Abstract
Background
The production of a new lateral root from parental root primary tissues has been investigated extensively, and the most important regulatory mechanisms are now well known. A first regulatory mechanism is based on the synthesis of small peptides which interact ectopically with membrane receptors to elicit a modulation of transcription factor target genes. A second mechanism involves a complex cross-talk between plant hormones. It is known that lateral roots are formed even in parental root portions characterized by the presence of secondary tissues, but there is not yet agreement about the putative tissue source providing the cells competent to become founder cells of a new root primordium.
Scope
We suggest models of possible regulatory mechanisms for inducing specific root vascular cambium (VC) stem cells to abandon their activity in the production of xylem and phloem elements and to start instead the construction of a new lateral root primordium. Considering the ontogenic nature of the VC, the models which we suggest are the result of a comparative review of mechanisms known to control the activity of stem cells in the root apical meristem, procambium and VC. Stem cells in the root meristems can inherit various competences to play different roles, and their fate could be decided in response to cross-talk between endogenous and exogenous signals.
Conclusions
We have found a high degree of relatedness among the regulatory mechanisms controlling the various root meristems. This fact suggests that competence to form new lateral roots can be inherited by some stem cells of the VC lineage. This kind of competence could be represented by a sensitivity of specific stem cells to factors such as those presented in our models.
Keywords: Lateral root, pericycle, plant hormones, procambium, root meristem, secondary growth, stem cells, vascular cambium
INTRODUCTION
Development of plant architecture is the result of the activity of two main meristems: an aerial meristem at the shoot tip (shoot apical meristem; SAM) and a below-ground meristem at the root apex (root apical meristem; RAM) (Garay-Arroyo et al., 2012). This review focuses mainly on meristems responsible for development of the root system. The root arises at the embryo stage and is known by a number of terms: radicle, primary root, embryonic root or taproot. From the radicle, all other roots originate by branching, enabling the plant to capture necessary resources and to secure a stable anchorage (Atkinson et al., 2014). Adventitious roots originate from any portion of the plant body with the exclusion of a root, while lateral roots (LRs) originate only from the pericycle of a parental root. From a histological point of view, Chiatante and Scippa (2006) proposed the division of LRs into two groups, namely primary and secondary lateral roots (PLRs/SLRs), depending on whether they are produced respectively from a parental root characterized by primary or by secondary tissues.
Chiatante et al. (2010) report that new LRs can be produced in the zone of the parental root characterized by the exclusive presence of secondary tissues (i.e. there is no pericycle!). Several different tissues have been suggested as possible alternatives to pericycle for LR formation, namely the parenchyma, the cortex, the phelloderm and the vascular cambium (VC). Our hypothesis is that these tissues can be induced, under particular conditions, to specify new founder cells (FCs) responsible for producing the necessary number of stem cells (SCs) to make a new lateral root primordium (LRP). We have collected anatomical data in a number of woody species (Chiatante et al., 2007, 2010) showing that the specification of FCs for the formation of a new SLR may occur in the VC zone after mechanical stimulation. First insights concerning the molecules involved in these events have been obtained (Scippa et al., 2008; Trupiano et al., 2012a; Rossi et al., 2015), although knowledge of regulatory networks is still lacking.
In this review, we suggest some possible regulatory networks that, in response to a mechanical stress, could regulate the steps leading from specification of new FCs to emergence of SLRs from the VC of its parental root. Our hypothetical regulatory networks are based on reviewing the principal regulatory mechanisms known to be responsible for SC maintenance and proliferation in all meristems involved in the development of the overall root architecture, i.e. the RAM, procambium, VC and LRP. We have observed the occurrence of a high degree of relatedness (Sieburth and Deyholos, 2006) between the regulatory networks controlling all these root meristems independently from the developmental phase of the plant: (1) embryogenesis; (2) primary structure; and (3) secondary structure. This relatedness represents the basis on which we have developed our proposal for the occurrence of regulatory networks in the VC to induce the production of new SLRs. In the pericycle, the degree of relatedness to the regulatory mechanisms hypothesized for VC is increased by the observation that this meristematic tissue is obtained by the union of pericycle cells in the xylem sector with residual procambium cells located between the primary xylem and phloem (Fig. 1). As discussed later, we suggest that only certain SCs in the VC of roots are competent to initiate SLRs, and it is possible that their competence is inherited directly from those competent pericycle cells which were located opposite xylem poles. This process is shown in Fig. 2. When pericycle cells opposite xylem poles divide tangentially, the inner cells maintain a pericycle nature whereas outer cells become components of the VC.
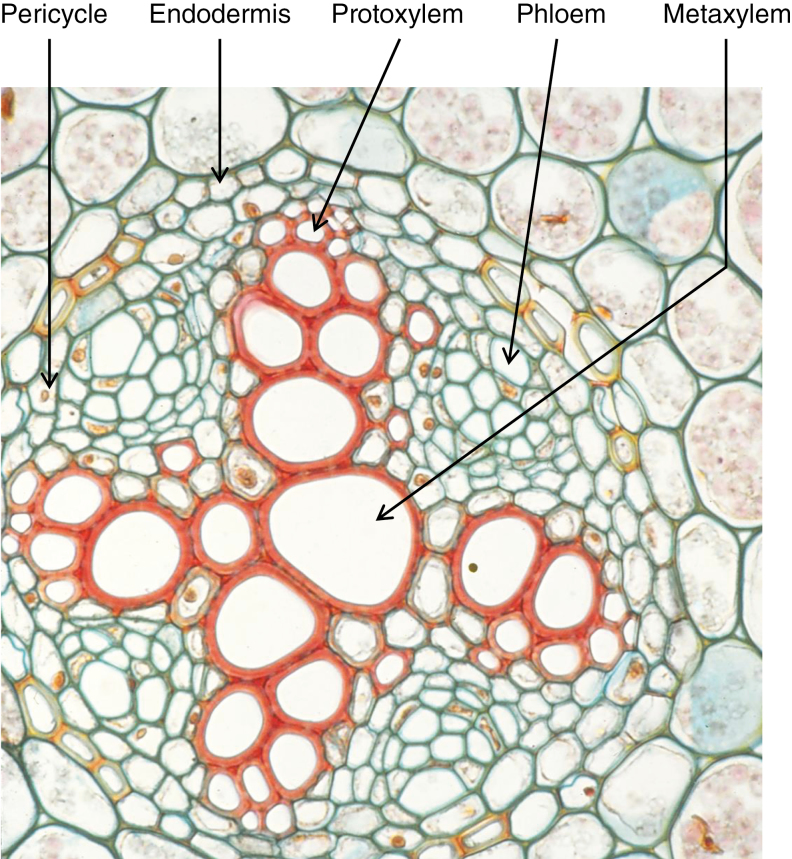
Fig. 1.
Primary growth of buttercup (Ranunculus sp.) root with all primary tissues differentiated. ×170. (Used with permission of the authors; http://www-plb.ucdavis.edu/courses/bis/1c/text/PLANTBIOLOGY1.htm)
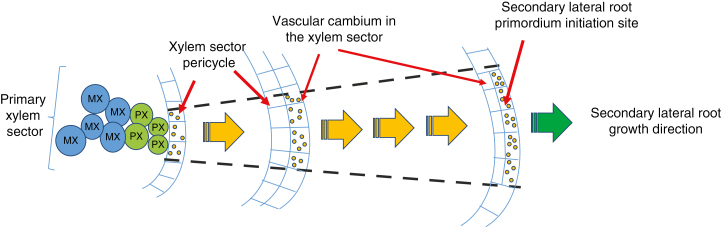
Fig. 2.
Hypothetical model for the derivation of the secondary lateral root initiation site in the ‘xylem’ sector. During VC formation, pericycle cells opposite xylem poles divide tangentially; the inner cells maintain a pericycle nature whereas outer cells become components of the VC. The model proposes that the VC initials derived from the pericycle which face the xylem poles inherit a competence to form new ‘lateral roots’. MX, metaxylem; PX, protoxylem.
We propose, therefore, that the VC initials derived from the pericycle which face the xylem poles inherit a competence to form (when necessary) new ‘lateral roots’ which should actually be called SLRs on the basis of their ontogenic origin. This ‘competence’ might be in the form of the ability to respond to a particular signal, or the activity of a particular gene or the presence of a transcription factor (TF) necessary to trigger a root initiation event. In what follows, we explore this idea starting first by recalling briefly the most important aspects of root meristem development and stem cell niche (SCN) maintenance. Later, similarities and common evolutionary origins characterizing the regulatory networks active in root meristems are considered. We then present our hypothesis regarding the way VC initials could produce SLRs.
ORIGIN AND PROPERTIES OF ROOT MERISTEM
During the initial stages of plant embryogenesis, an apical–basal axis is quickly set up (Teichmann and Muhr, 2015). At the opposite poles of this axis, the SAM and RAM are established as ‘foci’ of continuous development (Steeves and Sussex, 1989). At the earliest stage of root initiation, auxin is involved in the activation of the TF MONOPTERIS (MP) which in turn upregulates transcription of genes encoding an auxin transport protein, PIN-1 and a mobile TF Tmo 7. The asymmetric distribution of auxin and of Tmo 7 leads to the initiation of the embryonic root (Schlereth et al., 2010). This illustrates the general principle that asymmetric distribution of signalling molecules and regulatory proteins is involved when adjacent cells are routed to different developmental pathways However, there remains the larger question of what causes the asymmetric distributions.
By the 16-cell stage of embryo development in arabidopsis, it is already possible to detect the expression of marker genes such as WUSCHEL (WUS) in the SAM and PLETHORA (PLT) in the RAM, coding respectively for TFs WUS and PLT (Long and Barton, 1998). In early embryogenesis, ectopic expression of WUS in the root and PLT in the shoot demonstrates that these two genes regulate not only the formation of SAM and RAM, but also the acquisition of organ identity (Aida et al., 2004; Gallois et al., 2004). In these experiments, it was possible to form shoot tissues in root and root tissues in shoot.
The most important characteristics of a meristem tissue are first, the presence in a specific location of a SCN consisting of a microenvironment of the meristem which contains a sub-set of a few self-renewing SCs (Spradling et al., 2001). In the RAM, this corresponds to a group of cells on the edge of the quiescent centre (QC); indeed, some regard this SC population as being part of the QC. Secondly, there is the formation of cohorts of derivative stem cells (DVs) or transit-amplifying stem cells forming a population of dividing cells which give rise to all the tissues involved in building the plant body (Rieu and Laux, 2009). The SCs in the QC undergo cell division (albeit with a very long cell cycle) to renew their population and to generate DVs (Rahni et al., 2016). At each division, they generate two daughters with different fates. One is an SC and is committed to maintenance of the QC. The other is a DV and is committed to join the main proliferating zone of the meristem. The new SC is said to have inherited the ‘clononegic’ properties (Laux, 2003) of the original mother cell of the QC; this property is obviously only passed on to one of the two daughter cells at each division of a stem cell. DVs cease dividing when they enter a stage of differentiation which leads to the formation of all tissues necessary for organ construction. This occurs at different times in different cylinders of cells arising from the meristem, and thus the distal edge of the meristem is uneven.
It is now widely accepted that extracellular signals (as yet unknown) and TFs (Sablowski, 2007, 2011) in the zone of the meristem surrounding the SCN play a significant role in maintaining the SCs in an undifferentiated and dividing condition (Li and Xie, 2005). According to this hypothesis, the daughter cells displaced from the SCN are no longer affected by these extracellular signals and thus become DVs (van der Berg et al., 1995, 1997; Sablowski, 2007). It is possible in any meristem that there is a zone of ‘SCN competence’ so that when a daughter cell moves out from this zone it is forced to change its identity. The various tissue identities that DVs can adopt then depend on their specific location (Benfey, 2012).
These ideas highlight the importance of positional signals in SC maintenance as well as in the differentiation of DVs. Moreover, regulation of a meristem requires a considerable number of plasticity-based decisions made by cells in a co-ordinated way through a cross-talk between neighbouring and distant cells (Kiba et al., 2013; Stahl and Simon, 2013). Cell tracking and ablation experiments have demonstrated that the fate of a cell in a meristem depends on its position and not strictly on its lineage (van der Berg et al., 1995, 1997).
Communication in plants is mediated by mobile signals which move through plasmodesmata (Daum et al., 2014) (symplastic or selected pathway) in the case of short-distance signalling or through the vascular system (apoplastic or non-selected pathway) in long-distance signalling (Hirakawa et al., 2011, and references therein). With respect to movement through the plasmodesmata, the current opinion is that the size of these channels can be modified by callose and microtubules to adapt their gauge to the size of signalling molecules in order to ease or to arrest their passage (Jang and Lee, 2014). It was originally thought that all these signalling molecules were plant hormones. However, more recently, small peptides, microRNA (miRNA) and mobile TFs (Jang and Lee, 2014) have also been shown to be involved in signalling. Small peptides are thought to travel along both symplastic and apoplastic routes, whereas TFs travel along a symplastic route to induce or repress target genes (Jang and Lee, 2014).
There is thus a complex cross-talk between hormones and other signalling molecules for the regulation of meristem activities (Stahl and Simon, 2010). Three regulatory networks have been reported to be of fundamental importance for the maintenance and development of the SCN in the root (Sablowski, 2011; Azpeitia et al., 2013). These are: (1) auxin/PLT/WUSCHEL RELATED HOMEOBOX (WOX) (Ding and Friml, 2010; Garay-Arroyo et al., 2012); (2) SHORTROOT (SHR)/SCARECROW (SCR)/target genes/proteins (Welch et al., 2007); and (3) CLAVATA (CLE)/WUSCHEL RELATED HOMEOBOX (WOX) (Stahl et al., 2009).
REGULATORY NETWORK IN THE RAM
The RAM in the primary root body is divisible in two zones: the proliferation domain (PD) and the transition domain (TD) (Ivanov and Dubrovsky, 2013; Pacheco-Escobedo et al., 2016). This review, focuses on the PD and in particular on the region where a small group of SCs forms the QC.
The peptide signalling pathway
The literature of the last decade has thrown light upon another regulatory network responsible for homeostasis in the RAM. In this network, the receptor of the mobile signalling molecules seems to be CLAVATA1 (CLV1) (Stahl et al., 2013), a receptor-like kinase (RLK) expressed in the SCs. CLV1 forms a complex with ARABIDOPSIS CRINKLY 4 (ACR4), another RLK. CLV1 and ACR4 are both characterized by a functional motif also common to a number of leucine-rich repeat (LRR) RLKs (Czyzewicz et al., 2016). The CLV1/ACR4 complex localizes to specific plasma membrane domains associated with plasmodesmata (Stahl et al., 2013) where it can regulate the transport of signalling molecules by inducing variation of plasmodesmata apertures (Stahl and Faulkner, 2016).
It is suggested that small CLAVATA3/Embryo Surrounding Region-related (CLE) peptides are first synthesized in differentiated columella cells and then move to the RAM as mobile signalling candidates which bind to the CLV/ACR4 receptor (Stahl and Simon, 2013). In roots of arabidopsis and other plants, the CLE genes are: CLE40, CLE10 and CLE12 (Dodueva et al., 2013). This hypothesis is supported by the fact that ACR4 is required for CLE40 signalling activity in the RAM (Stahl et al., 2009) although direct binding has not yet been demonstrated (Czyzewicz et al., 2016). However, other CLE genes are expressed in the proximal differentiation zone of the root axis although their role remains unknown. A direct demonstration of the role played by CLE peptides in the RAM comes from experiments where CLE genes were overexpressed, resulting in a reduction in the size of the RAM (Ito, 2006). This reduction of RAM size is probably due to a premature cell differentiation of DVs (Meng et al., 2012). The fact that this reduction is restored by exogenous cytokinins suggests that cytokinins play an antagonist role in the RAM with respect to CLE peptides (Meng et al., 2012).
With regard to the possible target of the signalling module CLE40/ACR4/CLV1, one hypothesis is that it acts on the phosphorylation of WUSCHEL RELATED HOMEOBOX5 (WOX5) (an orthologue of WUS) expressed in the RAM (Sarkar et al., 2007). Phosphorylation of WOX5 would limit its diffusion to the surrounding cells and would maintain the SC identity in the QC (Sarkar et al., 2007), probably by repressing the TF CYCLIC DOF FACTOR 4 (CDF4). CDF4 has been shown to be responsible for cell differentiation of columella SCs (Pi et al., 2015). However, the possibility cannot be excluded that in the QC the WOX5 TF represses unknown cytokinin-inducible response regulators as happens in the SAM. Czyzewicz et al. (2016) have presented an interesting hypothesis that the binding of CLE40 by ACR4 could also have a protective function in limiting the concentration of CLE40 around the QC. These authors also suggest that differentiation of columella SCs could be dependent on ACR4 which is implicated in determining the asymmetric cell division. Pallakies and Simon (2014) suggested that CLE40 could also be active in the regulation of the proximal meristem size by interfering with several hormone signalling pathways.
The similarity of the regulatory network responsible for SCN homeostasis in the RAM and SAM, consisting of a signalling peptide, its specific kinase receptor and a TF target gene, suggests that a similar apo- and symplastic system of cell to cell movement of mobile molecules characterizes both apical meristems (Stahl and Simon, 2013; Drisch and Stahl, 2015). However, there are important differences regarding the nature of the signalling modules and in the source tissues of components of these modules. With regard to the first difference, it is now known that unlike the situation in the SAM, the CLE40 in the RAM is produced in differentiated tissues, namely the columella cells, and diffuses upward to the QC and surrounding SCs to exert its role (Stahl et al., 2009). Concerning the second difference, it is known that in the RAM, CLE40 does not need to be cleaved by CLV2, whereas, in the SAM it has been suggested by Pan et al. (2016) that CLV3 function most probably depends on its cleavage by heteromultimers of CLV2.
The plant hormone signalling pathway
In addition to the CLE40/ACR4/CLV1 regulatory network, auxin is also able to regulate several processes of root development including SCN formation and maintenance (Dinneny and Benfey, 2008; Zhou et al., 2010), proliferation of the proximal meristems, elongation and differentiation. The action of auxin in these processes depends on the formation of a concentration gradient mediated by the efflux carrier PIN (Vieten et al., 2005). More information about auxin transport and RAM development is available in Ding and Friml (2010) and Goh et al. (2014). The action of auxin is initiated after the degradation of its inhibitor AUX/IAA (BODENLOS or BDL1) that blocks the auxin response factors ARF/MP (Weijers et al., 2006). Removing the block allows the induction of the auxin-responsive elements of target genes (Benjamins and Scheres, 2008).
Important auxin target genes include those encoding TFs such as PLT 1/2/3 (Aida et al., 2004; Stahl and Simon, 2010) which regulate the formation of the QC and surrounding SCs in the RAM by means of expression of PIN genes (Iyer-Pascuzzi and Benfey, 2009). In particular, a maximum of endogenous auxin concentration observed in the QC supports the view that auxin has a role in positioning the SCN in the RAM (Petersson et al., 2009). Other transcriptional regulators such as TARGET OF MONOPTEROS 5 and 7 [TMO5 and TMO7 two basic-helix–loop–helix (bHLH) proteins] are also controlled by auxin and exert their role as cofactors of other bHLHs.
Auxin and cytokinin play antagonistic roles in establishment and maintenance of the SCN in the RAM (Müller and Sheen, 2008) where the former promotes proliferation while the latter pushes SCs toward differentiation. The action of cytokinin here is the repression of auxin signalling and migration (the latter via inhibition of PIN expression). This effect is achieved through the cytokinin receptor ARABIDOPSIS RESPONSE REGULATOR 1 (ARR1) which activates the expression of the SHORT HYPOCOTYL (SHY2) TF; this then inhibits signalling and PIN expression. In contrast, auxin promotes SHY2 degradation and therefore facilitates the maintenance of a high auxin concentration necessary for RAM maintenance (Ruzicka et al., 2009). The QC forms where the auxin maximum is present.
Finally, as with the SAM, maintenance of the undifferentiated state of SCs in the RAM depends on a number of factors, including the relationship between cyclins (Nieuwland et al., 2009) and cytokinins (Dewitte and Murray, 2003).
REGULATORY NETWORK IN THE PROCAMBIUM
The procambium is the primary meristem responsible for differentiation into the vascular system in the shoot, root and leaf. Procambium in the roots of dicots and gymnosperms is divided into 2–5 xylem poles (or archs) alternating with 2–5 phloem poles, giving rise to an architecture of vascular bundles called the actinostele (Esau, 1977). The protoxylem (the first vascular elements to differentiate) in each arch occupies a centrifugal position unlike metaxylems which differentiate in centripetal positions. Phloem poles occupy a perpendicular position in respect to the oblong protoxylem–xylem structure, with the protophloem of each pole in a centrifugal position and the corresponding metaphloem in a centripetal position.
The peptide signalling pathway
Most of what is known to date regarding the regulatory network of procambium derives from the analysis of tracheary element differentiation in cell cultures of Zinnia elegans. In these studies, a vascular tissue inhibitory protein has been identified and named TRACHEARY DIFFERENTIATION INHIBITOR FACTOR (TDIF) (Ito et al., 2006). A homology sequence search revealed that the 12 amino acids at the C-terminus of TDIF are the same as those of CLE41 (Clavata3/ESR-related protein 41) and CLE44 (references in Stahl and Simon, 2012), and are also similar to those of CLE42 found in arabidopsis.
In the search for a receptor of TDIF, CLE44 and CLE41, Hirakawa et al. (2008, 2010a) and Etchells and Turner (2010) found that these peptides bind to PHLOEM INTERCALATED WITH XYLEM (PXY) also known as TDIF RECEPTOR (TDR) (Etchells and Turner, 2010). PXY is an LRR-RLK expressed in the procambium SCs. More recent studies have shown that TDIF is produced mainly in phloem cells and secreted in the apoplast surrounding the mother SCs of phloem (Suer et al., 2011; Stahl and Simon, 2012), whereas TDR is located in the plasma membranes of procambial cells. These authors suggest that binding of TDIF to TDR would inhibit differentiation of procambial cells into xylem tracheary elements. This hypothesis is supported by the fact that in situ experiments with arabidopsis confirmed that in the procambium bundle, the addition of a synthetic peptide sequence similar to the TDIF sequence arrests xylem differentiation, whereas the differentiation of cambium and phloem remains unaltered (Hirakawa et al., 2008). Similarly, the overexpression of CLE41 and CLE44 affected tracheary element differentiation like TDIF (Hirakawa et al., 2010b; Dodueva et al., 2012).
It is therefore possible that TDIF represents the mobile signalling molecule enabling the cross-talk between phloem and xylem. In this case, the gradient of TDIF concentration (i.e. a higher signal in the phloem and a weak signal in the xylem) across the cambium represents positional information necessary for both procambium–cambium maintenance and the phloem–xylem differentiation. Thus, the putative ligand–receptor module CLE41–PXY controls vascular organization and proliferation in the procambium in a non-cell-autonomous way, by providing positional information similar to the module LRR-RLK/CLE which regulates cell–cell communications in the SAM and RAM (Stahl et al., 2009). This hypothesis is supported by the observation that pxy mutants present a disorganized disposition of the phloem and xylem elements in the vascular bundle (Fisher and Turner, 2007). Surprisingly TDIF also induces an increase of procambium stem cell proliferation. Therefore, the same peptide ligand is able to play two roles: (i) inhibition of tracheary element differentiation; and (2) stimulation of procambium SC proliferation (Ito et al., 2006). For all these reasons, it has been suggested that CLE41/CLE44, CLE45 and CLE40 (references in Czyzewicz and De Smet, 2015) play an important role in root architectural development despite the fact that most of the research on CLE41/CLE44 has focused on the hypocotyl. In the case of CLE45, a negative regulation of protophloem differentiation has been suggested by Depuydt et al. (2013).
With regard to the downstream target of the CLE41/44–PXY signalling module, it has been reported that procambium SC activity in arabidopsis and Solanum lycopersicum is promoted by overexpression of the WUSCHEL-RELATED HOMEOBOX4 (WOX4) gene (Hirakawa et al., 2010a, 2011). In particular, binding between CLE44 and TDR/PHY restricts downstream the expression of the WOX4 TF with the effect of maintaining the undifferentiated pluripotent status of the procambium SCs (Etchells and Turner, 2010; Hirakawa et al., 2010a; Suer et al., 2011). However, the fact that in pxy mutants WOX4 expression remains unaltered suggests that an alternative target for PXY must exist which acts redundantly with WOX4 (Hirakawa et al., 2010a).
There is also a surprising similarity between the role of WOX4 and the roles played by WUS and WOX5 in the SAM and RAM, respectively (Sarkar et al., 2007). The difference between the regulatory networks is that the one active in the SAM and RAM inhibits stem cell proliferation whereas the other present in procambium stimulates stem cell proliferation (Hirakawa et al., 2010a). The difference may be explained by the possibility that for the VC there is a second unknown module which can counteract the action of the PXY/CLE41/44 module. Support for this hypothesis comes from the finding that a cambium-specific LRR-RLK such as MORE LATERAL GROWTH (MOL1) plays an inhibitory role on the cambium SCs (Agusti et al., 2011). However, beside WOX4, another WUS-type gene (WOX14) seems to act redundantly to WOX4 on VC activity (Etchells et al., 2013).
BARELY ANY MERISTEM (BAM) 1/2/3 belong to the arabidopsis LRR-RLK family. It has been reported that BAM3 is expressed almost exclusively in the procambium of both shoot (Nimchuk et al., 2015) and root (Depuydt et al., 2013). However, this ligand–receptor seems more involved in protophloem differentiation (Rodriguez-Villalon et al., 2014) rather than in SC homeostasis as it is able to rescue a mutant defective in protophloem. The role played by BAM3 in the shoot remains less clear. Unlike the BAM TF gene ATHB15, a TF belonging to the class III HOMEODOMAIN-LEUCINE ZIPPER (HD-ZIPII) gene family is expressed in a narrow band corresponding to the stem procambial cells. It is thus not unreasonable to suggest the use of the ATHB15 gene as a marker for procambium SCs (Prigge et al., 2005).
The plant hormone pathway
It has been known for a long time that formation of new vascular bundles from procambium occurs after auxin treatment (Sachs, 1991). Strong evidence suggests that the MONOPTEROS (MP) locus regulates procambial development in roots and shoots of arabidopsis (Przemeck et al., 1996). In particular, it seems that procambium formation and its maintenance follows an accumulation of auxin controlled by the efflux carrier PIN1 (Ohashi-Ito and Fukuda, 2010). The auxin response factor MP then induces the expression of a number of TF genes belonging to the HD-ZIPIII gene family, including PHB (PHABULOSA/ATHB14), PHV (PHAVOLUTA/ATHB9), REV/IFL1 (REVOLUTA/INTERFASCUCULAR FIBERLESS), ATHB8 and CNA (CORONA/ATHB15) (Baucher et al., 2007; Ilegems et al., 2010). These same genes have been associated repeatedly with several other developmental processes in meristems (Baucher et al., 2007).
The effects of cytokinin treatment have been investigated in the vascular tissue of the embryo axis and in procambium at the seedling stage (Ueguchi, 2001). These studies suggest that cytokinins control cell proliferation during cambium development in Raphanus, Coleus and Helianthus (Helariutta and Bhalerao, 2003). More recently, in arabidopsis, it has been shown that cytokinins are necessary for cell proliferation and vascular differentiation (Mähönen et al., 2006), and this action seems to be mediated by the CRE1 gene coding for a cytokinin receptor.
REGULATORY NETWORK IN THE VC
The regulatory networks in the RAM and procambium (described above) present several similarities in the regulation of meristematic activity which seems to be independent from the developmental phase considered, These similarities support the hypothesis that a regulon (a group of genes regulated together) may be active and conserved in various organs for the whole life of a plant (Groover et al., 2006). The presence of proteins belonging to the same gene family (HD-ZIPIII) in both the SAM and VC suggests that regulatory mechanisms may be conserved even in different meristems (Baucher et al., 2007). Support for this hypothesis derives from the consideration that shoot poles can give rise to root poles, and vice versa, following the ectopic expression of genes such as PLT and HD-ZIPIII (Aida et al., 2004; Smith and Long, 2010). Therefore, it is reasonable to assume that procambium and the VC, two meristems involved in the production of vascular tissues, can share common elements of their regulatory networks despite their ontogenetic differences. This supports the suggestion that the VC may represent an efficient pipeline for signalling through the secondary structure of a plant (Brackmann and Greb, 2014).
With regard to the formation of the VC in the root, it is necessary to stress that residual procambium stem cells separate the central xylem from the external phloem (Baum et al., 2002). When these procambium SCs resume proliferation, the pericycle cells opposite xylem poles contemporaneously resume an SC identity and start to divide. The cell division rate of the procambium SCs is higher than in pericycle SCs, particularly in the zone where procambium separates the phloem from the xylem. This explains why, after a ew cell divisions, the procambium SCs and pericycle SCs form a continuous cylindrical uniseriate layer (a ring in transverse section) of SCs, the VC, that separates the primary xylem from the primary phloem (Lachaud et al., 1999).
Stem cells in VC are also named initial cells (ICs) and they renew their SC identities after cell division by suppressing differentiation, as also happens for the SCNs present in the RAM. The difference between the SCNs in the RAM and the SCNs in the VC is that the latter are formed by two types of ICs: fusiform and ray initials (Miyashima et al., 2013). Fusiform initials are long with a cylindrical shape and blunt ended; the ray initials are cubic and short (Fig. 3). Differences in transcriptomes between these two types of initials have been reported (Goué et al., 2008). Both fusiform and ray initials give rise after each division to a new SC and, alternatively, to an SC mother for xylem (centripetally) or an SC mother for phloem (centrifugally). The mother cells for xylem or phloem give rise to the proliferative tissue and therefore retain a ‘meristematic identity’ until they arrest cell division and start to differentiate (Chaffey et al., 1997). From a cytological point of view, it is impossible to distinguish the identity of initials and SC mothers for xylem or phloem. This explains why the term the VC zone is always used to indicate the cells characterized by an intense mitotic activity without distinguishing between SC initials and xylem or phloem mother cells. By adopting this approach, the VC zone must be considered as a series of concentric cylinders (rings in the transverse section) characterized by different identities and/or states of development.
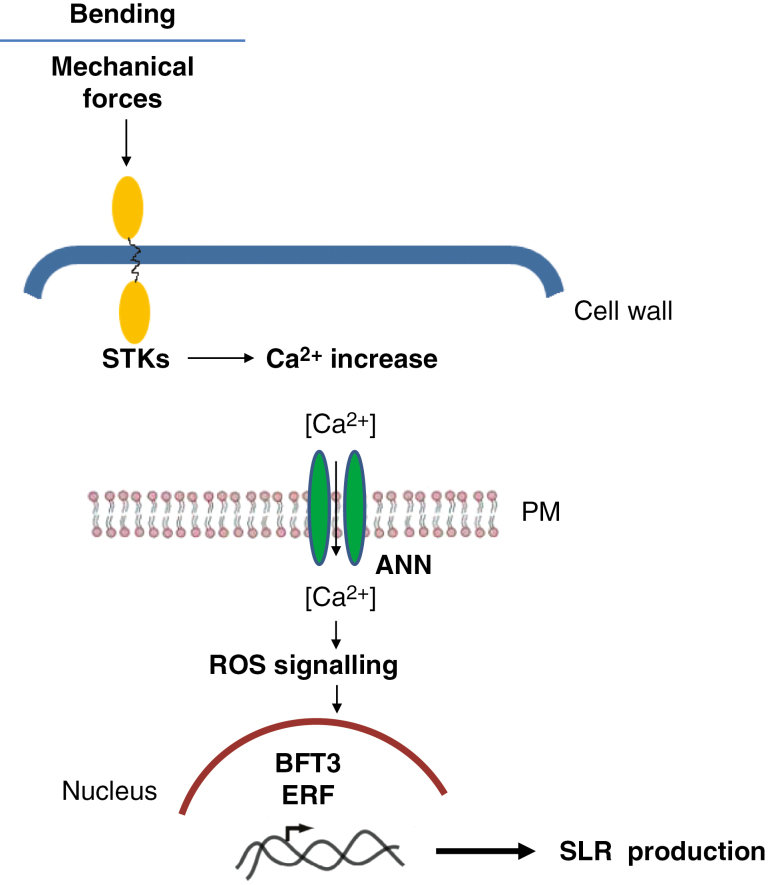
Fig. 3.
Summary of proteomic data. Possible role of protein factors identified by a proteomic approach in mechanical force signalling and lateral root formation from a secondary structure. STK, serine-threonine protein kinase plant-type; PM, plasma membrane; ANN, annexin; ROS, reactive oxygen species; BFT3, Basic Transcription Factor 3; ERF, ethylene-responsive factor.
The peptide pathway
There is no clear evidence of the presence in the VC zone of an SCN similar to those found in the organizing centre (OC) or QC, respectively, in the SAM and RAM (Miyashima et al., 2013; Brackmann and Greb, 2014). However, homologues of SAM regulators were found in VC of poplar stem, including PttCLV1, PttANT and PttKNOX genes (Schrader et al., 2004). Therefore, if a SCN also exists in VC it is interesting to understand how two types of SCs (fusiform and ray initials) preserve their independent identities and collaborate at the same time to produce a complex secondary tissue such as wood. In addition to the control exerted upon two types of SC initials, there is also the fact that VC must grow in girth to allow the centripetal accumulation of wood. Therefore, in addition to centripetal–centrifugal polarity, there is also the need to understand how this hypothetical SCN finely regulates the anticlinal division necessary for diameter growth (Doerner, 2003).
In the arabidopsis hypocotyl, in addition to PXY, another possible peptide LRR-RLK, ERECTA (ER), is present in VC (Etchells et al., 2013). The ligands of this receptor seem to be both EPFL4 and EPFL6 (Etchells et al., 2013). Further, two more RLKs have been reported by Agusti et al. (2011), named MORE LATERAL GROWTH1 (MOL1) and REDUCED IN LATERAL GROWTH (RUL1), which function in parallel on VC to balance its activity. According to this hypothesis, MOL1 acts as a repressor whereas RUL1 acts as an activator of VC activity. Moreover, the occurrence of a signalling module in controlling proliferation of the arabidopsis hypocotyl VC is supported by the presence of the peptides CLE41/44 that can bind to PXY (Hirakawa et al., 2008). By analogy with arabidopsis procambium, the accepted hypothesis is that in the VC zone CLE41/44 is also expressed in phloem and moves centripetally toward the VC SCs which express PXY. The formation of the ligand–receptor pair would maintain the SC population in VC probably by means of a negative feedback regulation of the signalling module. The target of the signalling module would be the same WOX4 gene shown to regulate positively the proliferation of procambium SCs (Hirakawa et al., 2010a). In addition to CLE41/44, the presence of CLE6 and CLE26 has been reported; these are highly expressed in arabidopsis on the phloem side of the junction between the VC of the hypocotyl and the secondary root (Zhao et al., 2005). A role for orthologues CLV3 (PttCLV3) and WUS (PttWUS) has been excluded in poplar stem VC (Schrader, 2004; Zhao et al., 2005), even though they are involved in regulation of the SAM of this plant species. For this reason, the occurrence of an alternative regulatory mechanism has been suggested (Baucher et al., 2007). The presence in the VC phloem of PttCLV1, an orthologue of CLV1, together with the high level of expression of two CLE genes (PttCLE;1 and PttCLE;3), coding for small peptides (Schrader, 2004), suggests the possible presence of two components of a potential signalling module pair. The occurrence of an alternative regulatory network is suggested by the overlapping expression on the xylem side of the VC of PttHB3-HB2 and PttRLK3, homologues of WOX4 and CLV1, respectively (Schrader, 2004; Dodueva et al., 2013; Brackmann and Greb, 2014). The opposing (i.e. xylem side vs. phloem side) expression profiles of PttCLV1 and PttRLK3, two membrane-bound receptor kinases, suggest the interesting possibility that in VC of poplar stem there are two separate regulatory loops each acting on one side of the stem cambium initials. In our opinion, the possible presence of two independent regulatory mechanisms could explain why the amount of secondary xylem and phloem production may be different and subject to frequent and independent variations. PttHB2 and PttHB3 are also involved in maintaining the undifferentiated pluripotent status of VC initial cells (Schrader et al., 2004).
The plant hormone pathway
At the outset of this discussion it needs to be said that the mode of action of hormones on the regulatory mechanisms of VC is still not clearly understood. It has been proposed that WOX4 expression in the VC is also induced by auxin independently from PXY (Suer et al., 2011). This result suggests that there are two types of induction of the WOX4 gene: (1) in response to long-distance-derived signalling via auxin; and (2) in response to short-distance signalling via the TDR/PHY/CLE41/44 module. However, the possibility cannot be excluded that regulation of VC activity by the TDR/PHY/CLE41/44 pathway acts downstream of auxin signalling (Zhang et al., 2014). Auxin involvement in VC patterning in poplar may be controlled by an interaction between the TF PtaBDL1 and PIN proteins, as suggested by Yordanov et al. (2010). This explains the variation of auxin concentration which shows a maximum in the VC zone (Petrasek and Friml, 2009) where auxin would inhibit LBD1 expression with the result of maintaining VC SC identity. The difference in auxin concentration between VC and secondary phloem, mediated through directional flux regulation by PIN proteins regulated by LBD1, would thus be the most important factor regulating VC patterning. The mechanism of action of auxin in the VC could be the same as observed in other meristems with the degradation of AUX/IAA to release ARF action. Support for this regulatory mechanism comes from data which show that mutants in polar auxin transport (PAT) show alterations in VC patterning (Baucher et al., 2007)
Cytokinins also seem to regulate the activity of the VC in both arabidopsis and Populus (Bishopp et al., 2011), probably through the action of WOX4 that represses cytokinin-inducible response regulators. Dodueva et al. (2012) suggest that in the VC there may be antagonistic roles played by auxins and cytokinins analogous to the one found in the regulation of the SAM and RAM. The importance of the role played by WOX4 for VC activity explains why the WOX4 gene is conserved among seed plants and pre-dates gymnosperm–angiosperm divergence (Nardmann and Werr, 2013).
Other plant hormones have also been reported to regulate the VC. In arabidopsis, for example, ETHYLENE RESPONSE FACTOR (ERF) TFs, such as ERF1, ERF108 and ERF109, promote cell division in the VC (Etchells et al., 2012) whereas in poplar PtaERF1 seems to be expressed more on the secondary phloem side (Van Raemdonck et al., 2005) and during the production of reaction secondary xylem (Vahala et al., 2013).
REGULATORY NETWORK IN LR FORMATION
The regulatory networks examined above in RAM, procambium and VC share a common pattern involving a plant hormone (mainly auxin and cytokinin) signalling pathway coupled with a module formed by a ligand, receptor kinase and/or target genes. By considering the ontogenetic origin and what is known to date with regard to the pericycle activity, it is not surprising that a regulatory network formed by the same elements seems to control LR formation.
Lateral roots originate from a xylem pericycle SC but, in maize, rice, wheat and carrot, it has been shown that phloem pericycle SCs are also able to produce LRs (Jansen et al., 2012). This highlights the importance of understanding how FCs are positioned (Dodueva et al., 2013). In this review, we will examine only the events occurring when an LR is formed in arabidopsis and therefore we will refer to LR formation when these are produced by xylem pericycle cells.
The cascade of events leading to LR formation from the pericycle cells has been well studied in arabidopsis and has been divided into eight stages preceded by a priming phase (Malamy and Benfey, 1997). Xylem pericycle cells responsible for LR branching are named FCs (De Smet et al., 2007; Moreno-Risueno et al., 2010) and their recruitment depends upon a transient spatio-temporal accumulation of auxins along the parental root axis (Benková et al., 2003; Geldner, 2003). However, it has not yet been conclusively demonstrated that auxin triggers FC specification (Jansen et al., 2012) even though it is widely accepted that auxin regulates not only FC specification, but also the development of LRPs from initiation to emergence (Péret et al., 2009).
The peptide pathway
The formation of LRs seems to be regulated by a network consisting of the same module formed by the same three components (ligand/receptor kinase/target gene) presented above for RAM, procambium and VC activity. With regard to this, during the past few years a number of small peptides have been shown in arabidopsis to be involved in LR development (Murphy et al., 2016). In particular, certain peptides have roles in regulating the number of LRs formed along the root axis, including CLE-LIKE (CLEL)/GOLVEN (GLV)/ROOT GROWTH FACTOR (RGF) involved in pericycle cell division inhibition (Fernandez et al., 2015), C-TERMINALLY ENCODED PEPTIDEs (CEPs) involved in reduction of the number of LRs (Roberts et al., 2016) and AUXIN-RESPONSIVE ENDOGENOUS POLYPEPTIDE 1 (AREP1) (Yang et al., 2014). Other peptides are involved in LR emergence through outer tissues (cortex and epidermis), including CLE (Araya et al., 2014), INFLUORESCENCE DEFICIENT IN ABSCISSION (IDA) (Kumpf et al., 2013) and RAPID ALKALINIZATION FACTOR1/19/23 (RALF1/19/23) (Atkinson et al., 2013; Bergonci et al., 2014). Further, the RALF peptide may be inhibited by ethylene and thus could act on LR development upstream of GATA23 (Murphy et al., 2016).
In experiments on nitrogen deficiency, it was shown that the inhibition of LR formation coincides with accumulation of CLE1, CLE2, CLE3, CLE4, CLE5 and CLE7 mRNAs (Araya et al., 2014). The suggestion is therefore made that these CLE peptides negatively regulate the number of LRs formed under such environmental conditions. Support for the hypothesis of the occurrence of a signalling module during LR formation also comes from experiments by Araya et al. (2014) where transgenic arabidopsis plants expressing a CLV1–green fluorescent protein (GFP) fusion protein showed the localization of this receptor in the companion cells of the phloem arc. The CLE peptides are synthesized in the pericycle cells; hence, to explain the binding with CLV1 to form the signalling module, it has been suggested that the CLE peptides diffuse from the pericycle to the phloem companion cells where their binding activates downstream signals to inhibit LRP formation.
Recently AtCLE26p has been reported to be involved in root architecture development not only in arabidopsis (Rodriguez-Villalon et al., 2015) but also in the monocots Brachypodium distachyon and Triticum aestivum (Czyzewicz and De Smet, 2015). Moreover, putative CLE26 orthologues seem to be present in several species, including S. lypersicum and Brassica napus (Czyzewicz and De Smet, 2015). It has been suggested that CLE26p alters auxin distribution to the RAM by inhibiting protophloem development and therefore auxin transport (Czyzewicz and De Smet, 2015; Rodriguez-Villalon et al., 2015; Czyzewicz et al., 2016). According to the hypothesis presented by these authors, for LR emission there could also be present a CLE26-activated module which would play an important role in enabling the plant to respond to environmental signals by altering its plant root architecture.
In relation to membrane-bound RLK, it has been reported that ACR4 is expressed in the two short cells resulting from division of the two FCs (De Smet et al., 2008). ACR4 (together with other family members) influences asymmetric cell division with the production of two daughter cells with different identities (De Smet et al., 2008). These two cells give rise to different cell lineages which are necessary for the formation of a new LRP. However, no expression of CLE40 has been reported so far in investigations of this process. Nevertheless, the possibility that there are different ligands that are able to bind to ACR4 cannot be excluded. De Smet et al. (2008) suggest that ACR4 could act cell autonomously for the initiation of LRs and non-cell autonomously for arresting the potential to form LRs in all the remaining pericycle cells. Furthermore, Chang et al. (2015) suggested that ACR4 has a positive impact on the cytokinin pathway, and vice versa.
For the passage from lateral root initiation (LRI) to LRP formation, a regulatory mechanism must intervene to change the position of PIN proteins in order to change the direction of auxin flux. In fact, during organogenesis of a new LR, a tissue patterning is needed in the perpendicular direction in respect to the existing parental root. In order to re-direct PIN proteins toward the tangential cell walls, the activation of the ARF GEF GNOM-dependent pathway is necessary (Kleine-Vehn et al., 2006).
Together with all the other TFs which form the target of the regulatory mechanisms involved in LRI, it has been suggested that the ALF4 gene coding for a nuclear protein could also positively regulate mitotic activity in FCs (De Smet et al., 2006) by controlling the G2 to M phase of the cell cycle transition. The target of a hypothetical signalling module in LRI could be the activation of the WOX5 gene which is expressed in FCs of the pericycle (Stahl et al., 2009). Finally, in order to control the timing of LRI, an as yet unknown signalling module must be also be present.
The plant hormone pathway in LRs
A functional role for auxin in LRI in arabidopsis is now widely accepted with the hypothesis that its accumulation in xylem pericycle can stimulate cyclin-dependent kinases (CDKA and CDKB1;1) and can relieve cell cycle inhibition (Beeckman et al., 2001). A TF, LBD9, controls cell cycle progression during LR formation through the regulation of PIN gene expression (Feng et al., 2012).
Auxin mutants suggest that different signalling modules (AUX/IAA–ARFs) regulate the specific step of LR formation, but the number of these modules remains unknown (Goh et al., 2012). A first module, AUX/IAA28–ARF, regulates FC specification (De Rybel et al., 2010), whereas a second module, SLR/IAA14–ARF7(ARF19), regulates nuclear migration in FCs and their asymmetric division leading to LRI, with ARF7 and ARF19 having a redundant function (Okushima et al., 2007). In the case of the SLR/IAA14–ARF7–ARF19 module, its putative action on LRI is supported by the observation that its mutant with a reduced sensitivity to auxin exhibits an absence of cell divisions in the pericycle and aborted LR formation (Vanneste et al., 2005). A third module, BODENLOS (BDL)/IAA12–MONOPTEROS (MP)/ARF5, seems to act in the same LRI process downstream of the previous modules and is likely to be necessary to correlate FC priming with LRI. This signalling module is the same one that is active in embryogenesis (De Smet et al., 2010); this supports the idea of a developmental conservation of these modules which can be reactivated when and wherever necessary. A fourth module, SHY2/IAA3–ARFs (Overvoorde et al., 2010), seems to be involved in LRP formation. In respect of this fourth module, Goh et al. (2012) suggest that the signalling SHY2/IAA3–ARF module may play a double role in the chain of events leading to LRP formation. It would first act positively on the transition from LRP formation to its emergence from the tissue of the parental root and it would, secondly, play an inhibitor role on the SLR/IAA14–ARF7–ARF19 module leading to the arrest of LRI. This is demonstrated by the arabidopsis mutant shy2/iaa3 that presents on one hand a decrease in the number of LRs formed and on the other an increase in the number of LRIs. However, the identity of the ARF component of the SHY2/IAA–ARF module remains to be elucidated (Goh et al., 2012).
Beside all these signalling modules described above, the patterning of LR seems to be also dependent upon other signalling modules based on inhibition of expression of ARF genes by miRNAs such as miR390 (Yoon et al., 2009; Marin et al., 2010) and miR167 (Gifford et al., 2008) as well as trans-acting short-interfering RNAs (tasiRNAs) (Marin et al., 2010). These molecules would act on LR development by negatively regulating auxin-related gene expression such as that of ARF4 in response to endogenous or environmental signals (Yoon et al., 2009). Expression of miR390 is related positively to increased auxin concentration, leading to a post-transcriptional gene silencing of ARF4 inhibitor through its binding to a tasiRNA-ARF. Further evidence for this regulatory mechanism comes from mutants of these miRNA genes in which there is a quantitative reduction of LRs.
The identity of FCs is established proximally to the border between the division zone and the developmental zone of the root parental axis (Beeckman et al., 2001). The number of FCs participating in this event is very limited, and the first stage leading to acquisition of FC identity (named ‘priming’ of FCs) seems to be related to an asymmetric division of two pericycle cells forming the two shortest initials flanked by two longer initials (Benková and Bielach, 2010). These two pericycle cells are stacked on top of each other, and their division seems to be preceded by a migration of their respective nuclei toward the same transverse cell wall (De Smet et al., 2007). This event is strongly related to the vicinity of the xylem pole (Parizot et al., 2008), but the molecular determinants responsible for assigning competence to become FCs remain to be discovered (Benková and Bielach, 2010). An arrest of specific pericycle cells in the G2 phase may represent the first step towards their acquisition of competence (priming) which needs to take place proximally to the border between the division zone and the developmental zone (Beeckman et al., 2001).
Priming of FCs is a rhythmically repetitive event that always takes place distally so that the new FCs are the always nearer to the RAM. With regard to plant hormone signalling involved in FC priming, a GATA-type TF gene (GATA23) has been recently identified that is specifically expressed in the primed pericycle cells, even before the occurrence of asymmetric divisions (De Rybel et al., 2010).
De Rybel et al. (2010) demonstrated that GATA23 expression depends on IAA28, ARF7 and ARF19. FC specification seems to depend upon ARF6–ARF8-mediated signalling with GATA23 as a target (Lavenus et al., 2013). The signalling pathway for FC priming starts with promotion by auxin of the interaction between Aux/IAA protein repressor with F-box TIR1 protein, an auxin receptor which is a component of the ubiquitin ligase complex. Following the interaction of Aux/IAA protein with the ubiquitin complex, the Aux/IAA protein is degraded and hence the inhibition of the ARF TF is relieved (Dharmasiri et al., 2005). The auxin signalling pathway described above represents the most studied pathway which is involved not only in LR formation in plants (Yoon et al., 2009) but also in other aspects of plant development such as VC development in poplar trees (Moyle et al., 2002). In arabidopsis, there are 23 known ARF genes (Guilfoyle and Hagen, 2007) and 29 AUX/IAA genes (Abel and Theologis, 1996), suggesting that the same signalling pathway could be organized with different components to play antagonistic roles. The fact that genes controlling the cell cycle are overexpressed during auxin accumulation indicates that the plant hormone induces the candidate FCs to re-enter the cell cycle from their G2 arrest.
After the first asymmetric division, a series of anticlinal and periclinal cell divisions take place under the control of auxins (De Smet et al., 2007), leading to the formation of an LRP (Dubrovsky et al., 2006). LRI takes place in a zone along the root axis at the border between the TD and the differentiation domain (DD). During this phase, a small meristem (eight cells) is formed which becomes the new RAM of the developing LR (Laskowski et al., 1995), and a dome structure (the new root primordium) becomes evident as a consequence of cells elongating in a centrifugal direction. The action of auxin seems to be dependent also on the interference of MONOPTEROS/ARF with the expression of PRE3/ATBS1/bHLH135, a bHLH TF gene (Castelain et al., 2012). This interference was demonstrated in experiments where overexpression of this gene induced a longer root with a reduction of the total number of LRs, a condition which could be rescued by addition of exogenous auxin.
The exact localization of LRI seems to be dependent on the type of growth pattern of the root that follows a wavy path induced by gravity (gravistimulation). LRI takes place preferentially in the convex side of the wave alternately on the left and right side of the root axis, contemporaneously with an increase of auxin concentration observable in the lower side of the same convex site (De Smet et al., 2007). The increase of auxin concentration in the pericycle cells follow an oscillatory pattern with an interval of 15 h (De Smet et al., 2006) due to a rhythmical localization of PIN proteins (De Smet et al., 2007). In particular, a modification of PIN1 protein distribution in xylem cells induced by gravity has been reported (Ditengou et al., 2008); this diverts the basal flux of auxin to specific positions around the pericycle. In addition, there is the observation that ex novo LRI induction or variations of spacing between LRI along the convex side are obtainable by an artificial bending, a mechanical obstruction and a number of tropic factors (Richter et al., 2009). An antagonistic role is played by cytokinins which disturb PIN protein function in regulating auxin movement (Ruzicka et al., 2009).
In all these cases, the possible role of Ca2+ cannot be excluded (Richter et al., 2009). Beside the gravistimulation, a number of additional chemical factors, e.g. nitrate and sucrose availability in the soil, also seem to be responsible for LR formation (Roycewicz and Malamy, 2012; Araya et al., 2014).
REGULATORY NETWORK IN SLR FORMATION
In the past 10 years, we have demonstrated that mechanical stresses induce the emission of new SLRs from roots characterized by the presence of secondary tissues (i.e. lacking pericycle) (Chiatante and Scippa, 2006; Chiatante et al., 2007; Trupiano et al., 2012a). This type of induction has been observed in a number of woody plant species and, in all cases, the VC seems to be the putative tissue producing the SLRs (Fig. 5). The molecular mechanisms involved in SLR induction by mechanical stress have been investigated and first insights in poplar (Populus nigra) have been obtained through a proteomic and hormone profiling approach.
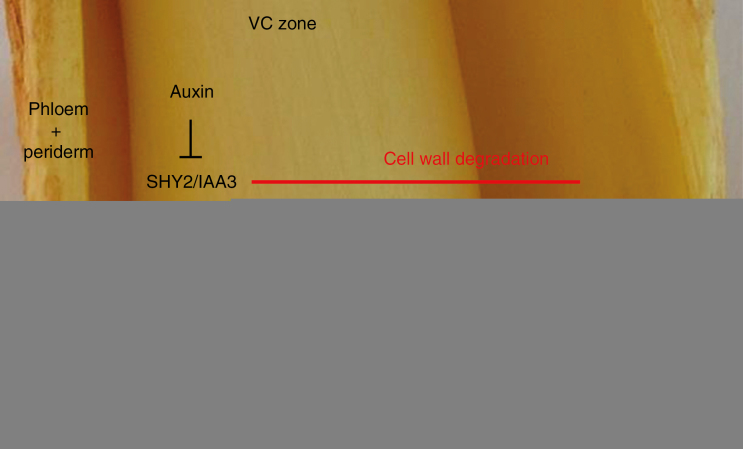
Fig. 5.
Phase II of SLR production: from an SCN to LRP formation within the secondary phloem. A cylindrical portion of a Populus nigra taproot after peeling off the periderm and the secondary phloem. Internally are visible (arrows) a number of newly formed LRPs of the SLRs produced in response to mechanical stress. The VC zone emerges when separating the two tissue flaps. The diagram shows two possible regulatory networks that could be involved in LRP formation. Both networks are based upon the initial action of auxin in the removal of SHY2/IAA3 repressor. The red line suggests that the same event is necessary to initiate the next phase that requires the degradation of the tissues surrounding the advancing new LRPs.
The proteomic investigations have revealed that a mechanical induction of SLR production may involve a putative serine-threonine protein kinase plant type (Trupiano et al., 2012b), associated with the cell wall; as shown in Fig. 3, it is proposed that this transduces mechanical forces into cellular signal (Peyronnet et al., 2014) mediated by Ca2+, as shown in arabidopsis. Indeed Trupiano et al. (2012b, 2013) and De Zio et al. (2016) reported that, in the bent woody root regions, the overexpression of proteins such as annexin and several reactive oxygen species scavenging factors that may be part of a Ca2+ signalling pathway occurs (Fig. 3). Important elements of this mechano-signalling module inducing SLRs may be represented by TFs, mechanically regulated miRNAs and the complex interplay among indole acetic acid (IAA), cytokinins, gibberellins (GAs), abscisic acid (ABA) and ethylene. Indeed, Basic Transcription Factor 3 (BFT3) and ERF have been found to be overexpressed in the bent woody root and, as proposed in Fig. 3, may be involved in SLR formation (Trupiano et al., 2012b, 2013, 2014), together with the Ptc-miR 164, Ptc-miR 172 and Ptc-miR 473 (Rossi et al., 2015).
In respect of the hormone pathway, we have also observed that when a woody root is bent, the mechanical stress induces a considerable quantitative and qualitative variation in hormonal profiling, indicating the occurrence of a complex interplay between auxin, GAs, ABA and ethylene which may regulate different stages of SLR development over time (Trupiano et al., 2012a). Moreover, the involvement of the PIN3 auxin efflux carrier in controlling auxin distribution has been suggested (Trupiano et al., 2014) probably by means of changes in its cellular location (Keuskamp et al., 2010).
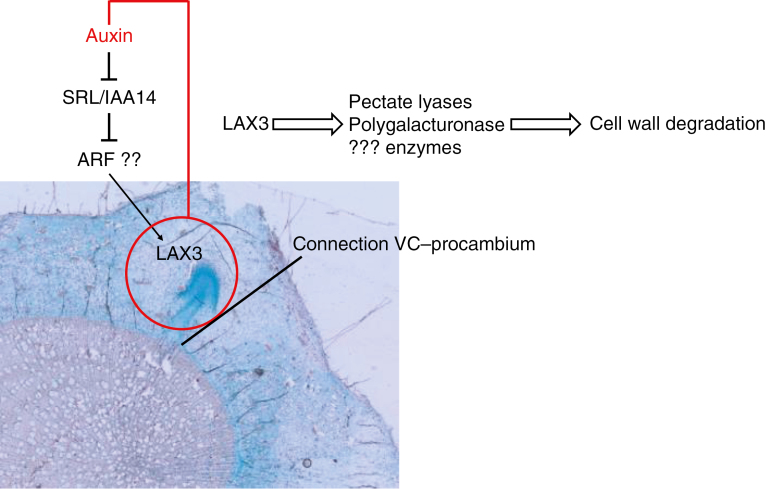
Despite the anatomical data accumulated by us which demonstrated clearly that VC in the root is able to produce new SLRs, the regulatory mechanisms controlling this event remain elusive: the proteomic analysis and the hormone profiling are still insufficient to suggest a complete list of all factors which could be involved in this event and to enable the modelling of a possible sequence of steps. Nevertheless, the above considerations regarding the RAM, procambium, VC and LRs clearly suggest that there is a possibility that some SCs of the VC have inherited from the xylem sector pericycle cells the competence to become FCs of a new SLR. Moreover, by considering the high degree of relatedness observed between the regulatory mechanisms controlling the various type of root meristems, we have attempted to model the regulatory mechanisms which could be active in the event of SLR production. In regard to this, we have divided the formation of a new SLR into a sequence of three different phases. In the first phase, we suggest that some SCs of VC are primed to become FCs in analogy with the events discussed above for LR formation from the pericycle. Two possible regulatory networks could be active in this event; these are shown in Fig. 4. The second phase involves the formation of a new SCN followed by the organization of a new LRP; this is represented in Fig. 5 where new LRPs have been made visible by peeling off the tissues surrounding the VC zone. Also in this case, two possible regulatory mechanisms are suggested which involve the same factors known to play similar roles during the formation of LRPs in roots characterized by primary structure. The third phase involves the protrusion of the new SLR from the parental root and requires the degradation of all the secondary tissues surrounding the VC (i.e. the secondary phloem, the phelloderm and the periderm). This phase is shown in Fig. 6; it involves the action of auxin on a gene (LAX3) (Swarup et al., 2008) able to influence the synthesis of enzymes active in cell wall degradation.
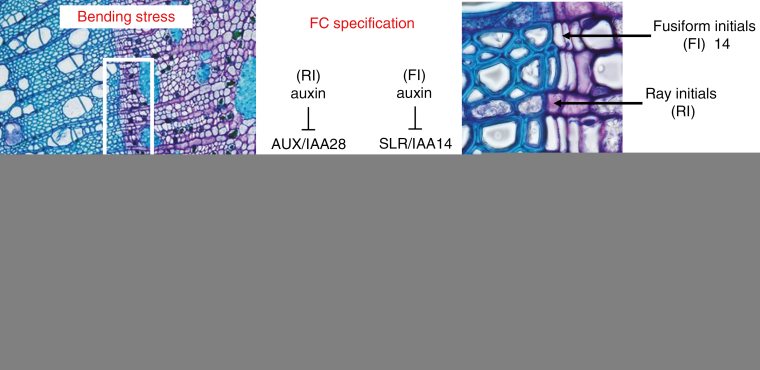
Fig. 4.
Phase I of SLR formation: FC specification, priming and SC proliferation to give rise to an SCN. The panel on the left shows the border zone between the secondary xylem and the secondary phloem of a 1-year-old Populus nigra taproot. The shadow area indicates the probable zone where the mechanical stress induced an accumulation of auxin produced in the SAM. The panel on the right shows a magnification of the same area with the arrows indicating the positions of fusiform initials (FI) and ray initials (RI). The regulatory network in the middle presents the two possible pathways followed by FI and RI to induce expression of GATA23 and the following FC specification.
Fig. 6.
Phase III of SLR production: from LRP to SLR protrusion. Transverse section of a Populus nigra taproot cut in the zone undergoing mechanical stress induction. In two places the formation of a new SLR is visible. The red circle indicates the position of the new LRP which is still developing internally to the parental woody root. Surrounding the new SLR it is possible that auxin achieves its maximum concentration. A possible regulatory mechanism is presented which is induced under the influence of auxin activation of the LAX3 gene. This gene promotes the activity of enzymes responsible for the metabolic degradation of the cell wall which could represent an obstacle for the emergence of the new SLR from its parental tissues.
The ability of woody plants to produce new SLRs (for nutritional and anchorage purposes) in portions of the root system characterized by the presence of a secondary structure modifies the root architecture and opens up the possibility to exploit again the soil which has been exploited already during the initial phase of plant development. Furthermore, the natural or induced (by pruning) death of root apices could thus be tolerated by a woody plant through the emergence of new SLRs. The implications for agriculture and forestry of a better knowledge of the regulatory mechanisms controlling SLR productions call for further investigations. The hypothetical models presented here suggest factors which could be tested for their involvement in this event.
ACKNOWLEDGEMENTS
The authors thank Antonio Monatgnoli, Dalila Trupiano, Mattia Terzaghi, Elena De Zio and Barbara Baesso for their contribution on the investigations on woody root biology.
LITERATURE CITED
- Abel S, Theologis A. 1996. Early genes and auxin action. Plant Physiology 111: 9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agusti J, Lichtenberger R, Schwarz M, Nehlin L, Greb T. 2011. Characterization of transcriptome remodelling during cambium formation identifies MOL1 and RUL1 as opposing regulators of secondary growth. PLoS Genetics 7: e1001312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aida M, Beis D, Heidstra R et al. . 2004. The PLETHORA genes mediate patterning of the Arabidopsis root stem cell niche. Cell 119: 119–120. [DOI] [PubMed] [Google Scholar]
- Araya T, von Wirén N, Takahashi H. 2014. CLE peptides regulate lateral root development in response to nitrogen nutritional status of plants. Plant Signaling and Behavior 9: e29302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson NJ, Lilley CJ, Urwin PE. 2013. Identification of genes involved in the response of Arabidopsis to simultaneous biotic and abiotic stresses. Plant Physiology 162: 2028–2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson JA, Rasmussen A, Traini R et al. . 2014. Branching out in roots: uncovering form, function, and regulation. Plant Physiology 166: 538–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azpeitia E, Weinstein N, Benitez M, Mendoza L, Alvarez-Buylla ER. 2013. Finding missing interactions of the Arabidopsis thaliana root stem cell niche gene regulatory network. Frontiers in Plant Science 4: 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baucher M, El Jaziri M, Vandeputte O. 2007. From primary to secondary growth: origin and development of the vascular system. Journal of Experimental Botany 58: 3485–3501. [DOI] [PubMed] [Google Scholar]
- Beeckman T, Burssens S, Inzé D. 2001. The peri-cell-cycle in Arabidopsis. Journal of Experimental Botany 52: 403–411. [DOI] [PubMed] [Google Scholar]
- Benfey PN. 2012. Toward a systems analysis of the root. Cold Spring Harbor Symposia on Quantative Biology 77: 91–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamins R, Scheres B. 2008. Auxin: the looping star in plant development. Annual Review of Plant Biology 59: 443–465. [DOI] [PubMed] [Google Scholar]
- Benková E, Bielach A. 2010. Lateral root organogenesis – from cell to organ. Current Opinion in Plant Biology 13: 677–683. [DOI] [PubMed] [Google Scholar]
- Benková E, Michniewicz M, Sauer M et al. . 2003. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115: 591–602. [DOI] [PubMed] [Google Scholar]
- van den Berg C, Willemsen V, Hage W, Weisbeek P, Scheres B. 1995. Cell fate in the Arabidopsis root meristem determined by directional signalling. Nature 378: 62–65. [DOI] [PubMed] [Google Scholar]
- van den Berg C, Willemsen V, Hendriks G, Weisbeek P, Scheres B. 1997. Short-range control of cell differentiation in the Arabidopsis root meristem. Nature 390: 287–289. [DOI] [PubMed] [Google Scholar]
- Bergonci T, Ribeiro B, Ceciliato PH, Guerrero-Abad JC, Silva-Filho MC, Moura DS. 2014. Arabidopsis thaliana RALF1 opposes brassinosteroid effects on root cell elongation and lateral root formation. Journal of Experimental Botany 65: 2219–2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishopp A, Help H, El-Showk S et al. . 2011. A mutually inhibitory interaction between auxin and cytokinin specifies vascular pattern in roots. Current Biology 21: 917–926. [DOI] [PubMed] [Google Scholar]
- Brackmann K, Greb T. 2014. Long- and short-distance signaling in the regulation of lateral plant growth. Physiologia Plantarum 151: 134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelain M, Le Hir R, Bellini C. 2012. The non-DNA-binding bHLH transcription factor PRE3/bHLH135/ATBS1/TMO7 is involved in the regulation of light signaling pathway in Arabidopsis. Physiologia Plantarum 145: 450–460. [DOI] [PubMed] [Google Scholar]
- Chaffey NJ, Barnett JR, Barlow PW. 1997. Visualization of the cytoskeleton within the secondary vascular system of hardwood species. Journal of Microscopy 187: 77–84. [DOI] [PubMed] [Google Scholar]
- Chang L, Ramireddy E, Schmülling T. 2015. Cytokinin as a positional cue regulating lateral root spacing in Arabidopsis. Journal of Experimental Botany 66: 4759–4768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiatante D, Scippa GS. 2006. Root architecture: influence of metameric organization and emission of lateral roots. Plant Biosystems 140: 307–320. [Google Scholar]
- Chiatante D, Scippa GS, Iorio AD, De Micco V, Sarnataro M. 2007. Lateral root emission in woody taproots of Fraxinus ornus L. Plant Biosystems 141: 204–213. [Google Scholar]
- Chiatante D, Beltotto M, Onelli E, Di Iorio A, Montagnoli A, Scippa SG. 2010. New branch roots produced by vascular cambium derivatives in woody parental roots of Populus nigra L. Plant Biosystems 144: 420–433. [Google Scholar]
- Czyzewicz N, De Smet I. 2015. The Arabidopsis thaliana CLAVATA3/EMBRYO-SURROUNDING REGION 26 (CLE26) peptide is able to alter root architecture of Solanum lycopersicum and Brassica napus. Plant Signaling and Behavior 11: e1118598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czyzewicz N, Nikonorova N, Meyer MR et al. . 2016. The growing story of (ARABIDOPSIS) CRINKLY 4. Journal of Experimental Botany 67: 4835–4847. [DOI] [PubMed] [Google Scholar]
- Daum G, Medzihradszky A, Suzaki T, Lohmann JU. 2014. A mechanistic framework for noncell autonomous stem cell induction in Arabidopsis. Proceedings of the National Academy of Sciences, USA 111: 14619–14624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Smet I, Vanneste S, Inze D, Beeckman T. 2006. Lateral root initiation or the birth of a new meristem. Plant Molecular Biology 60: 871–887. [DOI] [PubMed] [Google Scholar]
- De Smet I, Tetsumura T, De Rybel B et al. . 2007. Auxin-dependent regulation of lateral root positioning in the basal meristem of Arabidopsis. Development 134: 681–690. [DOI] [PubMed] [Google Scholar]
- De Smet I, Vassileva V, De Rybel B et al. . 2008. Receptor-like kinase ACR4 restricts formative cell divisions in the Arabidopsis root. Science 322: 594–597. [DOI] [PubMed] [Google Scholar]
- De Smet I, Lau S, Voss U et al. . 2010. Bimodular auxin response controls organogenesis in Arabidopsis. Proceedings of the National Academy of Sciences 107: 2705–2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depuydt S, Rodriguez-Villalon A, Santuari L, Wyser-Rmili C, Ragni L, Hardtke CS. 2013. Suppression of Arabidopsis protophloem differentiation and root meristem growth by CLE45 requires the receptor-like kinase BAM3. Proceedings of the National Academy of Sciences, USA 110: 7074–7079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rybel B, Vassileva V, Parizot B et al. . 2010. A novel Aux/IAA28 signaling cascade activates GATA23-dependent specification of lateral root founder cell identity. Current Biology 20: 1697–1706. [DOI] [PubMed] [Google Scholar]
- Dewitte W, Murray JAH. 2003. The plant cell cycle. Annual Review of Plant Biology 54: 235–264. [DOI] [PubMed] [Google Scholar]
- De Zio E, Trupiano D, Montagnoli A et al. . 2016. Poplar woody taproot under bending stress: the asymmetric response of the convex and concave sides. Annals of Botany 118: 865–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmasiri N, Dharmasiri S, Estelle M. 2005. The F-box protein TIR1 is an auxin receptor. Nature 435: 441–445. [DOI] [PubMed] [Google Scholar]
- Ding Z, Friml J. 2010. Auxin regulates distal stem cell differentiation in Arabidopsis roots. Proceedings of the National Academy of Sciences, USA 107: 12046–12051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinneny JR, Benfey PN. 2008. Plant stem cell niches: standing the test of time. Cell 132: 553–557. [DOI] [PubMed] [Google Scholar]
- Ditengou FA, Teale WD, Kochersperger P et al. . 2008. Mechanical induction of lateral root initiation in Arabidopsis thaliana. Proceedings of the National Academy of Sciences, USA 105: 18818–18823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerner P. 2003. Plant meristems: A merry-go-round of signals. Current Biology 13: 368–374. [DOI] [PubMed] [Google Scholar]
- Dodueva IE, Yurlova EV, Osipova MA, Lutova LA. 2012. CLE peptides are universal regulators of meristem development. Russian Journal of Plant Physiology 59: 14–27. [Google Scholar]
- Dodueva IE, Kiryushkin AS, Yurlova EV, Osipova MA, Buzovkina IS, Lutova LA. 2013. Effect of cytokinins on expression of radish CLE genes. Russian Journal of Plant Physiology 60: 388–395. [Google Scholar]
- Drisch RC, Stahl Y. 2015. Function and regulation of transcription factors involved in root apical meristem and stem cell maintenance. Frontiers in Plant Science 6: 505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubrovsky JG, Gambetta GA, Hernández-Barrera A, Shishkova S, González I. 2006. Lateral root initiation in Arabidopsis: developmental window, spatial patterning, density and predictability. Annals of Botany 97: 903–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esau K. 1977. Anatomy of seed plants, 2nd edn. Chichester, UK, 455–473. [Google Scholar]
- Etchells JP, Turner SR. 2010. The PXY–CLE41 receptor ligand pair defines a multifunctional pathway that controls the rate and orientation of vascular cell division. Development 137: 767–774. [DOI] [PubMed] [Google Scholar]
- Etchells JP, Provost CM, Turner SR. 2012. Plant vascular cell division is maintained by an interaction between pxy and ethylene signalling. PLoS Genetics 8: 1002997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etchells JP, Provost CM, Mishra L, Turner SR. 2013. WOX4 and WOX14 act downstream of the PXY receptor kinase to regulate plant vascular proliferation independently of any role in vascular organisation. Development 140: 2224–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Z, Sun X, Wang G, Liu H, Zhu J. 2012. LBD29 regulates the cell cycle progression in response to auxin during lateral root formation in Arabidopsis thaliana. Annals of Botany 110: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez A, Drozdzecki A, Hoogewijs K et al. . 2015. The GLV6/RGF8/CLEL2 peptide regulates early pericycle divisions during lateral root initiation. Journal of Experimental Botany 66: 5245–5256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher K, Turner S. 2007. PXY, a receptor-like kinase essential for maintaining polarity during plant vascular-tissue development. Current Biology 17: 1061–1066. [DOI] [PubMed] [Google Scholar]
- Gallois J.-L., Nora F. R., Mizukami Y, Sablowski R. 2004. WUSCHEL induces shoot stem cell activity and developmental plasticity in the root meristem. Genes and Development 18: 375–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garay-Arroyo A, De La Paz Sanchez M, Garcia-Ponce B, Azpeitia E, Alvarez-Buylla ER. 2012. Hormone symphony during root growth and development. Developmental Dynamics 241: 1867–1885. [DOI] [PubMed] [Google Scholar]
- Geldner N. 2003. Partial loss-of-function alleles reveal a role for GNOM in auxin transport-related, post-embryonic development of Arabidopsis. Development 131: 389–400. [DOI] [PubMed] [Google Scholar]
- Gifford ML, Dean A, Gutierrez RA, Coruzzi GM, Birnbaum KD. 2008. Cell-specific nitrogen responses mediate developmental plasticity. Proceedings of the National Academy of Sciences, USA 105: 803–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh T, Joi S, Mimura T, Fukaki H. 2012. The establishment of asymmetry in Arabidopsis lateral root founder cells is regulated by LBD16/ASL18 and related LBD/ASL proteins. Development 139: 883–893. [DOI] [PubMed] [Google Scholar]
- Goh T, Voβ U, Farcot E, Bennett MJ, Bishopp A. 2014. Systems biology approaches to understand the role of auxin in root growth and development. Physiologia Plantarum 151: 73–82. [DOI] [PubMed] [Google Scholar]
- Goué N, Lesage-Descauses MC, Mellerowicz EJ, Magel E, Label P, Sundberg B. 2008. Microgenomic analysis reveals cell type-specific gene expression patterns between ray and fusiform initials within the cambial meristem of Populus. New Phytologist 180: 45–56. [DOI] [PubMed] [Google Scholar]
- Groover AT, Mansfield SD, DiFazio SP et al. . 2006. The Populus homeobox gene ARBORKNOX1 reveals overlapping mechanisms regulating the shoot apical meristem and the vascular cambium. Plant Molecular Biology 61: 917–932. [DOI] [PubMed] [Google Scholar]
- Guilfoyle TJ, Hagen G. 2007. Auxin response factors. Current Opinion in Plant Biology 10: 453–460. [DOI] [PubMed] [Google Scholar]
- Hirakawa Y, Shinohara H, Kondo Y et al. . 2008. Non-cell-autonomous control of vascular stem cell fate by a CLE peptide/receptor system. Proceedings of the National Academy of Sciences, USA 105: 15208–15213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirakawa Y, Kondo Y, Fukuda H. 2010a. TDIF peptide signaling regulates vascular stem cell proliferation via the WOX4 homeobox gene in Arabidopsis. The Plant Cell 22: 2618–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirakawa Y, Kondo Y, Fukuda H. 2010b. Regulation of vascular development by CLE peptide–receptor systems. Journal of Integrative Plant Biology 52: 8–16. [DOI] [PubMed] [Google Scholar]
- Hirakawa Y, Kondo Y, Fukuda H. 2011. Establishment and maintenance of vascular cell communities through local signaling. Current Opinion in Plant Biology 14: 17–23. [DOI] [PubMed] [Google Scholar]
- Ilegems M, Douet V, Meylan-Bettex M et al. . 2010. Interplay of auxin, KANADI and class III HD-ZIP transcription factors in vascular tissue formation. Development 137: 975–984. [DOI] [PubMed] [Google Scholar]
- Ito Y, Nakanomyo I, Motose H, Iwamoto K, Sawa S, Dohmae N, Fukuda H. 2006. Dodeca-CLE peptides as suppressors of plant stem cell differentiation. Science 313: 842–845. [DOI] [PubMed] [Google Scholar]
- Ivanov VB, Dubrovsky JG. 2013. Longitudinal zonation pattern in plant roots: conflicts and solutions. Trends in Plant Science 18: 237–243. [DOI] [PubMed] [Google Scholar]
- Iyer-Pascuzzi AS, Benfey PN. 2009. Transcriptional networks in root cell fate specification. Biochimica et Biophysica Acta 1789: 315–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang G, Lee JY. 2014. Intercellular trafficking of transcription factors in the vascular tissue patterning. Physiologia Plantarum 151: 184–191. [DOI] [PubMed] [Google Scholar]
- Jansen L, Roberts I, De Rycke R, Beeckman T. 2012. Phloem-associated auxin response maxima determine radial positioning of lateral roots in maize. Philosophical Transactions of the Royal Society B: Biological Sciences 367: 1525–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keuskamp DH, Pollmann S, Voesenek LACJ, Peeters AJM, Pierik R. 2010. Auxin transport through PIN-FORMED 3 (PIN3) controls shade avoidance and fitness during competition. Proceedings of the National Academy of Sciences, USA 107: 22740–22744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiba T, Takei K, Kojima M, Sakakibara H. 2013. Side-chain modification of cytokinins controls shoot growth in Arabidopsis. Developmental Cell 27: 452–461. [DOI] [PubMed] [Google Scholar]
- Kleine-Vehn J, Dhonukshe P, Swarup R, Bennett M, Friml J. 2006. Subcellular trafficking of the Arabidopsis auxin influx carrier AUX1 uses a novel pathway distinct from PIN1. The Plant Cell 18: 3171–3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumpf RP, Shi CL, Larrieu A et al. . 2013. Floral organ abscission peptide IDA and its HAE/HSL2 receptors control cell separation during lateral root emergence. Proceedings of the National Academy of Sciences, USA 110: 5235–5240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachaud S, Catesson AM, Bonnemain JL. 1999. Structure and functions of the vascular cambium. Comptes Rendus de l’Academie des Sciences – Serie III 322: 633–650. [DOI] [PubMed] [Google Scholar]
- Laskowski MJ, Williams ME, Nusbaum HC, Sussex IM. 1995. Formation of lateral root meristems is a two-stage process. Development 121: 3303–3310. [DOI] [PubMed] [Google Scholar]
- Laux T. 2003. The stem cell concept in plants: a matter of debate. Cell 113: 281–283. [DOI] [PubMed] [Google Scholar]
- Lavenus J, Goh T, Roberts I et al. . 2013. Lateral root development in Arabidopsis: fifty shades of auxin. Trends in Plant Science 18: 450–458. [DOI] [PubMed] [Google Scholar]
- Li L, Xie T. 2005. Stem cell niche: structure and function. Annual Review of Cell and Developmental Biology 21: 605–631. [DOI] [PubMed] [Google Scholar]
- Long J, Barton MK. 1998. The development of apical embryonic pattern in Arabidopsis. Development 125: 3027–3035 [DOI] [PubMed] [Google Scholar]
- Mähönen AP, Higuchi M, Törmäkangas K et al. . 2006. Cytokinins regulate a bidirectional phosphorelay network in Arabidopsis. Current Biology 16: 1116–1122. [DOI] [PubMed] [Google Scholar]
- Malamy JE, Benfey PN. 1997. Organization and cell differentiation in lateral roots of Arabidopsis thaliana. Development 124: 33–44. [DOI] [PubMed] [Google Scholar]
- Marin E, Jouannet V, Herz A et al. . 2010. miR390, Arabidopsis TAS3 tasiRNAs, and their AUXIN RESPONSE FACTOR targets define an autoregulatory network quantitatively regulating lateral root growth. The Plant Cell 22: 1104–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng L, Buchanan BB, Feldman LJ, Luan S. 2012. CLE-like (CLEL) peptides control the pattern of root growth and lateral root development in Arabidopsis. Proceedings of the National Academy of Sciences, USA 109: 1760–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashima S, Sebastian J, Lee J-Y, Helariutta Y. 2013. Stem cell function during plant vascular development. EMBO Journal 32: 178–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Risueno MA, Van Norman JM, Moreno A, Zhang J, Ahnert SE, Benfey PN. 2010. Oscillating gene expression determines competence for periodic Arabidopsis root branching. Science 329: 1306–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyle R, Schrader J, Stenberg A et al. . 2002. Environmental and auxin regulation of wood formation involves members of the Aux/IAA gene family in hybrid aspen. The Plant Journal 31: 675–685. [DOI] [PubMed] [Google Scholar]
- Müller B, Sheen J. 2008. Cytokinin and auxin interaction in root stem-cell specification during early embryogenesis. Nature 453: 1094–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy E, Vu LD, Van Den Broeck L et al. . 2016. RALFL34 regulates formative cell divisions in Arabidopsis pericycle during lateral root initiation. Journal of Experimental Botany 67: 4863–4875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardmann J, Werr W. 2013. Symplesiomorphies in the WUSCHEL clade suggest that the last common ancestor of seed plants contained at least four independent stem cell niches. New Phytologist 199: 1081–1092. [DOI] [PubMed] [Google Scholar]
- Nieuwland J, Maughan S, Dewitte W, Scofield S, Sanz L, Murray JAH. 2009. The D-type cyclin CYCD4;1 modulates lateral root density in Arabidopsis by affecting the basal meristem region. Proceedings of the National Academy of Sciences, USA 106: 22528–22533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimchuk ZL, Zhou Y, Tarr PT, Peterson BA, Meyerowitz EM. 2015. Plant stem cell maintenance by transcriptional cross-regulation of related receptor kinases. Development 142: 1043–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi-Ito K, Fukuda H. 2010. Transcriptional regulation of vascular cell fates. Current Opinion in Plant Biology 13: 670–676. [DOI] [PubMed] [Google Scholar]
- Okushima Y, Fukaki H, Onoda M, Theologis A, Tasaka M. 2007. ARF7 and ARF19 regulate lateral root formation via direct activation of LBD/ASL genes in Arabidopsis. The Plant Cell 19: 118–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overvoorde P, Fukaki H, Beeckman T. 2010. Auxin control of root development. Cold Spring Harbor Perspectives in Biology 2: a001537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco-Escobedo MA, Ivanov VB, Ransom-Rodríguez I et al. . 2016. Longitudinal zonation pattern in Arabidopsis root tip defined by a multiple structural change algorithm. Annals of Botany 118: 763–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallakies H, Simon R. 2014. The CLE40 and CRN/CLV2 signaling pathways antagonistically control root meristem growth in Arabidopsis. Molecular Plant 7: 1619–1636. [DOI] [PubMed] [Google Scholar]
- Pan L, Lv S, Yang N, Yanting L, Liu Z, Wu J, Wand G. 2016. The multifunction of CLAVATA2 in plant development and immunity. Frontiers in Plant Science 7: 1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parizot B, Laplaze L, Ricaud L et al. . 2008. Diarch symmetry of the vascular bundle in Arabidopsis root encompasses the pericycle and is reflected in distich lateral root initiation. Plant Physiology 146: 140–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Péret B, De Rybel B, Casimiro I et al. . 2009. Arabidopsis lateral root development: an emerging story. Trends in Plant Science 14: 399–408. [DOI] [PubMed] [Google Scholar]
- Petersson SV, Johansson AI, Kowalczyk M et al. . 2009. An auxin gradient and maximum in the Arabidopsis root apex shown by high-resolution cell-specific analysis of IAA distribution and synthesis. The Plant Cell 21: 1659–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrasek J, Friml J. 2009. Auxin transport routes in plant development. Development 136: 2675–2688. [DOI] [PubMed] [Google Scholar]
- Peyronnet R, Tran D, Girault T, Frachisse J-M. 2014. Mechanosensitive channels: feeling tension in a world under pressure. Frontiers in Plant Science 5: 558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi L, Aichinger E, van der Graaff E et al. . 2015. Organizer-derived WOX5 signal maintains root columella stem cells through chromatin- mediated repression of CDF4 expression. Developmental Cell 33: 576–588. [DOI] [PubMed] [Google Scholar]
- Prigge MJ, Otsuga D, Alonso JM, Ecker JR, Drews GN, Clark SE. 2005. class III homeodomain-leucine zipper gene family members have overlapping, antagonistic, and distinct roles in Arabidopsis development. The Plant Cell 17: 61–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przemeck GKH, Mattsson J, Hardtke CS, Sung ZR, Berleth T. 1996. Studies on the role of the Arabidopsis gene MONOPTEROS in vascular development and plant cell axialization. Planta 200: 229–237. [DOI] [PubMed] [Google Scholar]
- Rahni R, Efroni I, Birnbaum KD. 2016. A case for distributed control of local stem cell behavior in plants. Developmental Cell. 38: 635–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter GL, Monshausen GB, Krol A, Gilroy S. 2009. Mechanical stimuli modulate lateral root organogenesis. Plant Physiology 151: 1855–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieu I, Laux T. 2009. Signaling pathways maintaining stem cells at the plant shoot apex. Seminars in Cell and Developmental Biology 20: 1083–1088. [DOI] [PubMed] [Google Scholar]
- Roberts I, Smith S, Stes E et al. . 2016. CEP5 and XIP1/CEPR1 regulate lateral root initiation in Arabidopsis. Journal Experimental Botany 67: 4889–4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Villalon A, Gujas B, Kang YH et al. . 2014. Molecular genetic framework for protophloem formation. Proceedings of the National Academy of Sciences, USA 111: 11551–11556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Villalon A, Gujas B, van Wijk R, Munnik T, Hardtke CS. 2015. Primary root protophloem differentiation requires balanced phosphatidylinositol-4,5-biphosphate levels and systemically affects root branching. Development 142: 1437–1446. [DOI] [PubMed] [Google Scholar]
- Rossi M, Trupiano D, Tamburro M et al. . 2015. MicroRNAs expression patterns in the response of poplar woody root to bending stress. Planta 242: 339–51. [DOI] [PubMed] [Google Scholar]
- Roycewicz P, Malamy JE. 2012. Dissecting the effects of nitrate, sucrose and osmotic potential on Arabidopsis root and shoot system growth in laboratory assays. Philosophical Transactions of the Royal Society B: Biological Sciences 367: 1489–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzicka K, Simaskova M, Duclercq J et al. . 2009. Cytokinin regulates root meristem activity via modulation of the polar auxin transport. Proceedings of the National Academy of Sciences, USA 106: 4284–4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sablowski R. 2007. The dynamic plant stem cell niches. Current Opinion in Plant Biology 10: 639–644. [DOI] [PubMed] [Google Scholar]
- Sablowski R. 2011. Plant stem cell niches: from signalling to execution. Current Opinion in Plant Biology 14: 4–9. [DOI] [PubMed] [Google Scholar]
- Sachs T. 1991. Cell polarity and tissue patterning in plants. Development 113: 83–93. [Google Scholar]
- Sarkar AK, Luijten M, Miyashima S et al. . 2007. Conserved factors regulate signalling in Arabidopsis thaliana shoot and root stem cell organizers. Nature 446: 811–814. [DOI] [PubMed] [Google Scholar]
- Schlereth A, Möller B, Liu W et al. . 2010. MONOPTEROS controls embryonic root initiation by regulating a mobile transcription factor. Nature 464: 913–916. [DOI] [PubMed] [Google Scholar]
- Schrader J. 2004. A high-resolution transcript profile across the wood-forming meristem of poplar identifies potential regulators of cambial stem cell identity. The Plant Cell 16: 2278–2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scippa GS, Trupiano D, Rocco M, Di Iorio A, Chiatante D. 2008. Unravelling the response of poplar (Populus nigra) roots to mechanical stress imposed by bending. Plant Biosystems 142: 401–413. [Google Scholar]
- Sieburth LE, Deyholos MK. 2006. Vascular development: the long and winding road. Current Opinion in Plant Biology 9: 48–54. [DOI] [PubMed] [Google Scholar]
- Smith ZR, Long JA. 2010. Control of Arabidopsis apical–basal embryo polarity by antagonistic transcription factors. Nature 464: 423–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spradling A, Drummond-Barbosa D, Kai T. 2001. Stem cells find their niche. Nature 414: 98–104. [DOI] [PubMed] [Google Scholar]
- Stahl Y, Faulkner C. 2016. Receptor complex mediated regulation of symplastic traffic. Trends in Plant Science 21: 450–459. [DOI] [PubMed] [Google Scholar]
- Stahl Y, Simon R. 2010. Plant primary meristems: shared functions and regulatory mechanisms. Current Opinion in Plant Biology 13: 53–58. [DOI] [PubMed] [Google Scholar]
- Stahl Y, Simon R. 2012. Peptides and receptors controlling root development. Philosophical Transactions of the Royal Society B: Biological Sciences 367: 1453–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl Y, Simon R. 2013. Gated communities: apoplastic and symplastic signals converge at plasmodesmata to control cell fates. Journal of Experimental Botany 64: 5237–5241. [DOI] [PubMed] [Google Scholar]
- Stahl Y, Wink RH, Ingram GC, Simon R. 2009. A signaling module controlling the stem cell niche in Arabidopsis root meristems. Current Biology 19: 909–914. [DOI] [PubMed] [Google Scholar]
- Stahl Y, Grabowski S, Bleckmann A et al. . 2013. Moderation of arabidopsis root stemness by CLAVATA1 and ARABIDOPSIS CRINKLY4 receptor kinase complexes. Current Biology 23: 362–371. [DOI] [PubMed] [Google Scholar]
- Steeves TA, Sussex IM. 1989. Patterns in plant development. New York: Cambridge University Press. [Google Scholar]
- Suer S, Agusti J, Sanchez P, Schwarz M, Greb T. 2011. WOX4 imparts auxin responsiveness to cambium cells in Arabidopsis. The Plant Cell 23: 3247–3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarup K, Benková E, Swarup R et al. . 2008. The auxin influx carrier LAX3 promotes lateral root emergence. Nature Cell Biology 10: 946–954. [DOI] [PubMed] [Google Scholar]
- Teichmann T, Muhr M. 2015. Shaping plant architecture. Frontiers in Plant Science 6: 233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trupiano D, Di Iorio A, Montagnoli A et al. . 2012a. Involvement of lignin and hormones in the response of woody poplar taproots to mechanical stress. Physiologia Plantarum 146: 39–52. [DOI] [PubMed] [Google Scholar]
- Trupiano D, Rocco M, Renzone G et al. . 2012b. The proteome of Populus nigra woody root: response to bending. Annals of Botany 110: 415–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trupiano D, Rocco M, Renzone G et al. . 2013. Poplar woody root proteome during the transition dormancy–active growth. Plant Biosystems 147: 1095–1100. [Google Scholar]
- Trupiano D, Rocco M, Renzone G et al. . 2014. Temporal analysis of poplar woody root response to bending stress. Physiologia Plantarum 150: 174–193. [DOI] [PubMed] [Google Scholar]
- Ueguchi C. 2001. The AHK4 gene involved in the cytokinin-signaling pathway as a direct receptor molecule in Arabidopsis thaliana. Plant and Cell Physiology 42: 751–755. [DOI] [PubMed] [Google Scholar]
- Vahala J, Felten J, Love J et al. . 2013. A genome-wide screen for ethylene-induced ethylene response factors (ERFs) in hybrid aspen stem identifies ERF genes that modify stem growth and wood properties. New Phytologist 200: 511–522. [DOI] [PubMed] [Google Scholar]
- Vanneste S, De Rybel B, Beemster GTS et al. . 2005. Cell cycle progression in the pericycle is not sufficient for SOLITARY ROOT/IAA14-mediated lateral root initiation in Arabidopsis thaliana. The Plant Cell 17: 3035–3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Raemdonck D, Pesquet E, Cloquet S et al. . 2005. Molecular changes associated with the setting up of secondary growth in aspen. Journal of Experimental Botany 56: 2211–2227. [DOI] [PubMed] [Google Scholar]
- Vieten A, Vanneste S, Wisniewska J et al. . 2005. Functional redundancy of PIN proteins is accopanied by auxin-dependednt cross-regulation of PIN expression. Development 132: 4521–4531. [DOI] [PubMed] [Google Scholar]
- Weijers D, Schlereth A, Ehrismann JS, Schwank G, Kientz M, Jurgens G. 2006. Auxin triggers transient local signaling for cell specification in Arabidopsis embryogenesis. Developmental Cell 10: 265–270. [DOI] [PubMed] [Google Scholar]
- Welch D, Hassan H, Blilou I, Immink R, Heidstra R, Scheres B. 2007. Arabidopsis JACKDAW and MAGPIE zinc finger proteins delimit asymmetric cell division and stabilize tissue boundaries by restricting SHORT-ROOT action. Genes and Development 21: 2196–2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Song Y, Yang H, Liu Z, Zhu G, Yang Y. 2014. An auxin responsive endogenous peptide regulates root development in Arabidopsis. Journal of Integrative Plant Biology 56: 635–647. [DOI] [PubMed] [Google Scholar]
- Yoon EK, Yang JH, Lim J, Kim SH, Kim SK, Lee WS. 2009. Auxin regulation of the microRNA390-dependent transacting small interfering RNA pathway in Arabidopsis lateral root development. Nucleic Acids Research 38: 1382–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yordanov YS, Regan S, Busov V. 2010. Members of the LATERAL ORGAN BOUNDARIES DOMAIN transcription factor family are involved in the regulation of secondary growth in Populus. The Plant Cell 22: 3662–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Zhang HJ, Zhao B et al. . 2014. The RNA-seq approach to discriminate gene expression profiles in response to melatonin on cucumber lateral root formation. Journal of Pineal Research 56: 39–50. [DOI] [PubMed] [Google Scholar]
- Zhao C, Craig JC, Petzold HE, Dickerman AW, Beers EP. 2005. The xylem and phloem transcriptomes from secondary tissues of the Arabidopsis root-hypocotyl. Plant Physiology 138: 803–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Wei L, Xu J et al. . 2010. Arabidopsis tyrosylprotein sulfotransferase acts in the auxin/PLETHORA pathway in regulating postembryonic maintenance of the root stem cell niche. The Plant Cell 22: 3692–3709. [DOI] [PMC free article] [PubMed] [Google Scholar]