Abstract
Objectives. To report demographics, regional variations, and indications for preexposure prophylaxis (PrEP) use for HIV prevention in the Veterans Health Administration (VHA).
Methods. We identified persons initiating tenofovir/emtricitabine for the PrEP indication in the United States between July 2012 and April 2016 in a VHA national database. We stratified PrEP use by provider type and VHA region. We calculated PrEP initiation rate for each region with VHA population data.
Results. Of the 825 persons who initiated PrEP during the observation period, 67% were White and 76% were men who have sex with men. People who inject drugs and transgender persons represented less than 1% each of the cohort. The majority of PrEP initiations were clustered in 3 states, leading with California (28%) followed by Florida (9%) and Texas (8%). The Southeast had one of the lowest PrEP rates at 10 PrEP initiations per 100 000 persons in care. Infectious disease specialists issued more than two thirds of index PrEP prescriptions.
Conclusions. Uptake of PrEP in the VHA is uneven along geographic and risk categories. Understanding the reasons behind these gaps will be key in expanding the use of this important prevention tool.
Once-daily tenofovir/emtricitabine (TDF/FTC), approved for human immunodeficiency virus (HIV) preexposure prophylaxis (PrEP) in 2012, is an effective HIV prevention tool in high-risk groups including men who have sex with men (MSM), transgender persons, heterosexual individuals, and people who inject drugs (PWID).1–4 Despite the advances in prevention, with nearly 40 000 new diagnoses in 2016, HIV remains a significant public health problem in the United States.5
According to the Centers for Disease Control and Prevention (CDC), of an estimated 1.1 million persons who had an indication for PrEP in the United States in 2015, 44% were Black and approximately a quarter of those who would benefit from PrEP were Hispanic.6 However, PrEP uptake in these racial and ethnic demographic groups has not been commensurate with the need.7 The same report also estimates that among the heterosexually active adults with a PrEP indication, 68% are women, yet PrEP remains underutilized in this risk group.8 Another risk group in which PrEP use can be expanded is PWID.9 The US National HIV/AIDS Strategy identifies PWID as a priority population for HIV prevention and the CDC recommends PrEP for high-risk PWID with an estimated 115 000 PWID believed to be PrEP-eligible in the United States.10 In addition, regional PrEP uptake in the United States has been incongruent with HIV incidence. For example, in 2017, southern states had the lowest ratio of active PrEP prescriptions per new HIV diagnosis compared with other US regions.11
The Veterans Health Administration (VHA) is the largest integrated provider of HIV care in the United States, offering a unique opportunity to assess PrEP uptake in a national health system.12 Although PrEP adherence in a VHA cohort has been previously described,13 uptake among various risk groups and regional patterns of use are not known. Herein we report demographic characteristics, regional variations, and indications for PrEP use in the VHA from 2012 to 2016. These data can help guide PrEP delivery not only within the VHA but also in other large health systems.
METHODS
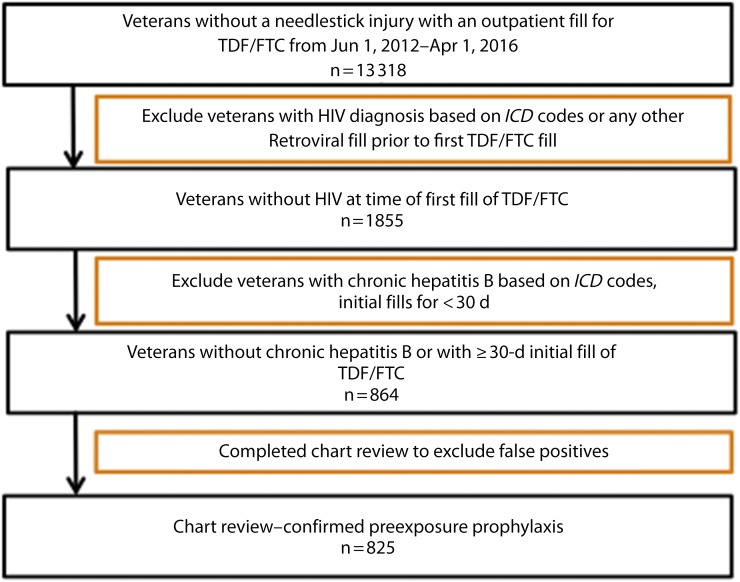
We queried the Corporate Data Warehouse, a national VHA patient database within a platform called the VA Informatics and Computing Infrastructure, to identify individuals in VHA care with initial TDF/FTC prescriptions between June 1, 2012, and April 1, 2016 (Figure 1). We used TDF/FTC fill dates and days supply for cohort definition. We excluded persons with International Classification of Diseases, Ninth Revision (ICD-9) and Tenth Revision (ICD-10) encounter codes for HIV (ICD-9: 042; ICD-10: B20–B24), chronic hepatitis B (ICD-9: 070.32; ICD-10: B18), or needlestick injury (ICD-9: V15.85; ICD-10: W46)14,15; those receiving other antiretrovirals before or at the time of the initial TDF/FTC prescription; and those who filled a single TDF/FTC prescription for 30 or fewer days as they were presumed to be on postexposure prophylaxis. We identified demographic data including age, gender, self-reported race and ethnicity, and laboratory data (HIV serology, HIV viral load, hepatitis B serology) directly from the Corporate Data Warehouse files. We stratified location of PrEP initiations by state and VHA geographic region: region 1 (Northeast and Atlantic), region 2 (Southeast), region 3 (Midwest), region 4 (South and Rocky Mountains), and region 5 (West Coast and noncontinental states).
FIGURE 1—
Algorithm for Identifying Unique Preexposure Prophylaxis Users in Veterans Health Administration
Note. ICD = International Classification of Diseases (includes ninth14 and tenth15 revisions); TDF/FTC = tenofovir/emtricitabine.
Chart Review
We validated this cohort by chart review with VHA’s national chart Compensation and Pension Record Interchange. We included only persons confirmed by clinical documentation to have filled index TDF/FTC for the PrEP indication in the final cohort. The study staff recorded the indication for PrEP use, whether the patient or provider initiated the PrEP discussion, and the provider type issuing the index prescription. Providers were characterized as primary care providers (PCP), infectious disease specialists (including physicians and physician extenders, namely nurse practitioners or physician assistants), or pharmacists. Authors hypothesized that infectious disease specialists issued the majority of index PrEP prescriptions. Therefore, we were interested in quantifying the index prescriptions by provider type, as one of the ways to address disparities in PrEP use may be to expand usage by non–infectious disease specialists if such an imbalance exists. For each PrEP initiation, we recorded whether an infectious disease consultation was completed before initiation. We classified the PrEP initiations into 5 groups on the basis of index prescription: infectious disease, PCP without infectious disease consultation, PCP with infectious disease consultation, PCP with infectious disease electronic consultation, and pharmacists. Electronic consultation is a form of consultation that uses chart review without an in-person visit.
Statistical Analysis
We evaluated demographic variables and geographic location of PrEP initiations among all PrEP recipients and by PrEP prescribers. We presented descriptive statistics as means and percentages. The state of the health care facility where the initial PrEP prescription was issued determined the VHA geographic region. We classified VHA facility types as medical centers or outpatient clinics. We used publicly available VHA population data16 and HIV surveillance data published by the VA National HIV Clinical Registry Reports17 to calculate HIV prevalence in the VHA by region. We used data from the year 2012 to coincide with the approval of TDF/FTC for PrEP and start year of this analysis. For each VHA region, we calculated HIV prevalence by using numbers of persons in care for HIV in 2012 per 100 000 persons in care in 2012. We calculated PrEP initiation rate for each region as the cumulative number of unique PrEP initiations during study period per 100 000 persons in care in 2012. Because of the difficulties in defining the population at risk for HIV by using the electronic medical record, we used total numbers in care as the denominator to approximate relative PrEP need.
RESULTS
We identified 864 potential unique PrEP recipients by using the algorithm described in Methods. Thirty-nine patients that met criteria for PrEP by the algorithm were found to have non-PrEP related indications for TDF/FTC, including repeated postexposure prophylaxis use, chemoprophylaxis against hepatitis B reactivation, and chronic hepatitis B that did not meet ICD-9 or -10 criteria for exclusion. We confirmed 825 to be on PrEP by chart review and included them in the analysis. Persons receiving PrEP were primarily male (97%) with a mean age of 41 years (range = 21–77 years; Table 1). The majority of the cohort was White (67%) and 168 (20%) were Black. Ethnicity is self-reported separately from race in the Corporate Data Warehouse. Of the cohort, 14% identified as Hispanic. The median age of White persons on PrEP was older than Black and Hispanic persons (40, 36, and 34 years, respectively). Within the VHA, uptake of PrEP was slow initially, with fewer than 30 PrEP initiations per quarter in the first 2 years after PrEP approval. Of PrEP initiations, 75% occurred in 2015 and 2016. A minority of charts contained documentation of how the candidates for PrEP were identified (39%). Out of these, 85% of PrEP discussions were patient-initiated and the remainders were provider-initiated.
TABLE 1—
Demographic Characteristics and Indications for Preexposure Prophylaxis (PrEP) Use for HIV Prevention in the Veterans Health Administration: United States, June 1, 2012–April 1, 2016
| Mean (Range) or No. (%) | |
| Age, y | 41.2 (21–77) |
| Men | 802 (97.2) |
| Race/ethnicity | |
| White | 552 (66.9) |
| Black | 168 (20.4) |
| Other | 105 (12.7) |
| Hispanic | 119 (14.4) |
| Indications for PrEP | |
| MSM | 626 (75.9) |
| Heterosexual men | 64 (7.6) |
| Bisexual men | 72 (8.7) |
| Heterosexual women | 19 (2.3) |
| Transgender | 5 (0.6) |
| PWID | 8 (0.9) |
| Missing data | 31 (3.7) |
Note. MSM = men who have sex with men; PWID = people who inject drugs.
The primary risk group was high-risk MSM exclusively (n = 626; 76%), followed by high-risk heterosexual men (n = 64; 7.6%; Table 1). A small fraction of the cohort was heterosexual women (n = 19; 2%), of which 66% were Black. Notably, of the 19 women receiving PrEP, 15 (79%) were in the context of serodiscordant relationships. Only 5 persons identified as transgender, constituting less than 1% of the cohort. Less than 1% of recipients reported injection drug use as the only reason for initiating PrEP. Indication for 32 of the PrEP initiations was undocumented. Overall, 265 (32%) of PrEP initiations were in serodiscordant relationships.
Initiations of PrEP were geographically clustered: greater than 27% of PrEP initiations were in California, with more than half of those attributable to San Francisco and San Diego. Region 5 (West Coast and noncontinental states) had the highest PrEP initiation rate at 26.4 PrEP initiations per 100 000 persons in care, with the third-highest HIV prevalence in the VHA of 487 persons with HIV per 100 000 persons in care (Table 2). Even though HIV prevalence rates in the VHA were highest in region 2 at 627 per 100 000, this region had a comparatively low PrEP initiation rate at 10.1 per 100 000. Rates of PrEP initiation were similar in region 1 (Northeast) at 10.4 per 100 000, with second-highest HIV prevalence rates (540 per 100 000). Region 3 (Midwest) had both the lowest PrEP initiation rates (5.4 per 100 000) and HIV prevalence rates in the VHA (239/100 000). The top 9 sites in PrEP prescription frequency accounted for 43% of all initiations. Florida (8.6%) and Texas (7.6%) were the second- and third-highest states for PrEP initiations. With the exception of Colorado, all interior US states contributed less than 2% each to total PrEP initiations. Outside of California, the majority of PrEP initiations were at facilities close to or within large cities. The majority of PrEP initiations occurred at major medical centers as opposed to outpatient clinics.
TABLE 2—
Preexposure Prophylaxis (PrEP) Use in the Veterans Health Administration (VHA) Stratified by VHA Regions and Index Prescription Provider: United States, July 2012–April 2016
| All, No. (%) | No. of Persons With HIV in Care/100 000 Persons in Carea | No. of Unique PrEP Starts/100 000 Persons in Careb | IDS,c No. (%) | PCP Without IDS Consultation,c No. (%) | PCP With IDS Consultation,c No. (%) | PCP With IDS Electronic Consultation,c No. (%) | Pharmacist,c No. (%) | |
| No. | 825 | 566 | 105 | 56 | 46 | 52 | ||
| Region 1d | 167 (20.2) | 540 | 10.4 | 115 (20.3) | 26 (24.8) | 14 (25) | 8 (17.4) | 4 (7.7) |
| Region 2e | 126 (15.3) | 627 | 10.1 | 83 (14.7) | 24 (22.9) | 8 (14.3) | 6 (13) | 5 (9.6) |
| Region 3f | 94 (11.4) | 239 | 5.4 | 81 (14.3) | 7 (6.7) | 1 (1.8) | 3 (6.5) | 2 (3.8) |
| Region 4g | 127 (15.4) | 443 | 11 | 68 (12) | 14 (13.3) | 7 (12.5) | 4 (8.7) | 34 (65.4) |
| Region 5h | 311 (37.7) | 487 | 26.4 | 219 (38.7) | 34 (32.4) | 26 (46.4) | 25 (54.3) | 7 (13.5) |
| Index PrEP—medical center | 730 (88.5) | 531 (93.8) | 60 (57.1) | 52 (92.9) | 36 (78.3) | 51 (98.1) | ||
| Index PrEP—outpatient center | 95 (11.5) | 35 (6.2) | 45 (42.9) | 4 (7.1) | 10 (21.7) | 1 (1.9) |
Note. IDS = infectious disease specialist; PCP = primary care provider.
Number of persons with HIV in care at VHA in 2012 per 100 000 persons in care at VHA in 2012. Data sources: Department of Veterans Affairs. VHA facility quality and safety report fiscal year 2012 data. 2013. Available at: https://www.va.gov/HEALTH/docs/QandS_Report_2013_data_tables_fy12_data.pdf. Accessed June 16, 2018; and HIV infected veterans in VHA care in 2011 through 2015, for the nation, by VISN and by station.17
Number of unique PrEP starts between June 1, 2012, and April 1, 2016, per 100 000 persons in care at VHA in 2012.
IDS consultation either face to face or electronic obtained for PrEP eligibility only.
CT, DE, DC, ME, MD, MA, NH, NJ, NY, NC, PA, RI, VT, VA, and WV.
AL, FL, GA, KY, SC, and TN.
IL, IN, IA, KS, MI, MN, MO, NE, ND, OH, SD, and WI.
AR, CO, LA, MS, MT, OK, TX, UT, and WY.
AK, AZ, CA, HI, ID, NV, NM, OR, and WA.
Across all VHA regions, infectious disease specialists accounted for the majority of PrEP initiations (69%; Table 2). Either a face-to-face or electronic consultation to determine PrEP eligibility occurred with an infectious disease practitioner before PrEP initiation in a small minority of cases (7% and 6%, respectively). Primary care providers initiated 13% of PrEP whereas a clinical infectious disease pharmacist initiated 7%.
DISCUSSION
Uptake of PrEP since the approval of TDF/FTC for HIV prevention in 2012 was disproportionate in VHA as key risk groups and US regions were underrepresented in this important prevention effort. Use of PrEP was predominantly reaching older White MSM whereas Black people constituted a disproportionately small fraction of the PrEP cohort. Relatively few PrEP initiations occurred in 2 high-risk groups; transgender persons and PWID. Recipients of PrEP tended to actively request PrEP from their providers rather than providers identifying them as being at an increased risk for HIV infection. In contrast to HIV prevalence rates in the VHA , PrEP initiation rates were low in the Southeast and highest on the West Coast. Infectious disease specialists initiated most PrEP prescriptions, and most recipients received infectious disease consultation to determine PrEP eligibility.
In 2016, 44% of new HIV infections in the United States occurred in Black people.5 Approximately 20% of PrEP recipients in the VHA were Black. Although the majority of all veterans in care at the VHA are White, 48% of all HIV-infected persons in care are Black, suggesting a disproportionately higher PrEP need than their White counterparts. This finding was concordant with studies in non-VHA populations that have demonstrated that PrEP uptake among Black people is lower than would be expected on the basis of incidence of HIV in that population.18 Several barriers, including low PrEP awareness and structural barriers (e.g., lack of health insurance), have been cited as potential reasons for this disparity.19 Conspiracy beliefs regarding HIV transmission and stigma have also been reported to play a role in low PrEP usage among those with high level of PrEP awareness.20 In addition, mistrust in medical providers and experiences with discrimination also contribute to low PrEP uptake.21 Targeted interventions that address these barriers are needed to reach this key demographic group.
Similarly, according to the CDC, in 2016, Black women were more than 3 times more likely to be diagnosed with HIV than their White counterparts.5 Because the vast majority of the VHA population is men, it is not surprising that our cohort contains few women. Though women were underrepresented at 2% of the total cohort, the vast majority of women receiving PrEP for HIV prevention were Black. However, it is notable that the primary indication for PrEP among heterosexual women who did receive PrEP was being in a serodiscordant relationship. Exploring barriers to PrEP use and how to expand use among other high-risk women such as those with multiple sexual partners represents an area of further study.
In 2013, 2567 persons in the VHA identified as transgender, with an estimated prevalence of 33 per 100 000 patients.22 Transgender women are among the highest-risk groups for HIV acquisition with an HIV prevalence of 22%.23 In sharp contrast to the disease burden in this population, in our cohort, only 0.6% of PrEP recipients identified as transgender. One previous study involving a cohort of transgender women in New York City reported significant barriers to both PrEP use and adherence, including concern about health impacts of PrEP, lack of access to routine testing, barriers to follow-up, and financial concerns.24 Even though financial barriers to PrEP are largely mitigated in the VHA, health disparities and barriers such as stigma and discrimination nevertheless exist in the transgender veteran population.25 How PrEP use can be expanded in this vulnerable population remains an important area of study.
According to the CDC, injection drug use remains a significant risk factor for HIV infection with nearly 1 in 10 new HIV infections occurring in PWID.26 Out of 825 PrEP users in the VHA, less than 1% reported injection drug use as their only HIV risk factor. With the rise of the opioid epidemic, there have also been outbreaks of HIV infection in the setting of risky injection practices such as needle sharing or reuse of personal needles.27 Prevalence of substance use disorders in VHA is high, suggesting that opportunities exist to increase usage of this important HIV prevention tool in this risk group.28 Outreach efforts that specifically target PWID for PrEP initiation, such as engagement of substance use specialists and social workers, are needed to improve HIV prevention in this population.
Similar to in the general US population, PrEP use in VHA was predominant in coastal cities and regions.29 Prescriptions for PrEP were clustered in facilities in California, Texas, and Florida. Not surprisingly, these 3 states are also the largest provider of HIV care within the VHA with 27%, 8%, and 9%, respectively, of proportions of veterans with HIV served.17 Despite the fact that HIV prevalence rates in the VHA were similar across regions at the start of PrEP use in 2012, the initiation rate over the next 4 years varied among regions. In fact, the region with the highest HIV prevalence, the Southeast, had the second-lowest PrEP initiation rate. Uptake of PrEP in this region was not commensurate with national HIV incidence either. Data from CDC demonstrate that HIV incidence in the southern states has been rising, accounting for nearly 52% new HIV diagnoses in 2016.5 Furthermore, 60% of Black MSM diagnosed with HIV in 2016 in the United States lived in the South, and Black women shared an even more disproportionate burden of disease compared with national rates at 69% of all women diagnosed.5 Many factors have been cited as contributing to this disparity, including income inequality, access to health insurance, cultural factors such as racial bias and inequalities, and limited adoption of HIV-prevention modalities.30 Awareness of and access to PrEP in the South remains low compared with that in coastal states.19 In a small survey of MSM in Atlanta, Georgia, only about 12% of those surveyed were using PrEP for HIV prevention in 2016.31 These data are in accordance with our findings of disproportionately low PrEP initiations in the Southeast and one of the lowest initiation rates among all VHA regions. The disproportionality high burden of disease, coupled with significant racial and gender disparities, underscores the importance of expanding prevention strategies including PrEP to more closely reflect the burden shared by various geographic regions.
A potential opportunity to address the gap may exist in PrEP delivery models. Although HIV-negative individuals are most likely to be seen in primary care settings, PCPs may not always be trained to prescribe PrEP. In contrast, infectious disease specialists who are more likely to have PrEP knowledge are less likely to see high-risk individuals. This concept, described in the literature as “purview paradox,” can result in added barriers to PrEP use.32 In the present study, infectious disease practitioners issued two thirds of PrEP prescriptions within VHA. These occurred upon consultation request or other locally implemented efforts by infectious disease specialists to identify high-risk patients such as those with sexually transmitted illnesses. In addition, they provided consultation on an additional 14% of PrEP recipients for whom the PCP issued the index prescription. This is in contrast to other smaller health care systems in which PCPs wrote the majority of PrEP prescriptions.33 The expertise of an infectious disease specialist may not be available at every health care facility and this may represent a barrier to PrEP access in the VHA. Where infectious disease expertise is not immediately available, electronic consultation to determine eligibility, as was done for a proportion of the cohort, may be an important means of overcoming this barrier. Expanded use of pharmacists, as is being done at certain VHA facilities, may also help enhance access in regions where infectious disease specialty care may not be available, such as rural areas. The majority of pharmacist-initiated PrEP occurred in region 4, suggesting a regional effort to promote PrEP access in areas where specialty services could be limited.
Lastly, engaging PCPs in PrEP delivery will be a crucial step in increasing PrEP uptake and filling these gaps. A survey of PCPs identified lack of knowledge as a potential barrier.34 Even in settings where awareness of PrEP exists, adoption can be low among PCPs. For example, in a survey of PCPs with prior knowledge of PrEP, those who had concerns about PrEP safety or risk compensation were less likely to prescribe it.35 We found that PrEP initiations primarily occurred at the request of the patient, instead of the provider. It is not known whether this is a unique finding in our cohort or if it is also occurring outside the VHA. This finding does suggest that high-risk individuals who are unaware of PrEP must rely on their provider to initiate the PrEP conversation. These data highlight the need for expansion of PrEP use beyond specialty care as well as further exploration into various PrEP delivery models to fit the needs of the specific population and health care system.
Limitations
Our study had several important limitations. The VHA represents a unique population and the results may not be generalizable to the US population as a whole. One consequence of this unique population is that we had few women in the cohort. In addition, the nationally integrated care model is unique to the VHA, potentially limiting the generalizability to other PrEP care delivery models in the United States. Because of the limitations of the electronic medical record, PrEP eligibility is difficult to define. We used HIV prevalence data in the VHA as a surrogate measure of PrEP need. Our algorithm for case definition utilized ICD-9 and -10 codes that may have led to possible exclusion of other PrEP recipients because of incorrect ICD coding. Only a minority of charts had information on how candidates for PrEP were identified; therefore, it is possible that the gender and risk category for the missing data may have had an impact on the results. However, we collected data on HIV serostatus as defined by negative serology or viral load and found ICD codes to yield high sensitivity, and our subsequent chart review provided high specificity. Validating the cohort via chart review strengthened our algorithm and ensured inclusion of only true PrEP recipients in the cohort. Chart review also offered the ability to identify PrEP indication, which is otherwise not discernable through the electronic medical records.
Conclusions
In this national cohort, PrEP use was variable among risk groups and geographic regions. Understanding the root causes behind these disparities and realigning PrEP resources accordingly to target key high-risk groups and regions is needed. Targeted interventions that address these gaps have the potential to prevent HIV infection not only among veterans but also nonveterans in their social network.
ACKNOWLEDGMENTS
P. Van Epps received funding from the HIV, Hepatitis, and Related Conditions Office within the Veterans Health Administration.
L. Beste and M. Maier received support from the HIV, Hepatitis, and Related Conditions Office within the Veterans Health Administration. M. E. Ohl received support from the Office of Rural Health, Veterans Health Administration. Authors thank the Louis Stokes Cleveland VA Geriatric Research Education and Clinical Center for the technical support for this study.
HUMAN PARTICIPANT PROTECTION
This study was approved by the institutional review board at the Cleveland VA Medical Center (IRB 15068H38).
REFERENCES
- 1.Baeten JM, Donnell D, Ndase P et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367(5):399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thigpen MC, Kebaabetswe PM, Paxton LA et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367(5):423–434. doi: 10.1056/NEJMoa1110711. [DOI] [PubMed] [Google Scholar]
- 3.Grant RM, Lama JR, Anderson PL et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363(27):2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choopanya K, Martin M, Suntharasamai P et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2013;381(9883):2083–2090. doi: 10.1016/S0140-6736(13)61127-7. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. HIV Surveillance Report, 2016: Volume 28. 2017. Available at: https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-report-2016-vol-28.pdf. Accessed June 16, 2018.
- 6. Smith DK, Handel, MV, Grey JA. By race/ethnicity, Blacks have highest number needing PrEP in the United States, 2015. Poster presentation at: Conference on Retroviruses and Opportunistic Infections; March 4–7, 2018; Boston, MA.
- 7.Marcus JL, Hurley LB, Hare CB, Silverberg MJ, Volk JE. Disparities in uptake of HIV preexposure prophylaxis in a large integrated health care system. Am J Public Health. 2016;106(10):e2–e3. doi: 10.2105/AJPH.2016.303339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blackstock OJ, Patel VV, Felsen U, Park C, Jain S. Pre-exposure prophylaxis prescribing and retention in care among heterosexual women at a community-based comprehensive sexual health clinic. AIDS Care. 2017;29(7):866–869. doi: 10.1080/09540121.2017.1286287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roth AM, Aumaier BL, Felsher MA et al. An exploration of factors impacting preexposure prophylaxis eligibility and access among syringe exchange users. Sex Transm Dis. 2018;45(4):217–221. doi: 10.1097/OLQ.0000000000000728. [DOI] [PubMed] [Google Scholar]
- 10.Smith DK, Van Handel M, Wolitski RJ et al. Vital Signs: Estimated percentages and numbers of adults with indications for preexposure prophylaxis to prevent HIV acquisition—United States, 2015. MMWR Morb Mortal Wkly Rep. 2015;64(46):1291–1295. doi: 10.15585/mmwr.mm6446a4. [DOI] [PubMed] [Google Scholar]
- 11.Siegler AJ, Mouhanna F, Giler RM Distribution of active PrEP prescriptions and PrEP-to-need ratio, US, Q2 2017. Poster presented at: Conference on Retroviruses and Opportunistic Infections; March 4–7, 2018; Boston, MA.
- 12.Backus LI, Boothroyd DB, Phillips BR et al. National quality forum performance measures for HIV/AIDS care: the Department of Veterans Affairs’ experience. Arch Intern Med. 2010;170(14):1239–1246. doi: 10.1001/archinternmed.2010.234. [DOI] [PubMed] [Google Scholar]
- 13.van Epps P, Maier M, Lund B et al. Medication adherence in a nationwide cohort of veterans initiating pre-exposure prophylaxis (PrEP) to prevent HIV infection. J Acquir Immune Defic Syndr. 2018;77(3):272–278. doi: 10.1097/QAI.0000000000001598. [DOI] [PubMed] [Google Scholar]
- 14.International Classification of Diseases, Ninth Revision. Geneva, Switzerland: World Health Organization; 1980. [Google Scholar]
- 15.International Classification of Diseases, Tenth Revision. Geneva, Switzerland: World Health Organization; 1992. [Google Scholar]
- 16. 2012 VHA facility quality and safety report. Veterans Health Administration. September 2012. Available at: https://www.va.gov/HEALTH/docs/2012_VHA_Facility_Quality_and_Safety_Report_FINAL508.pdf. Accessed June 16, 2018.
- 17. HIV infected veterans in VHA care in 2011 through 2015, for the nation, by VISN and by station. Department of Veterans Affairs National HIV Clinical Registry Reports. 2016. Available at: https://catalog.data.gov/dataset/hiv-infected-veterans-in-vha-care-in-2011-through-2015-for-the-nation-by-visn-and-by-stati. Accessed June 16, 2018.
- 18.Kuhns LM, Hotton AL, Schneider J, Garofalo R, Fujimoto K. Use of pre-exposure prophylaxis (PrEP) in young men who have sex with men is associated with race, sexual risk behavior and peer network size. AIDS Behav. 2017;21(5):1376–1382. doi: 10.1007/s10461-017-1739-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Strauss BB, Greene GJ, Phillips G, 2nd et al. Exploring patterns of awareness and use of HIV pre-exposure prophylaxis among young men who have sex with men. AIDS Behav. 2017;21(5):1288–1298. doi: 10.1007/s10461-016-1480-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eaton LA, Kalichman SC, Price D, Finneran S, Allen A, Maksut J. Stigma and conspiracy beliefs related to pre-exposure prophylaxis (PrEP) and interest in using PrEP among Black and White men and transgender women who have sex with men. AIDS Behav. 2017;21(5):1236–1246. doi: 10.1007/s10461-017-1690-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cahill S, Taylor SW, Elsesser SA, Mena L, Hickson D, Mayer KH. Stigma, medical mistrust, and perceived racism may affect PrEP awareness and uptake in Black compared to White gay and bisexual men in Jackson, Mississippi and Boston, Massachusetts. AIDS Care. 2017;29(11):1351–1358. doi: 10.1080/09540121.2017.1300633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kauth MR, Shipherd JC, Lindsay J, Blosnich JR, Brown GR, Jones KT. Access to care for transgender veterans in the Veterans Health Administration: 2006–2013. Am J Public Health. 2014;104(suppl 4):S532–S534. doi: 10.2105/AJPH.2014.302086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baral SD, Poteat T, Stromdahl S, Wirtz AL, Guadamuz TE, Beyrer C. Worldwide burden of HIV in transgender women: a systematic review and meta-analysis. Lancet Infect Dis. 2013;13(3):214–222. doi: 10.1016/S1473-3099(12)70315-8. [DOI] [PubMed] [Google Scholar]
- 24.Golub SA, Gamarel KE, Rendina HJ, Surace A, Lelutiu-Weinberger CL. From efficacy to effectiveness: facilitators and barriers to PrEP acceptability and motivations for adherence among MSM and transgender women in New York City. AIDS Patient Care STDS. 2013;27(4):248–254. doi: 10.1089/apc.2012.0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown GR, Jones KT. Mental health and medical health disparities in 5135 transgender veterans receiving healthcare in the Veterans Health Administration: a case–control study. LGBT Health. 2016;3(2):122–131. doi: 10.1089/lgbt.2015.0058. [DOI] [PubMed] [Google Scholar]
- 26.Wejnert C, Hess KL, Hall HI et al. Vital Signs: Trends in HIV diagnoses, risk behaviors, and prevention among persons who inject drugs—United States. MMWR Morb Mortal Wkly Rep. 2016;65(47):1336–1342. doi: 10.15585/mmwr.mm6547e1. [DOI] [PubMed] [Google Scholar]
- 27.Conrad C, Bradley HM, Broz D et al. Community outbreak of HIV infection linked to injection drug use of oxymorphone—Indiana, 2015. MMWR Morb Mortal Wkly Rep. 2015;64(16):443–444. [PMC free article] [PubMed] [Google Scholar]
- 28.Teeters JB, Lancaster CL, Brown DG, Back SE. Substance use disorders in military veterans: prevalence and treatment challenges. Subst Abuse Rehabil. 2017;8:69–77. doi: 10.2147/SAR.S116720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu H, Mendoza MC, Huang YA, Hayes T, Smith DK, Hoover KW. Uptake of HIV preexposure prophylaxis among commercially insured persons—United States, 2010–2014. Clin Infect Dis. 2017;64(2):144–149. doi: 10.1093/cid/ciw701. [DOI] [PubMed] [Google Scholar]
- 30.Reif S, Safley D, McAllaster C, Wilson E, Whetten K. State of HIV in the US deep south. J Community Health. 2017;42(5):844–853. doi: 10.1007/s10900-017-0325-8. [DOI] [PubMed] [Google Scholar]
- 31.Rolle CP, Rosenberg ES, Luisi N et al. Willingness to use pre-exposure prophylaxis among Black and White men who have sex with men in Atlanta, Georgia. Int J STD AIDS. 2017;28(9):849–857. doi: 10.1177/0956462416675095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoffman S, Guidry JA, Collier KL et al. A clinical home for preexposure prophylaxis: diverse health care providers’ perspectives on the “purview paradox.”. J Int Assoc Provid AIDS Care. 2016;15(1):59–65. doi: 10.1177/2325957415600798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bien CH, Patel VV, Blackstock OJ, Felsen UR. Reaching key populations: PrEP uptake in an urban health care system in the Bronx, New York. AIDS Behav. 2017;21(5):1309–1314. doi: 10.1007/s10461-016-1663-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clement ME, Seidelman J, Wu J et al. An educational initiative in response to identified PrEP prescribing needs among PCPs in the Southern US. AIDS Care. 2018;30(5):650–655. doi: 10.1080/09540121.2017.1384534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blackstock OJ, Moore BA, Berkenblit GV et al. A cross-sectional online survey of HIV pre-exposure prophylaxis adoption among primary care physicians. J Gen Intern Med. 2017;32(1):62–70. doi: 10.1007/s11606-016-3903-z. [DOI] [PMC free article] [PubMed] [Google Scholar]