCP method may help fill this gap.
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and consist of the following. Table S1: Results of independent experiments for determinations of T by the RCP method (n = 8, mean ± s.e.). Table S2: Cell cycle duration in wild type Arabidopsis thaliana L. (Heynh) root apical meristem determined by different methods.
ACKNOWLEDGEMENTS
V.B.I. and J.G.D. express their deepest gratitude to Peter Barlow for all his encouragement, understanding and invaluable support throughout more than 30 years of our friendship, scientific collaboration and idea exchange. We thank Аnna Kozhevnikova, Vadim Volkov, Anna Ivanova, Victoria Mironova and Sonia Barlow for their valuable comments on previous versions of the manuscript and two anonymous reviewers for their valuable comments. We very much appreciate the help of all those who donated the seeds used in this study, especially personnel from the Vavilov Research Institute of Plant Industry in Saint Petersburg, Russia; Tsytsin Main Botanical Garden of the Russian Academy of Sciences (RAS), Moscow, Russia; Botanical Institute of the RAS, Saint Petersburg, Russia; Royal Botanical Gardens, Kew, UK; Bonn University Botanic Gardens, Bonn, Germany; and Leibniz-Institute of Plant Genetics and Crop Plant Research IPK in Gatersleben, Germany. We also thank Kathleen Farquharson for editing the English text. This work was supported by the Russian Foundation for Basic Research (Grant RFBR- 15-04-02502a to V.B.I), Consejo Nacional de Ciencia y Tecnología, Mexico (CONACyT, Grant 237430 to J.G.D.), UNAM-DGAPA-PAPIIT (IN205315 to J.G.D.) and UNAM Academic Exchange Program.
LITERATURE CITED
- Antosiewicz D. 1990. Analysis of the cell cycle in the root meristem of Allium сера under the influence of ledakrin. Folia Histochemica et Cytobiologica 28: 79–95. [PubMed] [Google Scholar]
- Arcara PG, Nuti RV. 1967. Effect of ethyl alcohol on the mitotic cycle of Allium сера root meristems. Caryologia 20: 229–232. [Google Scholar]
- Balodis VA, Ivanov VB. 1970. Study of the multiplication of cells in the roots upon transfer from meristems to tension area. Tsitologiya 12: 983–992 (in Russian). [Google Scholar]
- Baluška F, Mancuso S, Volkmann D, Barlow PW. 2010. Root apex transition zone: a signalling-response nexus in the root. Trends in Plant Science 15: 402–408. [DOI] [PubMed] [Google Scholar]
- Barlow PW. 1973. Mitotic cycles in root meristems. In: Billett FS, ed. The cell cycle in development and differentiation. Cambridge: Cambridge University Press, 133–165. [Google Scholar]
- Barlow PW. 1976a. The effect of ethylene on root meristems of Pisum sativum and Zea mays. Planta 131: 235–243. [DOI] [PubMed] [Google Scholar]
- Barlow PW. 1976b. Towards an understanding of the behaviour of root meristems. Journal of Theoretical Biology 57: 433–451. [DOI] [PubMed] [Google Scholar]
- Barlow PW. 1976c. The concept of the stem cell in the context of plant growth and development. In: Lord BI, Potten CS, Cole RJ, eds. Stem cells and tissue homeostasis. Cambridge: Cambridge University Press, 87–113. [Google Scholar]
- Barlow PW. 1987. Cellular packets, cell division and morphogenesis in the primary root meristem of Zea mays L. New Phytologist 105: 27–56. [DOI] [PubMed] [Google Scholar]
- Barlow PW. 1992. The meristem and quiescent centre in cultured root apices of the gib-1 mutant of tomato (Lycopersicon esculentum Mill.). Annals of Botany 69: 533–543. [Google Scholar]
- Barlow PW. 1994. Structure and function at the root apex - phylogenetic and ontogenetic perspectives on apical cells and quiescent centres. Plant and Soil 167: 1–16. [Google Scholar]
- Barlow PW. 1997. Stem cells and founder zones in plants, particularly their roots. In: Potten CS, ed. Stem cells. London: Academic Press. [Google Scholar]
- Barlow PW. 2015a. The concept of the quiescent centre and how it found support from work with X-rays. I. Historical perspectives. Plant Root 9: 43–55. [Google Scholar]
- Barlow PW. 2015b. The concept of the quiescent centre and how it found support from work with X-rays. II. The molecular aftermath. Plant Root 9: 56–67. [Google Scholar]
- Barlow PW. 2016. Origin of the concept of the quiescent centre of plant roots. Protoplasma 253: 1283–1297. [DOI] [PubMed] [Google Scholar]
- Barlow PW, MacDonald PDM. 1973. An analysis of the mitotic cell cycle in the root meristem of Zea mays. Proceedings of the Royal Society of London B: Biological Sciences 183: 385–398. [Google Scholar]
- Barlow PW, Pilet PE. 1981. Hormonal regulation of cell proliferation in the quiescent centre of maize roots. Letcombe Laboratory Annual Report 62. [Google Scholar]
- Barlow PW, Pilet PE. 1984. The effect of abscisic acid on cell growth, cell division and DNA synthesis in the maize root meristem. Physiologia Plantarum 62: 125–132. [Google Scholar]
- Barlow PW, Woodiwiss HV. 1992. A comparison of the effectiveness of colchicine and oryzalin in estimating cell doubling times in a root meristem. Cytobios 72: 177–190. [Google Scholar]
- Barlow PW, Brain P, Parker JS. 1991. Cellular growth in roots of a gibberellin-deficient mutant of tomato (Lycopersicon esculentum Mill.) and its wild-type. Journal of Experimental Botany 42: 339–351. [Google Scholar]
- Baskin TI. 2000. On the constancy of cell division rate in the root meristem. Plant Molecular Biology 43: 545–554. [DOI] [PubMed] [Google Scholar]
- Baumann ТW. 1972. Der Mitosezyklus in diploiden und triploiden Wurzelmeristem von Scilla sibirica. Experientia 29: 860–862 (in German). [Google Scholar]
- Baуliss МW. 1975. The duration of the cell cycle of Daucus carota L. in vivo and in vitro. Experimental Cell Research 92: 31–38. [DOI] [PubMed] [Google Scholar]
- Beemster GTS, Baskin TI. 1998. Analysis of cell division and elongation underlying the developmental acceleration of root growth in Arabidopsis thaliana.Plant Physiology 116: 1515–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beemster GTS, Baskin TI. 2000. STUNTED PLANT 1 mediates effects of cytokinin, but not of auxin, on cell and expansion in the root of Arabidopsis. Plant Physiology 124: 1718–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benbadis MC. 1970. Proliferation et differentiation cellulaires dans le meristeme radicularie d’Allium sativum L.: action chloramphenicol. Bulletine de la Société Botanique de France 117: 125–144. [Google Scholar]
- Bennett MD, Finch RA. 1972. The mitotic cycle time of root meristem cells of Hordeum vulgare. Caryologia 25: 439–444. [Google Scholar]
- Berta G, Tagliasacchi AM, Fansconi A, Gerlero D, Trotta A, Scannerini S. 1991. The mitotic cycle in root apical meristems of Allium porrum L. is controlled by the endomycorrhizal fungus Glomus sp. strain E3. Protoplasma 161: 12–16. [Google Scholar]
- Bogdanov YF. 1967. Synthesis of DNA and the composition of cellular population in the embryos of seeds in connection with the types of chromosomal mutations (based on the example of Pisum sativum). PhD Thesis, University of Novosibirsk; (in Russian). [Google Scholar]
- Bogdanov YF, Lyapunova NA, Sherudilo AI. 1967. Cytophotometric and autoradiographic analysis of the composition of cellular population in the meristem of pea seeds and roots. Tsitologiya 9: 569–576 (in Russian). [Google Scholar]
- Bӧsen H, Nagl W. 1978. Short duration of the mitotic and endomitotic cell cycle in the heterochromatin-rich monocot Allium carinatum. Cell Biology International Reports 2: 565–571. [DOI] [PubMed] [Google Scholar]
- Bryant TR. 1969. DNA synthesis and cell division in germinating onion. I. Onset of DNA synthesis and mitosis. Caryologia 22: 127–137. [Google Scholar]
- Burholt R, Van’t Hof J. 1971. Quantitative thermal-induced changes in growth and cell population kinetics of Helianthus roots. American Journal of Botany 58: 386–393. [Google Scholar]
- Campilho A, Garcia B, Wijk HV, Campilho A, Scheres B. 2006. Time‐lapse analysis of stem‐cell divisions in the Arabidopsis thaliana root meristem. The Plant Journal 48: 619–627. [DOI] [PubMed] [Google Scholar]
- Choudhury HC. 1969. Late DNA replication pattern in sex chromosomes of Melandrium.Canadian Journal of Genetics and Cytology 11: 192–198. [DOI] [PubMed] [Google Scholar]
- Clowes FAL. 1971. The proportion of cells that divide in root meristem of Zea mays L. Annals of Botany 35: 249–261 [Google Scholar]
- Clowes FAL. 1976. Estimation of growth fractions in meristems of Zea mays L. Annals of Botany 40: 933–938. [Google Scholar]
- Comas L, Mueller K, Taylor L, Midford P, Callahan H, Beerling D. 2012. Evolutionary patterns and biogeochemical significance of angiosperm root traits. International Journal of Plant Sciences 173: 584–595. [Google Scholar]
- Cools T, Iantcheva A, Maes S, Van den Daele H, De Veylder L. 2010. A replication stress-induced synchronization method for Arabidopsis thaliana root meristems. The Plant Journal 64: 705–714. [DOI] [PubMed] [Google Scholar]
- Creber HMC, Davies MS, Francis D. 1993. Effects of temperature on cell division in root meristems of natural populations of Dactylis glomerata of contrasting latitudinal origins. Environmental and Experimental Botany 33: 433–442. [Google Scholar]
- Daviеs POL, Rees H. 1975. Mitotic cycle in Triticum species. Heredity 35: 337–345. [Google Scholar]
- Demchenko NP. 1976. Duration of mitotic cycle, its periods and mitosis in the cells of dermatogen and periblem of the roots of wheat. Tsitologiya 18: 16–21 (in Russian). [Google Scholar]
- Deweу DL, Howard A. 1963. Cell dynamics in the bean root tip. Radiation Botany 3: 259–263. [Google Scholar]
- Deysson G, Bonaly J. 1970. Modifications experimentales du cycle cellulaire etudies sur le meristeme radiculaire d’Allium sativum L. Annales Pharmaceutiques Françaises 28: 605–614. [PubMed] [Google Scholar]
- Dubrovsky JG, Barlow PW. 2015. The origins of the quiescent centre concept. New Phytologist 206: 493–496. [DOI] [PubMed] [Google Scholar]
- Dubrovsky JG, Ivanov VB. 2003. Celebrating 50 years of the cell cycle. Nature 426: 759. [DOI] [PubMed] [Google Scholar]
- Dubrovsky JG, Contreras-Burciaga L, Ivanov VB. 1998. Cell cycle duration in the root meristem of Sonoran Desert Cactaceae as estimated by cell-flow and rate-of-cell production methods. Annals of Botany 81: 619–624. [Google Scholar]
- Dubrovsky JG, Doerner PW, Colón-Carmona A, Rost TL. 2000. Pericycle cell proliferation and lateral root initiation in Arabidopsis. Plant Physiology 124: 1648–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essad S. 1973. Variations du cycle mitotique et des teneurs en ADN chez Vicia sativa L. mise en evidence de liaisons entre les durees des phases du cycle mitotique dans differents groups botaniques. Caryologia 26: 357–374. [Google Scholar]
- Essad S, Maunoury C. 1979. Kinetic and instantaneous characteristic related to heterosis and inbeeding in Zea mays L. Ann. Amelior. Plantes 29: 689–698. [Google Scholar]
- Evans GM, Rees H. 1971. Mitotic cycles in Dicotyledons and Monocotyledons. Nature 233: 350–351. [DOI] [PubMed] [Google Scholar]
- Evans GM, Rees H, Snell CL, Sun S. 1972. The relationship between nuclear DNA amount and time duration of the mitotic cycle. Chromosome Today 3: 24–31. [Google Scholar]
- Evans HJ, Savage JRK. 1963. The relation between DNA synthesis and chromosome structure as resolved by X-ray damage. Journal of Cell Biology 18: 525–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans HJ, Scott D. 1964. Influence of DNA synthesis on the production of chromatid aberrations by X-rays and maleic hydrazide in Vicia faba. Genetics 49: 17–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans LS, Van’t Hоf J. 1975. The age-distribution of cell cycle populations in plant root meristems. Experimental Cell Research 90: 401–410. [DOI] [PubMed] [Google Scholar]
- Filippenko VN. 1983. On the possibility of determining of lines and time parameters of cell differentiation in root meristem by radiomitostatic method (based on the example of cell differentiation in the rhizoderma). In: Gudkov IN, ed. Cell cycle in plants. Kiev: Naukova Dumka, 51–58 (in Russian). [Google Scholar]
- Fiorani F, Beemster G. 2006. Quantitative analyses of cell division in plants. Plant Molecular Biology 60: 963–979. [DOI] [PubMed] [Google Scholar]
- Francis D, Barlow PW. 1988. Temperature and the cell cycle. Symposia of the Society for Experimental Biology 181–201. [PubMed] [Google Scholar]
- Francis D, Davies MS, Barlow PW. 2008. A strong nucleotypic effect on the cell cycle regardless of ploidy level. Annals of Botany 101: 747–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furbank RT, Tester M. 2011. Phenomics–technologies to relieve the phenotyping bottleneck. Trends in Plant Science 16: 635–644. [DOI] [PubMed] [Google Scholar]
- Gahan PВ, Hurst PR. 1976. Effects of ageing on the cell cycle in Zea mays. Annals of Botany 40: 887–890. [Google Scholar]
- Gahan PB, Viola-Magni MP, Cave CF. 1986. Autoradiographic study of the turnover of chromatin associated phospholipids in Vicia faba L. Caryologia 39: 281–285. [Google Scholar]
- Ganassi EE. 1978. Radiation damage and the reparation of chromosomes. PhD Thesis, Puschino State Institute of Natural Sciences; (in Russian). [Google Scholar]
- Garay‐Arroyo A, Ortiz‐Moreno E, de la Paz Sánchez M et al. 2013. The MADS transcription factor XAL2/AGL14 modulates auxin transport during Arabidopsis root development by regulating PIN expression. The EMBO Journal 32: 2884–2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Generalova MV. 1969. Determination of the time parameters of the mitotic cycle of root meristems of Crepis capillaris. Genetika 5: 48–51 (in Russian). [Google Scholar]
- Gimenez-Abian MJ, De la Torre C, Lopez-Saez JE. 1987. Growth and cell proliferation in Allium roots at different oxygen tensions. Environmental and Experimental Botany 27: 233–23. [Google Scholar]
- Giménez-Martín G, González-Fernández A, López-Sáez J. 1965. A new method of labeling cells. The Journal of Cell Biology 26: 305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Fernández A, Giménez-Martín G, Díez JL, de la Torre C, López-Sáez JF. 1971. Interphase development and beginning of mitosis in the different nuclei of polynucleate homokaryotic cells. Chromosoma 36: 100–111. [Google Scholar]
- Grant CJ, Heslot H. 1965. Remaniements chromosomiques iduits par les nitrosamines chez Vicia faba et leur relation avec le cycle mitotique. Annales de Génétique 8: 98–104. [PubMed] [Google Scholar]
- Grif VG. 1981. Application of a coefficient of temperature dependence during the study of mitotic cycle in plants. Tsitologiya 23: 166–173 (in Russian). [Google Scholar]
- Grif VG, Machs EM. 1996. The influence of the rhythm of illumination on the mitotic cycle in the root meristem of plants. Tsitologiya 38: 710–725 (in Russian). [Google Scholar]
- Grif VG, Valovich EM. 1973a. Influence of low positive temperatures on cell growth and division in germinating seed development. Tsitologiya 15: 1362–1369 (in Russian). [Google Scholar]
- Grif VG, Valovich EM. 1973b. Mitotic cycle of plant cells at minimum temperature of mitosis. Tsitologia 15: 1510–1514 (in Russian). [Google Scholar]
- Grif VG, Ivanov VB, Machs EM. 2002. Cell cycle and its parameters in flowering plants. Cytologia 44: 936–980. [PubMed] [Google Scholar]
- Gudkov IN, Grodzinsky DM. 1972. On the study of the heterosensitivity of cell cycle phases in plant meristems. Radiobiologia 12: 260 (in Russian). [Google Scholar]
- Gudkov IN, Grodzinsky DM. 1976. Role of asynchronous cellular divisions and heterogeneity of meristems in the plant radioresistance. In: Troyan VM, ed. Plant cell cycle in ontogenesis. Kiev: Naukova Dumka, 110–137 (in Russian). [Google Scholar]
- Gudkov IN, Grodzinsky DM, Grupina SA. 1971. Method of autoradiography in the study of cell cycle kinetics in meristems of higher plants during the gamma-irradiation. Fisiologiya i Biochimiya kul’turnyh rasteniy 3: 115–121 (in Russian). [Google Scholar]
- Gudkov IN, Peterova SA, Zezina NV. 1974. Temperature effect on the duration of the phases of the mitotic cycle in meristematic cells of roots of pea and its radioresistance. Fisiologiya i Biochimiya kul’turnyh rasteniy 6: 257–262 (in Russian). [Google Scholar]
- Gupta SВ. 1969. Duration of mitotic cycle and regulation of DNA replication in Nicotiana plumbaginifolia and a hybrid derivative of N. tabacum showing chromosome instability. Canadian Journal of Genetics and Cytology 11: 133–142. [Google Scholar]
- Hayashi K, Hasegawa J, Matsunaga S. 2013. The boundary of the meristematic and elongation zones in roots: endoreduplication precedes rapid cell expansion. Scientific Reports 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hejnowicz Z. 1959. Growth and cell division in apical meristem of wheat root. Physiologia Plantarum 12: 124–136. [Google Scholar]
- Howard A, Pelс SR. 1953. Synthesis of desoxyribonucleic acid in normal and irradiated cells and its relation to chromosome breakage. Heredity suppl. 6: 261–273. [Google Scholar]
- Ivanov VB. 1968. The growth of corn seedling root cells irradiated with high x-ray doses. II. Cell growth with a complete blocking of mitoses. Tsitologiya 10: 1105–1117 (in Russian). [PubMed] [Google Scholar]
- Ivanov V. 1971. Critical size of the cell and its transition to division. l. sequence of transition to mitosis for sister cells in the corn seedling root tip. Soviet Journal of Developmental Biology 2: 421–428. [Google Scholar]
- Ivanov VB. 1974. The cellular bases of plant growth. Moscow: Nauka; (in Russian). [Google Scholar]
- Ivanov VB. 1987. Proliferatsia kletok v rasteniyakh (Cell proliferation in plants). Moscow: VINITI (in Russian). [Google Scholar]
- Ivanov VB. 1994. Root growth responses to chemicals. Soviet Scientific Review, Ser. Biol. 13: 1–70. [Google Scholar]
- Ivanov VB, Dubrovsky JG. 1997. Estimation of the cell-cycle duration in the root apical meristem: a model of linkage between cell-cycle duration, rate of cell production, and rate of root growth. International Journal of Plant Sciences 158: 757–763. [Google Scholar]
- Ivanov VB, Dubrovsky J. 2013. Longitudinal zonation pattern in plant roots: conflicts and solutions. Trends in Plant Science 18: 237–243. [DOI] [PubMed] [Google Scholar]
- Kaltsikes PJ. 1971. The mitotic cycle in an amphidiploid (triticale) and its parental species. Canadian Journal of Genetics and Cytology 13: 656–662. [Google Scholar]
- Kaltsikes PJ. 1972. Duration of the mitotic cycle in Triticale. Caryologia 25: 537–542. [Google Scholar]
- Kaznadzei VV. 1971. Dependence of the duration of the separate stages of the mitotic cycle in the acrospire of Crepis capillaris on the dose of X-ray irradiation. Tsitolologiya i Genetika 5: 416–420 (in Russian). [Google Scholar]
- Keusсh F. 1971. Chromosomenmutationen durch ein organisches Peroxyd. Berichte der Schweizerischen Botanischen Gesellschaft 81: 180–272. [Google Scholar]
- Kidd AD, Francis D, Bennett MD. 1987. Replicon size, mean rate of DNA replication and the duration of the cell cycle and its component phases in eight monocotyledonous species of contrasting DNA C-values. Annals of Botany 59: 603–609. [Google Scholar]
- Kirschner GW, Stahl Y, Von Korff M. 2017. Unique and conserved features of the barley root meristem. Frontiers in Plant Science 8: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langridge WHR, O’ Malleу ТA, Wallace H. 1970. Neutral amphiplasty and regulation of the cell cycle in Crepis Herbs. Proceedings of the National Academy of Sciences of the USA 67: 1894–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavrekha VV, Pasternak T, Ivanov VB, Palme K, Mironova VV. 2017. 3D analysis of mitosis distribution highlights the longitudinal zonation and diarch symmetry in proliferation activity of the Arabidopsis thaliana root meristem. The Plant Journal 92: 834–845. [DOI] [PubMed] [Google Scholar]
- López-Bucio JS, Dubrovsky JG, Raya-González J et al. 2014. Arabidopsis thaliana mitogen-activated protein kinase 6 is involved in seed formation and modulation of primary and lateral root development. Journal of Experimental Botany 65: 169–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucretti S, Nardi L, Trionfetti Nisini P, Moretti F, Gualberti G, Dolezel J. 1999. Bivariate flow cytometry DNA/BrdUrd analysis of plant cell cycle. Methods in Cell Science 21: 155–166. [DOI] [PubMed] [Google Scholar]
- MacLeod RD. 1968. Changes in the mitotic cycle in lateral root meristems of Vicia faba following kinetin treatment. Chromosoma 24: 177–187. [Google Scholar]
- MacLeod RD. 1971. The response of apical meristems of primary roots of Vicia faba L. to colchicine treatments. Chromosoma 35: 217–232. [Google Scholar]
- Maherali H. 2017. The evolutionary ecology of roots. New Phytologist 215: 1295–1297. [DOI] [PubMed] [Google Scholar]
- Maizel A, von Wangenheim D, Federici F, Haseloff J, Stelzer EHK. 2011. High-resolution live imaging of plant growth in near physiological bright conditions using light sheet fluorescence microscopy. The Plant Journal 68: 377–385. [DOI] [PubMed] [Google Scholar]
- Malamy JE, Benfey PN. 1997. Organization and cell differentiation in lateral roots of Arabidopsis thaliana. Development 124: 33–44. [DOI] [PubMed] [Google Scholar]
- Marciniak K, Olszewska MJ, Osiecka R, Bialas J. 1978. Relation between DNA content and rate of cell growth during interphase in four species of Angiospermae. Acta Societatis Botanicorum Poloniae 47: 297–305. [Google Scholar]
- Matagne R. 1968. Duration of mitotic cycle and patterns of DNA replication in chromosomes of Allium сера. Caryologia 21: 209–224. [Google Scholar]
- Mazzuka S, Bitony MB, Innocenti AM, Francis D. 2000. Inactivation of DNA replication origins by the cell cycle regulator, trigonelline, in root meristems of Lactuca sativa.Planta 211: 127–132. [DOI] [PubMed] [Google Scholar]
- Montezuma-de-Carvalho J. 1962. The period of DNA synthesis in the mitotic cycle of Luzula purpurea. Boletim da Sociedade Broteriana 36: 179–188. [Google Scholar]
- Morcillo G, Krimer DB, De la Torre C. 1978. Modification of nuclear components by growth temperature in meristems. Experimental Cell Research 115: 95–102. [DOI] [PubMed] [Google Scholar]
- Moretti F, Lucretti S, Dolezel J. 1992. Plant cell cycle analysis on isolated nuclei using a monoclonal antibody against Brd Urd. European Journal of Photochemistry 36: 367. [Google Scholar]
- Müller ML, Pilet PE, Barlow PW. 1993. An excision and squash technique for analysis of the cell cycle in the root quiescent centre of maize. Physiologia Plantarum 87: 305–312. [Google Scholar]
- Müller ML, Barlow PW, Pilet PE. 1994. Effect of abscisic acid on the cell cycle in the growing maize root. Planta 195: 10–16. [Google Scholar]
- Nagl W. 1974. Mitotic cycle time in perennial and annual plants with various amount of DNA and heterochromatin. Developmental Biology 39: 342–346. [DOI] [PubMed] [Google Scholar]
- Nagl W. 1978. Endopolyploidy and polyteny in differentiation and evolution. Amsterdam: North-Holland Publishing Company. [Google Scholar]
- Napsucialy-Mendivil S, Alvarez-Venegas R, Shishkova S, Dubrovsky JG. 2014. ARABIDOPSIS HOMOLOG of TRITHORAX1 (ATX1) is required for cell production, patterning, and morphogenesis in root development. Journal of Experimental Botany 65: 6373–6384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarrete MH, Cuadrado A, Canovas JL. 1983. Partial elimination of Gl and G2 periods in higher plant cell by increasing the S period. Experimental Cell Research 148: 273–280. [DOI] [PubMed] [Google Scholar]
- Olszewska MJ, Bilecka A, Kuran H, Marciniak K, Osiecka R. 1989. The lack of correlation between the rate of rRNA transport from nuclcoli into cytoplasm and the duration of Gl and G2 phases in root meristem cells. Folia Histochemica et Cytobiologica 27: 107–119. [PubMed] [Google Scholar]
- Olszewska MJ, Bilecka A, Kuran H, Marciniak K, Jakobinski J. 1990. Dry mass and protein increase during interphase as a possible factor regulating the cell cycle duration. Caryologia 43: 43–55. [Google Scholar]
- O’Toole AJ. 1970. Effect of 5-aminouracil on cellular population kinetics of Secale cereal (rye). American Journal of Botany 57: 765–769. [Google Scholar]
- Pacheco-Escobedo MA, Ivanov VB, Ransom-Rodríguez I et al. 2016. Longitudinal zonation pattern in Arabidopsis root tip defined by a multiple structural change algorithm. Annals of Botany 118: 763–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pachter BP, Mitra J. 1977. Mitotic cycle of diploid and autotetraploid Zea mays. Nucleus 20: 302–305. [Google Scholar]
- Pareyre C, Deysson G. 1975. Activity of tubulosine on the kinetics of root meristem cell population. Cell Tissue Kinetics 8: 67–79. [DOI] [PubMed] [Google Scholar]
- Ponce G, Barlow PW, Feldman LJ, Cassab GI. 2005. Auxin and ethylene interactions control mitotic activity of the quiescent centre, root cap size, and pattern of cap cell differentiation in maize. Plant, Cell and Environment 28: 719–732. [DOI] [PubMed] [Google Scholar]
- Powell MJ, Davies MS, Francis D. 1986. The influence of zinc on the cell cycle in the root meristem of zinc-tolerant and non-tolerant cultivar of Festuca rubra. New Phytologist 102: 419–428. [DOI] [PubMed] [Google Scholar]
- Prasad AB, Gоdward MBE. 1965. Comparison of the development responses of diploid and tetraploid Phalaris following irradiation of the dry seed. Radiation Botany 5: 465–474. [Google Scholar]
- Price HJ, Bachmann K. 1976. Mitotic cycle time and DNA content in annual and perennial Microseridinaea (Compositae, Cichoriaceae). Plant Systematics and Evolution 126: 323–330. [Google Scholar]
- Quastler H, Sherman F. 1959. Cell population kinetics in the intestinal epithelium of the mouse. Experimental Cell Research 17: 420–438. [DOI] [PubMed] [Google Scholar]
- Reckless DM. 1995. The effect of temperature on growth and development and cell division of Vicia faba and Glycine max roots. PhD Thesis, University of Wales, Cardiff. [Google Scholar]
- Seyhodjaev AI. 1971. Study of mitotic cycles in the primary roots in diploid and tetraploid buckwheats. Tsitologia 13: 62–68 (in Russian). [Google Scholar]
- Sharp RE, Silk WK, Hsiao TC. 1988. Growth of the maize primary root at low water potentials. I. Spatial distribution of expansive growth. Plant Physiology 87: 50–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shishkova S, Rost TL, Dubrovsky JG. 2008. Determinate root growth and meristem maintenance in angiosperms. Annals of Botany 101: 319–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk WK, Lord EM, Eckard KJ. 1989. Growth patterns inferred from anatomical records empirical tests using longisections of roots of Zea mays L. Plant Physiology 90: 708–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava HK, Levania UC. 1978. Duration of the mitotic cycle and its component phases in Brassica juncea. Journal of Heredity 69: 355–357. [Google Scholar]
- Svarinskaya RA, Gavrilova NS. 1976. The action of gibberelin on the duration of mitotic cycle and the intensity of DNA synthesis in cells of barley. Genetika 12: 30–36 (in Russian). [Google Scholar]
- Tagliasacchi AM, Berta G, Fasconi A. 1983. Duration of the mitotic cycle of Ornithogalum umbellatum as measured by H3-thymidine in the root meristem. Caryologia 36: 189–193. [Google Scholar]
- Tapia-López R, García-Ponce B, Dubrovsky JG et al. 2008. An AGAMOUS-related MADS-box gene, XAL1 (AGL12), regulates root meristem cell proliferation and flowering transition in Arabidopsis. Plant Physiology 146: 1182–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas HC. 1992. Effects of manganese and phosphorus on cellular aspects of growth in contrasting species. PhD Thesis, University of Wales, Cardiff. [Google Scholar]
- Tiţsu H. 1967. Durata cicluli mitotic şi a perioadei de sinteză a AND la tomatele haploide şi diploide (Lycopersicon esculentum Mill.). Studii şi cercatări de Biologie, seria botanică 19: 165–172. [Google Scholar]
- Titsu H, Pороviсi I. 1970. Duration of mitotic cycle phases in the radicular meristem of diploid and tetraploid sugar beet (Beta vulgaris). Review Roumain de Biologie, Serie de Botanique 15: 51–56. [Google Scholar]
- Tоdorоva JG, Ronchi VN. 1969. The mitotic cycle time of root meristem cells of Helianthus annuus. Caryologia 22: 331–337. [Google Scholar]
- Torre C, Clowes FAL. 1974. Thymidine and the measurement of rates of mitosis in meristems. New Phytologist 73: 919–925. [Google Scholar]
- Urbach VY. 1964. Biometric methods. Moscow: Nauka; (in Russian). [Google Scholar]
- Valverde‐Barrantes OJ, Freschet GT, Roumet C, Blackwood CB. 2017. A worldview of root traits: the influence of ancestry, growth form, climate and mycorrhizal association on the functional trait variation of fine‐root tissues in seed plants. New Phytologist 215: 1562–1573. [DOI] [PubMed] [Google Scholar]
- Van der Weele CM, Jiang HS, Palaniappan KK, Ivanov VB, Palaniappan K, Baskin TI. 2003. A new algorithm for computational image analysis of deformable motion at high spatial and temporal resolution applied to root growth. Roughly uniform elongation in the meristem and also, after an abrupt acceleration, in the elongation zone. Plant Physiology 132: 1138–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van’t Hof J. 1963. DNA, RNA and protein synthesis in the mitotic cycle of pea root meristem cells. Cytologia 28: 30–35. [Google Scholar]
- Van’t Hof J. 1965. Relationships between mitotic cycle duration, S period duration and the average rate of DNA synthesis in the root meristem cells of several plants. Experimental Cell Research 39: 48–58. [DOI] [PubMed] [Google Scholar]
- Van’t Hof J. 1966. Comparative cell population kinetics of tritiated thymidine labelled diploid and colchicine-induced tetraploid cells in the soma tissue of Pisum. Experimental Cell Research 41: 274–288. [DOI] [PubMed] [Google Scholar]
- Van’t Hof J. 1967. Studies on the relationships between cell population and growth kinetics of root meristem. Experimental Cell Research 46: 335–347. [DOI] [PubMed] [Google Scholar]
- Van’t Hof J, Sparrow AH. 1963. A relationship between DNA content, nuclear volume, and minimum mitotic cycle time. Proceedings of the National Academy of Sciences USA 49: 897–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van’t Hof J, Wilson G, Colon A. 1960. Studies on the control of mitotic activity. Chromosoma 11: 313–321. [DOI] [PubMed] [Google Scholar]
- Van’t Hof J, Kuniyuki A, Bjerknes C. 1978. The size and number of replicon families of chromosomal DNA of Arabidopsis thaliana. Chromosoma 68: 269–285. [Google Scholar]
- Verbelen JP, De Cnodder T, Le J, Vissenberg K, Baluška F. 2006. The root apex of Arabidopsis thaliana consists of four distinct zones of growth activities: meristematic zone, transition zone, fast elongation zone and growth terminating zone. Plant Signalling and Behaviour 1: 296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma RS. 1980. The duration of G1, S, G2 and mitosis at four different temperatures in Zea mays L. as measured with H3-thymidine. Cytologia 45: 327–333. [Google Scholar]
- Verma RS, Lin MS. 1978. Chemically induced alterations of the nuclear cycle and chromosomes in root meristem cells of maize. Journal of Heredity 69: 285–294. [Google Scholar]
- Verma RS, Lin MS. 1979. The duration of DNA synthetic (S) period in Zea mays: a genetic control. Theoretical and Applied Genetics 54: 277–282. [DOI] [PubMed] [Google Scholar]
- Walter A, Schurr U. 2005. Dynamics of leaf and root growth: endogenous control versus environmental impact. Annals of Botany 95: 891–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Wangenheim D, Hauschild R, Fendrych M, Barone V, Benkova E, Friml J. 2017a. Live tracking of moving samples in confocal microscopy for vertically grown roots. eLife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Wangenheim D, Hauschild R, Friml J. 2017b. Light sheet fluorescence microscopy of plant roots growing on the surface of a gel. Journal of Visualized Experiments: JoVE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster PL, Davidson D. 1968. Evidence from thymidine-H3 labeled meristems of Vicia faba of two cell populations. Journal of Cell Biology 39: 332–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster PL, MacLeod RD. 1980. Characteristics of root apical meristem cell population kinetics: a review of analyses and concepts. Environmental and Experimental Botany 20: 335–358. [Google Scholar]
- Wimber DE. 1960. Duration of the nuclear cycle in Tradescantia paludosa root tip as measured with H3-tymidine. American Journal of Botany 47: 828–843. [PubMed] [Google Scholar]
- Wimber DE. 1966. Duration of the nuclear cycle in Tradescantia root tips at three temperatures as measured with H3-thymidine. American Journal of Botany 53: 21–24. [PubMed] [Google Scholar]
- Wimber DE, Quastler A. 1963. A 14C- and 3H-thymidine double labelling technique in the study of cell proliferation in Tradescantia root tip. Experimental Cell Research 30: 8–22. [Google Scholar]
- Yang DP, Dodson EO. 1970. The amounts of nuclear DNA and the duration of DNA synthetic period (S) in related diploid and autotetraploid species of Oats. Chromosoma 31: 309–320. [Google Scholar]
- Yang X, Dong G, Palaniappan K, Mi G, Baskin TI. 2017. Temperature‐compensated cell production rate and elongation zone length in the root of Arabidopsis thaliana. Plant, Cell and Environment 40: 264–276. [DOI] [PubMed] [Google Scholar]
- Yin K, Ueda M, Takagi H et al. 2014. A dual‐color marker system for in vivo visualization of cell cycle progression in Arabidopsis. The Plant Journal 80: 541–552. [DOI] [PubMed] [Google Scholar]
- Zuk J. 1969. Autoradiographic studies in Rumex with special reference to sex chromosomes. In: Darlington CD, Lewis KR, eds. Chromosomes today, vol. 2 Edinburgh: Oliver and Boyd, 183–188. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


