Abstract
Human mucosal tissues and skin contain two distinct types of dendritic cell (DC) subsets, epidermal Langerhans cells (LCs) and dermal DCs, which can be distinguished by the expression of C-type lectin receptors, Langerin and DC-SIGN, respectively. Although peripheral blood monocytes differentiate into these distinct subsets, monocyte-derived LCs (moLCs) induced by coculture with GM-CSF, IL-4, and TGF-β1 coexpress both Langerin and DC-SIGN, suggesting that the environmental cues remain unclear. In this study, we show that LC differentiation is TGF-β1 dependent and that cofactors such as IL-4 and TNF-α promote TGF-β1–dependent LC differentiation into Langerin+DC-SIGN− moLCs but continuous exposure to IL-4 blocks differentiation. Steroids such as dexamethasone greatly enhanced TNF-α–induced moLC differentiation and blocked DC-SIGN expression. Consistent with primary LCs, dexamethasone-treated moLCs express CD1a, whereas monocyte-derived DCs (moDCs) express CD1b, CD1c, and CD1d. moDCs but not moLCs produced inflammatory cytokines after stimulation with CD1b and CD1d ligands mycolic acid and α-galactosylceramide, respectively. Strikingly, CD1a triggering with squalene on moLCs but not moDCs induced strong IL-22-producing CD4+ helper T cell responses. As IL-22 is an important cytokine in the maintenance of skin homeostasis, these data suggest that CD1a on LCs is involved in maintaining the immune barrier in the skin.
Introduction
Two distinct types of dendritic cells (DCs) are localized in the skin and mucosal barriers to prevent the intrusion of pathogens from outside and to alert and eliminate tumor growth within the epidermis. In the skin, Langerhans cells (LCs) (1) are predominantly situated within epidermal area among the stratum spinosum (2), whereas DCs are positioned within dermal region, and these skin DC subsets are separated by a basement membrane (3, 4).
The critical difference between epithelial LCs and subepithelial DCs is that LCs exclusively express the C-type lectin receptor (CLR) Langerin, whereas DCs express DC-SIGN (5). Indeed, human LCs are characterized by the expression of CD1a and Langerin, which is associated with Birbeck granules (6). Previously, when the induction of LC-like cells from peripheral blood monocytes was reported (7), LC-like cells expressed both Langerin and DC-SIGN when monocytes were cultured with GM-CSF, IL-4, and TGF-β1. However, we and others have reported that LCs in the epidermis uniformly express Langerin but not DC-SIGN, whereas DCs predominantly expressed DC-SIGN but not Langerin (8). Also, DC-SIGN expression on the monocyte-derived LCs (moLCs) is markedly decreased by E-cadherin/E-cadherin interaction (9). These studies suggest that monocytes differentiate into moLCs expressing both Langerin and DC-SIGN, whereas additional signals are required to decrease DC-SIGN expression. Indeed, an inhibitory role of IL-4 on LC differentiation has been described (10), whereas DC-SIGN is induced by IL-4 on monocyte-derived DCs (moDCs) (11). Therefore, we have investigated the differentiation program that leads to the development of Langerin+DC-SIGN− LCs and found that short-term (48 h) exposure of IL-4 at the initiation of the culture promoted LC differentiation, whereas prolonged IL-4 stimulation interfered with LC differentiation. As corticosteroids prevent generation of dermal DCs but do not inhibit LC development (12), we speculated that steroids such as dexamethasone (Dex) can promote LC differentiation from monocytes but inhibit dermal DC development. Strikingly, our data show that the Dex strongly decreased DC-SIGN expression on moLCs during differentiation with GM-CSF, IL-4, TNF-α, and TGF-β1. In contrast, treatment of monocytes with the Notch ligand (DLL1) did not affect LC differentiation, but the disparity with previous study in which DLL1 induces LC differentiation (13) remains unclear.
Finally, taking advantage of the established moLC culture protocol, we examined the function of the CD1 molecules on the DC subsets. CD1a molecules were detected on moLCs, primary LCs, and moDCs, whereas moDCs expressed both CD1b and CD1d. On the basis of our recent observations showing that murine DCs expressing CD1d molecules are activated to secrete inflammatory cytokines by stimulating with the known CD1d-specific glycolipid α-galactosylceramide (α-GalCer) (14–16), we examined responses of purified DC-SIGN+ moDCs and Langerin+ moLC against lipid/glycolipid Ags. Purified human moDCs strongly responded to mycolic acids (MA) via CD1b to produce inflammatory cytokines such as TNF-α and IL-12 and weakly responded to α-GalCer via CD1d to secrete IL-12 but not TNF-α, whereas they did not respond to squalene, a ligand for CD1a. In contrast, purified LCs did not respond to any of the lipid Ags to produce inflammatory cytokines. Strikingly, moLCs treated with squalene strongly induced IL-22–expressing T cells. And, as IL-22 is an important cytokine in the maintenance of skin homeostasis for the establishment of external immune barrier (17, 18), our findings, therefore, suggest that LCs within the epidermis may assist in establishing external barriers and in their defense and repair by inducing IL-22–expressing T helper cells, whereas DCs within the dermal region may eliminate pathogenic intruders or tumors through the secretion of inflammatory cytokines.
Materials and Methods
Cytokines and reagents
The following cytokines and regents were commercially obtained: recombinant human GM-CSF, recombinant human IL-4, recombinant human TNF-α, recombinant human IL-13, recombinant human IL-15, and recombinant human IL-6 were all from PeproTech (Rocky Hill, NJ). Recombinant human TGF-β1, recombinant human Notch ligand (DLL1), recombinant human IL-1β, and recombinant human thymic stromal lymphopoietin (TSLP) were all obtained from R&D Systems (Minneapolis, MN). Dex was purchased from Sigma-Aldrich (St. Louis, MO). α-GalCer (KRN7000; Kyowa Hakko Kirin, Tokyo, Japan) was dissolved in DMSO (Sigma-Aldrich). Squalene (Sigma-Aldrich) was dissolved in ethanol (Sigma-Aldrich).
Mycobacterium tuberculosis Aoyama B was provided by the Research Institute of Tuberculosis/Japan Anti-Tuberculosis Association, (Tokyo, Japan). MA was obtained as described previously with some modifications (19). Briefly, M. tuberculosis Aoyama B was grown at 37°C on 7H9 medium (Difco, Detroit, MI) for 4 wk. MA was prepared by alkaline hydrolysis of glycolipids. The glycolipids were hydrolyzed with 10% NaOH at 70°C for 1 h, and the resultant MA were then extracted with n-hexane (Kanto Chemical, Tokyo, Japan) after acidification with HCl.
Cells
PBMCs were isolated from buffy coats of adult healthy volunteers using Lymphocyte Separation Medium (PromoCell, Heidelberg, Germany), by gradient centrifugation. CD14+ monocytes were purified from PBMCs by negative selection using EasySep Human Monocyte Enrichment Kit (STEMCELL Technologies, Vancouver, CA). After magnetic separation, 90–95% of the cells were CD14+ monocytes as measured by flow cytometry. Monocytes were resuspended in RPMI 1640–based complete cell medium (20) supplemented with 10 mM HEPES, 50 mM 2-ME, 2 mM l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin (all from Life Technologies, Waltham, MA), and 10% heat-inactivated FCS (HyClone Laboratories, Logan, UT) and seeded in 24-well tissue-culture plates at density of 1 × 106 cells/ml. To induce the formation of moDCs, monocytes were cultured with 100 ng/ml GM-CSF and 10 ng/ml IL-4, and, to obtain Langerin+ cells, monocytes were cultured with different combinations of the following cytokines: IL-4 was added for the first 2 d of the culture in the presence of GM-CSF and 10 ng/ml TGF-β1. In some experiments, a mixture of cytokines such as 100 ng/ml GM-CSF + 10 ng/ml TGF-β1, ± 10 ng/ml IL-4, ± 5 μg/ml DLL1, ± 20 ng/ml IL-6, ± 10 ng/ml IL-13, or ± 200 ng/ml IL-15 was used. DLL1 was coated in 24-well culture plates for 5 h at 37°C and then washed with PBS.
To determine the effect of IL-4 on the induction of moLCs, IL-4 was depleted at different time point from the culture medium in the presence of 100 ng/ml GM-CSF and 10 ng/ml TGF-β1. On day 2, IL-4 was depleted from the culture medium in the presence of 100 ng/ml GM-CSF and 10 ng/ml TGF-β1. In addition, monocytes were cultured with 100 ng/ml GM-CSF, 10 ng/ml IL-4, 10 ng/ml TGF-β1, and 20 ng/ml TNF-α in the absence or presence of various concentrations of Dex (10−10 to 10−5 M). The different cytokine combinations were GM-CSF + IL-4 (moDCs), GM-CSF + IL-4 + TGF-β1 (LC-like cells), GM-CSF + IL-4 (depleted on day 2) + TGF-β1, GM-CSF + IL-4 (depleted on day 2) + TGF-β1 + TNF-α (TNF-α–induced moLCs), and GM-CSF + IL-4 (depleted on day 2) + TGF-β1 + TNF-α + Dex (Dex–TNF-α–induced moLCs). Half of the total volume of the culture medium was replaced with fresh medium containing cytokines on day 2 and 4. For some experiments, moDCs and Dex–TNF-α–induced moLCs were stained with a mouse anti-Langerin IgG1 Ab (clone DCGM4; Beckman Coulter, Brea, CA) or with anti–DC-SIGN IgG1 Ab (clone 120507; R&D Systems) and purified by anti-mouse IgG1 beads using MACS separation columns (Miltenyi Biotec, Bergisch Gladbach, Germany). Gating strategies before and after purified DCs and LCs are shown in the Supplemental Fig. 1C and 1D.
Primary LCs were isolated based on the following procedure (21), with slight modifications. Normal healthy adult skin obtained from plastic surgery was used within 3 h after the operation. Three-millimeter-thick slices of skin, containing the epidermis and dermis, were obtained by using a dermatome. The slices were incubated with Dispase II (1 mg/ml; Roche Diagnostics, Branford, CT) in IMDM (Invitrogen), 10% FCS, and gentamicin (10 mg/ml) for 2 h at 37°C. Epidermis was mechanically separated, washed in medium, and cut into 1 mm2 pieces. Emigrant LCs were generated by floating the epidermis on IMDM, 10% FCS, 10 mg/ml gentamicin, and 80 ng/ml GM-CSF. After 3 d, the migrated cells were layered on a Ficoll gradient and cultured at 5 × 105/ml in IMDM, 10% FCS, 10 mg/ml gentamicin, and 80 ng/ml GM-CSF. Immature primary LCs were isolated by incubating epidermal sheets in PBS containing DNase I (20 U/ml; Roche Applied Science, Indianapolis, IN) and either trypsin (0.05%; Beckton Dickinson, Franklin Lakes, NJ) or collagenase blend F (0.25%; Sigma-Aldrich) for 30 min at 20–22°C. FCS was used to inactivate trypsin digestion and generated a single-cell suspension. Then, LCs were selected from layered cells on a Ficoll gradient using CD1a-labeled immune-magnetic microbeads (Miltenyi Biotec).
Flow cytometry analysis
The phenotypical characteristics of human CD14+ monocyte-derived cells were assessed by flow cytometry. After washing, cells were suspended in 100 μl of FACS buffer (PBS with 2% heat-inactivated FCS and 10 mM sodium azide). Nonspecific binding was blocked using 10 μg human Ig polyglobin (Nippon Red Cross, Tokyo, Japan). The cells were stained for 30 min at 4°C with different combinations of specific Abs or their isotype-matched control Abs, washed twice, and resuspended in FACS buffer. Then, labeled cells were analyzed with a FACSCanto II (BD Biosciences) and LSRFortessa X-20 Cell Analyzer (BD Biosciences) using FlowJo software (TreeStar, Ashland, OR). Live cells were gated based on propidium iodide (PI) gating. Gating strategy for obtaining cells is shown in the Supplemental Fig. 1.
The following mAbs were used: FITC- and allophycocyanin/Cy7-conjugated anti-CD1a (clone HI149; BioLegend, San Diego, CA), allophycocyanin-conjugated anti–E-cad (clone 67A4; BioLegend), FITC- and allophycocyanin-conjugated anti–DC-SIGN (R&D Systems), PE-conjugated anti-Langerin (clone DCGM4; Beckman Coulter), PE-conjugated anti-CD14 (clone M5E2; BD Biosciences), PECy7-conjugated anti-CD19 (clone HIB19; eBioscience), allophycocyanin Cy7–conjugated anti–HLA-DR (clone L243; BioLegend), Alexa Fluor 488–conjugated anti-CD86 (clone IT2.2; BioLegend), BV711-conjugated anti-CD11b (clone HI149; BD Biosciences), CD3-allophycocynin-Cy7 (clone HIT3A; BioLegend), CD4-BV421 (clone RPA-T4; BD Biosciences), CD8-FITC (clone SK1; BioLegend), CD1b-FITC (clone M-T101; BD Biosciences), allophycocyanin-CD1d (clone 51.1; BioLegend), biotin-conjugated anti-CD3 (clone UCHT1; BioLegend) secondarily stained with streptavidin-BV421 (BioLegend), and biotin-conjugated anti–HLA-DR (clone L243; BioLegend) secondarily stained with streptavidin–BV-650 (BD Biosciences).
RT-PCR
To examine the expression of mRNA for C-type–lectin and CD1 molecules, total RNA was extracted from 3 × 105 cells of each cell preparation using an RNeasy Kit (Qiagen, Hilden, Germany), and first-strand DNA was synthesized as described previously (22). PCR was performed with the following primers: GAPDH sense, 5′-GCC TCA AGA TCA TCA GCA ATG C-3′; GAPDH antisense, 5′-ATG CCA GTG AGC TTC CCG TTC-3′; CD1a sense, 5′-TTT TGC TAC TTC CAT TGT TA-3′; CD1a antisense, 5′-AGC TTC CTG AGA CCT TTC CA-3′; CD1b sense, 5′-TCA CCC CGC ATC CAC ATC AC-3′; CD1b antisense, 5′-AGA GAT ATC GGG GGC AGG TT-3′; CD1c sense, 5′-CGG GAT CCATGC TGT TTC TGC GAT TT-3′; CD1c antisense, 5′-ATT TGC GGC CGC CAG GAT GTC CTG ATA TGA GC-3′; CD1d sense, 5′-GGG TGC CTG CTG TTT CTG CT-3′; CD1d antisense, 5′-AGT GGG GCC TCT TGG GTT GG-3′; DC-SIGN sense, 5′-GAG CTT AGC AGG GTG TCT TG-3′; DC-SIGN antisense, 5′-GCA GGC GGT GAT GGA GTC GT-3′; Langerin sense, 5′-CGC ACT TCA CTG TGG ACA AA-3′; Langerin antisense, 5′-GAA TCC AGG GTG CTG ATG TT-3′. The PCR produces were detected by electrophoresis on 1.5% agarose gel containing ethidium bromide.
Measurement of cytokine production by ELISA
Cytokine production in the culture medium either of purified or unpurified moDCs and Dex–TNF-α–induced moLCs or of autologous CD4+ T cells stimulated by those APCs for 48 h with 500 μg/ml MA, 2.0 μg/ml α-GalCer, or 500 μM squalene was measured by ELISA kit for human TNF-α, IL-12p40, and IL-6 (all from BioLegend), as well as IL-10 and IL-22 (R&D Systems). The control cells were treated with n-hexane, DMSO, or ethanol, respectively.
Short interfering RNA transfection
Short interfering RNAs (siRNAs) targeting human CD1b (ON-TARGET plus siRNA, identifier [ID]: J-01499-05, 06, 07, and 08), targeting human CD1d (ON-TARGET plus siRNA ID: J-019429-05, 06, 07, and 08), human GAPDH (ID: D-001830-10-05), and negative control siRNA (IDs: D-001810-10-20 and D-001810-02-05) were obtained from Dharmacon (Horizon Discovery, Cambridge, U.K.), and siRNA electroporation of moDCs were performed as described previously (23). Briefly, moDCs were pulsed with the siRNAs (final 500 mM) at 250 V and 950 μF using Gene Pulser II apparatus (Bio-Rad Laboratories, Hercules, CA).
mRNA quantification by real-time PCR
The levels of mRNAs were measured by quantitative real-time PCR using a commercial kit (TaqMan Gene Expression Master Mix; Thermo Fisher Scientific, Waltham, MA) according to the manufacturer’s instruction with minor modifications. Briefly, 5 μl of Master Mix, 0.5 μl TaqMan Assay (Thermo Fisher Scientific), and 2.5 μl nuclease-free water were mixed and then 2 μl of cDNA (corresponding to 5 ng of RNA template) was added to the reaction mixture. The reaction was performed using PIKOREAL96 (Thermo Fisher Scientific). TaqMan Assay IDs are Hs00957537_m1 (CD1b), Hs00939888_m1 (CD1d), and Hs02786624_g1 (GAPDH). The relative expression was calculated by the equation 2^(−Δ cycle threshold[Ct]) × 1000. The Δ Ct value was calculated by subtraction of Ct values (target gene – internal control gene) using GAPDH as an internal control.
Western blotting
Cells were lysed using RIPA buffer (Merck Millipore, Darmstadt, Germany) supplemented with protease and phosphatase inhibitors (Roche Diagnostics, Basel, Switzerland). Cell lysate with the amount corresponding to 5 × 105 cells was loaded and separated on polyacrylamide gels (12.5%) and blotted to PVDF membrane. The target proteins were detected using primary Abs specific to human CD1d (clone 51.1; BioLegend), and the secondary Ab was HRP-conjugated anti-mouse IgG (Cell Signaling Technology). Protein bands were with the ECL Prime Western Blotting Detection kit (GE Healthcare, Buckinghamshire, UK) and imaged with ImageQuant LAS 4000 mini (GE Healthcare). The band intensities were quantified using ImageJ version 1.46r software (National Institutes of Health, Bethesda, MD).
Intracellular cytokine staining
To examine intracellular cytokines such as IL-12p40, TNF-α, and IL-10 in moDCs, purified DC-SIGN+ cells were stimulated with the indicated CD1-related lipid Ags for 48 h. Cells were restimulated for 4 h with 25 ng/ml PMA and 1 μg/ml ionomycin in the presence of 10 μg/ml Brefeldin A (all from Sigma-Aldrich) at 37°C. EDTA (2 mM; Sigma-Aldrich) was added for 10 min on ice to stop activation. Cells were then treated with Zombie Aqua Fixable Viability Kit (BioLegend) at room temperature for 15 min for dead cell discrimination and subsequently fixed with 4% paraformaldehyde (BD Biosciences) on ice for 20 min. The cells were permeabilized in FACSPerm (BD Biosciences), blocked in 1:50 mouse serum, and incubated for 30 min on ice with the following anti-human, mouse mAbs: IL-12/23p40-PE (clone C8.6; eBioscience), TNF-α–PE (clone MAB11; eBioscience), and IL-10–PE (clone JES3-9D7; eBioscience).
Additionally, naive CD4+ T cells were stimulated either with allogeneic moDCs or with allogeneic moLCs for 7 d. Using the method mentioned above, the production of IL-17 and IL-22 in CD4+ T cells were measured with the following anti-human, mouse mAbs: IL-17–PerCp-Cy5.5 (clone eBio17B7; eBioscience) and IL-22–allophycocyanin (clone 14298; R&D Systems).
CFSE-MLR proliferation assay
Naive CD4+ T cells were purified from PBMCs using human naive CD4+ T Cell Isolation Kit (BioLegend, San Diego, CA). To evaluate proliferative responses, purified naive CD4+ T cells were labeled with 10 μM CFSE (Thermo Fisher Scientific) at 37°C for 1 h. Thereafter, CSFE-labeled purified naive CD4+ T cells (1 × 105 cells per well) were cultured with either moDCs or moLCs (2 × 103 cells per well) in 96-well round-bottom plates for 7 d. As a negative control, wells for T cells alone were used. T cell proliferation was analyzed on day 7 of culture. The cells were harvested and stained with CD3-PE (clone SP34-2; BD Biosciences) for 30 min on ice. Live cells were gated based on PI gating. PI-negative and CD4+ cells were first gated and then plotted as CFSE versus CD3. Samples were acquired using a FACSCanto II flow cytometer and analyzed with FlowJo software. The CFSE-low cells were quantified as a percentage of proliferating cells in the culture.
Statistical analyses
Statistical analyses were performed with Prism (GraphPad Software, San Diego, CA). The results were analyzed using Student t test and are presented as the mean ± SEM. Differences of p < 0.05 were considered significant.
Study approval
All human healthy blood donors gave assigned informed consent. This study was approved by the Review Board of Nippon Medical School.
Results
Transient IL-4 exposure induces Langerin+ cells from CD14+ human monocytes
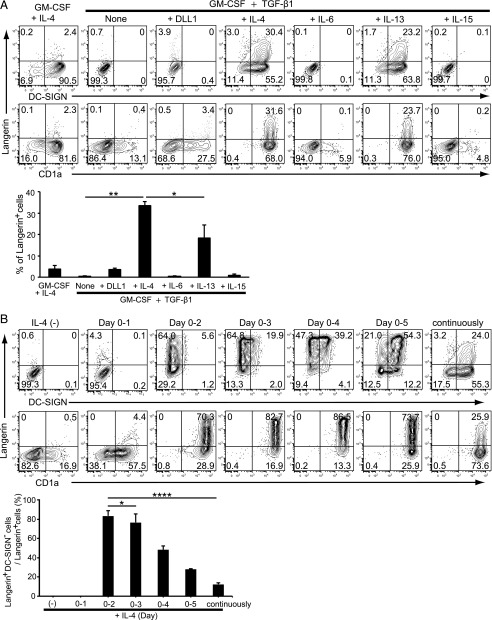
To determine which cytokines are required for the differentiation of human CD14+ monocytes into LCs, we first confirmed that GM-CSF, IL-4, and TGF-β1 induced the differentiation of CD14+ monocytes toward CD1a+ LC-like cells expressing both Langerin and DC-SIGN (7), whereas GM-CSF and IL-4 induced CD14+ monocytes differentiation into DCs (24) (Fig. 1A). Additionally, because IL-4 and IL-13 share the IL-4Rα-chain (25), we confirmed that IL-13, but neither IL-6 nor IL-15, could substitute for the effect of IL-4 (Fig. 1A). However, it should be noted that IL-4 has been reported as a DC-SIGN inducer (11), and thus it may inhibit LC differentiation from monocytes. Additionally, IL-4 has been shown to inhibit the induction of LCs from CD34+ hematopoietic stem cells (10). Indeed, it has recently been reported that Langerin+ cells could be generated with GM-CSF and TGF-β1 in serum-free condition without requiring IL-4 (26), whereas Langerin+ cells could not be induced by GM-CSF and TGF-β1 in the culture medium supplemented with 5% FCS (27). Moreover, it has been shown that Notch ligand Δ-1, in cooperation with GM-CSF and TGF-β1, promotes the differentiation of monocytes into LCs (13). We have compared the effect of 5 μg/ml Notch ligand (also called DLL1) with IL-4 on LC differentiation. However, LCs could not be generated from monocytes (Fig. 1A). These studies suggest that additional factors are involved in LC differentiation.
FIGURE 1.
Requirement of IL-4 for the induction of Langerin+ cells from CD14+ monocytes in the presence of GM-CSF and TGF-β1. (A) Requirement of IL-4–mediated stimulation for Langerin+ cells. Monocytes were cultured with 100 ng/ml GM-CSF and 10 ng/ml IL-4 or GM-CSF and TGF-β1 with or without 5 μg/ml DLL1, 10 ng/ml IL-4, 20 ng/ml IL-6, 10 ng/ml IL-13, or 200 ng/ml IL-15 for 6 d. The expression of Langerin and DC-SIGN was assessed by FACS analysis. The percentage of Langerin+ cells from four independent experiments is shown in the lower panel (+SEM). (B) Effects of IL-4 depletion from the culture condition on the differentiation of CD14+ monocytes into Langerin+ cells. Monocytes were cultured with 100 ng/ml GM-CSF and 10 ng/ml TGF-β1 without 10 ng/ml IL-4 or completely washed out from culture medium at different times on 6 d. The expression of Langerin and DC-SIGN was assessed at day 6 by FACS analysis. The percentage of Langerin+DC-SIGN− cells/Langerin+ cells from five independent experiments is shown in the lower panel (+SEM). *p < 0.05, **p < 0.01, ****p < 0.0001, Student t test.
In this study, we have confirmed that we could not elicit Langerin+ cells in the complete cell medium supplemented with 10% FCS (20) containing GM-CSF and TGF-β1 in the absence of IL-4 (9) (Fig. 1A). IL-4 is an essential element for LC differentiation as removal of IL-4 at day 1 leads to increased CD1a expression but no expression of either Langerin or DC-SIGN (Fig. 1B). However, when IL-4 was removed after 2 d (day 0–2), the percentage of Langerin+ cells was remarkably increased (Fig. 1B). Moreover, removal of IL-4 after 3–5 d increased the percentages of both Langerin+ and DC-SIGN+ cells (Fig. 1B). Interestingly, continuous presence of IL-4 decreased the percentage of Langerin+DC-SIGN− cells, whereas the number of DC-SIGN+ cells was markedly increased (Fig. 1B). Taken together, these findings clearly indicate that the Langerin+ LCs were efficiently induced by IL-4 when it was present during the first 2 d but that further exposure to IL-4 inhibited LC differentiation.
TNF-α augments Langerin while decreasing DC-SIGN expression
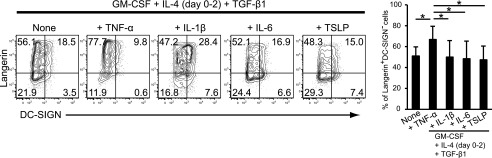
TNF-α induces Langerin expression on moLCs (28). TNF-α also stimulates the differentiation of LCs from CD34+ cord blood (29). Based on the findings, we examined the effect of TNF-α on Langerin and DC-SIGN expression on moLCs induced by GM-CSF, IL-4, and TGF-β1. Langerin and DC-SIGN expression were analyzed every 24 h for 6 d. IL-4 was removed from the culture medium 2 d after the initiation of culture (Fig. 1A). Expression of Langerin was predominantly enhanced, whereas expression of DC-SIGN was markedly suppressed in the presence of TNF-α (Supplemental Fig. 2A). However, expression of DC-SIGN was not completely inhibited on these so-called “TNF-α–induced moLCs.” We plotted the time course effect of TNF-α on the downmodulation of DC-SIGN (left panel of Supplemental Fig. 2B) and enhancement of Langerin expression (right panel of Supplemental Fig. 2B). The maximal Langerin expression induced by TNF-α was observed 4 d after initiating the culture. Therefore, we used moLCs 4 d after initiating the culture for the analysis of Langerin expression for future experiments.
Next, we investigated the effect of other inflammatory stimuli on the expression of Langerin and DC-SIGN on moLCs. In comparison with TNF-α, IL-1β, IL-6, and TSLP did not affect Langerin expression after 4 d of stimulation (left panels of Fig. 2). Thus, as reported previously, TNF-α is a unique and strong inflammatory stimulus for LC differentiation compared with IL-1β, IL-6, and TSLP stimuli (right panel of Fig. 2).
FIGURE 2.
Effect of TNF-α on the expression of Langerin and DC-SIGN in the presence of GM-CSF, TGF-β1, and IL-4. Effects of various proinflammatory cytokines on Langerin and DC-SIGN expression on cells from CD14+ monocytes in the presence of GM-CSF, IL-4 (day 0–2), and TGF-β1. Monocytes were cultured with GM-CSF, IL-4 (day 0–2), and TGF-β1 with or without 20 ng/ml TNF-α, 30 ng/ml IL-1β, 20 ng/ml IL-6, or 50 ng/ml TSLP for 4 d. The percentage of Langerin+DC-SIGN− cells from four independent experiments is shown in the right panel (+SEM). *p < 0.05, Student t test.
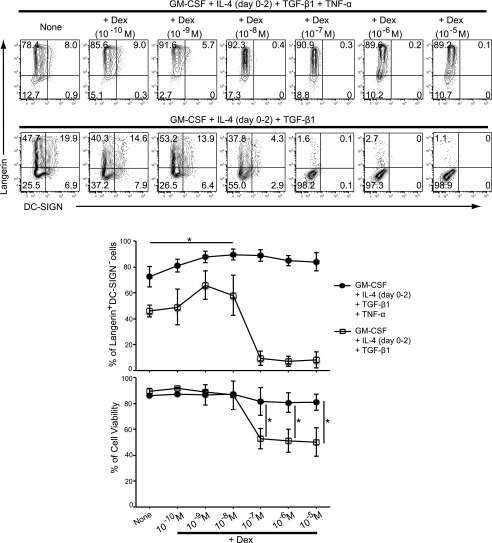
Effect of Dex on Langerin/DC-SIGN expression
Although moLCs induced with GM-CSF, IL-4 (day 0–2), TGF-β1, and TNF-α for 4 d were similar to primary LCs with regard to Langerin, they still expressed DC-SIGN. It has been reported that Dex induces downregulation of DC-SIGN (11). On the basis of these findings, we investigated the effect of Dex for further differentiation of TNF-α–induced moLCs by 4-d culture with GM-CSF, IL-4 (day 0–2), TGF-β1, and TNF-α by focusing on the DC-SIGN expression. We found that DC-SIGN expression on moLCs was significantly decreased in presence of more than 10−8 M Dex for 4 d (Fig. 3). Interestingly, LC differentiation was inhibited when TNF-α was removed from the medium (Fig. 3). Therefore, TNF-α is required for the LC differentiation by Dex. Moreover, we observed that Dex significantly affected Langerin+DC-SIGN− LC numbers (Fig. 3) and cell viability at concentration higher than 10−7 M (Fig. 3). Thus, we chose 10−8 M Dex in the generation of Dex–TNF-α–induced moLCs from monocytes.
FIGURE 3.
Effect of Dex on the expression of Langerin and DC-SIGN on cells from CD14+ monocytes in the presence of GM-CSF, IL-4, TGF-β1, and TNF-α. Monocytes were cultured in the presence of GM-CSF, IL-4, TGF-β1, and TNF-α with or without different concentrations of Dex (10−10–10−5 M) from initiating the culture for 4 d (upper panels). Monocytes were cultured in the presence of GM-CSF, IL-4, and TGF-β1 with or without different concentrations of Dex from initiating the culture for 4 d (lower panels). Surface expression of Langerin and DC-SIGN was analyzed by flow cytometry. Results shown are representative of four independent experiments, respectively. The percentage of Langerin+DC-SIGN− cells is shown in the middle panel (±SEM). Cell viability was assessed by PI exclusion. The percentage of cell viability from four independent experiments is shown in the bottom panel (±SEM). *p < 0.05, Student t test.
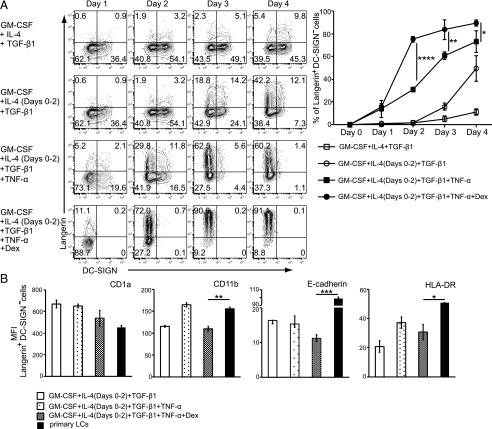
Next, we examined Langerin and DC-SIGN expression every day for 4 d in the different conditions (Fig. 4A). Dex–TNF-α–induced moLCs expressed high-levels of Langerin (as high as 91% at 96 h after starting the culture). Notably, the expression of DC-SIGN was seemingly negative throughout 4 d of Dex–TNF-α–induced moLCs. Consequently, the percentage of Langerin+DC-SIGN− LCs in each group was the highest on day 4 (Fig. 4A), and the cells also expressed CD1a, CD11b, E-cadherin, and HLA-DR (Fig. 4B). Thus, our data strongly suggest that the phenotype of the generated moLCs shows similar pattern with primary LCs.
FIGURE 4.
Phenotype of Langerin+ moLCs induced from CD14+ monocytes in the presence of GM-CSF and TGF-β1. (A) Monocytes were differentiated into Langerin+ cells using the following methods: GM-CSF, IL-4, and TGF-β1 (upper panel); GM-CSF, IL-4 (day 0–2), and TGF-β1 (second panel); GM-CSF, IL-4 (day 0–2), TGF-β1, and TNF-α (third panel); and GM-CSF, IL-4 (day 0–2), TGF-β1, TNF-α, and 10−8 M Dex (lower panel). The percentage of Langerin+DC-SIGN− cells from three independent experiments is shown in the right panel (±SEM). (B) The respective surface phenotype of moLCs compared with primary LCs. The surface expression of CD1a, CD11b, E-cadherin, and HLA-DR was analyzed by flow cytometry. Mean fluorescence intensity (MFI) from three independent experiments is shown in the right panel (+SEM). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, Student t test.
Phenotype and characteristics of Dex–TNF-α–induced moLCs
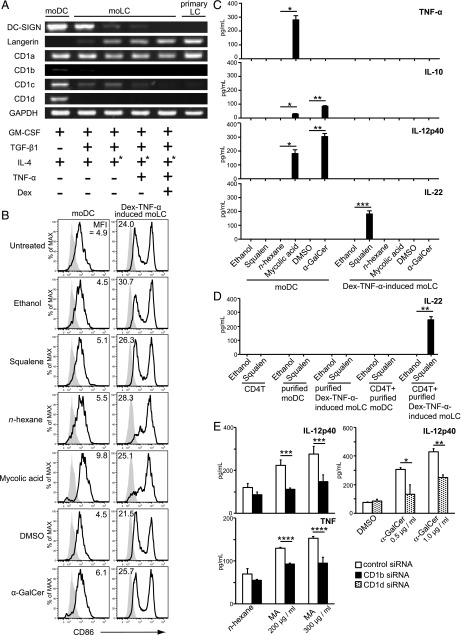
Using the obtained Dex–TNF-α–induced moLCs, which were quite similar to primary LCs, we compared their phenotype and characteristics with moDCs induced with GM-CSF and IL-4 and with LC-like cells induced with different stimuli for expression of DC-SIGN, Langerin, and the CD1 molecules (Fig. 5A). Similar to FACS analyses (Fig. 4), mRNA for DC-SIGN in Dex–TNF-α–induced moLC was undetectable, consistent with primary LCs from human skin (Fig. 5A). With regard to CD1 expression, DC-SIGN+Langerin− moDCs expressed CD1a, CD1b, CD1c, and CD1d expression, whereas moLCs did not express CD1d (Fig. 5A). Moreover, CD1b and CD1c decreased with downregulated DC-SIGN expression, whereas CD1a expression on moDCs was almost the same as primary LCs and Dex–TNF-α–induced moLCs.
FIGURE 5.
Analysis and comparison of the moDCs, moLCs established by various methods, and primary LC. (A) mRNA was isolated from moDCs, moLCs, and freshly isolated epidermal LCs. cDNA was amplified using the primers described in Materials and Methods. PCR products are shown in the figure. GAPDH was used as a control. IL-4 was washed out from culture medium at day 2 (+*). (B) moDCs and Dex–TNF-α–induced moLCs were resuspended in 24-well culture plates or test tubes at a concentration of 5 × 105 cells/ml. Squalene, MA, or α-GalCer was added, and the cells were incubated for 48 h. Subsequently, the cells were stained with anti-CD86 mAb and analyzed by flow cytometry compared with DMSO, n-hexane, or ethanol control, respectively. Gray histograms indicate the isotype-matched negative control. Results are representative of four independent experiments. (C) moDCs and Dex–TNF-α–induced moLCs were stimulated for 48 h with 500 μM squalene, 500 μg/ml MA, or 2.0 μg/ml α-GalCer. Levels of cytokines TNF-α, IL-10, IL-12p40, and IL-22 in the cell supernatants were examined by ELISA. Data are shown as the mean + SEM of results pooled from four independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001, Student t test. (D) Purified Dex–TNF-α–induced moLCs were cocultured with autologous CD4+ T cells for 48 h in the presence of 500 μM squalene. IL-22 production was measured in the culture supernatant using ELISA. Data are shown as the mean + SEM of results pooled from three independent experiments. **p < 0.01, Student t test. (E) Cytokine production of IL-12p40 and TNF-α by CD1b siRNA or CD1d siRNA transfected cells in response to the respective stimuli for 24 h was examined by ELISA. Data are shown as mean + SEM of the results pooled from five independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 by multiple comparisons using the Holm–Sidak Method.
Therefore, we examined the effect of the known human CD1-specific stimuli, squalene for CD1a, MA for CD1b (30), and α-GalCer for CD1d, on activation of moDCs or Dex–TNF-α–induced moLCs. Dex–TNF-α–induced moLCs showed enhanced CD86 expression even without activation, suggesting that treatment with Dex and TNF-α enhanced the expression of CD86 on moLCs (Fig. 5B). In comparison with the controls, squalene induced IL-22 production by CD1a-positive Dex–TNF-α–induced moLCs but not by CD1a-positive moDCs (Fig. 5C).
As Dex–TNF-α–induced moLC cultures also contain small numbers of autologous T cells, we investigated whether the LCs or T cells secreted IL-22. Upon squalene stimulation, IL-22 was not produced by purified CD4+ T cells, purified moDCs, Dex–TNF-α–induced moLCs, or in a coculture of autologous CD4+ T cells with purified moDCs. Strikingly, squalene induced IL-22 in a coculture of Dex–TNF-α–induced moLCs with autologous CD4+ T cells (Fig. 5D). These data strongly suggest that squalene binding to CD1a on Dex–TNF-α–induced moLCs leads to induction of IL-22–expressing CD4+ T cells. In contrast, CD1b or CD1d triggering on moDCs induced expression of various inflammatory cytokines by DCs alone, as shown by intracellular (Supplemental Fig. 3A) and extracellular (Supplemental Fig. 3B) analyses. Silencing of CD1b and CD1d on DCs by RNA interference (Supplemental Fig. 4) blocked the expression of proinflammatory cytokines (Fig. 5E). Collectively, as sentinels, epidermal LCs capture lipid Ags such as squalene via CD1a and activate IL-22–producing T cells, whereas dermal DCs were activated through CD1b or CD1d lipid Ag-presenting molecules with MA or α-GalCer and secreted various inflammatory cytokines.
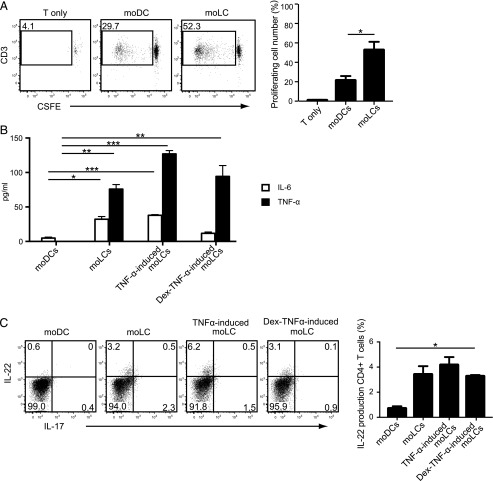
MoLC activate and induce IL-22–producing allogeneic T cells
These findings suggest that moLCs, in particular TNF-α–stimulated activated moLCs, seem to be more potent in stimulating T cells to produce IL-22. Thus, we then stimulated allogeneic T cells with either moLCs or moDCs by coculturing them in vitro to compare their ability for T cell expansion. The stimulatory potency for allogeneic T cell responses was far stronger in moLCs than moDCs (Fig. 6A). Moreover, the LC-activated allogeneic CD4+ T cells secreted IL-6 and TNF-α, which are cytokines required for IL-22 production (31) (Fig. 6B). Strikingly, IL-22 was produced by the CD4+ allogeneic T cells stimulated with moLCs even in the absence of squalene (right accompanied panel of Fig. 6C). These findings indicate that LC-activated IL-22–secreting CD4+ helper T cells seem to be the major effectors in skin defense and repair.
FIGURE 6.
Induction of allogeneic T cell proliferation by coculturing with TNF-α–stimulated activated moLC. (A) CSFE-labeled allogeneic naive CD4+ T cells (1 × 105 cells per well) were cultured with either moDCs or moLCs (2 × 103 cells per well) for 7 d. The number of proliferated cells is calculated and plotted. (B) The amount of IL-6 and TNF-α in the cell culture supernatants after coculture with moDCs or moLCs and naive allogeneic CD4+ T cells for 7 d was measured by ELISA. (C) Naive allogeneic CD4+ T cells stimulated by moDCs or moLCs were examined for intracellular cytokine expression of IL-22 and IL-17 by flow cytometry. Data are shown as the mean + SEM of results pooled from all three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001, Student t test.
Discussion
Two distinct types of DCs, epidermal LCs and dermal DCs, establish our skin barriers, and the more external LCs may provide protection for the more internal DCs. DCs are efficiently generated from monocytes by culturing with GM-CSF and IL-4, whereas the LC differentiation from monocytes is less efficient and the phenotype is not completely similar to primary LCs. This difficulty in inducing LC differentiation from monocytes has hindered investigation into the precise mechanisms underlying the interaction between LCs and DCs. Although direct isolation of primary epidermal LCs from the skin has been previously attempted (9, 21), it takes a significant amount of time and effort to obtain these primary LCs for their biological responses. Therefore, moLCs have typically been investigated even though their phenotype is not similar to primary LCs.
The critical difference between DCs and LCs is that DCs principally express DC-SIGN on their surface, whereas LCs predominantly express Langerin. DC-SIGN captures various Ags such as HIV-1 (5). Our observations in this study strongly indicated that the Langerin+ cells were efficiently induced when monocytes were stimulated for only the first 2 d with IL-4 in the presence of GM-CSF and TGF-β1. However, when IL-4 was continuously provided for the duration of the culture experiment, the percentage of Langerin+ cells was decreased and the number of DC-SIGN+ cells was remarkably increased. Therefore, initial transient stimulation of monocytes with IL-4 for 2 d seems to be critical and sufficient for the induction of Langerin+ cells.
Indeed, it has been reported that treatment of monocytes with IL-4 for 48 h is a critical time point when DC-SIGN induction occurs (11), and we found in this study that incubation with IL-4 more than 48 h will induce monocytes to become DC-SIGN+ cells. Therefore, removing the IL-4 stimulation within 48 h after culture initiation is critical for the induction of DC-SIGN− cells, and thus further stimulation with Langerin-inducing factors such as TGF-β1 (7, 27), TNF-α (28, 32), and Dex (11, 33) induces the DC-SIGN− monocytes to become Langerin+ LCs.
As shown in the current study, Langerin+ DC-SIGN− moLCs induced by GM-CSF, IL-4, TGF-β1, TNF-α, and Dex for 4 d were very similar to primary LCs in the expression of DC-SIGN and Langerin. In addition, the expression pattern of CD1 lipid Ag-presenting molecules was comparable between primary LCs and Dex–TNF-α–induced moLCs, which strongly expressed CD1a and weakly expressed CD1c but did not express CD1b and CD1d, whereas moDCs expressed all the four CD1s (CD1a, CD1b, CD1c, and CD1d). Stimulation of the Dex–TNF-α–induced moLCs with CD1a-specific ligand induced the differentiation of IL-22–producing CD4+ T cells. In contrast, moDCs did not induce differentiation of IL-22–producing T cells. moDCs secreted inflammatory cytokines such as TNF-α and IL-12p40 and a small amount of IL-10 by themselves when stimulated them with MA or α-GalCer, specific stimuli for CD1b and CD1d, respectively. These results suggest that DCs can be activated to secrete various inflammatory cytokines by stimulating via CD1b and CD1d molecules with their specific lipid ligands to fight against various pathogens and tumors, whereas LCs would not secrete cytokines by themselves but rather stimulate associated T cells to secrete IL-22 and thereby maintain skin homeopathy through CD1a-associated stimuli. In this study, we observed that moLCs were more potent to induce proliferation of allogeneic T cells to secrete IL-22. These findings are compatible to a recent report indicating that moLCs induce a stronger allogeneic T cell proliferation but release low amounts of inflammatory cytokines upon TLR stimulation (26). Thus, even though moLCs displayed a lower ability to respond to external foreign Ags by stimulation of CD1a molecules with lipid Ags than moDCs, they established a local defense and skin homeostasis through induction of IL-22–specific T cells.
Additionally, as we have reported recently using murine system, the known lipid Ag α-GalCer, which specifically stimulates invariant NKT cells, can also activate DCs through CD1d molecules and induce various cytokines to regulate the internal immune systems (16). Moreover, repetitive sequential stimulation with α-GalCer in the tumor-bearing mice preferentially activates DCs rather than invariant NKT cells, and the activated DCs elicit tumor-specific CD8+ CTLs to eliminate the tumor cells in vivo (14). Thus, tumor may be attacked after sequential stimulation of CD1d molecules on the DCs through their specific lipid ligands α-GalCer in the murine system.
In the current study, our data strongly suggest that IL-4 has a dual role in the differentiation of LCs; although it is required for their initiate differentiation, prolonged exposure inhibits LC differentiation. Steroids together with TNF-α also enhance LC differentiation and inhibit differentiation into DCs. The differentiation into LCs strongly affects the expression and activation of CD1 molecules. This study has identified important role for IL-4 and steroids in the differentiation of LCs, and this might be used in the generation of LCs from monocytes as well as understanding of LC-mediated immune inflammatory disorders.
Supplementary Material
Acknowledgments
We thank Drs. Yasuyuki Negishi and Mariko Ishibashi for helpful advice regarding experiments.
This work was supported by the Ministry of Education, Culture, Sports, Science and Technology (MEXT), the Ministry of Health and Labor and Welfare, Japan (Grants 25461715 and 16K09262 to H.T.), the Japanese Health Sciences Foundation, the Promotion and Mutual Aid Corporation for Private Schools of Japan, and a MEXT-supported Program for the Strategic Research Foundation at Private Universities, Japan. T.B.H.G. was supported by European Research Council Advanced Grant 670424.
The online version of this article contains supplemental material.
- Ct
- cycle threshold
- DC
- dendritic cell
- Dex
- dexamethasone
- α-GalCer
- α-galactosylceramide
- ID
- identifier
- LC
- Langerhans cell
- MA
- mycolic acid
- moDC
- monocyte-derived DC
- moLC
- monocyte-derived LC
- PI
- propidium iodide
- siRNA
- short interfering RNA
- TSLP
- thymic stromal lymphopoietin.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Merad M., Manz M. G., Karsunky H., Wagers A., Peters W., Charo I., Weissman I. L., Cyster J. G., Engleman E. G. 2002. Langerhans cells renew in the skin throughout life under steady-state conditions. [Published erratum appears in 2003 Nat. Immunol. 4: 92.] Nat. Immunol. 3: 1135–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khalil H. M., Nitiuthai S., Allen J. R. 1982. Alkaline phosphatase-positive Langerhans cells in the epidermis of cattle. J. Invest. Dermatol. 79: 47–51. [DOI] [PubMed] [Google Scholar]
- 3.Flacher V., Tripp C. H., Stoitzner P., Haid B., Ebner S., Del Frari B., Koch F., Park C. G., Steinman R. M., Idoyaga J., Romani N. 2010. Epidermal Langerhans cells rapidly capture and present antigens from C-type lectin-targeting antibodies deposited in the dermis. J. Invest. Dermatol. 130: 755–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van den Berg L. M., Cardinaud S., van der Aar A. M., Sprokholt J. K., de Jong M. A., Zijlstra-Willems E. M., Moris A., Geijtenbeek T. B. 2015. Langerhans cell-dendritic cell cross-talk via Langerin and hyaluronic acid mediates antigen transfer and cross-presentation of HIV-1. J. Immunol. 195: 1763–1773. [DOI] [PubMed] [Google Scholar]
- 5.Geijtenbeek T. B., Kwon D. S., Torensma R., van Vliet S. J., van Duijnhoven G. C., Middel J., Cornelissen I. L., Nottet H. S., KewalRamani V. N., Littman D. R., et al. 2000. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell 100: 587–597. [DOI] [PubMed] [Google Scholar]
- 6.Fehres C. M., Duinkerken S., Bruijns S. C., Kalay H., van Vliet S. J., Ambrosini M., de Gruijl T. D., Unger W. W., Garcia-Vallejo J. J., van Kooyk Y. 2017. Langerin-mediated internalization of a modified peptide routes antigens to early endosomes and enhances cross-presentation by human Langerhans cells. Cell. Mol. Immunol. 14: 360–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geissmann F., Prost C., Monnet J. P., Dy M., Brousse N., Hermine O. 1998. Transforming growth factor beta1, in the presence of granulocyte/macrophage colony-stimulating factor and interleukin 4, induces differentiation of human peripheral blood monocytes into dendritic Langerhans cells. J. Exp. Med. 187: 961–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Witte L., Nabatov A., Geijtenbeek T. B. 2008. Distinct roles for DC-SIGN+-dendritic cells and Langerhans cells in HIV-1 transmission. Trends Mol. Med. 14: 12–19. [DOI] [PubMed] [Google Scholar]
- 9.Mayumi N., Watanabe E., Norose Y., Watari E., Kawana S., Geijtenbeek T. B., Takahashi H. 2013. E-cadherin interactions are required for Langerhans cell differentiation. Eur. J. Immunol. 43: 270–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caux C., Massacrier C., Dubois B., Valladeau J., Dezutter-Dambuyant C., Durand I., Schmitt D., Saeland S. 1999. Respective involvement of TGF-beta and IL-4 in the development of Langerhans cells and non-Langerhans dendritic cells from CD34+ progenitors. J. Leukoc. Biol. 66: 781–791. [DOI] [PubMed] [Google Scholar]
- 11.Relloso M., Puig-Kröger A., Pello O. M., Rodríguez-Fernández J. L., de la Rosa G., Longo N., Navarro J., Muñoz-Fernández M. A., Sánchez-Mateos P., Corbí A. L. 2002. DC-SIGN (CD209) expression is IL-4 dependent and is negatively regulated by IFN, TGF-beta, and anti-inflammatory agents. J. Immunol. 168: 2634–2643. [DOI] [PubMed] [Google Scholar]
- 12.Woltman A. M., Massacrier C., de Fijter J. W., Caux C., van Kooten C. 2002. Corticosteroids prevent generation of CD34+-derived dermal dendritic cells but do not inhibit Langerhans cell development. J. Immunol. 168: 6181–6188. [DOI] [PubMed] [Google Scholar]
- 13.Hoshino N., Katayama N., Shibasaki T., Ohishi K., Nishioka J., Masuya M., Miyahara Y., Hayashida M., Shimomura D., Kato T., et al. 2005. A novel role for Notch ligand Delta-1 as a regulator of human Langerhans cell development from blood monocytes. J. Leukoc. Biol. 78: 921–929. [DOI] [PubMed] [Google Scholar]
- 14.Kogo H., Shimizu M., Negishi Y., Uchida E., Takahashi H. 2017. Suppression of murine tumour growth through CD8+ cytotoxic T lymphocytes via activated DEC-205+ dendritic cells by sequential administration of α-galactosylceramide in vivo. Immunology 151: 324–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murakami R., Nakagawa Y., Shimizu M., Wakabayashi A., Negishi Y., Hiroi T., Okubo K., Takahashi H. 2015. Effects of dendritic cell subset manipulation on airway allergy in a mouse model. Int. Arch. Allergy Immunol. 168: 219–232. [DOI] [PubMed] [Google Scholar]
- 16.Ichikawa T., Negishi Y., Shimizu M., Takeshita T., Takahashi H. 2016. α-Galactosylceramide-activated murine NK1.1(+) invariant-NKT cells in the myometrium induce miscarriages in mice. Eur. J. Immunol. 46: 1867–1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Jong A., Cheng T. Y., Huang S., Gras S., Birkinshaw R. W., Kasmar A. G., Van Rhijn I., Peña-Cruz V., Ruan D. T., Altman J. D., et al. 2014. CD1a-autoreactive T cells recognize natural skin oils that function as headless antigens. Nat. Immunol. 15: 177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim J. H., Hu Y., Yongqing T., Kim J., Hughes V. A., Le Nours J., Marquez E. A., Purcell A. W., Wan Q., Sugita M., et al. 2016. CD1a on Langerhans cells controls inflammatory skin disease. Nat. Immunol. 17: 1159–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kai M., Fujita Y., Maeda Y., Nakata N., Izumi S., Yano I., Makino M. 2007. Identification of trehalose dimycolate (cord factor) in Mycobacterium leprae. FEBS Lett. 581: 3345–3350. [DOI] [PubMed] [Google Scholar]
- 20.Takahashi H., Nakagawa Y., Leggatt G. R., Ishida Y., Saito T., Yokomuro K., Berzofsky J. A. 1996. Inactivation of human immunodeficiency virus (HIV)-1 envelope-specific CD8+ cytotoxic T lymphocytes by free antigenic peptide: a self-veto mechanism? J. Exp. Med. 183: 879–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Witte L., Nabatov A., Pion M., Fluitsma D., de Jong M. A., de Gruijl T., Piguet V., van Kooyk Y., Geijtenbeek T. B. 2007. Langerin is a natural barrier to HIV-1 transmission by Langerhans cells. Nat. Med. 13: 367–371. [DOI] [PubMed] [Google Scholar]
- 22.Yagi Y., Watanabe E., Watari E., Shinya E., Satomi M., Takeshita T., Takahashi H. 2010. Inhibition of DC-SIGN-mediated transmission of human immunodeficiency virus type 1 by Toll-like receptor 3 signalling in breast milk macrophages. Immunology 130: 597–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shinya E., Shimizu M., Owaki A., Paoletti S., Mori L., De Libero G., Takahashi H. 2016. Hemopoietic cell kinase (Hck) and p21-activated kinase 2 (PAK2) are involved in the down-regulation of CD1a lipid antigen presentation by HIV-1 Nef in dendritic cells. Virology 487: 285–295. [DOI] [PubMed] [Google Scholar]
- 24.Sallusto F., Lanzavecchia A. 1994. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J. Exp. Med. 179: 1109–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Izuhara K., Shirakawa T. 1999. Signal transduction via the interleukin-4 receptor and its correlation with atopy. Int. J. Mol. Med. 3: 3–10. [DOI] [PubMed] [Google Scholar]
- 26.Picarda G., Chéneau C., Humbert J. M., Bériou G., Pilet P., Martin J., Duteille F., Perrot P., Bellier-Waast F., Heslan M., et al. 2016. Functional Langerinhigh-expressing Langerhans-like cells can arise from CD14highCD16- human blood monocytes in serum-free condition. J. Immunol. 196: 3716–3728. [DOI] [PubMed] [Google Scholar]
- 27.Guironnet G., Dezutter-Dambuyant C., Vincent C., Bechetoille N., Schmitt D., Péguet-Navarro J. 2002. Antagonistic effects of IL-4 and TGF-beta1 on Langerhans cell-related antigen expression by human monocytes. J. Leukoc. Biol. 71: 845–853. [PubMed] [Google Scholar]
- 28.Geissmann F., Dieu-Nosjean M. C., Dezutter C., Valladeau J., Kayal S., Leborgne M., Brousse N., Saeland S., Davoust J. 2002. Accumulation of immature Langerhans cells in human lymph nodes draining chronically inflamed skin. J. Exp. Med. 196: 417–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arrighi J. F., Soulas C., Hauser C., Saeland S., Chapuis B., Zubler R. H., Kindler V. 2003. TNF-alpha induces the generation of Langerin/(CD207)+ immature Langerhans-type dendritic cells from both CD14-CD1a and CD14+CD1a- precursors derived from CD34+ cord blood cells. Eur. J. Immunol. 33: 2053–2063. [DOI] [PubMed] [Google Scholar]
- 30.Layre E., Collmann A., Bastian M., Mariotti S., Czaplicki J., Prandi J., Mori L., Stenger S., De Libero G., Puzo G., Gilleron M. 2009. Mycolic acids constitute a scaffold for mycobacterial lipid antigens stimulating CD1-restricted T cells. Chem. Biol. 16: 82–92. [DOI] [PubMed] [Google Scholar]
- 31.Hitzler M., Majdic O., Heine G., Worm M., Ebert G., Luch A., Peiser M. 2012. Human Langerhans cells control Th cells via programmed death-ligand 1 in response to bacterial stimuli and nickel-induced contact allergy. PLoS One 7: e46776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bechetoille N., André V., Valladeau J., Perrier E., Dezutter-Dambuyant C. 2006. Mixed Langerhans cell and interstitial/dermal dendritic cell subsets emanating from monocytes in Th2-mediated inflammatory conditions respond differently to proinflammatory stimuli. J. Leukoc. Biol. 80: 45–58. [DOI] [PubMed] [Google Scholar]
- 33.Piemonti L., Monti P., Allavena P., Leone B. E., Caputo A., Di Carlo V. 1999. Glucocorticoids increase the endocytic activity of human dendritic cells. Int. Immunol. 11: 1519–1526. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.