Abstract
Early secretion of IL-12 by mouse dendritic cells (DCs) instructs T cells to make IFN-γ. However, only activated, but not naive T cells are able to license DCs for IL-12 production. We hypothesized that it might be due to different levels of CD40L expression on the surface of these cells, as CD40 signals are required for IL-12 production. Using quantitative cell-free systems incorporating CD40L in lipid bilayers combined with total internal reflection fluorescence microscopy and flow cytometry, we show that as low as ∼200 CD40L molecules/μm2 in combination with IL-4 is sufficient to induce IL-12 production by DCs. Remarkably, CD40L alone is adequate to induce IL-23 secretion by DCs. Thus, although activated T cells have somewhat higher levels of CD40L, it is the combination of CD40L and the cytokines they secrete that licenses DCs and influences the effector class of the immune response.
Introduction
Dendritic cells (DCs) are known as “professional” APCs because of their ability to activate naive T cells (1) and to initiate IFN-γ–mediated responses (TH1) by their ability to secrete IL-12 (2). Thus, the decision-making partner in the DC/T cell interaction is thought to be the DC. IL-12 is a heterodimeric cytokine composed of two covalently linked subunits, namely p40 and p35 (3). The p40 subunit of IL-12 also pairs with p19 to form IL-23 (4). However, the mechanism of IL-12 production during the primary TH1 response has remained unclear.
The current model holds the following: 1) DC and T cell activation are temporally separated; 2) Ag-bearing DCs provide IL-12 to naive T cells during their cognate interaction; and 3) the presence of DC-derived IL-12 induces naive T cells to produce IFN-γ. However, we have previously shown that the decision to make IL-12 is not intrinsic to the DC, as external cues are critical factor. For example, in the absence of T cells, the early presence of IFN-γ is a prerequisite for IL-12 production when DCs are exposed to LPS (5). This early IFN-γ could be supplied by NK cells or by Ag-activated, but not by naive T cells (6), which is also consistent with data obtained with human cells (7, 8). In addition, Ag-activated T cells can license DCs to produce IL-12 in the absence of IFN-γ (9), and we have shown that CD40L is essential for this process (6).
CD40L/CD40 interactions play a pivotal role not only by licensing DCs to prime cytotoxic T cells (10), but it is also a critical signal to induce IL-12 production from DCs (11). Although naive T cells express CD40L (12), we reasoned that perhaps the inability of naive T cells to induce IL-12 from DCs could be due to insufficient expression of CD40L molecules compared with Ag-activated T cells.
Various strategies have been used to stimulate B cells or DCs through CD40, such as insect cells expressing CD40L (13) stable transfection of cell lines J558 (11), NIH-3T3 (14), and HEK-293 cells (15). There are limitations to these strategies. For instance, we showed previously that activated DCs stimulated with NIH-3T3, CD40L-expressing cells induce IL-12 production only in the presence of IL-4 (6). Therefore, we were concerned that cell lines transfected with CD40L express/secrete biologically active molecules that could potentially affect the outcomes of CD40-expressing cells. In addition, because the expression level of CD40L on transfected cells is supraphysiological, we decided to take a quantitative approach to examine the role of CD40L in triggering IL-12 production from DCs.
In this study, we used three quantitative systems to compare the number of CD40L molecules on naive and Ag-activated T cells and DC IL-12 production. We used flow cytometry, total internal reflection fluorescence (TIRF) microscopy, lipid bilayers carrying various amounts of CD40L (CD40L lipid beads), and beads coated with histidine-tagged soluble CD40L (sCD40L; CD40L beads) to provide CD40 signaling to DCs. We found that a minimum of ∼200 molecules/μm2 of CD40L is required to induce IL-12 production from LPS-activated DCs (LPS-DCs), but only in the presence of IL-4. Surprisingly, IL-23 was readily secreted from LPS-DCs in the presence of CD40L alone, and its secretion showed an inverse correlation with IL-12. Collectively, these data suggest that although to some extend naive T cells express CD40L, mere engagement of CD40L with CD40 is not sufficient to license DCs for IL-12 production and that the cytokine milieu is an important factor in determining the effector class of immune response.
Materials and Methods
Mice
Eight- to fourteen-week-old TCR/Cyt 5C.C7-1 RAG2−/− transgenic mice specific for peptide 88–103 of moth cytochrome c (MCC), B10.A RAG2−/−, and 5C.C7 CD40L−/− mice were generated at the National Institute of Allergy and Infectious Diseases. All studies were carried out and approved in accordance with the Institutional Animal Care and Use Committee of the National Institutes of Health.
Media, reagents, and bacteria
Recombinant cytokines were obtained from PeproTech LPS and staphylococcal enterotoxin A (SEA) (Sigma-Aldrich). Cells were cultured in complete medium as described (6).
Generation of bone marrow–derived DCs
Bone marrow cells were flushed out of the femurs and tibias of B10.A RAG2−/− mice into complete medium and cultured at 1 × 106 cells per well in a 24-well plate supplemented with GM-CSF and IL-4 as described (6).
Generation of resting or LPS-activated bone marrow–derived DCs
Six-day bone marrow–derived DCs (BMDCs) cultures were either left untreated (resting DCs) or were preactivated with LPS (LPS-DCs) at a final concentration of 200 ng/ml for 18–24 h. DCs in these cultures were 80–90% CD11c+ cells determined by flow cytometry and, >80% LPS-DCs expressed CD40 (6).
Generation of naive T cells
Splenocytes from 5C.C7-1 RAG2−/− mice were incubated with a mixture of mAbs (BD Pharmingen) followed by negative selection with Dynabeads (Dynal Biotech) as described (6).
In vitro generation of Ag-activated/primed CD4+ T cells
Splenocytes from 5C.C7-1 RAG2−/− mice were cultured at 1 × 106 cells/ml plus 1 μM MCC 88–103 at 37°C for 5 d as described (6).
Ni-NTA beads coupled with histidine-tagged CD40L
Recombinant, soluble, mouse histidine-tagged extracellular CD40L (Glu 61/Leu 260 catalogue no. P27548) and sCD40L were obtained from R&D Systems (catalogue no. 1163-CL/CF). One hundred microliters (∼2 × 105) of agarose Ni-NTA beads (QIAGEN) were washed twice with 1 ml of cold PBS and incubated with 20 μg of sCD40L in a final volume of 500 μl PBS at room temperature for 1 h on a rotor mixer. Unbound CD40L was removed by washing three times with 1 ml of PBS before being used.
Coculture of BMDCs with CD40L beads
Resting DCs or LPS-DCs were cocultured with Ni-NTA agarose beads with or without CD40L (at a ratio of ∼3:1 DC:bead), in 96-well round-bottom plates for 48 h in the presence or absence of various cytokines. Culture supernatant (CSN) from these coculture assays were tested for the presence of cytokines.
In vivo model of sepsis for measuring IL-12 and IL-2 secretion from wild type or CD40L−/− mice
Mice were injected i.v. with 100 μl saline containing 10 μg SEA. Six hours later, blood was collected through intracardiac puncture. The serum cytokines were measured using a multiplex assay.
Coculture of BMDCs with T cells
1 × 105 sort-purified or bead-depleted CD4+ naive or Ag-activated 5C.C7 T cells were incubated with 2 × 104 of BMDCs with or without 0.1 μM MCC peptide 88–103 in 96-well U-bottom plates for 48 h. The CSN was used for measuring the cytokines. Cytokine kinetics was done in a 24-well plate with the final volume of 2 ml. At various time points, 100 μl of CSN was removed without addition of fresh medium.
Cytokine measurements
The concentrations of IL-12 in the cell-free medium were determined using an ELISA Kit from R&D Systems (Quantikine). In addition, IL-12 and IL-23 in the CSN were detected using Multiplex Cytokine Array (SearchLight).
Determining CD40L expression on T cells
Naive or activated T cells were stained with anti-mouse TCR, CD4, CD44, CD62L, and CD40L (SouthernBiotech). The mean fluorescence intensity (MFI) of CD40L on live (7AADneg) TCRβ+, CD4+ naive (CD62LhiCD44lo), and activated (CD62LloCD44hi) T cells was used to calculate the average number of CD40L molecules per cell. A standard curve was generated using FITC standard beads (Quantum FITC-5; Bangs Laboratories) that were coated with defined numbers of FITC molecules on the surface. The ratio of FITC to labeled Ab was used to convert the number of FITC molecules to the number of Ab molecules bound on the surface of naive and activated T cells.
Confocal microscopy
Agarose beads+/− histidine-tagged CD40L were stained with anti-mouse CD40L-PE (BD Pharmingen), and images of agarose beads were acquired with a confocal microscope using a 20× objective lens.
Time-lapse video microscopy
Images were acquired using time-lapse microscopy for 20 h. The images were collected on a LAS X Widefield Microscope System.
Scanning electron microscopy
Briefly, agarose beads with adherent cells were fixed and processed as described (16).
Lipid bilayer containing CD40L coupled to silica/glass beads
Silica beads supporting DGS-NTA(Ni)/DOPC bilayers were prepared as described (17). Ni-NTA liposomes were mixed with silica beads (5 μm) after washing; they were incubated with various concentrations of soluble histidine-tagged CD40L. The number of CD40L molecules on the beads was calculated as described (17).
TIRF microscopy
Supported planar lipid bilayers were prepared on cover glass as described (18). Histidine-tagged ligands (MHC class II I-Ek-T102S, ICAM-1, and CD80) were loaded onto each supported bilayer using Ni-NTA lipids in the flow chamber system. The flow chamber system was then brought to 37°C and 1 × 106 naive or Ag-activated T cells were injected into the chamber along with Alexa Fluor 647 conjugated 6xHis CD40 (sCD40) and T cells were imaged as described (17).
Statistical analysis
Statistical analyses, Student t test, ANOVA, and linear regression were performed using Prism and JMP software.
Results and Discussion
Naive T cells do not induce IL-12 from LPS-DCs
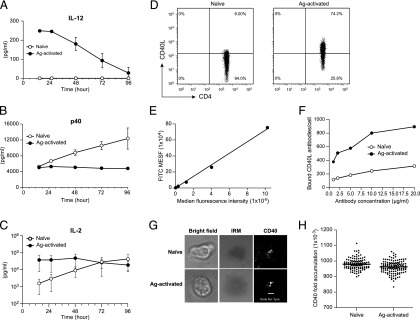
We have previously shown that Ag-activated, but not naive CD4+ T cells, can induce IL-12 secretion from LPS-DCs (44 independent experiments) (6). To investigate whether the inability of naive T cells to induce IL-12 from LPS-DCs reflected a kinetic delay, we cocultured naive T cells obtained from 5C.C7-1 RAG2−/− transgenic mice with LPS-DCs and measured IL-12 in the CSN at different time points. Although Ag-activated T cells rapidly induced IL-12 production from LPS-DCs, reaching a maximum at 12 h (Fig. 1A), continuous coculture of naive T cells with LPS-DCs for 4 d did not result in IL-12 production (Fig. 1A).
FIGURE 1.
Naive T cells do not induce IL-12 production from DCs. (A) BMDCs from B10.A-RAG2−/− mice were preactivated with 200 ng/ml LPS for 22 h after being washed and cocultured at 1 × 105 cells per well with 5 × 105 cells per well naive or Ag-activated 5C.C7-1 RAG2−/− T cells in the presence of 0.1 μm MCC in 24-well plates. CSN collected at various time points (without addition of fresh medium to the cells) were tested for the presence of IL-12 using cytokine array. These data are expressed as the mean ± SD of two independent experiments of single well. (B) Same conditions as (A), except the p40 subunit was measured in the CSN. (C) Same conditions as (A), except IL-2 was measured in the CSN. (D) Naive or Ag-activated T cells were stained with 1 μg of anti-CD40L and were analyzed using flow cytometry. (E) Standard curve generated using FITC standard beads with a previously determined number of FITC molecules on the surface of the beads. (F) Same conditions as (D), except naive or Ag-activated T cells were stained with various concentrations of anti-CD40L/FITC. The MFI of labeled cells was converted to the average number of FITC molecules per cell from a standard curve generated using FITC standard beads (E) representative of two independent experiments, single replicate. (G) A total of 1 × 106 naive or Ag-activated T cells along with Alexa Fluor 647 conjugated 6xHis CD40 (sCD40) were injected into the flow chamber systems (FCS2) that were loaded with MHC class II I-Ek-T102S, ICAM-1, and CD80 into Ni-NTA lipid–supported bilayer at 37°C. T cells were imaged for 30 min after stable synapse formation on the supported bilayer. Scale bar, 2 μm. (H) Same as (G), the average fluorescence intensities of naive and Ag-activated T cells determined by normalizing the images to the background to calculate the fold accumulation of CD40. Although the mean difference between naive and Ag-activated cells (H) was statistically significant, determined by Student t test. The magnitude of the difference between naive and Ag-activated T cells was quite small, and the statistical significance is entirely driven by the large sample sizes. Representative of two independent experiments of single replicate.
These findings are in agreement with human CD4+ T cells with a memory, but not naive phenotype (7). Furthermore, this is not specific to naive CD4+ T cells, as freshly isolated mouse splenic CD8+ T cells are unable to induce IL-12 production from CD8α+ DCs (19).
To confirm that LPS-DCs remained viable during their coculture with naive T cells, we measured the concentration of p40 subunit of IL-12 in the same CSN, which is secreted from DCs in response to damage-/pathogen-associated molecular patterns (20). Fig. 1B shows that naive T cells, but not Ag-activated T cells, induced p40, demonstrating that LPS-DCs were viable and able to secrete p40, but not the heterodimeric IL-12. The significance of the increased p40 production from LPS-DCs cocultured with naive T cells remains to be determined. Additionally, we measured IL-2 in the same CSN to confirm that the naive T cells were also viable (Fig. 1C). These results are at odds with the prevailing model of TH1 immune response, which emphasizes that interaction between naive T cells and DCs leads to IL-12 production. This poses an important conceptual question: how could IL-12 be the instigator of cell-mediated immunity to microbial pathogens if its induction is dependent on the prior presence of IFN-γ or activated T cells?
A possible explanation for the observed differences between naive and Ag-activated is, perhaps, the following: 1) surface molecule(s) preferentially expressed by Ag-activated, but not naive, T cells might be involved in IL-12 production; 2) these cells might differ in secretion of cytokine(s) that are conducive for IL-12 production; and 3) a combination of both. To dissect these possibilities, we started with CD40L, which has shown to play an important role in IL-12 production during the DC/T cell cross-talk (21). Previous studies have suggested that naive T cells do not express sufficient levels of CD40L (7, 12). Therefore, from the above data, we deduced that perhaps the inability of naive T cells to stimulate IL-12 production from LPS-DCs might be due to lower levels of CD40L expression. We took a quantitative approach to examine the expression of CD40L on naive and Ag-activated T cells by using flow cytometry (Fig. 1D). We used the MFI of beads with known number of FITC molecules to create a standard curve (Fig. 1E). This curve was used to calculate the number of CD40L molecules on T cells based on MFI of naive and Ag-activated T cells stained with anti-CD40L/FITC. Fig. 1F shows that Ag-activated T cells expressed 3-fold (∼600–900) more CD40L molecules per cell than naive T cells (∼200–300).
A 3-fold difference in cell surface expression of CD40L between naive and Ag-activated T cells is unlikely to explain the difference in IL-12 production from LPS-DCs. However, the distribution of CD40L on the surface of T cells has not been systematically investigated. We hypothesized that perhaps this may lead to difference in the distribution of CD40L in naive and Ag-activated T cells. We used TIRF microscopy of T cells interacting with supported lipid bilayers containing MHC–peptide Ag, CD80, ICAM-1, and used sCD40 protein as a probe for CD40L. Fig. 1G and 1H (quantification of the imaging data) shows that there are no major differences in distribution of CD40L and in CD40 accumulation by naive and Ag-activated T cells. To our knowledge, this is the first reported distribution of CD40L in microclusters when engaged with sCD40. Collectively, these data suggest that prior activation of T cells is an essential step for induction of IL-12 from DCs and the mere expression of CD40L on T cells is not sufficient to explain the differences between the naive and Ag-activated T cells.
CD40L−/− mice do not produce IL-12 in response to superantigen
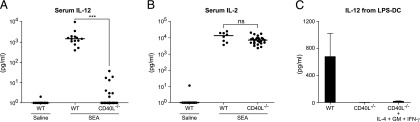
Thus, to better understand the role of CD40L/CD40 interaction and IL-12 production in vivo, we crossed 5C.C7-1 RAG2−/− transgenic mice with CD40L-deficient mice. We challenged these mice with the SEA and measured IL-12 production in their sera. We found a significant reduction in serum IL-12 in CD40L−/− mice in comparison with wild type (WT) (Fig. 2A). Additionally, we measured IL-2 in the same sera to determine T cell activation and found no significant difference in IL-2 levels between CD40L−/− and WT mice (Fig. 2B).
FIGURE 2.
Weak IL-12 response to superantigen in CD40L-deficient mice. (A) Serum IL-12 heterodimer from 5C.C7-1 RAG2−/− (WT) (n = 14) or 5C.C7-1 RAG2−/−CD40L−/− (n = 26) mice 6 h after i.v. injection with 10 μg SEA, or saline, as indicated. ***p < 0.001. (B) Same conditions as (A), except that serum IL-2 was measured. Student t tests were computed between WT and CD40L−/− groups for both IL-12 and IL-2. (C) BMDCs from B10.A-RAG2−/− mice were preactivated with 200 ng/ml LPS for 21 h, then washed and adjusted to 2 × 104 cells per well prior to coculture with 1 × 105 cells per well 5C.C7-1 RAG2−/− Ag-activated (WT) or CD40L−/− T cells plus 0.1 μm MCC in the presence or absence of cytokines at 100 ng/ml for 48 h in 96-well plates. CSN were tested for the presence of IL-12. These data are expressed as the mean ± SD in triplicate wells representative of two independent experiments.
To define the specific requirements for IL-12 production at the cellular levels, we examined the ability of purified Ag-activated T cells from CD40L−/− and WT mice to induce IL-12 production from DCs in vitro. Fig. 2C shows that only WT, but not CD40L−/− T cells cocultured with LPS-DCs induced high levels of IL-12 production. To rule out the possibility that CD40L−/− T cells were defective in producing those cytokines (IL-4, GM-CSF, and IFN-γ) normally secreted by WT T cells (Supplemental Fig. 1), which have been shown to stimulate IL-12 production from DCs, in particular IL-4 (22, 23), we added these cytokines to the coculture of CD40L−/− T cells with LPS-DCs to determine if they could reconstitute IL-12 production by DCs. Fig. 2C shows that in the absence of CD40L, combination of cytokines was not sufficient to induce IL-12 production. These data suggest that CD40/CD40L signaling is critical and cytokines cannot replace it. Thus, we decided to take a reductionist approach by addressing the role of CD40L presented on noncellular substrates to specifically define the requirements for induction of DC-derived IL-12 in the absence of any other confounding molecules.
LPS-DCs bind beads coated with sCD40L
Cells transfected with CD40L are commonly used to deliver signals to CD40-bearing cells. To prevent the possibility that CD40L-expressing cells might contribute to DCs activation by the expression of other immunostimulating molecules either as secreted product(s) or cell surface–associated molecules, we used a simple cell-free approach to deliver CD40L to CD40-expressing DCs. To test this, we used nickel agarose resins (Ni-NTA) that were coupled with sCD40L. We confirmed the immobilization of sCD40L to the beads by visualizing it using a confocal microscope after staining with anti-CD40L (Supplemental Fig. 2A, 2B).
Next, we examined the ability of LPS-DCs to bind CD40L beads. We visualized this binding using confocal microscope. Supplemental Fig. 2C shows that CD40 engagement with CD40L alone is strong enough to facilitate formation of stable conjugates between beads and LPS-DCs. Furthermore, a single DC (stained for CD11c+ cells) extended multiple processes that facilitated their binding simultaneously to more than one bead, mediating formation of bead/DC bead conjugates (Supplemental Fig. 2D, 2E). This binding was confirmed by scanning electron microscopy (Supplemental Fig. 2F). In addition, we used time-lapse microscopy to visualize interaction of LPS-DCs in the presence/absence of CD40L. Supplemental Videos 1 and 2 demonstrates that bead-adhering DCs move cellular extensions toward other CD40L beads, leading to the formation of bead DC/bead DC aggregates. Supplemental Fig. 2G shows the number of LPS-DCs bound to these beads.
Combination of CD40L beads and IL-4 induce IL-12 production by LPS-DCs
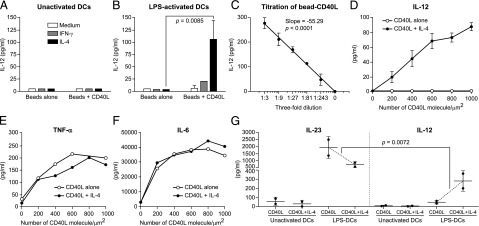
Next, we asked if this cell-free system (CD40L beads) could be used to deliver biological signals to DCs and induce IL-12 production. Fig. 3A and 3B demonstrate that IL-12 can be detected in the CSN after 48-h coculture of LPS-DCs (but not unactivated DCs) with CD40L bead only in the presence of IL-4, but not that of IFN-γ, and this response was dose dependent (Fig. 3C).
FIGURE 3.
Stimulation of DCs with CD40L beads. (A) BMDCs (unactivated) from B10.A-Rag2−/− mice were cocultured at 2 × 104 cells per well plus 50 μl of Ni-NTA agarose beads (∼3000 beads/well), which were either coated with ∼15 μg/ml sCD40L (CD40L+beads), or not (beads alone), in the presence or absence of cytokines at 100 ng/ml for 48 h in a 96-well U-bottom plate. CSN was tested for the presence of IL-12 using ELISA. (B) Same conditions as (A), except DCs were preactivated with 200 ng/ml LPS for 22 h, then washed and cocultured with beads alone or beads coated with CD40L. All pairwise comparisons were made using two-way ANOVA with Tukey post hoc tests of two independent experiments of duplicate wells. (C) Same condition as (B); LPS-DCs cocultured with serially diluted CD40L-beads (∼15 μg/ml) in the presence of constant (100 ng/ml) level of IL-4 for 48 h. CSN was tested for the presence of IL-12. Symbols and whiskers represent mean ± SD of triplicates, whereas line represents linear regression. (D) Same as (B); LPS-DCs cocultured with glass-supported Ni-NTA lipid bilayer beads that had been coated with the indicated number of histidine-tagged CD40L molecules in the presence or absence of IL-4 at 100 ng/ml for 48 h. CSN was tested for the presence of IL-12 heterodimer using ELISA and cytokine array. These data are expressed as the mean ± SD triplicate wells, representing two independent experiments. (E and F) Same conditions as (D), except CSN were tested for the presence of TNF-α and IL-6. (G) Same conditions as (A) and (B), except that IL-12 and IL-23 were measured in parallel. The impact of IL-4 on CD40L-induced IL-23 production by LPS-DC (a decrease) was compared with that of IL-4 on CD40L-induced IL-12 production by LPS-DC (an increase). The significance of this difference from a larger decrease in IL-23 to slightly smaller increase in IL-12 was analyzed with three-way ANOVA and a custom contrast to compare a difference of differences between the IL-12 increase and the IL-23 decrease using JMP software of two independent experiments of duplicate wells.
The stoichiometry of binding and signaling of the CD40/CD40L remain largely undefined. To investigate the effect of CD40L surface density, we adopted a quantitative system by using supported lipid bilayers (lipids with Ni-NTA head groups) formed on 5-μm–diameter silica beads. We incubated LPS-DCs with lipid-coated silica beads containing various number of CD40L molecules in the presence of constant level of IL-4. We found that CD40L alone, even at high concentrations, ∼1000 molecules/μm2, did not have any effect on IL-12 production from LPS-DCs (Fig. 3D). However, when combined with IL-4, beads containing a minimum of ∼200 molecules of CD40L/μm2 was sufficient to induce IL-12 production by LPS-DCs (Fig. 3D). The requirement for IL-4 was specific to IL-12, because no differences in TNF-α or IL- 6 production was observed in the same CSN (Fig. 3E, 3F).
Next, we asked if DCs need similar requirements for IL-23 production. We stimulated unactivated DCs or LPS-DCs with CD40L beads in the presence or absence of IL-4 and measured IL-23 and IL-12 in the same CSN after 48 h. Unexpectedly, CD40L alone was sufficient to induce high levels of IL-23 production by LPS-DCs and showed inverse correlation with IL-12 (Fig. 3G). Unlike IL-12, IL-4 reduced IL-23 production from LPS-DCs by ∼3-fold (Fig. 3G). Collectively, these data suggest that DCs have different requirements for IL-12 and IL-23 production and CD40/CD40L interactions differentially regulate these heterodimeric cytokines.
The prevailing view is that IL-4 inhibits, rather than inducing, IL-12 production. Therefore, why should IL-4–producing TH2 cells (albeit expressing CD40L and secrete IL-4) induce IL-12 production from DCs? We have previously shown that, because of the high levels of IL-10 secreted by TH2 cells (pushed to the extreme), which is known to be a potent inhibitor of IL-12 (24), TH2 cells cannot induce IL-12 production from DCs (6). However, when IL-10 was specifically removed from the CSN of TH2 cells, then the same CSN was conducive for IL-12 production (6). Furthermore, Ag-activated T cells that are used in this study (not pushed to the extreme) make both TH1 and TH2 cytokines and are able to induce IL-12 production from LPS-DCs (Supplemental Fig. 1). Interestingly, addition of IL-4 to the coculture of naive T cells with LPS-DCs resulted in IL-12 production (Supplemental Fig. 3), which is consistent with previous observations (25). Thus, the complexity of cytokine milieu (positive/negative feedback) and perhaps the plasticity associated with T cells during their interaction with DCs plays a critical role in influencing the effector class of immune responses. We propose that the initiation of IFN-γ response is like IL-4: it takes small amount of cytokine to jump-start more cytokine; one difference between IL-4 and of IFN-γ is that IL-12 comes later to amplify the response.
In summary, in addition to CD40L and TCR/MHC–peptide Ag signals, Ag-activated T cells must also provide IL-4 during their cognate interaction with Ag-bearing DC to license DCs for IL-12 production (Supplemental Fig. 4). Understanding the requirements for DC activation and secretion of IL-12 will help in designing more specific clinical protocols for cancer immunotherapies and vaccine developments.
Supplementary Material
Acknowledgments
We thank Dr. P. Matzinger for suggestions and critical review of the manuscript and Dr. N. Singh for help with T cells. We thank A. Hoofring and E. Tyler for illustrations, N. Quizon for help with lipid bilayer, and S. Ganesan for help with imaging.
This work was supported by the Intramural Research Program at the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
The online version of this article contains supplemental material.
- BMDC
- bone marrow–derived DC
- CSN
- culture supernatant
- DC
- dendritic cell
- LPS-DC
- LPS-activated DC
- MCC
- moth cytochrome c
- MFI
- mean fluorescence intensity
- sCD40L
- soluble CD40L
- SEA
- staphylococcal enterotoxin A
- TIRF
- total internal reflection fluorescence
- WT
- wild type.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Lassila O., Vainio O., Matzinger P. 1988. Can B cells turn on virgin T cells? Nature 334: 253–255. [DOI] [PubMed] [Google Scholar]
- 2.Trinchieri G. 2003. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 3: 133–146. [DOI] [PubMed] [Google Scholar]
- 3.Trinchieri G. 1995. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu. Rev. Immunol. 13: 251–276. [DOI] [PubMed] [Google Scholar]
- 4.Oppmann B., Lesley R., Blom B., Timans J. C., Xu Y., Hunte B., Vega F., Yu N., Wang J., Singh K., et al. 2000. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity 13: 715–725. [DOI] [PubMed] [Google Scholar]
- 5.Abdi K., Singh N., Matzinger P. 2006. T-cell control of IL-12p75 production. Scand. J. Immunol. 64: 83–92. [DOI] [PubMed] [Google Scholar]
- 6.Abdi K., Singh N. J., Matzinger P. 2012. Lipopolysaccharide-activated dendritic cells: “exhausted” or alert and waiting? J. Immunol. 188: 5981–5989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sallusto F., Lenig D., Förster R., Lipp M., Lanzavecchia A. 1999. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401: 708–712. [DOI] [PubMed] [Google Scholar]
- 8.Miro F., Nobile C., Blanchard N., Lind M., Filipe-Santos O., Fieschi C., Chapgier A., Vogt G., de Beaucoudrey L., Kumararatne D. S., et al. 2006. T cell-dependent activation of dendritic cells requires IL-12 and IFN-gamma signaling in T cells. J. Immunol. 177: 3625–3634. [DOI] [PubMed] [Google Scholar]
- 9.Abdi K., Singh N. J. 2010. Antigen-activated T cells induce IL-12p75 production from dendritic cells in an IFN-γ-independent manner. Scand. J. Immunol. 72: 511–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ridge J. P., Di Rosa F., Matzinger P. 1998. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature 393: 474–478. [DOI] [PubMed] [Google Scholar]
- 11.Cella M., Scheidegger D., Palmer-Lehmann K., Lane P., Lanzavecchia A., Alber G. 1996. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J. Exp. Med. 184: 747–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lesley R., Kelly L. M., Xu Y., Cyster J. G. 2006. Naive CD4 T cells constitutively express CD40L and augment autoreactive B cell survival. Proc. Natl. Acad. Sci. USA 103: 10717–10722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miyashita T., McIlraith M. J., Grammer A. C., Miura Y., Attrep J. F., Shimaoka Y., Lipsky P. E. 1997. Bidirectional regulation of human B cell responses by CD40-CD40 ligand interactions. J. Immunol. 158: 4620–4633. [PubMed] [Google Scholar]
- 14.Schultze J. L., Cardoso A. A., Freeman G. J., Seamon M. J., Daley J., Pinkus G. S., Gribben J. G., Nadler L. M. 1995. Follicular lymphomas can be induced to present alloantigen efficiently: a conceptual model to improve their tumor immunogenicity. Proc. Natl. Acad. Sci. USA 92: 8200–8204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ivanov R., Aarts T., Hagenbeek A., Hol S., Ebeling S. 2005. B-cell expansion in the presence of the novel 293-CD40L-sCD40L cell line allows the generation of large numbers of efficient xenoantigen-free APC. Cytotherapy 7: 62–73. [DOI] [PubMed] [Google Scholar]
- 16.Fischer E. R., Hansen B. T., Nair V., Hoyt F. H., Dorward D. W. 2012. Scanning electron microscopy. Curr. Protoc. Microbiol. 25: 2B.2.1–2B.2.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crites T. J., Padhan K., Muller J., Krogsgaard M., Gudla P. R., Lockett S. J., Varma R. 2014. TCR microclusters pre-exist and contain molecules necessary for TCR signal transduction. J. Immunol. 193: 56–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dustin M. L., Starr T., Varma R., Thomas V. K. 2007. Supported planar bilayers for study of the immunological synapse. Curr. Protoc. Immunol. 76: 18.13.1–18.13.35. [DOI] [PubMed] [Google Scholar]
- 19.Wong K. L., Lew F. C., MacAry P. A., Kemeny D. M. 2008. CD40L-expressing CD8 T cells prime CD8alpha(+) DC for IL-12p70 production. Eur. J. Immunol. 38: 2251–2262. [DOI] [PubMed] [Google Scholar]
- 20.Abdi K. 2002. IL-12: the role of p40 versus p75. Scand. J. Immunol. 56: 1–11. [DOI] [PubMed] [Google Scholar]
- 21.Banchereau J., Bazan F., Blanchard D., Brière F., Galizzi J. P., van Kooten C., Liu Y. J., Rousset F., Saeland S. 1994. The CD40 antigen and its ligand. Annu. Rev. Immunol. 12: 881–922. [DOI] [PubMed] [Google Scholar]
- 22.D’Andrea A., Ma X., Aste-Amezaga M., Paganin C., Trinchieri G. 1995. Stimulatory and inhibitory effects of interleukin (IL)-4 and IL-13 on the production of cytokines by human peripheral blood mononuclear cells: priming for IL-12 and tumor necrosis factor alpha production. J. Exp. Med. 181: 537–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hochrein H., O’Keeffe M., Luft T., Vandenabeele S., Grumont R. J., Maraskovsky E., Shortman K. 2000. Interleukin (IL)-4 is a major regulatory cytokine governing bioactive IL-12 production by mouse and human dendritic cells. J. Exp. Med. 192: 823–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.D’Andrea A., Aste-Amezaga M., Valiante N. M., Ma X., Kubin M., Trinchieri G. 1993. Interleukin 10 (IL-10) inhibits human lymphocyte interferon gamma-production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J. Exp. Med. 178: 1041–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abdi K., Herrmann S. H. 1997. CTL generation in the presence of IL-4 is inhibited by free p40: evidence for early and late IL-12 function. J. Immunol. 159: 3148–3155. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.