Abstract
Background
Sinomenine (SIN) is an extract of the Chinese medicinal herb Sinomenium acutum; it has various pharmacological properties, including immunosuppression and anti-inflammation. The present study aimed to investigate whether SIN has an anti-depressant-like effect in a mouse model of depression induced by chronic unpredictable mild stress (CUMS), and to explore the underlying molecular mechanisms.
Material/Methods
A mouse model of depression was established and treated with different concentrations of SIN (30, 100, or 300 mg/kg). Then, behavioral tests, including sucrose preference test (SPT), forced swimming test (FST), and the tail suspension test (TST), were performed. The levels of norepinephrine (NE), 5-hydroxytryptamine (5-HT), and proinflammatory cytokines (interleukin-1β [IL-1β] interleukin-6 [IL-6], and tumor necrosis factor-α [TNF-α]) in the hippocampus of mice were detected by ELISA assay. The levels of p-p38, p-p65, NLRP3, ASC, and caspase-1 were measured by Western blot or/and qRT-PCR.
Results
The results showed that SIN significantly relieved CUMSinduced depressive-like behaviors. Compared with the model mice, SIN treatment significantly increased the sucrose preference of the mice, and the immobility time in the forced swimming and the tail suspension test were shortened. In addition, SIN decreased CUMS-induced reduction in the concentrations of NE and 5-HT in the hippocampus of mice. SIN reduced CUMS-induced increases in the levels of IL-1β, IL-6, and TNF-α in the hippocampus of mice. Furthermore, activation of the p38MAPK-NF-κB pathway and the nucleotide binding and oligomerization domain-like receptor family pyrin domain-containing 3 (NLRP3) inflammasome induced by CUMS were inhibited by SIN treatment.
Conclusions
In conclusion, our results indicate the antidepressantlike effects of SIN on chronic unpredictable mild stress-induced depression in a mouse model.
MeSH Keywords: Depression, Inflammation, p38 Mitogen-Activated Protein Kinases, Sinomenium
Background
Depression is a complex affective disorder. Its main clinical features are marked and persistent depression, diminished mobility, and delayed thinking and cognitive function. The latest WHO statistics show a global prevalence of about 11% and predict that depression will be the second-leading medical disorder in the world by 2020 [1]. Depression impairs health and quality of life. It imposes a heavy burden on families, society, and government, which has seriously affected social and economic development. It has also become a serious social and medical problem.
The classic monoamine neurotransmitter hypothesis suggests that the occurrence of depression is mainly related to the reduction of 5-hydroxytryptamine (5-HT), dopamine (DA), norepinephrine (NE), and adrenaline (A) in the central nervous system, and most antidepressant drugs are developed based on this theory. The efficacy of these drugs is generally only 60% to 70%, they are associated with many adverse effects, and the clinical application is subject to certain restrictions [2]. Therefore, actively searching for drugs with special effects, low toxicity, and ability to easily pass through the blood-brain barrier for the treatment of depression has become an urgent task worldwide.
At present, developing innovative medicines is an urgent task for our country (China). Looking for antidepressant active ingredients in traditional Chinese medicines is an effective approach and has the advantage of developing antidepressant innovative drugs in our own country. Hypericin is a substance isolated from the plant Hypericum perforatum; it has been found to have a good antidepressant effect, and it has been developed as an antidepressant drug in Germany [3].
Sinomenine (SIN) (7,8-didehydro-4-hydroxy3,7-dimethoxy-17-methyl-9α, 13α, 14α-morphinan-6-one; SIN) is an extract of the Chinese medicinal herb Sinomenium acutum. In China, SIN is widely used to treat mesangial proliferative nephritis and rheumatoid arthritis treatment [4,5]. SIN has anti-cancer, cytoprotection, immunosuppression, and anti-inflammation effects [6–10]. A large body of researches shows that depression is an inflammatory disorder [11–14]. Nevertheless, the effects of SIN on development of depression and the potential underlying molecular mechanisms remain largely unclear.
Chronic socioenvironmental stressors affect the development of depression, and stress is known to be one of the causal factors for development of major depression [15]. Stress has been reported to lead to neuronal atrophy and loss in certain brain structures (mainly in the hippocampus), and exogenous stress can induce neuronal cell death in the hippocampus [16,17]. Stress-based animal models have been widely used to investigate biological mechanisms of depression. Most of these animal models resemble some of the observed dysfunctions of human depression, including significant weight loss, anhedonia, and increased anxiety [18,19]. Recently, unpredictable chronic mild stress (UCMS) has been developed as an experimental model of depression [20–22].
Therefore, in the present study, we aimed to investigate the effects of SIN administration in a mouse model of depression induced by chronic unpredictable mild stress (CUMS), and to explore the underlying molecular mechanisms.
Material and Methods
Mouse model of depression establishment
This study was approved by the ethics committee of Jiaxing University.
30 healthy young male ICR mice (~20 g; 5 weeks old) were obtained from the Vital River Company (Beijing, China). The mice were fed under a 12-h light/dark cycle in 55±5% humidity at 22±2°C with water and food provided freely. All animal experiments were conducted in line with the guidelines for the Care and Use of Laboratory Animals by the National Institute of Health.
Mice were randomly divided into 6 groups (n=5 per group): (1) Control group (unstressed + saline vehicle); (2) Model group (CUMS + saline vehicle); (3–5) 3 SIN treatment groups (CUMS + SIN); (6) fluoxetine treatment group (CUMS + FLU). From the 22th day, the rats were orally administered with SIN (30, 100 or 300 mg/kg) or fluoxetine (20 mg/kg) every day for 21 days. SIN was obtained from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). Fluoxetine treatment group was considered as the positive control group. Fluoxetine hydrochloride was purchased from Hubei Bangsun Chemical co., LTD (Wuhan, Hubei, China). At the last of the experiments, behavioral tests were carried out, and the hippocampal tissues of the mice were extracted as previously described [23,24].
Mouse model of depression induced by chronic unpredictable mild stress (CUMS) were established as previously described [23]. In brief, mice were group housed and allowed to adapt to the environment for one week. Then, the mice of the control group were not disturbed in their cages in a separated room throughout the following 42 days, while the mice of the other 4 groups were single housed and subjected to a variety of mild stressors for 42 days: (1) food deprivation for 24 h, (2) water deprivation for 24 h, (3) overnight illumination, (4) cage tilt (45°) for 7 h, (5) soiled cage (200 mL water in 100 g sawdust bedding), (6) foreign object exposure, (7) light/dark perversion, (8) overhang (10 min), (9) physical restraint for 3 h, (10) 1 min tail pinch (1 cm from the beginning of the tail), (11) 5 min oscillation, and (12) white noise. To ensure the unpredictability of the experiment, all stressors were performed randomly.
Sucrose preference test (SPT)
SPT was carried out every week according to the previous study [24]. The value of sucrose preference was assessed as follows: Preference value (%)=sucrose intake/(sucrose intake+water intake)×100%. Experiments were performed at least for 3 times.
Forced swimming test (FST)
FST was performed according to the previous study [25]. The immobility time was recorded as the length of time the mouse floated in the upright position without a struggle, and only slight movements were made to keep its head out of the water. The duration of immobility was recorded at the last 4 min of the total 6 min, which indicated the depressive state. Experiments were performed at least for 3 times.
Tail suspension test (TST)
TST was conducted based on a previous study [26]. In short, hang the mouse 25 cm above the ground by the tip of the tail (1 cm) tied up to the level. The immobility time was recorded in the test period of 6 minutes (first 1 min for adaptation and the remaining 5 min were recorded). It is considered as immobile only when the mouse is hung passively and completely suspended. Mice crawling to the tail were excluded from the experimental data analysis. Experiments were performed at least for 3 times.
NE and 5-HT levels detection
The expression levels of NE (Cat no. F02611; Shanghai Westang Bio-Tech Co., Ltd., Shanghai, China) and 5-HT (Cat no. F16311; Shanghai Westang Bio-Tech Co., Ltd., Shanghai, China) in the hippocampi of the mice were measured by using ELISA assay according to the manufacturer’s instructions of each kit. The levels of NE and 5-HT were expressed as ng/g tissues. Experiments were performed at least for 3 times.
Pro-inflammatory cytokine levels detection
The levels of interleukin (IL)-1β (Cat no. PI305, Beyotime, Shanghai, China), IL-6 (Cat no. PI330, Beyotime, Shanghai, China) and tumor necrosis factor-α (TNF-α) (Cat no. PT518, Beyotime, Shanghai, China) in the hippocampal tissues of the mice of different groups were measured by using ELISA assay according to the manufacturer’s instructions of each kit. The levels of IL-1β, IL-6 and TNF-α were expressed as pg/mg tissues. Experiments were performed at least for 3 times.
QRT-PCR
Total RNA from hippocampus tissues was extracted by using TRIzol (Invitrogen; Thermo Fisher Scientific Inc.) per as the manufacturer’s protocol. TaqMan microRNA Reverse Transcription kit (Invitrogen) was applied to perform the reverse transcription experiments according to the manufacturer’s protocol. cDNAs were analyzed by using the TaqMan® Universal PCR Master Mix kit (Thermo Fisher Scientific Inc.) under the ABI PRISM 7900 HT sequence-detection system (Applied Biosystems; Thermo Fisher Scientific, Inc.). The conditions for amplification were as follows: 95°C for 10 min, followed by 37 cycles of 95°C for 10 s and 60°C for 60 s. The primer sequences were obtained as required, and listed as following:
NLRP3-forward, 5′-GATCTTCGCTGCGATCAACAG-3′
reverse, 5′-CGTGCATTATCTGAACCCCAC-3′;
ASC-forward, 5′-GCAATGTGCTGACTGAAGGA-3′
reverse, 5′-TGTTCCAGGTCTGTCACCAA-3′;
caspase-1-forward, 5′-GCACAAGACCTCTGACAGCA-3′
reverse, 5′-TTGGGCAGTTCTTGGTATTC-3′;
GAPDH-forward, 5′-AAAATCAAGTGGGGCGATGC-3′
reverse, 5′-AGGAGGCATTGCTGATGATCT-3′.
Experiments were performed at least for 3 times. Relative gene expression was quantified using the 2−ΔΔCq method [27].
Western blot
After specific treatment, total proteins from the hippocampus were extracted using the RIPA buffer (Beijing Solarbio Science & Technology Co., Ltd., Beijing, China) and quantified by BCA assay (Thermo Fisher Scientific, Inc) according to the manufacturer’s protocol. Protein samples (2 μg/lane) were separated on 12% SDS-PAGE and then blotted onto PVDF membranes. After blocked with 5% no-fat milk for 2 h at room temperature, the membranes were then incubated with a primary antibody (all Cell Signaling Technology Inc., Danvers, MA, USA) against p-p38 (1: 1000; cat. no. 1170), p-p65 (1: 1000; cat. no. 3033), NLRP3 (1: 1000; cat. no. 13158), ASC (1: 1000; cat. no. 67824), caspase-1 (1: 1000; cat. no. 3866) or β-actin (1: 5,000; cat no. 4970) overnight at 4°C. Subsequently, the membranes were incubated with the horseradish peroxidase-conjugated secondary antibody, Anti-rabbit IgG, HRP-linked Antibody (1: 5000; cat. no. 7074; Cell Signaling Technology Inc., Danvers, MA, USA), for 4 h at room temperature. Bands were observed using a chemiluminescence detection kit (cat. no. 6883; Cell Signaling Technology Inc.) per as the manufacturer’s protocol.
Statistical analysis
Data were presented as the mean ± standard deviation (SD). Data analysis were performed using SPSS version 17.0 software (SPSS, Inc., Chicago, IL, USA). The significance between different groups was analyzed using one-way analysis of variance followed by Bonferroni’s multiple comparisons test. p<0.05 was considered have significant statistical significance.
Results
CUMS-induced mice body weight reduction was restored by SIN
As shown in Figure 1, at the beginning of the test, the body weight of mice in different groups was similar. However, compared with that of the control group, 3 weeks of CUMS significantly reduced the body weight of the mice (p<0.05). Different concentrations of SIN (30, 100 or 300 mg/kg) as well as fluoxetine significantly eliminated the decreased body weight induced by CUMS.
Figure 1.
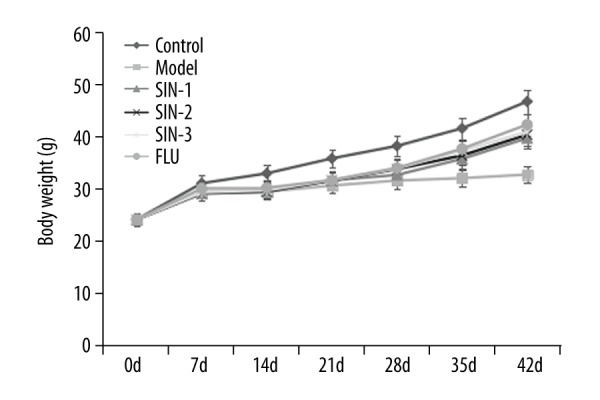
Effect of Sinomenine on body weight of mice. SIN1/2/3 – model mice treated with Sinomenine at 30, 100, or 300 mg/kg; FLU – depression mice treated with fluoxetine. Data are expressed as mean ±SD.
CUMS-induced depressive-like behavior was alleviated by SIN
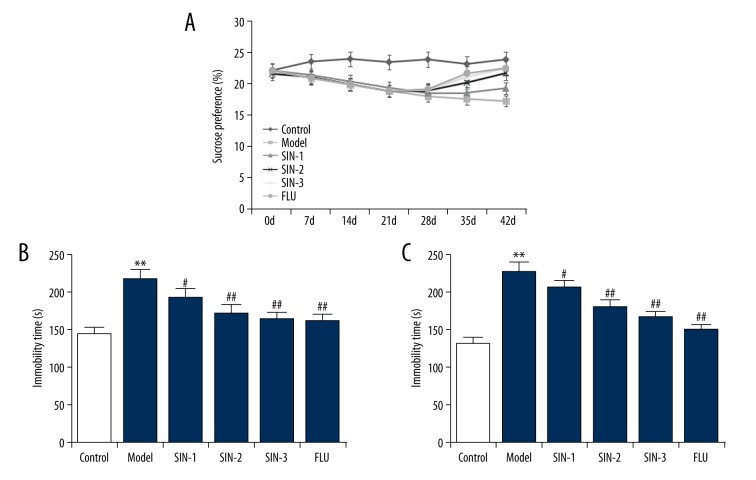
To investigate the effects of SIN on CUMS-induced depressive-like behaviors, SPT, FST and TST were performed after specific treatment. As shown in Figure 2, compared with the control group, the model group presented a significant decrease in sucrose consumption, and SIN and fluoxetine treatment notably increased the sucrose consumption. In addition, the immobility time in the FST and TST were significantly increased in mice of the model group, while SIN and fluoxetine treatment significantly reduced these increases. The data suggested that SIN and fluoxetine treatment significantly alleviated CUMS-induced depression.
Figure 2.
Effect of Sinomenine on chronic unpredictable mild stress-induced depressive-like behavior. (A) Sucrose preference test; (B) Forced swimming test; (C) Tail suspension test. SIN1/2/3 – depression mice treated with Sinomenine at 30, 100, or 300 mg/kg; FLU – depression mice treated with fluoxetine. Data are expressed as mean ±SD. ** p<0.01 vs. control group; #, ## p<0.05, 0.01 vs. model group.
CUMS-induced imbalances in hippocampal neurotransmitter levels were alleviated by SIN
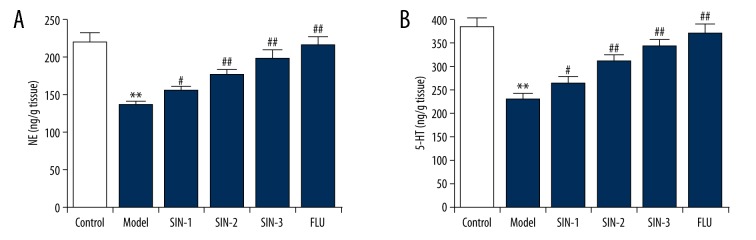
The findings showed that compared with the control group, NE and 5-HT levels in the hippocampus of mice were notably decreased after CUMS conduction. However, compared with the model group, SIN and fluoxetine treatment obviously increased NE and 5-HT levels in the hippocampus of these mice (Figure 3).
Figure 3.
Effect of Sinomenine on hippocampal 5-HT and NE levels in chronic unpredictable mild stress-induced mice. (A) Levels of NE in the hippocampus of mice; (B) Levels of 5-HT in the hippocampus of mice. SIN1/2/3 – depression mice treated with Sinomenine at 30, 100, or 300 mg/kg; FLU – depression mice treated with fluoxetine. Data are expressed as mean ±SD. ** p<0.01 vs. control group; #, ## p<0.05, 0.01 vs. model group. NE – norepinephrine; 5-HT – 5-hydroxytryptamine.
CUMS-induced increases in hippocampal pro-inflammatory cytokine levels were reduced by SIN
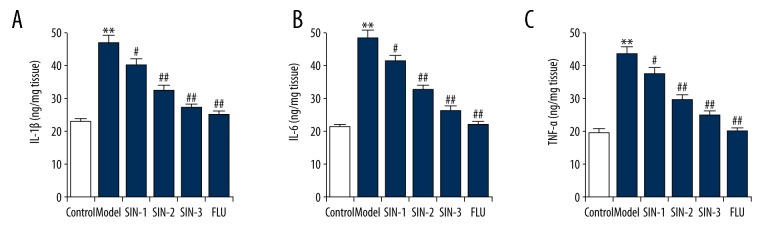
The effect of SIN on the levels of pro-inflammatory cytokines in the hippocampus of mice subjected to CUMS were determined. We found that compared with the control group, CUMS significantly increased the levels of IL-1β, IL-6, and TNF-α in the hippocampus of mice, while SIN and fluoxetine treatment markedly reduced these increases (Figure 4).
Figure 4.
Effect of Sinomenine on hippocampal IL-1β, IL-6, and TNF-α concentrations in chronic unpredictable mild stress-induced mice. (A) Levels of IL-1β in the hippocampus of mice; (B) Levels of IL-6 in the hippocampus of mice; (C) Levels of TNF-α in the hippocampus of mice. SIN1/2/3 – depression mice treated with Sinomenine at 30, 100, or 300 mg/kg; FLU – depression mice treated with fluoxetine. Data are expressed as mean ±SD. ** p<0.01 vs. control group; #, ## p<0.05, 0.01 vs. model group.
CUMS-induced activation of p38MAPK-NF-κB pathway in the hippocampus of mice were inhibited by SIN
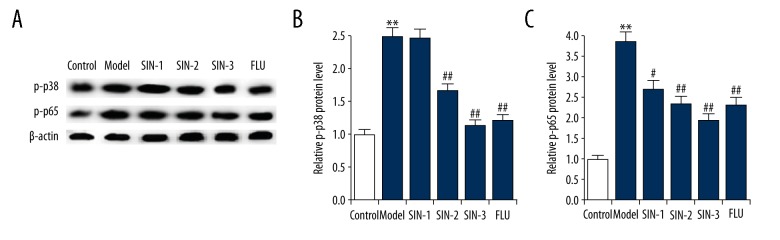
To explore the molecular mechanism of anti-depressant-like effects of SIN on CUMS-induced model of depression in mice, p38MAPK-NF-κB pathway was analyzed. As shown in Figure 5, compared with the control group, the level of p-p38 and p-p65 in the hippocampus of mice subjected to CUMS significantly enhanced, indicating the activation of p38MAPK-NF-κB pathway. This activation was markedly inhibited by SIN and fluoxetine treatment.
Figure 5.
(A–C) Effect of Sinomenine on p38MAPK-NF-κB pathway in the hippocampus of chronic unpredictable mild stress-induced mice. After treatment, the protein level of p-p38 and p-p65 in the hippocampus of mice was measured by Western blot, and the data were analyzed. SIN1/2/3 – depression mice treated with Sinomenine at 30, 100, or 300 mg/kg; FLU – depression mice treated with fluoxetine. Data are expressed as the mean ±SD. ** p<0.01 vs. control group; #, ## p<0.05, 0.01 vs. model group.
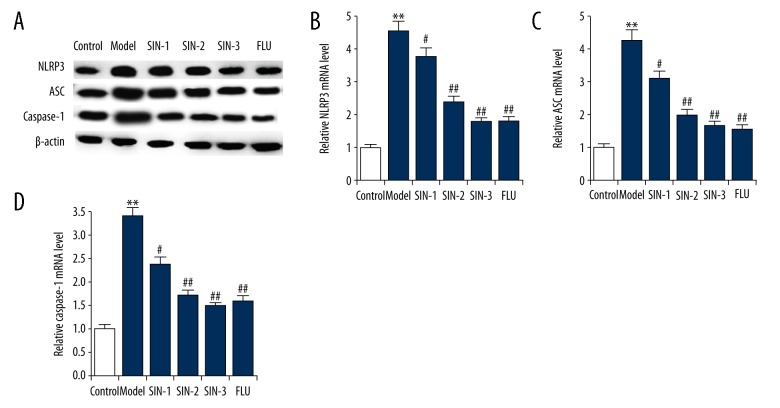
CUMS-induced increases of NLRP3 inflammasome in the hippocampus of mice were decreased by SIN
In addition, NLRP3 inflammasome was determined in the current study. Results suggested that compared with the control group, the protein and mRNA levels of NLRP3, ASC and caspase-1 in the hippocampus of mice subjected to CUMS were significantly up-regulated, and these up-regulations were eliminated by SIN and fluoxetine treatment (Figure 6).
Figure 6.
(A–D) Effect of SIN on NLRP3 inflammasome complex protein levels in the hippocampus of CUMS-induced mice. After treatment, protein and mRNA levels of NLRP3, ASC, and caspase-1 in the hippocampus of mice were measured by Western blot and qRT-PCR, respectively. SIN1/2/3 – depression mice treated with Sinomenine at 30, 100, or 300 mg/kg; FLU – depression mice treated with fluoxetine. Data are expressed as the mean ±SD. ** p<0.01 vs. control group; #, ## p<0.05, 0.01 vs. model group.
Discussion
Depression has become a serious social issue that cannot be ignored because of the serious impact of depression on the health and quality of life of the patients. Research shows that drug treatment is an effective treatment for depression [28]. Therefore, it is very urgent for us to search for novel and effective agents for treatment of depression.
SIN is a bioactive alkaloid extracted from Sinomenium acutum. A previous study suggested that SIN exerts antidepressant effects in mice with depression induced by chronic social defeat stress (CSDS) by promoting the hippocampal BDNF signaling pathway [29]. However, whether SIN has an anti-depressant-like effect on mice with depression induced by CUMS remains unclear, and the underlying molecular mechanisms are not fully understood. Therefore, we performed the present study, and a mouse model of depression induced by CUMS was conducted. Mice were treated with various concentrations of SIN (30, 100, or 300 mg/kg), and fluoxetine (20 mg/kg) was used as the positive control drug. We found that SIN and fluoxetine treatment relieved CUMS-induced depressive-like behaviors and alleviated CUMS-induced reductions in hippocampal NE and 5-HT levels, and increased the levels of proinflammatory cytokines (IL-1β, IL-6, TNF-α) in the hippocampus of mice. In addition, activation of the p38MAPK-NF-κB pathway and NLRP3 inflammasome complex induced by CUMS were repressed by SIN and fluoxetine treatment. To the best of our knowledge, this is the first study showing the anti-depressive activity of SIN in a mouse model of depression induced by CUMS.
To investigate the effects of SIN administration on depression, a mouse model of depression was established by chronic unpredictable mild stress (CUMS) induction. We observed that compared with the control group, a 3-week CUMS procedure markedly decreased the body weight gain of the mice. Our results also demonstrated that mice subjected to CUMS had depressive-like behaviors, including decreased SP and longer immobility time in FST and TST. It is noteworthy that these changes were significantly reduced by SIN and fluoxetine treatment, as evidenced by increased SP and shortened immobility time in FST and TST.
Increasing evidence indicates the critical roles of the neurotransmitters 5-HT and NE in the process of learning and memory [30,31], and reduction of 5-HT and NE in the central nervous system has been found during depression. The present study found that the levels of NE and 5-HT in the hippocampus of mice were significantly decreased after CUMS, and this is consistent with previous studies. However, SIN and fluoxetine treatment significantly reversed the reduction of NE and 5-HT levels caused by CUMS in the hippocampus of mice.
Inflammatory cytokines play an important role in the pathogenesis and development of depression [32]. Studies have reported that proinflammatory cytokines, including IL-1β, IL-6, and TNF-α, were significantly up-regulated during depression [12,14]. Similarly, our study found that CUMS significantly increased the levels of IL-1β, IL-6, and TNF-α in the hippocampus of mice, while SIN treatment markedly reduced these increases. The p38MAPK-NF-κB pathway, as well as NLRP3 inflammasome, play a key role in inflammation response, and various studies have found that the p38MAPK-NF-κB pathway and NLRP3 inflammasome were activated during depression [33–35]. The present study suggests that CUMS-induced activation of the p38MAPK-NF-κB pathway and increases of NLRP3 inflammasome complex (NLRP3, ASC, and caspase-1) in the hippocampus of mice were notably inhibited by SIN and fluoxetine treatment.
Taken together, the data of the present study indicate that SIN exerts an anti-depressive effect on the mouse model of depression induced by CUMS, and that the underlying molecular mechanism may be related to the prevention of p38MAPK-NF-κB pathway and NLRP3 inflammasome activation.
Conclusions
SIN significantly ameliorated CUMSinduced depressive-like behaviors, as evidenced by increased sucrose preference and shortened immobility time in the forced swimming and the tail suspension test. In addition, SIN inhibited CUMS-induced reduction of NE and 5-HT and increased levels of IL-1β, IL-6, and TNF-α in the hippocampus of mice. Therefore, SIN exerts an anti-depressive effect on the mouse model of depression induced by CUMS, and it may be a promising and effective agent for depression treatment. To provide a more reliable theoretical basis for clinical treatment of depression with SIN, much research needs to be carried out in the future.
Footnotes
Conflict of interest
None.
Source of support: This study was supported by the experimental animal science and technology plan projects of Zhejiang province (Grant no. 2018C37092, 2018) and the experimental animal science and technology plan projects of Zhejiang province (Grant no. 2014C37019, 2014)
References
- 1.Kendler KS, Gardner CO. Dependent stressful life events and prior depressive episodes in the prediction of major depression: The problem of causal inference in psychiatric epidemiology. Arch Gen Psychiatry. 2010;67:1120–37. doi: 10.1001/archgenpsychiatry.2010.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sanford M, Boyle M, McCleary L, et al. A pilot study of adjunctive family psychoeducation in adolescent major depression: Feasibility and treatment effect. J Am Acad Child Adolesc Psychiatry. 2006;45:386–495. doi: 10.1097/01.chi.0000198595.68820.10. [DOI] [PubMed] [Google Scholar]
- 3.da Conceição AO, Takser L, Lafond J. Effect of St. John’s Wort standardized extract and hypericin on in vitro placental calcium transport. J Med Food. 2010;13:934–42. doi: 10.1089/jmf.2009.0161. [DOI] [PubMed] [Google Scholar]
- 4.Cheng Y, Li F, Wang D, et al. Sinomenine inhibits the expression of PDL1 in the peripheral blood mononuclear cells of mesangial proliferative nephritis patients. Mol Med Rep. 2013;7:1223–28. doi: 10.3892/mmr.2013.1302. [DOI] [PubMed] [Google Scholar]
- 5.Xu M, Liu L, Qi C, et al. Sinomenine versus NSAIDs for the treatment of rheumatoid arthritis: A systematic review and meta-analysis. Planta Med. 2008;74:1423–29. doi: 10.1055/s-2008-1081346. [DOI] [PubMed] [Google Scholar]
- 6.Cheng Y, Zhang J, Hou W, et al. Immunoregulatory effects of sinomenine on the T-bet/GATA-3 ratio and Th1/Th2 cytokine balance in the treatment of mesangial proliferative nephritis. Int Immunopharmacol. 2009;9:894–99. doi: 10.1016/j.intimp.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 7.Qian L, Xu Z, Zhang W, et al. Sinomenine, a natural dextrorotatory morphinan analog, is antiinflammatory and neuroprotective through inhibition of microglial NADPH oxidase. J Neuroinflammation. 2007;4:23. doi: 10.1186/1742-2094-4-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu XL, Zeng J, Chen YL, et al. Sinomenine hydrochloride inhibits human hepatocellular carcinoma cell growth in vitro and in vivo: Involvement of cell cycle arrest and apoptosis induction. Int J Oncol. 2013;42:229–38. doi: 10.3892/ijo.2012.1704. [DOI] [PubMed] [Google Scholar]
- 9.Li X, Li PP, Liu C, et al. Sinomenine hydrochloride inhibits breast cancer metastasis by attenuating inflammation-related epithelial-mesenchymal transition and cancer stemness. Oncotarget. 2017;8:13560–74. doi: 10.18632/oncotarget.14593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Q, Li XK. Immunosuppressive and anti-inflammatory activities of sinomenine. Int Immunopharmacol. 2011;11:373–76. doi: 10.1016/j.intimp.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 11.Bluthé RM, Layé S, Michaud B, et al. Role of interleukin-1beta and tumour necrosis factor-alpha in lipopolysaccharide-induced sickness behaviour: A study with interleukin 1 type I receptor defcient mice. Eur J Neurosci. 2000;12:4447–56. [PubMed] [Google Scholar]
- 12.Mikova O, Yakimova R, Bosmans E, et al. Increased serum tumor necrosis factor alpha concentrations in major depression and multiple sclerosis. Eur Neuropsychopharmacol. 2001;11:203–8. doi: 10.1016/s0924-977x(01)00081-5. [DOI] [PubMed] [Google Scholar]
- 13.Maes M. Depression is an inflammatory disease, but cell mediated immune activation is the key component of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:664–75. doi: 10.1016/j.pnpbp.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 14.Adler UC, Marques AH, Calil HM. Inflammatory aspects of depression. Inflamm Allergy Drug Targets. 2008;7:19–23. doi: 10.2174/187152808784165216. [DOI] [PubMed] [Google Scholar]
- 15.Culverhouse RC, Saccone NL, Bierut LJ. The state of knowledge about the relationship between 5-HTTLPR, stress, and depression. J Affect Disord. 2017;228:205–6. doi: 10.1016/j.jad.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 16.Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biological Psychiatry. 2006;59:1116–27. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 17.Lee AL, Ogle WO, Sapolsky RM. Stress and depression: Possible links to neuron death in the hippocampus. Bipolar Disorders. 2002;4:117–28. doi: 10.1034/j.1399-5618.2002.01144.x. [DOI] [PubMed] [Google Scholar]
- 18.Cryan JF, Markou A, Lucki I. Assessing antidepressant activity in rodents: Recent developments and future needs. Trends Pharmacol Sci. 2002;23:238–45. doi: 10.1016/s0165-6147(02)02017-5. [DOI] [PubMed] [Google Scholar]
- 19.Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008;455:894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bondi CO, Rodriguez G, Gould GG, et al. Chronic unpredictable stress induces a cognitive deficit and anxiety-like behavior in rats that is prevented by chronic antidepressant drug treatment. Neuropsychopharmacology. 2008;33:320–31. doi: 10.1038/sj.npp.1301410. [DOI] [PubMed] [Google Scholar]
- 21.Zhu S, Shi R, Wang J, et al. Unpredictable chronic mild stress not chronic restraint stress induces depressive behaviours in mice. Neuroreport. 2014;25:1151–55. doi: 10.1097/WNR.0000000000000243. [DOI] [PubMed] [Google Scholar]
- 22.Zhu S, Wang J, Zhang Y, et al. Unpredictable chronic mild stress induces anxiety and depression-like behaviors and inactivates AMP-activated protein kinase in mice. Brain Res. 2014;1576:81–90. doi: 10.1016/j.brainres.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 23.Deng XY, Li HY, Chen JJ, et al. Thymol produces an antidepressant-like effect in a chronic unpredictable mild stress model of depression in mice. Behav Brain Res. 2015;291:12–19. doi: 10.1016/j.bbr.2015.04.052. [DOI] [PubMed] [Google Scholar]
- 24.Deng XY, Xue JS, Li HY, et al. Geraniol produces antidepressant-like effects in a chronic unpredictable mild stress mice model. Physiol Behav. 2015;152:264–71. doi: 10.1016/j.physbeh.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 25.Kurhe Y, Radhakrishnan M, Gupta D. Ondansetron attenuates depression co-morbid with obesity in obese mice subjected to chronic unpredictable mild stress; An approach using behavioral battery tests. Metab Brain Dis. 2014;29:701–10. doi: 10.1007/s11011-014-9574-8. [DOI] [PubMed] [Google Scholar]
- 26.Li M, Fu Q, Li Y, et al. Emodin opposes chronic unpredictable mild stress induced depressive-like behavior in mice by upregulating the levels of hippocampal glucocorticoid receptor and brain-derived neurotrophic factor. Fitoterapia. 2014;98:1–10. doi: 10.1016/j.fitote.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 27.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 28.Cho H, Son SJ, Kim S, Park J. A randomized comparison of medication and cognitive behavioral therapy for treating depression in low-income young minority women. Med Sci Monit. 2016;22:4947–53. doi: 10.12659/MSM.902206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li X, Liu C, Jiang B, et al. The antidepressant-like effects of sinomenine in mice: A behavioral and neurobiological characterization. Behav Pharmacol. :2017. doi: 10.1097/FBP.0000000000000350. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 30.Hou C, Jia F, Liu Y, Li L. CSF serotonin, 5-hydroxyindolacetic acid and neuropeptide Y levels in severe major depressive disorder. Brain Res. 2006;1095:154–58. doi: 10.1016/j.brainres.2006.04.026. [DOI] [PubMed] [Google Scholar]
- 31.Kuhn M, Popovic A, Pezawas L. Neuroplasticity and memory formation in major depressive disorder: An imaging genetics perspective on serotonin and BDNF. Restor Neurol Neurosci. 2014;32:25–49. doi: 10.3233/RNN-139005. [DOI] [PubMed] [Google Scholar]
- 32.Anisman H, Merali Z. Cytokines, stress and depressive illness: Brain-immune interactions. Ann Med. 2003;35:2–11. doi: 10.1080/07853890310004075. [DOI] [PubMed] [Google Scholar]
- 33.Li R, Wang X, Qin T, et al. Apigenin ameliorates chronic mild stress-induced depressive behavior by inhibiting interleukin-1β production and NLRP3 inflammasome activation in the rat brain. Behav Brain Res. 2016;296:318–25. doi: 10.1016/j.bbr.2015.09.031. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y, Liu L, Liu YZ, et al. NLRP3 inflammasome mediates chronic mild stress induced depression in mice via neuroinflammation. Int J Neuropsychopharmacol. 2015;18(8) doi: 10.1093/ijnp/pyv006. pii: pyv006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li H, Lin S, Qin T, et al. Senegenin exerts anti-depression effect in mice induced by chronic un-predictable mild stress via inhibition of NF-κB regulating NLRP3 signal pathway. Int Immunopharmacol. 2017;53:24–32. doi: 10.1016/j.intimp.2017.10.001. [DOI] [PubMed] [Google Scholar]