Abstract
We present a Veterans Affairs–sponsored pilot study of U500 concentrated insulin administered via disposable patch insulin pump (DPIP) vs twice-daily (BID) injections with an insulin pen in a case series format. We conducted a prospective, single-center, randomized, intent-to-treat pilot study. Ten participants were enrolled with poorly controlled diabetes, defined as hemoglobin A1C >8.0 and severe insulin resistance defined as total daily dose >200 units. Participants were randomized in a 1:1 ratio to either U500 DPIP or U500 BID insulin titration protocols for 14 weeks. A clinical pattern emerged where four participants randomized to the DPIP treatment arm were withdrawn early as the DPIP did not work well for the purpose studied. There was not a statistically significant difference in the rate of hypoglycemia between treatment arms. Based on our clinical experience and results, we argue against the general use of U500 DPIP in clinical practice.
Keywords: disposable insulin patch pump, severe insulin resistance, type 2 diabetes, U500
Individuals requiring >200 units of insulin per day, commonly defined as severe insulin resistance, are frequently treated with U500 insulin. U500 insulin is a concentrated form of regular insulin that contains 500 units of insulin per milliliter instead of the traditional 100 units per milliliter and has been available since the 1950s. Use of U500 resolves several practical issues, including the number of insulin injections required per day, subcutaneous depot size, and insulin storage space requirements. Some studies have also found U500 to be more cost-effective and provide improved patient satisfaction [1–3].
Transition to U500 insulin in patients who have diabetes with severe insulin resistance is associated with improved glycemic control. Granata et al. [4] published a retrospective chart review of Veterans Affairs patients treated with a twice-daily (BID) U500 protocol. Patients achieved a percent hemoglobin A1C (HbA1C) of 8.7 ± 1.7 compared with a baseline of 9.4 ± 1.9. Hood et al. [5] performed a multicenter unmasked randomized trial in 325 patients examining three times daily vs BID protocols of U500. At the end of the study period, average HbA1C was 7.5 ± 1.1 compared with a baseline of 8.7 ± 1.7. Furthermore, they found no difference in glycemic control between BID and three times daily dosing of U500.
A literature search of continuous subcutaneous insulin infusion (CSII) and U500 reveal several peer-reviewed publications about transition from multiple daily injections to CSII with U500 insulin. The most compelling was a prospective proof-of-concept study published by Lane et al. [6]. This group used U500 via CSII off-label in the “Omnipod” insulin pump. The study consisted of 21 adults. It significantly improved glycemic control without increased risk of hypoglycemia and on average lowered HbA1C by 1.23%. The group also reported improved treatment satisfaction among participants. A meta-analysis of U500 studies that includes 310 patients using U500 via BID injections and 55 patients using U500 via CSII by Reutrakul et al. [7] claims, “Overall, use of U-500R BID and U-500R via CSII appears to be equally effective in achieving glycemic control with HbA1c reduction of approximately 1.6% without significant increase in severe hypoglycemia.” CSII has also been associated with significant reductions in insulin requirements in U100 insulins compared with multiple daily injections. Some studies cite decreases of 20% to 30% [8, 9].
We therefore hypothesized that U500 CSII via disposable insulin patch pumps (DPIPs) may realize the same benefits demonstrated in U500 CSII via a digital insulin pump. Our study used the “V-Go,” manufactured by Valeritas. It is a once-daily DPIP approved for use in patients with diabetes by the US Food and Drug Administration. It administers a continuous infusion of 0.2 mL, 0.3 mL, or 0.4 mL of insulin over 24 hours with the ability to bolus 0.02 mL of insulin by clicking a button. It approximates insulin administration achieved by durable digital insulin pumps. DPIP use can be used to replace multiple daily injection treatment regimens, which are associated with poor patient compliance [10–12]. In fact, 72% of patients on basal/bolus insulin regimens report they never take injections outside of the home [13]. Indeed, a retrospective study of DPIP use demonstrated improved glycemic control, decreased insulin requirements, and decreased hypoglycemic events with use [14].
There is limited literature on using U500 via DPIP. A case report by Kennedy and Tannock [15] demonstrated improved HbA1C from 9.3 to 6.8 and total daily dose (TDD) of insulin from 415 to 280 units over 12 weeks. The authors also reported decreased frequency of hypoglycemia and improved satisfaction with the treatment regimen.
Achieving an HbA1C <8% in patients with severe insulin resistance poses a substantial clinical challenge. We hypothesized DPIP administration of U500 may achieve a more physiologic, continuous basal rate and rapid-acting mealtime compared with BID administration. We also hypothesized potential cost savings and prevention of weight gain with DPIP compared with BID given potentially decreased dosing requirements. Finally, we hypothesized DPIP could lead to improved patient compliance and treatment satisfaction. Therefore, we designed a pilot study to preliminarily assess efficacy and safety of DPIP compared with BID treatment with U500 and determine the feasibility of an appropriately powered future study.
1. Methods
We conducted a prospective, single-center, randomized, intent-to-treat pilot study of BID vs DPIP administration of U500 insulin. Informed consent was obtained from all participants. Research activities were approved and monitored by both our local institutional review board and the US Food and Drug Administration as an Investigator-Initiated Investigational New Drug study. Study criteria were notable for severe insulin resistance, defined as TDD >200 units of insulin per day, and poor glycemic control, defined as baseline HbA1C >8.0. All participants were presumed to have type 2 diabetes based on their medical record problem list and prior treatment histories. Participants were required to demonstrate proficiency and willingness to use both BID and DPIP as part of their initial screening visit prior to enrollment. History, physical exam, laboratories, and a treatment satisfaction questionnaire were obtained at baseline and upon completion of a 14-week treatment period. Participants were randomized in a 1:1 ratio to either BID or DPIP arms. Weekly phone visits were conducted during the treatment period with protocol-based insulin dose titration to achieve an average glucose of 80 to 130 mg/dL. Glucose values <80 mg/dL and <65 mg/dL were tracked continuously throughout the study for the purposes of down-titrating participant insulin dosing.
In analyzing hypoglycemia data, we used definitions laid out by the 2018 American Diabetes Association hypoglycemia classification system and Ademolus Classification of Hypoglycemia (ACH) [16, 17]. Data were considered in the scope of a longitudinal study and analyzed for significant differences between the two treatments while accounting for repeated measurements within each subject. A generalized estimating equations model was applied in SPSS 24 in which binary responses were used to assess differences in the frequencies at which patients reached two hypoglycemic benchmarks (≤70 mg/dL and <54 mg/dL). This model was chosen for ability to function with incomplete data sets. For this analysis, group effect P values (with 0.05 denoting significance) were reported.
2. Results
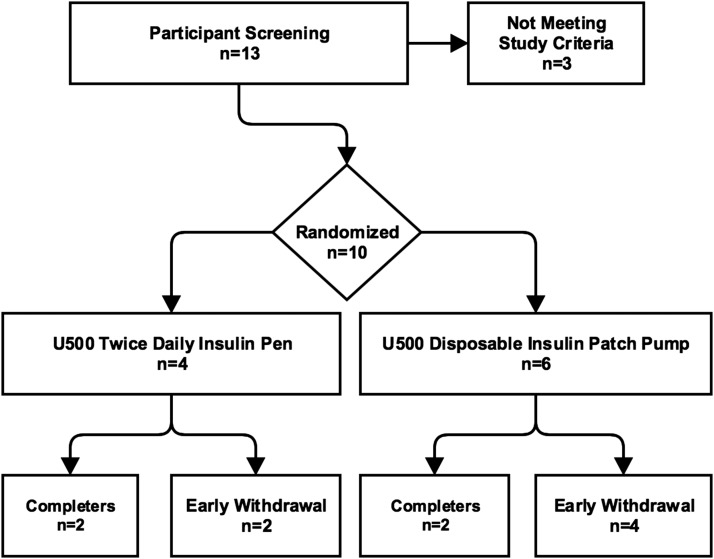
From March 2017 to September 2017, 10 participants from within the Minneapolis Veterans Affairs Health Care System were enrolled. Four participants were randomized to the BID arm. Six participants were randomized to the DPIP arm (Fig. 1).
Figure 1.
Overview of recruitment. Two participants from the BID arm were withdrawn early for adverse events unrelated to the study. Four participants from the DPIP arm were withdrawn early due to inability of the DPIP to match participant insulin requirements.
A. Individual Participant Data: DPIP Treatment Arm
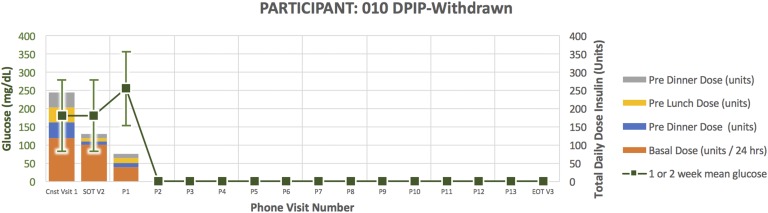
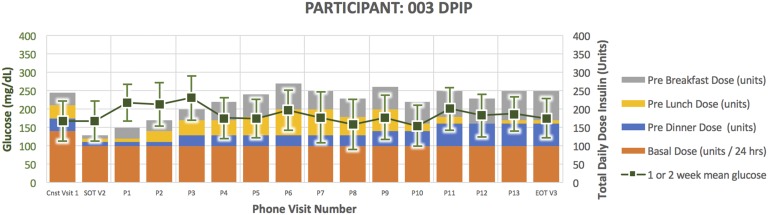
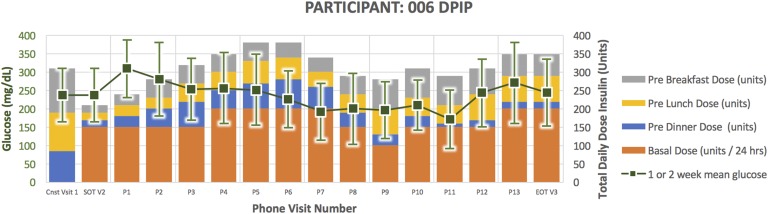
A clinical pattern emerged in the DPIP treatment arm. Four participants were withdrawn early due to inability of the DPIP to match subject insulin requirements (Figs. 2, 3, 4, and 5). More specifically, they required protocol-driven down-titration from U500 to U100 insulin in response to glucose <80 mg/dL and inability to further reduce evening bolus insulin. After subsequently down-titrating to U100 DPIP, they became hyperglycemic, necessitating early withdrawal for participant safety. In response to this clinical pattern, study enrollment was voluntarily stopped. Two participants randomized to the DPIP arm completed the study per protocol (Figs. 6 and 7; Table 1).
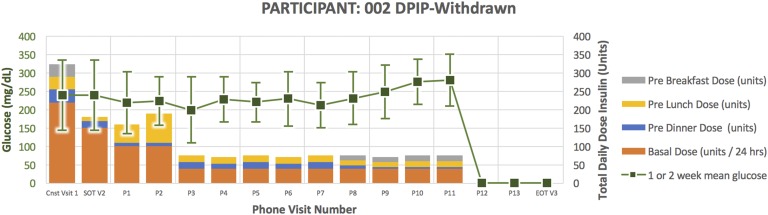
Figure 2.
Treatment course for participant 002.
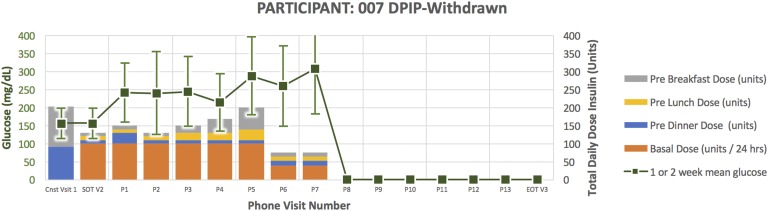
Figure 3.
Treatment course for participant 007.
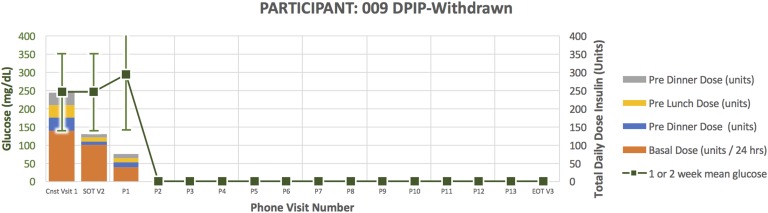
Figure 4.
Treatment course for participant 009.
Figure 5.
Treatment course for participant 010.
Figure 6.
Treatment course for participant 003.
Figure 7.
Treatment course for participant 006.
Table 1.
Results of Study Completers
| Participant |
Baseline
|
End of Treatment
|
Hypoglycemia
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TDD | DTSQ | HbA1C | Mean ± SD Glucose | TDD | DTSQ | HbA1C | Mean ± SD Glucose | <65 | 66–79 | |
| DPIP | ||||||||||
| 003 | 245 | 24 | 8.9 | 168 ± 55.4 | 250 | 33 | 8.5 | 176 ± 54.1 | 0 | 5 |
| 006 | 310 | 25 | 9 | 237 ± 72.8 | 350 | 45 | 8.9 | 244 ± 91 | 6 | 6 |
| BID | ||||||||||
| 004 | 360 | 20 | 9.3 | 244 ± 56.4 | 330 | 37 | 6.9 | 179 ± 64 | 1 | 2 |
| 008 | 220 | 32 | 8.3 | 149 ± 53.4 | 190 | 34 | 7.5 | 153 ± 41.9 | 5 | 5 |
HbA1C and glucose values displayed in mg/dL.
Abbreviation: DTSQ, Diabetes Treatment Satisfaction Questionnaire.
Participant 002 (Fig. 2) was a 68-year-old man randomized to DPIP. He had a 22-year duration of diabetes. His HbA1C at the time of enrollment was 8.9 and TDD 325 units. He required down-titration from U500 to U100 insulin per protocol in response to glucose <80 mg/dL and inability to further reduce evening bolus insulin. After subsequently down-titrating to U100 DPIP, he became hyperglycemic, necessitating early withdrawal for participant safety.
Participant 007 (Fig. 3) was a 79-year-old man randomized to DPIP. He had a 20-year duration of diabetes. His HbA1C at the time of enrollment was 9.2 and TDD 202.5 units. He required down-titration from U500 to U100 insulin per protocol in response to glucose <80 mg/dL and inability to further reduce evening bolus insulin. After subsequently down-titrating to U100 DPIP, he became hyperglycemic, necessitating early withdrawal for participant safety.
Participant 009 (Fig. 4) was a 72-year-old man randomized to DPIP. He had a 28-year duration of diabetes. His HbA1C at the time of enrollment was 9.3 and TDD 245 units. He required down-titration from U500 to U100 insulin per protocol in response to glucose <80 mg/dL and inability to further reduce evening bolus insulin. After subsequently down-titrating to U100 DPIP, he became hyperglycemic, necessitating early withdrawal for participant safety.
Participant 010 (Fig. 5) was a 69-year-old man randomized to DPIP. He had a 14-year duration of diabetes. His HbA1C at the time of enrollment was 10.8 and TDD 245 units. He required down-titration from U500 to U100 insulin per protocol in response to glucose <80 mg/dL and inability to further reduce evening bolus insulin. After subsequently down-titrating to U100 DPIP, he became hyperglycemic, necessitating early withdrawal for participant safety.
Participant 003 (Fig. 6) was a 47-year-old man randomized to DPIP. He had a 14-year duration of diabetes. His HbA1C at the time of enrollment was 8.9 and TDD 245 units. He successfully completed the study per protocol. He experienced zero documented episodes of glucose <65 mg/dL and five episodes of glucose 66 to 79 mg/dL. At the end of treatment, his HbA1C was 8.5 and TDD 250 units.
Participant 006 (Fig. 7) was a 62-year-old man randomized to DPIP. He had a 14-year duration of diabetes. His HbA1C at the time of enrollment was 9.0 and TDD 310 units. He successfully completed the study per protocol. He experienced six documented episodes of glucose <65 mg/dL and six episodes of glucose 66 to 79 mg/dL. At the end of treatment, his HbA1C was 8.9 and TDD 350 units.
B. BID Treatment Arm
Two participants randomized to the BID treatment arm were withdrawn early due to serious adverse events unrelated to the study occurring within the first 2 weeks of treatment. One participant was hospitalized for a chronic obstructive pulmonary disease exacerbation and prescribed a course of high-dose corticosteroids. The second withdrawn participant was hospitalized after a gust of wind caused a fall while raising a flag. He was diagnosed with vertebral fracture treated nonsurgically. Two participants randomized to the BID treatment successfully completed the study (Figs. 8 and 9; Table 1).
Figure 8.
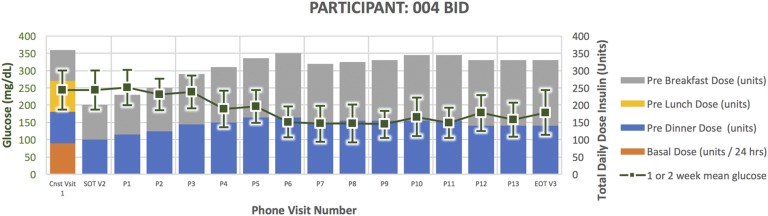
Treatment course for participant 004.
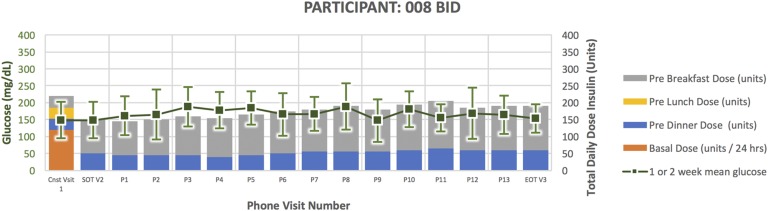
Figure 9.
Treatment course for participant 008.
Participant 004 (Fig. 8) was a 69-year-old man randomized to BID. He had a 14-year duration of diabetes. His HbA1C at the time of enrollment was 9.3 and TDD 360 units. He successfully completed the study per protocol. He experienced one documented episode of glucose <65 mg/dL and two episodes of glucose 66 to 79 mg/dL. At the end of treatment, his HbA1C was 6.9 and TDD 330 units.
Participant 008 (Fig. 9) was a 54-year-old man randomized to BID. He had a 15-year duration of diabetes. His HbA1C at the time of enrollment was 8.3 and TDD 220 units. He successfully completed the study per protocol. He experienced five documented episodes of glucose <65 mg/dL and five episodes of glucose 66 to 79 mg/dL. At the end of treatment, his HbA1C was 7.5 and TDD 190 units.
C. Hypoglycemia
There was not a statistically significant difference in the rate of hypoglycemia between treatment arms (Table 2). There were no American Diabetes Association severe hypoglycemic episodes requiring external assistance for recovery throughout the course of the study. There were no ACH grade 3 hypoglycemic episodes of 10 to 39.9 mg/dL or ACH grade 4 hypoglycemic episodes <10 mg/dL throughout the course of the study.
Table 2.
Statistical Analysis of Hypoglycemia
| Variable |
GEE Model
|
|
|---|---|---|
| Repeated, Binary <54 mg/dL | Repeated, Binary ≤70 mg/dL | |
| Group effect P value | 0.948 | 0.52 |
| Ratio <54/≥54 mg/dL | ||
| BID | 2:27 | |
| DPIP | 3:43 | |
| Ratio ≤70/>70 mg/dL | ||
| BID | 5:24 | |
| DPIP | 13:33 | |
There was not a statistically significant difference demonstrated between treatment arms.
Abbreviation: GEE, generalized estimating equation.
3. Discussion
The two participants who successfully completed the U500 BID treatment arm had outcomes consistent with previously published studies [4, 5]. They experienced improved HbA1C, reduced TDD of insulin, and improved patient satisfaction scores. Conversely, among the two participants who successfully completed the DPIP treatment arm, one did not have a significant change in HbA1C: 9.0 to 8.9. The other participant had a modest improvement in HbA1C: 8.9 to 8.5. Both DPIP completers had increased TDD of insulin compared with baseline. A retrospective chart review by Meade et al. [18] seems to be consistent with these observations. Their work studied 66 patients transitioned from U100 to either U500 via CSII or multiple daily injections. HbA1C in patients using U500 in multiple daily injections decreased by 1.8%, whereas patients using U500 CSII experienced an HbA1C decrease of 0.63% after 1 year. The difference in rate of hypoglycemia between BID and DPIP treatment arms was not statistically significant. This finding was also consistent with previously published work on U500 administration via CSII [6, 7].
Four DPIP treatment arm participants demonstrated a clinical pattern of U500 DPIP failure to match participant insulin requirements. A major contributing factor may have been that the DPIP used in the study was limited to only three basal rates of 100, 150, or 200 units per 24 hours. Therefore, participants with basal CSII requirements <100 units per 24 hours could not be successfully treated with DPIP. DPIP participant insulin requirements were also somewhat unpredictable compared with baseline TDD. One participant with a baseline TDD of 245 units successfully completed the study, whereas two other participants with a baseline TDD of 240 units required early withdrawal. Another participant with a baseline TDD of 325 units had two episodes of glucose 65 to 79 mg/dL on U100 delivered via DPIP. An additional contributing factor may have been the pharmacokinetics of U500. U500 has been shown to have a rapid onset of action over 30 minutes and a blunted, prolonged duration over at least 7 hours [19, 20]. We hypothesized a positive correlation between dose and duration of action. More specifically, we hypothesized that a small bolus of U500 via DPIP would provide mealtime glycemic control. Although there is a paucity of literature regarding the effect of dose size on U500 pharmacokinetics, a positive correlation between dose and duration of action has been demonstrated in other insulins. Plank et al. [21] showed insulin detemir has a duration of action equal to 5.7 hours when dosed at 0.1 units per kilogram vs 23.2 when dosed at 1.6 units per kilogram. Contrary to our hypothesis, we observed that DPIP participants tended to have more frequent and larger magnitude weekly protocol-driven insulin dose changes compared with BID participants during their respective treatment courses (Figs. 1–8). Given weekly insulin dose titrations were made in response to poor glycemic control, mealtime bolus delivery via DPIP may have failed to create a physiologic prandial response due to the unique pharmacokinetics of U500.
Finally, the specific DPIP used for this pilot study had logistical and patient education issues. It was designed for use with a proprietary insulin filling device. The filling device was not compatible with a 20-mL U500 insulin vial. Participants therefore required education on a multistep filling procedure using needles and syringe in lieu of the filling device. The U500 insulin pen required less steps, and most study participants had previous experience with use prior to enrollment.
There are several limitations regarding interpretation of these results. These include small sample size, homogeneous study population, and high early withdrawal. Using newly available insulin lispro U200 for use with DPIP instead of U500 may ameliorate the high early withdrawal rate observed in our study. We suggest this as an area of future research.
To our knowledge, this pilot study represents the first prospective research of U500 DPIP. Based on our clinical experience and results, we argue against the general use of U500 DPIP in clinical practice.
Acknowledgments
We thank the Minneapolis Veteran’s Affairs Health Care System, Minnesota Veterans Medical Education and Research Foundation, and military veterans who participated in the study. We acknowledge Valeritas and Eli-Lilly for donating study supplies.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- ACH
Ademolus Classification of Hypoglycemia
- BID
twice daily
- CSII
continuous subcutaneous insulin infusion
- DPIP
disposable insulin patch pump
- HbA1C
hemoglobin A1C
- TDD
total daily dose
References and Notes
- 1. Dailey AM, Gibert JA, Tannock LR. Durability of glycemic control using U-500 insulin. Diabetes Res Clin Pract. 2012;95(3):340–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Eby EL, Zagar AJ, Wang P, Curtis BH, Xie J, Haldane DC, Idris I, Peters AL, Hood RC, Jackson JA. Healthcare costs and adherence associated with human regular U-500 versus high-dose U-100 insulin in patients with diabetes. Endocr Pract. 2014;20(7):663–670. [DOI] [PubMed] [Google Scholar]
- 3. Dailey AM, Williams S, Taneja D, Tannock LR. Clinical efficacy and patient satisfaction with U-500 insulin use. Diabetes Res Clin Pract. 2010;88(3):259–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Granata JA, Nawarskas AD, Resch ND, Vigil JM. Evaluating the effect of u-500 insulin therapy on glycemic control in veterans with type 2 diabetes. Clin Diabetes. 2015;33(1):14–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hood RC, Arakaki RF, Wysham CH, Li YG, Settles JA, Jackson JA. A randomized clinical trial comparing efficacy and safety of 2 titration algorithms for human regular U-500 insulin in severely insulin-resistant patients with type 2 diabetes. In: Endocrine Society's 97th Annual Meeting and Expo; 5 March 2015; San Diego, CA. Abstract PP01-3. [Google Scholar]
- 6. Lane WS, Weinrib SL, Rappaport JM, Przestrzelski T. A prospective trial of U500 insulin delivered by Omnipod in patients with type 2 diabetes mellitus and severe insulin resistance. Endocr Pract. 2010;16(5):778–784. [DOI] [PubMed] [Google Scholar]
- 7. Reutrakul S, Wroblewski K, Brown RL. Clinical use of U-500 regular insulin: review and meta-analysis. J Diabetes Sci Technol. 2012;6(2):412–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bode BW, Sabbah HT, Gross TM, Fredrickson LP, Davidson PC. Diabetes management in the new millennium using insulin pump therapy. Diabetes Metab Res Rev. 2002;18(Suppl 1):S14–S20. [DOI] [PubMed] [Google Scholar]
- 9. Reznik Y, Cohen O, Aronson R, Conget I, Runzis S, Castaneda J, Lee SW; OpT2mise Study Group . Insulin pump treatment compared with multiple daily injections for treatment of type 2 diabetes (OpT2mise): a randomised open-label controlled trial. Lancet. 2014;384(9950):1265–1272. [DOI] [PubMed] [Google Scholar]
- 10. Asche CV, Bode B, Busk AK, Nair SR. The economic and clinical benefits of adequate insulin initiation and intensification in people with type 2 diabetes mellitus. Diabetes Obes Metab. 2011;14(1):47–57. [DOI] [PubMed] [Google Scholar]
- 11. García-Pérez LE, Alvarez M, Dilla T, Gil-Guillén V, Orozco-Beltrán D. Adherence to therapies in patients with type 2 diabetes. Diabetes Ther. 2013;4(2):175–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Peyrot M, Rubin RR, Kruger DF, Travis LB. Correlates of insulin injection omission. Diabetes Care. 2010;33(2):240–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Peyrot M, Barnett AH, Meneghini LF, Schumm-Draeger PM. Factors associated with injection omission/non-adherence in the Global Attitudes of Patients and Physicians in Insulin Therapy study. Diabetes Obes Metab. 2012;14(12):1081–1087. [DOI] [PubMed] [Google Scholar]
- 14. Lajara R, Fetchick DA, Morris TL, Nikkel C. Use of V-Go® insulin delivery device in patients with sub-optimally controlled diabetes mellitus: a retrospective analysis from a large specialized diabetes system. Diabetes Ther. 2015;6(4):531–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kennedy R, Tannock LR. A case report of continuous subcutaneous U-500 insulin administration in a patient with insulin resistant lipodystrophy. AACE Clin Case Rep. 2015;1(1):e45–e48. [Google Scholar]
- 16. Ademolu AB. Role of Ademolus Classification of Hypoglycemia in blood glucose and diabetes mellitus management. Gastroenterol Liver Clin Med Issues. 2017;1:GLCM 102.
- 17. American Diabetes Association Glycemic targets: standards of medical care in diabetes—2018. Diabetes Care. 2017;41(Suppl 1):S55–S64. [DOI] [PubMed] [Google Scholar]
- 18. Meade LT, Tart RC, Nuzum D. Clinical experience with U-500 regular insulin by multiple daily injections and continuous subcutaneous insulin infusion. Diabetes Technol Ther. 2017;19(4):220–225. [DOI] [PubMed] [Google Scholar]
- 19. Davidson MB, Navar MD, Echeverry D, Duran P. U-500 regular insulin: clinical experience and pharmacokinetics in obese, severely insulin-resistant type 2 diabetic patients. Diabetes Care. 2009;33(2):281–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. de la Peña A, Ma X, Reddy S, Ovalle F, Bergenstal RM, Jackson JA. Application of PK/PD modeling and simulation to dosing regimen optimization of high-dose human regular U-500 insulin. J Diabetes Sci Technol. 2014;8(4):821–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Plank J, Bodenlenz M, Sinner F, Magnes C, Görzer E, Regittnig W, Endahl LA, Draeger E, Zdravkovic M, Pieber TR. A double-blind, randomized, dose-response study investigating the pharmacodynamic and pharmacokinetic properties of the long-acting insulin analog detemir. Diabetes Care. 2005;28(5):1107–1112. [DOI] [PubMed] [Google Scholar]