Abstract
Introduction
Inadequate fetal growth has severe consequences for both neonatal and adult development. It is hypothesised that the feto-maternal interface associated with the lightest and male fetuses will undergo more apoptosis and less proliferation than those supplying the closest to mean litter weight (CTMLW) and female fetuses respectively.
Methods
Placental and endometrial samples associated with the lightest and CTMLW (gestational day (GD) 18 and 30), male and female (GD45, 60 and 90) Large White X Landrace conceptuses or fetuses were obtained. The mRNA expression of candidate genes involved in apoptosis or proliferation (BAX, BCL2, P53 and KI67) was quantified by qPCR. TUNEL staining was performed on placental samples supplying the lightest and CTMLW fetuses (GD45 and 60), of both sex (GD60).
Results
Placentas associated with the lightest fetuses had decreased P53 and KI67 expression compared to the CTMLW fetuses at GD45. At GD60, P53 expression was increased in placentas supplying the lightest compared to CTMLW fetuses. P53 expression was increased in endometrial samples associated with the lightest compared to the CTMLW fetuses at GD45. At GD30 and GD60 respectively, BAX expression was increased and BCL2, P53 and KI67 expression were decreased in endometrial samples associated with females compared to their male littermates. TUNEL staining revealed no association between fetal size or sex, and apoptotic cell number.
Discussion
This study has highlighted dynamic associations between fetal size, sex, and apoptosis and proliferation at the porcine feto-maternal interface. Further studies should be performed to improve the understanding of the mechanisms behind these findings.
Keywords: Placenta, Endometrium, Porcine, Apoptosis, Sexual dimorphism, Intrauterine growth restriction (IUGR)
Highlights
-
•
Gestational day influence feto-maternal interface apoptotic mRNA expression.
-
•
Fetal size is associated with feto-maternal interface apoptotic mRNA expression.
-
•
Sexual dimorphism exists in feto-maternal interface apoptotic mRNA expression.
1. Introduction
The establishment and maintenance of pregnancy requires significant changes in uterine structure and function, including the formation of new blood vessels in both the endometrium and placenta. Apoptosis can occur by activation of either extrinsic or intrinsic pathways [1]. The intrinsic apoptosis pathway is tightly regulated by pro- and anti-apoptotic members of the B-cell lymphoma 2 (BCL2) family of proteins [1]. In response to cell stressors, tumour suppressor protein 53 (P53) is upregulated which initiates the intrinsic pathway.
Apoptosis has been described in the human placenta where it is essential for trophoblast invasion, differentiation and survival [[2], [3], [4], [5], [6], [7], [8], [9]]. Similarly, apoptosis and proliferation have been suggested to play a role in implantation in the pig [[10], [11], [12], [13]]. As pigs exhibit non-invasive epitheliochorionic placentation, extensive remodelling must occur at the porcine feto-maternal interface to ensure that adequate nutrient transfer can occur to meet fetal demands [14]. This remodelling is likely to involve extensive apoptosis and proliferation. Cristofolini et al. [15], demonstrated that apoptotic cells are present in the porcine placenta throughout gestation and that the number of apoptotic cells relative to the total cell number in placental villi was associated with gestational day (GD). A high number of apoptotic cells was observed at GD28, presumably reflecting the extensive remodelling that occurs at the feto-maternal interface during placentation. They also suggested that BCL-2-associated X protein (BAX) is expressed at the feto-maternal interface in early and late gestation, which would provide further evidence for the activation of the intrinsic apoptotic pathway.
Appropriately regulated proliferation is essential for the establishment and maintenance of a successful pregnancy. Several factors known to be expressed at the feto-maternal interface during implantation have been shown to activate Phosphatidylinositol-3 kinase (PI3K) - protein kinase B (AKT) and Mitogen-activated Protein Kinase (MAPK) signaling pathways to induce proliferation and/or migration of porcine trophectoderm cells in vitro [reviewed by Ref. [16]]; highlighting the role of proliferation in pregnancy establishment in the pig.
Dysregulated apoptosis has been linked to pregnancy complications including gestational trophoblast disease [17,18], preeclampsia [[19], [20], [21], [22], [23]] and intrauterine growth restriction (IUGR) [21,[24], [25], [26], [27], [28], [29], [30]]. Placentas associated with term IUGR human infants have increased apoptosis compared to those supplying normally-grown infants [27,31]. Decreased placental BCL2 expression [29,30], accompanied by increased BAX [29] expression has been observed in term IUGR placentas compared to those supplying normally-grown infants. In the pig, it has been suggested on a protein level that components of the proliferation pathway are downregulated, accompanied by increased apoptotic stress in placentas associated with IUGR fetuses compared to normal-body weight fetuses at GD60, 90 and 110 [32].
Recent studies have revealed sexual dimorphism in human placentas [33,34], with fetal sex influencing the expression of placental genes and the inflammatory response [35,36]. Intriguingly, sexual dimorphism has been demonstrated in P53 knockout mice, with decreased implantation rate, pregnancy rate and litter size observed when matings were carried out using female P53 null mice, but not with male P53 null mice [37]. On occasions where human pregnancy is complicated by preeclampsia and IUGR, male offspring have increased perinatal mortality and morbidity [36,38]. Although it is proposed that male new-born piglets have a survival disadvantage compared to their female littermates [39], the possibility of sexual dimorphism in porcine placental development is poorly understood. However, recent investigations in our laboratory have revealed striking relationships between fetal sex and both placental and endometrial vascularity [40].
This study aimed to improve the understanding of the relationship between fetal size, sex and apoptosis and proliferation at the porcine feto-maternal interface. It is hypothesised that IUGR in the pig occurs due to aberrant conceptus attachment. Specifically, it is hypothesised that apoptosis and proliferation pathways will be up- and down-regulated respectively in the feto-maternal interface associated with the lightest compared to the closest to mean litter weight (CTMLW) conceptus or fetus and that the feto-maternal interface associated with male fetuses will have increased apoptosis and decreased proliferation compared to those supplying female fetuses throughout gestation.
2. Materials and methods
All procedures were performed with approval from The Roslin Institute (University of Edinburgh) Animal Welfare and Ethical Review Board and in accordance with the U.K. Animals (Scientific Procedures) Act, 1986.
2.1. Experimental animals and sample collection
Large White X Landrace gilts (age 11–14 months; n = 31) were observed daily for signs of oestrus and were housed in groups of 6–8 animals per pen. Oestrous cyclicity and ovarian function were controlled in accordance with routine normal practice at The Roslin Institute Large Animal Unit. In a subset of gilts (distribution between the GD investigated indicated in Supplementary Table 1) oestrus was synchronised by daily feeding of 20 mg Altrenogest (Regumate, Hoechst Roussel Vet Ltd., Milton Keynes, U.K.) for 18 days followed by injection of pregnant mare serum gonadotrophin (PMSG, Intervet UK Ltd, Milton Keynes, U.K.) and human chorionic gonadotrophin (hCG; Intervet UK Ltd, Milton Keynes, U.K.) [41]. All gilts were inseminated twice daily for the duration of oestrus with semen from one of four sires (Large White). The sires used were equally distributed throughout the GD to attempt to minimise the effect of sire. The first day of insemination was assigned as GD0 and samples were obtained at GD18, 30, 45, 60 and 90 (n = 5, 6, 6, 11, and 8 respectively). Gilts were euthanised at the GD of interest with sodium pentobarbitone 20% w/v (Henry Schein Animal Health, Dumfries, U.K.) at a dose of 0.4 ml/kg by intravenous injection via a cannula inserted in the ear vein. Following confirmation of death, mid-ventral incision revealed the reproductive tract. The tract was lifted from the body cavity and placed in a dissecting tray. Both uterine horns were dissected from the ovary towards the cervix.
At GD18, the uterine tract was rinsed with saline and pieces of string were used to tie the end of the right and left uterine horns at the bifurcation. The uterine horns were cut between the two pieces of string and each uterine horn was placed in a floatation device containing a solution to preserve the integrity of the RNA. This solution was prepared by dissolving 700 g ammonium sulphate (SLS, Nottingham, U.K.) in 935 ml of RNase free water. Once dissolved, 25 ml of 1 M sodium citrate (Fisher Scientific, Loughborough, U.K.) and 40 ml of 0.5 M ethylenediaminetetraacetic acid (EDTA) were added, and the solution was adjusted to pH 5.2 using concentrated sulphuric acid. Using dissection scissors, the uterine horn was opened along the mesometrial side, and the conceptuses floated upwards in the solution. The conceptuses were removed and weighed in a cryovial (Starlab, Milton Keynes, U.K.). The uterine lumen was occluded between each conceptus to ensure that endometrial samples associated with individual conceptuses could be identified. The lightest and CTMLW conceptus was identified based on weight, and endometrial samples were taken from each conceptus of interest. Samples were snap-frozen in liquid nitrogen and stored at −80 °C for RNA extraction or fixed in Bouin's (Sigma Aldrich, St Louis, Missouri, U.S.A.) for histology.
On the remaining GD investigated, the uterine lumen was occluded between each feto-placental unit by tying with string to ensure that tissues associated with individual conceptuses or fetuses could be identified later. Fetuses were identified as ‘live’ or ‘dead’ based on their morphology at the time of dissection and were weighed. At GD45, 60 and 90, sex was determined morphologically. DNA was isolated from the GD30 fetuses using the DNeasy Blood and Tissue DNA extraction kit (Qiagen, Manchester, U.K.), and PCR was performed for the sex-determining region Y (SRY) region of the Y chromosome [42]. The lightest and CTMLW fetus (GD30), of both sex (GD45, 60 and 90) were identified based on fetal weight. From the anti-mesometrial side, placental and endometrial samples were taken from each feto-placental unit of interest and snap-frozen in liquid nitrogen or fixed in Bouin's (Sigma Aldrich).
2.2. Analysis of candidate gene expression by qPCR
2.2.1. Total RNA extraction and cDNA synthesis
RNA was extracted from 20 to 50 μg of tissue from snap-frozen samples as previously described [43], with the addition of a DNase treatment step (RNase-free DNase, Qiagen, Manchester, U.K.). The RNA was quantified spectrophotometrically using a Nanodrop ND-1000 (Labtech International Ltd., Heathfield, U.K.), and the quality assessed electrophoretically using a Tapestation 2200 (Agilent Technologies, Edinburgh, U.K.) (Supplementary Table 2). If the RINe value obtained was lower than the desired ranges (Supplementary Table 2), the sample was excluded from the analyses.
Complementary DNA (cDNA) was prepared from 0.3 μg of RNA with SuperScript III reverse transcriptase (Life Technologies, ThermoFisher Scientific, Altrincham, U.K.). Each reaction contained 250 ng random primers (Promega, Southampton, U.K.) and 40 units RNaseIn (Promega, Southampton, U.K.). Negative controls without reverse transcriptase were included to check for genomic contamination. Reverse transcription was performed in duplicate for each sample and pooled.
2.2.2. Relative expression of candidate genes
Quantitative PCR was performed on a Stratagene MX3000 instrument using Platinum SYBR Green SuperMixUTG (Life Technologies, ThermoFisher Scientific, Altrincham, U.K.) using cDNA from placental samples at GD30, 45, 60 and 90, and endometrial samples at GD18, 30, 45, 60 and 90 (n = 5, 6, 6, 6 and 8 litters respectively). The samples were associated with the lightest and CTMLW conceptuses or fetuses at GD18 and 30, and the lightest and CTMLW fetuses of both sex at GD45, 60 and 90. The final concentrations of magnesium, ROX reference dye and each primer were 3 mM, 50 nM and 400 nM respectively in a 25 μl reaction volume. All qPCRs were carried out at an annealing temperature of 60 °C and dissociation curves consisting of single peaks were generated. The mRNA expression of BAX, BCL2, P53 and KI67 was quantified. In addition, the ratio of BAX:BCL2 expression was calculated. The reference genes hypoxanthine phosphoribosyl-transferase 1 (HPRT1) and TATA box binding protein 1 (TBP1), and TBP1 and YWHAZ (Tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, zeta polypeptide) were utilised to normalise placental and endometrial gene expression respectively. These reference genes were identified as having stable expression in the samples by analysis of eleven candidate reference genes [44,45] using geNORM V3.5 (Ghent University Hospital, Centre for Medical Genetics). Primer sequences are detailed in Supplementary Table 3.
Serial dilutions of pooled cDNA ranging from 1:5 to 1:640 in nuclease-free water were used as standards. Sample cDNA was diluted 1:25 and 5 μl of sample, standard or control were added per well. Each plate contained duplicate wells of a no template control, standards, sample cDNA and reverse transcriptase blanks. Data were analysed using qbase + software V3.0 (Biogazelle, Zwijnaarde, Belgium). A target and run specific strategy was employed and the results, normalised to the two reference genes, were scaled to the minimum sample. The mean slope, intercept, PCR efficiency and R2 values are detailed in Supplementary Table 4.
2.2.3. TUNEL staining of GD45 and 60 placentas
Placental samples supplying the overall lightest fetus compared with the overall CTMLW fetus (hereafter referred to as true lightest and true CTMLW) at GD45 (n = 4 litters (all male fetuses)) and GD60 (n = 6 litters (equal numbers of fetuses of both sex)) were used. These GD were investigated due to the dynamic changes in gene expression observed. The samples were fixed with Bouin's overnight at room temperature (RT) and changed daily for ∼1 week in 70% ethanol (Genta Medical, York, U.K.). The samples were placed in histological cassettes and dehydrated by passing through graded ethanol and xylene (Genta Medical, York, U.K.), before being embedded in paraffin wax and sectioned at 5 μm thickness. The TUNEL assay was performed using the ApopTag plus Peroxidase In Situ Apoptosis Detection Kit (S7101, Merck Millipore, Cork, Ireland). A slide of normal female rat mammary gland 3–5 days post-weaning was used as a positive control. As a negative control, CTMLW female GD45 and GD60 placental sections were treated with PBS instead of TDT enzyme.
The slides were imaged using the NanoZoomer slide scanner (Hamamatsu, Welwyn Garden City, U.K.). The placental stromal and the chorioallantoic membrane (CAM) regions were analysed separately, with 6 images taken of both regions at ×20 magnification from 2 sections per sample from the slide scans. For the stromal image analysis, all cells were counted in each image and the data were expressed as the number of positive cells per total number of cells counted. As the images were all taken at the same magnification, they occupied the same area. For the CAM, the positively stained cells in the CAM region were counted and the area of the CAM region in each image was measured. This was then expressed as the number of positively stained cells per 10,000 μm2 to allow direct comparison between samples.
2.3. Statistical analysis
All statistical analyses were performed using Minitab 17 (Pennsylvania, U.S.A.) or GenStat 13.1 (VSN International Ltd., Oxford, U.K.). Throughout the mean value was taken for each sample and the normality of the distribution of the data was assessed by an Anderson-Darling test. If a P value of ≤0.05 was obtained, then the data were not considered to have a normal distribution. Outliers were tested for using a Grubbs outlier test and were excluded systematically, with normality within each group being reassessed following each exclusion. Log10 transformations were carried out where appropriate to improve the normality of the distribution of the data. Fetal size was compared for the true lightest and true CTMLW, and fetal sex was compared using samples from the true lightest and true CTMLW fetuses at GD30, and the lightest and CTMLW fetuses of both sex at GD45, 60 and 90. Where data had a normal distribution, ANOVA for GD, fetal size or sex was performed, with a block for gilt to account for the common maternal environment. A post-hoc Tukey test was performed where appropriate. Where data did not have a normal distribution, Kruskal-Wallis and Mann Whitney tests were performed.
3. Results
There were significant temporal changes in the placental expression of BAX (P ≤ 0.001, Table 1) and BCL2 (P ≤ 0.001; Table 1) throughout gestation, with the lowest expression observed at GD30, the highest expression at GD45 and 90, and an intermediate expression level observed at GD60. No significant temporal changes in placental BAX:BCL2 ratio were observed (Table 1). P53 expression was decreased at GD30 compared to GD45 and 90 (P ≤ 0.01; Table 1). KI67 expression showed a moderate fluctuation throughout gestation (P ≤ 0.05; Table 1), with a statistically significant decrease in expression observed between GD45 and 60.
Table 1.
Temporal Changes in both Placental and Endometrial mRNA Expression were observed.
| Gene | GD18 | GD30 | GD45 | GD60 | GD90 | P Value |
|---|---|---|---|---|---|---|
| Relative Placental mRNA Expression (Mean ± S.E.M.) | ||||||
| BAX | n/a | 1.563 ± 0.186a | 2.891 ± 0.116 b | 2.149 ± 0.202c | 2.764 ± 0.193 b | P ≤ 0.001 |
| BCL2 | n/a | 6.495 ± 1.198a | 22.071 ± 3.254bc | 15.305 ± 2.609bd | 26.433 ± 4.539b | P ≤ 0.001 |
| BAX:BCL2 Ratio | n/a | 0.327 ± 0.068 | 0.190 ± 0.026 | 0.178 ± 0.015 | 0.189 ± 0.039 | ns |
| P53 | n/a | 2.126 ± 0.169a | 3.908 ± 0.145b | 3.213 ± 0.229bc | 3.081 ± 0.282c | P ≤ 0.01 |
| KI67 | n/a | 2.134 ± 0.182ab | 2.700 ± 0.140a | 1.945 ± 0.180b | 2.207 ± 0.206ab | P ≤ 0.05 |
| Relative Endometrial mRNA Expression (Mean ± S.E.M.) | ||||||
| BAX | 4.079 ± 0.634 | 4.298 ± 0.217 | 4.665 ± 0.446 | 3.812 ± 0.385 | 3.450 ± 0.863 | ns |
| BCL2 | 6.639 ± 0.640a | 2.930 ± 0.379b | 3.569 ± 0.460b | 3.630 ± 0.419b | 2.592 ± 0.871b | P ≤ 0.001 |
| BAX:BCL2 Ratio | 0.636 ± 0.093a | 1.670 ± 0.197b | 1.506 ± 0.156b | 1.141 ± 0.110ab | 1.354 ± 0.922b | P ≤ 0.05 |
| P53 | 6.511 ± 0.727a | 4.888 ± 0.560ab | 4.069 ± 0.411b | 4.161 ± 0.396b | 3.268 ± 0.869b | P ≤ 0.01 |
| KI67 | 2.408 ± 0.257 | 2.654 ± 0.226 | 2.264 ± 0.211 | 2.335 ± 0.190 | 1.958 ± 0.898 | ns |
P value indicates overall temporal change detected by ANOVA with a block for gilt or Kruskal-Wallis. Differing superscript letters indicate that group means differ from one another. n = 10–23 samples per group. ns=not significant.
No temporal changes in endometrial expression of BAX or KI67 (Table 1) were observed. Increased BCL2 expression was observed in GD18 endometrial samples compared to the other GD investigated (ANOVA P ≤ 0.001; Table 1). The BAX:BCL2 ratio was decreased at GD18 compared to GD30, 45 and 90 (ANOVA P ≤ 0.05; Table 1). Endometrial P53 expression decreased with advancing gestation (ANOVA P ≤ 0.01; Table 1), with a statistically significant decrease observed between GD18 and 45.
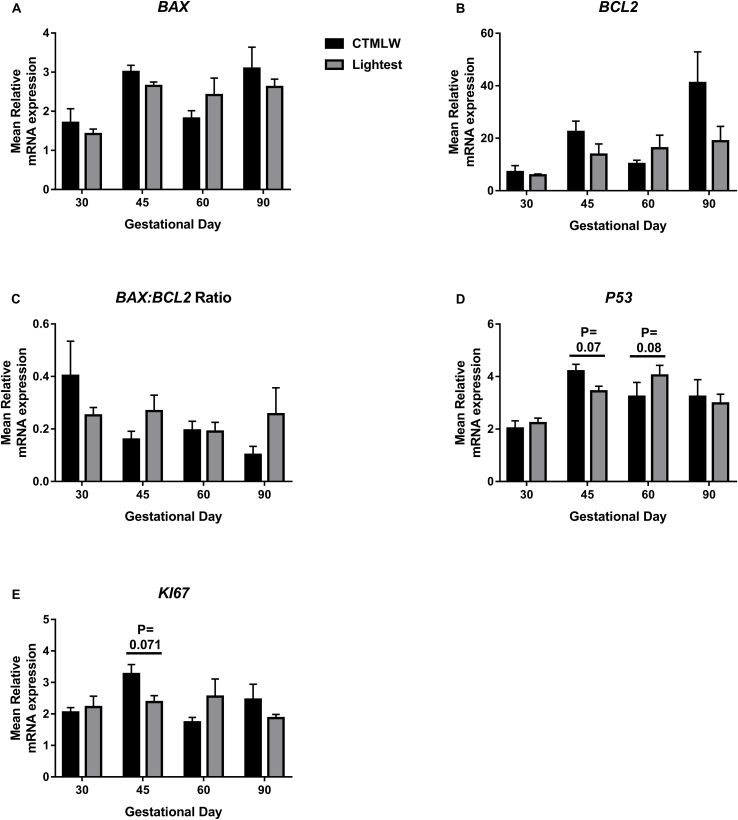
No association between fetal size and the placental expression of BAX, BCL2, or the BAX:BCL2 ratio was observed (Fig. 1A–C). P53 expression was decreased in placental samples associated with the lightest fetuses compared to the CTMLW fetuses at GD45 (ANOVA P = 0.07; Fig. 1D). Intriguingly, the direction of this difference switched at GD60 (ANOVA P = 0.08), with placentas associated with the lightest fetuses having increased P53 expression compared to the CTMLW fetuses. At GD45, the expression of KI67 was also decreased in placentas supplying the lightest fetuses compared to the CTMLW fetuses (ANOVA P = 0.07; Fig. 1E).
Fig. 1.
BAX, BCL2, BAX:BCL2 Ratio, P53 and KI67 mRNA expression in placental tissues associated with the lightest and CTMLW fetuses on days 30, 45, 60 and 90 of pregnancy. No relationship between fetal size and the placental expression of BAX (A), BCL2 (B), or the BAX:BCL2 ratio (C) were observed. D: The expression of P53 was decreased in placental samples associated with the lightest fetuses compared to the closest to mean litter weight (CTMLW) fetuses at gestational day (GD) 45 (P = 0.07). The direction of this difference switched at GD60 (P = 0.08), with placentas associated with the lightest fetuses having increased P53 expression compared to the CTMLW fetuses. E: At GD45, the expression of KI67 was decreased in placentas supplying the lightest fetuses compared to the CTMLW fetuses ( P = 0.071). Error bars represent S.E.M. n = 4–7 samples per group.
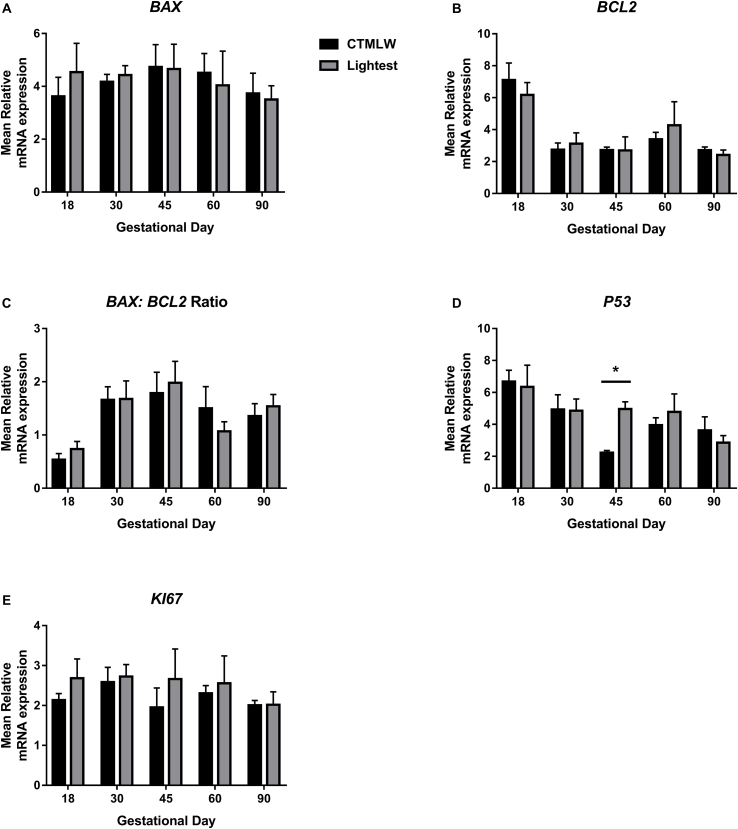
No association between fetal size and endometrial expression of BAX (Fig. 2A), BCL2 (Fig. 2B), BAX:BCL2 Ratio (Fig. 2C) or KI67 (Fig. 2E) was observed. Increased P53 expression was observed in endometrial samples supplying the lightest fetuses compared to the CTMLW fetuses at GD45 (P ≤ 0.05; Fig. 2D).
Fig. 2.
BAX, BCL2, BAX:BCL2 Ratio, P53 and KI67 mRNA expression in endometrial tissues associated with the lightest and CTMLW fetuses on days 18, 30, 45, 60 and 90 of pregnancy. No association between conceptus or fetal size and endometrial expression of BAX (A), BCL2 (B), BAX:BCL2 Ratio (C), or KI67 (E) were observed. D: The expression of P53 was increased in endometrial samples supplying the lightest fetuses compared to the closest to mean litter weight (CTMLW) fetuses at gestational day (GD) 45. Error bars represent S.E.M. *P ≤ 0.05. n = 4–6 samples per group.
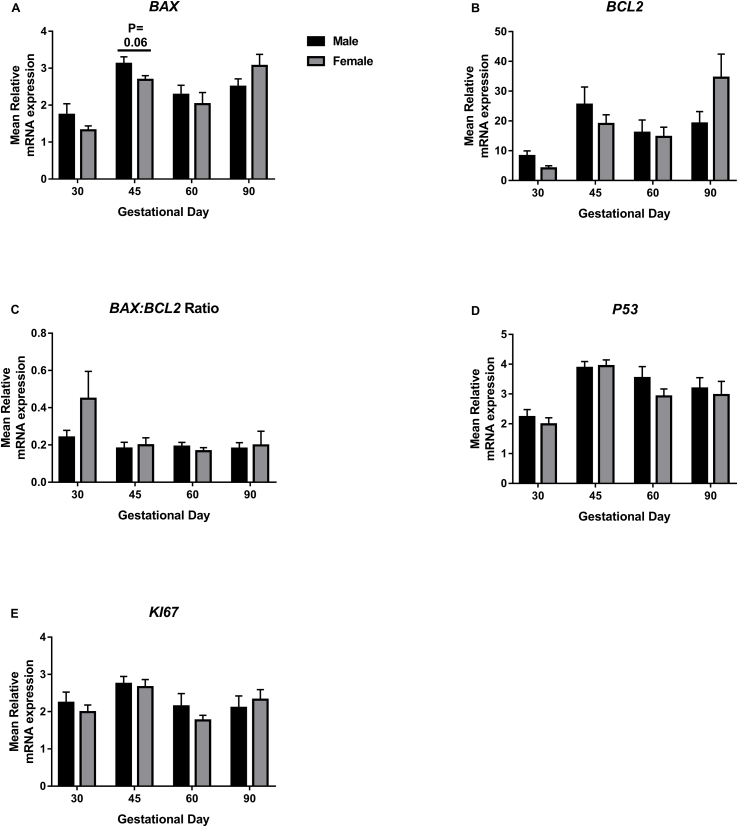
A trend towards decreased BAX expression in placental samples associated with female fetuses compared to their male littermates was observed at GD45 (P = 0.06; Fig. 3A). No statistically significant associations between fetal sex and placental BCL2 (Fig. 3B), BAX:BCL2 Ratio (Fig. 3C), P53 (Fig. 3D) or KI67 (Fig. 3E) expression were observed.
Fig. 3.
BAX, BCL2, BAX:BCL2 Ratio, P53 and KI67 mRNA expression in placental tissues associated with male and female fetuses on days 30, 45, 60 and 90 of pregnancy. A: A trend towards decreased BAX expression in placental samples associated with female fetuses compared to their male littermates was observed at GD45 (P = 0.06). No statistically significant associations between fetal sex and placental BCL2 (B), BAX:BCL2 Ratio (C), P53 (D) or KI67 (E) expression were observed. Error bars represent S.E.M. n = 5–12 samples per group.
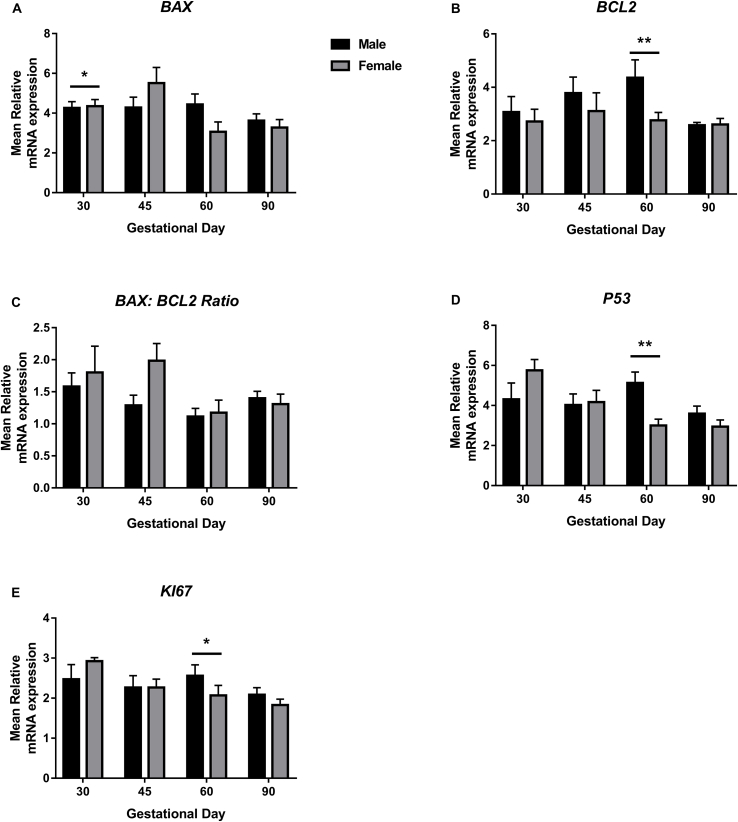
BAX expression was increased in endometrial samples supplying female fetuses compared to those supplying male fetuses at GD30 (P ≤ 0.05; Fig. 4A). Endometrial samples supplying female fetuses had decreased BCL2 expression compared to those supplying male fetuses at GD60 (P ≤ 0.001; Fig. 4B). Endometrial P53 (P ≤ 0.01; Figure 4D) and KI67 (P ≤ 0.05; Fig. 4E) expression were decreased in samples supplying female fetuses compared to those supplying male fetuses at GD60.
Fig. 4.
BAX, BCL2, BAX:BCL2 Ratio, P53 and KI67 mRNA expression in endometrial tissues associated with male and female fetuses on days 30, 45, 60 and 90 of pregnancy. A: BAX expression was increased in endometrial samples supplying female fetuses compared to those supplying male fetuses at GD30. B: Endometrial samples supplying female fetuses had decreased BCL2 expression compared to those supplying male fetuses at GD60. C: The BAX:BCL2 ratio was not associated with fetal sex. P53 (D) and KI67 (E) expression were decreased in endometrial samples supplying female fetuses compared to those supplying male fetuses at GD60. Error bars represent S.E.M. *P ≤ 0.05. **P ≤ 0.01. n = 4–12 samples per group.
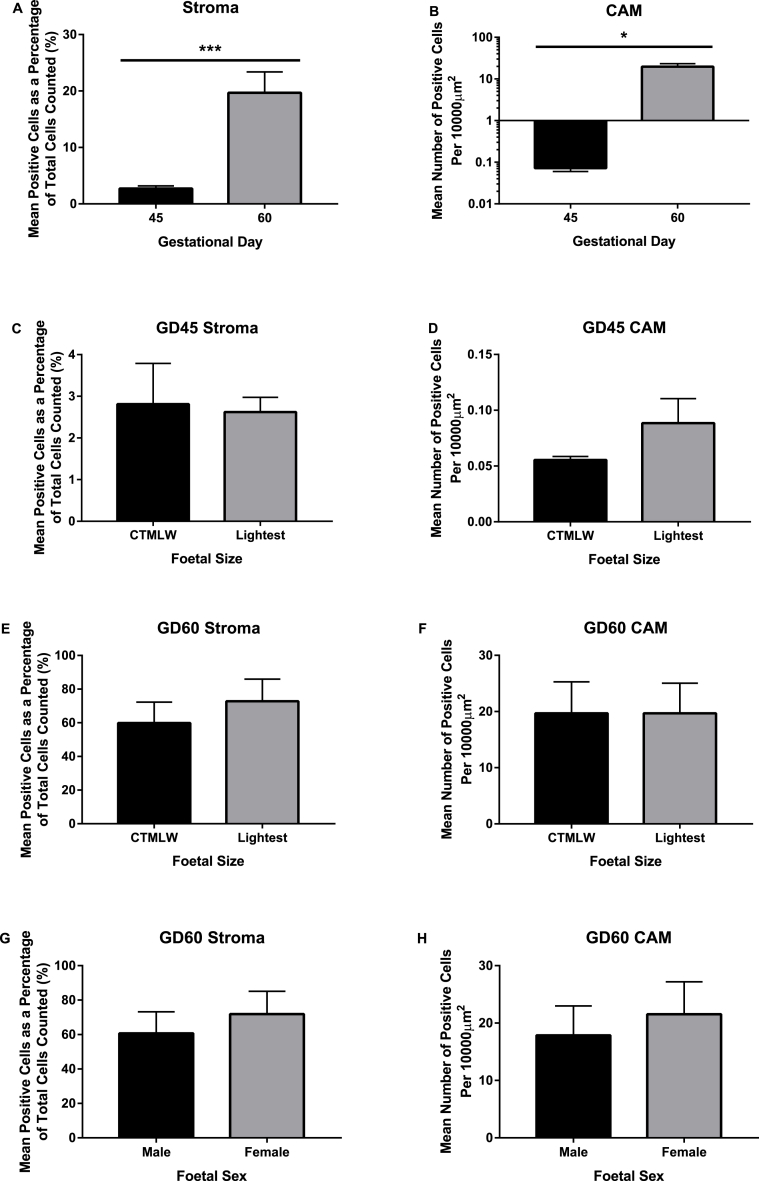
More TUNEL stained cells, indicating increased apoptosis, were observed in both the stroma (P ≤ 0.001; Fig. 5A; Supplementary Figure 1) and CAM (P ≤ 0.05; Fig. 5B; Supplementary Figure 1) of GD60 placentas compared to GD45 placentas.
Fig. 5.
TUNEL staining of placentas supplying fetuses of different size and sex at days 45 and 60 of pregnancy. An increased number of TUNEL stained cells, indicating an increase in placental apoptosis, were observed in both the stroma (P ≤ 0.001; A) and CAM (P ≤ 0.05; B) at gestational day (GD) 60 (n = 12) compared to GD45 (n = 8). No statistically significant relationships were observed between the number of TUNEL stained cells and fetal size in the placental stroma (C and E) or CAM (D and F) at GD45 (n = 8) or 60 (n = 12). No statistically significant associations were observed between fetal sex and TUNEL stained in the stroma or CAM at GD60 (n = 12) (G and H). Error bars represent the S.E.M.
No statistically significant relationships were observed between the number of TUNEL stained cells and fetal size, in the placental stroma or CAM at either GD investigated (Fig. 5C–F; Supplementary Figure 1).
No statistically significant associations were observed between fetal sex and TUNEL staining in the stroma or CAM at GD60 (Fig. 5G and H; Supplementary Figure 1).
4. Discussion
The experiments described in this study have highlighted novel relationships between fetal size, and more intriguingly fetal sex, and apoptosis and proliferation at the porcine feto-maternal interface.
The current study has reported temporal changes in mRNA expression in both the placenta and endometrium, accompanied by temporal changes in placental TUNEL staining. Intriguingly, different temporal profiles in mRNA expression were observed in the two tissues, reflecting the dynamic nature of the porcine feto-maternal interface and the differences in function of the two tissues. The significant decrease in placental KI67 expression observed between GD45 and 60 could reflect the change in the relationship between the placenta and the developing fetus that occurs at this stage of pregnancy. At GD45, placental growth occurs at a greater rate than fetal growth whereas at GD60, placental growth begins to plateau whilst the fetus undergoes exponential growth [[46], [47], [48], [49]]. Therefore, the observed decrease in cell proliferation, and increase in TUNEL staining observed at this GD may reflect the decrease in placental growth rate. It has previously been suggested that porcine endometrial samples have high expression of both anti- and pro-apoptotic, and proliferation associated genes at GD14 [10]. This, in combination with the observed high endometrial expression of P53 and BCL2 at GD18 in this study, suggests tight regulation of apoptosis at the feto-maternal interface during implantation. Further, the temporal and tissue-specific changes in P53 expression may be explained by the significant remodelling of the feto-maternal interface to meet increasing fetal demand [14].
Aberrant regulation of apoptosis has been heavily implicated in human IUGR. In the current study, placental KI67 expression was decreased, suggesting decreased proliferation, in samples associated with the lightest fetuses compared to the CTMLW at GD45. This supports the work published by Chen et al. [32], which suggested that components of the proliferation pathway were downregulated on a protein level in porcine placental and endometrial samples associated with IUGR fetuses compared to those associated with normal birth weight (NBW) fetuses at GD60, 90, and 110.
The increased expression of P53 observed in GD60 placentas associated with the lightest fetuses compared to those supplying the CTMLW fetuses reinforces the findings of Chen et al. [32], who demonstrated increased apoptotic stress in placentas associated with IUGR fetuses compared to NBW fetuses at GD60, 90 and 110. In contrast, at GD45, placental and endometrial P53 expression were decreased in samples associated with the lightest fetuses compared to the CTMLW fetuses. At GD45, placental growth occurs at a greater rate than fetal growth however, at GD60 placental growth begins to plateau whilst the fetus undergoes exponential growth. This period of development is likely to be highly dynamic which may reflect the changes in gene expression observed between GD45 and 60. In the current study, fetal size was not found to be associated with placental TUNEL staining at GD45 and 60, although a modest sample size was used to investigate this. Temporal changes in the activation of intrinsic and extrinsic apoptosis have been suggested at the porcine feto-maternal interface [15,50]. Considering this, further assessment of components of both the intrinsic and extrinsic pathway should be performed to ensure that placentas supplying the lightest fetuses are utilising the same programmed cell death pathway as those supplying the CTMLW fetuses.
Whilst temporal changes in the activation of intrinsic and extrinsic apoptosis have been suggested at the porcine feto-maternal interface [15,50], additional forms of cell death [51,52] such as necroptosis, which has been implicated in human IUGR [53], have not yet been investigated and may further the understanding of the mechanisms which regulate remodelling of the porcine feto-maternal interface.
Of particular interest, were relationships between placental and endometrial gene expression and fetal sex. The expression of the pro-apoptotic gene BAX was increased in endometrial samples supplying female fetuses compared to those supplying male fetuses at GD30. Interestingly, this was not observed in the placenta, where instead a trend towards decreased BAX expression in placental samples associated with female fetuses compared to their male littermates was observed at GD45. This intriguing finding may be explained by differential early signaling events between conceptuses of different sex and the endometrium, which should be investigated further.
In contrast, at GD60 endometrial samples supplying female fetuses had decreased expression of BCL2, P53, and KI67 compared to those supplying male fetuses. In this study males were heavier than their female littermates throughout gestation (data not presented) and could therefore place an increased demand on the placenta for nutrients. It could be hypothesised that increased remodelling of the feto-maternal interface is required to accommodate the increasing demands being placed on placentas supplying male fetuses compared to those supplying female fetuses. This would require increased apoptosis and proliferation, reflecting the relationship between fetal sex and gene expression illustrated in this study.
To date, there have been limited investigations into the relationship between fetal sex and placental and endometrial expression of apoptosis and proliferation related genes. Fetal sex was not found to influence BCL2 or BAX protein staining in term human placentas [54]. Although, in instances of preeclampsia, it has been demonstrated that placentas supplying male fetuses have increased apoptotic activity, with a greater number of TUNEL positive cells, and increased expression of PUMA and BAX than placentas supplying females [38]. In the current study, fetal sex was not associated with placental TUNEL staining at GD60, although a modest sample size was utilised. Given the considerable temporal changes in gene expression patterns observed, further analyses utilising an increased number of samples on both placental and endometrial samples at additional GDs would provide further insights into the relationship between fetal sex and cell death at the porcine feto-maternal interface.
This study has highlighted novel relationships between fetal size, and more intriguingly fetal sex, and placental and endometrial apoptosis and proliferation. It is hoped that further investigation into the roles of these processes at the feto-maternal interface will advance understanding of the mechanisms governing fetal growth.
Conflicts of interest
The authors have no conflicts of interest to declare.
Funding
The Roslin Institute receives Institute Strategic Grant funding from the BBSRC (BB/J004316/1). CS received a studentship from the University of Edinburgh.
Author contributions
CS and CJA designed the experiments described in this study. All authors were involved in the sample collection process. COH provided innovative technical advice and support. CS performed all experiments, analyses and prepared the manuscript with assistance from CJA. All authors approved the final manuscript.
Acknowledgements
We thank the staff of The Roslin Institute Large Animal Unit for skilled assistance, Chris Stenhouse for creating the conceptus floatation device and Dr Darren Shaw for statistical advice.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.placenta.2018.08.006.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- 1.Cory S., Adams J.M. The BCL2 family: regulators of the cellular life-or-death switch. Nat. Rev. Canc. 2002;2:647–656. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- 2.Straszewski-Chavez S.L., Abrahams V.M., Mor G. The role of apoptosis in the regulation of trophoblast survival and differentiation during pregnancy. Endocr. Rev. 2005;26:877–897. doi: 10.1210/er.2005-0003. [DOI] [PubMed] [Google Scholar]
- 3.Mayhew T.M., Leach L., McGee R., Wan Ismail W., Myklebust R., Lammiman M.J. Proliferation, differentiation and apoptosis in villous trophoblast at 13-41 Weeks of gestation (including observations on annulate lamellae and nuclear pore complexes) Placenta. 1999;20:407–422. doi: 10.1053/plac.1999.0399. [DOI] [PubMed] [Google Scholar]
- 4.Marzusch K., Ruckh P., Hornyb P., Dietl J., Kaiserling E. Short Communication : expression of the p53 tumour suppressor gene in human placenta: an immunohistochemical study. Placenta. 1995;16:101–104. doi: 10.1016/0143-4004(95)90086-1. [DOI] [PubMed] [Google Scholar]
- 5.Levy R., Smith S.D., Chandler K., Sadovsky Y., Nelson D.M. Apoptosis in human cultured trophoblasts is enhanced by hypoxia and diminished by epidermal growth factor. Am. J. Physiol. Cell Physiol. 2000;278:C982–C988. doi: 10.1152/ajpcell.2000.278.5.C982. [DOI] [PubMed] [Google Scholar]
- 6.Kang H.J., Rosenwaks Z. P53 and reproduction. Fertil. Steril. 2018;109:39–43. doi: 10.1016/j.fertnstert.2017.11.026. [DOI] [PubMed] [Google Scholar]
- 7.Huppertz B., Kadyrov M., Kingdom J.C.P. Apoptosis and its role in the trophoblast. Am. J. Obstet. Gynecol. 2006;195:29–39. doi: 10.1016/j.ajog.2005.07.039. [DOI] [PubMed] [Google Scholar]
- 8.Huppertz B., Kaufmann P. The apoptosis cascade in human villous trophoblast. Trophobl. Res. 1999;13:215–242. [Google Scholar]
- 9.Heazell A.E.P., Crocker I.P. Live and let die - regulation of villous trophoblast apoptosis in normal and abnormal pregnancies. Placenta. 2008;29:772–783. doi: 10.1016/j.placenta.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 10.Samborski A., Graf A., Krebs S., Kessler B., Bauersachs S. Deep sequencing of the porcine endometrial transcriptome on day 14 of pregnancy. Biol. Reprod. 2013;88:1–13. doi: 10.1095/biolreprod.113.107870. [DOI] [PubMed] [Google Scholar]
- 11.Østrup E., Bauersachs S., Blum H., Wolf E., Hyttel P. Differential endometrial gene expression in pregnant and nonpregnant sows. Biol. Reprod. 2010;83:277–285. doi: 10.1095/biolreprod.109.082321. [DOI] [PubMed] [Google Scholar]
- 12.Samborski A., Graf A., Krebs S., Kessler B., Reichenbach M., Reichenbach H.D., Ulbrich S.E., Bauersachs S. Transcriptome changes in the porcine endometrium during the preattachment phase. Biol. Reprod. 2013;89:1–16. doi: 10.1095/biolreprod.113.112177. [DOI] [PubMed] [Google Scholar]
- 13.Kiewisz J., Krawczynski K., Lisowski P., Blitek A., Zwierzchowski L., Ziecik A.J., Kaczmarek M.M. Global gene expression profiling of porcine endometria on Days 12 and 16 of the estrous cycle and pregnancy. Theriogenology. 2014;82:897–909. doi: 10.1016/j.theriogenology.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 14.Vallet J.L., Freking B.A. Differences in placental structure during gestation associated with large and small pig fetuses. J. Anim. Sci. 2007;85:3267–3275. doi: 10.2527/jas.2007-0368. [DOI] [PubMed] [Google Scholar]
- 15.Cristofolini A., Sanchis G., Moliva M., Alonso L., Chanique A., Koncurat M., Merkis C. Cellular remodelling by apoptosis during porcine placentation. Reprod. Domest. Anim. 2013;48:584–590. doi: 10.1111/rda.12130. [DOI] [PubMed] [Google Scholar]
- 16.Jeong W., Song G. EGF, IGF-I, VEGF and CSF2: effects on trophectoderm of porcine conceptus. Reprod. Dev. Biol. 2014;38:21–34. [Google Scholar]
- 17.Chiu P.M., Ngan Y.S., Khoo U.S., Cheung A.N.Y. Apoptotic activity in gestational trophoblastic disease correlates with clinical outcome: assessment by the caspase-related M30 CytoDeath antibody. Histopathology. 2001;38:243–249. doi: 10.1046/j.1365-2559.2001.01065.x. [DOI] [PubMed] [Google Scholar]
- 18.Halperin R., Peller S., Sandbank J., Bukovsky I., Schneider D. Expression of the p53 gene and apoptosis in gestational trophoblast disease. Placenta. 2000;21:58–62. doi: 10.1053/plac.1999.0442. [DOI] [PubMed] [Google Scholar]
- 19.Levy R. The role of apoptosis in preeclampsia. Isr. Med. Assoc. J. 2005;7:178–181. [PubMed] [Google Scholar]
- 20.Allaire A.D., Ballenger K.A., Wells S.R., McMahon M.J., Lessey B.A. Placental apoptosis in preeclampsia. Obstet. Gynecol. 2000;96:271–276. doi: 10.1016/s0029-7844(00)00895-4. [DOI] [PubMed] [Google Scholar]
- 21.Cali U., Cavkaytar S., Sirvan L., Danisman N. Placental apoptosis in preeclampsia, intrauterine growth retardation, and HELLP syndrome: an immunohistochemical study with caspase-3 and bcl-2. Clin. Exp. Obstet. Gynecol. 2013;40:45–48. [PubMed] [Google Scholar]
- 22.Can M., Guven B., Bektas S., Arikan I. Oxidative stress and apoptosis in preeclampsia. Tissue Cell. 2014;46:477–481. doi: 10.1016/j.tice.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 23.Leung D.N., Smith S.C., To K.F., Sahota D.S., Baker P.N. Increased placental apoptosis in pregnancies complicated by preeclampsia. Am. J. Obstet. Gynecol. 2001;184:1249–1250. doi: 10.1067/mob.2001.112906. [DOI] [PubMed] [Google Scholar]
- 24.Heazell A.E.P., Sharp A.N., Baker P.N., Crocker I.P. Intra-uterine growth restriction is associated with increased apoptosis and altered expression of proteins in the p53 pathway in villous trophoblast. Apoptosis. 2011;16:135–144. doi: 10.1007/s10495-010-0551-3. [DOI] [PubMed] [Google Scholar]
- 25.Ishihara N., Matsuo H., Murakoshi H., Laoag-Fernandez J.B., Samoto T., Maruo T. Increased apoptosis in the syncytiotrophoblast in human term placentas complicated by either preeclampsia or intrauterine growth retardation. Am. J. Obstet. Gynecol. 2002;186:158–166. doi: 10.1067/mob.2002.119176. [DOI] [PubMed] [Google Scholar]
- 26.Jeschke U., Schiessl B., Mylonas I., Kunze S., Kuhn C., Schulze S., Friese K., Mayr D. Expression of the proliferation marker Ki-67 and of p53 tumor protein in trophoblastic tissue of preeclamptic, HELLP, and intrauterine growth-restricted pregnancies. Int. J. Gynecol. Pathol. 2006;25:354–360. doi: 10.1097/01.pgp.0000225838.29127.6. [DOI] [PubMed] [Google Scholar]
- 27.Smith S.C., Baker P.N., Symonds E.M. Increased placental apoptosis in intrauterine growth restriction. Am. J. Obstet. Gynecol. 1997;177:1395–1401. doi: 10.1016/s0002-9378(97)70081-4. [DOI] [PubMed] [Google Scholar]
- 28.Longtine M.S., Chen B., Odibo A.O., Zhong Y., Nelson D.M. Villous trophoblast apoptosis is elevated and restricted to cytotrophoblasts in pregnancies complicated by preeclampsia, IUGR, or preeclampsia with IUGR. Placenta. 2012;33:352–359. doi: 10.1016/j.placenta.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karowicz-Bilińska A., Szczerba A., Kowalska-Koprek U., Nawrocka-Kunecka A. The evaluation of selected indices of apoptosis in placentas from pregnancies complicated by fetal growth restriction. Ginekol. Pol. 2007;78:521–526. [PubMed] [Google Scholar]
- 30.Börzsönyi B., Demendi C., Rigó J., Szentpéteri I., Rab A., Joó J.G. The regulation of apoptosis in intrauterine growth restriction: a study of Bcl-2 and Bax gene expression in human placenta. J. Matern. Neonatal Med. 2013;26:347–350. doi: 10.3109/14767058.2012.733770. [DOI] [PubMed] [Google Scholar]
- 31.Barrio E., Calvo M.T., Romo A., Alvarez R., Gutierrez J.I., Longás F. Restricted intrauterine growth: study of apoptosis in the placenta. An. Pediatr. 2003;58:51–54. [Google Scholar]
- 32.Chen F., Wang T., Feng C., Lin G., Zhu Y., Wu G., Johnson G., Wang J. Proteome differences in placenta and endometrium between normal and intrauterine growth restricted pig fetuses. PLoS One. 2015;10 doi: 10.1371/journal.pone.0142396. E0142396 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kalisch-Smith J., Simmons D.G., Dickinson H., Moritz K.M. Review: sexual dimorphism in the formation, function and adaptation of the placenta. Placenta. 2017;54:10–16. doi: 10.1016/j.placenta.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 34.Adibi J., Burton G.J., Clifton V., Collins S., Frias A.E., Gierman L., Grigsby P., Jones H., Lee C., Maloyan A., Markert U.R., Morales-Prieto D.M., Murthi P., Myatt L., Pollheimer J., Roberts V., Robinson W., Salafia C., Schabel M., Shah D., Sled J., Vaillancourt C., Weber M., O'Tierney-Ginn P.F. 2017. IFPA Meeting 2016 Workshop Report II: Placental Imaging, Placenta and Development of Other Organs, Sexual Dimorphism in Placental Function and Trophoblast Cell Lines, Placenta. Xxx; pp. 1–5. [DOI] [PubMed] [Google Scholar]
- 35.Ghidini A., Salafia C.M. Gender differences of placental dysfunction in severe prematurity. Br. J. Obstet. Gynaecol. 2005;112:140–144. doi: 10.1111/j.1471-0528.2004.00308.x. [DOI] [PubMed] [Google Scholar]
- 36.Challis J., Newnham J., Petraglia F., Yeganegi M., Bocking A. Fetal sex and preterm birth. Placenta. 2013;34:95–99. doi: 10.1016/j.placenta.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 37.Hu W., Feng Z., Teresky A.K., Levine A.J. p53 regulates maternal reproduction through LIF. Nature. 2007;450:721–724. doi: 10.1038/nature05993. [DOI] [PubMed] [Google Scholar]
- 38.Muralimanoharan S., Maloyan A., Myatt L. Evidence of sexual dimorphism in the placental function with severe preeclampsia. Placenta. 2013;34:1183–1189. doi: 10.1016/j.placenta.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baxter E.M., Jarvis S., Palarea-Albaladejo J., Edwards S.A. The weaker sex? the propensity for male-biased piglet mortality. PLoS One. 2012;7 doi: 10.1371/journal.pone.0030318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stenhouse C. University of Edinburgh; 2018. Investigating the Porcine Feto-maternal Interface throughout Gestation : Associations with Foetuses of Different Size and Sex. [Google Scholar]
- 41.Lillico S.G., Proudfoot C., Carlson D.F., Stverakova D., Neil C., Blain C., King T.J., Ritchie W.A., Tan W., Mileham A.J., McLaren D.G., Fahrenkrug S.C., Whitelaw C.B.A. Live pigs produced from genome. Zygotes, Sci. Rep. 2013;3:1–4. doi: 10.1038/srep02847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stenhouse C., Tennant P., Duncan W.C., Ashworth C.J. Doppler ultrasound can be used to monitor umbilical arterial blood flow in lightly sedated pigs at multiple gestational ages. Reprod. Fertil. Dev. 2018 doi: 10.1071/RD17298. (in press) [DOI] [PubMed] [Google Scholar]
- 43.Hernández S.C., Hogg C.O., Billon Y., Sanchez M.P., Bidanel J.P., Haley C.S., Archibald A.L., Ashworth C.J. Secreted phosphoprotein 1 expression in endometrium and placental tissues of hyperprolific large white and meishan gilts. Biol. Reprod. 2013;88:1–7. doi: 10.1095/biolreprod.112.104679. [DOI] [PubMed] [Google Scholar]
- 44.Erkens T., Van Poucke M., Vandesompele J., Goossens K., Van Zeveren A., Peelman L.J. Development of a new set of reference genes for normalization of real-time RT-PCR data of porcine backfat and longissimus dorsi muscle, and evaluation with PPARGC1A. BioMed Cent. Biotechnol. 2006;6:1–8. doi: 10.1186/1472-6750-6-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nygard A.B., Jørgensen C.B., Cirera S., Fredholm M. Selection of reference genes for gene expression studies in pig tissues using SYBR green qPCR. BioMed Cent. Mol. Biol. 2007;8:1–6. doi: 10.1186/1471-2199-8-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Knight J.W., Bazer F.W., Thatcher W.W., Franke D.E., Wallace D. Conceptus development in intact and unilaterally hysterectomized-ovariectomized Gilts : interrelations among hormonal status, placental development, fetal fluids and fetal growth. J. Anim. Sci. 1977;44:620–637. doi: 10.2527/jas1977.444620x. [DOI] [PubMed] [Google Scholar]
- 47.Wu G., Bazer F.W., Hu J., Johnson G.A., Spencer T.E. Polyamine synthesis from proline in the developing porcine placenta. Biol. Reprod. 2005;72:842–850. doi: 10.1095/biolreprod.104.036293. [DOI] [PubMed] [Google Scholar]
- 48.Marrable A.W. Pitman Medical; London: 1971. The Embryonic Pig: a Chronological Account. [Google Scholar]
- 49.Wright E.C., Miles J.R., Lents C.A., Rempel L.A. Uterine and placenta characteristics during early vascular development in the pig from day 22 to 42 of gestation. Anim. Reprod. Sci. 2016;164:14–22. doi: 10.1016/j.anireprosci.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 50.Merkis C., Cristofolini A., Sanchis E., Koncurat M. Expression of death cellular receptors FAS/CD95 and DR4 during porcine placentation. Int. J. Morphol. 2010;28:829–834. [Google Scholar]
- 51.Ke B., Tia M., Li J., Liu B., He G. Targeting programmed cell death using small-molecule compounds to improve potential cancer therapy. Med. Res. Rev. 2016;36:938–1035. doi: 10.1002/med.21398. [DOI] [PubMed] [Google Scholar]
- 52.Tait S.W.G., Ichim G., Green D.R. Die another way – non-apoptotic mechanisms of cell death. J. Cell Sci. 2014;127:2135–2144. doi: 10.1242/jcs.093575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hannan N.J., Beard S., Binder N.K., Onda K., Kaitu’u-Lino T.J., Chen Q., Tuohey L., De Silva M., Tong S. Key players of the necroptosis pathway RIPK1 and SIRT2 are altered in placenta from preeclampsia and fetal growth restriction. Placenta. 2017;51:1–9. doi: 10.1016/j.placenta.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 54.Gol M., Tuna B. Effect of fetal sex on apoptosis- regulating proteins in trophoblasts of full-term human placenta. Gynecol. Obstet. Invest. 2009;67:53–56. doi: 10.1159/000161570. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.