Abstract
Sixty-three children (1–14 years of age) newly diagnosed with T-cell acute lymphoblastic leukemia were treated from January 2001 to December 2014. Patient outcomes were evaluated based on the regimen received; Capizzi methotrexate (C-MTX) vs. high-dose methotrexate (HDMTX). Complete remission (CR) was achieved in 54 of 60 (90.0%) patients and 3 patients died during induction. The 5-year overall survival (OS) and disease-free survival (DFS) were 88.3 ± 6.5% and 85 ± 7.5%, respectively. Post-induction, 35 patients were treated with HDMTX and 25 with C-MTX. There was no difference in OS or DFS for patients treated with HDMTX vs. C-MTX (P > 0.05 for both). Central nervous system involvement (CNS3) was associated with inferior survival outcomes compared to Non-CNS3 patients (OS, CNS3 73.3 ± 9.1% vs.non-CNS3 93.2 ± 2.6%, (P = 0.045) and DFS, CNS3 66.7 ± 10.4% vs. non-CNS3 90.9 ± 3.1% (P = 0.0163)). Delayed radiation in CNS3 was associated with relapse (P = 0.0037) regardless of regimen. Thus optimization of CNS-directed therapy for patients with CNS3 is needed.
Keywords: Acute lymphoblastic leukemia, High dose methotrexate, Central nervous system, T cell, Children
1. Introduction
T-cell acute lymphoblastic leukemia (T-ALL) is an uncommon pediatric malignancy with a historically poor prognosis [1]. The prognosis of T-ALL has improved over the last 40 years with the introduction of high dose multi-agent pulse chemotherapy [2], [3]. Early intensification with methotrexate (MTX) is a key component of most modern treatment regimens used for patients with T-ALL [4]. Treatment doses have ranged from 33.6 g/m2/24 h intravenous infusion to oral 20 mg/m2/week [5]. Higher doses have been used to control testicular and medullary disease, but had limited effect in treating central nervous system (CNS) disease [6]. An optimal dose of methotrexate has not been identified. Long-term remissions are found in 70–75% of patients with T-ALL [7]. Outcomes of patients with T-ALL are commonly reported combined with the more common B-ALL [8]. We evaluated children with T-ALL treated before and after the substitution of high dose methotrexate (HDMTX) for standard escalating Capizzi methotrexate (C-MTX) in the first interim maintenance phase (IM1). We hypothesized that the outcomes of patients with T-ALL treated with a modified Children's Oncology Group (COG) backbone protocol [3], [9], [10] that included double delayed intensification and interim-maintenance (IM1 and IM2) phases while incorporating HDMTX would have superior outcomes compared to C-MTX. The effect of patient gender, age, white blood cell count (WBC), CNS involvement, testicular involvement, slow early response and treatment type on patient outcome was evaluated.
2. Materials and methods
2.1. Patients
Children 14 years of age or younger newly diagnosed with T-ALL were eligible for treatment at the Princess Noorah Oncology Center of the King Abdulaziz Medical City-Jeddah. Patients had T-cell ALL immunophenotype confirmed using flow cytometry studies. The protocol was approved by the Hospital Ethics and Review Committee of the King Abdullah International Medical Research Center. Sixty-four patients consented for treatment from January 2001 to December 2014. One patient treated with bone marrow transplantation after the first complete remission was excluded from analysis.
2.2. Study criteria
Study risk criteria included categorization of patients according to National Cancer Institute (NCI)-risk, extra-medullary disease involvement (CNS or testicular), or slow early response [11]. Patients diagnosed before January 2008 received C-MTX regardless of clinical risk factors. Patients with no high-risk (HR) features were designated as standard-risk (SR) and received C-MTX from January 2008 to December 2014. Patients with HR features were treated with a HDMTX regimen during this time period. Any patient with NCI-HR, CNS involvement (CNS3), testicular involvement, steroid pre-treated, or slow early response (defined as blasts ≥ 5% on the day 15 or end-of-induction bone marrow (BM) evaluation or positive minimal residual disease (MRD) ≥ 0.01% at the end-of-induction) were designated as HR.
A cerebrospinal fluid (CSF) blood cell count less than 5 WBC/mm3 with no leukemic blasts was defined as CNS1. CNS2 was defined as a CSF count of less than 5 WBC/mm3 with leukemic blasts present or a traumatic lumbar puncture with 10 or more red blood cells/mm3 and leukemic blasts present but not consistent with CNS3 using the Steinherz/Bleyer algorithm [12]. CNS3 was defined as a CSF WBC count greater than 5/mm3 with leukemic blasts in a non-traumatic lumbar puncture, a traumatic lumbar puncture but consistent with CNS3 by the Steinherz/Bleyer algorithm, or clinical signs of CNS involvement regardless of CSF results.
Response was assessed by BM morphology on day 15 and end-of-induction. Remission was defined as less than 5% blasts in the BM at end-of-induction. Patients with ≥5% blasts at any time point were considered slow early responders. MRD was assessed using flow cytometry after Dec 2007 and patients with MRD greater than or equal to 0.01% were classified as slow early responders.
Complete remission (CR) was defined as <5% blasts on BM exam with no extra-medullary disease at end-of-induction therapy. Induction failure was defined as >25% blasts on BM exam or proven residual extra-medullary disease at end-of-induction or at end-of-consolidation for patients who continued on the treatment regimen.
2.3. Treatment
Patients were treated using modified regimens based on the Children's Oncology Group (COG) experience (Table 1) [3], [9], [10]. Modifications included the use of dexamethasone instead of prednisone, and extended intensification using double delayed intensification (DI-1 and DI-2) and double interim maintenance (IM) phases. The difference between the two regimens was only in the IM-1 phase where standard escalating Capizzi MTX (C-MTX) without folinic acid rescue was used in the standard regimen and HDMTX with folinic acid rescue was used in the study group. HDMTX consisted of intravenous MTX, 5 gm/m2/day over 24 h with no maximum dose (Days 1, 15, 29, and 43) with folinic acid rescue starting 42 h after the start of HDMTX (starting dose of 15 mg/m2/dose intravenous/oral every 6 hours, adjusted according to MTX levels until level was less than 0.1 µMol/L) and C-MTX consisted of escalating intravenous MTX starting at a dose of 100 mg/m2/day (Days 1, 11, 21, 31, and 41) without folinic acid rescue. The IM-2 phase consisted of standard escalating C-MTX without folinic acid rescue in both groups (Table 1). No prophylactic cranial irradiation (pCRT) was given. [13] Therapeutic cranial radiation (CRT) was planned for patients with CNS3 status only, at a dose of 18 Gy divided in 10 daily fractions, at the start of consolidation for patients treated with C-MTX and during the DI-2 phase for patients treated with HDMTX.
Table 1.
Treatment regimens. High dose methotrexate versus standard dose methotrexate (Cycle duration in parentheses).
| C-MTX regimen | HDMTX regimen | ||
|---|---|---|---|
| Phase and treatment | Dose and schedule | Phase and treatment | Dose and schedule |
| Induction (4 weeks) | Induction (4 weeks)6 | ||
| IT cytarabine | Age adjusteda Day 1 | IT cytarabine | Age adjusteda Day 1 |
| Vincristine | 1.5 mg/m2 (2 mg max) IV Days 1, 8, 15, 22 | Vincristine | 1.5 mg/m2 (2 mg max) IV Days 1, 8, 15, 22 |
| Pegasparginaseb | 2500 U/m2 IM between Days 4 and 6 (one dose) | Pegasparginase2 | 2500 U/m2 IM between Days 4 and 6 (one dose) |
| Dexamethasone | 6 mg/m2/day in divided doses BID PO/IV Days 1–28 (No tapering) | Dexamethasone | 6 mg/m2/day in divided doses BID PO/IV Days 1–28 (no tapering) |
| IT MTXc | Age adjusteda Days 15, 29 | IT MTXc | Age adjusteda Days 15, 29 |
| Daunorubicin | 25 mg/m2 IV Days 1, 8, 15, 22 | Daunorubicin | 25 mg/m2 IV Days 1, 8, 15, 22 |
| Consolidation (7 weeks) | Consolidation (7 weeks) | ||
| Cyclophosphamide | 1000 mg/m2/day IV Days 1 and 29 | Cyclophosphamide | 1000 mg/m2/day IV Days 1 and 29 |
| Cytarabine | 75 mg/m2/day SQ/IV Days 1–4, 8–11, 29–32, 36–39 | Cytarabine | 75 mg/m2/day SQ/IV Days 1–4, 8–11, 29–32, 36–39 |
| Mercaptopurine | 60 mg/m2/day PO Days 1–14, 29–42 | Mercaptopurine | 60 mg/m2/day PO Days 1–14, 29–42 |
| IT MTXd | Age-adjusteda Days 1,8,15,22 | IT MTXd | Age-adjusteda Days 1,8,15,22 |
| Pegasparaginaseb | 2500 U/m2/day IM Days 18, 46 | Pegasparaginaseb | 2500 U/m2/day IM Days 18, 46 |
| Vincristine | 1.5 mg/m2/day IV Days 15,22,43,50 | Vincristine | 1.5 mg/m2/day IV Days 15,22,43,50 |
| IM-1 (7 weeks) | IM-1 (8 weeks) | ||
| Vincristine | 1.5 mg/m2/day IV Days 1, 11, 21, 31, 41 | Vincristine | 1.5 mg/m2/day IV Days 1, 15, 29, 43 |
| IV MTX | 100 mg/m2/day IV Days 1, 11, 21, 31, 41 (escalate by 50 mg/m2 per dose) | IV MTX (high dose) | 5 gm/m2/day IV Days 1, 15, 29, 43 |
| Pegasparaginase | 2500 U/m2/day IM Days 2, 22 | Mercaptopurine | 25 mg/m2/day PO Days 1–56 |
| IT MTX | Age-adjusteda Days 1, 31 | IT MTX | Age-adjusted1 Days 1, 29 |
| DI-1 (8 weeks) | DI-1 (8 weeks) | ||
| Re-induction (4 weeks) | Re-induction (4 weeks) | ||
| Dexamethasone | 10 mg/m2/day in divided doses BID PO/IV Days 1–7, 15–21 | Dexamethasone | 10 mg/m2/day in divided doses BID PO/IV Days 1–7, 15–21 |
| Vincristine | 1.5 mg/m2/day IV Days 1, 8, 15 | Vincristine | 1.5 mg/m2/day IV Days 1, 8, 15 |
| Doxorubicin | 25 mg/m2/day IV Days 1, 8, 15 | Doxorubicin | 25 mg/m2/d IV Days 1, 8, 15 |
| Pegasparaginase | 2500 IU/m2/day IM Day 4 | Pegasparaginase | 2500 IU/m2/day IM Day 4 |
| IT MTX | Age-adjusteda Day 1 | IT MTX | Age-adjusteda Day 1 |
| Reconsolidation (4 weeks) | Reconsolidation (4 weeks) | ||
| Cyclophosphamide | 1000 mg/m2/day IV Day 29 | Cyclophosphamide | 1000 mg/m2/day IV Day 29 |
| Thioguanine | 60 mg/m2/day PO Days 29–42 | Thioguanine | 60 mg/m2/day PO Days 29–42 |
| Cytarabine | 75 mg/m2/day SQ/IV Days 29–32, 36–39 | Cytarabine | 75 mg/m2/day SQ/IV Days 29–32, 36–39 |
| IT MTX | Age-adjusteda Days 29, 36 | IT MTX | Age-adjusteda Days 29, 36 |
| Vincristine | 1.5 mg/m2/day IV Days 43, 50 | Vincristine | 1.5 mg/m2/day IV Days 43, 50 |
| Pegasparaginase | 2500 U/m2/day IM Day 46 | Pegasparaginase | 2500 U/m2/day IM Day 46 |
| IM-2 (7 weeks) | Same as IM-1 (starting at 50 mg/m2 less than the maximum tolerated dose in IM-1) with IT MTX on Days 1, 31 | IM-2 (7 weeks) | Same as IM-1 of the C-MTX |
| DI-2 (8 weeks) | Same as DI-1 | DI-2 (8 weeks)d | Same as DI-1 |
| Maintenance (12 weeks)e | Maintenance (12 weeks)e | ||
| Vincristine | 1.5 mg/m2 (2 mg max) IV Days 1, 29, 57 | Vincristine | 1.5 mg/m2 (2 mg max) IV Days 1, 29, 57 |
| Dexamethasone | 6 mg/m2/day in divided doses BID PO Days 1–5, 29–33, 57–61 | Dexamethasone | 6 mg/m2/day in divided doses BID PO Days 1–5, 29–33, 57–61 |
| Mercaptopurine | 75 mg/m2/day PO daily | Mercaptopurine | 75 mg/m2/day PO daily |
| MTX | 20 mg/m2/dose PO weekly Days 8, 15, 22, 29, 36, 43, 50, 57, 64, 71, and 78 of each cycle | MTX | 20 mg/m2/dose PO weekly Days 8, 15, 22, 29, 36, 43, 50, 57, 64, 71, and 78 of each cycle |
| IT MTX | Age-adjusteda Days 1, 29 of the first 4 cycles, then Day 1 of each cycle thereafter. | IT MTX | Age-adjusteda Days 1, 29 of the first 4 cycles, then Day 1 of each cycle thereafter. |
C-MTX = standard escalating Cappizi methotrexate, HDMTX = high dose methotrexate, IT = intrathecal, IM = intramuscular, IV = intravenous, PO = oral, SQ = subcutaneous, MTX = methotrexate, IM = interim maintenance, DI = delayed intensification.
IT cytarabine was adjusted for age as follows: 1–1.99 years, 30 mg; 2–2.99 years, 50 mg; > 3 years, 70 mg. IT MTX was adjusted for age as follows; 1–1.99 years, 8 mg; 2–2.99 years, 10 mg; > 3 years, 12 mg.
Asparaginase preparation: pegylated asparaginase or L-asparaginase with dose and timing adjustment was used; Erwinia asparaginase replaced pegaspargase/l-asparaginase after severe allergic reactions.
For CNS2 and CNS3: 2 extra doses on days 8 and 22 were added.
Starting day 1 of consolidation for patients treated with C-MTX and day 29 of DI-2 for patients treated with HDMTX, patients with CNS3 at diagnosis received 1,800 cGy to the cranium. 6-Thioguanine oral doses are omitted in DI-2 for patients treated with HDMTX. Patients with testicular disease at diagnosis received 2,400 cGy bilateral testicular radiation in 8 fractions during consolidation therapy if testicular disease was persistent at end of induction. Patients with CNS3 disease at diagnosis did not receive IT methotrexate on days 15 and 22 consolidation therapy.
Total duration of treatment was 38 months for males and 24 months for females.
2.4. Statistical analysis
Patients were analyzed according to the treatment received (HDMTX vs. C-MTX) in IM-1. Patient gender, age, WBC, CNS status, NCI-risk, BM findings, testicular involvement, steroid pre-treatment, treatment regimen, death rate, and frequency of CR were evaluated for their effect on overall survival (OS) and disease-free survival (DFS) and as a source on variance in different treatment groups.
Continuous variables were presented as the mean ± standard deviation (SD). Count data was expressed as a number and percentage. A Mann-Whitney U test was used to compare two groups of quantitative data. A Chi-square test was used to compare count data. Fisher's exact test was used to compare categorical data when one of the values was 0. All P values presented were for 2-sided tests. Statistical significance was attributed to tests with a P value less than 0.05. No adjustments were made for multiple comparisons. OS and DFS were estimated using Kaplan Meier testing. A 1-sided log rank test was used to compare survival curves.
3. Results
3.1. Patient characteristics
3.1.1. All patients
Sixty-three children were evaluated (Table 2). The male to female ratio was 4.7. Twenty-four patients (38.1%) ≥ 10 years of age and 38 (60.3%) patients had WBC ≥ 50,000/μL. Thirty-five (56%) patients had CNS1 status, 12 (19%) had CNS2, and 15 (24%) CNS3. Two male patients had testicular involvement. One patient had history of steroid pre-treatment. Fifty-five patients were classified as HR and 8 as SR.
Table 2.
Treatment group characteristics and outcomes.
| Age (years) | Gender (M/F) | WBC (x10-3/μL) | CNS (1/2/3) | Response (CR/REL) | Deaths (CR/REL/ID) | 5 year disease free survival (%) | 5 year overall survival (%) | |
|---|---|---|---|---|---|---|---|---|
| All patients (N = 63) | 8.27 ± 2.85 | 51/12 | 148.6 ± 184.3 | 35/12/15 | 54/6 | 3/4/3 | 85.0 ± 78.5% | 88.3 ± 6.5% |
| Treatment groups | ||||||||
| HD-MTX (N = 35) | 8.47 ± 2.9629 | 29/6 | 190.8 ± 209.6 | 17/7/10 | 31/4 | 1/3 | 85.7 ± 8.9% | 88.6 ± 7.7% |
| C-MTX (N = 25) | 7.68 ± 2.6 | 21/4 | 97.8 ± 132. | 16/4/5 | 23/2 | 2/1 | 84.0 ± 10.7% | 88.0 ± 8.8% |
Not all numbers add up to the numbers of patients treated due to missing data points.
M = male.
F = female.
CNS = central nervous system involvement; CNS1, CNS2, or CNS3.
CR = complete response.
REL = relaspe.
ID = induction death.
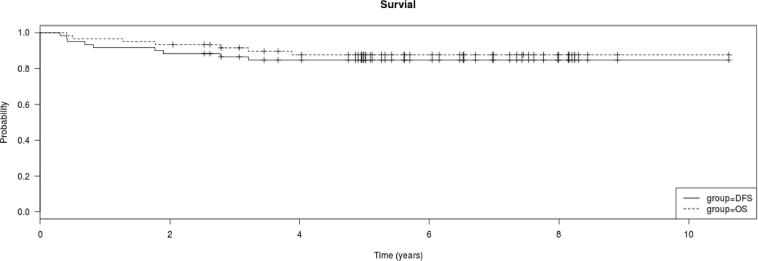
Three (4.8%) patients died during induction. Of the 60 patients surviving induction, 35 received HDMTX and 25 C-MTX. Mean follow-up was 5.24 ± 2.46 years (range: 0.02–10.6). The 5-year OS and DFS were 88.3% (6.5% SE) and 85% (7.5% SE), respectively (Fig. 1a). No second malignancies were diagnosed during follow-up.
Fig. 1.
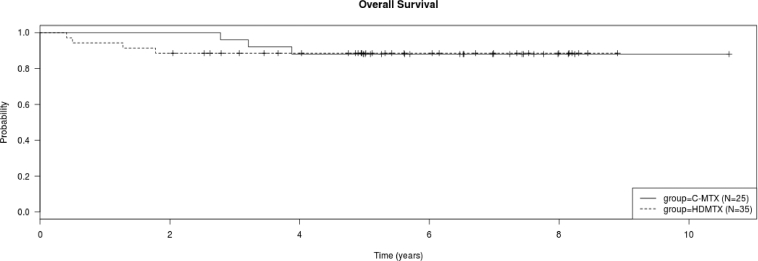
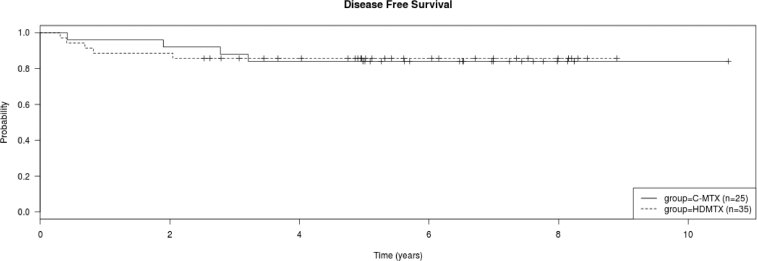
a. Kaplan Meier plot of overall survival and disease free survival of all treated patients. b. Kaplan Meier plot of overall survival of all patients treated with C-MTX and HDMTX. There was no difference in the overall survival of the two groups (log rank test, P > 0.05).c. Kaplan Meier plot of disease free survival of all patients treated with C-MTX and HDMTX. There was no difference in the disease free survival of the two groups (log rank test, P > 0.05).
CNS relapse was more frequent in patients with CNS3 (P = 0.00326). Five of the 6 (83.3%) patients who relapsed had CNS3 status and 5 out of 15 (33.3%) patients with CNS3 status had a CNS Relapse (Table 3). CNS3 status was found in similar proportion in both genders (P = 0.91), however, females were more likely to relapse than males (P = 0.0179) (Table 4).
Table 3.
Cranial radiation therapy (CRT) for CNS3 patients.
| # | Regimen | Gender (F/M) | CRT given (Y/N) | Phase CRT given | Relapse (Y/N) |
|---|---|---|---|---|---|
| 1 | C-MTX | F | N | (refused) | Y |
| 2 | C-MTX | F | Y | Consolidation | Y |
| 3 | C-MTX | M | Y | Consolidation | N |
| 4 | C-MTX | M | Y | Consolidation | N |
| 5 | C-MTX | M | Y | Consolidation | N |
| 6 | HDMTX | M | Y | DI-2 | N |
| 7 | HDMTX | M | N | (relapse)* | Y |
| 8 | HDMTX | M | Y | DI-2 | N |
| 9 | HDMTX | M | N | (relapse)* | Y |
| 10 | HDMTX | M | Y | DI-2 | N |
| 11 | HDMTX | M | Y | DI-2 | N |
| 12 | HDMTX | M | Y | DI-2 | N |
| 13 | HDMTX | M | Y | DI-2 | N |
| 14 | HDMTX | F | N | (relapse)* | Y |
| 15 | HDTMX | M | Y | DI-2 | N |
F = female, M = male.
Y = Yes, N = No.
DI-2 = delayed intensification-2.
Relapse before planned cranial radiation (CRT) given.
Table 4.
Gender related outcomes.
| Variable | Female | Male | P-value |
|---|---|---|---|
| Age | 8.53 | 8.20 | 0.89 |
| WBC | 116.7 | 156.1 | 0.61 |
| CNS3 (+/-) | 3/9 | 12/39 | 0.91 |
| Induction death (+/-) | 2/10 | 1/50 | 0.031 |
| Post-induction death (+/-) | 3/7 | 4/46 | 0.048 |
| Relapse (+/-) | 3/7 | 3/47 | 0.021 |
| Protocol | |||
| - C-MTX (+/-) | 6/6 | 21/30 | 0.56 |
| - HDMTX (+/-) | 6/6 | 30/21 | 0.58 |
| 5-year OS | 64.3 ± 17% | 91.9 ± 3.4% | 0.043 |
| 5-year DFS | 56. ± 17% | 90 ± 4.3% | 0.011 |
| 5-year DFS – C-MTX | 25 ± 22% | 95.2 ± 4.7% | <0.0001 |
| 5-year DFS – HDMTX | 83. ± 15.2% | 86 ± 6.4% | 0.86 |
(+/-) = (# with characteristic/# without characteristic).
3.1.2. HDMTX vs. C-MTX
Patients treated with HDMTX and C-MTX were evaluated (Table 2). Patients in the two groups had similar gender distribution (P > 0.05), age (P = 0.05), WBC (P > 0.05), CNS3 involvement (P > 0.05), and incidence of achieving CR (P > 0.05).
Patient WBC and NCI-risk category did not affect death or relapse rates in patients treated with C-MTX (P > 0.05 for both). However, patients treated with C-MTX older than 10.25 years had more deaths than younger patients (optimal cut-point, P = 0.0117). These older patients had a shorter OS and DFS than the younger patients (OS P = 0.0080, DFS P = 0.034). Patients with CNS3 more frequently relapsed than non-CNS3 patients (P = 0.033). Females treated with C-MTX had more frequent deaths than males (P = 0.011).
In contrast, gender, age, WBC, and NCI-risk did not affect death or relapse rate in patients treated with HDMTX. Of patients treated with HDMTX, those with CNS3 had higher death and relapse rates than non-CNS3 patients (P = 0.033). Patients with CNS3 had shorter OS than non-CNS3 patients (P = 0.039).
There were a similar number of CR (P > 0.05) and deaths in the two treatment groups (P > 0.05). Time to relapse was similar in the 2 treatment groups (P > 0.05). There was no difference in the OS or DFS of patients treated with the different regimens (P > 0.05 for both) (Fig. 1b and c). The 5-year DFS for the HDMTX vs. C-MTX regimen was 85.7% (8.9% SE) vs. 84.0% (8.7% SE), respectively.
3.1.3. Relapse
Relapses occurred in six (10%) patients; all relapses involved the CNS. Relapses occurred 0.98 ± 0.63 years after diagnosis (range: 0.31–1.9). Four of the patients who relapsed were treated with HDMTX and two with Capizzi MTX. All relapses, except one, occurred in patients with CNS3 status.
3.1.4. Subgroup analyses
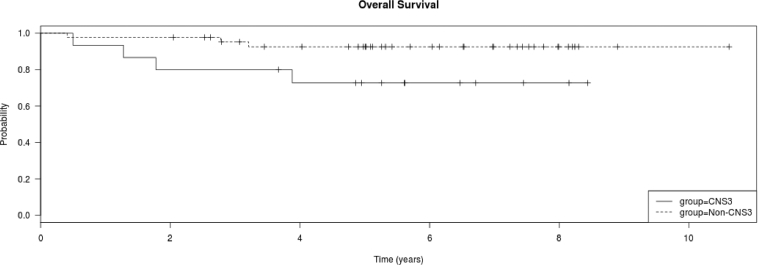
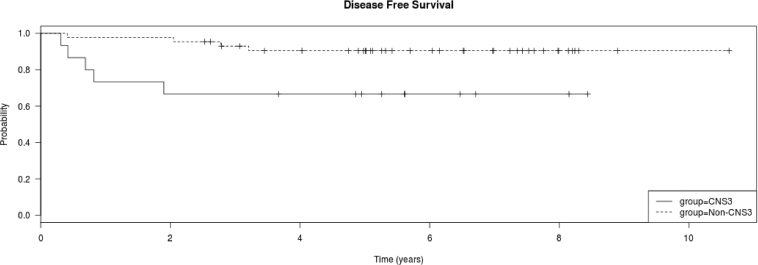
Fifteen patients had CNS3 status at diagnosis. The 5-year OS and DFS were shorter in patients with CNS3 than in non-CNS3 patients (OS, P = 0.045; non-CNS3 93.2% [2.6%] vs. CNS3 73.3% [9.1%]; DFS, P = 0.0163; non-CNS3 90.9% [SE 3.1%] vs. CNS3 66.7% [SE 10.4%]) (Fig. 2a and b). All but 4 of these 15 patients received CRT (Table 3). Of note, all the four patients who did not receive CRT relapsed, while only one out of 11 (9.1%) who received CRT relapsed. Thus delay in CRT was significantly associated with relapse (P = 0.0037). However, there was no difference in the OS and DFS of CNS3 vs. non-CNS3 patients treated with HDMTX or C-MTX (P > 0.05 for both). All relapses in CNS3 patients treated with the HDMTX regimen occurred before the start of their planned CRT date (week 16 to week 42.9). In contrast, both relapses in CNS3 patients treated with the C-MTX regimen occurred in females, one before receiving CRT (parents refused) and one after CRT, with the time of relapse occurring at week 21.7 in one and week 99.1 in the other patient.
Fig. 2.
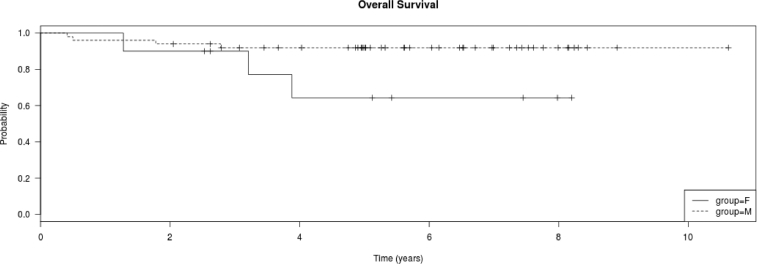
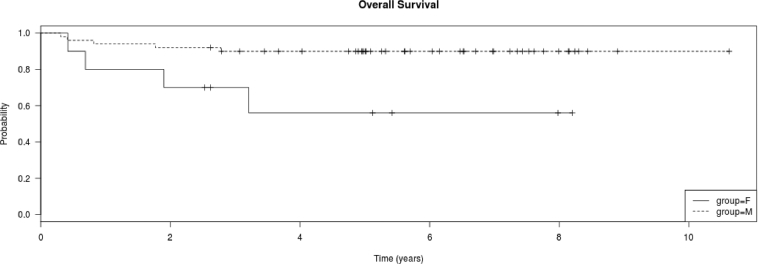
a. Kaplan Meier plot of overall survival of all treated patients, CNS3 vs. non-CNS3. CNS3 patients had shorter 5-year survival than non-CNS3 patients (non-CNS3 93.2% [2.6%] vs. CNS3 73.3% [9.1%]; log rank test P = 0.045). b. Kaplan Meier plot of disease free survival of all treated patients, CNS3 vs. non-CNS3. CNS3 patients had shorter 5-year survival than non-CNS3 patients (non-CNS3 90.9% [SE 3.1%] vs. CNS3 66.7% [SE 10.4%]); log rank test P = 0.0163). c. Kaplan Meier plot of overall survival of the 60 treated patients by gender. Females had shorter 5-year survival than males (F 64.3% [SE 17%] vs. M 91.9% [SE 3.4%]); log rank test P = 0.043). d. Kaplan Meier plot of disease free survival of the 60 treated patients by gender. Females had shorter 5-year survival than males (F 56% [SE 17%] vs. M 90% [SE 4.3%]); log rank test P = 0.0108).
Overall, female patients had more deaths than male patients (P = 0.0066) (Table 4). Evaluation of events showed a preponderance of female, compared to male, deaths (P = 0.048) and relapses (P = 0.021). The 5-year OS and DFS were higher in male than in female patients (Fig. 2c and d) (OS: 91.9 ± 3.4% vs. 64.3 ± 17%, P = 0.043; DFS: 90 ± 4.3% vs. 56 ± 17%, P = 0.011). This effect was mainly in patients treated with the C-MTX regimen (Table 4). Male children treated with C-MTX had a higher 5-year DFS survival than females (95.2 ± 4.7% vs. 25 ± 22%, P < 0.0001). However, there was no difference in the 5-years DFS of male and female children treated with HDMTX (P > 0.05).
4. Discussion
Sixty children with T-ALL treated with C-MTX or HDMTX from Jan 2001 to Dec 2014 were evaluated for outcome. The male to female ratio was 4.7, higher than previous reports where the ratio ranged from 3.0 −3.7 [5], [14]. Overall, 54 of 60 (90%) had a CR and 7 of 60 (11.7%) died, with female patients having the highest death rates. The overall death rate in our patients was similar to that of previous reports [15]. Fifty patients were still alive at last follow-up with a mean follow-up of 4.99 ± 2.39 years. The 5-year DFS was 85% (7.5% SE). These findings are comparable with results reported by major cooperative groups [2], [5], [16].
Patients with T-ALL have historically had a poor prognosis compared to B-ALL, largely due to CNS disease recurrence. The introduction of CRT and intrathecal (IT) chemotherapy has had a large impact on CNS disease, improving these outcomes. In the present study, all relapses occurred early, within 2 years of treatment, and all relapses had a CNS component. Relapse occurred in 33.3% of CNS3 patients. All patients with CNS3 that did not receive CRT relapsed while approximately 9% of patients with CNS3 who received CRT relapsed. Treatment with HDMTX did not eliminate the negative impact of CNS3 status, as approximately 65% of patients who relapsed were treated with the HDMTX regimen. Thus, further optimization of CNS-directed therapy is needed in patients with CNS3.
While pCRT has been used in patients with HR T-ALL, it has not gained widespread acceptance due to mixed positive findings and treatment related toxicity [13], [17]. pCRT was compared to IV HDMTX plus IT MTX in a randomized study of HR T-ALL patients and similar outcomes were found in both groups [18]. Patients receiving pCRT had a 10.6% lower incidence of CNS relapse and a 6.3% higher incidence of non-CNS relapse. No difference in 10-year EFS was seen. This was one of several studies that showed a decreased need for pCRT in T-ALL patients when IT therapy was used. We treated 44 patients with C-MTX or HDMTX that did not have CNS3 at initial diagnosis. These patients did not receive pCRT and had 5-year OS and DFS rates greater than 90%. Only one of these patients (2.3%) developed a CNS relapse. This finding reflects the improvement in outcomes of childhood T-ALL with current intensive therapy.
Different methods have been used to intensify CNS-directed therapy in ALL including the use of triple IT (ITT), HDMTX, intensified asparaginase, and dexamethasone [1]. The use of intensive ITT has been suggested to decrease the risk of CNS relapse [1]. ITT consisting of cytarabine, MTX, and hydrocortisone has been used to replace pCRT in intermediate risk patients with T-ALL [19]. ITT has been used for the prophylactic treatment of T-ALL patients and was associated with a 1.5–4.5% relapse rate [1]. CRT is usually reserved for patients with CNS3 disease or HR patients [20], [21]. Treatment regimens for CNS3 disease include ITT starting during induction and throughout treatment, with variable doses during maintenance [20]. Patients we treated did not receive ITT. Instead they were treated with IT MTX. The use of ITT may improve patient CNS outcomes. CRT was administered for patients with CNS3 status in our study. However, patients with CNS3 who experienced a relapse did not receive the planned CRT as their relapse occurred before the planned timing of CRT. Therefore, intensifying IT therapy using ITT may help better control CNS3 disease.
In our study systemic therapy was intensified with the use of dexamethasone instead of prednisone, double DI, double IM, and HDMTX. In addition, the total number of IT MTX doses ranged from 19–21 doses in females and 26–28 in males. These measures may explain the comparable outcomes of patients treated with HDMTX vs. C-MTX in our study.
Female gender was more frequently associated with death than male gender, in contrast to previous studies [5], [22], [23]. Females we treated with the C-MTX regimen had a significantly inferior 5-yr DFS compared to males. Studies suggest that methotrexate clearance is lower in females compared to males, resulting in higher drug levels and toxicities in females. Female children with ALL have been reported to have a higher incidence of treatment related toxicity, treatment delays, and deaths than males [24]. Pharmacokinetic studies in Norwegian children being treated for high grade osteosarcoma with HDMTX showed gender differences in MTX metabolism [25]. Pre-treatment erythrocyte folate concentrations were higher in males than in females and the highest peak treatment concentrations of MTX were seen in female children. Serum ALT concentrations were found to be related to clearance of MTX, gender, age and serum 7-OH-MTX concentrations, with younger female children having the strongest correlations. 7-OH-MTX concentration was felt to be related to hepatic toxicity and female children were affected more than male children. This finding was of particular interest as the C-MTX regimen we used did not include folinic acid rescue, while the HDMTX regimen did. Attention to intensive hydration, maintaining adequate renal function and monitoring serum MTX levels are standard practice to minimize MTX-related toxicities in patients receiving HDTMX but not the C-MTX regimen. As female patients treated with C-MTX had significantly more death and relapse events, utilizing the HDMTX regimen with folinic acid rescue, particularly in female patients, may be justified.
Several polymorphisms can affect MTX dosing and related toxicity. The MTHFR C677T polymorphism has been associated with an increased risk of MTX-induced all-grade and severe hepatic and gastrointestinal toxicities in Caucasian adults being treated for cancer [26]. The expression of multidrug resistance gene polymorphisms, like ABCC2, have been noted to affect folate metabolism and related MTX toxicity [27]. Polymorphism analysis in Arabs has shown a lower frequency of the MTHFR C677T polymorphism than that found in Caucasians [28]. The frequency of similar polymorphisms in the Saudi population is not known.
Several recent advances may contribute to improved outcomes in patients with T-ALL. Nelarabine is a promising new agent undergoing evaluation [16]. Early studies in newly diagnosed adults suggest there is low toxicity and good efficacy associated with its use. Experience in children is limited and nelarabine efficacy studies from the COG AALL0434 are still ongoing [15]. The evaluation of MRD is becoming an important prognostic indicator of relapse [29]. Polymerase chain reaction determination of genetic markers in MRD may better define high risk patients with T-ALL and allow individual adjustments to treatment, minimizing toxicity or relapse, depending on patient risk [30].
There were several limitations to this study. There were a small number of patients treated in each treatment arm, limiting the power to detect differences in outcomes. About 40% of patients had less than 5 years follow-up, although patients with T-cell ALL most frequently relapsed within 2 years of treatment [4]. Patients treated with C-MTX had longer follow-up than patients treated with HDMX. Despite this, survival outcomes between the two regimens were similar suggesting no added benefit to the incorporation of HDTMX, particularly for patients with CNS3 status.
5. Conclusions
Treatment of T-cell ALL is improving with intensification of therapy. The incorporation of HDMTX on a double DI and IM backbone did not impact survival outcomes. Patients with CNS3 disease should be considered for early intensification of IT therapy using ITT and/or early therapeutic CRT in order to prevent CNS relapse. MTX metabolism in female children needs further investigation. This report demonstrates the world-wide improvement in treating children with T-ALL and suggests the need to optimize CNS-directed therapy in patients with T-cell ALL and CNS3 status.
Acknowledgments
Contributors
All authors contributed sufficiently to this project to make them eligible for authorship.
Funding
The study was funded by the King Abdullah International Research Center (KAIMRC), King Abdulaziz Medical City, Ministry of National Guard Health Affairs, Saudi Arabia.
Conflicts of interest
The authors report no conflict of interest.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.lrr.2018.10.001.
Appendix. Supplementary materials
References
- 1.Pui C.-H., Evans W.E. Treatment of acute lymphoblastic leukemia. N. Engl. J. Med. 2006;354(2):166–178. doi: 10.1056/NEJMra052603. [DOI] [PubMed] [Google Scholar]
- 2.Hunger S.P., Lu X., Devidas M. Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: a report from the children's oncology group. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2012;30(14):1663–1669. doi: 10.1200/JCO.2011.37.8018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Winter S.S., Devidas M., Chen S. Capizzi-style methotrexate with pegasparagase (C-MTX) is superior to high-dose methotrexate (HDMTX) in T-Lineage acute lymphoblastic leukemia (T-ALL): results from Children's Oncology Group (COG) AALL0434. Blood. 2015;126(3):794. [Google Scholar]
- 4.Shuper A., Stark B., Kornreich L. Methotrexate treatment protocols and the central nervous system: significant cure with significant neurotoxicity. J. Child Neurol. 2000;15(9):573–580. doi: 10.1177/088307380001500902. [DOI] [PubMed] [Google Scholar]
- 5.Asselin B.L., Devidas M., Wang C. Effectiveness of high-dose methotrexate in T-cell lymphoblastic leukemia and advanced-stage lymphoblastic lymphoma: a randomized study by the Children's Oncology Group (POG 9404) Blood. 2011;118(4):874–883. doi: 10.1182/blood-2010-06-292615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clarke M., Gaynon P., Hann I. CNS-directed therapy for childhood acute lymphoblastic leukemia: childhood all collaborative group overview of 43 randomized trials. J. Clin. Oncol. 2003;21(9):1798–1809. doi: 10.1200/JCO.2003.08.047. [DOI] [PubMed] [Google Scholar]
- 7.Vora A., Goulden N., Wade R. Treatment reduction for children and young adults with low-risk acute lymphoblastic leukaemia defined by minimal residual disease (UKALL 2003): a randomised controlled trial. Lancet Oncol. 2013;14(3):199–209. doi: 10.1016/S1470-2045(12)70600-9. [DOI] [PubMed] [Google Scholar]
- 8.LeClerc J.M., Billett A.L., Gelber R.D. Treatment of childhood acute lymphoblastic leukemia: results of Dana-Farber ALL consortium protocol 87-01. J. Clin. Oncol. 2002;20(1):237–246. doi: 10.1200/JCO.2002.20.1.237. [DOI] [PubMed] [Google Scholar]
- 9.Seibel N.L., Steinherz P.G., Sather H.N. Early postinduction intensification therapy improves survival for children and adolescents with high-risk acute lymphoblastic leukemia: a report from the Children's Oncology Group. Blood. 2008;111(5):2548–2555. doi: 10.1182/blood-2007-02-070342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jastaniah W., Elimam N., Abdalla K. Identifying causes of variability in outcomes in children with acute lymphoblastic leukemia treated in a resource-rich developing country. Pediatr. Blood Cancer. 2015;62(6):945–950. doi: 10.1002/pbc.25374. [DOI] [PubMed] [Google Scholar]
- 11.Smith M., Arthur D., Camitta B. Uniform approach to risk classification and treatment assignment for children with acute lymphoblastic leukemia. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1996;14(1):18–24. doi: 10.1200/JCO.1996.14.1.18. [DOI] [PubMed] [Google Scholar]
- 12.The Lancet. Steinherz/Bleyer algorithm method of evaluating traumatic lumbar puncture. http://www.thelancet.com/cms/attachment/2025028614/2044736503/mmc1.pdf. October 2014.
- 13.Kelly M.J., Trikalinos T.A., Dahabreh I.J., Gianferante M., Parsons S.K. Cranial radiation for pediatric T-lineage acute lymphoblastic leukemia: a systematic review and meta-analysis. Am. J. Hematol. 2014;89(10):992–997. doi: 10.1002/ajh.23784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Willemse M.J., Seriu T., Hettinger K. Detection of minimal residual disease identifies differences in treatment response between T-ALL and precursor B-ALL. Blood. 2002;99(12):4386–4393. doi: 10.1182/blood.v99.12.4386. [DOI] [PubMed] [Google Scholar]
- 15.Winter S.S., Dunsmore K.P., Devidas M. Safe integration of nelarabine into intensive chemotherapy in newly diagnosed. Pediatr. Blood Cancer. 2015;62(7):1176–1183. doi: 10.1002/pbc.25470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muffly L., Larson R.A. Improving outcomes in childhood T-cell acute lymphoblastic leukemia: promising results from the Children's Oncology Group incorporating nelarabine into front-line therapy. Transl. Pediatr. 2012;1(2):120–122. doi: 10.3978/j.issn.2224-4336.2012.09.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kelly M.J., Pauker S.G., Parsons S.K. Using nonrandomized studies to inform complex clinical decisions: the thorny issue of cranial radiation therapy for T-cell acute lymphoblastic leukemia. Pediatr. Blood Cancer. 2015;62(5):790–797. doi: 10.1002/pbc.25451. [DOI] [PubMed] [Google Scholar]
- 18.Hill F.G.H., Richards S., Gibson B. Successful treatment without cranial radiotherapy of children receiving intensified chemotherapy for acute lymphoblastic leukaemia: results of the risk-stratified randomized central nervous system treatment trial MRC UKALL XI (ISRC TN 16757172) Br. J. Haematol. 2004;124(1):33–46. doi: 10.1046/j.1365-2141.2003.04738.x. [DOI] [PubMed] [Google Scholar]
- 19.Stark B., Avrahami G., Nirel R. Extended triple intrathecal therapy in children with T-cell acute lymphoblastic leukaemia: a report from the Israeli National ALL-Studies. Br. J. Haematol. 2009;147(1):113–124. doi: 10.1111/j.1365-2141.2009.07853.x. [DOI] [PubMed] [Google Scholar]
- 20.Cooper S.L., Brown P.A. Treatment of pediatric acute lymphoblastic leukemia. Pediatr. Clin. North Am. 2015;62(1):61–73. doi: 10.1016/j.pcl.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laver J.H., Barredo J.C., Amylon M. Effects of cranial radiation in children with high risk T cell acute lymphoblastic leukemia: a Pediatric Oncology Group report. Leukemia. 2000;14(3):369–373. doi: 10.1038/sj.leu.2401693. [DOI] [PubMed] [Google Scholar]
- 22.Silverman L.B., Stevenson K.E., O'Brien J.E. Long-term results of Dana-Farber Cancer Institute ALL Consortium protocols for children with newly diagnosed acute lymphoblastic leukemia (1985–2000) Leukemia. 2010;24(2):320–334. doi: 10.1038/leu.2009.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rives S., Estella J., Camos M. T-cell pediatric acute lymphoblastic leukemia: analysis of survival and prognostic factors in 4 consecutive protocols of the Spanish cooperative study group SHOP. Med. Clin. (BARC) 2012;139(4):141–149. doi: 10.1016/j.medcli.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 24.Meeske K.A., Ji L., Freyer D.R. Comparative toxicity by sex among children treated for acute lymphoblastic leukemia: a report from the Children's Oncology Group. Pediatr. Blood Cancer. 2015;62(12):2140–2149. doi: 10.1002/pbc.25628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holmboe L., Andersen A.M., Morkrid L., Slordal L., Hall K.S. High dose methotrexate chemotherapy: pharmacokinetics, folate and toxicity in osteosarcoma patients. Br. J. Clin. Pharmacol. 2012;73(1):106–114. doi: 10.1111/j.1365-2125.2011.04054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao M., Liang L., Ji L. MTHFR gene polymorphisms and methotrexate toxicity in adult patients with hematological malignancies: a meta-analysis. Pharmacogenomics. 2016;17(9):1005–1017. doi: 10.2217/pgs-2016-0004. [DOI] [PubMed] [Google Scholar]
- 27.Rau T., Erney B., Gores R., Eschenhagen T., Beck J., Langer T. High-dose methotrexate in pediatric acute lymphoblastic leukemia: impact of ABCC2 polymorphisms on plasma concentrations. Clin. Pharmacol. Ther. 2006;80(5):468–476. doi: 10.1016/j.clpt.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 28.Bu R., Gutierrez M.I., Al-Rasheed M., Belgaumi A., Bhatia K. Variable drug metabolism genes in Arab population. Pharmacogen. J. 2004;4(4):260–266. doi: 10.1038/sj.tpj.6500251. [DOI] [PubMed] [Google Scholar]
- 29.Berry D.A., Zhou S., Higley H. Association of minimal residual disease with clinical outcome in pediatric and adult acute lymphoblastic leukemia: a meta-analysis. JAMA Oncol. 2017;3(7) doi: 10.1001/jamaoncol.2017.0580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Y., Easton J., Shao Y. The genomic landscape of pediatric and young adult T-lineage acute lymphoblastic leukemia. Nat. Genet. 2017;49(8):1211–1218. doi: 10.1038/ng.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.