Abstract
Objectives. To assess the potential impact of preexposure prophylaxis (PrEP) on the HIV epidemic among Black and White adolescent sexual minority males (ASMM).
Methods. We used a network model and race-specific data from recent trials to simulate HIV transmission among a population of Black and White 13- to 18-year-old ASMM over 20 years. We estimated the number of infections prevented (impact) and the number needed to treat to prevent an infection (efficiency) under multiple coverage and adherence scenarios.
Results. At modeled coverage and adherence, PrEP could avert 3% to 20% of infections among Black ASMM and 8% to 51% among White ASMM. A larger number, but smaller percentage, of infections were prevented in Black ASMM in all scenarios examined. PrEP was more efficient among Black ASMM (number needed to treat to avert an infection = 25–32) compared with White ASMM (146–237).
Conclusions. PrEP can reduce HIV incidence among both Black and White ASMM but is far more efficient for Black ASMM because of higher incidence.
Public Health Implications. Black ASMM communities suffer disproportionate HIV burden; despite imperfect adherence, PrEP programs could prevent HIV efficiently in these communities.
Adolescent sexual minority males (ASMM)—those who identify as gay or bisexual, or are sexually active with other males—have significant HIV risk. An estimated 18% of new HIV diagnoses in the United States occur in men who have sex with men (MSM) younger than 25 years, with an unknown percentage reflecting incidence by age 18 years.1 Estimates of incidence for ASMM are hard to obtain because the population size at risk is not clearly defined and infections among youths often remain undiagnosed until later in life. A Chicago, Illinois, study found HIV incidence as high as 5.2 per 100 person-years at risk among 16- to 17-year-old ASMM.2 Additionally, several studies have found significant HIV burden among young adult MSM (7.0% prevalence among those aged 18–19 years3; 11% to 14% prevalence among those aged 18–22 years),4,5 suggesting nontrivial incidence before age 18 years.
As with adult MSM, this burden is not equal across racial and ethnic groups. Although race-specific estimates for ASMM are scarce, prevalence estimates among young adult MSM—reflecting cumulative incidence—show marked disparities. In 2014, the National HIV Behavioral Surveillance study estimated HIV prevalence among 18- to 24-year-old Black and White MSM as 26% and 3%, respectively.5 In the InvolveMENt cohort, baseline HIV prevalence among 18- to 24-year-old Black and White MSM in Atlanta was 30% and 5.5%, respectively.3
Preexposure prophylaxis (PrEP) with daily combined oral tenofovir and emtricitabine is a safe and effective intervention to prevent HIV.6–8 Initial PrEP clinical trials and demonstration projects for MSM included only adults aged 18 years and older,6–11 with adolescent data only recently published.12–14 Consequently, the US Centers for Disease Control and Prevention (CDC) published clinical practice guidelines indicating PrEP use for sexually active adult MSM at substantial HIV risk,15 and as of 2014 also recommends that for adolescents, the risks and benefits of PrEP be weighed carefully in the context of local regulations.15
Recent studies have focused on the unique challenges and opportunities for PrEP program implementation among both young adult MSM (Adolescent Trials Network [ATN] 110) and ASMM (ATN 113) in the United States. ATN 110 (ages 18–22 years) found greater than 50% biomarker-assessed PrEP adherence at the highest dosing level (equivalent to 4 or more doses/week) through 12 weeks, with a subsequent decline when clinical visits were reduced from 4- to 12-week intervals.11 ATN 113 (ages 15–17 years) found lower overall adherence than ATN 110, with a similar pattern of greater than 50% high adherence until visit frequency was reduced, after which high adherence declined to about 30%.12 In both studies, protocols included adherence counseling. ATN 110 also found significant racial disparities in adherence, and it is reasonable to assume that these would extend to ASMM; this could limit the impact of PrEP among Black ASMM and potentially increase the HIV incidence disparity, even if incidence overall declined.11 Other studies have found PrEP uptake to be higher among White adult MSM than among Black adult MSM, despite similar or higher interest in PrEP among Black men.16,17
In a previous modeling study,18 we found that PrEP use among sexually active 16- to 18-year-old ASMM in high-prevalence US settings could reduce new HIV infections considerably in this population, with number-needed-to-treat (NNT) estimates between 27 and 34, indicating that PrEP among ASMM could be a cost-effective intervention despite lower adherence than among adult MSM. However, that study did not explore differences by race. We expect that the inclusion of race in our model will have 2 impacts on HIV outcomes and thus on PrEP efficiency. First, if Black ASMM have higher HIV incidence than do White ASMM, as data among young adult MSM suggest,3,5 PrEP should be more efficient (i.e., have a lower NNT) for Black ASMM than for White ASMM. However, lower uptake16 and adherence11 among Black ASMM might attenuate PrEP effectiveness and substantially reduce efficiency in this population.19 These impacts are countervailing, so modeling can be useful in assessing their interactions.
In this study, we extend our model from a prior study to consider HIV transmission among Black and White ASMM and assess the potential impact of PrEP on HIV incidence and future disparities in higher prevalence areas of the United States, given differences in HIV burden and potential differences in PrEP uptake and adherence.
METHODS
We used a stochastic, dynamic, network model18 of an open cohort of 13- to 18-year-old ASMM that included 2 race groups of equal size. We chose these age bounds on the basis of assessments of reasonable risk, data availability, and an interest within the CDC in prevention programs covering the age range of youths in high school. ASMM could enter the modeled population at any age and become available for relationships immediately or at a future time point based on age-specific probabilities. These probabilities ensured that most sexual contacts were between individuals toward the top of the age range. We modeled the relationships composing the network using separable-temporal exponential-family random graph models,20 and we implemented the model using the EpiModel software platform (http://www.epimodel.org).21 Within each relationship, we determined anal intercourse (AI), condom use, and role selection stochastically. In addition, individuals could test for HIV, initiate or terminate treatment, and initiate or terminate PrEP. We also modeled intrahost viral dynamics and vital dynamics. For further details, see the Technical Supplement (available as a supplement to the online version of this article at http://www.ajph.org). Code for model specification, parameterization, and simulation is available at https://github.com/statnet/ASMMPreP_Two_Race_Groups.
Updates from our previous model18 specific to this analysis included the addition of a race attribute, assortative mixing by race, and race-specific PrEP coverage and adherence. We divided the population equally between non-Hispanic Black and White ASMM, reflecting the 2 largest racial/ethnic groups in a community similar to Atlanta, Georgia, the focus of our earlier work on racial disparities.22 The model included a hazard of infection from MSM above the modeled age range; differences in this parameter by race reflect reported differences in HIV prevalence among 18- to 24-year-old Black and White MSM (26% and 3%, respectively)5 and high rates of assortative mixing by race.
We calibrated the model using approximate Bayesian computation to estimate parameter values that produced 7% overall HIV prevalence among sexually active 18-year-old ASMM, the prevalence target for which we had the strongest empirical data,23 and a 2.1-to-1 race disparity. We used 3 parameters for calibration, each based on data that were outdated or subject to desirability bias: frequency of AI within ongoing relationships between 2 ASMM and 2 race-specific weekly probabilities of HIV infection from non-ASMM partners. We assumed a uniform prior distribution (range = 1–10) as multipliers for each.
For parameter sets yielding approximately 7% HIV prevalence among sexually active 18-year-old ASMM, the maximum Black–White prevalence ratio generated was 8.9, with a 95% credible interval (the middle 95% of simulated data) of 4.3 to 23.9. Three data sources provided estimates for the target ASMM Black–White prevalence ratio: the InvolveMENt study (12.4-fold disparity),3 the CDC surveillance rate ratio for 15- to 19-year-old youths (20.1-fold),1 and the same CDC report for 13- to 19-year-old youths, adjusted for the proportion of cases among MSM (both injection drug users and nonusers) by race (17.9-fold). All 3 estimates were higher than our generated mean, consistent with the literature reporting challenges in generating observed HIV disparities from reported behavioral data22; however, all estimates were within our 95% credible interval, so we accepted our parameters as reasonable but conservative estimates.
Preexposure Prophylaxis Implementation Scenarios
Our initial implementation scenario considered PrEP for ASMM aged 16 to 18 years who had initiated AI, with a 6-month delay between AI initiation and PrEP initiation, reflecting the average interval expected when sexual debut occurs between annual health care visits. We defined coverage as the proportion of those meeting PrEP eligibility criteria who were currently using PrEP at any adherence level. Individuals could terminate PrEP while still eligible; we modeled 50% PrEP discontinuation by 48 weeks, reflecting ATN 113 data on participants with no detectible PrEP at that time.12
Our research plan comprised 4 sets of analyses. First, we assumed no difference by race in PrEP coverage, discontinuation, or adherence. We modeled 40% coverage to match previous work, and included 4 levels of PrEP adherence corresponding to no measurable adherence, low (< 2 pills/week), medium (2–3 pills) and high (≥ 4 pills) adherence, with 20.9%, 24.4%, 13.1%, and 41.6% of PrEP users adhering at each level, respectively; these reflect adherence averaged across all visits (4–48 weeks) in ATN 113.12 Per-act transmission was reduced at each adherence level by 0%, 31%, 81%, and 95%, respectively.19,24
Second, we introduced disproportionate PrEP coverage by race. Applying a 2.1-fold difference16 to our base case with 40% coverage overall yielded race-specific coverage of 25.8% and 54.2% for Black and White ASMM, respectively. Note that (25.8%+54.2%)/2 = 40%, the target coverage for the population, and 54.2%/25.8% = 2.1, the race disparity. We then systematically varied average coverage from 10% to 60% while maintaining the 2.1 race ratio. Table 1 shows the race-specific coverage levels. (Note that weighting absolute uptake from a demonstration trial9 by that trial’s metropolitan demographic composition yielded a qualitatively similar 1.8-fold disparity.)
TABLE 1—
HIV Prevalence and Modeled Scenarios for Evaluating the Effects of Preexposure Prophylaxis (PrEP) on HIV Prevalence Among US Black and White Adolescent Sexual Minority Males
| Analysis | PreP Coverage, % |
HIV Prevalence (%) at Age 18 Years, Median (95% CrI) |
Black–White Prevalence Ratio, Median (95% CrI)a | Absolute Difference in Prevalence, Median (95% CrI)b | ||||
| All | Black | White | All | Black | White | |||
| Baseline | 0 | 7.0 (5.8, 8.6) | 12.4 (10.0, 15.1) | 1.4 (0.5, 2.4) | 8.9 (4.3, 23.9) | 11.0 (8.1, 13.9) | ||
| Analysis 1 | 40 | 5.6 (4.6, 6.9) | 10.2 (8.1, 12.3) | 1.0 (0.5, 1.7) | 10.2 (5.1, 21.6) | 9.2 (6.6, 1.7) | ||
| Analysis 2 | 10 | 6.5 | 13.6 | 6.8 (5.5, 7.9) | 12.1 (9.8, 14.7) | 1.3 (0.4, 2.1) | 9.3 (5.2, 32.5) | 10.9 (8.3, 13.6) |
| 20 | 12.6 | 27.1 | 6.4 (5.5, 7.8) | 11.8 (9.6, 13.8) | 1.3 (0.4, 2.2) | 9.2 (5.1, 25.4) | 10.6 (8.0, 12.6) | |
| 30 | 19.4 | 40.7 | 6.2 (5.0, 7.5) | 11.2 (8.8, 13.7) | 1.2 (0.5, 1.9) | 9.5 (5.3, 19.9) | 9.9 (7.4, 12.8) | |
| 40 | 25.8 | 54.2 | 6.0 (4.9, 7.2) | 10.9 (8.6, 13.2) | 1.0 (0.3, 1.9) | 11.0 (5.0, 27.8) | 9.9 (7.4, 12.2) | |
| 50 | 32.3 | 67.8 | 5.7 (4.6, 6.9) | 10.4 (8.4, 12.4) | 0.9 (0.3, 1.6) | 12.2 (5.9, 28.4) | 9.5 (6.6, 11.5) | |
| 60 | 38.7 | 81.3 | 5.5 (4.3, 6.8) | 10.1 (8.1, 12.5) | 0.8 (0.2, 1.5) | 12.6 (6.2, 34.7) | 9.1 (7.1, 11.6) | |
| Analysis 3 | 10 | 6.5 | 13.6 | 6.8 (5.2, 7.8) | 12.2 (9.6, 14.0) | 1.3 (0.5, 2.4) | 10.0 (4.1, 24.9) | 11.0 (8.2, 13.1) |
| 20 | 12.6 | 27.1 | 6.4 (5.2, 7.8) | 11.7 (9.7, 14.5) | 1.2 (0.4, 2.0) | 9.8 (5.7, 22.8) | 10.6 (7.9, 13.2) | |
| 30 | 19.4 | 40.7 | 6.4 (5.1, 7.8) | 11.4 (9.2, 13.9) | 1.0 (0.5, 1.8) | 11.0 (5.5, 23.1) | 10.5 (7.8, 12.5) | |
| 40 | 25.8 | 54.2 | 6.2 (4.8, 7.2) | 11.4 (9.0, 13.2) | 0.9 (0.3, 1.7) | 12.2 (5.1, 32.5) | 10.3 (7.9, 12.3) | |
| 50 | 32.3 | 67.8 | 5.8 (4.8, 7.0) | 10.7 (8.4, 12.6) | 0.8 (0.3, 1.5) | 13.5 (6.4, 34.6) | 10.0 (7.5, 12.1) | |
| 60 | 38.7 | 81.3 | 5.6 (4.5, 6.7) | 10.4 (8.3, 12.7) | 0.7 (0.3, 1.2) | 14.5 (7.4, 37.9) | 9.6 (7.5, 12.1) | |
Note. CrI = credible interval. The table shows the modeled population of adolescent sexual minority males where age is 13–18 years. Analysis 4 repeats analyses 1 through 3 with an alternative eligibility definition: age 16 to 18 years and having had 10 or more acts of condomless anal intercourse in the prior 6 months.
For the ratio of HIV prevalence column, the ratio is calculated first for each run and then the median and credible intervals are calculated across those; thus, the median ratio presented does not necessarily equal the ratio of the medians in the previous 2 columns.
Difference represents the absolute numerical difference (HIV prevalence among Black adolescent sexual minority males [ASMM] minus HIV prevalence among White ASMM).
Third, we modeled the additional impact of disparate adherence, maintaining the same overall adherence rates but applying race ratios using adult MSM data.9 Because we could not simultaneously match the overall proportion and race disparity at each coverage level, we fit the highest and lowest adherence levels (White–Black ratios of 1.62 and 0.35, respectively) and assumed a uniform distribution within race across the 2 middle levels. Table 2 shows resulting adherence profiles.
TABLE 2—
Preexposure Prophylaxis (PreP) Adherence Rates Among US Black and White Adolescent Sexual Minority Males for the 3 Sets of Analyses
| PreP Adherence, % |
||||
| None | Low | Moderate | High | |
| Analysis 1 | 20.9 | 24.4 | 13.1 | 41.6 |
| Analysis 2 | 20.9 | 24.4 | 13.1 | 41.6 |
| Analysis 3 | ||||
| All | 20.9 | 24.4 | 13.1 | 41.6 |
| Black | 30.9 | 18.7 | 18.7 | 31.7 |
| White | 10.9 | 18.8 | 18.8 | 51.5 |
Finally, we repeated all analyses with more focused risk-based eligibility criteria: 16- to 18-year-old ASMM with 10 or more acts of condomless AI in the previous 6 months. Our prior models found that this approach resulted in fewer infections averted but greater efficiency. This might help improve efficiency among White ASMM in particular, which we hypothesized would otherwise be low because of low incidence.
Simulations and Analysis
Our modeled population began with 10 000 ASMM and was run for 20 years; scenarios were simulated 100 times. We calculated 6 outcomes per scenario: HIV prevalence; Black–White prevalence ratio; Black–White prevalence difference; number of infections averted (NIA) per 100 000 person-years at risk compared with no PrEP; percentage of infections averted (PIA) compared with no PrEP; and number of person-years on PrEP per infection averted (NNT). We report median prevalence at age 18 years, which approximates cumulative incidence per 100 person-years at risk, given low mortality. We present both prevalence ratios and differences because they can change in different directions under interventions, complicating interpretations of effects on disparities. NIA and PIA incorporate cumulative incidence across the 20-year simulation. NNT represents person-time on PrEP divided by NIA. For each measure, we present medians and 95% credible intervals across simulations. These are distinct from standard confidence intervals, which can be difficult to conceptualize for simulation-based analyses; 95% credible intervals present the range of results across simulation runs for a population of the modeled size, not our confidence in the means of those runs.
RESULTS
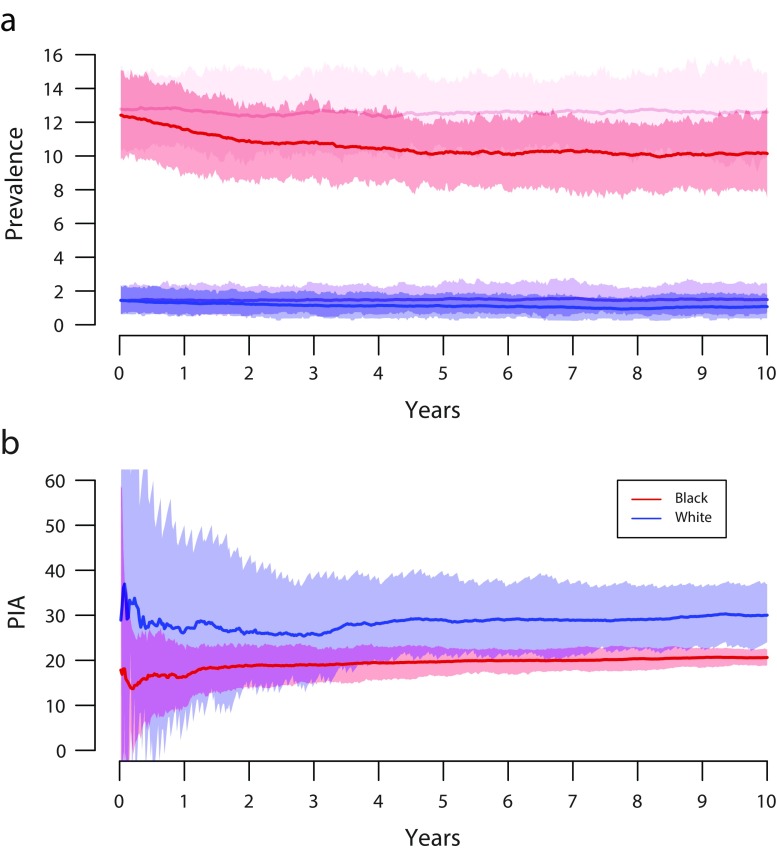
Our base model with no PrEP yielded median prevalence of 7.0% among those aged 18 years, and 12.4% and 1.4% for 18-year-old Blacks and Whites, respectively; median Black–White disparity was an 8.9-fold prevalence difference (Table 1). The PIA, NIA, and NNT for all scenarios are in Tables A and B of the Results Supplement (available as a supplement to the online version of this article at http://www.ajph.org). Overall, PrEP generated an epidemiologically meaningful reduction in HIV incidence. PIA was lower for Black ASMM (19.7%; 95% credible interval [CrI] = 15.2, 25.1) than for White ASMM (31.1%; 95% CrI = 20.4, 44.7), but this metric does not directly account for large differences in background incidence. By contrast, NIA does, and this metric indicated far more infections averted among Black ASMM (784.5 per 100 000 person-years at risk; 95% CrI = 606.5, 988.6) than among White ASMM (134.5 per 100 000 person-years at risk; 95% CrI = 87.3, 190.3), a 5.8-fold difference. NNT shows a similar pattern: among Black ASMM, 1 infection was averted for every 30.7 person-years on PrEP (95% CrI = 24.0, 39.0), versus 172.3 person-years on PrEP for White ASMM (95% CrI = 118.7, 261.0). Figure 1 shows HIV prevalence trajectories and PIA from the no-PrEP and PrEP scenarios over the first 10 years of simulation. These highlight the central paradox of PrEP’s effects by race in this setting: despite most infections being averted among Black ASMM, the PIA was slightly higher among White than among Black ASMM, resulting in the disparity (measured by prevalence ratio) increasing from 8.9 to 10.2 (Table 1). On the absolute scale, however, the disparity declined, from 11.0% to 9.2%.
FIGURE 1—
Time Series Results for the Base Model Simulation With and Without Preexposure Prophylaxis Among Black and White Sexual Minority Male Adolescents by (a) HIV Prevalence Among Those Aged 18 Years and (b) Cumulative Percentage of Infections Averted (PIA) Over 10 Years
Note. Base preexposure prophylaxis model eligibility criteria include age of 16 to 18 years with history of anal intercourse, initiation at 6 months after eligibility, and coverage at 40% of eligible. Panel a: HIV prevalence among Black (red) and White (blue) 18-year-old adolescent sexual minority males. Panel b: cumulative PIA among Black (red) and White (blue) adolescent sexual minority males.
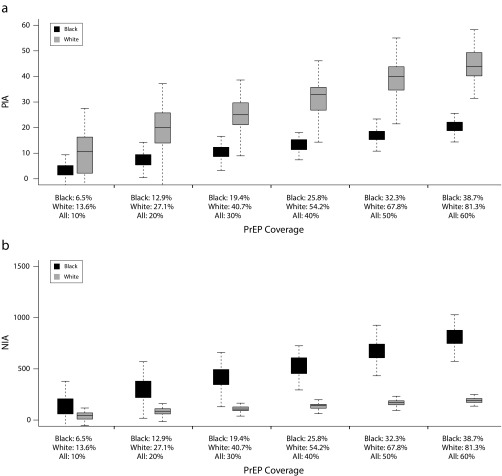
The second analyses included 6 coverage levels (10%–60%) for eligible ASMM overall, with a 2.1-fold coverage disparity by race (Figure 2). Across increasing coverage levels, PIA increased almost linearly for both Black (3.7%–20.1%) and White ASMM (10.6%–44.0%). Within each coverage scenario, PIA was lower for Black than for White ASMM, but NIA was greater for Black ASMM despite lower coverage. For example, at the highest coverage (overall = 60%, Black = 38.7%, White = 81.3%), PIA for White ASMM was 44.0% (95% CrI = 31.5, 55.4), roughly twice that for Black ASMM (20.1%; 95% CrI = 15.8, 24.9); however, NIA was 798.0 (95% CrI = 629.2, 985.5) for Black ASMM but just 188.7 (95% CrI = 136.0, 237.6) for White ASMM. The overall efficiency of PrEP also remained high among Black ASMM, with NNT of 26.9 to 29.2 across coverage levels, compared with 174.2 to 236 for White ASMM. This suggests that a large coverage disparity was not enough to offset differences in background incidence; with both of these factors present, PrEP continued to avert a much higher number of infections among Black than among White ASMM. Despite this, the racial disparity in prevalence again increased on the ratio scale but decreased on the absolute scale.
FIGURE 2—
Results for the Preexposure Prophylaxis Simulation Model Among Black and White Sexual Minority Male Adolescents by (a) Percentage of Infections Averted (PIA) and (b) Number of Infections Averted (NIA)
Note. Eligibility includes age of 16 to 18 years with history of anal intercourse; initiation is on average 6 months following eligibility. Row 1: percentage of infections averted among Black and White adolescent sexual minority males (ASMM). Row 2: number of infections averted per 100 000 years at risk among Black and White ASMM.
The third analyses added an adherence disparity to the coverage disparity, revealing a familiar pattern: PIA remained higher for White ASMM and increased linearly with coverage from 10.2% to 51.1%, compared with 3.6% to 18.2% for Black ASMM. However, NIA remained substantially higher for Black ASMM for all scenarios despite both lower coverage and lower adherence. For example, at 40% overall coverage (Black = 25.8%, White = 54.2%), 12.7% and 37.4% of infections were averted among Black and White ASMM, respectively. In terms of NIA, however, 504.1 infections were averted among Black ASMM compared with just 160.4 among White ASMM, a 3-fold difference. NNT among Black ASMM increased moderately with lower adherence (27.4–32.3) but remained well below that for White ASMM (180.7–202.9). The disparity once again behaved differently on the relative (declines) and absolute (increases) scales. Graphical results are in Figure A of the Results Supplement, available as a supplement to the online version of this article at http://www.ajph.org.
Finally, we changed PrEP eligibility criteria to ASMM aged 16 to 18 years with 10 or more condomless AI acts in the prior 6 months. As expected, the greater focus increased overall efficiency. When we compared the results from this set of analyses with those of the prior set, at 40% coverage, NNT changed from 31.4 (95% CrI = 23.1, 51.8) to 27.8 (95% CrI = 20.3, 43.4) for Black ASMM and from 193.7 (95% CrI = 142.4, 313.9) to 175.2 (95% CrI = 134.0, 272.8) for White ASMM. There was no systematic reduction in NIA, suggesting that the condomless-AI-based eligibility criterion did not remove high-risk ASMM from eligibility, whereas the NNT reduction suggests that this was an effective strategy for excluding some ASMM not at significant risk.
DISCUSSION
The implementation of a PrEP program among ASMM could result in meaningful reductions in new infections, particularly among Black ASMM who bear a disproportionate HIV burden. Indeed, efficiency for Black ASMM is comparable with that of adult MSM.19 Jurisdictions with large Black populations and high HIV prevalence may find it especially valuable to develop a program for PrEP among ASMM. Public health interventions are often structured to deploy resources in communities at highest risk, and this analysis presents compelling evidence that implementing PrEP programs in a way that maximizes access for Black ASMM appears reasonable from public health and efficiency perspectives. The US National HIV/AIDS Strategy lists as its first goal to “intensify HIV prevention efforts in the communities where HIV is most heavily concentrated.”25 Working closely with schools, providers, and community leaders who serve Black communities, along with outreach to ASMM, will all be required to translate our model’s results and facilitate uptake in these highly affected communities. Efforts will be especially challenging because they must deal with stigma and discrimination in terms of race, sexual orientation, and HIV risk—and do so for an adolescent population who might not identify as gay or engage with adult-focused gay community organizations. Additional challenges arise from including parental decision-making in sexual health matters, or in waiving that process.26 Recent data from the 2018 Conference on Retroviruses and Opportunistic Infections highlight the ongoing challenges of PrEP implementation in these communities.27–29 However, our results suggest that engaging early with ASMM populations, and Black ASMM populations specifically, could be impactful and efficient, and that waiting until age 18 years to begin providing comprehensive HIV prevention for MSM will be too late for many Black youths.
We note, however, that preventing new infections among ASMM through PrEP might have an unintended impact on racial disparities in HIV burden. In all scenarios investigated, far more infections were averted among Black than among White ASMM, yet counterintuitively, in every instance the racial disparity (measured by prevalence ratio) increased. The National HIV/AIDS Strategy lists the twin goals of reducing both infections and disparities25; our analyses demonstrate where these goals can stand in tension and that success in one (overall incidence) can result in losing ground on the other. However, they also confirm a common finding30 that 2 ways of measuring disparities—relative and absolute—are themselves often in tension, with increasing relative disparities reflecting decreasing absolute disparities. This is especially true when 1 group has low, declining disease burden, and relative differences become unstable. Each measure tells a piece of a complex story, and using both—as previously recommended30—provides more opportunity to assess both advances and setbacks on the road to health equity.
As our prior model demonstrated, coverage strongly affected PIA and NIA but had only modest impact on efficiency, which was driven primarily by acquisition risk in the population.18 Thus, here efficiency was high among Black ASMM at all coverage levels and low for White ASMM. The lower adherence modeled for Black ASMM reduced PIA and NIA, but the magnitude of the reduction was small relative to changes in coverage. Importantly, lower adherence did not substantially diminish efficiency over the range explored, and these changes were far smaller than the difference in efficiency between Black and White ASMM. Consequently, even at lower adherence, considerable impact and reasonable efficiency could be achieved when HIV prevalence is high (as among the Black ASMM in this study) and coverage is sufficient. However, comparison of PrEP adherence measures among ASMM indicates that considerable uncertainty in this crucial metric remains.14 If effective adherence is, in fact, substantially lower, PrEP’s impact will be diminished. Regardless, development of novel interventions to support PrEP adherence is needed; some interventions are currently under investigation by the ATN (http://atnweb.org/atnweb/studies).
Overall impact and efficiency of PrEP within any community directly depend on underlying HIV burden. Among adult MSM and ASMM, HIV burden varies across myriad dimensions, including but not limited to race and ethnicity. In our model, prevalence among youths aged 18 years was 7.0% overall, but 12.4% among Blacks and 1.4% among Whites. This difference generated an order of magnitude difference in PrEP efficiency. For communities of any type—ethnic, geographic, or otherwise—with prevalence closer to the 12.4% modeled for Black ASMM, PrEP may be efficient and effective enough (in terms of absolute number of infections averted) to justify adoption. This may include communities of Hispanic MSM,1 for whom estimated lifetime risk of HIV acquisition is about half that of Black MSM.31 Furthermore, there may be many local jurisdictions where HIV burden among ASMM overall will be sufficiently high for PrEP implementation to be efficient, even if the same is less likely at state or national levels. This highlights the need for robust local surveillance, especially among ASMM, to inform prevention efforts.
Our model has several limitations. We did not include cost-effectiveness analyses, an important step for evaluating PrEP scale-up in this population; however, this work provides numbers on population-level effectiveness that can be integrated with costing data, and we are currently in the process of conducting that analysis. We did not include risk compensation. Whereas early reports from adult MSM found little evidence of risk compensation,9,24,32 recent studies have reported more.8,33,34 These did not disaggregate risk compensation by race; differences in risk compensation by race would affect PrEP effectiveness differently by community, although the impacts of risk compensation on PrEP depend on its relationship with adherence.35 Regardless, any rollout of PrEP among adolescents would need to monitor risk compensation.
Our model did not account for changing HIV prevalence among ASMM beyond age 18 years, or for potential impacts on epidemic trajectories for ASMM. Consideration of how PrEP use might change the prevalence among ASMM beyond age 18 years would have 3 implications that should increase PrEP’s impact and efficiency. First, PrEP use among ASMM could reduce HIV prevalence among men recently aged out of the ASMM population to reduce risk for ASMM through reductions in prevalence among potential partners. Second, adolescent engagement with PrEP may set a lifelong norm, leading to higher retention and adherence. Third, if early engagement with PrEP facilitates development of norms for treatment-seeking and engagement with health care professionals more generally, PrEP may indirectly facilitate improvement at each step along the care continuum. These considerations might yield greater efficiency estimates than our current model, even in low-prevalence populations like White ASMM, justifying PrEP implementation in a broader range of communities.
Recent evidence suggests that uptake of and adherence to PrEP are lower among Black MSM than among White MSM in the United States despite higher HIV burden among the former; similar differences are likely among ASMM. Nevertheless, this work demonstrates that population-level PrEP effectiveness and efficiency remain high for Black ASMM, given relatively high HIV incidence. PrEP programs that address the unique needs, challenges, and strengths within this community could significantly reduce HIV incidence at the ages at which that burden currently begins, before it is too late.
ACKNOWLEDGMENTS
We acknowledge funding from National Center for HIV, Viral Hepatitis, STDs, and TB Prevention Epidemic and Economic Modeling Agreement (NEEMA; U38 PS004646-01) and the National Institutes of Health (grants R21HD075662, R01HD068395). Research reported in this publication was further supported by the National Institutes of Health via institutional support to the University of Washington Center for Studies in Demography and Ecology (R24 HD042828), the University of Washington Center for AIDS Research (P30AI027757), and the Emory University Center for AIDS Research (P30AI050409).
We thank members of the scientific and public health advisory groups of the Coalition for Applied Modeling for Prevention project for their input on this study, and specifically those members who reviewed a previous version of this article: Mary Ann Chiasson and Jane Kelly. We also thank the statnet development team and the team at Emory’s PRISM Health.
Note. The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or the National Institutes of Health.
HUMAN PARTICIPANT PROTECTION
No protocol approval was necessary because no human participants were involved in this research.
REFERENCES
- 1.Division of HIV/AIDS Prevention. Diagnoses of HIV Infection in the United States and Dependent Areas, 2015. Atlanta, GA: Centers for Disease Control; 2016. [Google Scholar]
- 2.Garofalo R, Hotton AL, Kuhns LM, Gratzer B, Mustanski B. Incidence of HIV infection and sexually transmitted infections and related risk factors among very young men who have sex with men. J Acquir Immune Defic Syndr. 2016;72(1):79–86. doi: 10.1097/QAI.0000000000000933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sullivan PS, Peterson J, Rosenberg ES et al. Understanding racial HIV/STI disparities in black and white men who have sex with men: a multilevel approach. PLoS One. 2014;9(3):e90514. doi: 10.1371/journal.pone.0090514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wejnert C, Le B, Rose CE et al. HIV infection and awareness among men who have sex with men—20 cities, United States, 2008 and 2011. PLoS One. 2013;8(10):e76878. doi: 10.1371/journal.pone.0076878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wejnert C, Hess KL, Rose CE et al. Age-specific race and ethnicity disparities in HIV infection and awareness among men who have sex with men—20 US Cities, 2008–2014. J Infect Dis. 2016;213(5):776–783. doi: 10.1093/infdis/jiv500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grant RM, Lama JR, Anderson PL et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363(27):2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grohskopf LA, Chillag KL, Gvetadze R et al. Randomized trial of clinical safety of daily oral tenofovir disoproxil fumarate among HIV-uninfected men who have sex with men in the United States. J Acquir Immune Defic Syndr. 2013;64(1):79–86. doi: 10.1097/QAI.0b013e31828ece33. [DOI] [PubMed] [Google Scholar]
- 8.McCormack S, Dunn DT, Desai M et al. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet. 2016;387(10013):53–60. doi: 10.1016/S0140-6736(15)00056-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu AY, Cohen SE, Vittinghoff E et al. Preexposure prophylaxis for HIV infection integrated with municipal- and community-based sexual health services. JAMA Intern Med. 2016;176(1):75–84. doi: 10.1001/jamainternmed.2015.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hosek S, Rudy D, Landovitz R ATN 110: an HIV PrEP demonstration project and phase II safety study for young men who have sex with men in the United States. Paper presented at: Conference of the International AIDS Society; July 19–22, 2015; Vancouver, British Columbia.
- 11.Hosek S, Rudy D, Landovitz R et al. An HIV preexposure prophylaxis demonstration project and safety study for young MSM. J Acquir Immune Defic Syndr. 2017;74(1):21–29. doi: 10.1097/QAI.0000000000001179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hosek S, Landovitz R, Rudy B An HIV pre-exposure prophylaxis demonstration project and safety study for adolescent MSM ages 15–17 in the US (ATN 113). Paper presented at: 21st International AIDS Conference; July 18–22, 2016; Durban, South Africa.
- 13.Hosek SG, Landovitz RJ, Kapogiannis B et al. Safety and feasibility of antiretroviral preexposure prophylaxis for adolescent men who have sex with men aged 15 to 17 years in the United States. JAMA Pediatr. 2017;171(11):1063–1071. doi: 10.1001/jamapediatrics.2017.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koss CA, Hosek SG, Bacchetti P et al. Comparison of measures of adherence to human immunodeficiency virus preexposure prophylaxis among adolescent and young men who have sex with men in the United States. Clin Infect Dis. 2018;66(2):213–219. doi: 10.1093/cid/cix755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.US Public Health Service. Preexposure prophylaxis for the prevention of HIV infection in the United States—2014: a clinical practice guideline. Available at: http://www.cdc.gov/hiv/pdf/guidelines/PrEPguidelines2014.pdf. Accessed January 8, 2018.
- 16.Hoots BE, Finlayson T, Nerlander L, Paz-Bailey G. National HIV Behavioral Surveillance Study Group. Willingness to take, use of, and indications for pre-exposure prophylaxis among men who have sex with men—20 US cities, 2014. Clin Infect Dis. 2016;63(5):672–677. doi: 10.1093/cid/ciw367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sullivan P, Sineath C, Kahle E, Sanchez T. Awareness, willingness and use of oral pre-exposure prophylaxis (PrEP) among a national sample of US men who have sex with men. Paper presented at: AIDS Impact Conference; July 28–31, 2015; Amsterdam, Netherlands.
- 18.Goodreau SM, Hamilton DT, Jenness SM et al. Targeting human immunodeficiency virus pre-exposure prophylaxis to adolescent sexual minority males in higher prevalence areas of the United States: a modeling study. J Adolesc Health. 2018;62(3):311–319. doi: 10.1016/j.jadohealth.2017.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jenness SM, Goodreau SM, Rosenberg E et al. Impact of the Centers for Disease Control’s HIV Preexposure Prophylaxis Guidelines for Men Who Have Sex With Men in the United States. J Infect Dis. 2016;214(12):1800–1807. doi: 10.1093/infdis/jiw223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krivitsky PN, Handcock MS. A separable model for dynamic networks. J R Stat Soc Series B Stat Methodol. 2014;76(1):29–46. doi: 10.1111/rssb.12014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jenness S, Goodreau SM, Morris M. EpiModel: an R package for mathematical modeling of infectious disease over networks. J Stat Softw. 2018;84:8. doi: 10.18637/jss.v084.i08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goodreau SM, Rosenberg ES, Jenness SM et al. Sources of racial disparities in HIV prevalence among men who have sex with men in Atlanta, GA: a modelling study. Lancet HIV. 2017;4(7):e311–e320. doi: 10.1016/S2352-3018(17)30067-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sullivan PS, Rosenberg ES, Sanchez TH et al. Explaining racial disparities in HIV incidence in black and white men who have sex with men in Atlanta, GA: a prospective observational cohort study. Ann Epidemiol. 2015;25(6):445–454. doi: 10.1016/j.annepidem.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grant RM, Anderson PL, McMahan V et al. Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. Lancet Infect Dis. 2014;14(9):820–829. doi: 10.1016/S1473-3099(14)70847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.White House Office of National AIDS Policy. National HIV/AIDS Strategy for the United States: updated to 2020. 2015. Available at: https://files.hiv.gov/s3fs-public/nhas-update.pdf. Accessed January 8, 2018.
- 26.Knopf AS, Ott MA, Liu N et al. Minors’ and young adults’ experiences of the research consent process in a phase II safety study of pre-exposure prophylaxis for HIV. J Adolesc Health. 2017;61(6):747–754. doi: 10.1016/j.jadohealth.2017.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morgan E, Ryan DT, Moran K, Newcomb ME, Mustanski B. Trends in PrEP uptake, adherence, and discontinuation among YMSM in Chicago. Program of the 25th Conference on Retroviruses and Opportunistic Infections (CROI); 2018; Boston, MA. Available at: http://www.croiconference.org/sites/default/files/posters-2018/1430_Morgan_1012.pdf. Accessed January 8, 2018.
- 28.Mayer KH, Grasso C, Levine K Increasing PrEP uptake, persistent disparities, in at-risk patients in a Boston community health center. Program of the 25th Conference on Retroviruses and Opportunistic Infections (CROI); 2018; Boston, MA. Available at: http://www.croiconference.org/sites/default/files/posters-2018/1430_Mayer_1014.pdf. Accessed January 8, 2018.
- 29.Scott HM, Nordell M, Hirozawa A Disparities in PrEP uptake among primary care patients screened for HIV and STIs in San Francisco. Program of the 25th Conference on Retroviruses and Opportunistic Infections (CROI); 2018; Boston, MA. Available at: http://www.croiconference.org/sites/default/files/posters-2018/1430_Scott_1015.pdf. Accessed January 8, 2018.
- 30.Moonesinghe R, Beckles GL. Measuring health disparities: a comparison of absolute and relative disparities. PeerJ. 2015;3:e1438. doi: 10.7717/peerj.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hess KL, Hu X, Lansky A, Mermin J, Hall HI. Lifetime risk of a diagnosis of HIV infection in the United States. Ann Epidemiol. 2017;27(4):238–243. doi: 10.1016/j.annepidem.2017.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marcus JL, Glidden DV, Mayer KH et al. No evidence of sexual risk compensation in the iPrEx trial of daily oral HIV preexposure prophylaxis. PLoS One. 2013;8(12):e81997. doi: 10.1371/journal.pone.0081997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Wit J, Murphy D, Lal L et al. Pre-exposure prophylaxis and risk compensation: evidence of decreased condom use at three-month follow-up among predominantly gay male participants in the vicprep study. Sex Transm Infect. 2015;91(suppl 2):A68. [Google Scholar]
- 34.Volk JE, Marcus JL, Phengrasamy T et al. No new HIV infections with increasing use of HIV preexposure prophylaxis in a clinical practice setting. Clin Infect Dis. 2015;61(10):1601–1603. doi: 10.1093/cid/civ778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jenness SM, Sharma A, Goodreau SM et al. Individual HIV risk versus population impact of risk compensation after HIV preexposure prophylaxis initiation among men who have sex with men. PLoS One. 2017;12(1):e0169484. doi: 10.1371/journal.pone.0169484. [DOI] [PMC free article] [PubMed] [Google Scholar]