ABSTRACT
Human brain development proceeds via a sequentially transforming stem cell population in the ventricular-subventricular zone (V-SVZ). An essential, but understudied, contributor to V-SVZ stem cell niche health is the multi-ciliated ependymal epithelium, which replaces stem cells at the ventricular surface during development. However, reorganization of the V-SVZ stem cell niche and its relationship to ependymogenesis has not been characterized in the human brain. Based on comprehensive comparative spatiotemporal analyses of cytoarchitectural changes along the mouse and human ventricle surface, we uncovered a distinctive stem cell retention pattern in humans as ependymal cells populate the surface of the ventricle in an occipital-to-frontal wave. During perinatal development, ventricle-contacting stem cells are reduced. By 7 months few stem cells are detected, paralleling the decline in neurogenesis. In adolescence and adulthood, stem cells and neurogenesis are not observed along the lateral wall. Volume, surface area and curvature of the lateral ventricles all significantly change during fetal development but stabilize after 1 year, corresponding with the wave of ependymogenesis and stem cell reduction. These findings reveal normal human V-SVZ development, highlighting the consequences of disease pathologies such as congenital hydrocephalus.
KEY WORDS: Stem cell niche, Human brain development, Ependymogenesis, Ventricular-subventricular zone
Highlighted Article: A systematic spatiotemporal analysis of cytoarchitectural changes within the developing human forebrain lateral ventricle stem cell niche connects stem cell loss and associated ependymogenesis with ventricle structural changes.
INTRODUCTION
During early brain development in humans, the lining of the neural tube and subsequently the cerebrospinal fluid (CSF)-filled ventricular system house a pseudostratified layer of proliferative cells that, in the forebrain, contributes to the robust expansion of the cerebral cortex. New neurons are initially generated by neuroepithelial cells, and then by descendant radial glia and outer radial glia via their progeny, intermediate progenitor cells (Hansen et al., 2010; LaMonica et al., 2012; Lui et al., 2011; Malik et al., 2013). Radial glia also generate a monolayer of ependymal cells that lines the ventricles (Jacquet et al., 2009; Mirzadeh et al., 2008; Spassky et al., 2005) and provides barrier and transport functions between the interstitial fluid of the brain parenchyma and the CSF (Bruni, 1998; Del Bigio, 1995, 2010; Roales-Buján et al., 2012). In mouse, formation of the epithelial ependymal cells displaces remaining radial glia/stem cell somata to the subventricular zone (SVZ). These remaining stem cells, referred to as ventricular-subventricular zone (V-SVZ) stem cells, are arrayed in clusters and maintain only a thin apical process at the ventricle surface (Alvarez-Buylla et al., 1998, 2001; Conover et al., 2000; Doetsch et al., 1999; Kriegstein and Alvarez-Buylla, 2009; Merkle et al., 2004). Stem cell apical processes surrounded by ependymal cells are referred to as ‘pinwheels’ (Mirzadeh et al., 2008) and represent regenerative units. Whether human V-SVZ stem cells are organized and maintained in similar units along the ventricle surface has not been reported.
After birth in humans, proliferative cells and neurogenesis have been observed along the lateral wall of the lateral ventricle, in the site of what was formerly the lateral ganglionic eminence. Perinatal V-SVZ stem cells appear to be restricted in their neurogenic potential and migration routes, which include three specific pathways within the anterior forebrain: (1) to the frontal lobe in which they distribute as interneurons within the cortical layers (arc pathway); (2) along the medial migratory stream (MMS) to the medial prefrontal cortex; (3) along the rostral migratory stream (RMS) to the olfactory bulb (Paredes et al., 2016a; Quiñones-Hinojosa et al., 2006; Sanai et al., 2011, 2004). Neurogenesis and frontal lobe migration is robust for the first several months after birth and then declines dramatically, so that by two years of age there is little, or no, observable neurogenesis or migration (Bergmann et al., 2012; Paredes et al., 2016b; Quiñones-Hinojosa et al., 2006; Sanai et al., 2011; Wang et al., 2011, 2014). Postnatal neurogenesis in the human forebrain deviates significantly from what is found in mice and even non-human primates (Kriegstein et al., 2006; LaMonica et al., 2012; Lui et al., 2011). Many mammals continue to generate new neurons via the V-SVZ stem cell niche throughout their lifetime, with the newly generated neurons migrating exclusively to the olfactory bulb via the RMS to function in olfaction (Alunni and Bally-Cuif, 2016; Conover and Shook, 2011; Lledo et al., 2008; Peretto et al., 1999). Although the exact function of postnatal inhibitory neurons in the human frontal cortex is unclear, it has been proposed that they contribute to neurocognitive maturation and plasticity that is required in infancy (Arshad et al., 2016; Paredes et al., 2016a; Sanai et al., 2011). Disease or injury that disrupts proliferation and differentiation of V-SVZ stem cells and migration of their progeny may contribute to sensorimotor and neurocognitive deficits that are frequently seen in cerebral palsy, autism and fetal-onset hydrocephalus (Arshad et al., 2016; Paredes et al., 2016a; Sanai et al., 2011).
Although the lateral ventricle neuroepithelium drives neurogenesis, overall brain development subsequently influences the contour of the ventricular system and therefore the V-SVZ stem cell niche. As the ventricles are filled with fluid and lined by a pseudostratified neuroepithelium in early fetal/embryonic development, the shape of the ventricle may initially be compliant. However, late in the second trimester in humans and around embryonic day (E)13-14 in mouse, V-SVZ stem cells (radial glia) generate a monolayer of ependymal cells that line the ventricle surface in an occipital-to-frontal gradient (Bruni, 1998; Bruni et al., 1985; Del Bigio, 1995; Jacquet et al., 2009; Kyrousi et al., 2015; Mirzadeh et al., 2008; Paez-Gonzalez et al., 2011; Spassky et al., 2005). Ependymal cells are multi-ciliated and tightly adherent. They provide several crucial functions (Johanson et al., 2011; Mirzadeh et al., 2008; Paez-Gonzalez et al., 2011; Spassky et al., 2005). Motile cilia at their apical surface contribute to laminar flow at the ventricle surface, and ependymal cells facilitate both barrier and transport functions between the interstitial fluid of the brain parenchyma and the CSF of the ventricular system (Bruni, 1998; Bruni et al., 1985; Del Bigio, 1995, 2010; Spassky et al., 2005). At the ventricle surface, ependymal cells are generally cuboidal in shape and tightly linked by adherens and tight junction protein complexes (Bruni, 1998; Bruni et al., 1985; Del Bigio, 1995; Mirzadeh et al., 2008; Spassky et al., 2005). Stem cells that retain a ventricle-contacting apical process also have apical adherens and tight junctions with neighboring ependymal cells and other stem cells (Jacquet et al., 2009; Mirzadeh et al., 2008; Paez-Gonzalez et al., 2011), supporting barrier and structural functions along the lateral wall.
Here, we sought to investigate changes to the V-SVZ stem cell niche over the course of human brain development and to determine the association between ependymogenesis, stem cell number and stem cell niche organization at the ventricle surface. Based on a comprehensive spatiotemporal analysis of cytoarchitectural changes along the ventricle surface, we found that ependymal cells were added to the ventricle lining of the frontal horn in a posterior-to-anterior wave beginning at ∼21 gestational weeks (gw). As more ependymal cells covered the ventricle, surface stem cell numbers were reduced and remaining stem cells were relegated to the subependymal zone, with only an apical process contacting the ventricle surface. Reduction of stem cell number corresponded to decreased neurogenesis within the SVZ. Stem cell reduction continued into postnatal development and no ventricle-contacting stem cells were observed in adolescent and adult lateral ventricle wall samples. Stability of the lateral ventricle volume, surface area and curvature (concavity/convexity) occurred after 1 year and corresponded temporally to the period of complete coverage of the ventricle surface by mature ependymal cells. Together, our findings link the timing of ependymogenesis and displacement of stem cells along the lateral ventricle wall with stabilization of the ventricle wall surface conformation.
RESULTS
Mouse ependymogenesis proceeds caudal to rostral and stem cells persist
Assessment of brain development in the mouse provides a model to compare and contrast with human brain development. We used serial coronal sections to generate three-dimensional (3D) reconstructions of both total brain and lateral ventricle volumes at five discrete stages of embryonic to postnatal brain development: E13, E16, postnatal day (P)1, P7 and P30 (Fig. 1, left column). In addition, whole-mount preparations of the lateral and medial wall of the lateral ventricles were prepared for each of the five stages of development (Doetsch et al., 1997; Mirzadeh et al., 2008; Shook et al., 2012). Changes in cell coverage along the entire extent of both the medial and lateral walls of the lateral ventricle were examined using immunohistochemistry to distinguish radial glia [γ-tubulin+ basal body of single cilium, GLAST+ (also known as SLC1A3), FOXJ1−], radial glia that are transitioning to immature ependymal cells (two to five γ-tubulin+ basal bodies of cilia, FOXJ1+), mature ependymal cells (multi-cilia γ-tubulin+ clusters, FOXJ1+) and neural stem cells (single cilium γ-tubulin+ basal body, GFAP+) (see Fig. S1A) (Jacquet et al., 2009; Mirzadeh et al., 2010b, 2008).
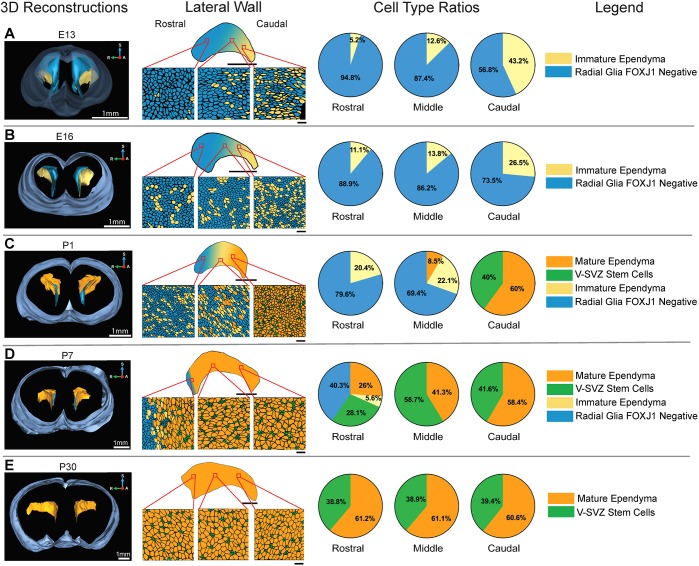
Fig. 1.
Ependymogenesis proceeds caudal to rostral along lateral ventricle wall during mouse brain development. (A-E) 3D reconstructions at E13 (A), E16 (B), P1 (C), P7 (D) and P30 (E) show lateral ventricles and whole-brain contours (left column). Schematics of representative microscope images (second column, lateral wall) highlight ependymal cell development along caudal, middle and rostral regions of the lateral ventricle wall. Wave of caudal-to-rostral ependymal cell formation is illustrated on 2D projections of the ventricle wall. Scale bars: 20 µm in top whole-view schematic; 1 mm in E13 and E16 2D projections; 500 µm in P1, P7 and P30 2D projections. Pie charts (third column) indicate average percentage of radial glia, immature ependymal cells, V-SVZ stem cells and mature ependymal cells along caudal, middle and rostral regions of the lateral ventricle wall (n=3) at each developmental stage. A, anterior; R, right; S, superior.
In Fig. 1, renderings of representative microscope images along the lateral wall detail cell organization at the ventricle surface (Fig. 1, second column, Fig. S1B). Cell type ratios (Fig. 1, third column), the average percentages of each cell type at three locations along the lateral wall for each developmental time point, were determined based on counts of a 13,567.59 µm2 area for each rostral, middle and caudal sample (n=3 animals). Before E13, radial glia cover the surface of the entire ventricular system surface (data not shown) (Kriegstein and Alvarez-Buylla, 2009). At E13 and E16 (Fig. 1A,B), immature ependymal cells, which make up ∼35% of total cells at the surface of the ventricle, were found primarily in the caudal-most aspects of the lateral ventricle lateral wall. Immature ependymal cells in the middle and rostral regions comprised only ∼11% and ∼7%, respectively, of the total cell number. By P1 (Fig. 1C), mature ependymal cells, which are characterized by a large tightly clustered array of multiple cilia, cover most of the caudal wall (60%) and stem cells that are organized in the core of pinwheel units made up the remainder (Fig. S1B). Immature and mature ependymal cells make up 34.2% of the middle lateral wall (22.1% immature ependymal cells and 8.5% mature ependymal cells), and only immature ependymal cells (20.4%) and radial glia (79.6%) line the rostral-most wall.
As the caudal-to-rostral wave of newly differentiated ependymal cells begins to cover the ventricle surface, clusters of radial glia/neural stem cells (V-SVZ stem cells) were found to retain only a small apical process at the ventricle surface, whereas stem cell somatas were displaced below the newly generated ependymal cell monolayer, as previously described (Mirzadeh et al., 2008). By P7 (Fig. 1D), only mature ependymal cells and clusters of stem cell apical processes, classic ‘pinwheel’ units (Mirzadeh et al., 2008), make up the caudal (58.4% mature ependymal cells, 41.6% stem cell processes) and middle (41.3% mature ependymal cells, 58.7% stem cell processes) aspect of the lateral wall. In the rostral-most aspect of the lateral wall, radial glia (40.3%) and immature ependymal cells (5.6%) were still detected. By P30 (Fig. 1E, Fig. S1B), all regions of the lateral wall were covered with organized pinwheel units. Cell counts at P30 indicate that the majority of cells at the ventricle surface are mature ependymal cells (∼60%), with stem cells making up ∼40% of the total cell count. However, as stem cell somatas are displaced to the SVZ, the ventricle-contacting apical process takes up only ∼10% of the ventricle surface area compared with ependymal cells (see also Spassky et al., 2005).
Ependymogenesis along the medial wall also proceeds as a caudal-to-rostral wave (Fig. S1C). At E13, the medial wall is covered by radial glia, with immature ependymal cells present only in the caudal-most region (not shown). By E16, differentiation of immature ependymal cells progresses rostrally along the medial wall and, after birth (P1), the caudal and middle regions were covered predominantly by mature multi-ciliated ependymal cells, whereas the rostral region was still lined primarily with radial glia. At P30, the medial wall was covered by mature multi-ciliated ependymal cells: stem cells were not observed along the medial wall. Others report small clusters of stem cells only along the rostral-most aspect of the medial wall in postnatal mice (Mirzadeh et al., 2008), but, as we have found, these are subsequently lost in early adulthood (Fig. S1C).
Here, we highlight the conversion of neuroepithelia to an ependymal monolayer that is interspersed with clusters of stem cells along only the lateral, not the medial, wall. These data support earlier findings that describe the caudal-to-rostral wave of ependymogenesis along the lateral ventricle lateral wall (Mirzadeh et al., 2008; Spradling et al., 2001).
Human ependymogenesis proceeds posterior to anterior along the lateral ventricle surface
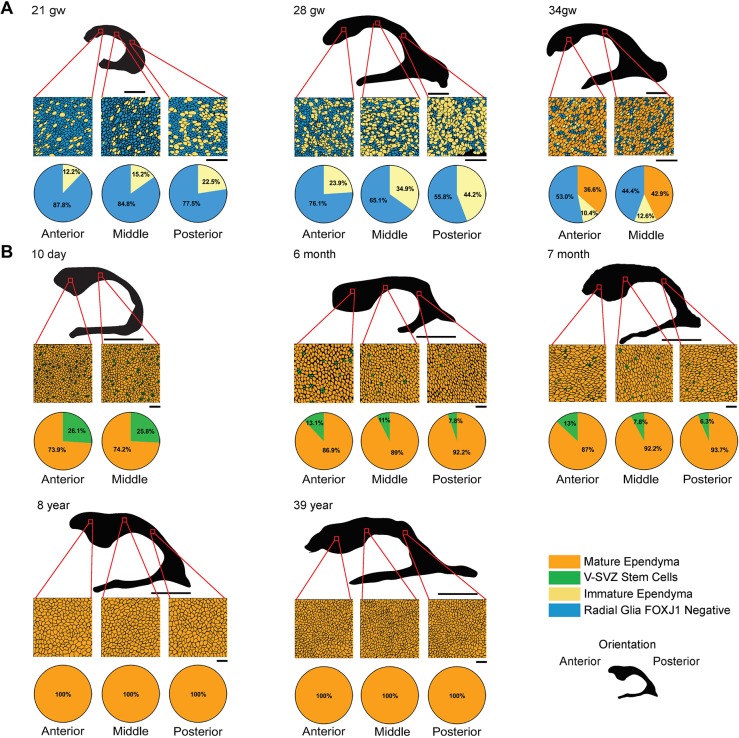
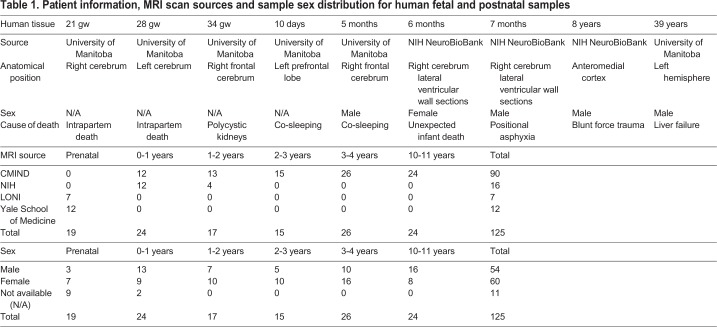
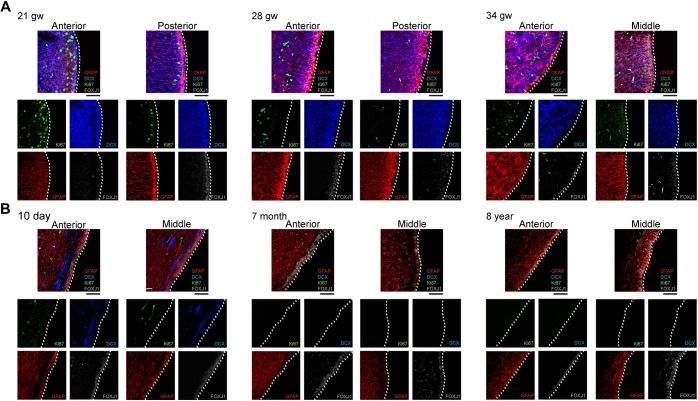
To characterize ependymogenesis and associated changes to the V-SVZ stem cell niche along the frontal horn of the lateral ventricles in humans, we prepared whole-mount sections of the lateral wall from fetal periventricular tissue at 21 gw, 28 gw and 34 gw (Fig. 2A). Wholemounts were also prepared at perinatal, adolescent and adult time points: 10 day (neonatal), 6 months, 7 months, 8 years and 39 years (Fig. 2B). Human fetal, perinatal, adolescent and adult tissues were obtained from the National Institutes of Health (NIH) NeuroBioBank (University of Maryland, MD, USA) and the University of Manitoba, Pathology Department (Winnipeg, Canada) (Table 1). Samples were without brain structural abnormalities or acquired lesions and were considered normal. Wholemounts of tissue from anterior (frontal horn over the caudate nucleus head), middle (frontal horn body near the interventricular foramen) and posterior (ventricular trigone/body) regions were prepared for immunohistochemistry. Cell composition, based on three samples within each anterior, middle and posterior region, was based on immunocytochemical criteria for radial glia, immature ependymal cells, V-SVZ stem cells and mature ependymal cells (see above and Fig. S2A). Representative microscope images for each developmental time point and region were rendered into schematic diagrams to show cell organization from each region, as indicated on two-dimensional (2D) reconstructions of the ventricle wall (Fig. 2A,B). Cell types were quantified within a 13,567.59 µm2 representative area for each anterior, middle and posterior sample and cell type ratios were indicated as pie charts.
Fig. 2.
Human ependymogenesis proceeds posterior to anterior along lateral ventricle wall during fetal-to-postnatal development and exhibits characteristic pinwheel organization. (A,B) Human fetal (A) and postnatal/adult (B) ependymal cell development was examined at 21 gw, 28 gw, 34 gw, 10 day, 6 months, 7 months, 8 years and 39 years (n=1). Representative schematics of microscope images of a 3391.90 µm2 (fetal) or 13,567.59 µm2 (postnatal) area of the lateral ventricle frontal horn are indicated by red squares on 2D ventricular surface projections (black). Pie charts below schematics indicate percentage of radial glia, immature ependymal cells, V-SVZ stem cells and mature ependymal cells along anterior, middle and posterior regions. Scale bars: 1 cm in A (2D ventricle wall renderings); 5 cm in B (2D ventricle wall renderings); 20 µm in A,B (tissue sections).
Table 1.
Patient information, MRI scan sources and sample sex distribution for human fetal and postnatal samples
Microscopic images and cell counts revealed a posterior-to-anterior developmental wave of ependymogenesis along the lateral wall of the frontal horn (Fig. 2A,B; Fig. S2B), similar to what was found in mouse (Fig. 1) (Spassky et al., 2005). At 21 gw, radial glia dominated in all regions; however, immature ependymal cells made up 22.5% of the total cell number in the posterior region of the body of the frontal horn. Fewer immature ependymal cells were found in the middle (15.2%) and anterior (12.2%) regions. By 28 gw, the number of immature ependymal cells increased in all regions, with the largest percentage in the posterior region (44.2% versus 34.9% and 23.9% immature ependymal cells in the middle and anterior regions, respectively). At 34 gw there was significant differentiation of immature ependymal cells into mature ependymal cells (36.6% and 42.9% in anterior and middle regions, respectively) with scattered large clusters of radial glial cells (blue cells) and few remaining immature ependymal cells (pale yellow cells). Following birth, at 10 days all ependymal cells along the surface were multi-ciliated mature ependymal cells, based on large clusters of basal bodies along their apical surface (73.9% and 74.2%, anterior and middle regions, respectively). V-SVZ stem cells made up ∼26% of the total cell number but retained only thin apical processes at the ventricle surface, which constituted ∼15% of the surface area of an ependymal cell. In striking similarity to mouse development (Mirzadeh et al., 2008; Spassky et al., 2005), the apical processes of stem cells were retained in clusters demonstrating classic ‘pinwheel’ organization along the lateral wall of the human frontal horn (Fig. S2B). During postnatal development, mature ependymal cell numbers increased as stem cell numbers declined (at 6 and 7 months). A reduction in stem cell numbers was observed to follow the same posterior-to-anterior pattern, with loss occurring in the posterior regions first. By 8 years, only mature ependymal cells were found lining the lateral ventricle surface; no stem cell processes were observed (Fig. 2B). This absence of stem cell apical processes along the lateral wall was also found in adult tissue, indicating that only mature ependymal cells line the lateral ventricle wall in adulthood (Fig. 2B).
The medial walls of the frontal horn of the lateral ventricles also show a posterior-to-anterior wave of ependymogenesis, but V-SVZ stem cells are not retained and, as a result, ependymal cells completely cover the medial wall (Fig. S2C). New immature ependymal cells were found scattered throughout the medial wall at 21 gw, and by 34 gw the middle regions of the wall were mostly covered by mature ependymal cells. By 10 days postpartum, an intact ependyma was found in all regions of the medial wall. We did not detect any remaining V-SVZ stem cells in any postnatal medial wall samples.
The above studies of fetal, perinatal, adolescent and adult human lateral wall tissue demonstrate a posterior-to-anterior transition from radial glia coverage (before 21 gw, data not shown) to a complete monolayer of ependymal cells (adolescent and adult tissue). As ependymal cells are generated (from 21 gw until perinatal time periods), radial glia and V-SVZ stem cell numbers (based on an apical process at the ventricle surface) decline. Pinwheel units initially contain many stem cell processes, but process number declines during postnatal development. Concurrent with ependymal cell maturation through postnatal development is an increase in apical surface area of ∼fivefold (Fig. S2D).
Human lateral ventricle volume and surface area changes correspond to curvature changes
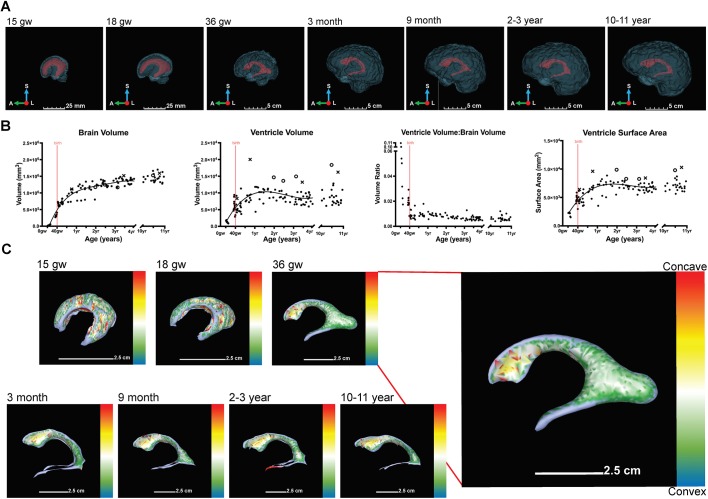
We next examined whether the spatiotemporal posterior-to-anterior progression of ependymogenesis and stem cell depletion along the lateral wall of the lateral ventricle were related to developmental changes in lateral ventricle volume and surface area. Using neurologically normal prenatal T2-weighted structural magnetic resonance imaging (MRI) scans (7T) and postnatal T1-weighted structural MRI scans (3T) from neurologically normal individuals (Table 1), we determined total brain and lateral ventricle volumes and lateral ventricle surface area at discrete developmental time points (Fig. 3A,B). Semi-automated segmentation using ITK-SNAP (Shook et al., 2014; Snippert et al., 2010; Todd et al., 2017; Yushkevich et al., 2006) followed by 3D reconstruction of the lateral ventricles and whole brain using 3D Slicer (Acabchuk et al., 2015; Shook et al., 2014) revealed that the average total brain volume increases rapidly from 15 gw to 1 year. Regression analysis predicts that from 19 gw to birth, the brain grows at a nearly linear rate of 1.2×106 mm3/year (23 cm3/week) and from birth to 1 year at 5.5×105 mm3/year (11 cm3/week) (see also Kinoshita et al., 2001). From 1 year to ∼3.5 years, brain volume growth slows to a nearly linear average rate of 1.3×105 mm3/year (2.5 cm3/week). These represent a 1100% increase in brain volume from 18 gw to birth, 110% increase from birth to 1 year, and 16% from 1 year to 2 years (Fig. 3A,B), similar to the findings of others (Knickmeyer et al., 2008). In contrast, lateral ventricle volume exhibits an increase, slight decrease and then a plateau across early postnatal development. Lateral ventricle volume increase occurs from 15 gw to ∼1.25 years. This occurs at an average rate of 8900 mm3/year (171 mm3/week) from 15 gw to birth, and 3800 mm3/year (73 mm3/week) from birth to 1.25 years. Ventricle volume decrease occurs at a nearly linear rate of 800 mm3/year (15 mm3/week) from 1.25 years to 3.5 years and is significant by ANOVA of a simple linear regression model across this data subset (P=0.032). A plateau appears to occur slightly after 3 years, stabilizing at ∼8400 mm3 and there is no significant evidence to indicate that the average ventricle volume from 3-4 years differs from that of 10-11 years, based on an outlier-resistant two-sample Mann–Whitney nonparametric test (P=0.66). In summary, ventricle volume increases 350% from 15 gw to birth, 86% from birth to 1.25 years and decreases 18% from 1.25 years to 3.5 years. Certain scans from the Cincinnati MR Imaging (C-MIND) database (www.cmind.research.cchmc.org) showed distinctly larger total ventricle volumes (see Fig. S3 for details). Some scans showed asymmetrical ventricles, with one ventricle being larger than the other (Fig. 3B, marked by ‘×’ on ventricle volume and surface area graphs) or symmetric and slightly enlarged ventricles (Fig. 3B, marked by ‘O’ on ventricle volume and surface area graphs).
Fig. 3.
Developmental changes in human lateral ventricle volume and surface area reflected in curvature changes. (A) 3D representations show lateral view of lateral ventricle (red) and whole-brain (blue) contours across fetal (15 gw, 18 gw, 36 gw) and postnatal (3 months, 9 months, 2-3 years, 10-11 years) development. (B) Scatterplots indicate changes in brain volume, ventricle volume, ventricle to whole brain volume ratio and ventricle surface area across fetal and postnatal human development. Brain volume was modeled as a fourth degree least-squares polynomial regression curve, ventricle volume and ventricle surface area were both modeled as third degree least-squares polynomial regression curves. Symmetrically (O) and asymmetrically (×) enlarged scans are noted. Birth is depicted by a red line. (C) Curvature heat maps of lateral ventricle surface across fetal (15 gw, 18 gw, 34 gw) and postnatal (3 months, 9 months, 2-3 years, 10-11 years) development. Heat map depicts the range of curvature from concave (red) to convex (blue). A, anterior; L, left; S, superior.
Plotting the ratio of ventricle to whole brain volume ratio shows that the lateral ventricles occupy ∼10% of the brain volume at 15 gw; this decreases rapidly to ∼2% at birth and stabilizes to ∼1% within a year (Fig. 3B). After 1 year, the brain continues to grow at a slow rate (2.5 cm3/week), which is responsible for any systemic decrease in the ventricle to whole brain volume ratio over time (Fig. 3B). There appears to be no significant correlation between sex and developmental age in our sample (n=114 individuals of known sex); therefore, sex was not included in our predictive models (see Materials and Methods, Statistical analysis section).
Lateral ventricle surface area exhibits a similar growth and plateau pattern. All expansion occurs from 15 gw to ∼1.25 years. The average growth from 15 gw to birth is 4700 mm2/year (90 mm2/week) and from birth to 1.25 years is 2200 mm2/year (42 mm2/week). Ventricle surface area decrease from 1.25 years to 3.5 years occurs at an average rate of 170 mm2/year (3.3 mm2/week). The ventricle surface area appears to plateau after 3 years, with an average surface area of 6800 mm2. Again, there is no significant evidence to indicate that the average ventricle surface area from 3-4 years differs from that of 10-11 years, based on an outlier-resistant two-sample Mann–Whitney nonparametric test (P=0.22). In summary, ventricle surface area increases 100% from 15 gw to birth, 62% from birth to 1.25 years and decreases 5.4% from 1.25 years to 3.5 years. There is no correlation between sex and developmental age in our sample (n=114 individuals of known sex); thus, sex was not included in our predictive models (see Materials and Methods, Statistical analysis section).
Analysis of surface area changes, unlike total volume changes, suggests changes in topography of the ventricle walls that may not correspond to minor volume changes (Del Bigio, 2014). Surface area increases emphasize a growing demand for ependymal cell coverage. We found that ventricle surface area increases during fetal development, plateauing at ∼1.5 years of age (Fig. 3B). Subjects with enlarged ventricle volumes also show similar trends. Increasing surface area results in the heightened need for ependymal cell coverage and may be responsible for the steady decline in stem cell number at the ventricle surface in postnatal development.
To characterize the changing topography associated with surface area increases along the ventricle wall, we examined how curvature of the lateral ventricles changed during fetal and postnatal development. From 15 gw to perinatal developmental stages, the curvature of the anterior horn (superior horn) of the lateral ventricles changes significantly from convex to concave (Fig. 3C, green to yellow) and this concavity appears to be maintained throughout postnatal development. In contrast, the temporal and posterior horn (occipital horn), although continuing to grow, remain concave from fetal through postnatal development. Based on these results, the posterior-to-anterior maturation of the ependymal cell monolayer at the ventricle surface also corresponds to the timing of the changing curvature of the anterior horn from convex to concave (Fig. 3C).
The V-SVZ stem cell niche and neurogenesis significantly decline during human brain development
To characterize the organization of the V-SVZ stem cell niche (frontal horn lateral wall of the lateral ventricle) across development, we examined tissue at fetal, perinatal, postnatal, adolescent and adult time points. Coronal sections from anterior, middle and posterior regions were prepared (see Table 1) and stained for GFAP (V-SVZ SCs), DCX (neuroblasts), Ki67 (cycling cells) and FOXJ1 [immature and mature ependymal cells, some late stage radial glia (Jacquet et al., 2009)] (Fig. 4A,B). At 21 gw, when the lateral ganglionic eminence (LGE) comprises the lateral wall of the lateral ventricles, immature ependymal cells (FOXJ1+) were detected primarily in the posterior region, similar to what was found in Fig. 2A. Radial glia (GFAP+) exhibited long radial processes and DCX+ neuroblasts were numerous throughout the SVZ and outer SVZ (oSVZ) regions (Hansen et al., 2010). Ki67+ proliferative cells were more numerous in the anterior versus posterior and/or middle regions. At 28 gw, the long radial processes of radial glia and widespread distribution of neuroblasts were still observed, and there are more proliferative cells observed in the anterior versus posterior region. FOXJ1+ ependymal cells were found at the ventricle surface in both anterior and posterior regions. By 34 gw, SVZ astrocytes (GFAP+) possessed shorter radial processes, neuroblasts were still numerous and a mature ependyma demarcated the ventricle lining. Proliferative cells were mainly found within the anterior SVZ/oSVZ region, extending a significant distance from the ventricle lining. By 10 days postpartum, Ki67+ cells were reduced in number, and the majority of DCX+ neuroblasts were restricted to a narrow pathway immediately subjacent and tangential to the ventricle surface. Both 6-month and 7-month samples revealed few neuroblasts or proliferative cells, the absence of long radial processes and a continuous monolayer of ependymal cells making up the ventricle lining. We detected no Ki67+ or DCX+ cells in the 8-year-old sample, with the exception of a few Ki67+ cells scattered along blood vessels. GFAP+ astrocytes consolidated as a ribbon parallel to, but separate from, an acellular zone that lay next to the ependymal monolayer, as previously reported (Sanai et al., 2011).
Fig. 4.
Human V-SVZ stem cell niche and neurogenesis diminish during fetal-to-postnatal development. (A,B) Human fetal (A) and postnatal (B) LGE and V-SVZ development across 21 gw, 28 gw, 34 gw, 10 day, 7 months and 8 years along the frontal horn lateral wall of the lateral ventricle (n=1). Anterior and posterior coronal sections of the anterior horn were examined for 21 gw and 28 gw, whereas anterior and middle regions were examined for 34 gw, 10 day, 7 months and 8 years. Composite image of all four channels is positioned above separated channels for each region. Dotted white line indicates the lateral ventricle wall edge. Glial fibrillary acidic protein (GFAP, red), doublecortin (DCX, blue), Ki67 (green), FOXJ1 (gray). Scale bars: 50 µm.
DISCUSSION
Organ-specific stem cell niches can have long-lasting effects on the development and function of the organ system. A vital stem cell niche within the developing brain is the V-SVZ. In addition to supporting neurogenesis, stem cells in the V-SVZ also generate cells that support their own niche. An essential, but understudied, contributor to the V-SVZ stem cell niche is the ependyma – a monolayer of multi-ciliated ependymal cells that are generated during mid- to late-gestation in humans. Ependymal cells anchor niche-associated stem cells and provide barrier and transport functions between the brain's interstitial fluid and the CSF. A mature ependymal cell lining is also required to support neurogenesis and neuroblast migration along the lateral ventricle wall in mice (Paez-Gonzalez et al., 2011).
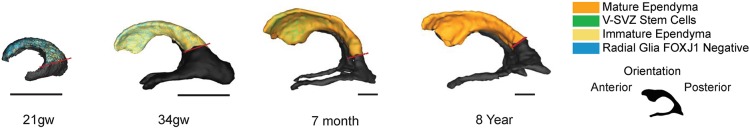
We have mapped the progression of ependymogenesis across the frontal horn of the lateral ventricles, along with changes in ventricle volume, surface area and curvature during human brain development (Fig. 5). Radial glia that initially line the ventricles in early fetal development were sequentially replaced by ependymal cells in an occipital-to-frontal wave across the ventricle surface. Significantly, only the lateral wall of the frontal horn retained radial glia/stem cells into infancy and these stem cells maintained only an apical process at the ventricle surface, whereas their somata were repositioned to the SVZ. Clusters of stem cell apical processes surrounded by ependymal cells, arrayed as classic ‘pinwheel’ units similar to those previously described in mouse brain (Mirzadeh et al., 2008), were found in whole-mount preparations of human perinatal frontal horn lateral wall tissue. The number of stem cells with ventricular contact steadily declined in a posterior-to-anterior gradient during postnatal development and no apical processes of stem cells were observed in whole-mount preparations of adolescent and adult lateral wall samples.
Fig. 5.
Summary of human ependymogenesis across fetal-postnatal development. Human ependymogenesis proceeds posterior to anterior along the lateral wall of the frontal horn of the lateral ventricle. At 21 gw, the majority of the lateral wall surface is covered by radial glial cells, as immature ependymal cells are forming in the posterior region. The wall is convex at this time. At 34 gw, a mixture of radial glial cells, immature ependymal cells and mature ependymal cells are found along the length of the wall and the anterior-most region is now concave. By 7 months, the lateral wall is composed of mature ependymal cells and stem cell clusters that are arranged in a pinwheel unit organization. Stem cells are replaced in a posterior-to-anterior manner. The concavity of the anterior horn is clearly evident. By 8 years, the lateral wall is comprised entirely of mature ependymal cells, with no pinwheels present. The conformation of the ventricle surface is stabilized. 3D representations of the lateral ventricle are shown (black), with a dotted red line marking the end of the frontal horn of the lateral ventricle, above the trigone region. Scale bars: 2.5 cm.
In contrast to the lateral wall, the medial wall of the frontal horn of the lateral ventricles, which also shows a similar posterior-to-anterior ependymogenesis progression, does not retain stem cells with ventricle contact after birth. Instead, an uninterrupted wall of ependymal cells eventually covers the entire medial wall.
Our findings related to ependymogenesis in humans during the fetal-to-postnatal transition mimics what has been demonstrated previously in mice [this study and others (Jacquet et al., 2009; Kyrousi et al., 2015; Mirzadeh et al., 2010b, 2008; Paez-Gonzalez et al., 2011; Spassky et al., 2005)]. However, in contrast to human, ventricle-contacting stem cells persist along the lateral wall throughout adulthood in mouse and other mammals (Alunni and Bally-Cuif, 2016; Lledo et al., 2008; Peretto et al., 1999; Conover and Shook, 2011) and although stem cell numbers decline over the course of aging, stem cells with ventricle contact are still found in elderly mice (Capilla-Gonzalez et al., 2015; Conover and Shook, 2011; Conover and Todd, 2017; Luo et al., 2006; Maslov et al., 2004; Shook et al., 2012).
The life-long retention of V-SVZ stem cells and accompanying neurogenesis found in adult mice and other mammals differs from the significant diminution of V-SVZ stem cells and neurogenesis that we, and others (Arshad et al., 2016; Hansen et al., 2010; Malik et al., 2013; Paredes et al., 2016a,b; Sanai et al., 2011; Wang et al., 2011, 2014), have observed by the time of adolescence in humans. This discrepancy has recently garnered special attention and driven theoretical models to explain retention or loss of stem cell populations in different organ systems and in different species (Hormoz, 2013). Hormoz (2013) proposed the division of stem cell population dynamics into two types, both supporting stem cell self-preservation and progenitor generation. (1) Stem cell population asymmetry occurs with heterogeneous proliferation rates, with some stem cells dividing symmetrically to give progenitor cells [‘consuming’ stem cell division (Obernier et al., 2018)] and neighboring slow-dividing stem cells divide symmetrically to yield two stem cells. (2) Asymmetric stem cell division occurs when stem cells divide to yield a stem cell (self-renewal) and a progenitor cell. Asymmetric cell division is the predominant form of division by radial glia in embryonic development (Kriegstein and Alvarez-Buylla, 2009), but it is a process that will not support the stem cell pool over extended periods of time because of stem cell exhaustion (Hormoz, 2013; Obernier et al., 2018; Shahriyari and Komarova, 2013). Population asymmetry would allow the slow-dividing stem cells to proliferate and purge the population of fast-dividing, older cells. This mechanism has been observed in adult V-SVZ stem cell-derived neurogenesis in mouse (Obernier et al., 2018) and may also explain V-SVZ stem cell retention into infancy in humans. It remains to be determined whether generation of ependymal cells is the final division product (through symmetric division) of stem cells with ventricle contact. A similar ‘disposable stem cell’ model was proposed by Encinas et al. (2011) to explain age-related loss of mouse hippocampal neural stem cells and the appearance of new astrocytes.
The extent of ependymogenesis is likely driven by alterations to the contours of the ventricle surface. In an attempt to correlate the spatiotemporal development of the ependymal lining with changing conformation of the ventricle wall, we assessed lateral ventricle volume and surface area changes over the course of fetal and postnatal development. Using MRI data from our human sources, we plotted volumes and surface area from 15 gw to 11 years. Rapid increases in total brain volumes and increases in lateral ventricle volumes were found from 15 gw until ∼1 year. After 1 year, the total brain volume rate of increase slows but continues at a linear rate. In contrast, lateral ventricle volume exhibits an increase, slight decrease, and then a plateau across early postnatal development; the ventricle volume at 3 years is not significantly different from that seen at 11 years. Others have reported similar trends for brain and lateral ventricle increases from the second trimester to birth (Kinoshita et al., 2001; Sakai et al., 2012) to 2 years of age (Knickmeyer et al., 2008; Leigh, 2004). To extend earlier studies, we found that early in the second trimester (15 gw) the lateral ventricles occupy ∼10% of the total brain volume, 2% of the total brain volume at birth and 1% of the total brain volume at 1 year. Lateral ventricle surface area showed similar gestational increases from 15 gw until birth, showing a slight decrease after 1 year and then reaching a plateau by 2-3 years of age. Volume and surface area are general measurements that do not reveal topographical changes at the ventricle surface [e.g. volume may increase through displacement (convexity) of the ventricle walls, whereas surface area remains unchanged (Del Bigio, 2014)]. To evaluate conformational changes along the ventricle surface, we used curvature analysis to reveal patterns of concavity and convexity along the extra-ventricle surface. These patterns are not outwardly apparent from MRI scan segments; however, they do become more obvious from 3D reconstructions. From 15 gw to late gestation (36 gw), the anterior horn exhibits significant conformational changes from convex to concave. Concavity of the anterior horn then stabilized through postnatal periods. The occipital horns grow and elongate extensively in late gestation, but remain concave from 36 gw through postnatal development. In the lateral ventricle frontal horn, we found that the eventual coverage of the lateral ventricle wall with mature ependymal cells corresponded to the period when ventricle volume, surface area and curvature stabilized (after ∼1.5 years). The mirroring of conformational stabilization and ependymogenesis suggests that deposition of ependymal cells along the ventricle walls likely contributes to the lateral ventricle wall stability.
Ependymogenesis in ventricle regions other than the lateral wall corresponds to completion of neurogenesis. V-SVZ neurogenesis along the frontal horn lateral wall in the postnatal human anterior forebrain populates the ‘arc’ pathway to the frontal cortex, and the MMS and RMS pathways (Hansen et al., 2010; Paredes et al., 2016a; Sanai et al., 2011, 2004). We documented changes to the V-SVZ and oSVZ in coronal sections along the lateral ventricle wall that showed substantial proliferation and neurogenesis during gestation, followed by decreased proliferation and neurogenesis after birth, in full support of the findings of others (Bergmann et al., 2012; Paredes et al., 2016b; Quiñones-Hinojosa et al., 2006; Sanai et al., 2011; Wang et al., 2011, 2014). In addition, we observed a reorganization of migratory neuroblasts to a narrow tangential pathway subjacent to the ependymal lining from birth until 7 months of age. By 8 years no proliferative cells or migratory neuroblasts were detected, instead a periventricular acellular gap region and associated astrocyte ribbon was detected, as previously described in the adult human brain (Sanai et al., 2011, 2004). A recent study provided strong evidence that human hippocampal dentate gyrus neurogenesis declines rapidly during the first year of life and persists at low levels into early adolescence, but does not continue into adulthood (Sorrells et al., 2018). This challenges previous reports (Dennis et al., 2016; Eriksson et al., 1998; Knoth et al., 2010; Spradling et al., 2001) and a new study (Boldrini et al., 2018) that find daily production of new neurons in adulthood (∼700/dentate gyrus/day). Although our study and others provide evidence that robust, or even moderate, levels of neurogenesis along the lateral ventricle surface do not continue into adulthood in the human brain, it remains unresolved, and highly controversial, whether this applies to other forms of adult human neurogenesis.
Concluding remarks
Full ependymal cell coverage of the ventricle walls supports both barrier and transport functions between the interstitial fluid and CSF and aids in maintaining brain homeostasis. Diseases resulting in ventriculomegaly, such as fetal-onset hydrocephalus, an abnormal enlargement of the lateral ventricles, places an extraordinary demand on the stem cell population to provide both crucial neurogenic functions and adequate ependymal cell coverage at the ventricle surface. Disruption, hypoproliferation or hyperproliferation of the ependymal layer is implicated in a variety of psychiatric, neurodegenerative and neurodevelopmental conditions and is understudied. The above studies define normal development of the V-SVZ stem cell niche and associated ependymal lining along the lateral ventricles and provide a developmental platform from which to assess disruption of this vital stem cell niche in diseases that result in infant ventriculomegaly or hydrocephalus.
MATERIALS AND METHODS
Animals
Male CD-1 mice (Mus musculus) (Charles River Laboratories, Wilmington, MA, USA) at E13, E16, P1, P7 and P30 (adult) were used. Housing, handling, care and processing of the animals were carried out in accordance with regulations approved by the Institutional Animal Care and Use Committee of the University of Connecticut.
Mouse brain tissue immunohistochemistry
All antibodies used in this study were used previously and validated by our group and others; the expression patterns were as expected and referenced. For coronal sections, P7 and P30 mice were anesthetized with isoflurane, then transcardially perfused with 0.9% saline followed by 4% paraformaldehyde (PFA). The extracted brains were fixed overnight in 4% PFA at 4°C. E13, E16, and P1 mice were anesthetized with isoflurane, heads were removed and fixed overnight in 4% PFA at 4°C. After removing the skin, embryonic and P1 brains were removed from the skull using a Leica MZ95 stereomicroscope. All brains were washed for 3×10 min in PBS before dissection and vibratome sectioning for 3D reconstructions.
Lateral ventricle wall wholemounts were prepared as described (Mirzadeh et al., 2008). Following isofluorane anesthesia and saline perfusion, P7 and P30 brains were extracted and whole-mount preparations were placed in PFA with 1% Triton X-100 overnight. E13, E16 and P1 mice were anesthetized with isofluorane, heads were extracted and placed in 4% PFA with 1% Triton X-100 overnight and wholemounts were then prepared (Mirzadeh et al., 2010a). Wholemounts were immunostained with the following primary antibodies: mouse monoclonal anti-β-catenin (1:250; BD Biosciences, #610154), rabbit polyclonal anti-β-catenin (1:100; Cell Signaling Technology, #9562), rabbit polyclonal anti-γ-tubulin (1:500; Sigma-Aldrich, #T5192), rabbit polyclonal anti-GLAST (1:200; Abcam, #ab416), goat polyclonal anti-GFAP (1:250; Abcam, #ab53554), rat monoclonal anti-GFAP (1:250; Invitrogen, #13-0300) and mouse monoclonal anti-FOXJ1 (1:250; Invitrogen, #14-9965-80). Alexa Fluor dye-conjugated polyclonal secondary antibodies (1:500, Invitrogen) were used: donkey anti-mouse 488 (#21202), donkey anti-mouse 546 (#A10036), donkey anti-rabbit (#A21206), donkey anti-goat-647(#A21447) and donkey anti-rat 647 (#A18744). Blocking solutions contained 1% Triton X-100. Validations of all commercial antibodies are available from manufacturer's datasheets. Lateral wall tissue wholemounts were coverslipped with Aqua-Poly/Mount (Polyscience) and imaged on a Leica TCS SP8 confocal laser scan microscope (Leica Microsystems).
Mouse lateral ventricle reconstruction and analysis
To generate 3D reconstructions of the mouse brain and ventricles, coronal sections from E13 (42 µm), E16, P1, P7 and P30 (all 50 µm) brains were sectioned on a vibratome (VT-1000S, Leica). Mouse brain tissue sections were stained with β-catenin overnight (rabbit polyclonal anti-β-catenin, 1:100; Cell Signaling Technology, #9562), secondary antibody for 1 h (donkey anti-rabbit 546, 1:500; Invitrogen, #A10040), nuclear stain DAPI (300 mM; Molecular Probes, #D-1306) for 10 min and imaged on a Zeiss Axio Imager M2 microscope with ApoTome (Carl Zeiss MicroImaging) with a Hamamatsu Photonics ORCA-R2 digital camera (C10600). Alternating coronal sections were imaged, and the contours of the lateral ventricle walls and surface of the brain were traced to generate 3D reconstructions, as described (Acabchuk et al., 2015). Volume and surface area analysis were performed using StereoInvestigator and Neurolucida Explorer software (MBF Bioscience).
Human brain tissue immunohistochemistry
Postmortem human brain tissue (whole hemispheres and regional portions of the lateral ventricle wall) ranging from 21 gw to 39 years of age were obtained from the NIH NeuroBioBank (University of Maryland, MD, USA) and the University of Manitoba, Pathology Department (Winnipeg, Canada). Tissues were acquired under protocol H2011-212, approved by the University of Manitoba Health Research Ethics Board. All tissue was archival and came de-identified. Hemispheres from 21 gw, 28 gw, 34 gw, 10 day and 39 year, and V-SVZ sections from 5 and 7 months were examined (Table 1). For lateral ventricle whole-mount preparations, several sections from the anterior (frontal horn over the caudate nucleus head), middle (frontal horn near the interventricular foramen) and posterior (frontal horn) lateral ventricle surfaces were dissected. Lateral ventricle wall wholemounts were coverslipped with AquaPoly/Mount and imaged on Leica TCS SP8 confocal laser scan microscope. Lateral ventricle wholemounts were imaged using a 100×/1.4 HC PL APO oil immersion objective lens, taken at scan zoom 1 (13,567.59 µm2 field area) or scan zoom 2 (3391.90 µm2 field area). The following antibodies were used: rabbit polyclonal anti-β-catenin (1:100; Cell Signaling Technology, #9562), rabbit polyclonal anti-γ-tubulin (1:500; Sigma-Aldrich, #T5192); rabbit polyclonal anti-GLAST (1:200; Abcam, #ab416), goat polyclonal anti-GFAP (1:250; Abcam, #ab53554), rat monoclonal anti-GFAP (1:250; Invitrogen, #13-0300) and mouse monoclonal anti-FOXJ1 (1:250; Invitrogen, #14-9965-80). Alexa Fluor dye-conjugated polyclonal secondary antibodies (1:500, Invitrogen) were used: donkey anti-mouse 488 (#21202), donkey anti-rabbit 546 (#A10040), donkey anti-goat-647 (#A21447) and donkey anti-rat 647 (#A18744). Validations of all commercial antibodies are available from manufacturer's datasheets. Representative areas (13,567.59 µm2) were imaged and cell types were identified based on basal body number (radial glia and V-SVZ stem cells, 1 basal body; immature ependymal cells, 2-5 basal bodies; mature ependymal cells, an array of many basal bodies) and immunostaining criteria. Schematics were generated by outlining an identified cell and assigning a ‘fill’ color.
Regions corresponding to the LGE in fetal tissue and the lateral wall of the lateral ventricle in postnatal tissue were dissected and sectioned coronally at 100 mm thickness. Immunohistochemistry was performed as described (Todd et al., 2017) using the following antibodies: goat polyclonal anti-GFAP (1:250; Abcam, #ab53554), mouse monoclonal anti-FOXJ1 (1:250; Invitrogen, #14-9965-80), guinea pig anti-doublecortin (DCX) (1:1000, EMD Millipore, #AB2253) and rabbit anti-Ki67 (1:1000; Novocastra, #6013874). Alexa Fluor dye-conjugated polyclonal secondary antibodies (1:500) were used: donkey anti-mouse 405 (Abcam, #ab175658), donkey anti-mouse 488 (Invitrogen, #21202), donkey anti-rabbit 546 (Invitrogen, #A10040), donkey anti-goat-647 (Invitrogen, #A21447) and donkey anti-rat 647 (Invitrogen, #A18744). Validations of all commercial antibodies are available from manufacturer's datasheets. Coronal sections were imaged on a Leica TCS SP8 confocal laser scan microscope using a 40×/1.3 HC PL APO oil immersion objective lens.
Human MRI lateral ventricle reconstruction and analysis
De-identified archival MRI scans ranging from 15 gw-10 years were used in this study. T1 or T2 structural scans were obtained from the C-MIND database with a 3T MRI scanner, the NIH National Institute of Mental Health (NIMH) data archive (https://ndar.nih.gov/), Laboratory of Neuro Imaging (LONI) database of the University of Southern California (www.loni.usc.edu, 7T MRI scanner) and Yale School of Medicine (3T MRI scanner) (Table 1). Lateral ventricle and whole-brain semi-automated segmentation and volume/surface area analyses were completed using ITK-SNAP and 3D Slicer software as previously described (Acabchuk et al., 2015; Todd et al., 2017). To determine accurate whole-brain volume and surface area measurements, skulls were digitally removed from MRI scans using Brain Suite software (www.brainsuite.org). De-skulled masks of MRI scans were automatically generated using the skull stripping tool under the cortical surface extraction sequence function of Brain Suite. Brain surface extractor mechanism was set to trim brain stem and spinal cord and dilate the final mask. Extractor settings that were kept constant include five automated iterations, three diffusion iterations and a diffusion constant of 25. Edge constant and erosion size were manually modified as necessary with each MRI scan. Manual edits using the edit mask function of Brain Suite were made following automatic skull stripping as needed.
Curvature was analyzed in Meshmixer software (www.meshmixer.com). 3D OBJ files created from lateral ventricle segmentations were imported into Meshmixer. Mean curvature was calculated and generated on the 3D model as a heat map using the mesh query analysis function. Meshmixer automatically calculates the mean curvatures based on a system of triangles (faces) and vertices on the 3D model, providing curvature values at each vertex of the model.
Statistical analysis
Data are reported as mean±s.e.m. Statistical analysis was performed in GraphPad Prism software (www.graphpad.com). One-way ANOVA with Bonferroni's multiple comparisons post-test was used. All statistical regression analysis was performed in SAS 9.1. The minimum level of significance for all tests was P<0.05. All regression models were fitted using least-squares polynomial regression and analyzed with sequential-addition (type-I sum of squares) partial F-tests of terms with increasing degree. All terms in our models, including the intercept term, are significant based on type-III sum of squares, partial F-tests. The following regression models were obtained, with ‘x’ denoting developmental age in years, brain and ventricle volume in mm3, and ventricle surface area in mm2.
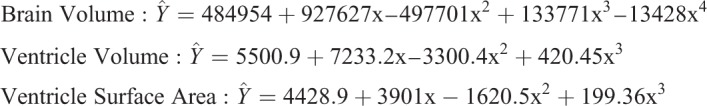 |
Supplementary Material
Acknowledgements
We gratefully acknowledge the NIH NeuroBioBank and the University of Manitoba, Pathology Department, for providing human tissue. We also thank and gratefully acknowledge the LONI database, the University of Southern California, C-MIND, NIH NIMH Data Archive and Dr Dustin Scheinost (Yale School of Medicine) for providing MRI data. This manuscript reflects the views of the authors and may not reflect the opinions or views of the NIH or of the Submitters submitting original data to NDAR.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: D.S., S.K., T.N.S., M.R.D.B., J.C.C.; Methodology: A.M.C., D.S., S.K., T.N.S., P.J.B., B.F.B., D.P., E.S.N., E.C.B., J.C.C.; Software: D.S., S.K.; Validation: A.M.C., D.S., S.K., T.N.S., P.J.B., B.F.B., D.P., E.S.N., E.C.B., J.C.C.; Formal analysis: A.M.C., D.S., S.K., T.N.S., P.J.B., B.F.B., D.P., E.S.N.; Investigation: A.M.C., D.S., S.K., T.N.S., P.J.B.; Resources: A.M.C., D.S., S.K., K.T.K., M.R.D.B., J.C.C.; Data curation: A.M.C., D.S., S.K., T.N.S., P.J.B., B.F.B., D.P., E.C.B.; Writing - original draft: A.M.C., D.S., S.K., K.T.K., M.R.D.B., J.C.C.; Writing - review & editing: A.M.C., D.S., S.K., T.N.S., P.J.B., B.F.B., K.T.K., M.R.D.B., J.C.C.; Visualization: A.M.C., D.S., S.K., P.J.B., B.F.B., D.P., E.S.N., E.C.B., J.C.C.; Supervision: K.T.K., M.R.D.B., J.C.C.; Project administration: J.C.C.; Funding acquisition: J.C.C.
Funding
This research was funded by the National Institutes of Health (NS090092 and NS098091 to J.C.C.; instrument grant S10ODO16435), the Hydrocephalus Association (to J.C.C.), the University of Connecticut Institute for Brain and Cognitive Sciences (to A.M.C., D.S., B.F.B., S.K. and J.C.C.). Dr Del Bigio holds the Canada Research Chair in Developmental Neuropathology. Deposited in PMC for release after 12 months.
Data availability
Data used in the preparation of this article were obtained from the following studies: (1) Brain Suite, LONI software (NIH-NINDS R01 NS074980, NIH-NIBIB R01 EB002010, NIH-NIBIB P41-EB015922). (2) The C-MIND Data Repository created by the C-MIND study of Normal Brain Development (CMINDS data set version 1.720). This is a multisite, longitudinal study of typically developing children from ages newborn through young adulthood conducted by Cincinnati Children’s Hospital Medical Center and UCLA and supported by the National Institute of Child Health and Human Development (Contract HHSN275200900018C). A listing of the participating sites and a complete listing of the study investigators can be found at https://research.cchmc.org/c-mind. (3) The NIH-supported National Database for Autism Research (NDAR). NDAR is a collaborative informatics system created by the National Institutes of Health to provide a national resource to support and accelerate research in autism.
Supplementary information
Supplementary information available online at http://dev.biologists.org/lookup/doi/10.1242/dev.170100.supplemental
References
- Acabchuk R. L., Sun Y., Wolferz R. Jr, Eastman M. B., Lennington J. B., Shook B. A., Wu Q. and Conover J. C. (2015). 3D modeling of the lateral ventricles and histological characterization of periventricular tissue in humans and mouse. J. Vis. Exp. 99, e52328 10.3791/52328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alunni A. and Bally-Cuif L. (2016). A comparative view of regenerative neurogenesis in vertebrates. Development 143, 741-753. 10.1242/dev.122796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Buylla A., García-Verdugo J. M., Mateo A. S. and Merchant-Larios H. (1998). Primary neural precursors and intermitotic nuclear migration in the ventricular zone of adult canaries. J. Neurosci. 18, 1020-1037. 10.1523/JNEUROSCI.18-03-01020.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Buylla A., García-Verdugo J. M. and Tramontin A. D. (2001). A unified hypothesis on the lineage of neural stem cells. Nat. Rev. Neurosci. 2, 287-293. 10.1038/35067582 [DOI] [PubMed] [Google Scholar]
- Arshad A., Vose L. R., Vinukonda G., Hu F., Yoshikawa K., Csiszar A., Brumberg J. C. and Ballabh P. (2016). Extended production of cortical interneurons into the third trimester of human gestation. Cereb. Cortex 26, 2242-2256. 10.1093/cercor/bhv074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann O., Liebl J., Bernard S., Alkass K., Yeung M. S. Y., Steier P., Kutschera W., Johnson L., Landén M., Druid H. et al. (2012). The age of olfactory bulb neurons in humans. Neuron 74, 634-639. 10.1016/j.neuron.2012.03.030 [DOI] [PubMed] [Google Scholar]
- Boldrini M., Fulmore C. A., Tartt A. N., Simeon L. R., Pavlova I., Poposka V., Rosoklija G. B., Stankov A., Arango V., Dwork A. J. et al. (2018). Human hippocampal neurogenesis persists throughout aging. Cell Stem Cell 22, 589-599.e585. 10.1016/j.stem.2018.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruni J. E. (1998). Ependymal development, proliferation, and functions: a review. Microsc. Res. Tech. 41, 2-13. [DOI] [PubMed] [Google Scholar]
- Bruni J. E., Del Bigio M. R. and Clattenburg R. E. (1985). Ependyma: normal and pathological. A review of the literature. Brain Res. 356, 1-19. 10.1016/0165-0173(85)90016-5 [DOI] [PubMed] [Google Scholar]
- Capilla-Gonzalez V., Herranz-Pérez V. and García-Verdugo J. M. (2015). The aged brain: genesis and fate of residual progenitor cells in the subventricular zone. Front. Cell Neurosci. 9, 365 10.3389/fncel.2015.00365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conover J. C. and Shook B. A. (2011). Aging of the subventricular zone neural stem cell niche. Aging Dis. 2, 49-63. [PMC free article] [PubMed] [Google Scholar]
- Conover J. C. and Todd K. L. (2017). Development and aging of a brain neural stem cell niche. Exp. Gerontol. 94, 9-13. 10.1016/j.exger.2016.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conover J. C., Doetsch F., Garcia-Verdugo J.-M., Gale N. W., Yancopoulos G. D. and Alvarez-Buylla A. (2000). Disruption of Eph/ephrin signaling affects migration and proliferation in the adult subventricular zone. Nat. Neurosci. 3, 1091-1097. 10.1038/80606 [DOI] [PubMed] [Google Scholar]
- Del Bigio M. R. (1995). The ependyma: a protective barrier between brain and cerebrospinal fluid. Glia 14, 1-13. 10.1002/glia.440140102 [DOI] [PubMed] [Google Scholar]
- Del Bigio M. R. (2010). Ependymal cells: biology and pathology. Acta Neuropathol. 119, 55-73. 10.1007/s00401-009-0624-y [DOI] [PubMed] [Google Scholar]
- Del Bigio M. R. (2014). Neuropathology of human hydrocephalus. In Adult Hydrocephalus (ed. Rigamonti D.), pp. 14-27. Cambridge University Press. [Google Scholar]
- Dennis C. V., Suh L. S., Rodriguez M. L., Kril J. J. and Sutherland G. T. (2016). Human adult neurogenesis across the ages: an immunohistochemical study. Neuropathol. Appl. Neurobiol. 42, 621-638. 10.1111/nan.12337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F., Garcia-Verdugo J. M. and Alvarez-Buylla A. (1997). Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J. Neurosci. 17, 5046-5061. 10.1523/JNEUROSCI.17-13-05046.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F., Garcia-Verdugo J. M. and Alvarez-Buylla A. (1999). Regeneration of a germinal layer in the adult mammalian brain. Proc. Natl. Acad. Sci. USA 96, 11619-11624. 10.1073/pnas.96.20.11619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Encinas J. M., Michurina T. V., Peunova N., Park J.-H., Tordo J., Peterson D. A., Fishell G., Koulakov A. and Enikolopov G. (2011). Division-coupled astrocytic differentiation and age-related depletion of neural stem cells in the adult hippocampus. Cell Stem Cell 8, 566-579. 10.1016/j.stem.2011.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson P. S., Perfilieva E., Björk-Eriksson T., Alborn A.-M., Nordborg C., Peterson D. A. and Gage F. H. (1998). Neurogenesis in the adult human hippocampus. Nat. Med. 4, 1313-1317. 10.1038/3305 [DOI] [PubMed] [Google Scholar]
- Hansen D. V., Lui J. H., Parker P. R. L. and Kriegstein A. R. (2010). Neurogenic radial glia in the outer subventricular zone of human neocortex. Nature 464, 554-561. 10.1038/nature08845 [DOI] [PubMed] [Google Scholar]
- Hormoz S. (2013). Stem cell population asymmetry can reduce rate of replicative aging. J. Theor. Biol. 331, 19-27. 10.1016/j.jtbi.2013.04.018 [DOI] [PubMed] [Google Scholar]
- Jacquet B. V., Salinas-Mondragon R., Liang H., Therit B., Buie J. D., Dykstra M., Campbell K., Ostrowski L. E., Brody S. L. and Ghashghaei H. T. (2009). FoxJ1-dependent gene expression is required for differentiation of radial glia into ependymal cells and a subset of astrocytes in the postnatal brain. Development 136, 4021-4031. 10.1242/dev.041129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanson C., Stopa E., McMillan P., Roth D., Funk J. and Krinke G. (2011). The distributional nexus of choroid plexus to cerebrospinal fluid, ependyma and brain: toxicologic/pathologic phenomena, periventricular destabilization, and lesion spread. Toxicol. Pathol. 39, 186-212. 10.1177/0192623310394214 [DOI] [PubMed] [Google Scholar]
- Kinoshita Y., Okudera T., Tsuru E. and Yokota A. (2001). Volumetric analysis of the germinal matrix and lateral ventricles performed using MR images of postmortem fetuses. AJNR Am. J. Neuroradiol. 22, 382-388. [PMC free article] [PubMed] [Google Scholar]
- Knickmeyer R. C., Gouttard S., Kang C., Evans D., Wilber K., Smith J. K., Hamer R. M., Lin W., Gerig G. and Gilmore J. H. (2008). A structural MRI study of human brain development from birth to 2 years. J. Neurosci. 28, 12176-12182. 10.1523/JNEUROSCI.3479-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoth R., Singec I., Ditter M., Pantazis G., Capetian P., Meyer R. P., Horvat V., Volk B. and Kempermann G. (2010). Murine features of neurogenesis in the human hippocampus across the lifespan from 0 to 100 years. PLoS ONE 5, e8809 10.1371/journal.pone.0008809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegstein A. and Alvarez-Buylla A. (2009). The glial nature of embryonic and adult neural stem cells. Annu. Rev. Neurosci. 32, 149-184. 10.1146/annurev.neuro.051508.135600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegstein A., Noctor S. and Martínez-Cerdeño V. (2006). Patterns of neural stem and progenitor cell division may underlie evolutionary cortical expansion. Nat. Rev. Neurosci. 7, 883-890. 10.1038/nrn2008 [DOI] [PubMed] [Google Scholar]
- Kyrousi C., Arbi M., Pilz G.-A., Pefani D.-E., Lalioti M.-E., Ninkovic J., Gotz M., Lygerou Z. and Taraviras S. (2015). Mcidas and GemC1 are key regulators for the generation of multiciliated ependymal cells in the adult neurogenic niche. Development 142, 3661-3674. 10.1242/dev.126342 [DOI] [PubMed] [Google Scholar]
- LaMonica B. E., Lui J. H., Wang X. and Kriegstein A. R. (2012). OSVZ progenitors in the human cortex: an updated perspective on neurodevelopmental disease. Curr. Opin. Neurobiol. 22, 747-753. 10.1016/j.conb.2012.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh S. R. (2004). Brain growth, life history, and cognition in primate and human evolution. Am. J. Primatol. 62, 139-164. 10.1002/ajp.20012 [DOI] [PubMed] [Google Scholar]
- Lledo P.-M., Merkle F. T. and Alvarez-Buylla A. (2008). Origin and function of olfactory bulb interneuron diversity. Trends Neurosci. 31, 392-400. 10.1016/j.tins.2008.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui J. H., Hansen D. V. and Kriegstein A. R. (2011). Development and evolution of the human neocortex. Cell 146, 18-36. 10.1016/j.cell.2011.06.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J., Daniels S. B., Lennington J. B., Notti R. Q. and Conover J. C. (2006). The aging neurogenic subventricular zone. Aging Cell 5, 139-152. 10.1111/j.1474-9726.2006.00197.x [DOI] [PubMed] [Google Scholar]
- Malik S., Vinukonda G., Vose L. R., Diamond D., Bhimavarapu B. B. R., Hu F., Zia M. T., Hevner R., Zecevic N. and Ballabh P. (2013). Neurogenesis continues in the third trimester of pregnancy and is suppressed by premature birth. J. Neurosci. 33, 411-423. 10.1523/JNEUROSCI.4445-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslov A. Y., Barone T. A., Plunkett R. J. and Pruitt S. C. (2004). Neural stem cell detection, characterization, and age-related changes in the subventricular zone of mice. J. Neurosci. 24, 1726-1733. 10.1523/JNEUROSCI.4608-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkle F. T., Tramontin A. D., Garcia-Verdugo J. M. and Alvarez-Buylla A. (2004). Radial glia give rise to adult neural stem cells in the subventricular zone. Proc. Natl. Acad. Sci. USA 101, 17528-17532. 10.1073/pnas.0407893101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzadeh Z., Merkle F. T., Soriano-Navarro M., Garcia-Verdugo J. M. and Alvarez-Buylla A. (2008). Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell Stem Cell 3, 265-278. 10.1016/j.stem.2008.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzadeh Z., Doetsch F., Sawamoto K., Wichterle H. and Alvarez-Buylla A. (2010a). The subventricular zone en-face: wholemount staining and ependymal flow. J. Vis. Exp. 39, e1938 10.3791/1938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzadeh Z., Han Y.-G., Soriano-Navarro M., Garcia-Verdugo J. M. and Alvarez-Buylla A. (2010b). Cilia organize ependymal planar polarity. J. Neurosci. 30, 2600-2610. 10.1523/JNEUROSCI.3744-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obernier K., Cebrian-Silla A., Thomson M., Parraguez J. I., Anderson R., Guinto C., Rodas Rodriguez J., Garcia-Verdugo J. M. and Alvarez-Buylla A. (2018). Adult neurogenesis is sustained by symmetric self-renewal and differentiation. Cell Stem Cell 22, 221-234.e228. 10.1016/j.stem.2018.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paez-Gonzalez P., Abdi K., Luciano D., Liu Y., Soriano-Navarro M., Rawlins E., Bennett V., Garcia-Verdugo J. M. and Kuo C. T. (2011). Ank3-dependent SVZ niche assembly is required for the continued production of new neurons. Neuron 71, 61-75. 10.1016/j.neuron.2011.05.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredes M. F., James D., Gil-Perotin S., Kim H., Cotter J. A., Ng C., Sandoval K., Rowitch D. H., Xu D., McQuillen P. S. et al. (2016a). Extensive migration of young neurons into the infant human frontal lobe. Science 354, aaf7073 10.1126/science.aaf7073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredes M. F., Sorrells S. F., Garcia-Verdugo J. M. and Alvarez-Buylla A. (2016b). Brain size and limits to adult neurogenesis. J. Comp. Neurol. 524, 646-664. 10.1002/cne.23896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peretto P., Merighi A., Fasolo A. and Bonfanti L. (1999). The subependymal layer in rodents: a site of structural plasticity and cell migration in the adult mammalian brain. Brain Res. Bull. 49, 221-243. 10.1016/S0361-9230(99)00037-4 [DOI] [PubMed] [Google Scholar]
- Quiñones-Hinojosa A., Sanai N., Soriano-Navarro M., Gonzalez-Perez O., Mirzadeh Z., Gil-Perotin S., Romero-Rodriguez R., Berger M. S., Garcia-Verdugo J. M. and Alvarez-Buylla A. (2006). Cellular composition and cytoarchitecture of the adult human subventricular zone: a niche of neural stem cells. J. Comp. Neurol. 494, 415-434. 10.1002/cne.20798 [DOI] [PubMed] [Google Scholar]
- Roales-Buján R., Páez P., Guerra M., Rodríguez S., Vío K., Ho-Plagaro A., García-Bonilla M., Rodríguez-Pérez L.-M., Domínguez-Pinos M.-D., Rodríguez E.-M. et al. (2012). Astrocytes acquire morphological and functional characteristics of ependymal cells following disruption of ependyma in hydrocephalus. Acta Neuropathol. 124, 531-546. 10.1007/s00401-012-0992-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai T., Hirata S., Fuwa K., Sugama K., Kusunoki K., Makishima H., Eguchi T., Yamada S., Ogihara N. and Takeshita H. (2012). Fetal brain development in chimpanzees versus humans. Curr. Biol. 22, R791-R792. 10.1016/j.cub.2012.06.062 [DOI] [PubMed] [Google Scholar]
- Sanai N., Tramontin A. D., Quiñones-Hinojosa A., Barbaro N. M., Gupta N., Kunwar S., Lawton M. T., McDermott M. W., Parsa A. T., Manuel-García Verdugo J. et al. (2004). Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature 427, 740-744. 10.1038/nature02301 [DOI] [PubMed] [Google Scholar]
- Sanai N., Nguyen T., Ihrie R. A., Mirzadeh Z., Tsai H.-H., Wong M., Gupta N., Berger M. S., Huang E., Garcia-Verdugo J.-M. et al. (2011). Corridors of migrating neurons in the human brain and their decline during infancy. Nature 478, 382-386. 10.1038/nature10487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahriyari L. and Komarova N. L. (2013). Symmetric vs. asymmetric stem cell divisions: an adaptation against cancer? PLoS ONE 8, e76195 10.1371/journal.pone.0076195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shook B. A., Manz D. H., Peters J. J., Kang S. and Conover J. C. (2012). Spatiotemporal changes to the subventricular zone stem cell pool through aging. J. Neurosci. 32, 6947-6956. 10.1523/JNEUROSCI.5987-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shook B. A., Lennington J. B., Acabchuk R. L., Halling M., Sun Y., Peters J., Wu Q., Mahajan A., Fellows D. W. and Conover J. C. (2014). Ventriculomegaly associated with ependymal gliosis and declines in barrier integrity in the aging human and mouse brain. Aging Cell 13, 340-350. 10.1111/acel.12184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snippert H. J., van der Flier L. G., Sato T., van Es J. H., van den Born M., Kroon-Veenboer C., Barker N., Klein A. M., van Rheenen J., Simons B. D. et al. (2010). Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell 143, 134-144. 10.1016/j.cell.2010.09.016 [DOI] [PubMed] [Google Scholar]
- Sorrells S. F., Paredes M. F., Cebrian-Silla A., Sandoval K., Qi D., Kelley K. W., James D., Mayer S., Chang J., Auguste K. I. et al. (2018). Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature 555, 377-381. 10.1038/nature25975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spassky N., Merkle F. T., Flames N., Tramontin A. D., Garcia-Verdugo J. M. and Alvarez-Buylla A. (2005). Adult ependymal cells are postmitotic and are derived from radial glial cells during embryogenesis. J. Neurosci. 25, 10-18. 10.1523/JNEUROSCI.1108-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spradling A., Drummond-Barbosa D. and Kai T. (2001). Stem cells find their niche. Nature 414, 98-104. 10.1038/35102160 [DOI] [PubMed] [Google Scholar]
- Todd K. L., Brighton T., Norton E. S., Schick S., Elkins W., Pletnikova O., Fortinsky R. H., Troncoso J. C., Molfese P. J., Resnick S. M. et al. (2017). Ventricular and Periventricular Anomalies in the Aging and Cognitively Impaired Brain. Front. Aging Neurosci. 9, 445 10.3389/fnagi.2017.00445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Liu F., Liu Y.-Y., Zhao C.-H., You Y., Wang L., Zhang J., Wei B., Ma T., Zhang Q. et al. (2011). Identification and characterization of neuroblasts in the subventricular zone and rostral migratory stream of the adult human brain. Cell Res. 21, 1534-1550. 10.1038/cr.2011.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., You Y., Qi D., Zhou X., Wang L., Wei S., Zhang Z., Huang W., Liu Z., Liu F. et al. (2014). Human and monkey striatal interneurons are derived from the medial ganglionic eminence but not from the adult subventricular zone. J. Neurosci. 34, 10906-10923. 10.1523/JNEUROSCI.1758-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yushkevich P. A., Piven J., Hazlett H. C., Smith R. G., Ho S., Gee J. C. and Gerig G. (2006). User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage 31, 1116-1128. 10.1016/j.neuroimage.2006.01.015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.