Abstract
Flowers of Anacardiaceae and other Sapindales typically produce nectar, but scent, often associated with a reward for pollinators, has surprisingly been mentioned only rarely for members of the family and order. However, flowers of Anacardium humile and Mangifera indica produce a strong sweet scent. The origin and composition of these floral scents is the subject of this study. Screening of potential osmophores on the petals and investigations of their anatomy were carried out by light, scanning and transmission electron microscopy. The composition of the floral fragrance was characterized by gas chromatography–mass spectrometry. In both species, the base of the adaxial side of each petal revealed specialized secretory epidermal cells which are essentially similar in structure and distinct from all other neighbouring cells. These cells also showed evidence of granulocrine secretory mechanisms and slight specific variations in their subcellular apparatus coinciding with the respective composition of the floral fragrance, predominantly composed of sesquiterpenes in A. humile and monoterpenes in M. indica. This study reports the presence of osmophores for the first time in flowers of Anacardiaceae and confirms the link between the ultrastructural features of their secretory cells and the volatiles produced by the flowers. The flowers of most Sapindales, including Anacardiaceae, are nectariferous. However, the presence of osmophores has only been described for very few genera of Rutaceae and Sapindaceae. Both the occurrence of osmophores and fragrance may have largely been overlooked in Anacardiaceae and Sapindales until now. Further studies are needed to better understand the nature and diversity of the interactions of their nectariferous flowers with their pollinators.
Keywords: Cashew family, floral scent, osmophores, pollinator attraction, secretory structures
This study focuses on the first report of osmophores in flowers of the economically important family Anacardiaceae. Flowers of Anacardium and Mangifera emit a strong and sweet scent suggesting the possible presence of osmophores. Surprisingly, we realized that scent glands were never mentioned in the family. Our study confirms the presence of osmophores in both genera, which are structurally similar. However, the composition of the fragrance shows varies widely between species, with sesquiterpenes dominating the scents of Anacardium humile and monoterpenes in Mangifera indica. Slight variations in their subcellular apparatus are consistent with those found in the composition of their fragrance.
Introduction
Pleasant or not, the fragrance of a flower typically serves as a sensory cue for pollinators, often indicative of some kind of reward, notably nectar (Vogel 1990; Endress 1994). Like nectar, floral scents are produced by specialized structures commonly referred to as scent glands or osmophores, which are located mainly on petals and other floral organs, such as sepals or stamens, or other specialized reproductive structures (Vogel 1990; Sazima et al. 1993; Vogel and Hadacek 2004; Guimarães et al. 2008; Melo et al. 2010; Marinho et al. 2014; Possobon et al. 2015; Gagliardi et al. 2016).
Osmophores typically consist of an epidermis of specialized secretory cells and/or secretory parenchyma. They are concentrated in certain regions of the floral organs and can have different shapes, sizes and colours (Vogel 1990; Vogel and Hadacek 2004; Guimarães et al. 2008; Melo et al. 2010; Marinho et al. 2014; Possobon et al. 2015; Gagliardi et al. 2016; Gonçalves-Souza et al. 2017). Osmophores are found in many unrelated orders of flowering plants, occurring on the distal portion of the spadix of some Araceae (Alismatales) and petals of Iridaceae or Orchidaceae (Asparagales) in monocots, on petals of Euphorbiaceae and the corona of some Passifloraceae (Malpighiales), on petals of Fabaceae (Fabales) as well as the anthers of Solanaceae (Solanales) or the staminal appendages (‘horns’) of Asclepiodoideae (Gentianales), among other eudicots (Vogel 1990; Evert 2006; Teixeira et al. 2014; Marinho et al. 2014, 2018; Gagliardi et al. 2016; Demarco 2017a).
Flowers of most members of Sapindales are nectariferous and often have a conspicuous nectary (Kubitzki 2011; Ronse De Craene and Haston 2006). However, out of the nine families and ca. 6550 species (Stevens 2001 onwards), osmophores have only been reported and studied in two genera of Rutaceae (Bussel et al. 1995; Marques et al. 2015), while those reported in two species of a genus of Sapindaceae were not studied in detail and lacked chemical characterization of their volatile compounds (Lima et al. 2016).
Anacardiaceae are one of the largest families of Sapindales and comprise 82 genera and ~800 species distributed mainly in tropical areas (Pell et al. 2011; Weeks et al. 2014). As in all other members of the order, the flowers of most genera of the family show features typical of melittophily, such as diurnal anthesis, small dish-shaped flowers and a conspicuous nectary often in the shape of a fleshy intrastaminal disk (Pell et al. 2011). During previous studies we noticed that, aside from producing nectar, the flowers of Anacardium humile, A. occidentale and Mangifera indica also emitted a strong and sweet scent suggesting the possible presence of osmophores (Bachelier and Endress 2009; Tolke et al. 2015,2018). Surprisingly, we realized that such secretory structures were never mentioned, not even in A. occidentale and M. indica, which are two economically important and extensively studied fruit crop species (Mukherjee 1972; Johnson 1973; Schnell et al. 1995; Freire et al. 2002).
In this study, we localized and characterized previously unrecognized specialized epidermal cells located at the base of the adaxial side of each petal of A. humile and M. indica and based on their secretory function, interpret them here as osmophores. Their ultrastructural features were studied before and during anthesis in order to understand their mechanism of secretion and whether there is a link between their subcellular apparatus and the composition of the floral fragrance by examining flowers of each species by gas chromatography–mass spectrometry (GC-MS). Based on our results, we also discuss the importance of diversified strategies on the attraction of pollinators in Anacardiaceae and the entire Sapindales, despite the scarcity of reports of osmophores in the order.
Materials and Methods
Plant material
Floral buds and flowers of A. humile were collected in the ‘Reserva Biológica e Estação Experimental de Mogi-Guaçu’, state of São Paulo, Brazil. Floral buds and flowers of M. indica were collected from plants cultivated at the gardens of the ‘Universidade Estadual de Campinas’, state of São Paulo, Brazil. Voucher specimens were deposited in the herbarium UEC (A. humile, UEC 119573; M. indica, UEC 119571).
Location and structure of the osmophores
All collected material was fixed in BNF (buffered neutral formalin) for 48 h (Lillie 1965) and stored in 70 % ethanol. The material was dehydrated through a tertiary butyl alcohol series and embedded in Paraplast® (Merck KGaA, Darmstadt, Germany) (Johansen 1940). Transverse and longitudinal sections 8–10 µm thick were obtained using a Leica RM2245 rotary microtome (Leica Microsystems Richmond, Inc., Wetzlar, Germany) and stained with Astra blue (Merck KGaA) and safranin (C.I. 50240, Merck KGaA) (Gerlach 1984). In order to detect the presence of lipids, some sections were stained with Sudan black B (C.I. 26150; Pearse 1985). All slides were mounted in Entellan® synthetic resin (Merck KGaA), and the images were obtained with an Olympus DP71 digital camera coupled to an Olympus BX51 microscope.
The anthetic flowers were dissected under a Leica M80 stereomicroscope (Leica Biosystems Richmond, Inc., Wetzlar, Germany) and dehydrated in an ethanol dilution series before being critically point-dried with CO2 and sputter-coated with gold (SCD-050 sputter coater, Bal-Tec AG, Balzers, Liechtenstein). Observations were carried out using a JEOL JSM 5800 LV scanning electron microscope (JEOL, Tokyo, Japan).
Ultrastructural organization of the osmophores
Petals of flowers in pre-anthesis and anthesis were fixed in 2.5 % glutaraldehyde in 0.1 M phosphate buffer, pH 7.3 for 24 h at 4 °C. They were post-fixed in 1 % osmium tetroxide in the same buffer for 1 h at room temperature, dehydrated in an acetone dilution series and embedded in Araldite resin (Machado and Rodrigues 2004). Ultrathin sections were obtained with a diamond knife and counterstained with uranyl acetate (Merck KGaA) (Watson 1958) and lead citrate (Merck KGaA) (Reynolds 1963). The material was observed with a Tecnai G2 Spirit Bio TWIN transmission electron microscope (FEI Company, Hillsboro, OR, USA).
Floral bouquet composition
Entire fresh flowers of A. humile (27.49 g) and M. indica (16.2 g) were submitted (separately) to hydrodistillation in a Clevenger-type apparatus for 4 h. The crude oils were extracted with dichloromethane, dried (anhydrous) Na2SO4, filtered, and the solvent removed at room temperature. The oils obtained were kept at –20 °C in amber glass bottles until the identification of their chemical composition. The extraction yield of each essential oil was expressed in % (w/w) of the fresh flowers.
Gas chromatography–mass spectrometry analysis of the volatile oils was performed with an Agilent 6850 gas chromatograph (Palo Alto, CA, USA) equipped with a HP-5MS capillary column (30 m × 0.25 mm i.d.; 0.25 μm) and connected to an Agilent 5975C mass spectrometer operating in the positive ion electron impact ionization (70 eV; m/z 50–800; source temperature 280 °C; quadruple temperature 180 °C). The column temperature was initially maintained at 50 °C for 5 min, increased to 100 °C at 3 °C min−1, and subsequently increased to 325 °C at 10 °C min−1, and maintained for 7 min at 325 °C. The carrier gas was helium at a flow rate of 1.0 mL min−1. The inlet temperature was maintained at 320 °C at a split mode of 50:1. The compounds were identified from their retention indices (Adams 2001) and by interpreting their fragmentation patterns in the mass spectra, further confirmed by comparing with fragmentation patterns of authentic compounds and the relevant spectral data from the NIST and Wiley libraries.
Results
Location and structure of the osmophores
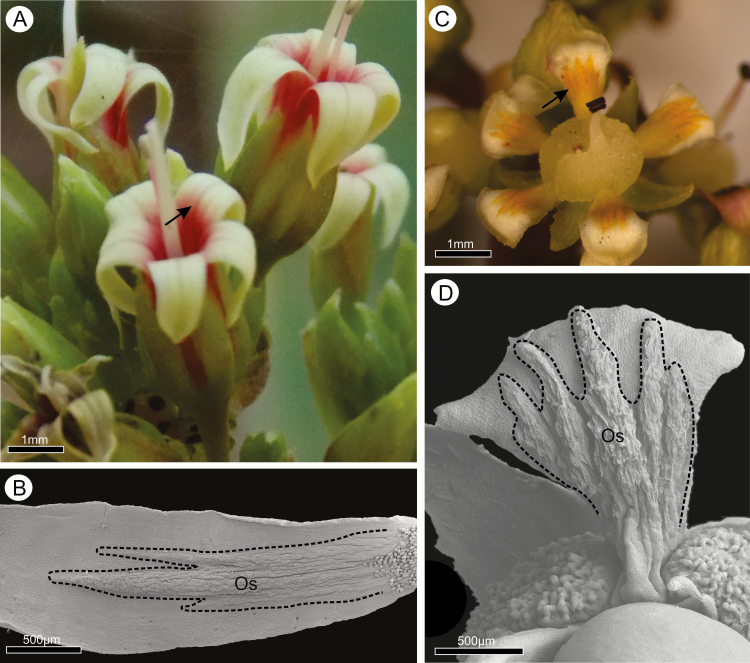
In both species, the adaxial surface at the base of each petal displayed prominent longitudinal ridges covered with distinct epidermal cells that were here interpreted as osmophores (Fig. 1). In A. humile, the osmophores were found to be located on narrow ridges following the main vascular bundles from the base up to the longitudinal midpoint of the petals. Their colour was recorded as white in anthetic flowers like the other regions of the petals, but turning distinctly pink later (Fig. 1A and B). In M. indica, the osmophores were found to be located not only on the ridges but also covering most of the lower half of the petals. Their colour was yellow at anthesis, turning brown later (Fig. 1C and D).
Figure 1.
Flowers of Anacardium humile (A and B) and Mangifera indica (C and D) in fresh material and scanning electron microscope images showing osmophores on the ridges of the adaxial surface (arrow) of petals. Os, osmophore.
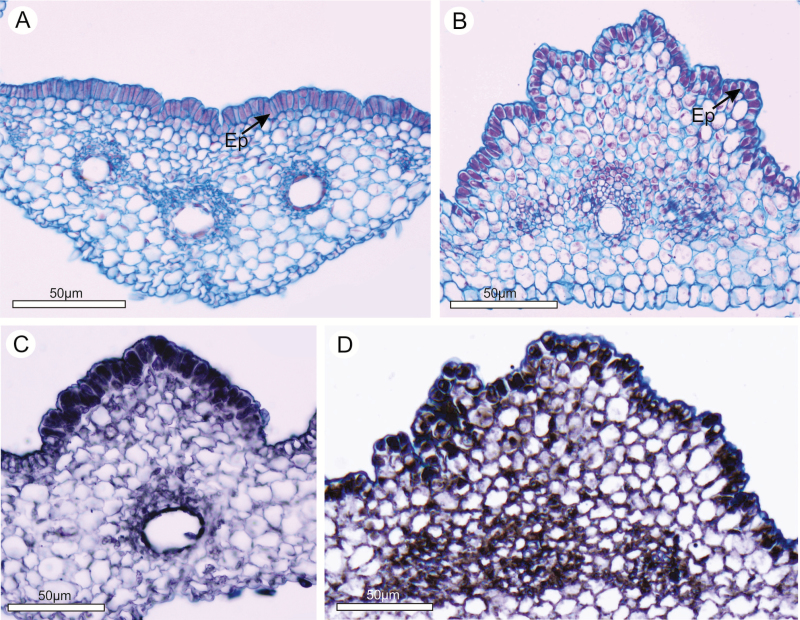
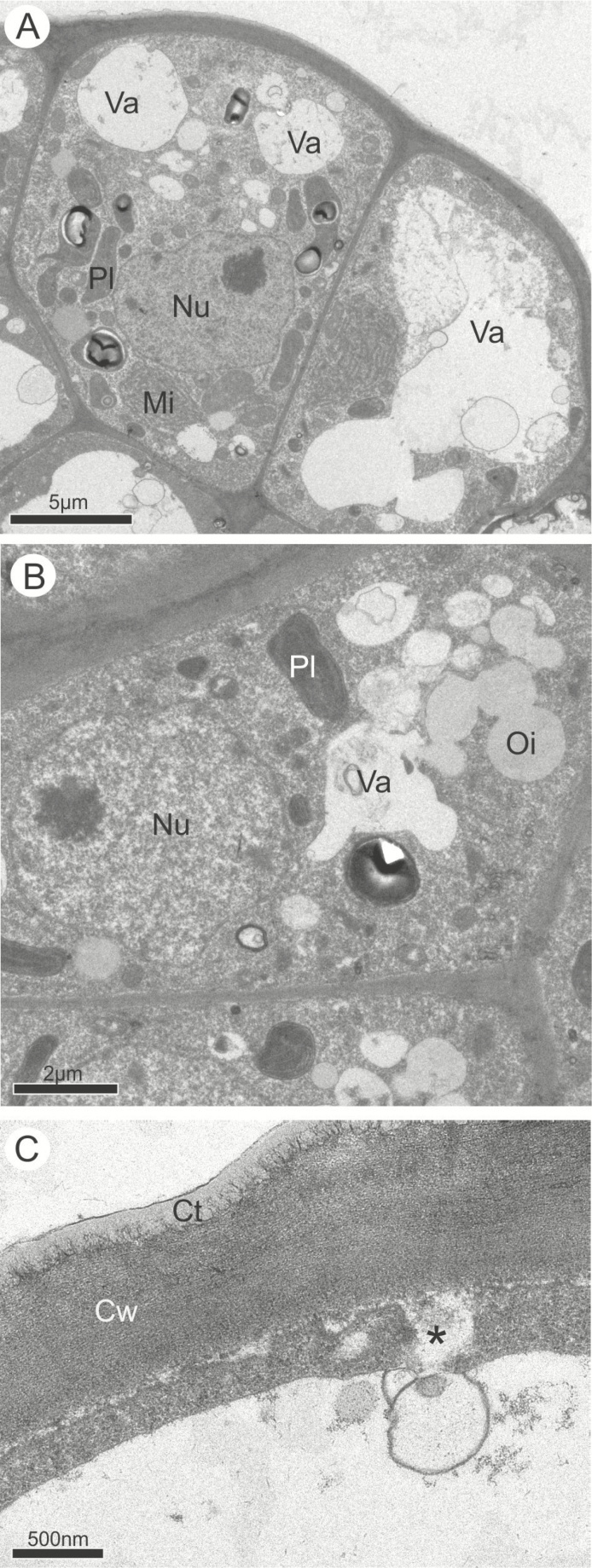
The osmophores were composed of only one layer of epidermal cells characterized by a prominent nucleus and a densely stained cytoplasm (Fig. 2A and B). A positive reaction for lipids confirmed the oil content in both species (Fig. 2C and D). In A. humile, the epidermis appeared as distinctly palisadic cells (Fig. 2A), whereas in M. indica their shapes were more irregular, ranging from cubic to elongated, like those of the mesophyll beneath (Fig. 2B). There was no evidence of stomata or vascular supply directed towards the osmophore.
Figure 2.
Anatomy (A and B) and histochemical tests (C and D) in cross-sections. (A) Palisade-like osmophore of Anacardium humile. (B) Osmophore of Mangifera indica formed by epidermal cells; note that the shape is like the underlying cells. (C) Positive reaction to Sudan black B on the epidermal cells of the petals of A. humile. (D) Positive reaction to Sudan black B on the epidermal cells of the petals of M. indica. Ep, epidermis.
Ultrastructural organization of the osmophores
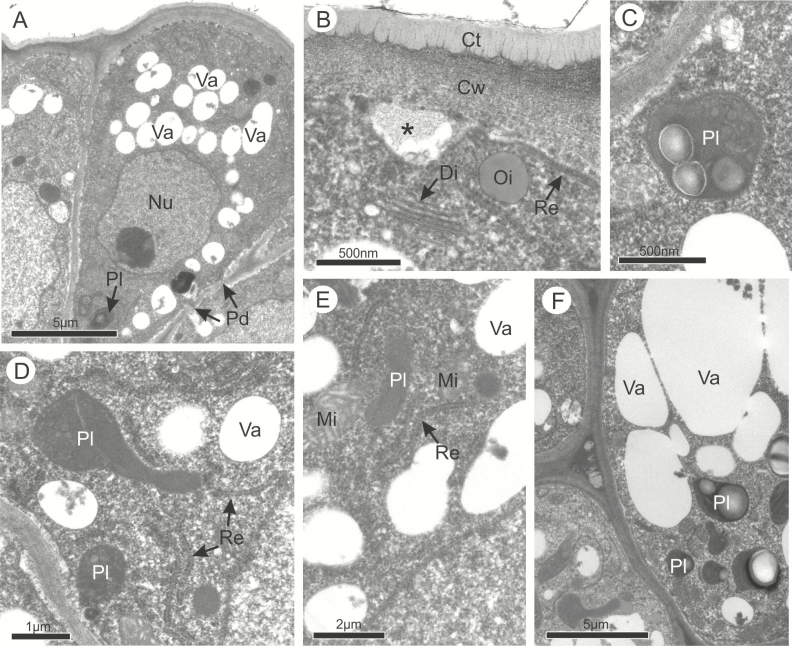
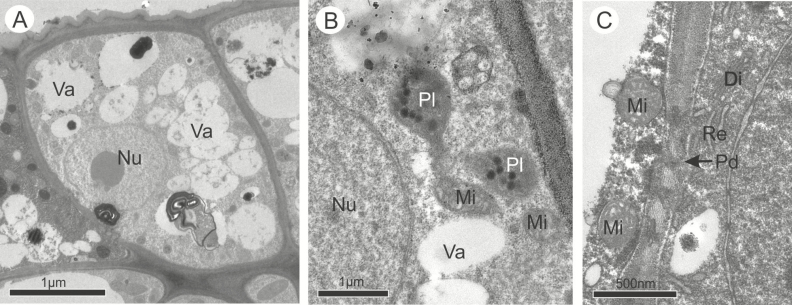
Despite their differences in shape and size, the cells of the osmophores in both species had the same subcellular bipolar organization and characteristics that indicate their similar secretory function (Figs 3A–F and 4A–F). Before anthesis, TEM observations revealed that in both species the epidermal cells have a thicker outer periclinal wall covered by a thin cuticle (Figs 3B and 4B), and dense and organelle-rich cytoplasm interconnected via plasmodesmata in the anticlinal walls (Figs 3A, D, E and 4A, C, D). In the proximal region, where the fragrant compounds are synthetized, all the cells had a large nucleus surrounded by a rough endoplasmic reticulum, and a high number of polyribosomes, mitochondria, small vacuoles and plastids containing starch grains and numerous oil droplets free in the cytosol (Figs 3A–F and 4A–F). In both species, some of the cells contained larger vacuoles (Fig. 3E and F) and a larger population of plastids containing starch grains (Figs 3F and 4A, C). In the apical portion where the secretions are released, the cytoplasm appeared as less dense than in the proximal region due to a greater number of vesicles and vacuoles of various sizes (Figs 3A and 4A). In A. humile, dictyosomes occurred in larger quantity with more vesicles than in M. indica (Fig. 3B), and the oil droplets were more common, always located in a peripheral position near the dictyosomes and near the endoplasmic reticulum (Figs 3B and 4C). In both species, secretion was observed in the periplasmic space and released by vesicles and vacuoles which merge with the plasma membrane on the apical side of the cell (Figs 3B and 4B). The cuticle displayed pectin projections in both species, being slightly thicker in A. humile than in M. indica (Figs 3B and 4B).
Figure 3.
Ultrastructural features of the osmophores in Anacardium humile in pre-anthesis. (A) General view of the epidermal cells. (B) Cuticle. Note the presence of vacuoles, oil droplets and dictyosomes near to the plasma membrane. The secretion is observed in the periplasmic space (*). (C) Plastid containing starch. (D and E) Epidermal cell showing polyribosomes, mitochondria, vacuoles, plastids and rough endoplasmic reticulum. (F) Large vacuoles and plastids containing starch. Ct, cuticle; Cw, cell wall; Di, dictyosomes; Mi, mitochondria; Nu, nucleus; Oi, oil droplet; Pd, plasmodesma; Pl, plastid; Re, rough endoplasmic reticulum; Va, vacuole.
Figure 4.
Ultrastructural features of the osmophores in Mangifera indica in pre-anthesis. (A) General view of the epidermal cells. (B) Cuticle. Asterisks indicate the secretions stored between the plasma membrane and the cell wall. (C) Epidermal cell with polyribosomes, mitochondria, plastids containing starch, vacuoles and oil droplets. (D) Plasmodesma between epidermal cells. Ct, cuticle; Cw, cell wall; Mi, mitochondria; Ni, nucleolus; Nu, nucleus; Oi, oil droplet; Pd, plasmodesma; Pl, plastid; Re, rough endoplasmic reticulum; Va, vacuole.
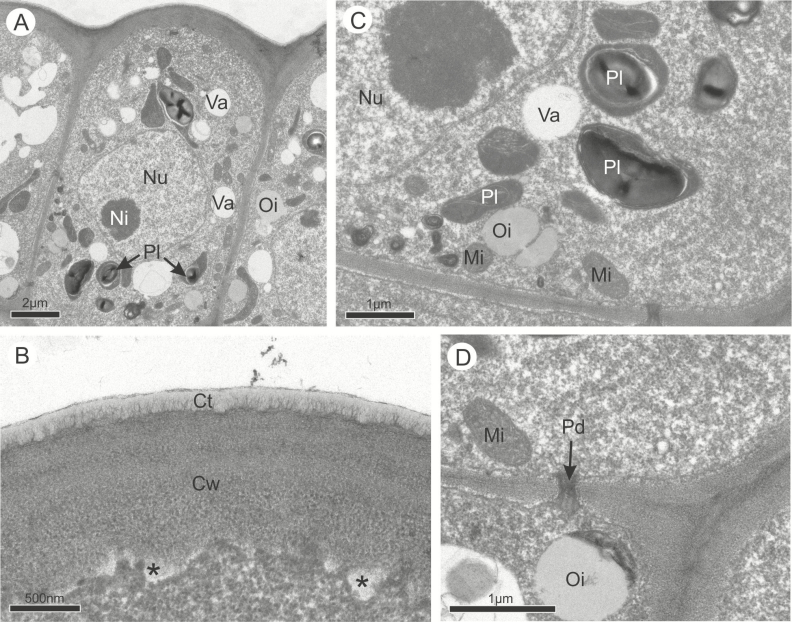
During anthesis, the vacuoles were larger than during the previous phase and in both species some cells were entirely occupied by a single and large vacuole filled with membranous and flocculent material while others were at different stages of secretion (Figs 5A–C and 6A–C). There was no difference in types of organelles found in the previous phase, and only their numbers were quite reduced (Figs 5A–C and 6A–C). In the distal portion of the active cells, vesicles and small vacuoles continued to merge with the plasma membrane and released their secretions to the periplasmic space which crossed the outer periclinal cell wall and the cuticle without disruption (Fig. 6C). Only in A. humile there was a total depletion of starch with a concomitant emergence of plastoglobules within plastids (Fig. 5B).
Figure 5.
Ultrastructural features of the osmophores in Anacardium humile at anthesis. (A) General view of the osmophores. (B) Epidermal cell showing the plastids containing oil droplets. (C) Plasmodesmata. Note also the presence of mitochondria, dictyosomes and endoplasmic reticulum. Di, dictyosomes; Mi, mitochondria; Nu, nucleus; Pd, plasmodesma; Pl, plastid; Re, endoplasmic reticulum; Va, vacuole.
Figure 6.
Ultrastructural features of the osmophores in Mangifera indica at anthesis. (A) General view of the osmophore during anthesis. Most of the cells contain a single large vacuole occupying almost the entire cytoplasm and the organelles are in a peripheral position; the cells are in different stages of secretion. (B) Small vacuoles and oil droplets dispersed in the cytoplasm of the epidermal cells. (C) Fusion of the vacuole with the cell wall before the release of the secretion (*). Ct, cuticle; Cw, cell wall; Mi, mitochondria; Nu, nucleus; Oi, oil droplet; Pl, plastid; Va, vacuole.
Floral bouquet composition
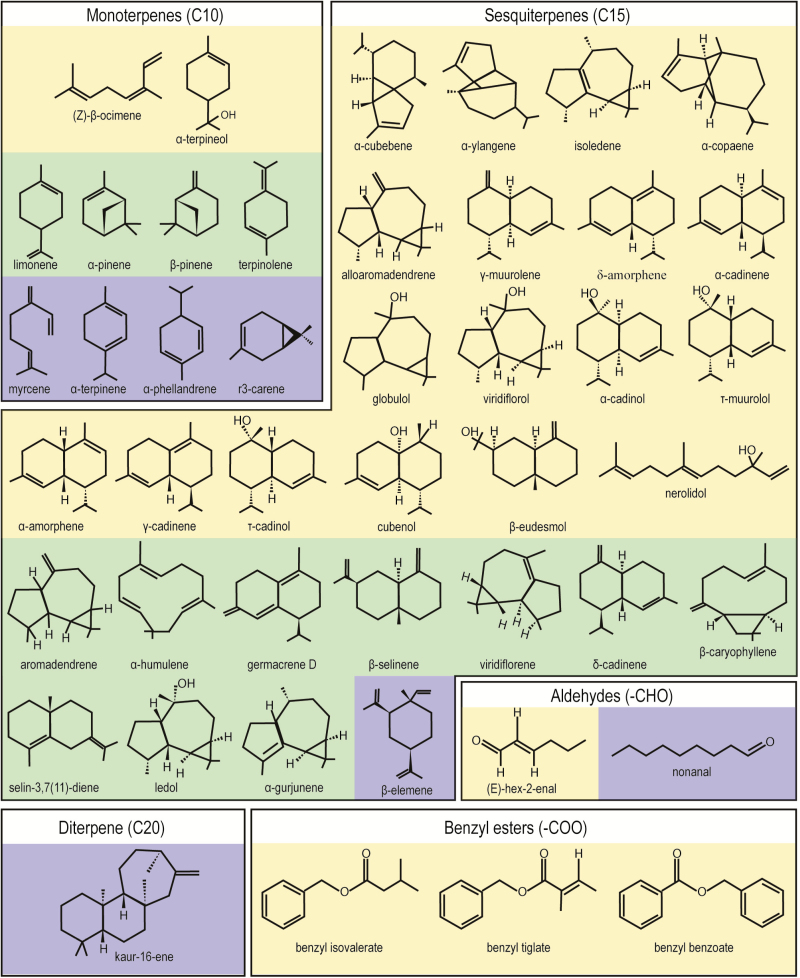
Our GC-MS analyses revealed that out of the 39 volatile compounds produced by the flowers in A. humile and 21 in M. indica, 14 are identical (10 sesquiterpenes and 4 monoterpenes). Diverse sesquiterpenes and monoterpenes occurred in both species and these dominate the composition of the fragrance in both cases (Fig. 7). They represent a total of 84.03 % of the compounds identified in A. humile and 96.78 % in M. indica. However, their relative concentrations differed markedly (e.g. the sesquiterpene β-caryophyllene or the monoterpene α-pinene) as well as their general presence/absence in each species (e.g. the sesquiterpene isoledene and the monoterpene (Z)-β-ocimene in A. humile and the sesquiterpene elemene and the monoterpene myrcene in M. indica) (Table 1). The sesquiterpenes represent 73.24 % of the fragrance in A. humile and are dominated by β-caryophyllene (29.36 %), followed by α-humulene (11.76 %) and δ-cadinene (7.05 %). In contrast, M. indica produced mainly monoterpenes (75.64 %), dominated by terpinolene (55.95 %), α-pinene (5.84 %) and Δ3-carene (5.12 %) (Table 1). In addition to sesqui- and monoterpenes, benzyl esters and oxygenated monoterpenes were also detected but only in A. humile, and diterpenes only in M. indica (Table 1; Fig. 7).
Figure 7.
Diversity of volatile compounds produced by the flowers of Anacardium humile (24 in yellow) and Mangifera indica (7 in blue). The compounds produced by both species are represented in green (14).
Table 1.
Relative yields (%) of the volatile oils from flowers of Anacardium humile and Mangifera indica organized by chemical classes and RI. aRI: retention indices relative to C6–C30n-alkanes on the same column; components identified through RI and MS.
| Component | Chemical class | RIa | A. humile | M. indica |
|---|---|---|---|---|
| (E)-Hex-2-enal | Aldehyde | 855 | 1.36 | – |
| α-Pinene | MonHy | 938 | 8.89 | 5.84 |
| β-Pinene | MonHy | 979 | 0.43 | 0.77 |
| Myrcene | MonHy | 990 | – | 3.30 |
| Δ3-Carene | MonHy | 1011 | – | 5.12 |
| α-Terpinene | MonHy | 1017 | – | 0.44 |
| α-Phellandrene | MonHy | 1030 | – | 0.79 |
| Limonene | MonHy | 1031 | 0.76 | 3.43 |
| (Z)-β-Ocimene | MonHy | 1040 | 0.41 | – |
| Terpinolene | MonHy | 1087 | 0.30 | 55.95 |
| Nonanal | Aldehyde | 1102 | – | 0.40 |
| α-Terpineol | OxyMo | 1188 | 0.24 | – |
| α-Cubebene | SesHy | 1351 | 0.82 | – |
| α-Ylangene | SesHy | 1372 | 0.31 | – |
| Isoledene | SesHy | 1373 | 0.38 | – |
| α-Copaene | SesHy | 1375 | 3.49 | – |
| Benzyl isovalerate | BenEs | 1382 | 0.54 | – |
| β-Elemene | SesHy | 1391 | – | 0.35 |
| α-Gurjunene | SesHy | 1408 | 0.48 | 6.00 |
| β-Caryophyllene | SesHy | 1418 | 29.36 | 3.66 |
| Aromadendrene | SesHy | 1439 | 1.14 | 0.49 |
| α-Humulene | SesHy | 1454 | 11.76 | 2.04 |
| Alloaromadendrene | SesHy | 1461 | 2.47 | – |
| γ-Muurolene | SesHy | 1477 | 2.84 | – |
| Germacrene D | SesHy | 1480 | 0.37 | 0.14 |
| β-Selinene | SesHy | 1485 | 0.97 | 5.96 |
| Viridiflorene | SesHy | 1493 | 5.51 | 2.10 |
| Benzyl tiglate | BenEs | 1496 | 4.07 | – |
| δ-Amorphene | SesHy | 1497 | 0.69 | – |
| α-Muurolene | SesHy | 1499 | 0.85 | – |
| α-Amorphene | SesHy | 1506 | 1.43 | – |
| γ-Cadinene | SesHy | 1513 | 1.86 | – |
| δ-Cadinene | SesHy | 1524 | 7.05 | 0.19 |
| α-Cadinene | SesHy | 1538 | 1.11 | – |
| Selin-3,7(11)-diene | SesHy | 1542 | 0.35 | 0.21 |
| Nerolidol | OxySe | 1564 | 1.31 | – |
| Ledol | OxySe | 1565 | 0.20 | 0.27 |
| Globulol | OxySe | 1583 | 1.42 | – |
| Viridiflorol | OxySe | 1590 | 0.81 | – |
| τ-Cadinol | OxySe | 1640 | 1.14 | – |
| τ-Muurolol | OxySe | 1641 | 1.31 | – |
| Cubenol | OxySe | 1642 | 0.45 | – |
| β-Eudesmol | OxySe | 1649 | 0.33 | – |
| α-Cadinol | OxySe | 1653 | 0.44 | – |
| Benzyl benzoate | BenEs | 1762 | 1.93 | – |
| Kaur-16-ene | Diterpene | 2034 | – | 0.23 |
| Total identified | 99.58 | 97.68 | ||
| MonoterpenesMonHy | 10.79 | 75.64 | ||
| Oxygenated monoterpenesOxyMo | 0.24 | – | ||
| SesquiterpenesSesHy | 73.24 | 21.14 | ||
| Oxygenated sesquiterpenesOxySe | 7.41 | 0.27 | ||
| Others (including aldehydes, benzyl estersBenEs and diterpenes) | 7.90 | 0.63 | ||
| Volatile oil yield [% (w/w)] | 0.0051 % | 0.0093 % | ||
Discussion
Diversity of osmophores in flowers of Anacardiaceae and other Sapindales
In the present study, we describe the presence of specialized secretory epidermal cells concentrated at the base of the petals of A. humile and M. indica—interpreted here as osmophores (see below subcellular apparatus). Coincidentally, both species were previously placed in Mangiferae (sensuEngler 1892) and now, together with most other former members of this tribe, form a well-supported monophyletic clade nested in Anacardioideae, the largest of the two subfamilies of Anacardiaceae (Weeks et al. 2014). In both species, the osmophores are conspicuously situated along ridges of the adaxial (ventral) side of petals; a similar structure is also sometimes visible in pictures and sections of other members of the family, such as Pleiogynium solandri in Spondioideae, and Semecarpus riparius and S. australiensis in Anacardioideae (Wannan and Quinn 1991; Bachelier and Endress 2009). However, a careful re-examination of the sections of these genera and those studied by Bachelier and Endress (2009; see list of material therein) confirmed the secretory activity of the epidermal cells on these ridges only in Anacardium occidentale and M. indica, and its absence in all other taxa. Only one previous study using neutral red suggested that osmophores may also be present on the adaxial side and tips of the petals of Tapirira guianensis (Fernandes et al. 2012), which belong to the subfamily Spondioideae. However, they did not report any scent, and staining with neutral red is not always conclusive (Gonçalves-Souza et al. 2017; Demarco 2017b). Our study is thus the first to provide anatomical evidence of the production of volatiles in Anacardiaceae, and more studies are necessary to confirm the presence of scented flowers and potential osmophores in more members of the family.
In other families of the Sapindales, the presence of osmophores was reported only in a few other species. In Rutaceae, osmophores have been described in a few species of Citrus on the tips of the petals and consist of a distinctly papillose epidermis with glandular trichomes (Marques et al. 2015). Scents have also been identified in flowers of Boronia; however, the authors could not confirm whether osmophores were responsible for its production (Bussel et al. 1995). In Sapindaceae, osmophores have been reported in Paullinia weinmanniifolia on the stamens and sepals (Lima et al. 2016), but neither with information on their structure, nor, like in Citrus, with details on their ultrastructure. Therefore, comparisons of osmophore diversity and evolution in Sapindales are still hampered by the lack of detailed studies.
Synthesis and release of volatile compounds produced by the osmophores
The secretory nature of the osmophores of A. humile and M. indica was here clearly demonstrated by the presence of cells with high metabolic activity. Their subcellular apparatus is essentially similar to that typically described in osmophores of other flowering plants (Fahn 1979, 2000; Davies et al. 2003; Ascensão et al. 2005; Antón et al. 2012; Pansarin et al. 2014). In the epidermis, the accumulation of starch grains observed here in both species is a very common feature of osmophores (Stern et al. 1987; Pansarin et al. 2009; Melo et al. 2010). These carbohydrates provide the main source of energy for the mitochondria and for the production of the volatiles (Fahn 1979; Vogel 1990; Melo et al. 2010; Kowalkowska et al. 2015; Gonçalves-Souza et al. 2017). Smooth endoplasmic reticulum, leucoplasts, polyribosomes and dictyosomes are also very common in cells that, like those of both species, produce terpenes (Turner and Croteau 2004; Melo et al. 2010; Marinho et al. 2018). According to these studies, plastids and rough endoplasmic reticulum are the main organelles responsible for the production of terpenes, and small vacuoles or plastids with plastoglobuli are indicative of their production. In addition, the presence of cytoplasmic oil droplets in both species confirms the production of oils (Effmert et al. 2006; Fahn 1979, 2000; Turner and Croteau 2004; Melo et al. 2010; Gonçalves-Souza et al. 2017; Marinho et al. 2018).
There are some slight variations in the subcellular apparatus of each species studied here, which seem to be consistent with the variation of the composition of the floral fragrance. Sesquiterpenes, such as those dominant in A. humile, are produced in the cytosol, whereas monoterpenes and diterpenes, such as those found more often in M. indica, are mainly produced by the plastids which are more numerous in this species (Aharoni et al. 2005; Effmert et al. 2006). However, the isoprenoid precursors found in both genera may be available in different cell compartments and may be used to generate terpenoids in more than one cell compartment (Aharoni et al. 2005; Effmert et al. 2006). In addition, benzyl esters are primarily synthesized in the plastids through the arogenate pathway and modified by the enzymes present in the cytosol (Maeda and Dudareva 2012), and the fatty acid derivatives, such as aldehydes, alcohols and their esters are produced by both plastids and peroxisomes (Borghi et al. 2017). Only plastoglobules were found exclusively in A. humile and may thus play a role in the dominant production of the sesquiterpenes (Pacek et al. 2012; Gonçalves-Souza et al. 2017).
Our study also demonstrates that in both species, all secretions, including highly lipophilic organic compounds such as terpenoids, are released by a granulocrine mechanism across the plasma membrane and into the periplasmic space via active transport, and then cross the cell wall and cuticle as it is usually observed in the release of lipophilic compounds (Curry et al. 1988; Fahn 2000; Riederer 2006; Pansarin et al. 2014; Gama et al. 2015; Kowalkowska et al. 2015; Possobon et al. 2015; Paiva 2016; Gonçalves-Souza et al. 2017). Ultrastructural studies like this one have not been conducted before in Sapindales. The granulocrine mechanism has been described for osmophores of other unrelated families of eudicots, such as Fabaceae and Passifloraceae, or for monocots such as Araceae and Orchidaceae (Pridgeon and Stern 1983; Skubatz et al. 1995; García et al. 2007; Gonçalves-Souza et al. 2017; Marinho et al. 2018). Moreover, the extensive production and release of scents is suggested by the presence of cells at different stages of secretion in anthetic flowers (Fahn 1979, 2000), an important strategy to attract pollinators over a longer period of time.
Diversity of volatile compounds in the floral bouquet of Anacardiaceae
Our study demonstrates that the flowers of both species produce sesquiterpenes and monoterpenes as primary volatile components of their fragrance, like most of the angiosperms that contain osmophores (Metcalf and Kogan 1987; Knudsen et al. 2006). The fragrance is dominated by sesquiterpenes in A. humile, especially β-caryophyllene, and, as previously reported by Wang et al. (2010), monoterpenes, especially terpinolene, in M. indica. The same types of substances are also identified as main components of essential oils in fruits and leaves of both species. However, in these organs, the predominant sesquiterpenes in A. humile are α-bulnesene and γ-cadinene (Cardoso et al. 2010; Winck et al. 2010), and in leaves of M. indica the principal monoterpene is cyperene (Gebara et al. 2011).
Some of the sesquiterpenes present in both species have been shown to be insect kairomones, which are efficient attractants of Hymenoptera (γ-cadinene) and Coleoptera (β-caryophyllene, α-copaene, α-pinene, among others) (Metcalf and Kogan 1987; Knudsen et al. 2006). This supports previous studies demonstrating that the main pollinators in both species are honeybees and beetles, as well as flies, which are more likely attracted by these compounds (Free and Williams 1976; Anderson et al. 1982; Jirón and Hedstrom 1985; Mitchell and Mori 1987; Heard et al. 1990; Freitas and Paxton 1996; Senchina and Summerville 2007; Almeida et al. 2011). Such diversity of pollinators and subtle differences in the sets of compounds comprised in the floral scents of both species likely reflects different functions and additional levels of specialization regarding pollinator behaviour and attraction (Azuma et al. 1997; Anderson et al. 2002). For instance, some components, such as benzenoids and terpenoids, are known to have stronger effects over long distances than other volatiles, that often only excerpt their action in small local ranges in order to induce feeding or to indicate the presence of floral rewards, especially nectar (e.g. aldehydes) (Azuma et al. 1997; Dötterl and Jürgens 2005; Knudsen et al. 2006; Raguso 2008; Wright and Schiestl 2009; Junker and Blüthgen 2010).
Further studies should focus on compounds produced by isolated parts of flowers in order to determine whether other floral secretory structures, aside from osmophores, also participate in the synthesis of scents. Gonçalves-Souza et al. (2017) demonstrated that different floral organs of Araceae may produce scents in a same flower. However, in Anacardiaceae there seems to be a clear contribution to the overall scent by secretions produced by the ducts, complicating the possible analysis of scents produced by individual separated floral organs.
Scent production in nectariferous flowers of Anacardiaceae and other Sapindales
The floral scents are an important way of communication between the flowers and their pollinators. They indicate the presence of a reward offered by the flowers, most of the time nectar (Vogel 1990; Endress 1994; Raguso 2008). The association between nectar and fragrance production can be quickly learned by social bees (Wright 2009), which are major pollinators of Anacardiaceae and Sapindales in general (Kubitzki 2011). In all families of the Sapindales for which the presence of osmophores has been reported, nectaries are also present as in the two species studied here (Bussel et al. 1995, Marques et al. 2015; Lima et al. 2016). Indeed, most members of the order share generalist to melittophilous insect pollination syndromes, with numerous but relatively small nectariferous flowers with a polysymmetric perianth (and androecium) (Engler 1892; Wannan and Quinn 1991; Bachelier and Endress 2009; Kubitzki 2011; Tölke et al. 2015). Since most other members of the order produce nectar, it is surprising that there are to date only a few reports of scented flowers mainly in Meliaceae, Rutaceae and Sapindaceae (Kubitzki 2011). In addition, most members of Sapindales produced (terminal or distal) inflorescences with numerous flowers which sometimes have a conspicuous fleshy nectary disc, but individual flowers are often small and their perianth inconspicuous, and appear, at least to the human eye, to not present any other obvious visual cues that indicate a reward (Endress 2010; Kubitzki 2011). Therefore, scented flowers and osmophores in flowers of Anacardiaceae and other sapindalean families have either been overlooked until now, or rely on other strategies to attract pollinators, which can only become evident with further studies.
Conclusion and Perspectives
This is the first study confirming the presence of osmophores in members of Anacardiaceae and providing details on their structure and function. It also reveals that while in both species the presence of a reward is advertised with a strong fragrance, still little is known on the evolution of scent and other cues signalling the presence of a reward present in most other members of Anacardiaceae and Sapindales. The results encourage further investigations on the possible presence of fragrances in other genera of the family and order, into the origin and composition of such fragrances and the relationship between their diversity and their pollination mechanisms.
Sources of Funding
This research was financially supported by grants from ‘Fundação de Amparo à Pesquisa do Estado de São Paulo’ (FAPESP - proc. nº 2014/18002-2) and CNPq (proc. nº 420417/2016-8). The first author also thanks CAPES for a scholarship provided for a semester abroad at FU Berlin (PDSE proc. nº 88881.133676/2016-01).
Contributions by the Authors
E.D.T. and D.D. posed the central questions; E.A.L. carried out the field work; E.D.T., D.D. and S.M.C. performed the microscopic analysis; M.J.P.F. performed the chemistry analysis; the authors analysed the data together; E.D.T. wrote the original manuscript and D.D. and J.B.B. edited for content and provided guidance on structure and style.
Conflict of Interest
None declared.
Acknowledgements
We thank CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) and CNPq (Conselho Científico de Desenvolvimento Científico e Tecnológico) for the PhD scholarship to the first author, granted during the development of this work. T. C. H. Cole and J. Gravendyck kindly provided support with language editing and provided valuable comments.
Literature Cited
- Adams RP. 2001. Identification of essential oil components by gas chromatography/quadrupole mass spectrometry. Carol Stream, IL: Allured Publishing Corporation. [Google Scholar]
- Aharoni A, Jongsma MA, Bouwmeester HJ. 2005. Volatile science? Metabolic engineering of terpenoids in plants. Trends in Plant Science 10:594–602. [DOI] [PubMed] [Google Scholar]
- Almeida ALS, Albuquerque UP, Castro CC. 2011. Reproductive biology of Spondias tuberosa Arruda (Anacardiaceae), an endemic fructiferous species of the caatinga (dry forest), under different management conditions in northeastern Brazil. Journal of Arid Environments 75:330–337. [Google Scholar]
- Anderson S, Nilsson LA, Groth I, Bergström G. 2002. Floral scents in butterfly-pollinated plants: possible convergence in chemical composition. Botanical Journal of the Linnean Society 140:129–153. [Google Scholar]
- Anderson DL, Sedgley M, Short JRT, Alwood AJ. 1982. Insect pollination of mango in northern Australia. Australian Journal of Agricultural Research 33:541–548. [Google Scholar]
- Antón S, Kamińska M, Stpiczyńska M. 2012. Comparative osmophore structure in the flower of Stanhopea graveolens Lindley and Cycnoches chlorochilon Klotzsch (Orchidaceae). Acta Agrobotanica 65:11–22. [Google Scholar]
- Ascensão L, Francisco A, Cotrim H, Pais MS. 2005. Comparative structure of the labellum in Ophrys fusca and O. lutea (Orchidaceae). American Journal of Botany 92:1059–1067. [DOI] [PubMed] [Google Scholar]
- Azuma H, Toyota M, Asakawa Y, Yamaoka R, Garcia-Franco JG, Dieringer G, Thien LB, Kawano S. 1997. Chemical divergence in floral scents of Magnolia and allied genera (Magnoliaceae). Plant Species Biology 12:69–83. [Google Scholar]
- Bachelier JB, Endress PK. 2009. Comparative floral morphology and anatomy of Anacardiaceae and Burseraceae (Sapindales), with a special focus on gynoecium structure and evolution. Botanical Journal of the Linnean Society 159:499–571. [Google Scholar]
- Borghi M, Fernie AR, Schiestl FP, Bouwmeester HJ. 2017. The sexual advantage of looking, smelling, and tasting good: the metabolic network that produces signals for pollinators. Trends in Plant Science 22:338–350. [DOI] [PubMed] [Google Scholar]
- Bussel BM, Considine JA, Spadek ZE. 1995. Flower and volatile oil ontogeny in Boronia megastigma. Annals of Botany 76:457–463. [Google Scholar]
- Cardoso CAL, Jeller AH, Ré-Poppi N, Coelho RM, Yasunaka DS, Schleder JD. 2010. Identification of the volatile compounds of fruit oil of Anacardium humile (Anacardiaceae). Journal of Essential Oil Research 22:469–470. [Google Scholar]
- Curry KJ, Stern WL, McDowell LM. 1988. Osmophore development in Stanhopea anfracta and S. pulla (Orchidaceae). Lindleyana 3:212–220. [Google Scholar]
- Davies KL, Turner MP, Gregg A. 2003. Lipoidal labellar secretions in Maxillaria Ruiz & Pav. (Orchidaceae). Annals of Botany 91:439–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demarco D. 2017a. Floral glands in asclepiads: structure, diversity and evolution. Acta Botanica Brasilica 31:477–502. [Google Scholar]
- Demarco D. 2017b. Histochemical analysis of plant secretory structures. In: Pellicciari C, Biggiogera M, eds. Histochemistry of single molecules. Methods in molecular biology, vol. 1560 New York: Humana Press, 313–330. [DOI] [PubMed] [Google Scholar]
- Dötterl S, Jürgens A. 2005. Spatial fragrance patterns in flowers of Silene latifolia: lilac compounds as olfactory nectar guides?Plant Systematics and Evolution 255:99–109. [Google Scholar]
- Effmert U, Buss D, Rohrbeck D, Piechulla B. 2006. Localization of the synthesis and emission of scent compounds within the flower. In: Dudareva N, Pichersky E, eds. Biology of floral scent. London: Taylor & Francis Group, 105–124. [Google Scholar]
- Endress PK. 1994. Diversity and evolutionary biology of tropical flowers. New York: Cambridge University Press. [Google Scholar]
- Endress PK. 2010. Flower structure and trends of evolution in eudicots and their major subclades. Annals of the Missouri Botanical Garden 97:541–583. [Google Scholar]
- Engler A. 1892. Anacardiaceae. In: Engler A, Prantl K, eds. Die natürlichen Pflanzenfamilien III. Leipzig, Germany: W. Engelmann, 138–178. [Google Scholar]
- Evert RF. 2006. Esau’s plant anatomy: meristems, cells, and tissues of the plant body: their structure, function, and development, 3rd edn. Hoboken, NJ: John Wiley & Sons, Inc. [Google Scholar]
- Fahn A. 1979. Secretory tissue in plants. London: Academic Press. [Google Scholar]
- Fahn A. 2000. Structure and function of secretory cells. Advances in Botanical Research 31:37–75. [Google Scholar]
- Fernandes MM, Venturieri GC, Jardim MAG. 2012. Biologia, visitantes florais e potencial melífero de Tapirira guianensis (Anacardiaceae) na Amazônia oriental. Amazonian Journal of Agricultural and Environmental Sciences 55:167–175. [Google Scholar]
- Free JB, Williams IH. 1976. Insect pollination of Anacardium occidentale L., Mangifera indica L., Blighia sapida Koenig and Persea americana Mill. Tropical Agriculture 53:125–139. [Google Scholar]
- Freire FCO, Cardoso JE, dos Santos AA, Viana FMP. 2002. Diseases of cashew nut plants (Anacardium occidentale L.) in Brazil. Crop Protection 21:489–494. [Google Scholar]
- Freitas BM, Paxton RJ. 1996. The role of wind and insects in cashew (Anacardium occidentale) pollination in NE Brazil. Journal of Agricultural Science 126:319–326. [Google Scholar]
- Gagliardi KB, Cordeiro I, Demarco D. 2016. Protection and attraction: bracts and secretory structures in reduced inflorescences of Malpighiales. Flora 220:52–62. [Google Scholar]
- Gama TSS, Demarco D, Aguiar-Dias ACA. 2015. Calicinal trichomes of Adenocalymma magnificum (Bignoniaceae) producing lipophilic substances: ultrastructural and functional aspects. Revista de Biología Tropical 63:537–544. [Google Scholar]
- García MTA, Galati BG, Hoc PS. 2007. Ultrastructure of the corona of scented and scentless flowers of Passiflora spp. (Passifloraceae). Flora 202:302–315. [Google Scholar]
- Gebara SS, de Oliveira Ferreira W, Ré-Poppi N, Simionatto E, Carasek E. 2011. Volatile compounds of leaves and fruits of Mangifera indica var. coquinho (Anacardiaceae) obtained using solid phase microextraction and hydrodistillation. Food Chemistry 127:689–693. [DOI] [PubMed] [Google Scholar]
- Gerlach D. 1984. Botanische Mikrotechnik: eine Einführung, 3rd edn. Stuttgart, Germany: Georg Thieme. [Google Scholar]
- Guimarães E, di Stasi LC, Maimoni-Rodella Rde C. 2008. Pollination biology of Jacaranda oxyphylla with an emphasis on staminode function. Annals of Botany 102:699–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves-Souza P, Schlindwein C, Dötterl S, Paiva EA. 2017. Unveiling the osmophores of Philodendron adamantinum (Araceae) as a means to understanding interactions with pollinators. Annals of Botany 119:533–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heard TA, Vithanage V, Chacko EK. 1990. Pollination biology of cashew in the northern territory of Australia. Australian Journal of Agricultural Research 41:1101–1114. [Google Scholar]
- Jirón LF, Hedstrom I. 1985. Pollination ecology of mango (Mangifera indica L.) (Anacardiaceae) in the neotropic region. Turrialba 35:269–277. [Google Scholar]
- Johansen DA. 1940. Plant microtechnique. New York: McGraw-Hill. [Google Scholar]
- Johnson D. 1973. The botany, origin, and spread of the cashew Anacardium occidentale L. Journal of Plantation Crops 1:1–7. [Google Scholar]
- Junker RR, Blüthgen N. 2010. Floral scents repel facultative flower visitors, but attract obligate ones. Annals of Botany 105:777–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen JT, Eriksson R, Gershenzon J, Stahl B. 2006. Diversity and distribution of floral scent. Botanical Review 72:1. [Google Scholar]
- Kowalkowska AK, Kozieradzka-Kiszkurno M, Turzyński S. 2015. Morphological, histological and ultrastructural features of osmophores and nectary of Bulbophyllum wendlandianum (Kraenzl.) Dammer (B. section Cirrhopetalum Lindl., Bulbophyllinae Schltr., Orchidaceae). Plant Systematics and Evolution 301: 609–622. [Google Scholar]
- Kubitzki K. 2011. The families and genera of vascular plants. Vol. X. Flowering plants. Eudicots. Sapindales, Cucurbitales, Myrtales. Berlin: Springer. [Google Scholar]
- Lillie RD. 1965. Histopathologic technique and practical histochemistry. New York: McGraw-Hill. [Google Scholar]
- Lima HA, Somner GV, Giulietti AM. 2016. Duodichogamy and sex lability in Sapindaceae: the case of Paullinia weinmanniifolia. Plant Systematics and Evolution 302:109–120. [Google Scholar]
- Machado SR, Rodrigues TM. 2004. Anatomia e ultra-estrutura do pulvino primário de Pterodon pubescens Benth. (Fabaceae – Faboideae). Brazilian Journal of Botany 27:135–147. [Google Scholar]
- Maeda H, Dudareva N. 2012. The shikimate pathway and aromatic amino acid biosynthesis in plants. Annual Review of Plant Biology 63:73–105. [DOI] [PubMed] [Google Scholar]
- Marinho CR, Martucci MEP, Gobbo-Neto L, Teixeira SP. 2018. Chemical composition and secretion biology of the floral bouquet in legume trees (Fabaceae). Botanical Journal of the Linnean Society 187:5–25. [Google Scholar]
- Marinho CR, Souza CD, Barros TC, Teixeira SP. 2014. Scent glands in legume flowers. Plant Biology 16:215–226. [DOI] [PubMed] [Google Scholar]
- Marques JPR, Amorim L, Silva-Júnior GJ, Spósito MB, Apezzato-da-Glória B. 2015. Structural and biochemical characteristics of citrus flowers associated with defense against a fungal pathogen. AoB Plants 7:plu090; doi: 10.1093/aobpla/plu090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo MC, Borba EL, Paiva EAS. 2010. Morphological and histological characterization of the osmophores and nectaries of four species of Acianthera (Orchidaceae: Pleurothallidinae). Plant Systematics and Evolution 286:141–151. [Google Scholar]
- Metcalf RL, Kogan M. 1987. Plant volatiles as insect attractants. Critical Reviews in Plant Sciences 5:251–301. [Google Scholar]
- Mitchell JD, Mori SA. 1987. The cashew and its relatives (Anacardium: Anacardiaceae). Memoirs of the New York Botanical Garden 42:1–76. [Google Scholar]
- Mukherjee SK. 1972. Origin of mango (Mangifera indica). Economic Botany 26:260–264. [Google Scholar]
- Pacek A, Stpiczynska M, Davies KL, Szymczak G. 2012. Floral elaiophore structure in four representatives of the Ornithocephalus clade (Orchidaceae: Oncidiinae). Annals of Botany 110:809–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paiva EAS. 2016. How do secretory products cross the plant cell wall to be released? A new hypothesis involving cyclic mechanical actions of the protoplast. Annals of Botany 117:533–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pansarin LM, Castro MM, Sazima M. 2009. Osmophore and elaiophores of Grobya amherstiae (Catasetinae, Orchidaceae) and their relation to pollination. Botanical Journal of the Linnean Society 159:408–415. [Google Scholar]
- Pansarin LM, Pansarin ER, Sazima M. 2014. Osmophore structure and phylogeny of Cirrhaea (Orchidaceae, Stanhopeinae). Botanical Journal of the Linnean Society 176:369–383. [Google Scholar]
- Pearse AGE. 1985. Histochemistry: theoretical and applied, vol. 2, 4th edn. Edinburgh: Churchill Livingstone. [Google Scholar]
- Pell SK, Mitchell JD, Miller AJ, Lobova TA. 2011. Anacardiaceae. In: Kubitzki, K, ed. The families and genera of vascular plants. Vol. X. Flowering plants. Eudicots. Sapindales, Cucurbitales, Myrtales. Berlin: Springer, 7–50. [Google Scholar]
- Possobon CCF, Guimarães E, Machado SR. 2015. Structure and secretion mechanisms of floral glands in Diplopterys pubipetala (Malpighiaceae), a neotropical species. Flora 211:26–39. [Google Scholar]
- Pridgeon AM, Stern WL. 1983. Ultrastructure of osmophores in Restrepia (Orchidaceae). American Journal of Botany 70:1233–1243. [Google Scholar]
- Raguso RA. 2008. Wake up and smell the roses: the ecology and evolution of floral scent. Annual Review of Ecology, Evolution, and Systematics 39:549–569. [Google Scholar]
- Reynolds ES. 1963. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. The Journal of Cell Biology 17:208–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riederer M. 2006. Biology of the plant cuticle. In: Riederer M, Müller C, eds. Biology of the plant cuticle (annual plant reviews), vol. 23 Oxford: Blackwell Publishing, 1–10. [Google Scholar]
- Ronse De Craene LP, Haston E. 2006. The systematic relationships of glucosinolate‐producing plants and related families: a cladistic investigation based on morphological and molecular characters. Botanical Journal of the Linnean Society 151:453–494. [Google Scholar]
- Sazima M, Vogel S, Cocucci A, Hausner G. 1993. The perfume flowers of Cyphomandra (Solanaceae): pollination by euglossine bees, bellows mechanism, osmophores, and volatiles. Plant Systematics and Evolution 187:51–88. [Google Scholar]
- Senchina D, Summerville KS. 2007. Great diversity of insect floral associates may partially explain ecological success of poison ivy (Toxicodendron radicans subsp. negundo [Greene] Gillis, Anacardiaceae). The Great Lakes Entomologist 40:120–128. [Google Scholar]
- Schnell RJ, Ronning CM, Knight RJ Jr. 1995. Identification of cultivars and validation of genetic relationships in Mangifera indica L. using RAPD markers. Theoretical and Applied Genetics 90:269–274. [DOI] [PubMed] [Google Scholar]
- Skubatz H, Kunkel DD, Patt J, Howald W, Rothman T, Meeuse BJD. 1995. Pathway of terpene excretion by the appendix of Sauromatum guttatum. Proceedings of the National Academy of Sciences of the United States of America 92:1084–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern WL, Curry KJ, Pridgeon AM. 1987. Osmophores of Stanhopea (Orchidaceae). American Journal of Botany 74:1323–1331. [Google Scholar]
- Stevens PF. 2001. onwards. Angiosperm phylogeny website. Version 14, July 2017 [and more or less continuously updated since] http://www.mobot.org/MOBOT/research/APweb/. (21 September 2018).
- Teixeira SP, Marinho CR, Paulino JV. 2014. A flor: aspectos morfofuncionais e evolutivos. In: Rech AR, Agostini K, Oliveira PE, Machado IC, eds. Biologia da Polinização. Rio de Janeiro, Brazil: Projeto Cultural, 45–69. [Google Scholar]
- Tölke ED, Bachellier JB, Lima EA, Galetto L, Demarco D, Carmello-Guerreiro SM. 2018. Diversity of floral nectary secretions and structure, and implications for evolution in Anacardiaceae. Botanical Journal of the Linnean Society 187:209–231. [Google Scholar]
- Tölke ED, Galetto L, Machado SR, Lacchia APS, Carmello-Guerreiro SM. 2015. Stages of development of the floral secretory disk in Tapirira guianensis Aubl. (Anacardiaceae), a dioecious species. Botanical Journal of the Linnean Society 179:533–544. [Google Scholar]
- Turner GW, Croteau R. 2004. Organization of monoterpene biosynthesis in Mentha. Immunocytochemical localizations of geranyl diphosphate synthase, limonene-6-hydroxylase, isopiperitenol dehydrogenase, and pulegone reductase. Plant Physiology 136:4215–4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel S. 1990. The role of scent glands in pollination: on the structure and function of osmophores. New Delhi, India: Amerind. [Google Scholar]
- Vogel S, Hadacek F. 2004. Contributions to the functional anatomy and biology of Nelumbo nucifera (Nelumbonaceae) III. An ecological reappraisal of floral organs. Plant Systematics and Evolution 249:173–189. [Google Scholar]
- Wang HW, Liu YQ, Wei SL, Yan ZJ, Lu K. 2010. Comparison of microwave-assisted and conventional hydrodistillation in the extraction of essential oils from mango (Mangifera indica L.) flowers. Molecules 15:7715–7723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wannan BS, Quinn CJ. 1991. Floral structure and evolution in the Anacardiaceae. Botanical Journal of the Linnean Society 107:349–385. [Google Scholar]
- Watson ML. 1958. Staining of tissue sections for electron microscopy with heavy metals. II. Application of solutions containing lead and barium. The Journal of Biophysical and Biochemical Cytology 4:727–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks A, Zapata F, Pell SK, Daly DC, Mitchell JD, Fine PV. 2014. To move or to evolve: contrasting patterns of intercontinental connectivity and climatic niche evolution in “Terebinthaceae” (Anacardiaceae and Burseraceae). Frontiers in Genetics 5:409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winck CR, Cardoso CAL, Jeller AH, Ré-Poppi N, Coelho RM, Schleder EJD. 2010. Identification of the volatile compounds of leaf oil of Anacardium humile (Anacardiaceae). Journal of Essential Oil Research 22:11–12. [Google Scholar]
- Wright GA, Schiestl FP. 2009. The evolution of floral scent: the influence of olfactory learning by insect pollinators on the honest signalling of floral rewards. Functional Ecology 23:841–851. [Google Scholar]