Abstract
Mitral valve prolapse is a common disorder, but severe mitral regurgitation (MR) as a result of rupture of mitral valve chordae tendineae is a rare manifestation of thyrotoxic heart disease. There are limited reports with respect to the onset of severe MR as a complication of Graves disease. We report a case of a 60-year-old woman with Graves disease and thyroid-associated ophthalmopathy as her past history. She had signs of congestive heart failure, a loud murmur as a result of MR, clinical cardiomegaly, and peripheral edema. Echocardiographic and angiographic data were consistent with moderate to severe MR. She also had thyrotoxicosis caused by the recurrence of Graves disease. She was taking methiamazole, a beta-blocker, hydrocortisone, and potassium iodide. Ultimately, thyroidectomy was performed to improve her hyperthyroid state. After normalization of her thyroid status, she continued to have moderate to severe MR, and mitral valve repair was performed. The present case had severe MR as a result of rupture of mitral valve chordae tendineae, which is considered rare in a patient with Graves disease.
1. Case Report
A 53-year-old woman visited Kanazawa University Hospital because of Graves disease and thyroid-associated ophthalmopathy. Her mother also had thyroid disease. Her thyroid function became normal by low-dose methimazole (MMI). She was treated with intravenous methylprednisolone pulse therapy, and this improved her diplopia. She did not have a systolic murmur or Marfan syndrome (MFS)-like physical characteristics. Furthermore, echocardiography showed no mitral valve prolapse (MVP) or other abnormalities. MMI was terminated after 6 years because of normalization of serum thyroid hormone levels and thyroid-stimulating hormone (TSH) antibody levels.
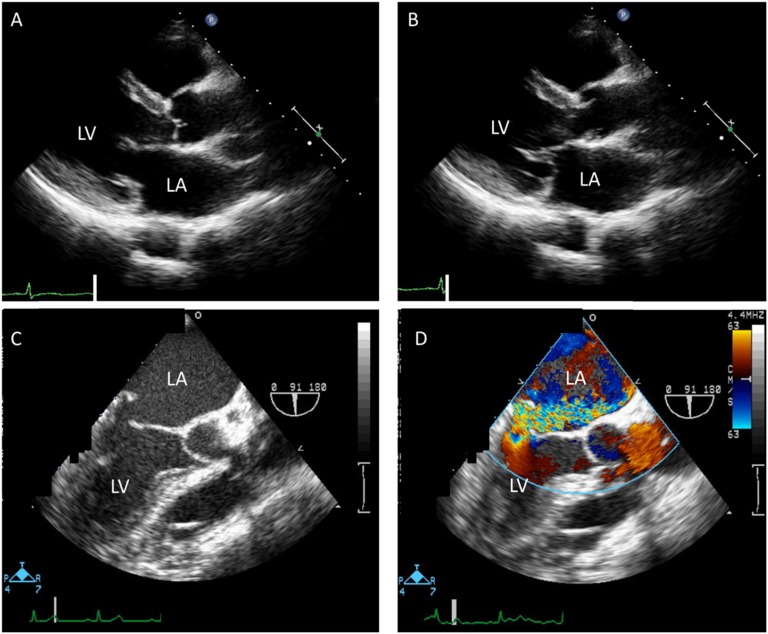
Nine months after MMI was stopped, she presented with dyspnea on exertion, hyperthermia, increased sweating, and diarrhea. Her Glasgow Coma Scale was 15 (E4V5M6). An apical systolic murmur (Levine scale 4/6) was audible. She had no obvious thyroid swelling or exophthalmos. However, she had pitting edema in both lower extremities. Her physical parameters and thyroid hormone profile are shown in Table 1. Laboratory data, including a complete blood count, were within normal limits. Cardiac enzymes, liver enzymes, renal function, and electrolyte levels were also within normal limits or slightly elevated. Her blood cultures were all negative. Thyroid ultrasonography was performed and showed results compatible with Graves disease. An electrocardiogram on admission showed atrial fibrillation at a rate of 100 beats/minute and no ST-T changes. Echocardiography revealed severe mitral regurgitation (MR) as a result of prolapse of the posterior mitral leaflet and moderate tricuspid regurgitation, which had not been detected before, and mild dilatation of the left atrium and ventricle. Left ventricular wall thickness was normal, and systolic function was preserved. Transesophageal echocardiography showed no vegetation or calcification on the valves (Fig. 1).
Table 1.
Course of Physical Data, Echocardiographic Findings, and Thyroid Hormone Profiles
| Variables | Day 1 | Day 30 | After Cardiac Surgery | |
|---|---|---|---|---|
| Physical data | Blood pressure, mm Hg | 160/75 | 88/49 | 102/63 |
| Heart rate, rhythm, beats/min | 100, AF | 94, SR | 82, SR | |
| Body temperature, °C | 40.0 | 36.9 | 36.7 | |
| Echocardiography | LAD, mm | 50.4 | 50.1 | 40.3 |
| LVDd/LVDs, mm | 55/31 | 53/32 | 44/32 | |
| LVEF (Teichholz), % | 74 | 70 | 61 | |
| TRPG, mm Hg | 67.2 | 31.4 | 23.0 | |
| Estimated RAP, mm Hg | 82 | 60 | 5 | |
| IVC, mm | 22 | – | 5 | |
| Hormones | BNP, pg/mL | 1343.4 | 247.9 | 30.1 |
| FT3, pg/mL | >25.0 | 3.09 | 2.61 | |
| FT4, ng/dL | >8.0 | 1.25 | 1.23 | |
| TSH, µU/mL | <0.01 | <0.01 | <0.01 | |
| TRAb, TSAb, IU/L, % | 96, 424 | – | -, 381 | |
| TgAb, TPOAb, IU/mL, IU/mL | <10, 2.3 | – | <10.0 |
Abbreviations: AF, atrial fibrillation; BNP, brain natriuretic peptide; FT3, free triiodothyronine; FT4, free thyroxine; IVC, inferior vena cava; LAD, left atrial dimension; LVDd, left ventricular diameter at end diastole; LVDs, left ventricular diameter at end systole; LVEF, left ventricular ejection fraction; RAP, right atrial pressure; SR, sinus rhythm; TgAb, thyroglobulin antibody; TPOAb, thyroperoxidase antibody; TRAb, TSH receptor antibody; TRPG, transtricuspid pressure gradient; TSAb, TSH-stimulating receptor antibody.
Figure 1.
Transthoracic echocardiography showed MVP at the posterior mitral leaflet [(A) parasternal long-axis view in the diastolic phase and (B) systolic phase]. (C and D) Transesophageal echocardiography showed severe MR from MVP. (D) The color bar shows flow velocity. LA, left atrium; LV, left ventricle.
She was diagnosed with definite thyroid storm as a result of the recurrence of Graves disease, according to the Japan Thyroid Association guideline [1]. She met the prerequisites for thyrotoxicosis, based on the following criteria: (i) fever (38°C or higher), (ii) congestive heart failure (class IV by the New York Heart Association classification) and MVP, and (iii) diarrhea.
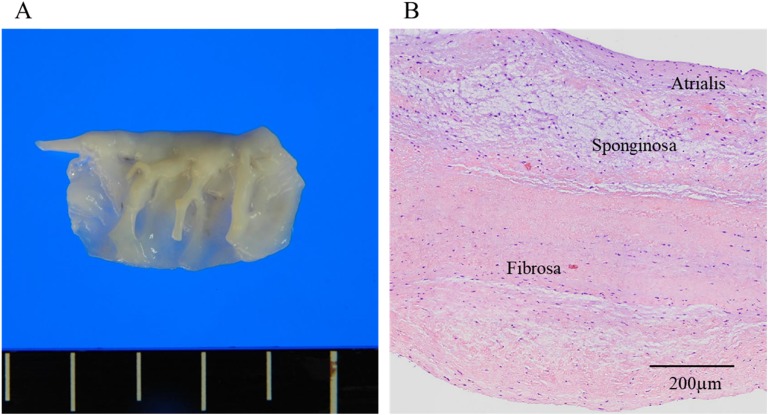
The patient was given MMI, a beta-blocker, hydrocortisone, and potassium iodide for thyroid storm. In addition, furosemide, human atrial natriuretic polypeptide, olmesartan, spironolactone, and heparin were given as a standard treatment of acute heart failure. Ceftriaxone was also administrated only for 5 days as a result of suspicion of infectious endocarditis. Her dyspnea, leg edema, and diarrhea gradually improved, and her body temperature declined to 36°C to 37°C. Blood pressure and pulse rate also normalized. On day 30 after admission, her serum thyroid hormones had returned to almost normal (Table 1), but echocardiography still showed severe MR, moderate tricuspid regurgitation, and mild dilatation of the left atrium and ventricle. On day 45, total thyroidectomy was performed. After thyroidectomy and the administration of 100 µg/day levothyroxine, her free triiodothyronine, free thyroxine, and TSH levels were within normal ranges. On day 59, minimally invasive cardiac surgery was performed for mitral valve repair. Rupture of mitral valve chordae tendineae (P3) was observed in the operative view. Histology revealed a disorganized fragmentation and tear of collagen and elastin fibers and myxomatous changes in the resected mitral valve, which was soft and flappy. There were no inflammatory cells (Fig. 2). After the operation, the grade of MR improved to mild, and there was a decrease in left atrial dimension and serum brain natriuretic peptide levels (Table 1).
Figure 2.
Histological findings on the mitral valve. (A) Rupture of mitral valve chordae tendineae (P3) was observed. The mitral valve was slightly thickened and soft. (B) Histology (hematoxylin-eosin stain) shows a disorganized fragmentation and tear of collagen and elastin fibers and myxomatous changes. There is no inflammatory cell invasion or granuloma.
2. Discussion
We report a 60-year-old female with severe MR who was also diagnosed with recurrence of Graves disease. The resected mitral valve had a disordered arrangement of collagen and elastin fibers and myxomatous changes. The mitral valve degeneration and rupture of mitral valve chordae tendineae were thought to cause severe MR and acute heart failure.
MVP is a common disorder, afflicting 2% to 3% of the general population [2]. MVP can be associated with substantial MR, bacterial endocarditis, congestive heart failure, and even sudden death [3]. It is characterized by typical fibromyxomatous changes in the mitral leaflet tissue with superior displacement of one or both leaflets into the left atrium [4]. Histological examination of myxomatous MVP leaflets characteristically demonstrates activated interstitial myofibroblast-like cells, disorganized fragmentation of collagen and elastin fibers, and expansion of the spongiosa layer as a result of the accumulation of proteoglycans and glycosaminoglycans, which extend into the load-bearing fibrosa [5].
Autoimmune thyroid disease (ATD) is frequently linked to autoimmune cardiovascular involvement [6–8] and is associated with an increased prevalence of MVP [8]. MVP is also observed in the presence of connective tissue disorders, such as MFS, Loeys-Dietz syndrome, Ehlers-Danlos syndrome, osteogenesis imperfecta, and pseudoxanthoma elasticum [4]. The patients with ATD generally have increased glycosaminoglycan production in the orbital space, pretibial region, and cardiac valves. The mechanism of glycosaminoglycan accumulation in the mitral valve was determined in the same way as in the orbit (for example, induction of TGF-β and IGF) [9]. The augmented secretion and accumulation of glycosaminoglycans in the cardiac valve led to thickening of the leaflets and additional disturbance of collagen synthesis and finally, disruption of the fibrosa. Kahaly and Dillmann [8] reported the prevalence of myxomatous valves in patients with Graves disease, Hashimotos thyroiditis, and toxic nodular goiter. Myxomatous mitral valve was present in 33% of patients with Graves disease and 36% of those with Hashimotos thyroiditis but was not found in patients with a toxic nodular goiter [9]. They demonstrated that thyroid function has no influence on the incidence and intensity of the myxomatous valve degeneration. However, increased triiodothyronine leads to enhancement of cardiac contraction and high cardiac output, which could act on the vulnerable and fragile myxomatous mitral valve, resulting in rupture of mitral chordae tendinae. This was thought to cause exacerbation of MR and acute heart failure in the present case. Immediate improvement of thyroid function and normalization of cardiac contraction were needed to prevent acute MR, which is usually a result of acute disruption of the valve-restraining forces.
Currently, the mechanism of MVP in patients with thyroid autoimmunity is unclear. Reports on the effect of thyroid autoimmunity on interstitial myofibroblast-like cells and the formation of collagen and elastin fibers are limited. Furthermore, there are few case reports written in English on spontaneous rupture of mitral chordae tendineae in patients with hyperthyroidism [10]. The rupture of mitral chordae tendineae may be a rare complication, but ATD is a common disease. Patients with ATD should have regular follow-up to assess heart valve function, and treatment should be started if MVP is detected.
This case report has some limitations. We did not examine specific reported gene mutations that have been found in MVP (for example, FBN1 and TGFBR1 and TGFBR2 or the TGF-β2 ligand or TGFB, which characterize MFS) to exclude incidental combinations. However, our patient did not have such connective tissue disorders, as there were no characteristic physical findings.
In summary, the present case illustrated a possible cause for rupture of mitral valve chordae tendineae in a patient with Graves disease. Although rupture of mitral valve chordae tendineae is a rare complication, physicians should pay attention to heart murmurs and abnormal valve function on echocardiography in patients with ATD, which is clinically important because of the risk of life-threatening heart failure.
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Abbreviations:
- ATD
autoimmune thyroid disease
- MFS
Marfan syndrome
- MMI
methimazole
- MR
mitral regurgitation
- MVP
mitral valve prolapse
- TSH
thyroid-stimulating hormone
Contributor Information
Kenshi Hayashi, Email: kenshi@med.kanazawa-u.ac.jp.
Takashi Yoneda, Email: endocrin@med.kanazawa-u.ac.jp.
References and Notes
- 1. Satoh T, Isozaki O, Suzuki A, Wakino S, Iburi T, Tsuboi K, Kanamoto N, Otani H, Furukawa Y, Teramukai S, Akamizu T. 2016 Guidelines for the management of thyroid storm from The Japan Thyroid Association and Japan Endocrine Society (First edition). Endocr J. 2016;63(12):1025–1064. [DOI] [PubMed] [Google Scholar]
- 2. Freed LA, Levy D, Levine RA, Larson MG, Evans JC, Fuller DL, Lehman B, Benjamin EJ. Prevalence and clinical outcome of mitral-valve prolapse. N Engl J Med. 1999;341(1):1–7. [DOI] [PubMed] [Google Scholar]
- 3. Delling FN, Vasan RS. Epidemiology and pathophysiology of mitral valve prolapse: new insights into disease progression, genetics, and molecular basis. Circulation. 2014;129(21):2158–2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Levine RA, Hagége AA, Judge DP, Padala M, Dal-Bianco JP, Aikawa E, Beaudoin J, Bischoff J, Bouatia-Naji N, Bruneval P, Butcher JT, Carpentier A, Chaput M, Chester AH, Clusel C, Delling FN, Dietz HC, Dina C, Durst R, Fernandez-Friera L, Handschumacher MD, Jensen MO, Jeunemaitre XP, Le Marec H, Le Tourneau T, Markwald RR, Mérot J, Messas E, Milan DP, Neri T, Norris RA, Peal D, Perrocheau M, Probst V, Pucéat M, Rosenthal N, Solis J, Schott JJ, Schwammenthal E, Slaugenhaupt SA, Song JK, Yacoub MH; Leducq Mitral Transatlantic Network . Mitral valve disease--morphology and mechanisms. Nat Rev Cardiol. 2015;12(12):689–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Padang R, Bagnall RD, Semsarian C. Genetic basis of familial valvular heart disease. Circ Cardiovasc Genet. 2012;5(5):569–580. [DOI] [PubMed] [Google Scholar]
- 6. Biondi B, Kahaly GJ. Cardiovascular involvement in patients with different causes of hyperthyroidism. Nat Rev Endocrinol. 2010;6(8):431–443. [DOI] [PubMed] [Google Scholar]
- 7. Biondi B. Mechanisms in endocrinology: heart failure and thyroid dysfunction. Eur J Endocrinol. 2012;167(5):609–618. [DOI] [PubMed] [Google Scholar]
- 8. Kahaly GJ, Dillmann WH. Thyroid hormone action in the heart. Endocr Rev. 2005;26(5):704–728. [DOI] [PubMed] [Google Scholar]
- 9. Bahn RS. Thyrotropin receptor expression in orbital adipose/connective tissues from patients with thyroid-associated ophthalmopathy. Thyroid. 2002;12(3):193–195. [DOI] [PubMed] [Google Scholar]
- 10. Aronson RJ, Hoffman M, Algueti-Margulis A, Yust I. Spontaneous rupture of mitral chordae tendineae in hyperthyroidism. Am J Cardiol. 1987;59(5):475–476. [DOI] [PubMed] [Google Scholar]