Abstract
Background and Aims
Helicocytic stomata are characterized by an inward spiral of mesogenous cells surrounding a central stomatal pore. They represent a relatively rare feature that occurs in some drought-tolerant angiosperm species. In some Begonia species with thick leaves, the stomata are not only helicocytic but also clustered into groups that are spaced apart by at least one cell. This paper presents a detailed ontogenetic study of this characteristic non-contiguous stomatal patterning in a developmental and phylogenetic context.
Methods
Light microscopy and both scanning and transmission electron microscopy were used to examine stomatal development in several species of Begonia. Published reports of stomatal development in Begonia and other angiosperms were reviewed to provide a comprehensive discussion of the evolution of stomatal patterning.
Key Results
Helicocytic stomata develop from meristemoids that undergo a series of oriented asymmetric divisions to produce a spiral of mesogene stomatal lineage ground cells (SLGCs) surrounding a stoma. A clear developmental similarity between anisocytic and helicocytic stomata is positively correlated with the number of iterations of amplifying divisions that result in SLGCs. Stomatal clusters develop from asymmetric divisions in neighbouring SLGCs. Within each cluster, non-contiguous spacing of meristemoids is maintained by asymmetric divisions oriented away from each developing meristemoid.
Conclusions
Formation of non-contiguous stomatal clusters in Begonia relies on two primary developmental factors in the epidermis: an inwardly spiralling series of amplifying divisions that result in helicocytic stomata, and the development of a variable number of meristemoids from neighbouring SLGCs within each cluster. Optimization of these features on an angiosperm phylogeny indicates that the occurrence of amplifying divisions could be pre-adaptive for these factors. Both factors have been thoroughly studied in terms of developmental genetics in Arabidopsis, suggesting gene orthologues that could be implicated in Begonia stomatal patterning.
Keywords: Amplifying divisions, anisocytic stomata, helicocytic stomata, Begonia, meristemoids, non-contiguous stomatal clusters, stomatal development
INTRODUCTION
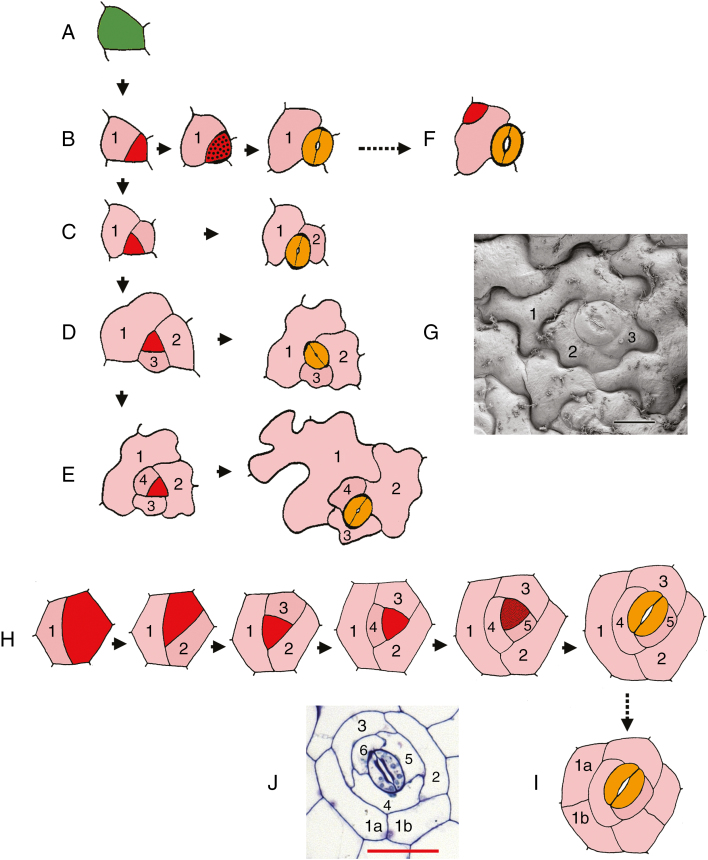
Stomatal patterning on leaves is governed by a series of developmental constraints that are directly linked with leaf expansion (Rudall et al., 2013, 2017). The well-documented pattern of stomatal development that occurs in Arabidopsis involves a series of asymmetric mitoses in each stomatal cell lineage wherein all the resulting cells are derived from the same initial meristemoid mother cell (Zhao and Sack, 1999). About two-thirds of meristemoids divide asymmetrically two or more times (Fig. 1D, E), so that the mature stomata are entirely surrounded by mesogene neighbour cells (stomatal lineage ground cells: SLGCs); each complex is therefore monoclonal (Nadeau and Sack, 2002). Such iterative asymmetric divisions in a single cell lineage are termed amplifying divisions; they result in an inward spiral with the stomatal pore at its centre.
Fig. 1.
Stomatal lineage diagrams. (A–F) Development of stomata in Arabidopsis from an epidermal initial cell, a meristemoid mother cell (A), showing stomata with either (B) a single mesogene stomatal lineage ground cell (SLGC), (C) two SLGCs, (D) three SLGCs (anisocytic type) or (E) four SLGCs. Types shown in D and E occur in about two-thirds of cases, and result in stomata that are entirely surrounded by either three or four mesogene SLGCs. In F, a secondary or ‘satellite’ meristemoid has formed by an asymmetric division of an SLGC oriented away from the initial meristemoid. (G) Scanning electron micrograph of anisocytic stoma in Arabidopsis thaliana, numbered as in D. (H, I) Development of helicocytic stomata with five mesogene SLGCs. In I, one of the initial cells has enlarged and secondarily divided, resulting in a ring of cells enclosing the entire complex. (J) Helicocytic stoma in Begonia calderonii, numbered as in H and I. Diagrams redrawn and coloured in A–F are from Zhao and Sack (1999), in H and I from Payne (1970). Numbers indicate sequence of development of SLGCs in inward spiral; the highest number is the sister cell to the meristemoid. Colours: green, meristemoid-mother cell (stomatogenic cell); red, meristemoid; red stippled, guard-mother cell; yellow, guard cell; pink, SLGC. Scale bars: G = 10 µm, J = 50 µm.
In some eudicots (e.g. Begonia L.), helicocytic stomata are formed when amplifying divisions become even more extensive; the guard-cell–mother cell (GMC) is preceded by a series of divisions that create several (often five or more) SLGCs that surround the stoma in a spiral arrangement (Payne, 1970). In most eudicot species, stomata are distributed relatively uniformly across intercostal regions of the leaf surface. However, some drought-tolerant species possess thick leaves and dense hypodermal tissue, effectively internalizing the photosynthesizing mesophyll tissues; the stomata remain spaced apart by at least one cell, but are clustered into groups that each share a common substomatal cavity (Gan et al., 2010). Such non-contiguous stomatal clusters have long been described in Begonia (since Strasburger, 1867), but there has been surprisingly little work on their early development, perhaps partly because the stomata are very small. One exception is Tang et al.’s (2002) study of development in B. peltatifolia using light microscopy of nail-polish peels. In this paper, we use both light microscopy and transmission electron microscopy to examine the development of the clusters of helicocytic stomata that occur in some species of Begonia. Our primary goal is to place this striking developmental pattern in a broader phylogenetic context and hence to present a predictive hypothesis on its possible adaptive significance and underlying genetic pathways.
Previous authors have documented the diversity of leaf anatomy and stomatal patterning in Begonia (e.g. Neubauer and Beissler, 1971; Boghdan and Barkley, 1972; Tang et al., 2002), and others have examined ecophysiological aspects of their anatomy (Hoover, 1986; Gan et al., 2010; Papanatsiou et al., 2017). Begonia is a large pantropical genus of about 1900 species of mostly understorey herbs from the moist tropics, although some more xerophytic species grow in rocky areas at altitude, often in arid or semi-arid habitats (Brennan et al., 2012; Harrison et al., 2016). Many xerophytic Begonia species possess a well-developed hypodermis beneath both abaxial and adaxial epidermises (wrongly reported as a multiple epidermis by Tang et al., 2002); in these species, stomata are confined to clusters on the abaxial surface, and different species are characterized by different numbers of stomata per cluster (from two to many). In mesophytic species that lack a hypodermis, stomata occur singly rather than in clusters. Here, we examine development in species both with and without stomatal clusters.
MATERIALS AND METHODS
Material
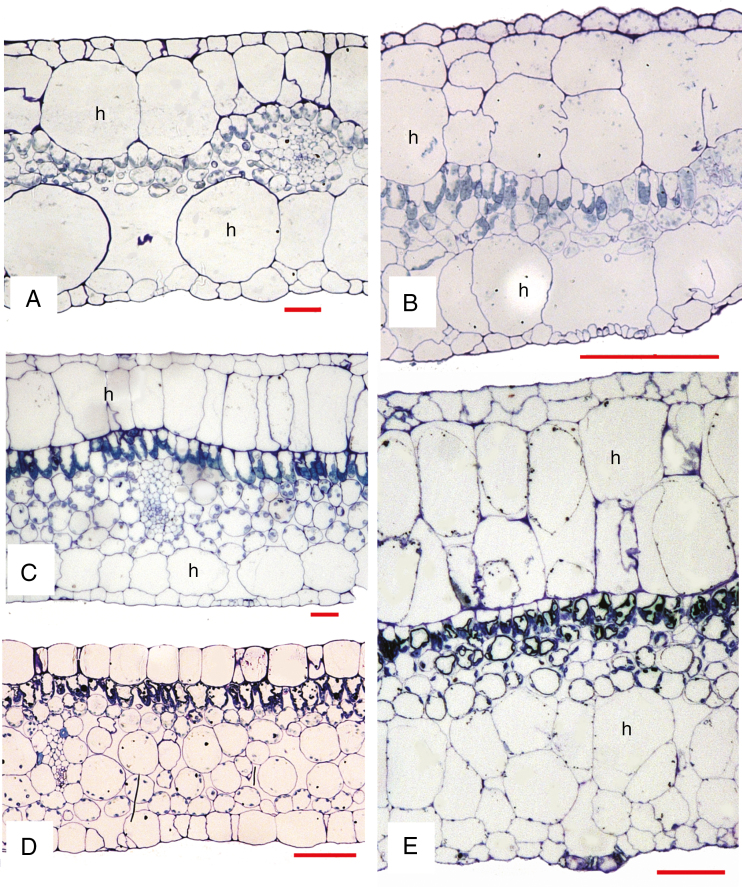
Samples were collected from five species cultivated in the extensive Begonia collections grown at the Royal Botanic Garden Edinburgh (RBGE). Two leaves were sampled from each individual: a large leaf and the smallest leaf available on the plant. Larger leaves were cut approximately midway between the midrib and leaf margin. Four species (B. calderonii Carpenter, B. conchifolia A.Dietr., B. heracleifolia Cham. & Schltdl., B. nelumbiifolia Cham. & Schltdl.) are native to Central America, occurring in rocky habitats at altitudes of up to 2000 m; they possess relatively thick xeromorphic leaves with abaxial stomatal clusters. A further species, B. venusta King, is native to more mesic habitats in Malaysia; it possesses comparatively thin, mesomorphic leaves. Finally, a widely cultivated species, B. fuchsioides Hook., was also imaged from a microscope slide available in the collection at RBG Kew, originally prepared from material grown at Kew (Fig. 2C). A leaf surface of Arabidopsis thaliana (L.) Heynh. was imaged from material also grown at Kew (Fig. 1G).
Fig. 2.
Light micrographs of leaf transverse sections. (A) Begonia nelumbiifolia. (B) B. heracleifolia. (C) B. calderonii. (D) B. venusta. (E) B. conchifolia. h, hypodermis (absent from D). Scale bars: A, C, E = 50 µm, B = 200 µm, D = 100 µm.
Methods
A combination of both transmission electron microscopy (TEM) and light microscopy (LM) is desirable for effective imaging of early developmental stages of the relatively small stomata of Begonia. For both TEM and LM, leaf samples were fixed in Karnovsky’s fixative (50 mL 0.1 m phosphate buffer, 20 mL 10 % paraformaldehyde solution, 10 mL 25 % glutaraldehyde solution and 20 mL deionized water), stained using osmium tetroxide and embedded in LR-white resin. Transverse sections of leaves and paradermal sections of the abaxial surfaces were prepared using a Reichert-Jung Ultracut ultramicrotome and DDK 5 -mm diamond knife to make both ultra-thin (approx. 0.1 µm) and semi-thin (approx. 0.5 µm) sections. Ultra-thin sections of two species (B. nelumbiifolia, B. venusta) were mounted on Formvar-coated copper grids and imaged using a Hitachi H-7650 transmission electron microscope. Semi-thin sections of all species except B. fuchsioides were stained in 0.5 % Toluidine Blue and mounted on microscope glass slides using DPX (a mixture of distyrene, a plasticizer and xylene). Sections were imaged using a Leica DM LB 100T light microscope and Zeiss AxioCam HRc digital camera. For scanning electron microscopy (SEM) of all species except B. fuchsioides, samples were fixed in formalin–acetic acid–alcohol (FAA), then passed through an ethanol series to 100 % ethanol, and critical-point dried using a Tousimis supercritical Autosamdri 815B dryer. Samples were then sputter-coated with platinum using a Quorum Q150TES sputter coater and imaged using a Hitachi S-4700 II cold-field emission scanning electron microscope.
RESULTS
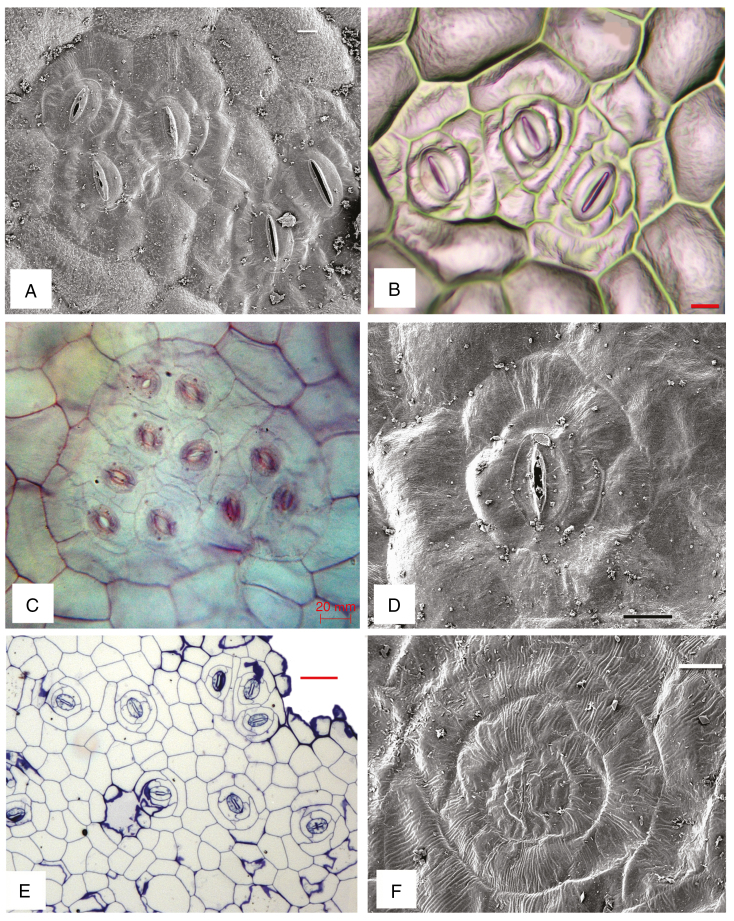
All species examined possess a hypodermis beneath both adaxial and abaxial epidermises (Fig. 2A–C, E), except B. venusta (Fig. 2D), which lacks a hypodermis. The hypodermis ranges in thickness from a single cell (Fig. 2A, C) to two or more cell layers that are often elongated (Fig. 2B, E). Mature stomatal patterns in the species examined are given in Table 1. Stomata are invariably helicocytic, and occur either in groups of up to six or more (Fig. 3A–C) or predominantly as single stomata (Fig. 3D–F). Some species have stomatal clusters of 10–20 (Fig. 3C). There is typically a distinct ring of cells surrounding each complex, and each individual stoma is surrounded by a spiral of SLGCs. Mature stomata have evenly thickened walls and contain starch grains (Fig. 4A).
Table 1.
Data on relevant leaf features in species examined
| No. of stomata per cluster | Hypodermis (no. of cell layers) | |
|---|---|---|
| B. calderonii | 1−2 (−3) | Both adaxial and abaxial hypodermis 1 cell layer |
| B. conchifolia | 1−2 (−3) | Adaxial 3–4, abaxial 2−3 |
| B. heracleifolia | 2−4+ | Both adaxial and abaxial 1 (−2) |
| B. nelumbiifolia | 3−6+ | Both adaxial and abaxial 1 cell layer |
| B. venusta | 1 (−2) | Absent |
Fig. 3.
Abaxial surface views under SEM (A, D, F) and LM (B, C, E). (A, B) Begonia nelumbiifolia, clusters with (A) five and (B) three stomata. (C) B. fuchsioides, cluster with ten stomata. (D) B. venusta, single stoma. (E, F) B. calderonii, both single and clustered stomata (E) and single stoma (F). Scale bars: A, B, F = 10 µm, C, D = 20 µm, E = 50 µm.
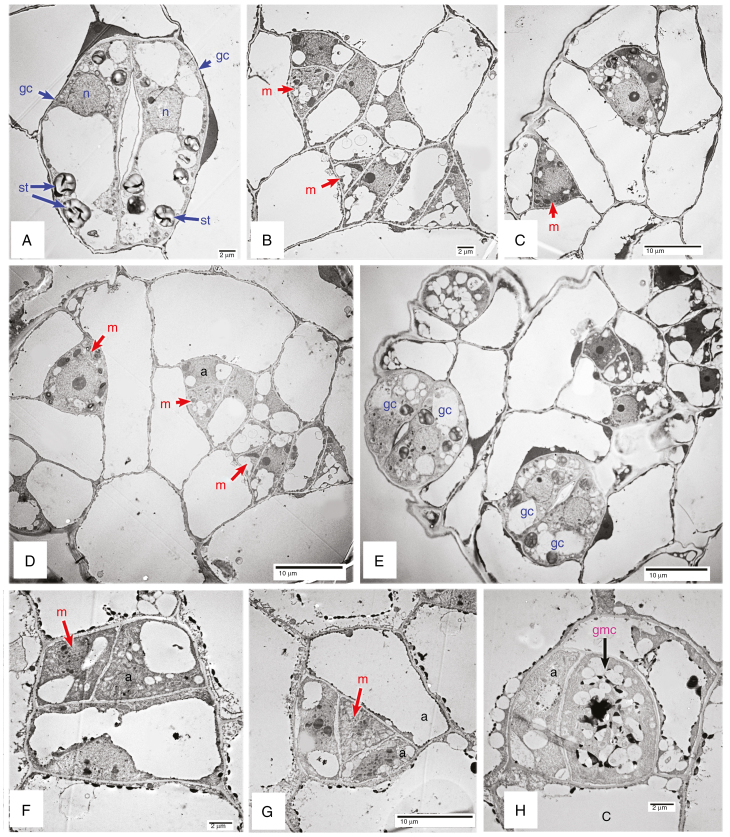
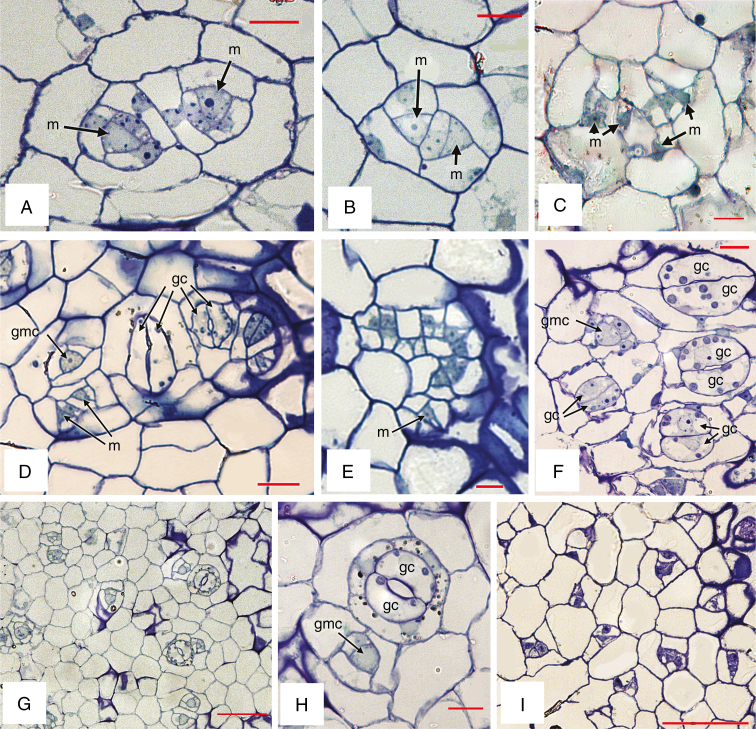
Fig. 4.
Transmission electron micrographs of mature (A) and developing stomata (B–H). (A–E) Begonia nelumbiifolia. (F–H) B. venusta. a, meristemoid sister cell; gc, guard cell; m, meristemoid; st, starch granule.
Formation of helicocytic stomata
Examples of stomatal development are shown in Figs 4 and 5. Helicocytic stomata develop from meristemoids that undergo a series of asymmetric divisions to produce several mesogene SLGCs surrounding a stoma (Fig. 1G, J). Within each complex, each succeeding division is oriented at an angle to the preceding one, resulting in a spiral arrangement. Identification of precursor cells can be problematic; stomatal meristemoids are generally identifiable by their relatively small size, small or absent vacuole, and sometimes darker appearance, but especially by their location and shape, which is typically triangular, the longest wall being adjacent to its sister cell. In the Begonia species examined here, we distinguish the meristemoid primarily by its shape and location; it does not always appear darker or more densely cytoplasmic than its sister cell and sometimes possesses small vacuoles, especially in larger stages (e.g. Fig. 4B). The meristemoid can be either the larger or the smaller of the two daughter cells resulting from an asymmetric division, and is typically located in the centre of a cell spiral (e.g. Fig. 4D). In most cases, the meristemoid is triangular in transverse section (e.g. Figs 4G, 5A–E), although it is occasionally polygonal (Fig. 4F). In cases where a meristemoid itself divides asymmetrically, the new meristemoid is formed at the narrower angle, resulting in a triangular meristemoid and a polygonal SGLC (e.g. Fig. 4B–D, G). The meristemoid ultimately assumes a rounded shape to form a GMC that has increased vacuolation (Fig. 4H). The GMC subsequently divides symmetrically into a pair of guard cells (Fig. 4C, 5H). Cell walls are relatively evenly thickened at all stages of development. In mature stomata, the guard cells possess thickenings at the pore, and other parts of the anticlinal walls have thickened; there is also extensive starch accumulation (Figs 4A, 5H).
Fig. 5.
Light micrographs of developing stomata. (A, B) Begonia calderonii. (C–F) B. nelumbiifolia. (G, H) B. conchifolia. (I) B. venusta. gc, guard cell; gmc, guard-mother cell; m, meristemoid. Scale bars: A–C, E, F, H = 10 µm, D = 20 µm, G, I = 50 µm.
Formation of non-contiguous stomatal clusters
In B. venusta, the stomata are isolated rather than in clusters; they develop more or less concurrently across the leaf, bearing meristemoids of similar size at each developmental stage (Fig. 5I). In the other species examined, formation of non-contiguous stomatal clusters occurs as secondary meristemoids are formed from adjacent SLGCs in the same cell lineage. Within each cluster, the sequence of development appears to differ in different species. In some species, we found stomata at different stages within a developing complex, often with a single older, fully formed stoma flanked by two or more meristemoids (Figs 4E, 5D). In other species, all stomata within a cluster are formed more-or-less simultaneously, so that the terms ‘secondary’ and ‘satellite’ are not readily applicable (e.g. Fig. 5A–C, E). Secondary meristemoids are apparently initiated at an early stage, so it is difficult to determine whether the cell division that gives rise to each secondary stomatal initial cell is symmetric or asymmetric. Two secondary stomatal initial cells are sometimes formed adjacent to each other (e.g. Fig. 5A), but one-cell spacing is maintained by asymmetric divisions oriented away from each developing secondary meristemoid, resulting in two helicocytic stomata. The ring of cells surrounding each stomatal cluster is apparently part of the same developmental complex, but it is impossible to determine their developmental sequence because in many cases early-formed SLGCs in the outer part of the spiral have subsequently divided symmetrically (Fig. 1I, J).
DISCUSSION
Evolution and development of helicocytic stomata
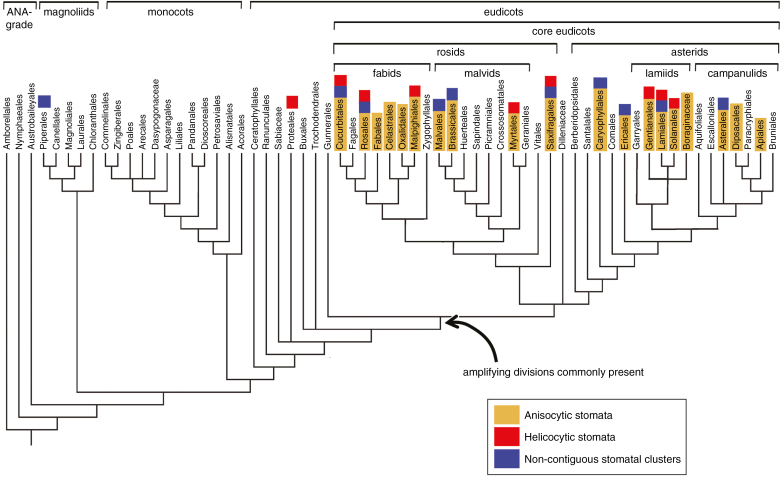
Helicocytic stomatal patterning (alternatively termed ‘helico-mesogenous’ by Fryns-Claessens and Van Cotthem, 1973) is most frequent in families in which anisocytic stomata are also present (Wilkinson, 1979). Anisocytic stomata possess three neighbour cells of different sizes (Fig. 1G); one cell is often noticeably smaller than the other two, reflecting their mesogenous development in an inward spiral (Payne, 1979; Rudall et al., 2013). Thus, there is a clear developmental similarity between anisocytic stomata and helicocytic stomata that is positively correlated with the number of iterations of amplifying divisions that result in SLGCs, as shown in Fig. 1. Iterative amplifying divisions are highly characteristic of eudicots. In contrast, they are apparently absent from monocots and rare or absent from some early-divergent (ANA-grade) angiosperms such as Amborella and Nymphaeaceae, although they are present in Austrobaileyales (Carpenter, 2005; Rudall and Knowles, 2013; Rudall et al., 2017). The relatively high incidence of amplifying divisions in the eudicot clade (Fig. 6) could influence not only stomatal patterning but also other aspects of leaf development. Indeed, stomatal patterning in eudicots develops during a specific phase of leaf growth, although its rate and duration are highly diverse (Barlow and Lück, 2009).
Fig. 6.
Angiosperm tree diagram of order-level relationships adapted from APG III (2009), with superimposed distributions of three stomatal traits: anisocytic stomata (yellow), helicocytic stomata (red) and non-contiguous stomatal clusters (blue). Stomatal data from literature summarized by Metcalfe and Chalk (1979); data on non-contiguous stomatal clusters from Metcalfe and Chalk (1979) and Gan et al. (2010).
Anisocytic stomata represent the characteristic stomatal type of Arabidopsis (Fig. 1D, G) and other Brassicaceae (Pant and Kidwai, 1967; Zhao and Sack, 1999; Nadeau and Sack, 2002). They are extremely common in eudicots; Metcalfe and Chalk (1979) listed 45 eudicot families in which anisocytic stomata have been recorded. When placed in a modern phylogenetic context (APG III, 2009), these 45 anisocytic families represent several orders of both asterid and rosid eudicots, but anisocytic stomata are not recorded in either core eudicots or any other extant angiosperms (ANA-grade, magnoliids and monocots). This phylogenetic distribution indicates that anisocytic patterning represents a relatively derived feature in the angiosperm clade that is clearly correlated with the phylogenetic distribution of amplifying divisions (Fig. 6). Similarly, helicocytic stomata are recorded only in eudicots, with the sole possible exception of a magnoliid family, Piperaceae; reports of this stomatal type in Piperaceae are rare and require confirmation using modern methods (Metcalfe and Chalk, 1979).
Evolution and development of non-contiguous stomatal clusters
Non-contiguous stomatal clusters were termed ‘compound helicocytic stomata’ by Payne (1970), who also noted that they are formed as a result of secondary meristemoid formation within the original complex during the early stages of helicocytic development. Like helicocytic stomata, non-contiguous stomatal clustering also constitutes a eudicot feature that relies on iterative amplifying divisions. There are records of non-contiguous stomatal clusters in 15 angiosperm families: Apocynaceae, Begoniaceae, Brassicaceae, Crassulaceae, Gesneriaceae, Ixonanthaceae, Melastomataceae, Moraceae, Ochnaceae, Proteaceae, Rubiaceae, Saxifragaceae, Simaroubaceae, Theaceae and Verbenaceae (reviewed by Metcalfe and Chalk, 1979; Gan et al., 2010), all representing eudicots, as we have shown in Fig. 6. In many of these eudicot families, this feature is correlated with the presence of uniformly thick, succulent leaves, although exceptions include some Proteaceae (species of Banksia, Dryandra and Lambertia), in which clusters of stomata occur in abaxial hair-lined depressions (Metcalfe and Chalk, 1950). Not all succulent-leafed taxa possess non-contiguous stomatal clusters; for example, Peperomia (Piperaceae), a magnoliid with thick, succulent leaves, lacks either stomatal clusters or amplifying divisions; in this genus, divisions in neighbour cells that produce radiating patterns of subsidiary cells are apparently exclusively perigenous (Sachs and Novplansky, 1993).
Thus, our optimization (Fig. 6) indicates not only a strong phylogenetic signal but also a positive correlation between the iterative origins of non-contiguous stomatal clusters and the presence of anisocytic/helicocytic stomata resulting from amplifying divisions. As stated above, amplifying divisions represent a highly characteristic feature (and possible synapomorphy) in core eudicots, and are a likely prerequisite for development of non-contiguous stomatal clusters. The ecophysiological significance of stomatal clustering in Begonia and other taxa has been widely discussed and to some extent tested in comparative studies (Strasburger, 1867; Hoover, 1986; Gan et al., 2010; Papanatsiou et al., 2017).
However, a strong correlation with anisocytic/helicocytic stomata does not fully explain the iterative origins of non-contiguous stomatal clusters. Many authors have suggested that stomatal clustering is related to increased leaf thickness (and hence to leaf succulence) because non-contiguous stomatal clustering is widespread in succulent plants with thick leaves. This stomatal feature is widely considered to reduce water loss through transpiration while maintaining efficient CO2 uptake (e.g. Hoover, 1986; Gan et al., 2010; Papanatsiou et al., 2017). Furthermore, a strong correlation with leaf succulence does not necessarily imply causation of non-contiguous stomatal clusters, which could represent a developmental product resulting from enlarged substomatal cavities and increased hypodermal thickness. Boghdan and Barkley (1972) and Tang et al. (2002) reported a relationship between the thickness of the multiseriate hypodermis and the prevalence of stomatal clustering in Begonia, an observation that is supported by our own study. However, the possible causal relationship between leaf anatomy and epidermal features is not addressed by our data; it has been discussed for Arabidopsis (e.g. Serna and Fenoll, 2000), but rarely for other taxa and remains largely unexplored. Studies in Begonia have demonstrated a link between relative stomatal density and environmental factors such as light intensity and water availability (Hoover, 1986; Papanatsiou et al., 2017), but it is unknown to what extent stomatal density is directly influenced by leaf thickness.
Contiguous (as opposed to non-contiguous) stomatal clustering characterizes mutants of the TOO MANY MOUTHS (TMM) gene of Arabidopsis (Geisler et al., 2000; Nadeau and Sack, 2003). TMM not only controls the orientation of asymmetric divisions (including amplifying divisions) but also helps to regulate cell proliferation and differentiation; hence, it is especially strongly implicated in the formation of satellite (or subsidiary) meristemoids. Satellite meristemoids are essential for maintenance of at least one intervening cell between stomata, ensuring that they remain non-contiguous even when they are so closely grouped together (the one-cell spacing rule: Geisler et al., 2000; Nadeau and Sack, 2002; Pillitteri and Torii, 2012).
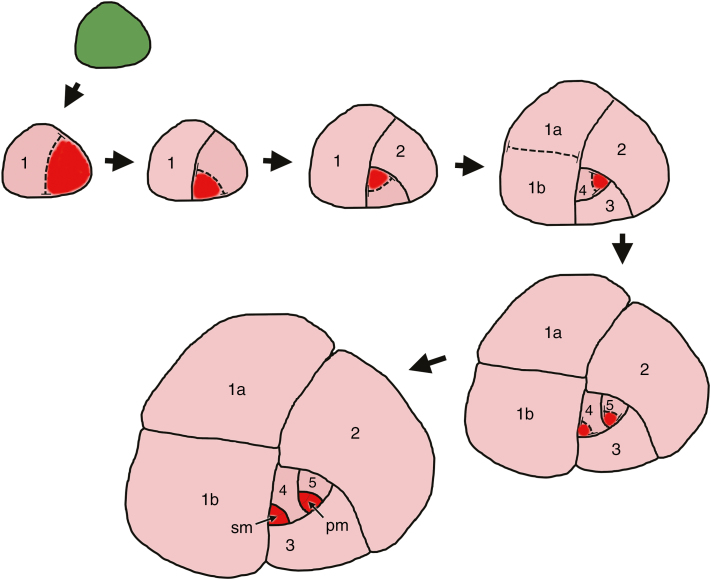
In Arabidopsis (Fig. 1F), a satellite meristemoid is formed directly by an asymmetric division of an SLGC oriented away from the initial meristemoid that itself has often already formed a GMC or even guard cells. In contrast, in Begonia, secondary meristemoids are apparently initiated at an earlier stage in the stomatal lineage, perhaps two or three mitoses before the symmetric GMC mitosis, reflecting the more extensive SLGC series (Fig. 7). As a result, it is difficult to determine whether the cell division that gives rise to each secondary stomatal initial cell is symmetric or asymmetric. One-cell spacing is maintained by asymmetric divisions oriented away from each developing secondary meristemoid, resulting in secondary helicocytic stomata. In the case of a relatively simple cluster consisting of only two stomata illustrated in Fig. 7, we interpret the secondary meristemoid as the sister cell to SLGC number 4; it develops from a SLGC that is not at the centre of the spiral. In many developing clusters, the spiral is disrupted by cell division and enlargement, and it is difficult to determine the precise sequence. As we have shown, in some species, the stomata in a single cluster are formed almost simultaneously (e.g. Fig. 5A–C, E). Bünning and Sagromsky (1948) and Barlow and Lück (2009) suggested that this type of patterning arises from a quartet of stomatogenic cells, which represents a common type of epidermal prepatterning in Begonia, although not confirmed by our data. A similar ‘quartet’ type of prepatterning is widespread in flowering plants; it is present in Amborella, the sister taxon to all other angiosperms (Rudall and Knowles, 2013). Thus, in Begonia, the terms ‘secondary’, ‘satellite’ and ‘subsidiary’ meristemoid are potential misnomers because multiple stomata can arise more or less simultaneously within a single cluster.
Fig. 7.
Diagram showing the development of non-contiguous stomatal cluster. Numbers indicate the sequence of development of SLGCs in an inward spiral; the highest number is the sister cell to the primary meristemoid. Colours: green, meristemoid-mother cell; red, meristemoid; pink, SLGC. pm, primary meristemoid; sm, secondary meristemoid.
CONCLUSIONS
Our results demonstrate that the manufacture of non-contiguous stomatal clusters in the epidermis of some Begonia species relies on two primary developmental factors: an inwardly spiralling series of amplifying divisions that result in helicocytic stomata, and the secondary development of a variable number of meristemoids. Our optimization of these features on an angiosperm phylogeny leads us to predict that the occurrence of amplifying divisions could be pre-adaptive for these factors. Although some differences exist, both factors occur in Arabidopsis, and both have been thoroughly studied in terms of developmental genetics, allowing us to suggest gene orthologues that could be implicated in Begonia stomatal patterning. In Arabidopsis, the bHLH protein SPEECHLESS (SPCH) has a primary role in promoting amplifying divisions (Bergmann and Sack, 2007; MacAlister et al., 2007), and therefore is an obvious and likely candidate in helicocytic patterning. A second likely candidate, TMM, is potentially implicated in the close formation of secondary meristemoids.
Factors that remain to be fully explored include the influences of leaf anatomy and genome size on stomatal patterning. Interactions between the developing epidermis and underlying mesophyll tissues, especially the hypodermis, could influence the formation of stomatal clusters. Furthermore, genome size in Begonia displays a broad range of variation, but is generally relatively small (approx. 0.75 pg in B. heracleifolia: Dewitte et al., 2009), consistent with the relatively small stomatal size. Since genome size is widely correlated with cell size and stomatal density in angiosperms (Beaulieu et al., 2008), it would be interesting to examine whether it also influences other epidermal traits such as the relative number of stomata per cluster. Future studies will compare gene expression in closely related Begonia species with contrasting stomatal patterning.
ACKNOWLEDGEMENTS
We thank Richard Bateman for critically reading the manuscript. The laboratory work was undertaken at Kew by A.C.M.J. towards completion of her MSc at RBG Edinburgh We acknowledge lab support at Kew from Tom Gregory and Elizabeth Chen.
LITERATURE CITED
- APG III 2009. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APGIII. Botanical Journal of the Linnean Society 161: 105–121. [Google Scholar]
- Barlow P, Lück J. 2009. Transformations of cellular pattern: progress in the analysis of stomatal cellular complexes using L-systems. Progress in Botany 71: 61–99. [Google Scholar]
- Bergmann DC, Sack FD. 2007. Stomatal development. Annual Review of Plant Biology 58: 163–181. [DOI] [PubMed] [Google Scholar]
- Beaulieu J, Leitch I, Patel S, Pendharkar A, Knight C. 2008. Genome size is a strong predictor of cell size and stomatal density in angiosperms. New Phytologist 179: 975–986. [DOI] [PubMed] [Google Scholar]
- Boghdan S, Barkley A. 1972. Stomatal patterns in the genus Begonia. Phytologia 23: 327–333. [Google Scholar]
- Brennan AC, Bridget S, Ali MS et al. . 2012. Genomic resources for evolutionary studies in the large, diverse, tropical genus, Begonia. Tropical Plant Biology 5: 261–276. [Google Scholar]
- Bünning E, Sagromsky H. 1948. Die bildung des spaltöffnungsmusters in der blattepidermis. Zeitschrift für Naturforschung 3b: 203–216. [DOI] [PubMed] [Google Scholar]
- Carpenter KJ. 2005. Stomatal architecture and evolution in basal angiosperms. American Journal of Botany 92: 1595–1615. [DOI] [PubMed] [Google Scholar]
- Dewitte A, Leus L, Eeckhaut T, Vanstechelman I, Van Huylenbroek J, Van Bockstaele E. 2009. Genome size variation in Begonia. Genome 52: 829–838. [DOI] [PubMed] [Google Scholar]
- Fryns-Claessens E, Van Cotthem W. 1973. A new classification of the ontogenetic types of stomata. Botanical Review 39: 71–138. [Google Scholar]
- Gan Y, Zhou L, Shen ZJ, Shen ZX, Zhang YQ, Wang GX. 2010. Stomatal clustering, a new marker for environmental perception and adaptation in terrestrial plants. Botanical Studies 51: 325–336. [Google Scholar]
- Geisler M, Nadeau J, Sack FD. 2000. Oriented asymmetric divisions that generate the stomatal spacing pattern in Arabidopsis are disrupted by the too many mouths mutation. The Plant Cell 12: 2075–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison N, Harrison RJ, Kidner C. 2016. Comparative analysis of Begonia plastid genomes and their utility for species-level phylogenetics. PLOS One 11: e0153248 DOI:10.1371/journal.pone.0153248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover WS. 1986. Stomata and stomatal clusters in Begonia: ecological response in two Mexican species. Biotropica 18: 16–21. [Google Scholar]
- MacAlister CA, Ohashi-Ito K, Bergmann DC. 2007. Transcription-factor control of asymmetric divisions that establish the stomatal lineage. Nature 445: 537–540. [DOI] [PubMed] [Google Scholar]
- Metcalfe CR, Chalk L. 1950. Anatomy of the Dicotyledons. 2 volumes Oxford, UK: Clarendon Press. [Google Scholar]
- Metcalfe CR, Chalk L. 1979. Anatomy of the dicotyledons, 2nd edn, vol. 1 Oxford, UK: Clarendon Press. [Google Scholar]
- Nadeau JA, Sack FD. 2002. Stomatal development in Arabidopsis. The Arabidopsis Book 1: e0066 DOI: http://dx.doi.org/10.1199/tab.0066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeau JA, Sack FD. 2003. Stomatal development: cross talk puts mouths in place. Trends in Plant Science 8: 294–299. [DOI] [PubMed] [Google Scholar]
- Neubauer HF, Beissler I. 1971. Uber stomata und stomatagruppen an verschiedenen blattanten bei Begonia. Gartenbau-Wissenschaft 36: 45–50. [Google Scholar]
- Pant DD, Kidwai PF. 1967. Development of stomata in some Cruciferae. Annals of Botany 31: 513–521. [Google Scholar]
- Papanatsiou M, Amtmann A, Blatt MR. 2017. Stomatal clustering in Begonia associates with the kinetics of leaf gaseous exchange and influences water use efficiency. Journal of Experimental Botany 68: 2309–2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne WW. 1970. Helicocytic and allelocytic stomata: unrecognized patterns in the Dicotyledonae. American Journal of Botany 57: 140–147. [Google Scholar]
- Payne WW. 1979. Stomatal patterns in embryophytes: their evolution, ontogeny and interpretation. Taxon 28: 117–132. [Google Scholar]
- Pillitteri LJ, Torii KU. 2012. Mechanisms of stomatal development. Annual Reviews in Plant Biology 63: 591–614. [DOI] [PubMed] [Google Scholar]
- Rudall PJ, Knowles EVW. 2013. Ultrastructure of stomatal development in early-divergent angiosperms reveals contrasting patterning and pre-patterning. Annals of Botany 112: 1031–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudall PJ, Hilton J, Bateman RM. 2013. Several developmental and morphogenetic factors govern the evolution of stomatal patterning in land plants. New Phytologist 200: 598–614. [DOI] [PubMed] [Google Scholar]
- Rudall PJ, Chen ED, Cullen E. 2017. Evolution and development of monocot stomata. American Journal of Botany 104: doi 10.3732/ajb.1700086. [DOI] [PubMed] [Google Scholar]
- Sachs T, Novoplansky N. 1993. The development and patterning of stomata and glands in the epidermis of Peperomia. New Phytologist 123: 567–574. [DOI] [PubMed] [Google Scholar]
- Serna L, Fenoll C. 2000. Stomatal development and patterning in Arabidopsis leaves. Physiologia Plantarum 109: 351–358. [Google Scholar]
- Strasburger E. 1867. Ein Beitrag zur Entwicklungsgeschichte der Spaltöffnungen. Jahrbücher fur Wissenschaftliche Botanik 5: 297–342. [Google Scholar]
- Tang M, Hu YX, Lin JX, Jin XB. 2002. Developmental mechanism and distribution pattern of stomatal clusters in Begonia peltatifolia. Acta Botanica Sinica 44: 384–390. [Google Scholar]
- Wilkinson HP. 1979. The plant surface (mainly leaf). Part 1: stomata. In: Metcalfe CR, Chalk L, eds. Anatomy of the dicotyledons, 2nd edn, vol. 1 Oxford, UK: Clarendon Press, 97–117. [Google Scholar]
- Zhao L, Sack FD. 1999. Ultrastructure of stomatal development in Arabidopsis (Brassicaceae) leaves. American Journal of Botany 86: 929–939. [PubMed] [Google Scholar]