Abstract
Background and Aims
Anaesthesia for medical purposes was introduced in the 19th century. However, the physiological mode of anaesthetic drug actions on the nervous system remains unclear. One of the remaining questions is how these different compounds, with no structural similarities and even chemically inert elements such as the noble gas xenon, act as anaesthetic agents inducing loss of consciousness. The main goal here was to determine if anaesthetics affect the same or similar processes in plants as in animals and humans.
Methods
A single-lens reflex camera was used to follow organ movements in plants before, during and after recovery from exposure to diverse anaesthetics. Confocal microscopy was used to analyse endocytic vesicle trafficking. Electrical signals were recorded using a surface AgCl electrode.
Key Results
Mimosa leaves, pea tendrils, Venus flytraps and sundew traps all lost both their autonomous and touch-induced movements after exposure to anaesthetics. In Venus flytrap, this was shown to be due to the loss of action potentials under diethyl ether anaesthesia. The same concentration of diethyl ether immobilized pea tendrils. Anaesthetics also impeded seed germination and chlorophyll accumulation in cress seedlings. Endocytic vesicle recycling and reactive oxygen species (ROS) balance, as observed in intact Arabidopsis root apex cells, were also affected by all anaesthetics tested.
Conclusions
Plants are sensitive to several anaesthetics that have no structural similarities. As in animals and humans, anaesthetics used at appropriate concentrations block action potentials and immobilize organs via effects on action potentials, endocytic vesicle recycling and ROS homeostasis. Plants emerge as ideal model objects to study general questions related to anaesthesia, as well as to serve as a suitable test system for human anaesthesia.
Keywords: Anaesthetics, Arabidopsis roots, cress seeds, chlorophyll accumulation, endocytic vesicle recycling, Drosera leaf trap, Mimosa seedlings, pea tendrils, plant movements, plant action potentials, reactive oxygen species, Venus flytrap
INTRODUCTION
The use of ether for medical purposes, such as anaesthesia, was introduced and first described in 1818 by Michael Faraday, famous for his work on electromagnetic fields (Bergman, 1992). In 1846, its utility was first demonstrated during a surgical procedure to painlessly remove a tumour from the neck of a patient who had inhaled ether vapour. Before that, ‘surgery’ and ‘pain’ were synonymous (Rinaldi, 2014). Many different chemicals have since been found to induce anaesthesia. These include diethyl ether, chloroform, halothane, isoflurane and xenon. In the current pharmaceutical market, an enormous number of anaesthetic drugs are being produced industrially. However, although anaesthetics have been used over a 150-year period, the exact mode of anaesthetic drug action on the animal nervous system remains controversial. One of these mysteries is how these different compounds with no structural similarities, even chemically inert elements such as xenon (a noble gas), behave as anaesthetic agents inducing loss of consciousness (Lawrence et al., 1946; Cullen and Gross, 1951; Pauling, 1961; Sonner, 2008; Sonner and Cantor, 2013; Turin et al., 2014). In the early history of anaesthesia research, the theory of the Meyer–Overton correlation was proposed (Meyer, 1899; Overton, 1901). This explains that the magnitude of anaesthesia of different compounds correlates well with their lipid-solubility. This ‘general’ theory has been abandoned and researchers are now attempting to identify specific receptors or neurons responding to anaesthetics (Franks, 2008). Remarkably, anaesthesia extends to plants. Claude Bernard demonstrated that the sensitive plant, Mimosa pudica L., was unresponsive in closing leaves upon touch under diethyl ether. He concluded that plants and animals must share a common biological essence that is disrupted by anaesthetics (Bernard, 1878; Perouansky, 2012; Grémiaux et al., 2014). After the work of Claude Bernard, many plant physiologists reported similar effects of anaesthetics on plants (Bancroft and Rutzler, 1931; Bünning, 1934; De Luccia, 2012; Perouansky, 2012; Grémiaux et al., 2014). However, it is still not known whether the effects of anaesthetics in plants are related to plant action potentials and which cellular processes are affected by these compounds in plant cells.
The relevance of anaesthetics for plants is also clear given that they generate their own endogenous anaesthetics such as ethanol, divinyl ether and ethylene, especially when under stress (Dillard, 1930; Finer, 1965; Stumpe et al., 2008; Fammartino et al., 2010). For example, Kimmerer and Kozlowski (1982) reported that stressed pine and birch seedlings, as well as 11 other analysed plants, release ethylene, ethane, acetaldehyde and ethanol, all compounds that have anaesthetic actions in animals and humans (Koppanyi, 1945; Eger and Laster, 2001). In fact, plants emit huge amounts of low-molecular-mass hydrocarbons and other volatile compounds (Sharkey, 1996; Niinemets et al., 2004; Loreto et al., 2006), and many of these compounds have anaesthetic properties (Eger and Laster, 2001; Baluška et al., 2016). Interestingly, plants release volatile compounds not only to cope with stress but also to perform better in plant competition, many of the compounds acting as allelochemicals (Kegge and Pierik, 2009). Plants produce both general and local anaesthetics such as diverse alkaloids and flavonoids, menthol, cocaine, atropine, monoterpenes and phenylpropanes (Ghelardini et al., 2001; Ruetsch et al., 2001; Facanha and Gomes, 2005; Watt et al., 2008; de Lima Silva et al., 2013; Behcet, 2014; Tsuchiya, 2017). We can expect that this list will become much longer in the future. Recently, dozens of anaesthetics and anaesthesia-related compounds isolated from plants were reviewed, including essential oils, terpenoids and alkaloids (Tsuchiya, 2017).
In addition to plant stress adaptation, other plant processes are known to be under control of endogenous compounds having anaesthetic actions. Ethanol and other anaesthetics overcome dormancy of Panicum dichotomiflorum and other plant species seeds and this effect can be reversed by pressure of >1 MPa during seed exposure to these anaesthetics (Taylorson and Hendricks, 1979, 1980). Moreover, secondary dormancy induction via high-temperature incubation of giant foxtail (Setaria faberi) seeds was prevented by anaesthetics (Taylorson, 1982). The pressure reversal of the impacts of anaesthetics on plants was also confirmed in their effects on lipid composition in barley root cells (Jackson and John, 1984). As in cells of animal and humans, anaesthetics fluidize membranes and pressure reverses these impacts. This feature suggests strongly that anaesthetics induced genuine anaesthetic actions on plants as pressure is well known to reverse all actions of anaesthetics, including loss of consciousness, in animals and humans (Johnson and Miller, 1970; Wlodarczyk et al., 2006; Heimburg and Jackson, 2007; Græsbøll et al., 2014).
In the present study, we have used a wider range of plants compared with published studies. We show that anaesthetics stop both action potentials and plant movements. Moreover, the anaesthetics tested affect endocytic vesicle recycling and homeostasis of reactive oxygen species (ROS). These results suggest that the action of anaesthetics is similar in plants and animals and that they target some general molecules and/or processes related to cellular membranes rather than specific receptors. Finally, anaesthetics also affect plant-specific aspects such as chlorophyll accumulation and seed germination.
MATERIALS AND METHODS
Plant materials
Sensitive plant (Mimosa pudica L.), Venus flytrap (Dionaea muscipula Ellis), Cape sundew (Drosera capensis L.), pea (Pisum sativum L.) and garden cress (Lepidium sativum L.) were obtained from a local garden store. These plants were maintained in a growth chamber at 23 °C several days before the anaesthetic experiments. Arabidopsis (Arabidopsis thaliana L.) Columbia wild type (Col-0) seeds were soaked in a sterilizing solution containing 12 % sodium hypochlorite and 0.1 % Triton X-100 for 15 min and washed at least five times with sterile distilled water. Sterilized seeds were planted on a 0.4 % phytagel-fixed half-strength Murashige-Skoog (MS) growth medium without vitamin B. Petri dishes were incubated vertically at 23 °C under 16-h/8-h light–dark cycle.
Impacts of anaesthetics on plant movements
These plants were acclimated in a glass chamber under a fume hood. A certain volume of diethyl ether (Carl Roth GmbH, Karlsruhe, Germany) reaching 15 % of vapour was poured into a small beaker, and Mimosa and Dionaea were treated for 1 h in the sealed glass chamber. The same procedure was made for the pea plant experiment. The approximate volume of diethyl ether was calculated using ideal gas constant (standard state of gas as 22.4 l–1 mol). For instance, to obtain 15 % vapour in a 1-litre test chamber, a liquid phase of 700 µL diethyl ether (74.12 g mol–1, 0.71 g cm–3) was poured into a beaker and allowed to evaporate inside the sealed glass chamber. There are no toxic impacts of this concentration of diethyl ether, as well as of the other anaesthetics used, and the effects are fully reversible after their removal.
For the lidocaine experiment, Mimosa roots were gently washed to remove soil and cultured in water-filled Erlenmeyer flasks for several days for adaptation to hydroponic growth conditions. A 1 % lidocaine hydrochloride monohydrate (Sigma-Aldrich, Schelldorf, Germany) solution was replaced with water in the flask, and only the roots of Mimosa were treated for 4 h. Leaves of Mimosa were stimulated with a paintbrush by stroking along petioles. Trigger hairs in traps of Dionaea were touched at least twice with the tip of the metal needle. Trap movement p was recorded with a single-lens reflex camera (Canon EOS Kiss X7i). The sequences of plant responses were followed in the same individuals. The responses of anaesthetic-treated plants were observed at least three times and representative images and movies are shown here.
The Cape sundew plants (Drosera capensis L.) were enclosed in a small jar (volume of 5 litres) in an atmosphere of 15 % diethyl ether. At the same time the control plants were enclosed in another jar without diethyl ether. After 90 min, three crushed dead fruit flies (Drosophila melanogaster) were put on individual sticky adhesive traps of sundew in control and diethyl ether-treated traps. In this way, four or five traps were fed on the same plant. We used dead crushed prey because in a diethyl ether atmosphere, the flies were anaesthetized (they do not move and do not provide mechanical stimuli) in comparison to control plants. The plants were kept for another 3 h in an atmosphere of diethyl ether. The effectiveness of the anaesthetic was observed and quantified as tentacle and trap bending reaction. After 3 h, the diethyl ether was removed to observe speeds of recovery after treatments.
Measurement of action potentials
Venus flytrap plants were incubated in 15 % diethyl ether for 2 h in a polypropylene bag with attached electrodes inside. The bag was then opened and the trigger hair was touched repeatedly every 100 s. The action potentials were measured on the trap surface inside a Faraday cage with non-polarizable Ag–AgCl surface electrodes (Scanlab systems, Prague, Czech Republic) fixed with a plastic clip and moistened with a drop of conductive EV gel (VUP, Prievidza, Slovakia) commonly used in electrocardiography. The reference electrode was taped to the side of the plastic pot containing the plant submerged in 1–2 cm of water in a dish beneath the pot. The electrodes were connected to an amplifier made in-house [gain 1–1000, noise 2–3 µV, bandwidth (–3 dB) 105 Hz, response time 10 µs, input impedance 1012 Ω]. The signals from the amplifier were transferred to an analog–digital PC data converter (eight analog inputs, 12-bit converter, ±10 V, PCA-7228AL, supplied by TEDIA, Plzeň, Czech Republic), collected every 6 ms.
Effects of anaesthetics on seed germination and chlorophyll accumulation
Garden cress seeds were directly placed on damp double-layered filter paper with 4 mL of distilled water in 9-cm circular Petri dishes. Lidocaine was dissolved in distilled water. For gaseous compounds, diethyl ether or ethyl vinyl ether was applied as liquid phase into a small container made from a lid of a 1.5-mL Eppendorf tube, and the Petri dish was immediately sealed to obtain 15 % vapour inside the dish. For xenon treatment, xenon and oxygen (both from Sigma-Aldrich) were premixed at a ratio of 80: 20 % in a 60-mL plastic syringe. The xenon gas was then insufflated into the tightly sealed Petri dish through a silicon tube. The dishes were incubated for 24 h at room temperature under continuous light conditions. For recovery experiments, the anaesthetic compounds were removed either by exchanging air or distilled water. For extraction of chlorophyll, the leaf samples were collected from the 2-d-old anaesthesia-treated seedlings (24 h of anaesthesia treatment followed by 24 h of incubation). The sample was weighed and ground in 1.5 mL of cold acetone. Crude extract was centrifuged at 10 000 g for 5 min. The supernatant was collected and mixed with 2.5 mm K-phosphate buffer (pH 7.4) to obtain 80 % acetone-extract solution. Absorbance of the extracted solution was measured at wavelengths of 663.6 and 646.6 nm with a spectrophotometer. The content of chlorophyll a and chlorophyll b in each sample was calculated using the equations given by Porra et al. (1989).
Histochemical ROS staining
Nitro blue tetrazolium salt (NBT; Sigma-Aldrich) was dissolved in 0.1 m Tris-HCl buffer (pH 9.5) with 0.1 m NaCl and 0.05 m MgCl2 to obtain 5 mm of stock solution. For maize and Arabidopsis roots, 500 µm and 5 mm of NBT was used for staining, respectively. Roots were treated with xenon (Xe 80 %: O2 20 %) or 15 % diethyl ether or 1 % lidocaine (w/v) in the same manner as described above for 1 h at room temperature. The samples were then incubated in NBT solution for 15 min prior to microscopic observation.
Effects of anaesthetics on endocytic vesicle recycling in root apex cells
Five days after germination, Arabidopsis seedlings were stained with 4 µmN-(3-triethylammoniumpropyl)-4-(6-(4-(diethylamino) phenyl) hexatrienyl) pyridinium dibromide (FM4-64; Sigma-Aldrich) dye dissolved in distilled water for 10 min. Seedlings were then rinsed and soaked in either 0 or 1 % lidocaine solution for 30 min. For gaseous anaesthetic treatments, seedlings were placed back into 1/2 MS plates after FM4-64 staining and treated. For diethyl ether treatment, approximately 25 µL diethyl ether was dropped onto the surface of phytagel and the lid of the Petri dish was quickly closed to obtain 15 % diethyl ether vapour. For xenon treatment, xenon and oxygen were mixed at a ratio of 80: 20 % in a 60-mL syringe. The xenon gas was then insufflated into the tightly sealed Petri dish with seedlings. In total, 200 mL of gas was used for each dish. The diethyl ether and xenon treatments were conducted for 30 and 90 min, respectively. After treatment with these anaesthetics, all seedlings were treated with 35 µm Brefeldin A (BFA; Sigma-Aldrich), which blocks endocytic vesicle recycling (Lippincott-Schwartz et al., 1991; Baluška et al., 2002), for 30 min.
Confocal microscopy
Images of FM4-64-stained BFA-treated cells were taken by confocal laser microscopy (Fluoview FV1000; Olympus, Germany). The FM4-64 was excited at 545 nm by an He–Ne laser. Fluorescence emissions were collected between 630 and 700 nm. The summation of BFA compartment areas visualized with FM4-64 fluorescence in a unit area (50 µm2) was calculated from the confocal image of root epidermal cells. The square contains about 20–30 BFA compartments. They were then averaged from independent root samples with ImageJ software (Mac OSX version 1.43r).
Statistical analysis
For statistical analyses of the quantification of chlorophyll contents (see Fig. 3B) and BFA compartment size (see Fig. 4D), P-values were calculated with a two-tailed Student’s t-test. P-values <0.05 were considered significant.
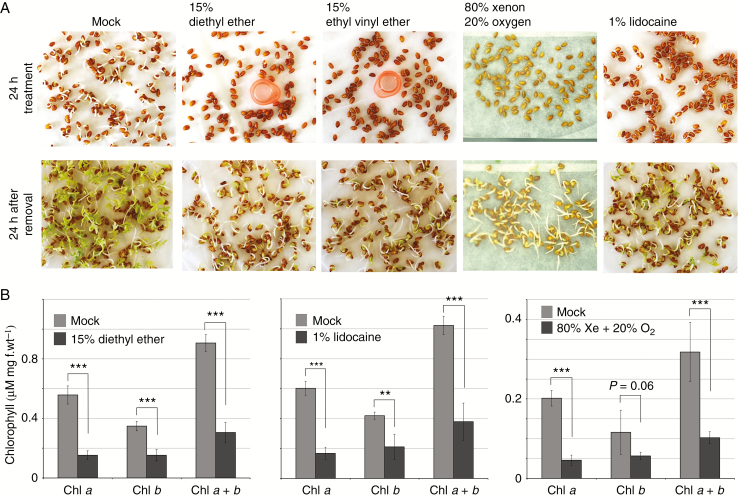
Fig. 3.
Inhibition of dormancy breaking and chlorophyll accumulation under anaesthetics. (A) Inhibition of cress seed germination under anaesthetic treatment. After 24 h, all anaesthetics inhibited germination. Seed germination recovered completely 24 h after removal of anaesthetics. (B) Anaesthetic treatment attenuates chlorophyll accumulation. Chlorophyll was extracted from the leaves of cress seedlings germinated after 24 h of anaesthetic treatment. The values are averaged from five independent treatments. The error bars represent the standard deviation (***P < 0.001; **P < 0.01)
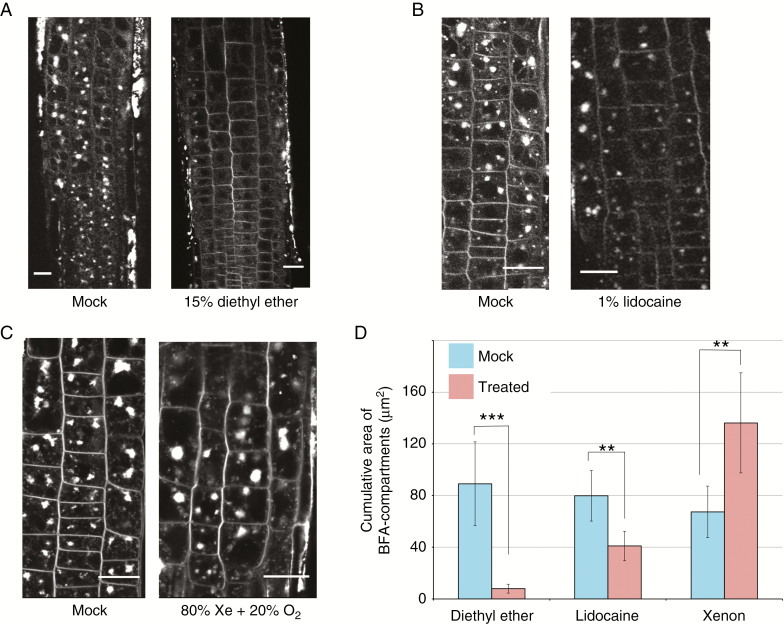
Fig. 4.
Anaesthetics modify vesicle recycling in Arabidopsis root epidermal cells. (A) Treatment with 15 % diethyl ether for 30 min; (B) 1 % lidocaine for 30 min; (C) 80 % xenon mixed with 20 % oxygen for 90 min. (D) A comparison of the size of BFA-induced compartments between mock and treated cells. The total area of BFA-induced compartments located inside a square of 50 μm2 was calculated and averaged from 7–9 independent roots. Error bars represent the standard deviation (***P < 0.001; **P < 0.01).
RESULTS
Effects of anaesthetics on plant organ movements
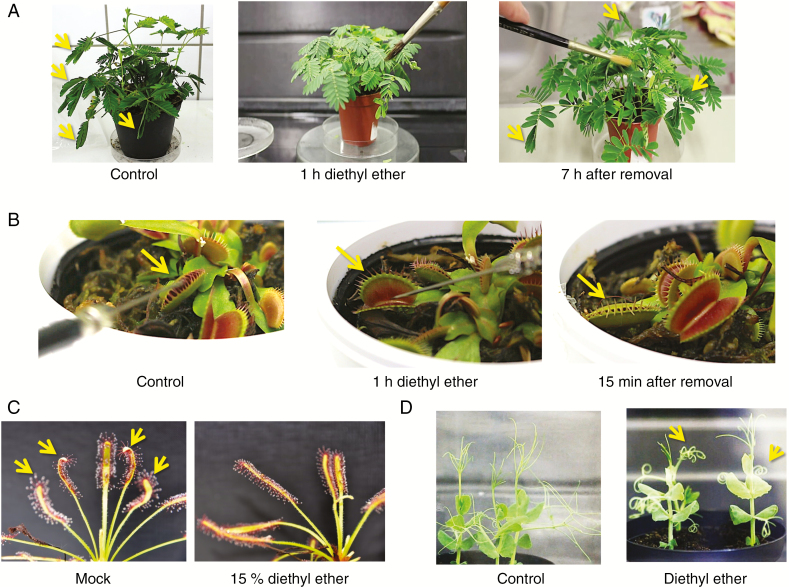
Diethyl ether vapour immobilized the leaf-closing reaction of sensitive plants Mimosa pudica (Fig. 1A). Leaf closure was observed by gently stroking the petiole with a paintbrush (Supplementary Data Video S1). After 1 h of 15 % diethyl ether treatment, the Mimosa plants completely lost their response to touch stimuli (Video S2). Once diethyl ether was removed by exchanging the air in a treatment chamber, leaf response was gradually recovered, and returned to normal 7 h after its removal (Video S3).
Fig. 1.
Effects of a volatile anaesthetic agent, diethyl ether, on plant movements. (A) The leaf-closing movement of Mimosa pudica under 15 % diethyl ether. After 1 h of treatment, leaves completely lost the response to touch stimuli. All leaves gradually recovered closure movement after 7 h following the removal of diethyl ether. Arrows indicate closed leaves. (B) The rapid trap movement of Dionaea muscipula disappeared after 1 h of 15 % diethyl ether treatment. The arrow indicates the leaf stimulated. (C) Sundew plant (Drosera capensis) showed no prey reaction under 15 % diethyl ether atmosphere. Arrows show normal trap bending reaction. (D) The movement of tendrils disappears with 15 % diethyl ether. The relevant movies are available as Supplementary Data.
Another moving plant, Venus flytrap (Dionaea muscipula), was also introduced in order to monitor the effect of diethyl ether. As shown in Fig. 1B, control plants closed the leaf trap after two or three stimulations of the trigger hair inside the leaves (Video S4). One hour of 15 % diethyl ether treatment completely abolished the response even though trigger hairs were stimulated many times (Video S5). The response recovered 15 min after the removal of diethyl ether (Video S6).
Cape sundew (Drosera capensis) is a well-known carnivorous plant that captures its prey by moving leaves covered with sticky tentacles. As shown in Fig. 1C, all leaves tested in the control plants had a strong leaf and tentacle bending reaction within 1 h in response to being fed crushed dead fruit flies. By contrast, leaves treated with 15 % diethyl ether showed no bending reaction. The reaction was not merely slowed but completely inhibited. After the removal of diethyl ether, the leaf bending reaction was recovered within a few hours (not shown).
Under normal conditions, growing pea tendrils show a rotating trajectory in free space (Fig. 1D and Video S7) (Jaffe and Galston, 1966). When the pea plants were exposed to 15 % diethyl ether, tendrils completely stopped their autonomous circumnutations immediately and were immobilized in a curled shape (Video S8). Interestingly, the application of a 1 % lidocaine solution only to the root part (local anaesthesia) of sensitive plant leaves also abolished the response to touch stimulus after 5 h of treatment (Video S9). Leaf responsiveness recovered 17 h after the solution was replaced with distilled water (Video S10).
Loss of action potentials in trigger hairs of Venus flytrap under diethyl ether
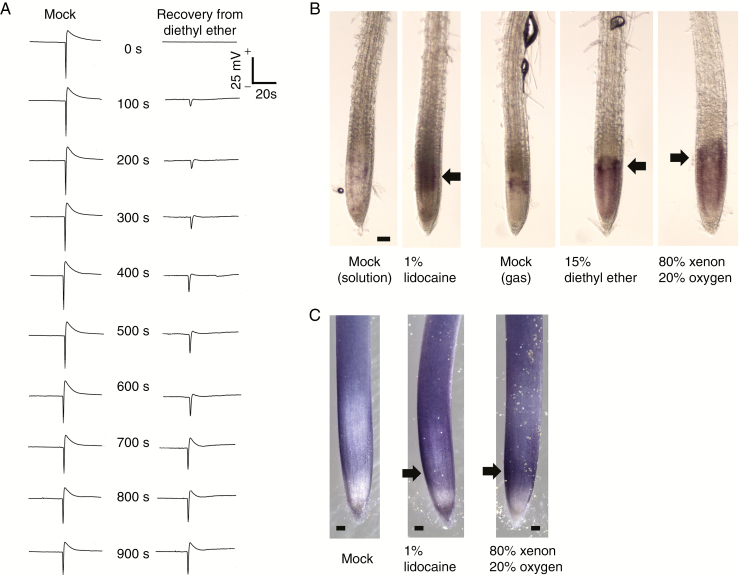
The 15 % diethyl ether treatment completely attenuated action potentials in response to trigger hair stimulation (Fig. 2A). After removal of diethyl ether, the amplitude of recorded action potentials gradually recovered (recorded every 100 s) and returned to the normal state after 900 s. Importantly, this recovery time of action potential is remarkably consistent with the observed leaf movements shown in Fig. 1B.
Fig. 2.
Disappearance and recovery of action potentials in Dionaea muscipula, and the production of reactive oxygen species in Arabidopsis roots under anaesthesia. (A) Recovery of diethyl ether inhibition of action potential on leaves of Venus flytrap in response to trigger hair stimulation recorded every 100 s after removal of diethyl ether. (B) NBT histochemical staining to detect superoxide production in Arabidopsis root apex. The treatment with anaesthetics promoted the generation of superoxide between the meristem and transition zones. (C) NBT staining in maize roots under lidocaine and xenon treatment. The purple–blue colour represents the area of superoxide generation. The black arrows indicate the position of strong NBT staining pattern. Representative images are shown from 7–9 stained samples.
Anaesthetics induce exaggerated production of ROS in Arabidopsis and maize root apices
We used the NBT histochemical staining procedure to detect superoxide production in Arabidopsis root apex. The treatment of roots with diethyl ether for 1 h promoted exaggerated generation of superoxide in the meristem and the root apex transition zones (Fig. 2B). Similarly high ROS production was observed in maize root apices under the lidocaine and xenon 1 h exposure. The purple–blue colour represents the area of superoxide generation (Fig. 2C).
Anaesthetics inhibit dormancy breaking and chlorophyll accumulation
Intriguingly, seeds of garden cress (Lepidium sativum) failed to break dormancy under the anaesthetic treatments. Seeds were incubated on moist filter paper under continuous light. Petri dishes were tightly sealed and filled with individual treatments of 15 % diethyl ether, 15 % ethyl vinyl ether or 80 % xenon with 20 % oxygen. For lidocaine treatment, filter paper was moistened with 1 % lidocaine solution instead of distilled water. As shown in Fig. 3A, dormancy was prolonged for 24 h under all the anaesthetics whereas the mock treatment revealed seed germination. Once the anaesthetics were removed, all seeds broke their dormancy over the following 24 h. Cotyledons of germinated seedlings treated with anaesthetics exhibited a distinct yellowish colour. Treatment with diethyl ether, lidocaine and xenon reduced the chlorophyll content in leaves (Fig. 3B). These results suggest that the anaesthetics used here impede dormancy breaking and chlorophyll synthesis and/or assembly in the thylakoid membranes. As anaesthetics also impair mitochondrial functions (Sanchez et al., 2011; Boscolo et al., 2012), these compounds might also have negative effects on the formation of thylakoid membranes of chloroplasts.
Anaesthetics affect membrane dynamics and vesicle trafficking in root apex cells
It is currently proposed that many anaesthetics are likely to interfere with lipid membranes. We checked the effect of anaesthetics on Arabidopsis root cells in terms of membrane trafficking, which is based on an elaborate maintenance of cellular membrane dynamics. The 15 % diethyl ether and 1 % lidocaine treatments slowed the rate of endocytic vesicle recycling, a process that was, in particular, completely diminished in the presence of diethyl ether (Fig. 4A, B). By contrast, xenon treatment increased the size of BFA-induced compartments in root epidermal cells. Although the mechanism involved remains unclear, these results indicate that anaesthetics alter normal membrane properties and vesicle trafficking in plant root cells.
DISCUSSION
The fact that plant cells responded to these compounds in a similar manner to animals and humans is intriguing. It has previously been demonstrated that Mimosa plants lose their leaf-closing response to touch stimulus under exposure to lidocaine and xenon (Weigl, 1968; Milne and Beamish, 1999). Importantly, as we show here, exposure of just roots of Mimosa seedlings to lidocaine is sufficient to block the movements of their shoots. Plant sensitivity to anaesthetics might help to reveal elusive mechanisms of their actions. It is puzzling how such chemically and physically diverse compounds, including the chemically inert gas xenon, the volatile organic solvent ether and water-soluble molecule such as lidocaine, can induce very similar impacts in both plants and animals.
Our present data not only expand on the plant systems tested for anaesthetics by using pea tendrils (Jaffe and Galston, 1966), seeds of garden cress and cape sundew leaf traps, but also show for the first time that the immobilization of plant organ movements is based on inhibition of action potentials. In other words, as in animals and humans, bioelectricity and action potentials animate not only humans and animals but also plants. In Venus flytrap, action potentials are also necessary to close the trap and to initiate the digestive processes (Hodick and Sievers, 1989; Böhm et al., 2016). The number of action potentials is translated via gland cells into the touch-inducible jasmonate signalling that promotes the formation of acidic secretory vesicles, which drive development of this ‘green stomach’ (Escalante-Pérez et al., 2011; Scherzer et al., 2017; Pavlovič et al., 2017). The ultimate prediction from our present data is that anaesthetics will prevent closure of the Venus flytrap and its subsequent transformation into the ‘green stomach’. That animals/humans and also plants are animated via action potentials is of great importance for our ultimate understanding of the elusive nature of plant movements and plant-specific cognition/intelligence based plant behaviour (Pollan, 2013; Gagliano, 2014; Baluška and Levin, 2016; Calvo et al., 2016; Gagliano et al., 2016; Gross, 2016; Trewavas, 2016, 2017; van Loon, 2016; Calvo and Friston, 2017; Krausko et al., 2017). It should be not surprising that plants are sensitive to anaesthetics as they express and use similar critical proteins that have been discussed as possible targets of anaesthetics in animals and humans, including glutamate and γ-aminobutyric acid (GABA) receptors (Price et al., 2012; Ramesh et al., 2015, 2017; Weiland et al., 2016; Žárský, 2015; Chen et al., 2016; De Bortoli et al., 2016). Saltveit (1993) reported that the anaesthetics halothane and methoxyflurane reduced chilling injury in cucumber cotyledons, cucumber hypocotyls and tomato pericarp. The relative effectiveness of the anaesthetics in reducing chilling injury was similar to their relative effectiveness in inducing anaesthesia in animals and their relative lipid solubility.
Although there is a strong consensus that anaesthesia results in loss of consciousness, it remains unclear how different kinds of chemical compounds bring about the same anaesthetic state. It is logical to expect that any cellular system that is affected or disrupted by an anaesthetic compound must be important for maintaining neural activities. Extensive work has been performed to investigate specific receptors or mechanisms perceiving anaesthetic compounds (Franks, 2008). However, many controversies remain, for example lipid (membrane) theory versus protein (receptor) theory (Rinaldi, 2014). One of main reasons behind this problem is the limited access to living tissues under anaesthesia treatment. Here we showed that the sensitive plant, Venus flytrap and Cape sundew plants, as well as pea tendrils, were immobilized with diethyl ether. Under anaesthesia treatment, Venus flytrap lost the ability to generate action potentials in response to touch, whereas pea tendrils stopped their autonomous searching movements and were immobilized in a curled stature. Xenon was effective at different levels of responses, such as seed germination, chlorophyll accumulation, ROS production and vesicle recycling. Importantly, seismonastic movements of Mimosa leaves are also based on action potentials in pulvinus motor cells (Volkov et al., 2010a, b). These electrical signals in pulvinus motor cells are closely associated with the actin cytoskeleton and calcium signalling (Kanazawa et al., 2006; Visnovitz et al., 2007; Yao et al., 2008). In future, patch clamp analysis of these excitable motor cells might prove to be useful. All these results suggest that critical molecules and/or lipids of membranes are targets of anaesthetics. As in animals, they block action potentials due to their actions on membranes and their lipids also in plants.
Although the current mainstream prefers the receptor theory of the action of anaesthetics, the fact that all life can be anaesthesized (Bernard, 1878; Wolfe et al., 1998; Eckenhoff, 2008; Sonner, 2008; La Monaca and Fodale, 2012; Perouansky, 2012; Sonner and Cantor, 2013; Rinaldi, 2014; Baluška et al., 2016) and the general validity of high-pressure reversal of anaesthesia speak strongly against this anaesthetic receptor theory (Johnson and Miller, 1970; Taylorson and Hendricks, 1979, 1980; Wlodarczyk et al., 2006; Heimburg and Jackson, 2007; Græsbøll et al., 2014). The physical action of anaesthetics was also supported recently by Turin et al. (2014) who reported connections between electron spin and anaesthesia. It is possible that the ultimate target of anaesthetics will be shown to be the electronic structure of critical proteins embedded within lipid bilayers of membranes (Ueda et al., 1977; Kamaya et al., 1981; Heimburg and Jackson, 2007; Booker and Sum, 2013; Græsbøll et al., 2014). Anaesthetics impact lipid bilayer thickness and mechanical properties, both of which are well known to control protein functions (Andersen and Koeppe, 2007). Lipid rafts, in particular, which have central roles in intracellular communication and signalling (Lingwood and Simons, 2010; Simons and Sampaio, 2011; Head et al., 2014; Sezgin et al., 2017), are particularly sensitive to anaesthetics (Morrow and Parton, 2005; Bandeiras et al., 2013).
Importantly, Arabidopsis expresses a homologue of the lipid raft organizer flotillin Flot1, which is involved in the clathrin-independent endocytic pathway (Li et al., 2012; Yu et al., 2017) in root apex cells (Li et al., 2012). Interestingly in this respect, lipid rafts are very abundant at the Arabidopsis root apex cross walls (Zhao et al., 2015a, b), and are active in endocytic vesicle recycling (Zhao et al., 2015b), which is a typical feature for this root apex zone (Baluška et al., 2002; Baluška et al., 2010; Baluška and Mancuso, 2013). Arabidopsis thaliana respiratory burst oxidase homologue D (RbohD) was reported to be localized to lipid rafts and we have shown that ROS regulate endocytic vesicle recycling (Hao et al., 2014; Yokawa et al., 2016). As our present data show that anaesthetics inhibit endocytic vesicle recycling in Arabidopsis root apex cells and disrupt ROS homeostasis, one possible scenario would be that anaesthetics primarily target the physical properties of membranes, especially lipid rafts. Consequently, this would then cause aberrant functions of membrane proteins and vesicle trafficking, inhibiting action potentials, and precluding plant organ movements.
In contrast to all the other anaesthetics used in our study, water-soluble lidocaine is a local anaesthetic known to act in animals via inhibition of the non-selectively voltage-gated sodium channels (Bean et al., 1983; Chevrier et al., 2004; Fozzard et al., 2011). The relevance of our data in this regard is still unclear and will await further experimental studies.
Arabidopsis model systems, with their excellent tools and mutant collections, represent an ideal in aims to illuminate the elusive mechanisms underlying both anaesthetic and the phenomenon of consciousness (Trewavas and Baluška, 2011; Mashour and Alkire, 2013; Baluška et al., 2016; Calvo et al., 2017). As plants in general, and the model plant A. thaliana in particular, are suitable to experimental manipulation (they do not run away) and allow easy electrical recordings, we propose them as ideal model objects to study anaesthesia and to serve as a suitable test system for human anaesthesia.
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and consist of the following. Videos S1–S10. Effects of anaesthetics on plant organ movements.
AUTHOR CONTRIBUTIONS
K.Y. and F.B. conceived the work and prepared the draft. K.Y. and T.K. conducted most of the experiments presented. A.P. performed the experiment using sundew plants and measured the action potential in Venus flytrap plants. S.G. recorded the time-lapse movie of the movement of pea tendrils. M.W. conducted the treatments with diethyl ether and lidocaine. S.M. provided the apparatus necessary for xenon treatment and evaluated the procedures and results. All authors edited the manuscript. The author have no competing financial interests.
Supplementary Material
ACKNOWLEDGMENTS
We thank the referees and editors for their comments and suggestions, which were very useful in preparation of the final version of the paper. K.Y. was supported by a JSPS (Japanese Society for the Promotion of Science) Postdoctoral Fellowship. This work was supported in part by JSPS KAKENHI, Grant-in-Aid for JSPS fellows, No. 261654 and by the Grant Agency of the Czech Republic No. 16-07366Y and LO1204 from The National Program of Sustainability I. We are very grateful to Dr. Luca Turin for his valueable suggestions and discussions.
LITERATURE CITED
- Andersen OS, Koeppe RE 2nd. 2007. Bilayer thickness and membrane protein function: an energetic perspective. Annual Review of Biophysics and Biomolecular Structure 36:107–130. [DOI] [PubMed] [Google Scholar]
- Baluška F, Levin M. 2016. On having no head: cognition throughout biological systems. Frontiers in Psychology 7:902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluška F, Mancuso S. 2013. Root apex transition zone as oscillatory zone. Frontiers in Plant Science 4:354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluška F, Hlavacka A, Samaj J et al. . 2002. F-actin-dependent endocytosis of cell wall pectins in meristematic root cells: insights from brefeldin A-induced compartments. Plant Physiology 130:422–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluška F, Yokawa K, Mancuso S, Baverstock K. 2016. Understanding of anesthesia – why consciousness is essential for life and not based on genes. Communications and Integrative Biology 9:e1238118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluška MS, Volkmann D, Barlow PW, Barlow PW. 2010. Root apex transition zone: a signalling-response nexus in the root. Trends in Plant Science 15:402–408. [DOI] [PubMed] [Google Scholar]
- Bancroft WD, Rutzler JE. 1931. Irritability and anesthesia in plants. Journal of Physical Chemistry 36:273–285. [Google Scholar]
- Bandeiras C, Serro AP, Luzyanin K, Fernandes A, Saramago B. 2013. Anesthetics interacting with lipid rafts. European Journal of Pharmaceutical Sciences 48:153–165. [DOI] [PubMed] [Google Scholar]
- Bean BP, Cohen CJ, Tsien RW. 1983. Lidocaine block of cardiac sodium channels. Journal of General Physiology 81:613–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behcet Al. 2014. The source-synthesis: history and use of atropine. Journal of Academic Emergency Medicine 13:2–3. [Google Scholar]
- Bergman NA. 1992. Michael Faraday and his contribution to anesthesia. Anesthesiology 77:812–816. [DOI] [PubMed] [Google Scholar]
- Bernard C. 1878. Leçons sur les phénomènes de la vie communs aux animaux et aux végétaux. (Librairie J.-B. Baillière et Fils).
- Böhm J, Scherzer S, Krol E et al. . 2016. The Venus flytrap Dionaea muscipula counts prey-induced action potentials to induce sodium uptake. Current Biology 26:286–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booker RD, Sum AK. 2013. Biophysical changes induced by xenon on phospholipid bilayers. Biochimica et Biophysica Acta 1828:1347–1356. [DOI] [PubMed] [Google Scholar]
- Boscolo A, Starr JA, Sanchez V et al. . 2012. The abolishment of anesthesia-induced cognitive impairment by timely protection of mitochondria in the developing rat brain: the importance of free oxygen radicals and mitochondrial integrity. Neurobiology of Disease 45:1031–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bünning E. 1934. Refraktärstadium, Ermüdung und Narkose bei der Seismonastie. Zeitschrift Wissenschaft Biol Abteilung E Planta 21:324–352. [Google Scholar]
- Calvo P, Baluška F, Sims A. 2016. “Feature Detection” vs. ‘Predictive Coding’ models of plant behavior. Frontiers in Psychology 7:1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo P, Friston K. 2017. Predicting green: really radical (plant) predictive processing. Journal of the Royal Society, Interface 14:20170096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo P, Sahi V, Trewavas A. 2017. Are plants sentient?Plant, Cell and Environment doi: 10.1111/pce.13065. [DOI] [PubMed] [Google Scholar]
- Chen J, Jing Y, Zhang X et al. . 2016. Evolutionary and expression analysis provides evidence for the plant glutamate-like receptors family is involved in woody growth-related function. Scientific Reports 6:32013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevrier P, Vijayaragavan K, Chahine M. 2004. Differential modulation of Nav1.7 and Nav1.8 peripheral nerve sodium channels by the local anesthetic lidocaine. British Journal of Pharmacology 142:576–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen SC, Gross EG. 1951. The anesthetic properties of xenon in animals and human beings, with additional observations on krypton. Science 113:580–582. [DOI] [PubMed] [Google Scholar]
- De Bortoli S, Teardo E, Szabò I, Morosinotto T, Alboresi A. 2016. Evolutionary insight into the ionotropic glutamate receptor superfamily of photosynthetic organisms. Biophysical Chemistry 218:14–26. [DOI] [PubMed] [Google Scholar]
- de Lima Silva L, Thomas da Silva D, Garlet QI et al. . 2013. Anesthetic activity of Brazilian native plants in silver catfish (Rhamdia quelen). Neotropical Ichthyology 11:443–451. [Google Scholar]
- De Luccia TP. 2012. Mimosa pudica, Dionaea muscipula and anesthetics. Plant Signaling and Behavior 7:1163–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillard MM. 1930. Ethylene – the new general anesthetic. Journal of the National Medical Association 22:10–11. [PMC free article] [PubMed] [Google Scholar]
- Eckenhoff RG. 2008. Why can all of biology be anesthetized?Anesthesia and Analgesia 107:859–861. [DOI] [PubMed] [Google Scholar]
- Eger EI 2nd Laster MJ. 2001. The effect of rigidity, shape, unsaturation, and length on the anesthetic potency of hydrocarbons. Anesthesia & Analgesia 92:1477–1482. [DOI] [PubMed] [Google Scholar]
- Escalante-Pérez M, Krol E, Stange A et al. . 2011. A special pair of phytohormones controls excitability, slow closure, and external stomach formation in the Venus flytrap. Proceedings of the National Academy of Sciences of the United States of America 108:15492–15497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facanha MF, Gomes LC. 2005. Efficacy of menthol as an anesthetic for tambaqui (Colossoma macropomum, Characiformes: Characidae). Acta Amazon 35:71–75 [Google Scholar]
- Fammartino A, Verdaguer B, Fournier J, Tamietti G, Carbonne F, Esquerré-Tugayé MT, Cardinale F. 2010. Coordinated transcriptional regulation of the divinyl ether biosynthetic genes in tobacco by signal molecules related to defense. Plant Physiology and Biochemistry 48:225–231. [DOI] [PubMed] [Google Scholar]
- Fozzard HA, Sheets MF, Hanck DA. 2011. The sodium channel as a target for local anesthetic drugs. Frontiers in Pharmacology 2:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finer B. 1965. Divinyl ether. British Journal of Anaesthesia 37:661–666. [DOI] [PubMed] [Google Scholar]
- Franks NP. 2008. General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nature Reviews. Neuroscience 9:370–386. [DOI] [PubMed] [Google Scholar]
- Gagliano M. 2014. In a green frame of mind: perspectives on the behavioural ecology and cognitive nature of plants. AoB Plants 7:plu075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagliano M, Vyazovskiy VV, Borbély AA, Grimonprez M, Depczynski M. 2016. Learning by association in plants. Scientific Reports 6:38427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghelardini C, Galeotti N, Mazzanti G. 2001. Local anaesthetic activity of monoterpenes and phenylpropanes of essential oils. Planta Medica 67:564–566. [DOI] [PubMed] [Google Scholar]
- Græsbøll K, Sasse-Middelhoff H, Heimburg T. 2014. The thermodynamics of general and local anesthesia. Biophysical Journal 106:2143–2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grémiaux A, Yokawa K, Mancuso S, Baluška F. 2014. Plant anesthesia supports similarities between animals and plants: Claude Bernard’s forgotten studies. Plant Signaling and Behavior 9:e27886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross M. 2016. Could plants have cognitive abilities?Current Biology 26:R181–RR184. [DOI] [PubMed] [Google Scholar]
- Hao H, Fan L, Chen T et al. . 2014. Clathrin and membrane microdomains cooperatively regulate RbohD dynamics and activity in Arabidopsis. Plant Cell 26:1729–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head BP, Patel HH, Insel PA. 2014. Interaction of membrane/lipid rafts with the cytoskeleton: impact on signaling and function: membrane/lipid rafts, mediators of cytoskeletal arrangement and cell signaling. Biochimica et Biophysica Acta 1838:532–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimburg T, Jackson AD. 2007. The thermodynamics of general anesthesia. Biophysical Journal 92:3159–3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodick D, Sievers A. 1989. On the mechanism of trap closure of Venus flytrap [Dionaea muscipula Ellis]. Planta 179:32–42. [DOI] [PubMed] [Google Scholar]
- Jackson PC, John St JB. 1984. Anesthetics alter the lipid composition of barley-root membranes. Planta 162:415–421. [DOI] [PubMed] [Google Scholar]
- Jaffe M, Galston A. 1966. Physiological studies on pea tendrils. I. Growth and coiling following mechanical stimulation. Plant Physiology 41:1014–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SM, Miller KW. 1970. Antagonism of pressure and anaesthesia. Nature 228:75–76. [DOI] [PubMed] [Google Scholar]
- Kamaya H, Suezaki Y, Ueda I, Eyring H. 1981. Anesthetics and high-pressure interaction upon elastic properties of a polymer membrane. Proceedings of the National Academy of Sciences of the United States of America 78:3572–3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanazawa N, Hoshino Y, Chiba M et al. . 2006. Change in the actin cytoskeleton during seismonastic movement of Mimosa pudica. Plant and Cell Physiology 47:531–539. [DOI] [PubMed] [Google Scholar]
- Kegge W, Pierik R. 2010. Biogenic volatile organic compounds and plant competition. Trends in Plant Science 15:126–132. [DOI] [PubMed] [Google Scholar]
- Kimmerer TW, Kozlowski TT. 1982. Ethylene, ethane, acetaldehyde, and ethanol production by plants under stress. Plant Physiology 69:840–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppanyi T. 1945. Acetaldehyde, a volatile anesthetic and sympathetic stimulant. Anesthesiology 6:603–611. [DOI] [PubMed] [Google Scholar]
- Krausko M, Perutka Z, Šebela M et al. . 2017. The role of electrical and jasmonate signalling in the recognition of captured prey in the carnivorous sundew plant Drosera capensis. New Phytologist 213:1818–1835. [DOI] [PubMed] [Google Scholar]
- La Monaca E, Fodale V. 2012. Effects of anesthetics on mitochondrial signaling and function. Current Drug Safety 7:126–139. [DOI] [PubMed] [Google Scholar]
- Lawrence JH, Loomis WF, Tobias CA, Turpin FH. 1946. Preliminary observations on the narcotic effect of xenon with a review of values for solubilities of gases in water and oils. Journal of Physiology 105:197–204. [PMC free article] [PubMed] [Google Scholar]
- Li R, Liu P, Wan Y et al. . 2012. A membrane microdomain-associated, protein, Arabidopsis Flot1, is involved in a clathrin-independent endocytic pathway required for seedling development. Plant Cell 24:2105–2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingwood D, Simons K. 2010. Lipid rafts as a membrane-organizing principle. Science 327:46–50. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz J, Yuan L, Tipper C, Amherdt M, Orci L, Klausner RD. 1991. Brefeldin A’s effects on endosomes, lysosomes, and the TGN suggest a general mechanism for regulating organelle structure and membrane traffic. Cell 67:601–616. [DOI] [PubMed] [Google Scholar]
- Loreto F, Barta C, Brilli F, Nogues I. 2006. On the induction of volatile organic compound emissions by plants as consequence of wounding or fluctuations of light and temperature. Plant, Cell and Environment 29:1820–1828. [DOI] [PubMed] [Google Scholar]
- Mashour GA, Alkire MT. 2013. Evolution of consciousness: phylogeny, ontogeny, and emergence from general anesthesia. Proceedings of the National Academy of Sciences of the United States of America 110(Suppl 2):10357–10364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer H. 1899. Zur Theorie der Alkoholnarkose. Arch Exp Pathol Pharmakol 42:109–118. [Google Scholar]
- Milne A, Beamish T. 1999. Inhalational and local anesthetics reduce tactile and thermal responses in Mimosa pudica. Canadian Journal of Anesthesia 46:287–289. [DOI] [PubMed] [Google Scholar]
- Morrow IC, Parton RG. 2005. Flotillins and the PHB domain protein family: rafts, worms and anaesthetics. Traffic 6:725–740. [DOI] [PubMed] [Google Scholar]
- Niinemets U, Loreto F, Reichstein M. 2004. Physiological and physicochemical controls on foliar volatile organic compound emissions. Trends in Plant Science 9:180–186. [DOI] [PubMed] [Google Scholar]
- Overton CE. 1901. Studien über die Narkose zugleich ein Beitrag zur Allgemeinen Pharmakologie. Fischer Verlag. [Google Scholar]
- Pauling L. 1961. A molecular theory of general anesthesia. Science 134:15–21. [DOI] [PubMed] [Google Scholar]
- Pavlovič A, Jakšová J, Novák O. 2017. Triggering a false alarm: wounding mimics prey capture in the carnivorous Venus flytrap [Dionaea muscipula Ellis]. New Phytologist 216:927–938. [DOI] [PubMed] [Google Scholar]
- Perouansky M. 2012. The quest for a unified model of anesthetic action: a century in Claude Bernard’s shadow. Anesthesiology 117:465–474. [DOI] [PubMed] [Google Scholar]
- Pollan M. 2013. The intelligent plant. New Yorker 23–30 December. [Google Scholar]
- Porra RJ, Thompson WA, Kriedemann RE. 1989. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochimica et Biophysica Acta – Bioenergetics 975:384–394. [Google Scholar]
- Price MB, Jelesko J, Okumoto S. 2012. Glutamate receptor homologs in plants: functions and evolutionary origins. Frontiers in Plant Science 3:235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesh SA, Tyerman SD, Xu B et al. . 2015. GABA signalling modulates plant growth by directly regulating the activity of plant-specific anion transporters. Nature Communications 6:7879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesh SA, Tyerman SD, Gilliham M, Xu B. 2017. γ-Aminobutyric acid (GABA) signalling in plants. Cellular and Molecular Life Sciences 74:1577–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldi A. 2014. Reawakening anaesthesia research. EMBO Reports 15:1113–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruetsch YA, Böni T, Borgeat A. 2001. From cocaine to ropivacaine: the history of local anesthetic drugs. Current Topics in Medicinal Chemistry 1:175–182. [DOI] [PubMed] [Google Scholar]
- Saltveit ME. 1993. Effect of high-pressure gas atmospheres and anaesthetics on chilling injury of plants. Journal of Experimental Botany 44:1361–1368. [Google Scholar]
- Sanchez V, Feinstein SD, Lunardi N et al. . 2011. General anesthesia causes long-term impairment of mitochondrial morphogenesis and synaptic transmission in developing rat brain. Anesthesiology 115:992–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherzer S, Shabala L, Hedrich B et al. . 2017. Insect haptoelectrical stimulation of Venus flytrap triggers exocytosis in gland cells. Proceedings of the National Academy of Sciences of the United States of America 114:4822–4827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sezgin E, Levental I, Mayor S, Eggeling C. 2017. The mystery of membrane organization: composition, regulation and roles of lipid rafts. Nature Reviews. Molecular Cell Biology 18:361–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey TD. 1996. Emission of low molecular mass hydrocarbons from plants. Trends in Plant Science 1:78–82. [Google Scholar]
- Simons K, Sampaio JL. 2011. Membrane organization and lipid rafts. Cold Spring Harbor Perspectives in Biology 3:a004697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonner JM. 2008. A hypothesis on the origin and evolution of the response to inhaled anesthetics. Anesthesia and Analgesia 107:849–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumpe M, Carsjens JG, Göbel C, Feussner I. 2008. Divinyl ether synthesis in garlic bulbs. Journal of Experimantal Botany 59:907–915. [DOI] [PubMed] [Google Scholar]
- Sonner JM, Cantor RS. 2013. Molecular mechanisms of drug action: an emerging view. Annual Review of Biophysics 42:143–167. [DOI] [PubMed] [Google Scholar]
- Taylorson RB. 1982. Anesthetic effects on secondary dormancy and phytochrome responses in Setaria faberi seeds. Plant Physiology 70:882–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylorson RB, Hendricks SB. 1979. Overcoming dormancy in seeds with ethanol and other anesthetics. Planta 145:507–510. [DOI] [PubMed] [Google Scholar]
- Taylorson RB, Hendricks SB. 1980. Reversal by pressure of seed germination promoted by anesthetics. Planta 149:108–111. [DOI] [PubMed] [Google Scholar]
- Trewavas A. 2016. Intelligence, cognition, and language of green plants. Frontiers in Psychology 7:588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trewavas A. 2017. The foundations of plant intelligence. Interface Focus 7:20160098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trewavas AJ, Baluška F. 2011. The ubiquity of consciousness. EMBO Reports 12:1221–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya H. 2017. Anesthetic agents of plant origin: a review of phytochemicals with anesthetic activity. Molecules 22:E1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turin L, Skoulakis EM, Horsfield AP. 2014. Electron spin changes during general anesthesia in Drosophila. Proceedings of the National Academy of Sciences of the United States of America 111:E3524–3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda I, Tashiro C, Arakawa K. 1977. Depression of phase-transition temperature in a model cell membrane by local anesthetics. Anesthesiology 46:327–332. [DOI] [PubMed] [Google Scholar]
- van Loon LC. 2016. The intelligent behavior of plants. Trends in Plant Science 21:286–294. [DOI] [PubMed] [Google Scholar]
- Visnovitz T, Világi I, Varró P, Kristóf Z. 2007. Mechanoreceptor cells on the tertiary pulvini of Mimosa pudica L. Plant Signaling and Behavior 2:462–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkov AG, Foster JC, Markin VS. 2010a. Molecular electronics in pinnae of Mimosa pudica. Plant Signaling and Behavior 5:826–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkov AG, Foster JC, Markin VS. 2010b. Signal transduction in Mimosa pudica: biologically closed electrical circuits. Plant, Cell and Environment 33:816–827. [DOI] [PubMed] [Google Scholar]
- Watt EE, Betts BA, Kotey FO et al. . 2008. Menthol shares general anesthetic activity and sites of action on the GABA(A) receptor with the intravenous agent, propofol. European Journal of Pharmacology 590:120–126. [DOI] [PubMed] [Google Scholar]
- Weigl J. 1968. [Membrane structure in plants: effect of xenon on the Mimosa reaction]. Zeitschrift fur Naturforschung. Teil B, Chemie, Biochemie, Biophysik, Biologie und Verwandte Gebiete 23:1253–1255. [PubMed] [Google Scholar]
- Weiland M, Mancuso S, Baluška F. 2016. Signalling via glutamate and GLRs in Arabidopsis thaliana. Functional Plant Biology 43:1–25. [DOI] [PubMed] [Google Scholar]
- Wlodarczyk A, McMillan PF, Greenfield SA. 2006. High pressure effects in anaesthesia and narcosis. Chemical Society Reviews 35:890–898. [DOI] [PubMed] [Google Scholar]
- Wolfe D, Hester P, Keil RL. 1998. Volatile anesthetic additivity and specificity in Saccharomyces cerevisiae: implications for yeast as a model system to study mechanisms of anesthetic action. Anesthesiology 89:174–181. [DOI] [PubMed] [Google Scholar]
- Yao H, Xu Q, Yuan M,. 2008. Actin dynamics mediates the changes of calcium level during the pulvinus movement of Mimosa pudica. Plant Signaling & Behavior 3:954–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokawa K, Kagenishi T, Baluška F. 2016. UV-B induced generation of reactive oxygen species promotes formation of BFA-induced compartments in cells of Arabidopsis root apices. Frontiers in Plant Science 6:1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M, Liu H, Dong Z et al. . 2017. The dynamics and endocytosis of Flot1 protein in response to flg22 in Arabidopsis. Journal of Plant Physiology 215:73–84. [DOI] [PubMed] [Google Scholar]
- Žárský V. 2015. GABA receptor found in plants. Nature Plants 1:15115. [DOI] [PubMed] [Google Scholar]
- Zhao X, Li R, Lu C, Baluška F, Wan Y. 2015a. Di-4-ANEPPDHQ, a fluorescent probe for the visualisation of membrane microdomains in living Arabidopsis thaliana cells. Plant Physiology and Biochemistry 87:53–60. [DOI] [PubMed] [Google Scholar]
- Zhao X, Zhang X, Qu Y, Li R, Baluška F, Wan Y. 2015b. Mapping of membrane lipid order in root apex zones of Arabidopsis thaliana. Frontiers in Plant Science 6:1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.