Abstract
Background
Roots are continuously exposed to mechanical pressure and this often results in their morphological modification. Most obvious are changes in the overall form of the root system as well as in the shapes of particular roots. These changes are often accompanied by modifications of the cell pattern and cell morphology.
Scope
This review focuses on the morphological responses of roots to mechanical stress. Results of early and recent experiments in which roots have been exposed to mechanical pressure are assembled, analysed and discussed. Research applying different experimental sets, obstacles, media of various compactness and structure are reviewed. An effect of the combination of mechanical stresses with other abiotic stresses on roots, and results of estimating the force exerted by the roots are briefly discussed. Possible consequences of the cell pattern rearrangements are considered.
Conclusions
Several modifications in root morphology are commonly reported: (1) decreased root size, (2) radial swelling accompanied by increased radial dimension of the cortex cell layers and (3) enhanced cap cell sloughing. Nevertheless, because of differences between species and individual plants, a universal scenario for root morphological changes resulting from externally applied pressures is not possible. Thus, knowledge of the root response to mechanical impedance remains incomplete. Studies on the mechanical properties of the root as well as on possible modifications in cell wall structure and composition as the elements responsible for the mechanical properties of the plant tissue are required to understand the response of root tissue as a biomaterial.
Keywords: Plant root deformation, altered root branching, root swelling, effect of mechanical impedance, response to stress, cell pattern modification
INTRODUCTION
In its natural environment – soil – a growing root tip encounters obstacles and soil particles that in some cases might be displaced while in other cases might inhibit further growth. In either case, however, the tip experiences mechanical pressure that to some degree affects all of root development and morphology. Root development and the general condition of the root system clearly have a crucial influence on whole plant growth and productivity (Lynch, 1995). For maximum use of plants we still need to learn more about the roots and about the ways to utilize this knowledge in agriculture and other areas of human activity. We therefore need to understand the different processes leading to proper development of the root, including its response to mechanical stresses.
As both a living object and a physical body the root may respond to mechanical pressure in a complex way. In mechanics a physical body deforms under mechanical stress. For example, an elastic rod undergoes buckling and/or becomes thicker in response to compression while it becomes thinner in response to stretching during application of an axially orientated force. The body is termed elastic if the change of the form is reversible – as long as the change is instant. A viscous body also deforms under mechanical stress, but in this case deformation is not immediate. If the change in form is not reversible after removing the source of the mechanical stress the physical body is termed plastic (Meyers and Chawla, 2009). Most materials, plant tissues among them, are neither completely plastic nor completely elastic or viscous (Niklas, 1992) but have features of all three kinds of materials. However, we should see the difference between deformation of an inanimate object as a passive reaction to mechanical stress and the morphological response of a living plant organ as an active process that involves adaptive and/or defensive mechanisms. Usually, it is difficult to determine if the reaction is passive or active, and in most cases it may be a combination of the two. Here we attempt to consider the character of the root response.
There are no literature reviews focusing on the morphological aspects of roots as either biological or physical objects, especially those reported in recent years. In their comprehensive review, Barley and Greacen (1967) considered soil and its properties as a source of mechanical impedance and discussed soil’s influence on the growth of roots. The excellent review by Atwell (1993) covers morphological and physiological responses of roots. However, it refers to studies from more than 20 years ago. The work by Clark et al. (2003) only briefly reviews morphological responses. Contemporary surveys mostly consider other aspects, such as different techniques for studying the influence of soil compaction on root growth (Tracy et al., 2011), the effect of various types of stress on root elongation (Bengough et al., 2011) or correlation between soil conditions and cereal root system architecture (Rich and Watt, 2013).
Roots are physical bodies; however, as living plant organs they sense any change in the environment and are able to activate various processes of adaptation. Our goal here is to assemble data on morphological modifications of roots in response to mechanical stress with respect to the dual character of roots. We discuss the results concerning changes in the morphology of roots and the root system, modifications to the root internal structure, the root cap response to mechanical stress, the role of the growth regulatory factor ethylene, interactions between mechanical stress and other abiotic stress factors and estimation of root growth pressure. Finally, we draw conclusions on the role and possible advantages of the change in root form and anatomy.
MECHANICAL FORCE CHANGES THE PHYSICAL BODY FORM: EFFECT ON MORPHOLOGY OF ROOT AND ROOT SYSTEM
Morphological traits of the root system, such as its size, the number of branches and their spatial distribution depend on the plant species and environmental conditions. But these features also influence the acquisition of soil resources. Moreover, a developed and properly branched root system provides the whole plant with stability.
Medium density and structure
Experiments in which plants had been grown in compact medium showed that changes usually involved a reduction in the speed of axial growth (Abdalla et al., 1969; Azam et al., 2014; Colombi et al., 2017), in the length of individual roots (Cook et al., 1996; Konôpka et al., 2009; Bécel et al., 2012; Lipiec et al., 2012; Loades et al., 2013; Chen et al., 2014; Colombi et al., 2017) and consequently in root system size (Mulholland et al., 1996a; Grzesiak, 2009). Commonly observed was radial swelling of the roots (Atwell, 1988; Bengough and Mullins, 1991; Sarquis et al., 1991; Kirby and Bengough, 2002; Alameda et al., 2012; Azam et al., 2014; Chen et al., 2014; Loades et al., 2015; Colombi et al., 2017) and occasionally a decrease in the number of roots occurred (Iijima and Kono, 1991; Grzesiak, 2009) (for details see Table 1). These morphological responses of the root to dense medium play an important role in facilitating growth under such unfavourable conditions. For example, swelling of the root leads to reducing stress in the front of the root apex and suppresses root buckling (Abdalla et al., 1969; Bengough et al., 2006). Some specific effects of compacted soil on root system architecture and individual root morphology depend on the plant species and the variant of the experiment applied. In various crop plants (Tsegaye and Mullins, 1994; Grzesiak, 2009; Konôpka et al., 2009; Bécel et al., 2012) and in lupin (Atwell, 1988; Chen et al., 2014) an altered pattern of root branching was observed. In maize individual roots formed local bends (Konôpka et al., 2009) and in wheat various genotypes showed significant diversity in root tip geometry under mechanical stress (Colombi et al., 2017). As was shown by Materechera et al. (1991), the roots of dicotyledons elongate faster and penetrate a dense soil more easily than those of monocotyledons, possibly because roots of the latter are characterized by smaller diameters. In general, the elongation rate is usually negatively correlated with the density of the medium (Atwell, 1988; Croser et al., 1999, 2000; Clark et al., 2001; Benigno et al., 2012) and with root penetration resistance (Bengough and Mullins, 1991; Tsegaye and Mullins, 1994).
Table 1.
Detailed specification of the effects of mechanical stress on root and root system morphology
| Effect | Stress conditions | Species | References |
|---|---|---|---|
| Reduced root system size | compacted soil |
Avena sativa
|
Schuurman (1965)
|
| compacted soil | Oryza sativa, Sorghum bicolor, Zea mays, Coix lacryma-jobi | Iijima and Kono (1991) | |
| compacted sand | Lolium perenne, Trifolium repens, Agrostis capillaris |
Cook et al. (1996) |
|
| compacted soil | Hordeum vulgare | Mulholland et al. (1996a) |
|
| compacted soil | Z. mays, ×Triticosecale | Grzesiak (2009) | |
| compacted soil | Triticum aestivum, Secale cereale, Triticosecale Wittmack, Z. mays | Lipiec et al. (2012) | |
| compacted soil |
Pisum sativum, Acacia salicina, Eucalyptus camaldulensis, E. leucoxylon, E. kochii |
Azam et al. (2014) | |
| compacted soil |
T. aestivum | Chen et al. (2014) | |
| glass beads | Z. mays | Groleau-Renaud et al. (1998) |
|
| large soil aggregates | Z. mays |
Donald et al. (1987); Alexander and Miller (1991) |
|
| pressurized glass beads | H. vulgare | Goss and Drew (1972) | |
| Reduced individual root length | compacted soil compacted sand compacted soil compacted soil compacted soil compacted sand compacted soil large soil aggregates glass beads pressurized soil/glass beads pressurized glass beads pressurized soil pressurized glass beads compression (apparatus) changed orientation |
O. sativa, S. bicolor, Z. mays, C. lacryma-jobi L. perenne, T. repens, A. capillaris Z. mays Prunus persica H. vulgare Lupinus angustifolius T. aestivum Z. mays Z. mays H. vulgare, O. sativa H. vulgare, T. aestivum, Z. mays, Beta vulgaris P. sativum Z. mays Z. mays Arabidopsis thaliana |
Iijima and Kono (1991)
Cook et al. (1996) Konôpka et al. (2009) Bécel et al. (2012) Loades et al. (2013) Chen et al. (2014) Colombi et al. (2017) Donald et al. (1987) Moss et al. (1988), Groleau-Renaud et al. (1998) Abdalla et al. (1969) Goss (1977) Castillo et al. (1982) Veen (1982) Barley (1962) Okamoto et al. (2008) |
| Increased root diameter (root swelling) | compacted soil/sand compacted soil compacted soil compacted soil/sand compacted soil compacted soil compacted soil compacted soil compacted soil large soil aggregates glass beads pressurized soil/glass beads pressurized glass beads pressurized soil/glass beads pressurized sand axial loading (apparatus) rigid tubes changed orientation |
L. angustifolius
Z. mays 22 monocotyledonous and dicotyledonous species P. sativum Glycine max Nicotiana tabacum H. vulgare P. sativum, A. salicina, E. camaldulensis, E. leucoxylon, E. kochii T. aestivum Z. mays Z. mays H. vulgare, O. sativa H. vulgare Z. mays L. angustifolius Z. mays Z. mays A. thaliana |
Atwell (1988); Chen et al. (2014) Bengough and Mullins (1991); Konôpka et al. (2009) Materechera et al. (1991) Croser et al. (1999); Kirby and Bengough (2002) Ramos et al. (2010) Alameda et al. (2012) Loades et al. (2013, 2015) Azam et al. (2014) Colombi et al. (2017) Donald et al. (1987); Logsdon et al. (1987) Moss et al. (1988) Abdalla et al. (1969) Goss and Russell (1980) Veen (1982); Sarquis et al. (1991) Hanbury and Atwell (2005) Kuzeja et al. (2001) Potocka et al. (2011) Okamoto et al. (2008) |
| Flattened roots | compacted soil compacted soil rigid tubes |
G. max
H. vulgare, Triticosecale Wittmack Z. mays |
Ramos et al. (2010)
Lipiec et al. (2012) Potocka et al. (2011) |
| Root bending/buckling | compacted soil two-layer phytagel medium two-layer gel medium artificial obstacle (horizontal barrier) rigid tubes artificial obstacle (vertical barrier) |
Z. mays (bending) A. thaliana (bending) Medicago truncatula (helical buckling) A. thaliana (bending) Z. mays (buckling) Populus deltoides × P. nigra (bending) |
Konôpka et al. (2009)
Yamamoto et al. (2008); Yan et al. (2017) Silverberg et al. (2012) Massa and Gilroy (2003) Potocka et al. (2011) Bizet et al. (2016) |
| Wavy root phenotype | changed orientation | A. thaliana | Okada and Shimura (1990); Rutherford and Masson (1996); Buer et al. (2000); Thompson and Holbrook (2004) |
|
Altered branching
Increased number of lateral roots Increased branching density Reduced number of lateral roots Decreased branching density Altered distribution of lateral roots Laterals formed close to root tip |
pressurized glass beads bending bending bending compacted soil compacted soil pressurized glass beads compacted sand compacted soil pressurized glass beads compacted sand compacted soil slope soil conditions compacted soil compacted soil pressurized glass beads |
Z. mays
Fraxinus ornus P. nigra A. thaliana P. sativum Z. mays H. vulgare L. perenne, T. repens, A. capillaris Z. mays, ×Triticosecale H. vulgare T. repens L. angustifolius Spartium junceum L. angustifolius G. max H. vulgare |
Veen (1982)
Chiatante et al. (2007) Scippa et al. (2008) Ditengou et al. (2008); Richter et al. (2009) Tsegaye and Mullins (1994) Konôpka et al. (2009) Goss and Drew (1972) Cook et al. (1996) Grzesiak (2009) Goss (1977) Cook et al. (1996) Chen et al. (2014) Chiatante et al. (2003); Lombardi et al. (2017) Atwell (1988) Ramos et al. (2010) Goss and Drew (1972); Goss (1977) |
| Reduced cap size | compacted sand pressurized glass beads artificial obstacle (horizontal barrier) |
Z. mays
H. vulgare Z. mays |
Iijima et al. (2003a) Wilson and Robards (1979) Souty and Rode (1987) |
Application of different density of topsoil and subsoil showed that roots could grow even in a very dense topsoil, although they did not penetrate into a very dense subsoil (Schuurman, 1965). Similar results were obtained by Yamamoto et al. (2008), who used two-layer phytagel medium of different concentration. In their experiment the roots did not penetrate into the high-concentration lower layer, but bent along the boundary between the layers (as is typical of an inanimate physical body at a solid barrier), while the roots penetrated the lower layer when its concentration was low (Yamamoto et al., 2008; Yan et al., 2017). Introducing another layer of phytagel of moderate concentration between the upper soft and the lower hard layers increased the degree of root penetration (Yan et al., 2017). This suggests that the moderate-concentration layer may function as an acclimation zone which allows the root tip to grow into harder layers (Yan et al., 2017). Roots of Medicago truncatula grown in two-layer hydrogel (softer upper layer and stiff lower layer) formed helices above the boundary between the layers (Silverberg et al., 2012). Such a helical shape results from a combination of mechanical buckling of the root whose growth has been halted by the hard surface and twisting of the root tip trying to penetrate the stiff medium. This specific morphological response allows axial loads to be converted to transverse loads (Silverberg et al., 2012).
Another important factor affecting root morphology is structure of the medium and its aggregate size. In a loose and porous medium roots grow rapidly and maintain their cylindrical shape, although such a medium may limit contact of the root with nutrients. In a hard medium, root growth is slower and the root itself becomes deformed, although access to potential sources of water and nutrients is easier (Passioura, 1991). Moreover, in the natural environment the structure of the medium may undergo dynamic changes due to weather, and animal and human activity. Deep ripping leading to decompaction of soil and to the change of its structure will clearly influence root architecture (Chen et al., 2014). In general, roots growing in coarse soil aggregates usually have greater diameters and are shorter than those growing in media consisting of finer aggregates (Donald et al., 1987; Logsdon et al., 1987; Alexander and Miller, 1991).
In some experiments soil has been replaced by glass beads, application of which not only simulates mechanical impedance (Groleau-Renaud et al., 1998), but also allows researchers to maintain unchanged aggregate and pore sizes as well as to quantify both impedance and growth rate (Goss and Drew, 1972). Root system architecture depends strongly on the beads’ diameter (and consequently on pore size): roots growing in larger beads are longer and form shorter laterals while those growing in smaller beads are shorter and develop a greater number of laterals (Goss and Drew, 1972). In some cases, the roots curve around the beads and lateral root primordia then form on the convex side and root hairs on the concave side (Goss and Russell, 1980). Pressure from individual beads on the root may result in local distortion on the root’s surface (Wilson and Robards, 1978). Unfortunately, the results do not allow us to infer if this local change in root shape is reversible (elastic) or permanent (plastic). Application of a controlled external mechanical pressure through the media or directly to the roots results in similar effects as for compacted soil, i.e. slower axial growth (Hanbury and Atwell, 2005) and consequently reduced length of roots (Barley, 1962; Goss, 1977; Castillo et al., 1982; Veen, 1982; Lindberg and Pettersson, 1985) as well as in root swelling (Abdalla et al., 1969; Veen, 1982; Kuzeja et al., 2001; Hanbury and Atwell, 2005).
The morphological changes, especially those concerning root system architecture (reduced root system size, modified branching pattern), but also modifications of the form of individual roots (increased diameter) probably lead to better adaptation of the living organ to unfavourable environmental conditions. On the other hand, deformations such as swelling or bending to some degree resemble deformation of a rod under compression. The root thus responds to mechanical pressure in a way similar to an inanimate physical body.
Artificial obstacles
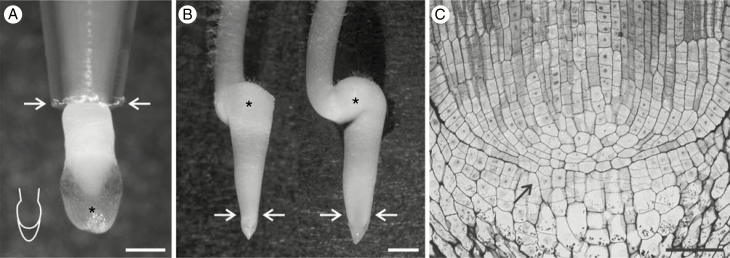
Over 60 years ago, Wiersum (1957) stated that roots are not able to grow into pores that are smaller than the root tip diameter. However, later experiments showed a high morphological adaptability of roots to extremely unfavourable growth conditions, namely pipes thinner than the roots (Scholefield and Hall, 1985; Potocka et al., 2011). In the Scholefield and Hall (1985) experiment roots of grasses were grown through sheets of steel mesh and through glass capillaries that formed a system of rigid pores. The transverse dimension of the roots was about twice the pore size, yet the roots were able to penetrate the pores, although local constriction was observed in the region where it passed through the pore. Potocka et al. (2011) forced maize root tips to grow either into tight plastic tubes of conical endings or into plastic tubes whose cross section was locally changed to an oval by use of a clip. In both variants the root tips managed to push through the narrowest part, although the morphology of the root apex was strongly modified (Fig. 1A, B). Arabidopsis roots whose vertical growth was impeded by a glass barrier underwent bending (Massa and Gilroy, 2003) as in the above-described application of a two-layered medium (Yamamoto et al., 2008). According to Bizet et al. (2016) a zone of mechanical weakness critical to the bending process is localized in the region between the growing and the mature zones of the root.
Fig. 1.
Morphology of maize root growing through tight tubes with circular (A) or oval (B) cross sections. Arrows indicate the narrowest region of the tubes. Root tip in A is covered with mucilage (asterisk); schematic drawing in the left lower corner shows the real shape of the apex (root proper + root cap). In B the front (left) and the side (right) view of the same root are shown, and root buckling is indicated by asterisks. (C) Axial section of the maize root. The root-cap boundary is broken by the RAM cells growing onto the cap side (arrow). Scale bars: 0.5 mm (A), 1 mm (B), 50 μm (C).
Changed orientation in the gravity field
Significant alterations in root system morphology occur when a growing plant experiences a changed orientation in relation to the gravity vector. Seedlings of Spartium junceum grown in containers tilted at 45° formed asymmetrical root systems whose branches failed to develop horizontally, growing up-slope and down-slope instead. Total root length increased in comparison with control plants (Chiatante et al., 2003). Arabidopsis roots respond to a changed orientation of 45° by forming a wavy growth pattern (Okada and Shimura, 1990), whose character is modulated by the growth conditions (Rutherford and Masson, 1996; Buer et al., 2000, 2003; Thompson and Holbrook, 2004). Lateral root primordia are always formed on the convex side (Fortin et al., 1989; Lucas et al., 2008), as found in mechanically bent roots (Goss and Russell, 1980; Ditengou et al., 2008; Richter et al., 2009). When grown horizontally Arabidopsis roots exhibit traits typical of organs exposed to mechanical force: they become thicker and shorter (Okamoto et al., 2008). As in Arabidopsis (Ditengou et al., 2008; Richter et al., 2009), bending tap roots of seedlings of woody plants (Chiatante et al., 2007; Scippa et al., 2008) results in a greater number of laterals. From a mechanical point of view, the convex side of the waving or bent root undergoes tension while the concave side undergoes compression. This means that when the curvature is formed the distribution of mechanical stresses within the tissue changes locally (Szymanowska-Pułka, 2013), which is followed by a chain of reactions on the cellular and molecular levels (see Richter et al., 2009 and Laskowski et al., 2008, respectively).
Effect on plant morphology
The non-impeded organs as well as whole plants whose roots are mechanically impeded do not remain unaffected (Tardieu, 1994). The plant becomes smaller (Iijima and Kono, 1991) and shoot growth slows (Cook et al., 1996; Mulholland et al., 1996a; Roberts et al., 2002), explaining the reduced shoot length (Kobaissi et al., 2013) and shoot dry weight (Donald et al., 1987; Alexander and Miller, 1991; Iijima and Kono, 1991; Grzesiak, 2009). The most frequently reported changes concern leaves. Numerous characteristics such as leaf number (Iijima and Kono, 1991; Grzesiak, 2009), leaf area (Alexander and Miller, 1991; Cook et al., 1996; Mulholland et al., 1996b; Grzesiak, 2009; Bingham et al., 2010; Kobaissi et al., 2013) and leaf elongation rates (Young et al., 1997) are decreased and stomata closure is observed (Roberts et al., 2002). The last was shown to be regulated by a root-sourced abscisic acid signal (Mulholland et al., 1996a, b; Roberts et al., 2002). Only the most important responses have been discussed in this section; the effect of mechanical stress on the morphology of above-ground plant parts is another wide topic that needs a separate review paper.
INTERNAL STRUCTURE OF THE PHYSICAL BODY CHANGES UNDER MECHANICAL STRESS: EFFECT ON CELL MORPHOLOGY AND CELL PATTERN
An internal structure of a deformed physical object undergoes modification through displacement of atoms and changes in their mutual distances. The specific character of the modification depends strictly on the material type. Similarly, alterations in root morphology are accompanied by internal structural changes (for details see Table 2). Swollen roots either have an increased number of cell layers (Wilson et al., 1977) or their cells show modified sizes (Atwell, 1988; Materechera et al., 1991; Hanbury and Atwell, 2005). Reduced root elongation usually accompanies decreasing length of cells (Bengough et al., 2006; Okamoto et al., 2008) and slower cell production (Croser et al., 1999). In some cases changes in the size or cellular organization of the root apical meristem (RAM) are observed (Wilson and Robards, 1979; Potocka et al., 2011).
Table 2.
Detailed specification of the effects of mechanical stress on cell morphology and cell pattern
| Effect | Stress conditions | Species | References |
|---|---|---|---|
| Increased radial size of cortical cells | compacted soil compacted soil compacted soil pressurized glass beads pressurized glass beads pressurized sand |
22 monocotyledonous and dicotyledonous species Lupinus angustifolius Triticum aestivum Hordeum vulgare Zea mays L. angustifolius |
Materechera et al. (1991)
Atwell (1988) Colombi et al. (2017) Wilson et al. (1977) Veen (1982) Hanbury and Atwell (2005) |
| Increased number of cortical layers | compacted sand compacted soil compacted soil pressurized glass beads |
P. sativum Z. mays, P. sativum, Gossypium hirsutum T. aestivum H. vulgare |
Croser et al. (1999)
Iijima and Kato (2007) Colombi et al. (2017) Wilson et al. (1977) |
| Decreased radial size of cortical cells | rigid pores |
Lolium perenne
Z. mays (plus epidermis) Cicer arietinum |
Scholefield and Hall (1985)
Bengough et al. (1997) Kolb et al. (2012) |
| Increased cell length | rigid pores | L. perenne (cortex) | Scholefield and Hall (1985) |
| Decreased cell length | compacted soil compacted sand pressurized glass beads pressurized glass beads pressurized sand changed orientation |
L. angustifolius (epidermis, cortex) P. sativum (cortex) H. vulgare (epidermis, cortex, endodermis) Z. mays (cortex) L. angustifolius (epidermis) Arabidopsis thaliana (epidermis) |
Atwell (1988)
Croser et al. (1999) Wilson et al. (1977) Veen (1982) Hanbury and Atwell (2005) Okamoto et al. (2008) |
| Deformed cells | compacted soil compacted soil pressurized glass beads rigid pores |
Glycine max (cortex, root hairs) T. aestivum (cortex), H. vulgare (cortex, vascular cylinder), Secale cereale (cortex), Triticosecale Wittmack (cortex, vascular cylinder), Z. mays (cortex) H. vulgare (epidermis, cortex, endodermis) C. arietinum (cortex) |
Ramos et al. (2010)
Lipiec et al. (2012) Goss and Drew (1972) Kolb et al. (2012) |
| Increased diameter/area of the stele | compacted soil compacted soil compacted soil pressurized glass beads axial loading (apparatus) |
Z. mays, P. sativum, G. hirsutum H. vulgare, S. cereale, Triticosecale Wittmack, Z. mays T. aestivum H. vulgare Z. mays |
Iijima and Kato (2007)
Lipiec et al. (2012) Colombi et al. (2017) Wilson et al. (1977) Kuzeja et al. (2001) |
| Decreased diameter/area of the stele | compacted soil rigid pores |
T. aestivum Z. mays |
Lipiec et al. (2012)
Bengough et al. (1997) |
|
Altered organization of the vascular tissue
Increased xylem thickness Increased xylem fibre area Increased diameter of xylem vessels Decreased diameter of xylem vessels |
bending slope soil conditions compacted soil pressurized glass beads rigid pores |
Populus nigra
Spartium junceum Z. mays, P. sativum H. vulgare Z. mays |
De Zio et al. (2016)
Lombardi et al. (2017) Iijima and Kato (2007) Wilson et al. (1977) Bengough et al. (1997) |
| Enhanced cell proliferation | compacted sand pressurized glass beads bending |
Z. mays (lateral cap) H. vulgare (stele, pericycle) P. nigra (cambium) |
Iijima et al. (2003a) Wilson et al. (1977); Wilson and Robards (1978) De Zio et al. (2016) |
| Aberrantly orientated divisions | pressurized glass beads rigid tubes |
H. vulgare (endodermis, root cap) Z. mays (root cap) |
Wilson and Robards (1978, 1979) Potocka et al. (2011) |
| Reduced size of the meristem | compression (apparatus) artificial obstacle (horizontal barrier) |
Z. mays Z. mays |
Barley (1962)
Souty and Rode (1987) |
| Meristem opening | pressurized glass beads rigid tubes |
H. vulgare Z. mays |
Wilson and Robards (1979)
Potocka et al. (2011) |
| Ectopic root hair formation | pressurized glass beads pressurized sand rigid tubes changed orientation |
H. vulgare L. angustifolius Z. mays A. thaliana |
Goss and Drew (1972); Goss and Russell (1980) Hanbury and Atwell (2005) Potocka et al. (2011) Okamoto et al. (2008) |
| Enhanced sloughing of root cap cells and mucilage secretion | compacted sand/soil rigid tubes |
Z. mays Z. mays |
Iijima and Kono (1992); Iijima et al. (2000, 2003a); Somasundaram et al. (2008) Potocka et al. (2011) |
| Ultrastructural changes in root cells | compacted soil pressurized glass beads |
Z. mays (root cap) H. vulgare (endodermis, pericycle, root meristem, root cap) |
Iijima and Kono (1992)
Wilson and Robards (1978, 1979) |
| Cell wall modification | compacted soil compacted soil pressurized glass beads pressurized glass beads |
Z. mays H. vulgare H. vulgare Z. mays |
Degenhardt and Gimmler (2000)
Bingham et al. (2010) Wilson and Robards (1978) Veen (1982) |
The most significant morphological changes are found in the cortex cells, of which some biometric traits are thought to correspond to the mechanical properties of the root tissues (Chimungu et al., 2015) as well as in the stele cells (Wilson et al., 1977). In response to high soil compaction and densely packed glass beads, the cortex becomes thicker due to an increased number of cells (Colombi et al., 2017), an increased radial dimension of cells (Atwell, 1988) or both (Wilson et al., 1977). In the stele the number of cells increases related to its larger diameter (Wilson et al., 1977). In some cases, the stele diameter remains unchanged independently of the level of compaction (Atwell, 1988). A similar effect occurs in roots that have been constricted radially (Scholefield and Hall, 1985) or from the sides (Kolb et al., 2012) whose cortex cells become significantly compressed while the dimension of the stele does not change. In axially loaded roots the vasculature increases (Kuzeja et al., 2001) as in roots grown in a dense medium (Wilson et al., 1977). The above results suggest that the direction of the mechanical force exerted on the root influences the character of the change in cell dimension and consequently in the cell pattern.
Other anatomical changes may be seen in endodermis and pericycle cells. In barley both tissues demonstrated secondary features earlier than in unimpeded roots (Wilson and Robards, 1978). The pericycle formed two layers which resulted from tangential divisions in the protoxylem pole mother cells. In the endodermis atypical oblique divisions occurred in cells that had entered the later stages of development (state III cells; Wilson and Robards, 1978). Aberrantly orientated division walls were also reported in the root cap columella of barley (Wilson and Robards, 1979) and maize roots (Potocka et al., 2011). Bent roots of woody plants showed significant differences in cambial cell number, xylem and phloem thickness, and vessel area on both the convex and the concave side of the bend (De Zio et al., 2016).
There have been few attempts to examine the potential effect of mechanical stress on the organization of the RAM. Barley (1962) and Souty and Rode (1987) mentioned that the RAM of mechanically impeded maize root apices was reduced in size. There are also reports concerning the lack of a distinct boundary between the root meristem and cap (Wilson and Robards, 1979). In the above-mentioned experiment by Potocka et al. (2011) the boundary was broken and the cells localized in the root proper pole grew onto the cap side, so that the typical closed organization of the RAM in this species changed to open (Fig. 1C) which led to strong modification to the cell arrangement. When the mechanical force had been removed the arrangement of the cells became more regular and returned to the closed type (Potocka et al., 2011). Nevertheless, the question arises, is the change from closed to open organization a simple consequence of the applied force or is it an element of the root adaptation? It appears that the latter is the case as the cell pattern eventually recovers.
A change in cell pattern meaning de facto a rearrangement of the cell wall network may indicate a tendency to adjust to the new distribution of stress within the root. Being a highly organized structure stiffening the whole plant body and responsible for protection of the interior of cells, it is the cell wall network that mostly responds to and transduces mechanical stimuli affecting the living plant organ. Unfortunately, there are few reports on the effect of mechanical stress on root cell wall structure and composition. One of the few observations concerns barley roots whose endodermal cells were characterized by suberin lamellae and thick a tertiary cellulosic wall due to an atypically early transition to later stages of development (Wilson and Robards, 1978). Another report comes from Veen’s (1982) study on crown roots of maize exposed to mechanical stress, in which there was reorientation of some cellulose microfibrils of cortex cells from transverse to longitudinal. Such a change may be correlated with radial thickening of the root because of a smaller number of transverse microfibrils that typically inhibit growth in the radial direction. Relatively recent research on barley roots revealed significant decreases of cellulose and hemicellulose content and increased lignin concentration in root tissue (Bingham et al., 2010). The last was also observed in endodermal cell walls of impeded maize roots (Degenhardt and Gimmler, 2000). To understand the mutual relationship between the cell pattern and root growth more research on both the reorganization of the cell wall network and possible changes in the structure and chemical composition of the cell walls in roots is needed.
Some authors report the increased exudation of deformed roots (Groleau-Renaud et al., 1998; Oliveros-Bastidas et al., 2012), suggesting that there might be a correlation between deformation and exudation as the effects of the same factor, namely mechanical impedance (Oliveros-Bastidas et al., 2012). Root exudation leads on to consideration of root cap behaviour when affected by mechanical stress.
ROOT CAP FACING MECHANICAL IMPEDANCE
The root cap covers the root tip and thus it is the region of the first contact of the root with soil. It plays multiple roles in root biology and in root interactions with the environment (Barlow, 2003; Iijima et al., 2008) and its functioning depends on the type of RAM (Hamamoto et al., 2006). Among the most evident functions of the root cap are protecting the root tip from environmental impulses, determining the direction of root growth and reducing the friction between soil and the growing root by sloughing of peripheral root cap cells and secretion of mucilage (Bengough and McKenzie, 1997). The lubricating effect of the root cap was shown by Iijima et al. (2003b, 2004) who estimated the contribution of mucilage to the decrease in root penetration resistance of maize roots grown in compact soil (Iijima et al., 2004). Decapped roots showed significantly reduced elongation either with or without mucilage treatment (Iijima et al., 2004). Interestingly, in experiments with decapped Zea roots grown in ballotini no effect of the decreased elongation rate was observed (Goss and Russell, 1980). Decapping not only changes the shape of the apical part of the root, which clearly results in an altered distribution of mechanical stress in the region, but it also may change the strategy of adaptation under conditions of a temporal lack of root tip protection.
In mechanically impeded roots mucilage production is enhanced (Potocka et al., 2011) and the pattern of its accumulation is modified (Wilson and Robards, 1979), possibly correlated with some ultrastructural effects, such as the increased number of dictyosomes and secretory vesicles in cap cells (Iijima and Kono, 1992). Enhanced cap exudation is also accompanied by a significant increase in the number of cap cells sloughed into the medium (Iijima et al., 2000, 2003a; Somasundaram et al., 2008). The size of the root cap and the number of cells of which it is composed change in response to mechanical impedance. The caps of stressed roots are usually shorter and narrower at the base (Wilson and Robards, 1979; Souty and Rode, 1987; Iijima et al., 2003a) and their total cell number is reduced despite the fact that the rate of cell production in flank areas is slightly enhanced (Iijima et al., 2003a). As was shown by Bengough and McKenzie (1997), the enhanced release of the outermost cells lessens mechanical impedance to root penetration. We thus infer that this kind of response (not observed in inanimate physical objects) may be adaptive. Decrease in the transverse dimension of the cap may also play the adaptive role as it makes the cap thinner and thus better adjusted to penetration through a dense medium. On the other hand, the decreased length of the root cap resembles shortening of an inanimate elastic rod under axial stress.
There is little information about the anatomical changes that occur in the cap under mechanical stress. In caps of impeded roots of barley, Wilson and Robards (1979) observed vacuolation of meristematic cells, a decrease in the number and atypical location of amyloplasts in the columella cells, and an atypical orientation of the cellular divisions. The last observation was also reported by Potocka et al. (2011) in the maize root cap.
ETHYLENE IN RELATION TO THE MORPHOLOGICAL RESPONSE OF ROOTS
Morphological alteration in mechanically impeded roots is preceded by enhanced ethylene production 1 h after stress application (Kays et al., 1974; Sarquis et al., 1991, 1992; He et al., 1996). In maize roots subjected to a cycle of alternating mechanical impulses, ethylene production increased and decreased rapidly during the on and off phase of the impulse, respectively (Sarquis et al., 1991). Inhibition of the action of ethylene partially restores the length and diameter of the impeded roots (Sarquis et al., 1991;Zacarias and Reid, 1992) which indicates a mediating role of this hormone in the morphological response of the root. Yamamoto et al. (2008) suggested that ethylene may regulate root responses to stress through softening or hardening the root tip (thus changing its mechanical properties) and through altering the growth rate of the root. In support of this are the recent results by Santisree et al. (2011), who showed that roots are unable to penetrate growing medium under conditions of lower ethylene production.
Application of ethylene results in root deformation similar to that induced by mechanical stress. In response to exogenous ethylene, unimpeded maize roots show increased diameter and decreased length (Moss et al., 1988) while Arabidopsis roots change their wavy growth pattern (Buer et al., 2000, 2003). Interestingly, in ethylene-treated decapped roots, elongation growth is not inhibited indicating that the cap may be a site of perception and response to this hormone (Hahn et al., 2008).
The response of treated roots to ethylene is accompanied by changes in the auxin response. In impeded Arabidopsis roots enhanced ethylene synthesis affects the pattern of auxin distribution (Okamoto et al., 2008) while in tomato roots in the presence of the ethylene inhibitor 1-methylcyclopropene a reduced ethylene synthesis along with a decrease of the DR5::GUS reporter activity were observed (Santisree et al., 2011, 2012). It has been also shown that ethylene regulates auxin distribution in the root cap and the interaction of the two hormones regulates the size of the cap (Ponce et al., 2005).
EFFECT OF A COMBINATION OF MECHANICAL STRESS AND OTHER ABIOTIC STRESSES ON ROOT MORPHOLOGY
In its natural environment a root is exposed to long-lasting or permanent stresses of various types to which it needs to adapt. Thus, the total of the environmental inputs to which a root adapts are shaped by both the stress experienced at that point and the impulses that have been previously experienced (Bengough et al., 1997). In lab experiments roots are generally subjected to one or more types of stress applied in a controlled way, for a set and usually short time in relation to the life of the plant. The roots adapt to new conditions ranging from morphological changes to modification of the composition of the cell wall (Degenhardt and Gimmler, 2000).
Various abiotic stresses such as low/high water availability (Cairns et al., 2004; Iijima and Kato, 2007; Konôpka et al., 2008; Kobaissi et al., 2013) or hypoxia (Barley, 1962; Iijima and Kato, 2007), applied together with mechanical stress, may induce various morphological root responses. The character of the responses usually resembles a typical reaction of roots to mechanical stress, namely decreased length and increased diameter. The interaction among the stresses alters root morphology depending on the character and the levels of particular stresses (Alameda et al., 2012), but it is the mechanical stress that seems to have the strongest influence (Cairns et al., 2004; Konôpka et al., 2008; Benigno et al., 2012; Kobaissi et al., 2013). There are few reports on alleviating the effects of mechanical stress on root deformation in combination with other abiotic stresses. A high aluminium (Al) concentration decreases root morphological parameters such as length and number, while application of mechanical stress along with Al partially overcomes the effect and induces enhanced mucilage production, which eventually decreases Al accumulation in the root tip (Horst et al., 1990; Okamoto and Yano, 2017).
The anatomical effects of combined stresses are mainly seen in the root cortex. Mechanical stress applied with drought and low oxygen supply result in a changed thickness of the tissue associated with an altered number and size of cortical cells (Iijima and Kato, 2007) and in aerenchyma formation (He et al., 1996), although the specific response depends on the plant species (Iijima and Kato, 2007). Effects at the cellular level are rarely reported and relate to altered tissue composition observed in roots grown in highly compacted soil with different types of stress such as low nitrogen (Bingham et al., 2010), high salinity, high pH and high concentrations of heavy metals (Degenhardt and Gimmler, 2000). The inputs of different abiotic characters may then interact with mechanical stress although even when applied in a controlled way precise interpretation of their effects is difficult (Alameda et al., 2012; Suzuki et al., 2014).
Mechanical stress may affect growth conditions by inducing stresses of different character, as with soil compaction that may reduce access to water and/or air (Drew et al., 2000; Tracy et al., 2011; Chimungu et al., 2015). An opposite action may also take place: drought stress may increase mechanical stress to the root (Iijima and Kato, 2007). As consequence of wetting and drying weather cycles, cyclical swelling and shrinkage of the soil occur (Striker et al., 2007) which eventually results in an alternating distribution of mechanical stress sensed by the roots. For more information on the impact of other abiotic stresses on root development, the reader is reffered to the recent reviews by Franco et al. (2011) and Sánchez-Calderón et al. (2013) and to the latest works in the field (Kadam et al., 2015; Líška et al., 2016).
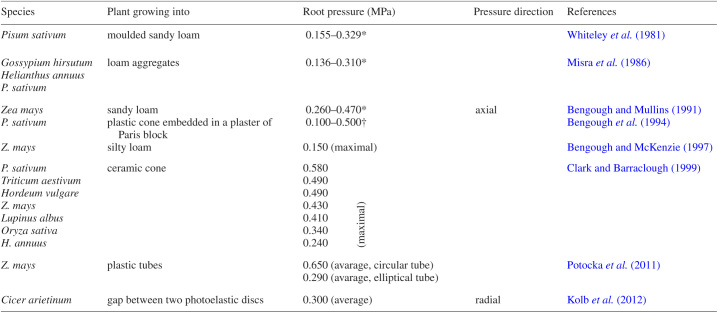
ESTIMATION OF ROOT GROWTH PRESSURE
The level of root growth pressure in soil clearly affects the degree of root tip deformation. That is why in some cases study of the morphological effects of mechanical stress on roots involves estimation of the root force. For technical reasons it is very difficult to measure this force and some researchers use penetrometer probes whose readings, however, appear significantly overestimated (Bengough and Mullins, 1990). Direct measurements show that root pressure for various plant species ranges from 0.10 to 0.65 MPa (Table 3) and depends strongly on growth conditions (Whiteley et al., 1981; Misra et al., 1986; Bengough and Mullins, 1991; Bengough et al., 1994; Bengough and McKenzie, 1997; Clark and Barraclough, 1999) as well as on the cultivar and the measurement technique (reviewed by Clark et al., 1999). Potocka et al. (2011) showed that the force exerted by the root growing through a plastic pipe depended on the space available above the tightest region of the pipe, which may be related to variability in the structure of the soil and its porosity in the field conditions. Another method was proposed by Kolb et al. (2012) who made a growing root push between photoelastic discs. Application of this material enabled visualization and registration of the optical fringes on the basis of which the value and direction of radial mechanical force exerted by the root might be estimated (Table 3). In studies determining root growth pressure a mathematical approach has also been applied. Kirby and Bengough (2002) presented a predicted distribution of the stresses around the tip of a root penetrating soil, based on the critical-state finite-element model. The model showed that the stress depends on the distance from the root tip, on soil conditions and on root diameter.
Table 3.
Measured root pressure
*Depending on soil conditions.
†Data from the plot in Figure 4 of the reference.
CONCLUDING REMARKS AND PERSPECTIVES
Based on the many descriptions of the root responses to mechanical stimuli of various types we may draw a portrait of the root as an easily adapting plant organ, quickly responding to changes in the environment. Nevertheless, a universal scenario of root responses is not possible as roots of various species at various stages of development may react in various ways to the same stimulus. Typically, the most often observed features of the impeded roots are (1) reduced axial growth and consequently decreased root length, (2) radial swelling in many cases associated with increased radial dimension of the cortex cell layers and (3) enhanced root cap cell sloughing (Table 1, Table 2). A reduction in root elongation seems to be a direct effect of the axial components of the applied force (Kolb et al., 2012; for detailed analysis see Bengough, 2012). This is explainable from the point of view of kinematics where a growing root may be considered a moving physical body whose movement is reduced or even stopped by a potential obstacle. Root thickening may be an element of both the physical reaction and the adaptation process while enhanced cap cell sloughing seems to be (a part of) an adaptation to stress conditions. The morphological response of the root to mechanical stress is more than a reaction of the physical object because being a living organ the root is able to sense and adapt to altering conditions of the environment.
The changed form is often accompanied by a rearrangement of the cell pattern in which either time (Goss, 1977) or the level of the applied mechanical impulse (Cook et al., 1996) might have a significant effect. The most spectacular examples concern modifications in RAM organization (Potocka et al., 2011) that may dramatically change cell fate within the root apex. Eventually, reorganization of the RAM is reversible, but the further fates of the cells have not been studied. To explore the issue, additional studies concerning the possible relationship between cell fates and experimental conditions are needed.
To give a sense of the significance of modification to the cell pattern in the meristematic area we need to consider the character of growth of the root apex. As with other plant organs, root apices grow in a highly coordinated way, designated ‘symplastic’ (Erickson, 1986). This coordination is recognizable through a stable and regular cellular pattern that is preserved during growth. Hejnowicz (1984) assumed that within the growing plant organ the so-called principal directions of growth (directions of either minimal or maximal growth rates) are present. These directions are tangential to periclines and anticlines (von Sachs, 1887) that are visible in the cell wall pattern. In plants growing in soil it is the root apex that experiences the strongest mechanical impulse from the environment. This usually alters its morphology and cell arrangement, changing the pattern of periclines and anticlines and consequently the principal directions of growth. As shown by Lynch and Lintilhac (1997) there is a direct relationship between the principal directions of growth and the principal directions of stress in the plant organ. This explains why in the morphologically changed root there is a rearranged distribution of mechanical stress.
The pronounced ability of roots to adapt to different stimuli and to moderate their effects is often described as root plasticity (Suralta et al., 2018; Riedelsberger and Blatt, 2017). This colloquial term is not precise from the point of view of mechanics because it does not fully mirror the mechanical properties of this plant organ. The mechanical properties of a material, such as Young’s modulus or viscosity coefficient, are estimated through rheological and other mechanical tests. Rheological tests on pea lateral roots (Tanimoto et al., 2000) show a viscoelastic and plastic characteristics of the root tissue material. Some recent work (Loades et al., 2013, 2015) has shown that environmental conditions, such as mechanical impedance coming from compacted soil, alter mechanical properties and their distribution along the root axis. More experiments on the mechanical properties of roots are needed to advance our understanding of their reaction as either physical objects or living organs.
ACKNOWLEDGEMENTS
We are grateful to Professor Lewis Feldman from UC Berkeley, USA, for critically reviewing the manuscript and for his valuable comments. We also thank the Referees for comments on a previous version of the text.
LITERATURE CITED
- Abdalla AM, Hettiaratchi DRP, Reece AR. 1969. The mechanics of root growth in granular media. Journal of Agricultural Engineering Research 14: 236–248. [Google Scholar]
- Alameda D, Anten NPR, Villar R. 2012. Soil compaction effects on growth and root traits of tobacco depend on light, water regime and mechanical stress. Soil and Tillage Research 120: 121–129. [Google Scholar]
- Alexander KG, Miller MH. 1991. The effect of soil aggregate size on early growth and shoot-root ratio of maize (Zea mays L.). Plant and Soil 138: 189–194. [Google Scholar]
- Atwell BJ. 1988. Physiological responses of lupin roots to soil compaction. Plant and Soil 111: 277–281. [Google Scholar]
- Atwell BJ. 1993. Response of roots to mechanical impedance. Environmental and Experimental Botany 33: 27–40. [Google Scholar]
- Azam G, Grant CD, Murray RS, Nuberg IK, Misra RK. 2014. Comparison of the penetration of primary and lateral roots of pea and different tree seedlings growing in hard soils. Soil Research 52: 87–96. [Google Scholar]
- Barley KP. 1962. The effects of mechanical stress on the growth of roots. Journal of Experimental Botany 13: 95–110. [Google Scholar]
- Barley KP, Greacen EL. 1967. Mechanical resistance as a soil factor influencing the growth of roots and underground shoots. Advances in Agronomy 19: 1–43. [Google Scholar]
- Barlow PW. 2003. The root cap: cell dynamics, cell differentiation and cap function. Journal of Plant Growth Regulation 21: 261–286. [Google Scholar]
- Bécel C, Vercambre G, Pagès L. 2012. Soil penetration resistance, a suitable soil property to account for variations in root elongation and branching. Plant and Soil 353: 169–180. [Google Scholar]
- Bengough AG. 2012. Root elongation is restricted by axial but not by radial pressures: so what happens in field soil?Plant and Soil 360: 15–18. [Google Scholar]
- Bengough AG, McKenzie BM. 1997. Sloughing of root cap cells decreases the frictional resistance to maize (Zea mays L.) root growth. Journal of Experimental Botany 48: 885–893. [Google Scholar]
- Bengough AG, Mullins CE. 1990. Mechanical impedance to root growth: a review of experimental techniques and root growth responses. European Journal of Soil Science 41: 341–358. [Google Scholar]
- Bengough AG, Mullins CE. 1991. Penetrometer resistance, root penetration resistance and root elongation rate in two sandy loam soils. Plant and Soil 131: 59–66. [Google Scholar]
- Bengough AG, Mackenzie CJ, Elangwe HE. 1994. Biophysics of the growth responses of pea roots to changes in penetration resistance. Plant and Soil 167: 135–141. [Google Scholar]
- Bengough AG, Croser C, Pritchard J. 1997. A biophysical analysis of root growth under mechanical stress. Plant and Soil 189: 155–164. [Google Scholar]
- Bengough AG, Bransby MF, Hans J, McKenna SJ, Roberts TJ, Valentine TA. 2006. Root responses to soil physical conditions; growth dynamics from field to cell. Journal of Experimental Botany 57: 437–447. [DOI] [PubMed] [Google Scholar]
- Bengough AG, McKenzie BM, Hallett PD, Valentine TA. 2011. Root elongation, water stress, and mechanical impedance: a review of limiting stresses and beneficial root tip traits. Journal of Experimental Botany 62: 59–68. [DOI] [PubMed] [Google Scholar]
- Benigno SM, Cawthray GR, Dixon KW, Stevens JC. 2012. Soil physical strength rather than excess ethylene reduces root elongation of Eucalyptus seedlings in mechanically impeded sandy soils. Plant Growth Regulation 68: 261–270. [Google Scholar]
- Bingham IJ, Bengough AG, Rees RM. 2010. Soil compaction–N interactions in barley: Root growth and tissue composition. Soil and Tillage Research 106: 241–246. [Google Scholar]
- Bizet F, Bengough AG, Hummel I, Bogeat-Triboulot M-B, Dupuy LX. 2016. 3D deformation field in growing plant roots reveals both mechanical and biological responses to axial mechanical forces. Journal of Experimental Botany 67: 5605–5614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buer CS, Masle J, Wasteneys GO. 2000. Growth conditions modulate root-wave phenotypes in Arabidopsis. Plant and Cell Physiology 41: 1164–1170. [DOI] [PubMed] [Google Scholar]
- Buer CS, Wasteneys GO, Masle J. 2003. Ethylene modulates root-wave responses in Arabidopsis. Plant Physiology 132: 1085–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns JE, Audebert A, Townend J, Price AH, Mullins CE. 2004. Effect of soil mechanical impedance on root growth of two rice varieties under field drought stress. Plant and Soil 267: 309–318. [Google Scholar]
- Castillo SR, Dowdy RH, Bradford JM, Larson WE. 1982. Effects of applied mechanical stress on plant growth and nutrient uptake. Agronomy Journal 74: 526–530. [Google Scholar]
- Chen YL, Palta J, Clements J, Buirchell B, Siddique KHM, Rengel Z. 2014. Root architecture alteration of narrow-leafed lupin and wheat in response to soil compaction. Field Crops Research 165: 61–70. [Google Scholar]
- Chiatante D, Baraldi A, Di Iorio A, Sarnataro M, Scippa GS. 2003. Root response to mechanical stress in plants growing on slopes: an experimental system for morphological, biochemical and molecular analysis. In: Abe J, ed. Roots: The Dynamic Interface between Plants and the Earth. Dordrecht: Springer, 427–437. [Google Scholar]
- Chiatante D, Scippa GS, Di Iorio A, De Micco V, Sarnataro M. 2007. Lateral root emission in woody taproots of Fraxinus ornus L. Plant Biosystems 141: 204–213. [Google Scholar]
- Chimungu JG, Loades KW, Lynch JP. 2015. Root anatomical phenes predict root penetration ability and biomechanical properties in maize (Zea mays). Journal of Experimental Botany 66: 3151–3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark LJ, Barraclough PB. 1999. Do dicotyledons generate greater maximum axial root growth pressures than monocotyledons?Journal of Experimental Botany 50: 1263–1266. [Google Scholar]
- Clark LJ, Bengough AG, Whalley WR, Dexter AR, Barraclough PB. 1999. Maximum axial root growth pressure in pea seedlings: effects of measurement techniques and cultivars. Plant and Soil 209: 101–109. [Google Scholar]
- Clark LJ, Whalley WR, Barraclough PB. 2001. Partial mechanical impedance can increase the turgor of seedling pea roots. Journal of Experimental Botany 52: 167–171. [PubMed] [Google Scholar]
- Clark LJ, Whalley WR, Barraclough PB. 2003. How do roots penetrate strong soil?Plant and Soil 255: 93–104. [Google Scholar]
- Colombi T, Kirchgessner N, Walter A, Keller T. 2017. Root tip shape governs root elongation rate under increased soil strength. Plant Physiology 174: 2289–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook A, Marriott CA, Seel W, Mullins CE. 1996. Effects of soil mechanical impedance on root and shoot growth of Lolium perenne L., Agrostis capillaris and Trifolium repens L. Journal of Experimental Botany 47: 1075–1084. [Google Scholar]
- Croser C, Bengough AG, Pritchard J. 1999. The effect of mechanical impedance on root growth in pea (Pisum sativum). I. Rates of cell flux, mitosis, and strain during recovery. Physiologia Plantarum 107: 277–286. [Google Scholar]
- Croser C, Bengough AG, Pritchard J. 2000. The effect of mechanical impedance on root growth in pea (Pisum sativum). II. Cell expansion and wall rheology during recovery. Physiologia Plantarum 109: 150–159. [Google Scholar]
- De Zio E, Trupiano D, Montagnoli A et al. 2016. Poplar woody taproot under bending stress: the asymmetric response of the convex and concave sides. Annals of Botany 118: 865–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt B, Gimmler H. 2000. Cell wall adaptations to multiple environmental stresses in maize roots. Journal of Experimental Botany 51: 595–603. [DOI] [PubMed] [Google Scholar]
- Ditengou FA, Teale WD, Kochersperger P et al. 2008. Mechanical induction of lateral root initiation in Arabidopsis thaliana. Proceedings of the National Academy of Sciences of the USA 105: 18818–18823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donald RG, Kay BD, Miller MH. 1987. The effect of soil aggregate size on early shoot and root growth of maize (Zea mays L.). Plant and Soil 103: 251–259. [Google Scholar]
- Drew MC, He C-J, Morgan PW. 2000. Programmed cell death and aerenchyma formation in roots. Trends in Plant Science 5: 123–127. [DOI] [PubMed] [Google Scholar]
- Erickson RO. 1986. Symplastic growth and symplasmic transport. Plant Physiology 82: 1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin M-C, Pierce FJ, Poff KL. 1989. The pattern of secondary root formation in curving roots of Arabidopsis thaliana (L.) Heynh. Plant, Cell and Environment 12: 337–339. [DOI] [PubMed] [Google Scholar]
- Franco JA, Bañón S, Vicente MJ, Miralles J, Martínez-Sánchez JJ. 2011. Root development in horticultural plants grown under abiotic stress conditions – a review. Journal of Horticultural Science and Biotechnology 86: 543–556. [Google Scholar]
- Goss MJ. 1977. Effects of mechanical impedance on root growth in barley (Hordeum vulgare L.). I. Effects on the elongation and branching of seminal root axes. Journal of Experimental Botany 28: 96–111. [Google Scholar]
- Goss MJ, Drew MC. 1972. Effect of mechanical impedance on growth of seedlings. Agricultural Research Council Letcombe Laboratory Annual Report 1971: 35–42. [Google Scholar]
- Goss MJ, Russell RS. 1980. Effects of mechanical impedance on root growth in barley (Hordeum vulgare L.). III. Observations on the mechanism of response. Journal of Experimental Botany 31: 577–588. [Google Scholar]
- Groleau-Renaud V, Plantureux S, Guckert A. 1998. Influence of plant morphology on root exudation of maize subjected to mechanical impedance in hydroponic conditions. Plant and Soil 201: 231–239. [Google Scholar]
- Grzesiak MT. 2009. Impact of soil compaction on root architecture, leaf water status, gas exchange and growth of maize and triticale seedlings. Plant Root 3: 10–16. [Google Scholar]
- Hahn A, Zimmermann R, Wanke D, Harter K, Edelmann HG. 2008. The root cap determines ethylene-dependent growth and development in maize roots. Molecular Plant 1: 359–367. [DOI] [PubMed] [Google Scholar]
- Hamamoto L, Hawes MC, Rost TL. 2006. The production and release of living root cap border cells as a function of root apical meristem type in dicotyledonous angiosperm plants. Annals of Botany 97: 917–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanbury CD, Atwell BJ. 2005. Growth dynamics of mechanically impeded lupin roots: does altered morphology induce hypoxia?Annals of Botany 96: 913–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C, Finlayson SA, Drew MC, Jordan WR, Morgan PW. 1996. Ethylene biosynthesis during aerenchyma formation in roots of maize subjected to mechanical impedance and hypoxia. Plant Physiology 112: 1679–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hejnowicz Z. 1984. Trajectories of principal directions of growth, natural coordinate system in growing plant organ. Acta Societatis Botanicorum Poloniae 53: 29–42. [Google Scholar]
- Horst WJ, Klotz F, Szulkiewicz P. 1990. Mechanical impedance increases aluminium tolerance of soybean (Glycine max) roots. Plant and Soil 124: 227–231. [Google Scholar]
- Iijima M, Kato J. 2007. Combined soil physical stress of soil drying, anaerobiosis and mechanical impedance to seedling root growth of four crop species. Plant Production Science 10: 451–459. [Google Scholar]
- Iijima M, Kono Y. 1991. Interspecific differences of the root system structures of four cereal species as affected by soil compaction. Japanese Journal of Crop Science 60: 130–138. [Google Scholar]
- Iijima M, Kono Y. 1992. Development of Golgi apparatus in the root cap cells of maize (Zea mays L.) as affected by compacted soil. Annals of Botany 70: 207–212. [Google Scholar]
- Iijima M, Griffiths B, Bengough AG. 2000. Sloughing of cap cells and carbon exudation from maize seedling roots in compacted sand. New Phytologist 145: 477–482. [DOI] [PubMed] [Google Scholar]
- Iijima M, Barlow PW, Bengough AG. 2003a. Root cap structure and cell production rates of maize (Zea mays) roots in compacted sand. New Phytologist 160: 127–134. [DOI] [PubMed] [Google Scholar]
- Iijima M, Higuchi T, Barlow PW, Bengough AG. 2003b. Root cap removal increases root penetration resistance in maize (Zea mays L.). Journal of Experimental Botany 54: 2105–2109. [DOI] [PubMed] [Google Scholar]
- Iijima M, Higuchi T, Barlow PW. 2004. Contribution of root cap mucilage and presence of an intact root cap in maize (Zea mays) to the reduction of soil mechanical impedance. Annals of Botany 94: 473–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima M, Morita S, Barlow PW. 2008. Structure and function of the root cap. Plant Production Science 11: 17–27. [Google Scholar]
- Kadam N, Yin X, Bindraban P, Struik PC, Jagadish KSV. 2015. Does morphological and anatomical plasticity during the vegetative stage make wheat more tolerant of water-deficit stress than rice?Plant Physiology 167: 1389–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kays SJ, Nicklow CW, Simons DH. 1974. Ethylene in relation to the response of roots to physical impedance. Plant and Soil 40: 565–571. [Google Scholar]
- Kirby JM, Bengough AG. 2002. Influence of soil strength on root growth: experiments and analysis using a critical-state model. European Journal of Soil Science 53: 119–127. [Google Scholar]
- Kobaissi AN, Kanso AA, Kanbar HJ, Kazpard VA. 2013. Morpho-physiological changes caused by soil compaction and irrigation on Zea mays. Eurasian Journal of Soil Science 2: 114–121. [Google Scholar]
- Kolb E, Hartmann C, Genet P. 2012. Radial force development during root growth measured by photoelasticity. Plant and Soil 360: 19–35. [Google Scholar]
- Konôpka B, Pagès L, Doussan C. 2008. Impact of soil compaction heterogeneity and moisture on maize (Zea mays L.) root and shoot development. Plant, Soil and Environment 54: 509–519. [Google Scholar]
- Konôpka B, Pagès L, Doussan C. 2009. Soil compaction modifies morphological characteristics of seminal maize roots. Plant, Soil and Environment 55: 1–10. [Google Scholar]
- Kuzeja PS, Lintilhac PM, Wei C. 2001. Root elongation against a constant force: experiment with a computerized feedback-controlled device. Journal of Plant Physiology 158: 673–676. [DOI] [PubMed] [Google Scholar]
- Laskowski M, Grieneisen VA, Hofhuis H et al. 2008. Root system architecture from coupling cell shape to auxin transport. PLoS Biology 6: e307. doi:10.1371/journal.pbio.0060307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg S, Pettersson S. 1985. Effects of mechanical stress on uptake and distribution of nutrients in barley. Plant and Soil 83: 295–309. [Google Scholar]
- Lipiec J, Horn R, Pietrusiewicz J, Siczek A. 2012. Effects of soil compaction on root elongation and anatomy of different cereal plant species. Soil and Tillage Research 121: 74–81. [Google Scholar]
- Líška D, Martinka M, Kohanová J, Lux A. 2016. Asymmetrical development of root endodermis and exodermis in reaction to abiotic stresses. Annals of Botany 118: 667–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loades KW, Bengough AG, Bransby MF, Hallett PD. 2013. Biomechanics of nodal, seminal and lateral roots of barley: effects of diameter, waterlogging and mechanical impedance. Plant and Soil 370: 407–418. [Google Scholar]
- Loades KW, Bengough AG, Bransby MF, Hallett PD. 2015. Effect of root age on the biomechanics of seminal and nodal roots of barley (Hordeum vulgare L.) in contrasting soil environments. Plant and Soil 395: 253–261. [Google Scholar]
- Logsdon SD, Parker JC, Reneau RB. 1987. Root growth as influenced by aggregate size. Plant and Soil 99: 267–275. [Google Scholar]
- Lombardi F, Scippa GS, Lasserre B, Montagnoli A, Tognetti R, Marchetti M, Chiatante D. 2017. The influence of slope on Spartium junceum root system: morphological, anatomical and biomechanical adaptation. Journal of Plant Research 130: 515–525. [DOI] [PubMed] [Google Scholar]
- Lucas M, Godin C, Jay-Allemand C, Laplaze L. 2008. Auxin fluxes in the root apex co-regulate gravitropism and lateral root initiation. Journal of Experimental Botany 59: 55–66. [DOI] [PubMed] [Google Scholar]
- Lynch J. 1995. Root architecture and plant productivity. Plant Physiology 109: 7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch TM, Lintilhac PM. 1997. Mechanical signals in plant development: a new method for single cell studies. Developmental Biology 181: 246–256. [DOI] [PubMed] [Google Scholar]
- Massa GD, Gilroy S. 2003. Touch modulates gravity sensing to regulate the growth of primary roots of Arabidopsis thaliana. The Plant Journal 33: 435–445. [DOI] [PubMed] [Google Scholar]
- Materechera SA, Dexter AR, Alston AM. 1991. Penetration of very strong soils by seedling roots of different plant species. Plant and Soil 135: 31–41. [Google Scholar]
- Meyers MA, Chawla KK. 2009. Mechanical behavior of materials. New York: Cambridge University Press. [Google Scholar]
- Misra RK, Dexter AR, Alston AM. 1986. Penetration of soil aggregates of finite size. II. Plant roots. Plant and Soil 94: 59–85. [Google Scholar]
- Moss GI, Hall KC, Jackson MB. 1988. Ethylene and the responses of roots of maize (Zea mays L.) to physical impedance. New Phytologist 109: 303–311. [Google Scholar]
- Mulholland BJ, Black CR, Taylor IB, Roberts JA, Lenton JR. 1996a. Effect of soil compaction on barley (Hordeum vulgare L.) growth. I. Possible role for ABA as a root-sourced chemical signal. Journal of Experimental Botany 47: 539–549. [Google Scholar]
- Mulholland BJ, Taylor IB, Black CR, Roberts JA. 1996b. Effect of soil compaction on barley (Hordeum vulgare L.) growth. II. Are increased xylem sap ABA concentrations involved in maintaining leaf expansion in compacted soils?Journal of Experimental Botany 47: 551–556. [Google Scholar]
- Niklas KJ. 1992. Plant biomechanics: an engineering approach to plant form and function. Chicago: The University of Chicago Press. [Google Scholar]
- Okada K, Shimura Y. 1990. Reversible root tip rotation in Arabidopsis seedlings induced by obstacle-touching stimulus. Science 250: 274–276. [DOI] [PubMed] [Google Scholar]
- Okamoto K, Yano K. 2017. Al resistance and mechanical impedance to roots in Zea mays: Reduced Al toxicity via enhanced mucilage production. Rhizosphere 3: 60–66. [Google Scholar]
- Okamoto T, Tsurumi S, Shibasaki K et al. 2008. Genetic dissection of hormonal responses in the roots of Arabidopsis grown under continuous mechanical impedance. Plant Physiology 146: 1651–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveros-Bastidas AJ, Macías FA, Molinillo JMG. 2012. Mechanical impedance effects on growth, phenolics and 2,4-dihydroxy-7-methoxy-2H-1, 4-benzoxazin-3-one (DIMBOA) in root exudates of Zea mays L. International Journal of Agriculture and Forestry 2: 225–234. [Google Scholar]
- Passioura JB. 1991. Soil structure and plant growth. Australian Journal of Soil Research 29: 717–728. [Google Scholar]
- Ponce G, Barlow PW, Feldman LJ, Cassab GI. 2005. Auxin and ethylene interactions control mitotic activity of the quiescent centre, root cap size, and pattern of cap cell differentiation in maize. Plant, Cell and Environment 28: 719–732. [DOI] [PubMed] [Google Scholar]
- Potocka I, Szymanowska-Pułka J, Karczewski J, Nakielski J. 2011. Effect of mechanical stress on Zea root apex. I. Mechanical stress leads to the switch from closed to open meristem organization. Journal of Experimental Botany 62: 4583–4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos JC, Imhoff SDC, Pilatti MÁ, Vegetti AC. 2010. Morphological characteristics of soybean root apexes as indicators of soil compaction. Scientia Agricola 67: 707–712. [Google Scholar]
- Rich SM, Watt M. 2013. Soil conditions and cereal root system architecture: review and considerations for linking Darwin and Weaver. Journal of Experimental Botany 64: 1193–1208. [DOI] [PubMed] [Google Scholar]
- Richter GL, Monshausen GB, Krol A, Gilroy S. 2009. Mechanical stimuli modulate lateral root organogenesis. Plant Physiology 151: 1855–1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedelsberger J, Blatt MR. 2017. Editorial: Roots – the hidden provider. Frontiers in Plant Science 8: 1021. doi:10.3389/fpls.2017.01021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JA, Hussain A, Taylor IB, Black CR. 2002. Use of mutants to study long-distance signalling in response to compacted soil. Journal of Experimental Botany 53: 45–50. [PubMed] [Google Scholar]
- Rutherford R, Masson PH. 1996. Arabidopsis thaliana sku mutant seedlings show exaggerated surface-dependent alteration in root growth vector. Plant Physiology 111: 987–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Calderón L, Ibarra-Cortés ME, Zepeda-Jazo I. 2013. Root development and abiotic stress adaptation. In: Vahdati K, Leslie C, eds. Abiotic stress – plant responses and applications in agriculture. Rijeka: InTech, 135–168. [Google Scholar]
- Santisree P, Nongmaithem S, Vasuki H, Sreelakshmi Y, Ivanchenko MG, Sharma R. 2011. Tomato root penetration in soil requires a coaction between ethylene and auxin signaling. Plant Physiology 156: 1424–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santisree P, Nongmaithem S, Sreelakshmi Y, Ivanchenko MG, Sharma R. 2012. The root as a drill. An ethylene-auxin interaction facilitates root penetration in soil. Plant Signalling and Behavior 7: 151–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarquis JI, Jordan WR, Morgan PW. 1991. Ethylene evolution from maize (Zea mays L.) seedling roots and shoots in response to mechanical impedance. Plant Physiology 96: 1171–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarquis JI, Morgan PW, Jordan WR. 1992. Metabolism of 1-aminocyclopropane-1-aarboxylic acid in etiolated maize seedlings grown under mechanical impedance. Plant Physiology 98: 1342–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholefield D, Hall DM. 1985. Constricted growth of grass roots through rigid pores. Plant and Soil 85: 153–162. [Google Scholar]
- Schuurman JJ. 1965. Influence of soil density on root development and growth of oats. Plant and Soil 22: 352–374. [Google Scholar]
- Scippa GS, Trupiano D, Rocco M, Di Iorio A, Chiatante D. 2008. Unravelling the response of poplar (Populus nigra) roots to mechanical stress imposed by bending. Plant Biosystems 142: 401–413. [Google Scholar]
- Silverberg JL, Noar RD, Packer MS et al. 2012. 3D imaging and mechanical modeling of helical buckling in Medicago truncatula plant roots. Proceedings of the National Academy of Sciences of the USA 109: 16794–16799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somasundaram S, Fukuzono S, Iijima M. 2008. Dynamics of root border cells in rhizosphere soil of Zea mays L.: crushed cells during root penetration, survival in soil, and long term soil compaction effect. Plant Production Science 11: 440–446. [Google Scholar]
- Souty N, Rode C. 1987. Aspect mécanique de la croissance des racines. I. - Mesure de la force de pénétration. Agronomie 7: 623–630. [Google Scholar]
- Striker GG, Insausti P, Grimoldi AA, Vega AS. 2007. Trade-off between root porosity and mechanical strength in species with different types of aerenchyma. Plant, Cell and Environment 30: 580–589. [DOI] [PubMed] [Google Scholar]
- Suralta RR, Kano-Nakata M, Niones JM et al. 201. 8. Root plasticity for maintenance of productivity under abiotic stressed soil environments in rice: Progress and prospects. Field Crops Research, in press. doi:10.1016/j.fcr.2016.06.023. [Google Scholar]
- Suzuki N, Rivero RM, Shulaev V, Blumwald E, Mittler R. 2014. Abiotic and biotic stress combinations. New Phytologist 203: 32–43. [DOI] [PubMed] [Google Scholar]
- Szymanowska-Pułka J. 2013. Form matters: morphological aspects of the lateral root development. Annals of Botany 112: 1643–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimoto E, Fujii S, Yamamoto R, Inanaga S. 2000. Measurement of viscoelastic properties of root cell walls affected by low pH in lateral roots of Pisum sativum L. Plant and Soil 226: 21–28. [Google Scholar]
- Tardieu F. 1994. Growth and functioning of roots and of root systems subjected to soil compaction. Towards a system with multiple signalling?Soil and Tillage Research 30: 217–243. [Google Scholar]
- Thompson MV, Holbrook NM. 2004. Root-gel interactions and the root waving behavior of Arabidopsis. Plant Physiology 135: 1822–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy SR, Black CR, Roberts JA, Mooney SJ. 2011. Soil compaction: a review of past and present techniques for investigating effects on root growth. Journal of the Science of Food and Agriculture 91: 1528–1537. [DOI] [PubMed] [Google Scholar]
- Tsegaye T, Mullins CE. 1994. Effect of mechanical impedance on root growth and morphology of two varieties of pea (Pisum sativum L.). New Phytologist 126: 707–713. [Google Scholar]
- Veen BW. 1982. The influence of mechanical impedance on the growth of maize roots. Plant and Soil 66: 101–109. [Google Scholar]
- von Sachs J. 1887. Lecture XXVII. Relations between growth and cell-division in the embryonic tissues. In: Lectures in plant physiology. Oxford: Clarendon Press, 431–459. [Google Scholar]
- Whiteley GM, Utomo WH, Dexter AR. 1981. A comparison of penetrometer pressures and the pressures exerted by roots. Plant and Soil 61: 351–364. [Google Scholar]
- Wiersum LK. 1957. The relationship of the size and structural rigidity of pores to their penetration by roots. Plant and Soil 9: 75–85. [Google Scholar]
- Wilson AJ, Robards AW. 1978. The ultrastructural development of mechanically impeded barley roots. Effects on the endodermis and pericycle. Protoplasma 95: 255–265. [Google Scholar]
- Wilson AJ, Robards AW. 1979. Some observations of the effects of mechanical impedance upon the ultrastructure of the root caps of barley. Protoplasma 101: 61–72. [Google Scholar]
- Wilson AJ, Robards AW, Goss MJ. 1977. Effects of mechanical impedance on root growth in barley, Hordeum vulgare L. II. Effects on cell development in seminal roots. Journal of Experimental Botany 28: 1216–1227. [Google Scholar]
- Yamamoto C, Sakata Y, Taji T, Baba T, Tanaka S. 2008. Unique ethylene-regulated touch responses of Arabidopsis thaliana roots to physical hardness. Journal of Plant Research 121: 509–519. [DOI] [PubMed] [Google Scholar]
- Yan J, Wang B, Zhou Y. 2017. A root penetration model of Arabidopsis thaliana in phytagel medium with different strength. Journal of Plant Research 130: 941–950. [DOI] [PubMed] [Google Scholar]
- Young IM, Montagu K, Conroy J, Bengough AG. 1997. Mechanical impedance of root growth directly reduces leaf elongation rates of cereals. New Phytologist 135: 613–619. [Google Scholar]
- Zacarias L, Reid MS. 1992. Inhibition of ethylene action prevents root penetration through compressed media in tomato (Lycopersicon esculentum) seedlings. Physiologia Plantarum 86: 301–307. [Google Scholar]