Abstract
One-step solid-phase extraction (SPE) using a multiwalled carbon nanotube (MWCNT) for simultaneous analysis of 21 mycotoxins, including nine trichothecenes, zearalenone (ZEN) and its derivatives, four aflatoxins, and two ochratoxins, in corn and wheat was developed. Several key parameters affecting the performance of the one-step SPE procedure—types of MWCNT, combinations with five sorbents (octadecylsilyl (C18), hydrophilic–lipophilic balance (HLB), mixed-mode cationic exchange (MCX), silica gel, and amino-propyl (NH2)), and filling amounts of the MWCNTs—were thoroughly investigated. The combination of 20 mg carboxylic MWCNT and 200 mg C18 was proven to be the most effective, allowing the quantification of all analyzed mycotoxins in corn and wheat. Under the optimized cleanup procedure prior to ultraperformance liquid chromatography–tandem mass spectrometry (UPLC–MS/MS) analysis, the method was validated by analyzing samples spiked at the limit of quantification (LOQ), two-times LOQ, and 10-times LOQ. Satisfactory linearity (r2 ≥ 0.9910), high sensitivity (LOQ in different ranges of 0.5–25 μg L−1), good recovery (75.6–110.3%), and acceptable precision (relative standard deviation (RSD), 0.3–10.7%) were obtained. The applicability of the method was further confirmed using raw samples of corn and wheat. In conclusion, the established method was rapid, simple and reliable for simultaneous analysis of 21 mycotoxins in corn and wheat.
Keywords: MWCNT, one-step cleanup, mycotoxin, UPLC–MS/MS, corn and wheat
1. Introduction
Corn and wheat are cereal grains widely consumed as food and feed and used as industrial raw materials worldwide. However, they are easily infected by spoilage fungi that cause the deterioration of grains, including discoloration, musty odors, tissue disintegration and loss of nutritional quality. Meanwhile, mycotoxins are produced under suitable temperature and humidity conditions, especially fusarium toxins, aflatoxins and ochratoxins, in corn and wheat [1,2]. Ingestion of these contaminated materials may be pathogenic in humans, because they may lead to serious health problems, such as liver, kidney or nervous system damage, immunosuppression, biphasic cellular response, and carcinogenesis [3,4]. Therefore, rapid, accurate and high-throughput analysis methods are needed to determine the global contamination level, assess the risk, and monitor the detoxification strategies of mycotoxins.
As we know, more than one mycotoxin may occur in cereal grains; for instance, deoxynivalenol (DON), zearalenone (ZEN) and nivalenol (NIV) co-occur in 74% of wheat samples [5], and at least eight fusarium toxins and ochratoxin A (OTA) were simultaneously detected in winter wheat [6]. Similarly, co-occurrence of OTA and ZEN was also reported in breakfast cereals [7]. As a result, it is an urgent problem to develop simultaneous analytical methods for multiclass mycotoxins. To date, numerous methods have been established to simultaneously analyze multiclass mycotoxins in different matrices by high-performance liquid chromatography–tandem mass spectrometry (HPLC–MS/MS) [8,9,10,11,12,13,14], but efficient pretreatment is still the main challenge. Due to the tedious process and matrix interference of quick, easy, cheap, effective, rugged and safe (QuEChERS) methods, poor stability and repeatability of liquid-phase microextraction (LPME), high cost of immunoaffinity chromatography (IAC), and poor selectivity of magnetic separation, solid-phase extraction (SPE) is still the main pretreatment method for analysis of multiclass mycotoxins [15,16].
For commercial SPE, silica gel, octadecylsilyl (C18), hydrophilic–lipophilic balance (HLB), and amino-propyl (NH2) can be obtained as adsorbent materials, but most of the commercial cartridges are not suitable for high-throughput screening for multiclass mycotoxins. Recently, multiwalled carbon nanotubes (MWCNTs), a new type of SPE absorbent, have been applied to sample pretreatment of mycotoxins due to their strong adsorption capacity, unique structure, and ease of being functionally modified as carboxylic MWCNT (MWCNT–COOH) and hydroxyl MWCNT (MWCNT–OH) [17,18]. As previously reported, MWCNTs have been used for the cleanup of type A trichothecenes and ZEN in cereals [19,20,21]. However, the procedure of sample pretreatment, including washing and eluting, is not convenient and also consumes significant time, especially when applied to analyze a great numbers of samples.
The purpose of our study is to establish a rapid, simple, sensitive and reliable procedure on the basis of one-step SPE cleanup using MWCNTs as sorbents followed by UPLC–MS/MS analysis, to simultaneously determine 21 mycotoxins in corn and wheat, including nine trichothecenes, ZEN and its derivatives, four aflatoxins, and two ochratoxins. The most important advantage of this method is that the cleanup is simple and fast, using a homemade MWCNT–COOH +C18 SPE cartridge as a filter, and the matrix effects are significantly reduced. This simple and rapid method can be used to simultaneously determine the targeted mycotoxins in corn and wheat with excellent efficiency.
2. Results and Discussion
2.1. UPLC–MS/MS
CORTECS C18 (1.6 μm, 2.1 × 100 mm, Waters, Milford, MA, USA) was employed as the separation column, and sharp chromatographic peaks, which means high sensitivity for each targeted mycotoxin in a spiked solvent sample at 10-times the limit of quantification (LOQ) level, are shown in Supplementary Figure S1. The results of MS/MS parameters demonstrated that ZEN and its derivatives, α-zearalenol (α-ZOL), β-ZOL, zearalanone (ZAN), α-zearalanol (α-ZAL) and β-ZAL, displayed more desirable results in negative electrospray ionization mode (ESI−), and the other 15 analytes showed much higher sensitivity in the positive mode of ESI (ESI+). The optimized parameters for each analyte—ionization mode, precursor and productions, collision energy and cone voltage—are shown in Supplementary Table S1.
2.2. Optimization of the SPE Cartridge
Effective sample pretreatment is critical for reducing matrix effects, especially trace amounts of mycotoxins existing in complex matrices [22]. MWCNTs can greatly reduce the matrix effect, owing to their strong adsorption capacity, and one can combine the features of MWCNTs with other adsorbent materials, thus forming a promising sorbent material for SPE in recent years. In our work, a simple and rapid homemade SPE procedure was developed to purify the prevalent multiclass mycotoxins, and also to minimize the matrix effects. Compared to the conventional SPE procedures, there was only one step, allowing the analytes to pass through and the co-extractive interference to be removed. Therefore, the SPE cartridge served as a rapid and convenient chemical filter. In this study, types of MWCNT, combinations with five sorbents and filling amounts were evaluated for optimization of the cleanup procedure. Matrix effects of the 21 analytes were investigated using corn, because it has a more complex matrix and more common mycotoxin contamination.
2.2.1. Types of MWCNT
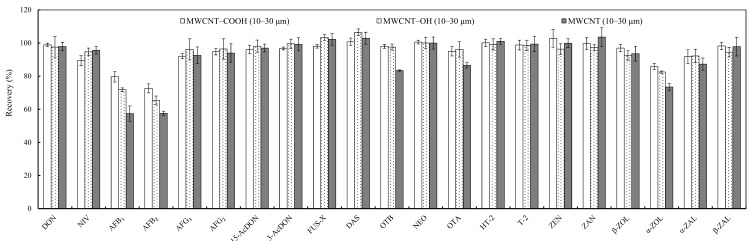
Initially, the effects of the type of MWCNT were investigated with 20 mg of the three multiwalled carbon nanotubes, MWCNT, MWCNT–COOH and MWCNT–OH, plus 200 mg silica gel. Compared to MWCNT–COOH, a relatively higher recovery of mycotoxins NIV, 3-acetyldeoxynivalenol (3-AcDON) and 15-acetyldeoxynivalenol (15-AcDON) was obtained when using unmodified MWCNT or MWCNT–OH as the sorbent, but the recovery of aflatoxins AFB1 and AFB2 was much lower, and even unacceptable (Figure 1). As a result, satisfactory recovery of 72.6–102.8% was observed using MWCNT–COOH as a sorbent for all the analytes. In addition, there was no significant difference in matrix effects for all mycotoxins in corn among the different types of MWCNT (Supplementary Figure S2); therefore, MWCNT–COOH was used for further study.
Figure 1.
Effects of three MWCNT sorbents on recovery (%) of mycotoxins in corn. MWCNT, multiwalled carbon nanotube; MWCNT–COOH, carboxylic MWCNT; MWCNT–OH, hydroxyl MWCNT; DON, deoxynivalenol; NIV, nivalenol; AFB1, AFB2, AFG1, AFG2, aflatoxins; 15-AcDON, 15-acetyldeoxynivalenol; 3-AcDON, 3-acetyldeoxynivalenol; FUS-X, fusarenon X; DAS, diacetoxyscirpenol; OTA, ochratoxin A; OTB, ochratoxin B; T-2, T-2 toxin; HT-2, HT-2 toxin; NEO, neosolaniol; ZEN, zearalenone; α-ZOL, α-zearalenol; β-ZOL, β-zearalenol; ZAN, zearalanone; α-ZAL, α-zearalanol; β-ZAL, β-zearalanol. Vertical bar represents ± standard error (n = 3).
2.2.2. Different Combinations of Sorbents
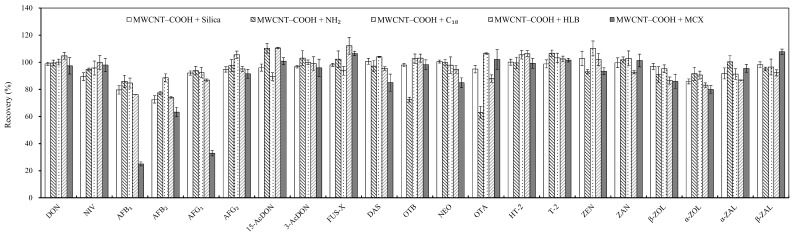
Different combinations of MWCNT–COOH with five sorbents, C18, HLB, NH2, mixed-mode cationic exchange (MCX) and silica gel, were used to investigate the cleanup efficiency of the 21 analytes. MCX is a common sorbent applied to both polar and nonpolar compounds [23]; however, recovery of MWCNT–COOH + MCX was significantly lower than that of other combinations, especially AFB1 (25.0%) and AFG1 (32.9%). NH2 is also a common sorbent that can remove similar types of compounds by interacting with chemicals, including some sugars, organic acids and pigments, through hydrogen bonding [24], but lower recovery of OTA (63.1%) was obtained here, and the recovery of OTB (72.4%) was much lower than those using other combinations; therefore, NH2 is not an eligible sorbent for the cleanup of ochratoxins (OT). Using silica gel and HLB achieved acceptable recovery for all the targeted mycotoxins; however, lower recovery of AFB1 and AFB2 was obtained compared to C18, which is commonly used for mycotoxin cleanup [25]. C18 achieved satisfactory recovery for all analytes, ranging from 84.7% to 110.2%, thus the combination of MWCNT–COOH and C18 was selected for further study (Figure 2).
Figure 2.
Effects of different solid-phase extraction (SPE) sorbents on the recovery (%) of mycotoxins in corn. For each of the five sorbents, 200 mg of the sorbent was well mixed with 20 mg of MWCNT–COOH and packed into the cartridge. NH2, aminopropyl; C18, octadecylsilyl; HLB, hydrophilic–lipophilic balance; MCX, mixed-mode cationic exchange. Vertical bar represents ± standard error (n = 3).
2.2.3. Amounts of MWCNT–COOH
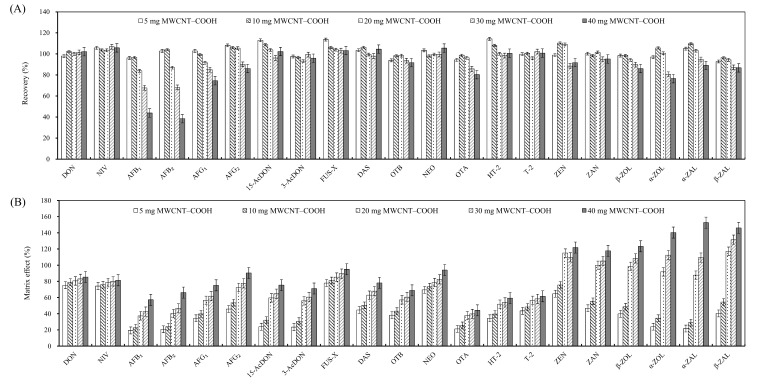
The amount of absorbent was an important factor in the purification of targeted compounds, and in our study, different amounts of MWCNT–COOH (5, 10, 20, 30 and 40 mg) were applied to evaluate the cleanup efficiency of all the analytes, so as to achieve the highest recovery with the lowest amount of sorbent. As shown in Figure 3, the results indicated that there was no significant difference in recovery for the target mycotoxins as the amount of MWCNT–COOH increased from 5 mg to 20 mg; however, a significant decrease in the recovery of some analytes (aflatoxins, ochratoxins and zearalenones) was observed from 20 mg to 40 mg of MWCNT–COOH, although the matrix effects were dramatically removed by the increasing amounts due to the strong absorption of MWCNT–COOH (Figure 3). As a result, the combination of 20 mg MWCNT–COOH and 200 mg C18 was proven to be the most effective cleanup, and satisfactory recovery ranging from 84.0% to 109.1% was achieved for all analyzed mycotoxins in corn. In previous reports, the amount of MWCNT was always a key parameter to be optimized, and 100 mg of MWCNT was chosen to achieve complete adsorption in the dispersive or magnetic solid-phase extraction [20,26]. Here, only 20 mg of MWCNT–COOH was used to obtain the best results at lower cost, and meanwhile, the potential health hazard from exposure to MWCNT was also a consideration [27].
Figure 3.
Effects of different amounts of MWCNT–COOH and 200 mg C18 on the recovery and matrix effect of mycotoxins in corn. Vertical bar represents ± standard error (n = 3). (A) Recovery (%); (B) Matrix effect (%).
2.3. Evaluation of the Homemade One-Step Cleanup Method
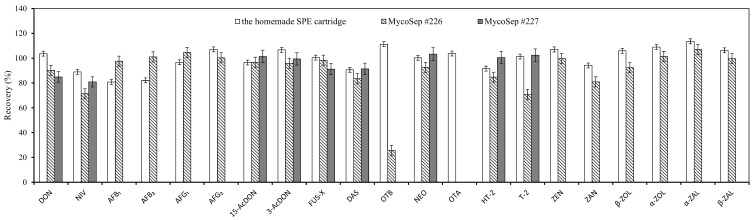
A comparison between homemade SPE and commercial multifunction cleanup (MFC) columns was performed using spiked samples (five-times LOQ) to evaluate the purification efficiency of the established one-step cleanup method. MycoSep #227 MFC columns are frequently utilized for purification of trichothecenes [28,29,30], and this was further supported by the present study based on the results that the recovery of trichothecenes with MycoSep #227 purification was 80.9–103.3% (Figure 4). MycoSep #226 MFC columns are also frequently applied to clean up aflatoxins and ZENs [31,32], and good recovery of aflatoxins and ZEN and its derivatives was also observed in our study (Figure 4); however, the above commercial MFCs could not be applied to the high-throughput determination of all the selected mycotoxins in cereal regulated by China, especially for OTA. According to our results, satisfactory recovery of all 21 mycotoxins, ranging from 80.8% to 113.6%, was obtained by our homemade SPE cartridge, suggesting that our SPE appeared to be a good choice for the simultaneous cleanup of prevalent mycotoxins in cereals with excellent performance and at much lower cost.
Figure 4.
Efficiency comparison among the homemade SPE cartridge, MycoSep #226 column (Romer Labs Inc., Union, MO, USA), and MycoSep #227 column (Romer) on the recovery (%) of mycotoxins in corn. Vertical bar represents ± standard error (n = 3).
2.4. Method Validation
Method validation was performed in terms of LOQ, linearity, accuracy and precision for the 21 targeted mycotoxins in the selected model cereals, including corn and wheat, which are important cereals in northern China and easily exposed to mycotoxin contamination.
Calculated LOQs were set to 0.5–25 μg L−1 for the targeted mycotoxins with signal-to-noise ratios of 10 or more. As shown in Table 1, eight targeted mycotoxins, including the four AFs, OTA, OTB, ZEN and T-2, were quantified at 0.5 μg L−1, neosolaniol (NEO) and NIV were quantified at 25 μg L−1 because of low signal responses in the matrix, and the other mycotoxins were quantified at 10 μg L−1. Matrix-matched standard calibration of 21 analytes showed good linear relationships with correlation coefficients ≥0.9910 over the range of LOQ to 200 LOQ in corn and wheat.
Table 1.
Accuracy and precision of the analytical method for determining mycotoxins in corn and wheat spiked at three different concentration levels.
| Mycotoxins | LOQ (μg L−1) | Spiked Levels | Corn | Wheat | ||||
|---|---|---|---|---|---|---|---|---|
| Recovery (%) | Intraday RSD (%) | Interday RSD (%) | Recovery (%) | Intraday RSD (%) | Interday RSD (%) | |||
| DON | 10 | LOQ | 93.4 | 8.2 | 9.6 | 94.7 | 4.1 | 6.3 |
| 2LOQ | 103.6 | 2.7 | 3.2 | 96.5 | 1.3 | 3.1 | ||
| 10LOQ | 101.9 | 4.1 | 6.4 | 98.3 | 0.8 | 2.2 | ||
| NIV | 25 | LOQ | 90.7 | 5.4 | 6.8 | 92.3 | 2.9 | 4.3 |
| 2LOQ | 99.4 | 4.8 | 5.4 | 99.3 | 4.4 | 4.8 | ||
| 10LOQ | 90.7 | 1.1 | 2.5 | 97.2 | 2.7 | 3.5 | ||
| AFB1 | 0.5 | LOQ | 78.5 | 3.9 | 5.6 | 85.1 | 2.6 | 4.2 |
| 2LOQ | 80.5 | 1.7 | 3.4 | 82.4 | 4.4 | 6.5 | ||
| 10LOQ | 85.6 | 4.1 | 5.4 | 86.9 | 2.9 | 4.9 | ||
| AFB2 | 0.5 | LOQ | 82.8 | 2.9 | 4.8 | 78.0 | 5.1 | 10.7 |
| 2LOQ | 87.5 | 1.8 | 3.6 | 80.3 | 4.6 | 9.2 | ||
| 10LOQ | 92.8 | 2.2 | 4.9 | 81.9 | 7.2 | 8.2 | ||
| AFG1 | 0.5 | LOQ | 90.9 | 3.5 | 5.6 | 97.0 | 3.9 | 8.1 |
| 2LOQ | 87.8 | 7.1 | 9.8 | 87.8 | 4.2 | 6.5 | ||
| 10LOQ | 92.7 | 3.7 | 6.7 | 94.8 | 5.4 | 6.9 | ||
| AFG2 | 0.5 | LOQ | 90.8 | 3.1 | 4.2 | 95.9 | 3.3 | 4.2 |
| 2LOQ | 103.6 | 2.8 | 3.5 | 90.5 | 4.0 | 4.8 | ||
| 10LOQ | 99.7 | 2.4 | 3.7 | 100.9 | 5.0 | 6.1 | ||
| 15-AcDON | 10 | LOQ | 91.8 | 2.8 | 4.3 | 100.1 | 2.0 | 3.1 |
| 2LOQ | 103.3 | 3.8 | 4.8 | 95.3 | 2.3 | 3.3 | ||
| 10LOQ | 98.9 | 4.8 | 5.7 | 97.9 | 0.8 | 2.1 | ||
| 3-AcDON | 10 | LOQ | 89.2 | 3.7 | 4.8 | 100.0 | 2.0 | 3.2 |
| 2LOQ | 105.1 | 5.8 | 7.1 | 106.8 | 3.6 | 4.5 | ||
| 10LOQ | 93.8 | 3.9 | 5.1 | 100.4 | 0.4 | 1.8 | ||
| FUS-X | 10 | LOQ | 97.1 | 5.8 | 7.2 | 92.8 | 2.9 | 4.3 |
| 2LOQ | 96.8 | 2.3 | 3.4 | 86.6 | 3.9 | 5.8 | ||
| 10LOQ | 84.1 | 3.2 | 4.0 | 101.5 | 1.1 | 3.7 | ||
| DAS | 10 | LOQ | 100.5 | 3.5 | 4.2 | 75.6 | 7.8 | 10.4 |
| 2LOQ | 84.6 | 2.3 | 4.3 | 93.3 | 2.0 | 4.7 | ||
| 10LOQ | 86.8 | 6.0 | 8.7 | 93.2 | 3.1 | 6.5 | ||
| T-2 | 0.5 | LOQ | 79.8 | 4.4 | 9.6 | 80.8 | 4.7 | 9.4 |
| 2LOQ | 104.3 | 6.0 | 6.6 | 104.8 | 3.3 | 4.3 | ||
| 10LOQ | 84.9 | 4.9 | 7.3 | 98.8 | 4.6 | 5.8 | ||
| HT-2 | 10 | LOQ | 81.4 | 6.8 | 8.9 | 82.2 | 3.3 | 5.7 |
| 2LOQ | 99.4 | 6.3 | 7.5 | 99.3 | 2.9 | 5.3 | ||
| 10LOQ | 89.2 | 3.2 | 6.7 | 96.2 | 3.7 | 6.8 | ||
| NEO | 25 | LOQ | 88.0 | 7.2 | 8.9 | 97.6 | 4.7 | 5.9 |
| 2LOQ | 101.0 | 1.9 | 2.5 | 97.9 | 6.0 | 7.6 | ||
| 10LOQ | 92.3 | 2.9 | 4.1 | 97.9 | 2.2 | 3.6 | ||
| OTA | 0.5 | LOQ | 83.2 | 2.9 | 5.4 | 105.5 | 3.0 | 4.2 |
| 2LOQ | 110.3 | 4.4 | 4.8 | 108.3 | 4.9 | 5.4 | ||
| 10LOQ | 82.0 | 4.9 | 6.3 | 92.0 | 5.6 | 6.4 | ||
| OTB | 0.5 | LOQ | 100.7 | 2.7 | 3.9 | 99.6 | 3.4 | 4.8 |
| 2LOQ | 101.9 | 3.6 | 4.7 | 100.5 | 3.5 | 3.8 | ||
| 10LOQ | 92.2 | 3.9 | 5.1 | 93.5 | 3.6 | 4.1 | ||
| ZEN | 0.5 | LOQ | 96.5 | 2.2 | 3.2 | 93.1 | 3.0 | 4.1 |
| 2LOQ | 98.5 | 3.6 | 4.8 | 102.0 | 3.6 | 4.5 | ||
| 10LOQ | 95.3 | 3.8 | 4.6 | 96.7 | 2.0 | 2.8 | ||
| ZAN | 10 | LOQ | 108.1 | 5.0 | 5.6 | 99.6 | 4.6 | 6.2 |
| 2LOQ | 106.6 | 4.1 | 4.9 | 103.8 | 3.2 | 5.4 | ||
| 10LOQ | 105.4 | 2.9 | 3.5 | 95.7 | 3.9 | 4.8 | ||
| β-ZOL | 10 | LOQ | 102.2 | 2.8 | 4.1 | 99.0 | 3.1 | 3.8 |
| 2LOQ | 103.0 | 3.4 | 4.9 | 94.0 | 0.3 | 1.8 | ||
| 10LOQ | 101.7 | 1.9 | 3.5 | 92.5 | 3.2 | 4.1 | ||
| α-ZOL | 10 | LOQ | 93.8 | 3.1 | 5.3 | 81.2 | 6.1 | 9.5 |
| 2LOQ | 96.8 | 1.8 | 2.4 | 88.0 | 4.5 | 6.3 | ||
| 10LOQ | 96.5 | 2.0 | 3.1 | 89.7 | 3.5 | 5.2 | ||
| α-ZAL | 10 | LOQ | 91.8 | 2.6 | 4.2 | 89.5 | 5.2 | 7.8 |
| 2LOQ | 93.8 | 1.6 | 3.2 | 96.2 | 6.3 | 8.2 | ||
| 10LOQ | 104.6 | 8.0 | 7.6 | 91.8 | 1.5 | 4.3 | ||
| β-ZAL | 10 | LOQ | 91.0 | 7.9 | 9.2 | 94.3 | 3.1 | 6.2 |
| 2LOQ | 99.1 | 5.2 | 7.5 | 84.6 | 8.1 | 9.8 | ||
| 10LOQ | 106.3 | 3.0 | 5.4 | 95.8 | 3.4 | 5.2 | ||
For each concentration level, mean recovery and relative standard deviation (RSD) were calculated on n = 5. LOQ, limit of quantification; DON, deoxynivalenol; NIV, nivalenol; AFB1, AFB2, AFG1, AFG2, aflatoxins; 15-AcDON, 15-acetyldeoxynivalenol; 3-AcDON, 3-acetyldeoxynivalenol; FUS-X, fusarenon X; DAS, diacetoxyscirpenol; OTA, ochratoxin A; OTB, ochratoxin B; T-2, T-2 toxin; HT-2, HT-2 toxin; NEO, neosolaniol; ZEN, zearalenone; α-ZOL, α-zearalenol; β-ZOL, β-zearalenol; ZAN, zearalanone; α-ZAL, α-zearalanol; β-ZAL, β-zearalanol.
Accuracy and precision of the method were evaluated by means of recovery and relative standard deviation (RSD) with five replicates at three levels: LOQ, two-times LOQ, and 10-times LOQ. The experimental data of recovery and RSD for each mycotoxin are summarized in Table 1. Overall, acceptable recovery ranging from 75.6% to 110.3% was obtained, and the intra- and interday RSDs of the 21 targeted mycotoxins were in the satisfactory range of 0.3–8.2% and 1.8–10.7%, respectively (Table 1), which indicated that this method had good repeatability and reproducibility.
All the validation data showed that the analytical method here was accurate and repeatable and could be applied to simultaneously analyze the 21 analytes in corn and wheat. So far as we know, limited information is available on cleanup of all major mycotoxins found in cereals using MWCNTs as sorbent. According to our results, a one-step cleanup procedure using MWCNT–COOH +C18 as a sorbent of the SPE cartridge was developed, which is feasible and efficient, and greatly simplified the sample pretreatment of 21 mycotoxins in cereals.
2.5. Method Application
As shown in Table 2, AFB1 was found in 19 corn samples at concentrations of 0.5–26 μg kg−1. ZEN was found in all corn samples at concentrations of 27.5–525 μg kg−1 and 13 wheat samples at concentrations of 26.8–602 μg kg−1. DON was found in seven corn samples and 27 wheat samples at higher concentrations. 3-AcDON and 15-AcDON were found in 11 and 12 wheat samples, respectively. The other 15 mycotoxins were not detected from the collected samples.
Table 2.
Mycotoxins detected in raw samples of corn and wheat using the homemade one-step cleanup procedure with ultraperformance liquid chromatography–tandem mass spectrometry (UPLC–MS/MS) analysis.
| Sample (n) | Mycotoxin | Positives (n) | Occurrence (%) | Mean (μg kg−1) | Range (μg kg−1) |
|---|---|---|---|---|---|
| Corn (59) | AFB1 | 19 | 32.2 | 2.18 | 0.5–26 |
| ZEN | 59 | 100 | 69.3 | 27.5–525 | |
| DON | 7 | 11.9 | 410.1 | 65.4–5100 | |
| Wheat (53) | ZEN | 13 | 24.5 | 50.7 | 26.8–602 |
| DON | 27 | 50.9 | 557.5 | 19.0–4050 | |
| 3-AcDON | 11 | 20.8 | 68.3 | 13.3–270 | |
| 15-AcDON | 12 | 22.6 | 49.2 | 10.1–141 |
Mean concentration of each mycotoxin was calculated on n = 3.
3. Conclusions
A novel and rapid one-step cleanup procedure using MWCNT–COOH +C18 as SPE sorbent was developed for purification of 21 mycotoxins in corn and wheat. In comparison with the conventional SPE method, this one-step cleanup method shortened the time for sample pretreatment with good recovery and minimized matrix effects. The homemade SPE cartridge also greatly reduced cost compared with the expensive immune-affinity column and various MycoSep multifunctional cleanup columns. The combination of the one-step cleanup procedure with UPLC–MS/MS analysis provided a rapid, sensitive and reliable method to simultaneously determine the 21 targeted mycotoxins, which was validated in corn and wheat, suggesting an application of the proposed method in the analysis of the targeted mycotoxins in various crops.
4. Material and Methods
4.1. Reagents
MS-grade acetonitrile and methanol were purchased from Thermo Fisher Scientific (Fair Lawn, NJ, USA). Ammonium acetate (NH4Ac), acetic acid and citric acid were purchased from Sigma-Aldrich (Buchs, Switzerland). Milli-Q quality water was obtained from Millipore (Billerica, MA, USA). Sorbents of C18, HLB, NH2, MCX and silica gel were purchased from Beijing Solarbio Science and Technology Company Limited (Beijing, China). MWCNT adsorbent materials of 10–30 μm length (8 nm i.d., 500 m2 g−1) were obtained from Nanjing XFNano Materials Technology Company Limited (Nanjing, China).
4.2. Standards
Certified standards of 4 aflatoxins (AFs: AFB1, AFB2, AFG1, AFG2), OTA, OTB, DAS, NIV, NEO, T-2, HT-2, DON, 3-AcDON, 15-AcDON, ZEN, α-ZOL, β-ZOL and fusarenon X (FUS-X) were obtained from Romer Labs Inc. (Union, MO, USA). ZAN and α-ZAL were purchased from Sigma-Aldrich (Buchs, Switzerland), and β-ZAL was purchased from Pribolab Private Limited (Immunos, Singapore).
The individual stock solutions of AFs, ZEN, T-2, OTA and OTB were dissolved in acetonitrile at 10 mg L−1 and the other 13 standards were prepared in acetonitrile at 100 mg L−1. AFs, ZEN, T-2, OTA and OTB were prepared in enough acetonitrile at 0.5 mg L−1 as an intermediate mixed solution (Mix A). A similar procedure was carried out for Mix B including DAS, DON, FUS-X, HT-2, 3-AcDON, 15-AcDON, ZAN, α-ZAL, β-ZAL, α-ZOL and β-ZOL toxins, at a stock concentration of 10 mg L−1. Mix C, of NEO and NIV at a concentration of 25 mg L−1, was obtained in the same way. Then, 1 mL of each mixture, Mix A, Mix B and Mix C, and 7 mL acetonitrile, were mixed to obtain 10 mL of total mixed stock of the standard solution, which was stored at −20 °C in the dark. Working solutions were freshly diluted with the blank matrix or acetonitrile before UPLC–MS/MS analysis.
4.3. Samples
Approximately 1 kg of corn and wheat samples, which were confirmed to be free of the 21 analytes, were purchased from local markets in Beijing, China. In total, 59 corn niblets and 53 wheat kernels were collected in Hebei Province, China, for method application. All the samples were ground into fine powder with a blender. Before extraction, these samples were stored at −20 °C.
4.4. Preparation of SPE Cartridges
Five SPE cartridges were obtained by placing MWCNT–COOH plus each of 5 sorbents (silica gel, NH2, HLB, MCX and C18) inside 6 mL polypropylene tubes with a frit at the 2 sides. For a combination of sorbents, 200 mg of each of the 5 sorbents was well mixed with 20 mg MWCNT–COOH and packed into the cartridge. Then, the cartridges were treated with 5 mL of acetonitrile and 5 mL of Milli-Q quality water.
4.5. Extraction
Mycotoxins were extracted according to a previous report [13] with minor revision. Briefly, 5.0 g of fine homogenized cereal was mixed with 4 mL water and 21 mL acetonitrile containing 100 mM citric acid. After being shaken for 30 min and centrifuged for 10 min at 10,000 rpm, the supernatant was passed through the homemade SPE cartridge, and 5 mL outflow was collected directly into a centrifuge tube. During this process, large matrix interferences were retained on the SPE column. The cleanup extract in the centrifuge tube was then evaporated to almost dryness at 50 °C under a gentle nitrogen stream, and the residue was dissolved in 1 mL acetonitrile/water (3:7 v/v). After being forced through a 0.22 μm polytetrafluoroethylene (PTFE) filter membrane (Pall, MI, USA), 5 μL of the final solution was analyzed by UPLC–MS/MS.
4.6. UPLC–MS/MS Conditions
UPLC–MS/MS conditions were optimized according to a previous report [13]. The UPLC system consisted of an Acquity UPLCTM from Waters (Milford, MA, USA). The analyte separation was carried out on a CORTECS® UPLC C18 column with particle size of 1.6 μm (2.1 × 100 mm; Waters), and the column temperature was maintained at 40 °C. Then, 1 mM NH4Ac in Milli-Q quality water (solvent A) and methanol (solvent B) served as the mobile phases at a flow rate of 0.3 mL min−1 for 9 min with the following gradient elution: 0–0.5 min (95% A), 6.0 min (25% A), 7.5 min (10% A), 7.6–9.0 min (95% A). The samples were maintained at 10 °C in the instrument during all runs until injection, which volume was 5 μL.
The UPLC system was coupled with a Xevo TQ-S equipped with an ESI source (Waters, Milford, MA, USA). Mass spectrometry was performed via both positive and negative modes of electrospray ionization due to the different structural properties of the analytes. Optimized parameters for the mass spectrometry were as follows: capillary voltage, +2.5 kV/−0.8 kV; desolvation temperature, 500 °C; source temperature, 150 °C; desolvation gas flow, 1000 L h−1; and cone gas flow, 150 L h−1. The analytes were detected in multiple-reaction monitoring (MRM) mode and the optimized parameters for each analyte are shown in Supplementary Table S1. Data acquisition and processing were performed using MassLynxTM 4.1 software (Waters, Milford, MA, USA).
4.7. Method Validation
The method was validated for sensitivity (LOQ), linearity, accuracy (recovery) and precision (%RSD). For that, method validation was performed according to SANCO guideline 12571/2013. Recovery and RSD were determined intra- and interday by analyzing spiked blank samples with 5 replicates. Each sample was spiked 10–12 h before the extraction and left at 4 °C. Spiked samples were extracted, purified using the homemade SPE, and then analyzed using the same UPLC–MS/MS conditions as described above. RSDs at 3 spiked levels on the same day and 5 consecutive days were used for evaluation of the intra- and interday precision, respectively. The concentrations of mycotoxins in the spiked samples were measured using the matrix-matched calibration curves, and the recovery was calculated as the percentage of the measured analyte concentration divided by the spiked concentration. LOQ was based on a signal-to-noise ratio of 10 or more observed in a sample spiked at 0.5–25.0 μg L−1 showing a lower response. Linearity and matrix effects were calculated using solvent- and matrix-matched calibrations in triplicate at 7 concentration levels ranging from LOQ to 200 LOQ. Matrix effect (%) was calculated according to the formula (Am/As) × 100, in which As is the slope of the standard calibration line and Am is the slope of the matrix-matched calibration line.
4.8. Statistical Analysis
All data in this study were analyzed using the Statistical Package for Social Science (SPSS) 15.0 for Windows (SPSS Inc., Chicago, IL, USA), and the level of statistical significance was set at p ≤ 0.05.
Abbreviations
The following abbreviations are used in this manuscript:
| 15-AcDON | 15-acetyldeoxynivalenol |
| 3-AcDON | 3-acetyldeoxynivalenol |
| AFB1 | aflatoxin B1 |
| AFB2 | aflatoxin B2 |
| AFG1 | aflatoxin G1 |
| AFG2 | aflatoxin G2 |
| DAS | diacetoxyscirpenol |
| DON | deoxynivalenol |
| ESI− | negative mode of electrospray ionization |
| ESI+ | positive mode of electrospray ionization |
| FUS-X | fusarenon X |
| HLB | hydrophilic–lipophilic balanced |
| HPLC–MS/MS | high-performance liquid chromatography–tandem mass spectrometry |
| IAC | immunoaffinity chromatography |
| LOQ | limit of quantification |
| MCX | mixed-mode cationic exchange |
| MFC | multifunction cleanup columns |
| MRM | multiple-reaction monitoring |
| MWCNT | multiwalled carbon nanotube |
| MWCNT–COOH | carboxylic MWCNT |
| MWCNT–OH | hydroxyl MWCNT |
| NEO | neosolaniol |
| NH2 | amino-propyl |
| NH4Ac | ammonium acetate |
| NIV | nivalenol |
| OTA | ochratoxin A |
| OTB | ochratoxin B |
| QuEChERS | quick, easy, cheap, effective, rugged, safe |
| RSD | relative standard deviation |
| SPE | solid-phase extraction |
| SPSS | Statistical Package for Social Science |
| UPLC–MS/MS | ultraperformance liquid chromatography–tandem mass spectrometry |
| ZAN | zearalanone |
| ZEN | zearalenone |
| α-ZAL | α-zearalanol |
| α-ZOL | α-zearalenol |
| β-ZAL | β-zearalanol |
| β-ZOL | β-zearalenol |
Supplementary Materials
The following are available online at http://www.mdpi.com/2072-6651/10/10/409/s1, Figure S1: UPLC–MS/MS chromatograms of 21 targeted mycotoxins in a spiked (10-times LOQ) solvent sample, Figure S2: Effects of three MWCNT sorbents on matrix effect (%) of mycotoxins in corn. Vertical bar represents ± standard error (n = 3), Table S1: Optimized multiple-reaction monitoring (MRM) parameters for the 21 targeted mycotoxins.
Author Contributions
M.W. conceived and designed the experiments; D.J. wrote the paper; D.W. and L.W. performed the experiments; S.M. and Y.D. contributed reagents/materials.
Funding
This research was funded by the National Natural Science Foundation (Project No. 31701709), National Program for Quality and Safety Risk Assessment of Agricultural Products of China (Project No. GJFP2018001) and the Innovation and Capacity-building Projects by Beijing Academy of Agriculture and Forestry Sciences (Project No. KJCX20180408).
Conflicts of Interest
The authors declare no conflict of interest.
Key Contribution
A simple and effective sample pretreatment technique using MWCNT–COOH +C18 as one-step cleanup sorbent for purification of 21 mycotoxins in corn and wheat was developed.
References
- 1.Richard J.L. Some major mycotoxins and their mycotoxicoses—An overview. Int. J. Food Microbiol. 2007;119:3–10. doi: 10.1016/j.ijfoodmicro.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 2.Aiko V., Mehta A. Occurrence, detection and detoxification of mycotoxins. J. Biosci. 2015;40:943–954. doi: 10.1007/s12038-015-9569-6. [DOI] [PubMed] [Google Scholar]
- 3.Boevre M., Mavungu J.D., Landshchoot S., Audenaert K., Eeckhout M., Maene P. Natural occurrence of mycotoxins and their masked forms in food and feed products. World Mycotoxin J. 2012;5:207–219. doi: 10.3920/WMJ2012.1410. [DOI] [Google Scholar]
- 4.Pereira V.L., Fernandes J.O., Cunha S.C. Mycotoxins in cereals and related foodstuffs: A review on occurrence and recent methods of analysis. Trends Food Sci. Technol. 2014;36:96–136. doi: 10.1016/j.tifs.2014.01.005. [DOI] [Google Scholar]
- 5.Calori-Domingues M.A., Bernardi C.M., Nardin M.S., de Souza G.V., Dos Santos F.G., Stein Mde A., Gloria E.M., Dias C.T., de Camargo A.C. Co-occurrence and distribution of deoxynivalenol, nivalenol and zearalenone in wheat from Brazil. Food Addit. Contam. B. 2016;9:142–151. doi: 10.1080/19393210.2016.1152598. [DOI] [PubMed] [Google Scholar]
- 6.Bryła M., Waśkiewicz A., Podolska G., Szymczyk K., Jędrzejczak R., Damaziak K., Sułek A. Occurrence of 26 mycotoxins in the grain of cereals cultivated in Poland. Toxins. 2016;8:160. doi: 10.3390/toxins8060160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beltrána E., Ibáñez M., Portolés T., Ripollés C., Sancho J.V., Yusà V., Marín S., Hernández F. Development of sensitive and rapid analytical methodology for food analysis of 18 mycotoxins included in a total diet study. Anal. Chim. Acta. 2013;783:39–48. doi: 10.1016/j.aca.2013.04.043. [DOI] [PubMed] [Google Scholar]
- 8.Wang L., Wang Z., Gao W., Chen J., Yang M., Kuang Y., Huang L., Chen S. Simultaneous determination of aflatoxin B1 and ochratoxin A in licorice roots and fritillary bulbs by solid-phase extraction coupled with high-performance liquid chromatography-tandem mass spectrometry. Food Chem. 2013;138:1048–1054. doi: 10.1016/j.foodchem.2012.11.066. [DOI] [PubMed] [Google Scholar]
- 9.Walravensa J., Mikula H., Rychlik M., Asam S., Ediage E.N., Di Mavungua J.D., Van Landschoot A., Vanhaecke L., De Saeger S. Development and validation of an ultra-high-performance liquid chromatography tandem mass spectrometric method for the simultaneous determination of free and conjugated Alternaria toxins in cereal-based foodstuffs. J. Chromatogr. A. 2014;1372:91–101. doi: 10.1016/j.chroma.2014.10.083. [DOI] [PubMed] [Google Scholar]
- 10.Sun W., Han Z., Aerts J., Nie D., Jin M., Shi W., Zhao Z., De Saeger S., Zhao Y., Wu A. A reliable liquid chromatography-tandem mass spectrometry method for simultaneous determination of multiple mycotoxins in fresh fish and dried seafoods. J. Chromatogr. A. 2015;1387:42–48. doi: 10.1016/j.chroma.2015.01.071. [DOI] [PubMed] [Google Scholar]
- 11.Yang Y., Wen J., Kong W., Liu Q., Luo H., Wang J., Yang M. Simultaneous determination of four aflatoxins and ochratoxin A in ginger after inoculation with fungi by ultra-fast liquid chromatography–tandem mass spectrometry. J. Sci. Food Agric. 2016;96:4160–4167. doi: 10.1002/jsfa.7618. [DOI] [PubMed] [Google Scholar]
- 12.Juan C., Covarelli L., Beccari G., Colasante V., Mañes J. Simultaneous analysis of twenty-six mycotoxins in durum wheat grain from Italy. Food Control. 2016;62:322–329. doi: 10.1016/j.foodcont.2015.10.032. [DOI] [Google Scholar]
- 13.Wang M., Jiang N., Xian H., Wei D., Shi L., Feng X. A single-step solid phase extraction for the simultaneous determination of 8 mycotoxins in fruits by ultra-high performance liquid chromatography tandem mass spectrometry. J. Chromatogr. A. 2016;1429:22–29. doi: 10.1016/j.chroma.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 14.Zhou J., Xu J.J., Huang B.F., Cai Z.X., Ren Y.P. High-performance liquid chromatographic determination of multi-mycotoxin in cereals and bean foodstuffs using interference-removal solid-phase extraction combined with optimized dispersive liquid-liquid microextraction. J. Sep. Sci. 2017;40:2141–2150. doi: 10.1002/jssc.201601326. [DOI] [PubMed] [Google Scholar]
- 15.Ridgway K. Sample preparation for food contaminant analysis. LCGC Eur. 2012;25:60–71. [Google Scholar]
- 16.Senyuva H.Z., Gilbert J. Immunoaffinity column clean-up techniques in food analysis: A review. J. Chromatogr. B. 2010;878:115–132. doi: 10.1016/j.jchromb.2009.05.042. [DOI] [PubMed] [Google Scholar]
- 17.Hussain C.M., Saridara C., Mitra S. Modifying the sorption properties of multi-walled carbon nanotubes via covalent function alization. Analyst. 2009;134:1928–1933. doi: 10.1039/b823316k. [DOI] [PubMed] [Google Scholar]
- 18.Moazzen M., Ahmadkhaniha R., Gorji M.E.H., Yunesian M., Rastkari N. Magnetic solid-phase extraction based on magnetic multi-walled carbon nanotubes for the determination of polycyclic aromatic hydrocarbons in grilled meat samples. Talanta. 2013;115:957–965. doi: 10.1016/j.talanta.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 19.Dong M., Si W., Jiang K., Nie D., Wu Y., Zhao Z., De Saeger S., Han Z. Multi-walled carbon nanotubes as solid-phase extraction sorbents for simultaneous determination of type A trichothecenes in maize, wheat and rice by ultra-high performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. A. 2015;1423:177–182. doi: 10.1016/j.chroma.2015.10.068. [DOI] [PubMed] [Google Scholar]
- 20.Dong M., Si W., Wang W., Bai B., Nie D., Song W., Zhao Z., Guo Y., Han Z. Determination of type A trichothecenes in coix seed by magnetic solid-phase extraction based on magnetic multi-walled carbon nanotubes coupled with ultra-high performance liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2016;408:1–9. doi: 10.1007/s00216-016-9809-0. [DOI] [PubMed] [Google Scholar]
- 21.Jiang K., Huang P., Luan L., Fan K., Guo W., Zhao Z., Wu Y., Han Z. Iron (II, III) oxide/multi-walled carbon nanotube composite as solid-phase extraction sorbent followed by ultra-high performance liquid chromatography tandem mass spectrometry for simultaneous determination of zearalenone and type A trichothecenes in Salviae miltiorrhizae Radix et Rhizoma (Danshen) J. Chromatogr. A. 2017;1482:1–10. doi: 10.1016/j.chroma.2016.12.058. [DOI] [PubMed] [Google Scholar]
- 22.Van Eeckhaut A., Lanckmans K., Sarre S., Smolders I., Michotte Y. Validation of bioanalytical LC–MS/MS assays: Evaluation of matrix effects. J. Chromatogr. B. 2009;877:2198–2207. doi: 10.1016/j.jchromb.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 23.Park Y., Choe S., Lee H., Jo J., Park Y., Kim E., Pyo J., Jung J.H. Advanced analytical method of nereistoxin using mixed-mode cationic exchange solid-phase extraction and GC/MS. Forensic Sci. Int. 2015;252:143–149. doi: 10.1016/j.forsciint.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 24.Schenck F.J., Lehotay S.J., Vega V. Comparison of solid-phase extraction sorbents for cleanup in pesticide residue analysis of fresh fruits and vegetables. J. Sep. Sci. 2002;25:883–890. doi: 10.1002/1615-9314(20021001)25:14<883::AID-JSSC883>3.0.CO;2-7. [DOI] [Google Scholar]
- 25.Capriotti A.L., Cavaliere C., Foglia P., Samperi R., Stampachiacchiere S., Ventura S., Laganàt A. Multiclass analysis of mycotoxins in biscuits by high performance liquid chromatography-tandem mass spectrometry. Comparison of different extraction procedures. J. Chromatogr. A. 2014;1343:69–78. doi: 10.1016/j.chroma.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 26.Ying Y.F., Wu Y.L., Wen Y., Yang T., Xu X.Q., Wang Y.Z. Simultaneous determination of six resorcylic acid lactones in feed using liquid chromatography-tandem mass spectrometry and multi-walled carbon nanotubes as a dispersive solid phase extraction sorbent. J. Chromatogr. A. 2013;1307:41–48. doi: 10.1016/j.chroma.2013.07.079. [DOI] [PubMed] [Google Scholar]
- 27.Takagi A., Hirose A., Nishimura T., Fukumori N., Ogata A., Ohashi N., Kitajima S., Kanno J. Induction of mesothelioma in p53+/− mouse by intraperitoneal application of multi-wall carbon nanotube. J. Toxicol. Sci. 2008;33:105–116. doi: 10.2131/jts.33.105. [DOI] [PubMed] [Google Scholar]
- 28.Klötzel M., Gutsche B., Lauber U., Humpf H.U. Determination of 12 type A and B trichothecenes in cereals by liquid chromatography−electrospray ionization tandem mass spectrometry. J. Agric. Food Chem. 2005;53:8904–8910. doi: 10.1021/jf051501c. [DOI] [PubMed] [Google Scholar]
- 29.Nathanail A.V., Sarikaya E., Jestoim M., Godula M., Peltonen K. Determination of deoxynivalenol and deoxynivalenol-3-glucoside in wheat and barley using liquid chromatography coupled to mass spectrometry: On-line clean-up versus, conventional sample preparation techniques. J. Chromatogr. A. 2014;1374:31–39. doi: 10.1016/j.chroma.2014.11.046. [DOI] [PubMed] [Google Scholar]
- 30.Bernhardt K., Valenta H., Kersten S., Humpf H.U., Dänicke S. Determination of T-2 toxin, HT-2 toxin, and three other type A trichothecenes in layer feed by high-performance liquid chromatography-tandem mass spectrometry (LC-MS/MS)—Comparison of two sample preparation methods. Mycotoxin Res. 2016;32:89–97. doi: 10.1007/s12550-016-0244-z. [DOI] [PubMed] [Google Scholar]
- 31.Berthiller F., Schuhmacher R., Buttinger G., Krska R. Rapid simultaneous determination of major type A- and B-trichothecenes as well as zearalenone in maize by high performance liquid chromatography–tandem mass spectrometry. J. Chromatogr. A. 2005;1062:209–216. doi: 10.1016/j.chroma.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 32.Fu Z., Huang X., Min S. Rapid determination of aflatoxins in corn and peanuts. J. Chromatogr. A. 2008;1209:271–274. doi: 10.1016/j.chroma.2008.09.054. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.