ABSTRACT
Nuclear RNA interference provides a unique approach to the study of RNA-mediated transgenerational epigenetic inheritance. A paradox in the field is that expression of target loci is necessary for the initiation and maintenance of their silencing. How expression and repression are coordinated during animal development is poorly understood. To resolve this gap, we took imaging, deep-sequencing and genetic approaches towards delineating the developmental regulation and subcellular localization of RNA transcripts of two representative endogenous targets, the LTR retrotransposons Cer3 and Cer8. By examining wild-type worms and a collection of mutant strains, we found that the expression and silencing cycle of Cer3 and Cer8 is coupled with embryonic and germline development. Strikingly, endogenous targets exhibit a hallmark of nuclear enrichment of their RNA transcripts. In addition, germline and somatic repressions of Cer3 have different genetic requirements for three heterochromatin enzymes, MET-2, SET-25 and SET-32, in conjunction with the nuclear Argonaute protein HRDE-1. These results provide the first comprehensive cellular and developmental characterization of nuclear RNAi activities throughout the animal reproductive cycle.
KEY WORDS: Epigenetic inheritance, Histone methyltransferase, Nuclear RNA localization, Nuclear RNAi, smFISH, Transposon
Highlighted Article: A detailed examination of the expression-and-silencing cycle of nuclear RNAi-targeted retrotransposon transcripts in C. elegans reveals tight coupling with germline development and a striking nuclear enrichment of these transcripts.
INTRODUCTION
Besides the classical RNA interference (RNAi) pathway, in which a target gene is silenced by mRNA degradation, small RNA can also guide heterochromatin formation and transcriptional silencing through the nuclear RNAi pathway in a diverse set of eukaryotic organisms (for reviews, see Castel and Martienssen, 2013; Holoch and Moazed, 2015). In Caenorhabditis elegans, nuclear RNAi can be conveniently triggered by exogenous double-strand RNA (dsRNA), resulting in highly specific heterochromatic responses (H3K9me3 and H3K27me3) at a target gene (Gu et al., 2012; Mao et al., 2015). Interestingly, both the transcriptional repression and heterochromatin marks at a germline-expressed target gene can persist for multiple generations after the dsRNA exposure has been removed (Gu et al., 2012; Mao et al., 2015). These features, together with the ∼3-day reproductive cycle (23-25°C) and the powerful genetics, make C. elegans a highly tractable system in which to study RNA-mediated chromatin regulation and transgenerational epigenetics in animals.
In order to establish a framework in which to study the native functions and mechanisms of nuclear RNAi in C. elegans, we previously identified the endogenous targets of the germline nuclear RNAi pathway. These endogenous targets were defined by the presence of abundant 22G-class small interfering RNAs (siRNAs), and the H3K9me3 or the transcription repressive state that is dependent on the germline nuclear AGO protein HRDE-1 (heritable RNAi defective 1; also known as WAGO-9). The most prominent endogenous targets include LTR retrotransposons in the C. elegans genome (Ni et al., 2014, 2016). These LTR retrotransposons have strong transcription potentials, as shown by their high RNA polymerase II (Pol II) occupancy and pre-mRNA transcripts levels in the germline nuclear RNA-defective mutants (Ni et al., 2014, 2016). In wild-type animals, the transcription activities of these LTR retrotransposons are repressed by nuclear RNAi to an extremely low level (Ni et al., 2014, 2016). Despite the low expression, transcription of these endogenous targets is expected to play an essential role for their silencing because (1) the RNA transcripts are needed as templates for the siRNA biogenesis (Sijen et al., 2001; Smardon et al., 2000), and (2) the nascent transcripts also function as the scaffold for binding of siRNA-guided silencing complexes (Guang et al., 2010, 2008). These features form an intriguing paradox: the silencing of nuclear RNAi targets is dependent on the expression of the same loci. In plants and Schizosaccharomyces pombe, this paradox is solved by coupling the expression of the nuclear RNAi target loci to distinct cell type or cell cycle stages (Chen et al., 2008; Kloc et al., 2008; Slotkin et al., 2009). Some small RNA-targeted and epigenetically silenced transposable elements (TEs) are transiently expressed at specific developmental stages and in specific cells during normal development of wild-type plants and animals (Crichton et al., 2014; Muotri et al., 2007; Rebollo et al., 2012), a phenomenon referred to as developmental relaxation of TE silencing (DRTS) (Martínez and Slotkin, 2012). DRTS is crucial for the maintenance of TE silencing by providing siRNAs to reinforce TE silencing (Ibarra et al., 2012; Schoft et al., 2009; Slotkin et al., 2009); in addition, it also benefits the host by providing innate immunity against similar TEs or viruses (Blanco-Melo et al., 2017; Grow et al., 2015).
When and where the endogenous targets of nuclear RNAi are expressed in the wild-type C. elegans, as well as in nuclear RNAi-defective mutant strains, is virtually unknown at the cellular level. In addition, although many nrde and hrde genes (named after the nuclear RNAi- and heritable RNAi-defective phenotypes) have been identified in recent years, the dynamics of this pathway at the subcellular level, as well as in the context of animal development is not well understood. We are particularly interested in determining how the two seemly conflicting activities, HRDE-1-dependent transcriptional repression and expression of the target transcripts, are coordinated during the reproductive cycle. C. elegans is well suited for this owing to the transparent body, a well-organized developmental pattern, and a large collection of mutant strains.
We and others recently determined that H3K9me3 enrichment at HRDE-1 targets requires three histone methyltransferases (HMTs): MET-2, SET-25 and SET-32 (Ashe et al., 2012; Kalinava et al., 2017; Mao et al., 2015; Spracklin et al., 2017). Combined genetic and genomic analyses revealed a highly complex relationship between H3K9 HMTs and HRDE-1 with respect to their roles in nuclear RNAi. Mutant animals that lack H3K9me3 as a result of combined mutations in all three H3K9 HMT genes do not exhibit any activation of the endogenous targets of HRDE-1, indicating that HRDE-1-dependent transcriptional repression can occur in an H3K9me3-independent manner. On the other hand, mutant animals with combined loss of H3K9 HMTs and HRDE-1 have much higher transcription activities of the endogenous targets than do hrde-1 mutant animals (Kalinava et al., 2018 preprint). This indicates that H3K9me3 does play a repressive role at the endogenous targets, at least when certain HRDE-1-dependent functions are compromised. However, when and where the H3K9me3-dependent repression occurs during animal development is unknown.
Here, by taking the single-molecule fluorescence in situ hybridization (smFISH) approach we delineated the developmental expression of the Cer3 and Cer8 RNA transcripts at single-cell resolution in wild-type C. elegans and a variety of mutant strains. We found that both relaxation (observed in wild type) and repression (inferred by using various silencing defective mutants) of the endogenous targets are tightly linked to animal development. Somatic and germline silencing have distinct requirements for H3K9 HMTs. In addition, we identified nuclear enrichment as a hallmark for nuclear RNAi-targeted endogenous transcripts.
RESULTS
Cer3 and Cer8 LTR retrotransposons as model systems in which to study nuclear RNAi
LTR retrotransposons belong to a major group of retrotransposons that are present in fungi, plant and animal genomes. They have two identical long terminal repeats (LTRs) flanking internal open reading frames that encode structural proteins and enzymes (GAG, POL, IN) that are necessary for replication and transposition (Curcio et al., 2015; Sandmeyer et al., 2015). LTR retrotransposons share a similar lifecycle and sequence structure with retroviruses, with the exception that LTR retrotransposons lack a functional env gene, which encodes the envelope protein necessary for the intercellular trafficking of the virus-like particles (Malik et al., 2000). As a result, replication and retrotransposition of LTR retrotransposons are primarily intracellular and they are also known as endogenous retroviruses. They are the major endogenous silencing targets for nuclear RNAi in C. elegans (Ni et al., 2014, 2016).
The gypsy-family LTR retrotransposon Cer3 was chosen as the primary target in this study for the following reasons. (1) Cer3 is a prominent endogenous target of the germline nuclear RNAi pathway. It is transcriptionally repressed in a HRDE-1-dependent manner and exhibits a high level of H3K9me3 (Ni et al., 2014; Ni et al., 2016). Cer3 is also associated with abundant endogenous siRNAs (endo-siRNAs), which are bound by HRDE-1 (Fig. 1B and Fig. S1). (2) There is only one allele of Cer3 in the haploid genome of the N2 laboratory strain (∼8.7 kb, ChrIV:912948-921667, WS190) (Bowen and McDonald, 1999; Ganko et al., 2001), which makes it highly tractable for molecular and imaging analyses. (3) The 5′ and 3′ LTRs (424 bases) are identical, suggesting that its entry into the C. elegans genome is sufficiently recent and the original cis regulatory elements are likely to be intact (Bowen and McDonald, 1999; Ganko et al., 2001). (4) A Cer3-deleted wild isolate (JU533) is available to serve as a negative control for smFISH experiment (Fig. 2B) (Barriere and Felix, 2005).
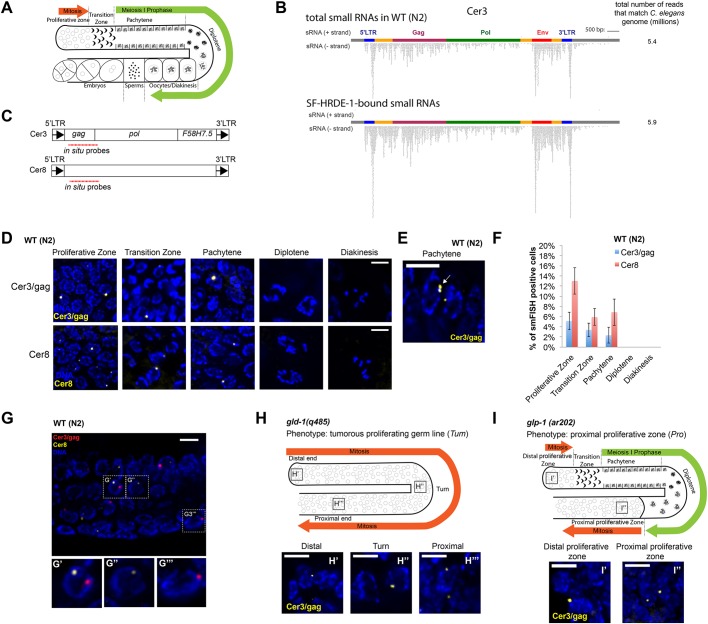
Fig. 1.
smFISH analysis of Cer3 and Cer8 RNA transcripts in WT adult germline. (A) Schematic of the gonad in C. elegans hermaphrodite adult. (B) Total siRNAs and HRDE-1-co-IP siRNAs at Cer3. Each dot represents a sequenced siRNA. Sense and antisense siRNAs were plotted separately as indicated. 5′- and 3′-LTR siRNAs cannot be distinguished due to the identical DNA sequences of the two LTRs. (C) Design of Cer3/gag and Cer8 smFISH probe sets. (D) Cer3/gag and Cer8 smFISH signals at different stages of adult germ cells. Wild-type (N2) hermaphrodite animals (23°C) were used (also for panels E-G). (E) An example of a pair of adjacent Cer3/gag smFISH dots (white arrow) in a pachytene nucleus. (F) Percentage of Cer3/gag or Cer8 smFISH-positive nuclei observed at different developmental stages of adult germ cells. Five biological replicates. Mean±s.d. is plotted. (G-G‴) Two-color smFISH analysis of Cer3/gag and Cer8 at the pachytene stage. G′-G‴ show magnifications of the boxed areas in G. (H-H‴) Schematic of the gld-1(q485) tumorous germline phenotype (H) and Cer3/gag smFISH in different regions of the gld-1(q485) gonad (H′-H‴). (I-I″) Schematic of the glp-1(ar202) proximal proliferative zone (I) and Cer3/gag smFISH in different regions of glp-1(ar202) gonad (25°C) (I′,I″). See Fig. S4 for smFISH profiles of the entire gonads. Scale bars: 5 μm.
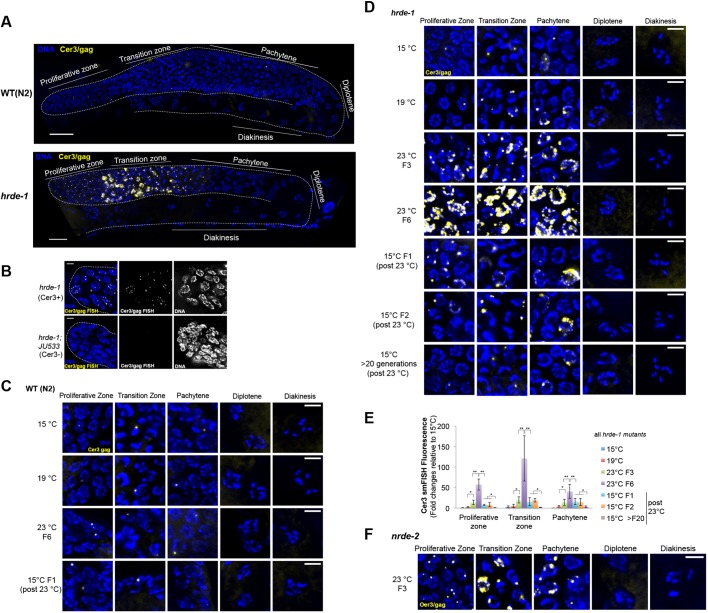
Fig. 2.
Cer3 smFISH analysis in germline nuclear RNAi-defective mutants and the effect of temperature on Cer3 expression. (A) Cer3/gag smFISH in WT (N2) and hrde-1 mutant adult (23°C F3). Gonads are outlined. (B) Cer3/gag smFISH signal is absent in a Cer3-lacking C. elegans strain (JU533). JU533 animals with WT or mutant hrde-1 (23°C F3) were used for this analysis. The proliferative zones at the distal gonad tips are shown. (C) Cer3/gag smFISH at different germline developmental stages of WT (N2) animals that were cultured at 15°C, 19°C, 23°C and 15°C F1 (the first generation after culturing at 23°C for six generations). (D) Cer3/gag smFISH at different germline developmental stages of hrde-1 adults that were cultured at 15°C, 19°C, 23°C F3/F6 (F3 and F6 at 23°C after shifting from 15°C) and then back to 15°C (F1, F2 and >20 generations at 15°C after shifting from six generations of growth at 23°C). (E) Quantification of the Cer3/gag smFISH fluorescence shown in D. Three biological replicates. hrde-1 15°C (no heat-stress experience) is set to 1. Mean±s.d. is plotted. *P<0.05, **P<0.005 (t-test). (F) Cer3/gag smFISH for nrde-2 mutant adult animals cultured at 23°C for three generations. Scale bars: 20 μm (A); 5 μm (B-D,F).
The second exemplary target used in this study is the Pao-family LTR retrotransposon Cer8. There are two full-length Cer8 copies in C. elegans genome, with one having a Tc1 DNA transposon insertion in its internal sequence. The two copies are both endogenous nuclear RNAi targets (Ni et al., 2014), and have non-homologous sequence around the Tc1 insertion site. We designed smFISH probes that are specific to the unique sequence in the Tc1-lacking Cer8 (ChrV: 5179680-5191222, WS190). The 5′ and 3′ LTRs (574 bases) of Cer8 are 98.8% identical, suggesting a relatively recent transposition (Bowen and McDonald, 1999; Ganko et al., 2001). No Cer8 deletion strain is currently available. The difference in the Cer8 smFISH signals between the wild-type (WT) and hrde-1 mutant animals (see ‘HRDE-1-dependent transcriptional silencing occurs at specific germline developmental stages’ section) was similar to that observed in our previous RNA-seq and Pol II-ChIP analyses (Ni et al., 2014), suggesting that Cer8 smFISH probes are specific.
Cer3 is transcribed in a small subset of germline nuclei in WT adults
C. elegans is a highly tractable model organism in which to study germline nuclear RNAi at single-cell resolution and in the context of animal development. C. elegans germline development begins with the birth of the founder primordial germ cell (PGC) after the initial four embryonic cell divisions (Kimble and Crittenden, 2005). The founder PGC divides only once to produce two daughter PGCs during embryogenesis. Starting from the end of the first larval (L1) stage, PGCs undergo continuous proliferation. When a hermaphrodite animal reaches the L3 stage, germ cells at the proximal region of the gonad enter meiosis and spermatogenesis, whereas germ cells at the distal region of the gonad continue to divide mitotically. Such polarity of the germline is maintained in L4 and adult hermaphrodite animals, except that the proximal germ cells switch to oogenesis. An adult hermaphrodite has two symmetric U-shaped gonad arms. Different developmental stages of germ cells – proliferation, transition zone, pachytene, diplotene and diakinesis – are temporally and spatially organized along the distal-to-proximal axis of each gonad arm, and can be distinguished by their locations in the gonad, cellular morphology and chromosome morphology (Fig. 1A).
To characterize the expression pattern of Cer3 RNA transcripts at single-cell resolution, we performed smFISH (Ji and van Oudenaarden, 2012; Raj et al., 2008) against the Cer3 gag gene (Fig. 1C). To evaluate the experimental design, we initially used hrde-1 mutant animals [Cer3(+)] and a Cer3-deleted wild isolate JU533 [Cer3(–)] (Barriere and Felix, 2005) for the smFISH analysis. We detected robust signals in the germline, but not the somatic cells, of hrde-1;Cer3(+) adults (Fig. 2A,B; see ‘HRDE-1-dependent transcriptional silencing occurs at specific germline developmental stages’ section for a more detailed characterization) and no signal in the hrde-1;Cer3(−) animals (Fig. 2B). Therefore, our Cer3 smFISH signals are specific to the Cer3 RNA transcripts. We will refer to cells that are positive for the Cer3 smFISH signals as Cer3+ cells hereafter.
In WT adult hermaphrodite animals (23°C), the Cer3 smFISH signals were restricted to a small fraction of germ cell nuclei and absent in any somatic cells (n=10 animals). The distribution of the Cer3+ cells in the adult germline is not uniform. They were present in approximately 5.1%, 3.4% and 2.3% of the nuclei in the proliferative zone, transition zone and pachytene zone, respectively, and were absent in the post-pachytene germ nuclei, including diplotene and oocytes (n=5 gonads) (Fig. 1D,F). Such polarization was also present in the male germline (Fig. S2). In both male and female germlines, the Cer3 smFISH signal was absent in the shared cytoplasm core of the gonad.
There was usually just one smFISH ‘dot’ in a Cer3+ nucleus, and occasionally a pair of adjacent dots in the pachytene nuclei (Fig. 1E). We never observed nuclei with three or more dots. These observations suggest that the Cer3 smFISH signals in WT animals correspond to the nascent Cer3 RNAs at the transcription sites.
Co-smFISH analysis of two different endogenous targets (Cer3 and Cer8) of germline nuclear RNAi
We also examined a second endogenous target of the germline nuclear RNAi pathway, the Pao-family LTR retrotransposon Cer8. Although Cer3 (gypsy family) and Cer8 (Pao family) belong to different clades of LTR retrotransposons (Bowen and McDonald, 1999; Ganko et al., 2001), the Cer8 smFISH signals were highly similar to Cer3 in the overall morphology and abundance of smFISH dots (one or two per Cer8+ cell), and the tissue specificity (Fig. 1C-F). Cer8+ cells were approximately 2- to 3-fold more abundant than the number of Cer3+ cells (Fig. 1F).
We then investigated how often Cer3 and Cer8 are expressed in the same cells. We performed two-color smFISH against Cer3 and Cer8 transcripts in WT adult animals (Fig. 1G). We found that Cer3+Cer8+ germ cells were rare (1.2%, 0.5% and 0.9% of the germ nuclei in proliferative zone, transition zone and pachytene, respectively; Fisher exact test: P>0.05 at each zone). The null hypothesis of the test is that Cer3 and Cer8 expressions are independent of each other in any given cell. Because P>0.05, the null hypothesis cannot be rejected. This analysis supports that the expressions of Cer3 and Cer8 are independent of each other. In the few double-positive cells, Cer3 and Cer8 smFISH dots did not colocalize (Fig. 1G).
Based on these results, we propose the following model. Cer3 and Cer8 LTR retrotransposons are not fully silenced in WT animals. Developmental relaxation of Cer3 and Cer8 occurs in at least a subset of proliferating germ cells and early meiotic cells. Cer3 and Cer8 RNA transcripts do not accumulate beyond one or two foci in the nucleus, likely the transcription sites. In addition, the transcripts do not persist in late meiotic cells, suggesting that Cer3 and Cer8 are subject to nuclear degradation. A previous study (Knutson et al., 2017) examined several somatic-specific transcripts by smFISH in the WT germline, none of which is similar to the Cer3 or Cer8 smFISH profile, suggesting that the observed smFISH profile for Cer3 and Cer8 is not a general feature of inactive genes in the germline. Cer3 or Cer8 are completely silenced in adult somatic cells. Consistent with this germline-specific expression, our analysis of published sRNA-seq data (Gent et al., 2010) shows that the Cer3 and Cer8 siRNAs, as well as siRNAs of other endogenous targets of HRDE-1, are enriched in WT and fem-1 mutant (23°C, sterile as a result of defective spermatogenesis) adults over the glp-1 mutant (23°C, largely depleted for germ cells) adults (Fig. S3), indicating that these siRNAs are germline specific.
Cer3 expression is linked to mitotic proliferation
To explore further whether the developmental relaxation of Cer3 is linked to mitotic proliferation, we performed Cer3/gag smFISH in the gld-1(q485) mutant strain, in which germ cells are defective in the mitotic-to-meiotic transition and all germ cells undergo mitotic cycling. This results in a tumorous germline that contains predominantly the mitotic nuclei throughout the gonad (Francis et al., 1995a,b). We found that the small number of Cer3+ nuclei were distributed throughout the entire length of the gld-1 tumorous gonad (Fig. 1H and Fig. S4). We also examined a second germline mutant strain, glp-1(ar202), in which proximal germ cells exit meiotic differentiation and re-enter mitotic cycling at the restrictive temperature (25°C) (Pepper et al., 2003). We found that Cer3+ nuclei in this mutant were again correlated with the proliferative cells: they were present in the proximal end of gonad arms where meiotic prophase germ cells switch back to the mitotic fate (Fig. 1I and Fig. S4), in addition to the distal germ nuclei. We did not observe any Cer3+ nuclei at the diplotene stage in glp-1(ar202). These results, together with observations in WT animals (Fig. 1D and Fig. 2A), indicate that Cer3 expression is developmentally regulated in the germline: Cer3 transcription is partially activated in mitotic proliferating cells and cells in the early stages of meiotic prophase I till pachytene, but completely repressed in post-pachytene meiotic cells.
HRDE-1-dependent transcriptional silencing occurs at specific germline developmental stages
HRDE-1 protein is localized in all germ cell nuclei in a hermaphrodite adult, except sperms (Ashe et al., 2012; Buckley et al., 2012; Shirayama et al., 2012) (Fig. S1). However, it was unknown when and where HRDE-1-mediated silencing occurs. Does HRDE-1-dependent silencing occur in all HRDE-1-expressing germ cells? Does HRDE-1-dependent silencing occur in non-HRDE-1-expressing cells? To answer these questions, we performed Cer3 smFISH analysis in hrde-1 mutant adults (cultured at 23°C for three generations after shifting from 15°C, referred to as ‘23°C F3’). Consistent with our previous RNA-seq analysis (Ni et al., 2014, 2016), Cer3 smFISH signals were dramatically increased in hrde-1 mutants compared with WT (Fig. 2A). Strikingly, the smFISH signals in hrde-1 mutant adult gonads were exclusively localized in the nuclei (Fig. 2D and Fig. 3A). No Cer3 smFISH signals were detected in the shared cytoplasmic core (Fig. 3A and Fig. S5A). As a control experiment, we detected abundant mRNA of the germline-specific gene oma-1 in the cytoplasmic core by smFISH (Fig. 3A and Fig. S5B).
Fig. 3.
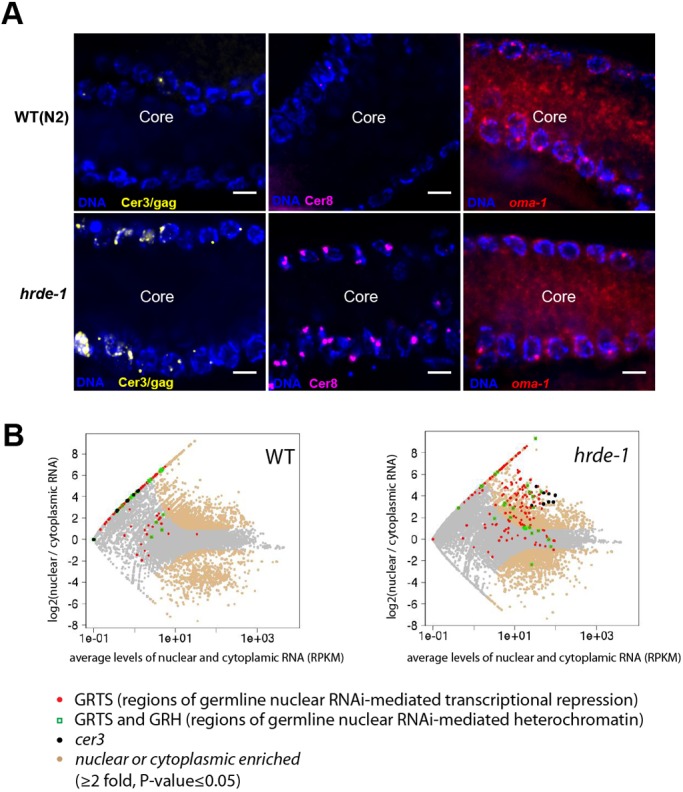
Transcripts of endogenous nuclear RNAi targets are nuclear enriched. (A) smFISH against Cer3/gag, Cer8 and oma-1 in WT (N2) and hrde-1 (23°C F3) adult pachytene germlines. Images show a representative longitudinal section through the gonad core. Note that the Cer3/gag and Cer8 smFISH signals were only present in the nuclei, and absent in the cytoplasm core. The same images without background reduction are in Fig. S5. oma-1 smFISH of an oma-1 deletion mutant strain is shown in Fig. S5 as well. (B) RNA-seq analysis of nuclear and cytoplasmic RNA samples from WT (N2) and hrde-1 mutant adults (23°C F3-4). Whole animals were used, allowing both somatic and germline RNAs to be captured in this analysis. The average nuclear and cytoplasmic RNA expression levels (RPKM, x-axis) and the log2 ratios of nuclear to cytoplasmic RNA level (y-axis) are plotted for all 1-kb regions in the genome. Regions with at least 2-fold difference between nuclear and cytoplasm RNA (P≤0.05) are beige.
Most of the distal germ nuclei (proliferative zone, transition zone and early pachytene nuclei) in hrde-1 mutants are positive for Cer3 smFISH signals (Fig. 2A,D). In addition, the average smFISH signal in Cer3+ nuclei was much stronger in hrde-1 mutants than in WT. The strongest signals appeared in the transition zone, where larger dots or irregular patches of Cer3/gag signals were frequently observed.
Despite the strong Cer3 RNA expression in the distal regions of the gonad, Cer3 smFISH signals were absent in the late pachytene, diplotene and diakinesis stages in hrde-1 mutants. No Cer3 RNA transcript was detected in hrde-1 adult somatic cells (data not shown). The same profile was observed for Cer8 (Fig. S6A,B). We also examined Cer3 in a second germline nuclear RNAi defective strain that has the nrde-2 loss-of-function mutation (Guang et al., 2010). We previously found that endogenous HRDE-1 targets are also de-repressed in the nrde-2 mutants (Ni et al., 2014). Here, we found that the nrde-2 mutant strain exhibited the same Cer3 smFISH profile in the gonad as that observed in the hrde-1 mutant (Fig. 2F). These results indicate that the germline nuclear RNAi pathway is essential for repression of Cer3 and Cer8 in the distal germline regions (proliferative zone, transition zone and early pachytene) in adult germline tissue, but is dispensable in the proximal germline regions (late pachytene, diplotene and diakinesis). A germline nuclear RNAi-independent mechanism is likely the cause of the absence of Cer3 and Cer8 transcripts in the proximal germline regions.
Heat stress-induced transgenerational accumulation of Cer3 transcripts
hrde-1 and other nuclear RNAi defective mutants display a mortal germline phenotype (Mrt) (Buckley et al., 2012). These mutants are fertile at the permissive temperature (15°C-20°C), but when cultured at a higher temperature (23°C-25°C), the population progressively lose their fertility over multiple generations and eventually become sterile. We previously found that heat stress enhances the transcription of a subset of endogenous targets (including Cer3) in hrde-1 mutant animals (Ni et al., 2016) (Fig. S7A). To determine the cellular origin of the heat-induced activation, we performed Cer3 smFISH using hrde-1 mutants that had been cultured at different temperatures: two permissive temperatures (15°C and 19°C) and a restrictive temperature (23°C). To capture the transgenerational effect caused by heat stress, we used hrde-1 mutants that had been continuously cultured at 23°C for three and six generations after shifting from 15°C (23°C F3 and 23°C F6, respectively).
Consistent with our previous RNA-seq analysis (Ni et al., 2016) (Fig. S7A), hrde-1 mutant animals exhibited much higher Cer3 smFISH signals at 23°C than at 15°C or 19°C (Fig. 2D,E). We did not detect any heat-induced enhancement of Cer3 expression in WT animals using smFISH (Fig. 2C). The 23°C F6 hrde-1 mutant animals also showed much stronger smFISH signals than the 23°C F3 mutant adults. Our smFISH results revealed that the heat stress-enhanced Cer3 expression was primarily due to higher expression in individual Cer3+ nuclei in the distal gonad (proliferative zone, transition zone and early pachytene), rather than more Cer3+ nuclei at later developmental stages (Fig. 2D). These results, together with our previous ChIP-seq analysis showing increased Pol II occupancy at Cer3 by heat stress (Ni et al., 2016), indicate that Cer3 transcription is enhanced at the distal end of the germline by heat in hrde-1 mutants, and that the temperature effect is transgenerationally progressive.
Not all female germ cells become oocytes in WT C. elegans; some are eliminated by apoptosis at the late pachytene stage (near the turn of gonad arm) (Gumienny et al., 1999). Reduced fertility in hrde-1 mutants at high temperature could conceivably be caused by an increase in germ cell apoptosis. To investigate this possibility, we examined germ cell corpses using a CED-1:GFP reporter (Zhou et al., 2001). In 23°C F3 hrde-1 mutants, apoptotic germ cells localized at the gonad turn, similar to observations in WT (Fig. S7B,C). The number of apoptotic germ cells per gonad was not significantly different between WT and hrde-1 (Fig. S7B,C). This result indicates that hrde-1 mutation does not promote germ cell apoptosis at the high temperature.
Transcripts of endogenous HRDE-1 targets are enriched in the nuclei of germ cells
Our smFISH analysis found that Cer3 and Cer8 RNA transcripts were only present in the nuclei, not in the cytoplasm, in both WT and hrde-1 mutant adult germ cells (Fig. 3A, Fig. S5 and Fig. S8C). To determine whether this nuclear localization is a general feature for the germline nuclear RNAi-targeted transcripts, we performed RNA-seq in isolated nuclei, cytoplasmic extracts and whole animals of WT and hrde-1 mutant adults (23°C for three or four generations). To capture both polyadenylated and non-polyadenylated RNA, RNA was sequenced without using the oligo(dT)-based enrichment (referred to as RNA-seq in this work). Many endogenous HRDE-1 targets are located in unannotated regions of the genome, so we calculated the nuclear enrichment index (RNAnuclear/RNAcytoplasmic) for each 1 kb region throughout the genome. By using a two-fold cutoff (P≤0.05), we found that transcripts from 4909 kb and 3669 kb regions were enriched in the nucleus in WT and hrde-1 mutants, respectively. Introns, particularly the long ones (>2 kb), were highly enriched in the nuclear RNA compared with the cytoplasmic RNA, confirming our fractionation procedure (Fig. S8A).
We previously classified the endogenous HRDE-1 targets based on the effect of the hrde-1 mutation at these loci: regions termed germline nuclear RNAi-dependent transcriptional repression (or GRTS) become transcriptionally de-repressed in the hrde-1 mutant, and regions termed germline nuclear RNAi-dependent heterochromatin (or GRH) lose H3K9me3 in the hrde-1 mutant (Ni et al., 2014). We found that many GRTS transcripts were enriched in the nucleus over cytoplasm for both WT and hrde-1 mutant samples (Fig. 3B). This feature was much more prominent in hrde-1 mutant than in WT animals. In hrde-1 mutants, 94.8% of the GRTS transcripts were enriched in the nucleus (cutoff: two-fold enrichment and P≤0.05). The average nuclear enrichment index value for the GRTS regions in hrde-1 mutant was 11.0 (P<2.2×10−16, Wilcoxon rank-sum test). In WT, 27.7% of the GRTS regions showed the nuclear enrichment using the same cutoff. The lower percentage is likely due to the lower expression of the GRTS loci in WT, which results in higher P-values. Nevertheless, GRTS transcripts were generally enriched in the nucleus over the cytoplasm in WT animals with an average nuclear enrichment index value of 8.1 (P<2.2×10−16, Wilcoxon rank-sum test).
We found that many GRH transcripts were also enriched in the isolated nuclei of WT and hrde-1 mutants (Fig. S8B). The average nuclear enrichment index values for these regions were 8.9 and 4.5, respectively (both P<2.2×10−16, Wilcoxon rank-sum test). However, GRH transcripts had a lower degree of nuclear enrichment than GRTS transcripts; 20.9% and 34.0% of GRH regions in WT and hrde-1 mutant, respectively, showed at least 2-fold nuclear enrichment (P≤0.05).
These results indicate that a large fraction of the endogenous germline nuclear RNAi targets are enriched in the nucleus, particularly the GRTS transcripts. This nuclear localization feature is not dependent on HRDE-1.
Distinct genetic requirements of Cer3 silencing in the germline and soma
In our recent study using RNA-seq and Pol II ChIP-seq, we found that H3K9 HMT;hrde-1 compound mutants exhibit stronger Cer3 expression than the hrde-1 single mutant (Kalinava et al., 2018 preprint). This was unexpected because the mutations in the H3K9 HMT genes, either single or in combination, do not cause any silencing defects at the endogenous HRDE-1 targets, including Cer3. To investigate further the synthetic effect at single-cell resolution, we performed Cer3 smFISH in WT, hrde-1 single mutant, set-32; met-2 set-25 triple mutant and set-32; met-2 set-25 hrde-1 quadruple mutant adult animals (23˚C F2 for all strains). Consistent with our sequencing results, Cer3 smFISH signals in set-32; met-2 set-25 triple mutants were very low, similar to WT (Fig. 4A,C). In contrast, set-32; met-2 set-25 hrde-1 quadruple mutant animals exhibited dramatically enhanced Cer3 smFISH signals compared with the hrde-1 single mutant (Fig. 4A,C). The Cer3 smFISH signals in the quadruple mutant germline were limited to the proliferative zone, transition zone and pachytene. Interestingly, intestine nuclei exhibited robust Cer3 smFISH signals in the quadruple mutant (Fig. 4B). Cer3 smFISH signal is also observed in a subset of somatic cells near the head, tail and epidermis of the quadruple mutant (data not shown), but the identity of these somatic cells cannot be further defined based on our DAPI stain of nuclear morphology. Cer3 smFISH signals in adult somatic cells were not observed in the WT, hrde-1 single mutant, or set-32;met-2 set-25 triple mutant (Fig. 4B).
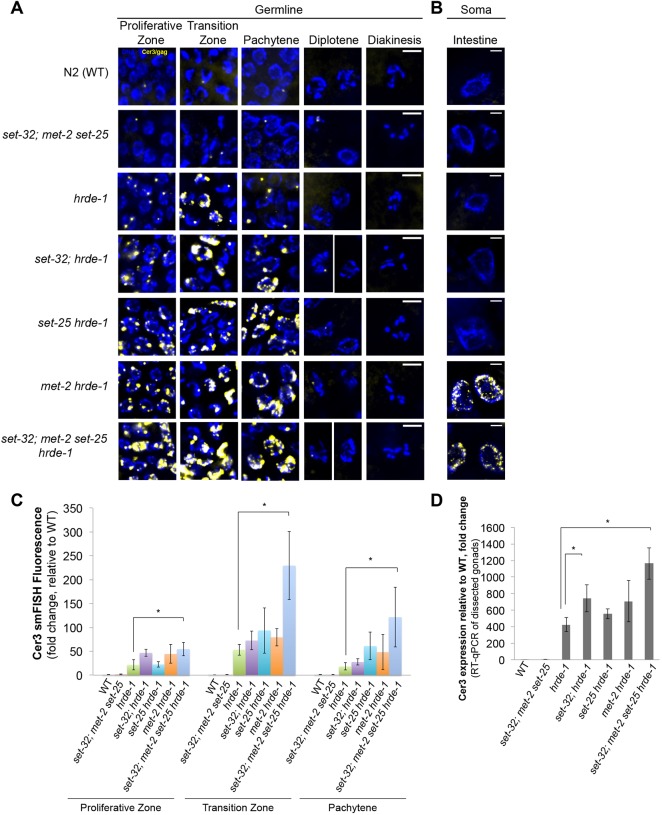
Fig. 4.
Requirement of different putative H3K9 HMTs for Cer3 silencing in the germline and soma. (A,B) Cer3/gag smFISH analysis in adult germline (A) and adult intestinal cells (B) of WT and different mutant strains as indicated. All strains were cultured at 23°C for two generations for this experiment. Scale bars: 5 μm. (C) Quantification of the Cer3/gag smFISH fluorescence shown in A. Three biological replicates. WT (N2) is set to 1. Mean±s.d. is plotted. *P<0.05 (t-test). (D) RT-qPCR measurement of Cer3 transcripts in dissected gonads. Three biological replicates. WT (N2) is set to 1. Mean±s.d. is plotted. *P<0.05 (t-test).
To determine which H3K9 HMT contributes to the enhanced Cer3 de-repression in the germline or soma, we performed Cer3 smFISH in different H3K9 HMT;hrde-1 double mutants (23°C F2 for all strains) (Fig. 4A-C). met-2 hrde-1 is the only double mutant strain that exhibited Cer3 depression in the intestinal cells (Fig. 4B). All three H3K9 HMT;hrde-1 double mutants exhibited at most modest enhancement in the germline over hrde-1 single mutant, suggesting a synergistic relationship of the three HMT mutations. Similar conclusions were obtained by RT-qPCR analysis of Cer3 transcripts from dissected gonads of different H3K9 HMT;hrde-1 mutants (Fig. 4D).
Together, these data indicate that H3K9 HMTs are involved in the transcriptional repression of Cer3. However, the underlying mechanisms are likely to be complex for the following reasons. (1) The requirement of H3K9 HMTs in Cer3 silencing is conditional on the loss of HRDE-1 activity. (2) The tissue specificities of different H3K9 HMTs' functions are not identical. In the intestinal cells, only MET-2, an H3K9me2 HMT, is required to maintain the repressive state of Cer3 in the hrde-1 mutant. In the germline, all three H3K9 HMTs contribute to the Cer3 repression in the absence of HRDE-1.
Dynamic expression of Cer3 RNA transcripts during embryogenesis
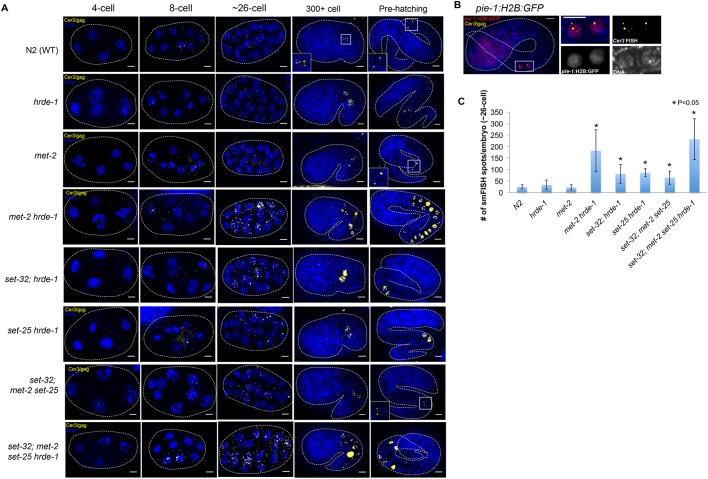
To investigate Cer3 expression during embryogenesis, we performed Cer3/gag smFISH in WT embryos (23°C F2) (Fig. 5A). We did not observe any Cer3 smFISH signals in the fertilized eggs or during the first two rounds of cell division, indicating that Cer3 is not expressed during these stages. This quiescent state was followed by a burst of Cer3 RNA expression in most, if not all, cells. Broad expression of Cer3 RNA began at the 8-cell stage and peaked at around the 30-cell stage, when the cells are still pluripotent blastomeres. Cer3 RNA expression ceased after the organogenesis/morphogenesis stages (Fig. 5A). Afterwards, Cer3 RNA largely disappeared in all of the embryonic cells except the two PGCs (Fig. 5A,B). These results indicate that (1) Cer3 RNA is expressed during embryogenesis, (2) the expression is highly dynamic, and (3) there are two modes of Cer3 RNA expression: a transient, non-cell-specific expression in the pluripotent blastomeres and a subsequent PGC-specific expression. Interestingly, Cer3 RNA in blastomeres was localized in both the cytoplasm and nuclei, whereas Cer3 RNA in PGCs was restricted to the nuclei, similar to observations in the post-embryonic germ cells.
Fig. 5.
Cer3 RNA expression during embryogenesis. (A) Cer3/gag smFISH in 4-cell, 8-cell, ∼26-cell, 300+ cell, and pre-hatching stages of WT and different mutant embryos as indicated. Insets show enlargements of the boxed areas. (B) Simultaneous detection of pie-1 promoter-driven GFP::H2B (immunofluorescence with anti-GFP) and Cer3/gag RNA with smFISH in a pre-hatching embryo. This strain carries the Ppie-1::GFP::H2B, but otherwise is wild type. Right-hand panels show enlargements of the boxed area in the left-hand panel. (C) Quantification of Cer3/gag smFISH spots in ∼26-cell embryos. Scale bars: 5 μm. Ten biological replicates. Mean±s.d. is plotted. *P<0.05 (t-test).
To investigate the role of HRDE-1 in Cer3 regulation during embryogenesis, we performed Cer3 smFISH in hrde-1 embryos (23°C F2). During early embryonic development, before the two PGCs were born, Cer3 expression in hrde-1 mutants was similar to that in WT (Fig. 5A). Cer3 RNA was not expressed at the 1- to 4-cell stage of hrde-1 mutant embryos, and then was expressed in both nuclei and cytoplasm in many cells from 8-cell to ∼30-cell stages. hrde-1 mutants did not appear to have a higher Cer3 RNA expression at this stage than the WT (Fig. 5C). During late embryonic development of the hrde-1 mutant, Cer3 RNA expression disappeared in most of the cells, as observed in WT. Compared with WT, the hrde-1 mutant did have higher Cer3 RNA expression in the two PGCs (Fig. 5A). These results indicate that the repressive role of HRDE-1, at least at Cer3, is limited to the PGCs during embryogenesis. An HRDE-1-independent mechanism(s) is likely to repress Cer3 in other embryonic cells.
We then investigated whether any of the three H3K9 HMTs represses Cer3 expression in the somatic or germline lineage in embryos (23°C F2 for all strains). Compared with WT embryos, set-32; met2 set-25 embryos displayed a modest increase in Cer3 RNA at 8-cell to ∼30-cell stages, but did not exhibit Cer3 depression in either the somatic lineages or PGCs in later embryos (Fig. 5A,C). This suggests that H3K9me3 plays a role in Cer3 repression in the blastomeres during early embryogenesis, but loss of H3K9me3 alone is not sufficient to activate Cer3 in either the somatic cells or PGCs of late embryos.
We then examined H3K9 HMT/hrde-1 compound mutant embryos (23°C F2 for all strains). All three double mutants (met-2 hrde-1, set-25 hrde-1, and set-32; hrde-1) and the quadruple mutant (set-32; met2 set-25 hrde-1) exhibited enhanced Cer3 expression in early embryos (8-cell to ∼30 cells) compared with WT, suggesting that H3K9 HMTs and HRDE-1 in combination play a repressive role for Cer3 expression at this stage (Fig. 5A,C).
In late embryos of met-2 hrde-1 and set-32; met2 set-25 hrde-1 mutants (23°C F2), Cer3 transcripts were strongly expressed in a group of nuclei along the epithelial tube where the intestine lineage and PGCs reside (McGhee, 2007) (Fig. 5A), which is consistent with enhanced Cer3 expression in the adult intestinal cells of the same mutant strains. However, met-2 mutation on its own did not cause Cer3 activation in either early or late embryos (Fig. 5A,C).
DISCUSSION
HRDE-1-dependent repression and developmental relaxation of LTR retrotransposons occur in the same set of germ nuclei
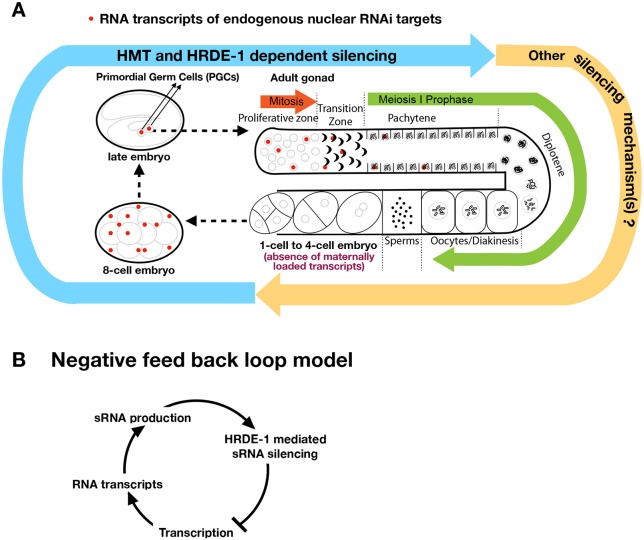
Given that RNA transcripts are required for both siRNA biogenesis (Sijen et al., 2001; Smardon et al., 2000) and siRNA-guided targeting by nuclear RNAi complexes (Guang et al., 2010, 2008), developmental relaxation at endogenous HRDE-1 targets is likely to play an important role in reinforcement of their silencing at each generation. When and where it occurs in the WT germline was unknown before this study. Developmental relaxation may occur in either all germline developmental stages or only some of them. This study found that the latter is true for the two prominent HRDE-1 targets, Cer3 and Cer8 LTR retrotransposons. Developmental relaxations of Cer3 and Cer8 in WT adult germline are limited to the proliferative germ cells and the first few steps of meiosis prophase I, including leptotene, zygotene and early pachytene, and does not occur in the subsequent meiotic stages. Because Cer3 (gypsy family) and Cer8 (Pao family) are in two different clades of LTR retrotransposons, the developmental relaxation mechanism is likely to be common to other nuclear RNAi-targeted retrotransposons. By examining the Cer3 and Cer8 RNA transcripts in the hrde-1 mutant, we determined that HRDE-1-dependent repression occurs in the same set of germ nuclei as developmental relaxation. These results suggest that developmental relaxation occurs at the germline development stages when most of the cells are repressed by nuclear RNAi (summarized in Fig. 6A). This is different from plants or the fission yeast, in which transcriptional relaxation and repression occur in different cell types or at different stages of the cell cycle (Chen et al., 2008; Kloc et al., 2008; Slotkin et al., 2009). Although the mechanisms for the developmental regulation of germline nuclear RNAi in C. elegans are largely unknown at this point, this study provides several important insights (outlined below).
Fig. 6.
A model of the developmentally regulated germline nuclear RNAi (endogenous targets). (A) Expression and silencing cycle of endogenous targets is coupled to the C. elegans reproductive cycle. Developmental relaxation of endogenous targets (Cer3 and Cer8) occurs in primordial germ cells (PGCs) of embryos, and in a small subset of adult germline nuclei in the proliferative zone, transition zone and pachytene zone. HRDE-1-dependent transcriptional silencing occurs in the same set of nuclei as developmental relaxation. Histone methyltransferases (MET-2, SET-25 and SET-32) function synergistically with HRDE-1 to repress endogenous targets. Because Cer3 and Cer8 transcripts are absent in post-pachytene stages and gametes, they cannot be the physical carriers of the transgenerational epigenetic memory. Cer3 and Cer8 are transiently and ubiquitously expressed in early embryos, and later their expression is restricted to the nuclei of PGCs in late embryos. (B) A negative-feedback loop provides a balance between transcription and repression of endogenous nuclear RNAi targets in the same germ cells. In this way, an increase in transcriptional activity will generate more RNA transcripts, which can lead to a higher siRNA production and more targets with which the HRDE-1-siRNA complex can engage. These changes will lead to a stronger repression. This negative-feedback loop avoids complete silencing because the repressive activity will stop acting on the target gene when there are no transcripts for HRDE-1-siRNA complexes to engage with. This is when transcriptional relaxation will occur.
Because the target RNA transcripts are required as RNA-dependent RNA polymerase (RdRP) templates for 22G endo-siRNAs biogenesis, our results suggest that the biogenesis of Cer3 and Cer8 siRNA in germline cells is limited to the distal regions of the gonad (from proliferative cells to early pachytene). These siRNAs are very likely to persist in subsequent germline developmental stages and be transmitted to the next generation because of their interactions with HRDE-1 and the presence of HRDE-1 in all female germline developmental stages, including oocytes (Ashe et al., 2012; Buckley et al., 2012; Shirayama et al., 2012).
Cer3 and Cer8 have strong transcription potentials in distal germ cells. This is shown by the Pol II ChIP-seq, RNA-seq (Ni et al., 2014, 2016) and smFISH analyses in hrde-1 single mutant and hrde-1 H3K9 HMT compound mutants. Our previous studies and this study also showed that heat stress can further enhance the transcription of Cer3 in hrde-1 mutants, and that this effect is transgenerationally progressive (Ni et al., 2016). It will be of great interest to determine the promoter and other cis-regulatory elements that are required for Cer3 expression and heat activation, and the molecular mechanism that causes transgenerational accumulation of Cer3 transcripts under heat stress.
Developmental relaxation and HRDE-1-mediated repression could, in principle, form a negative-feedback loop (Fig. 6B). Our results suggest that such a negative-feedback loop occurs in the same cells. This system could provide stable silencing of the target loci, even for those that have strong transcriptional potentials. For example, an increase in transcription activity would generate more RNA transcripts, which would lead to higher siRNA production and more targets for the HRDE-1-siRNA complex to engage. These changes would in turn lead to a stronger repression. On the other hand, such a system avoids complete silencing (i.e. a complete lack of transcription) because the repressive activity would stop acting on the target gene when there are no transcripts for HRDE-1-siRNA complexes to engage, and then transcription relaxation would occur.
Although HRDE-1 is present in all adult germ nuclei, our analysis indicates that HRDE-1-dependent repression, at least for Cer3 and Cer8, is not needed for repression in post-pachytene meiotic cells. This could be caused by (1) a global transcription quiescence state as meiotic cells progress into the late stages (Kelly et al., 2002; Starck, 1977), (2) rapid Cer3 and Cer8 nuclear RNA turnover at post-pachytene stages, or (3) LTR-retrotransposon life-cycle/promoter activities. Future studies will be required to test these hypotheses.
Is nuclear accumulation a sign of ‘foreign’ genetic material?
Here, we found that, unlike normal mRNAs that are enriched in cytoplasm, transcripts of Cer3, Cer8 and other germline nuclear RNAi targets are enriched in the germline nuclei. This provides a first important cytological hallmark for endogenous HRDE-1 targets. Nuclear RNA export is coupled with transcription and RNA processing (Nabih et al., 2017). Aberrant RNAs that fail to be exported are subjected to nuclear RNA degradation. We speculate that nuclear RNA accumulation of endogenous HRDE-1 targets may reflect aberrant structures or deficiencies in RNA processing of these transcripts. Such connections have been found in C. elegans and other model organisms. In S. pombe, splicing factors were identified as players in sRNA-mediated centromeric heterochromatin and silencing (Bayne et al., 2014, 2008). In the fungal pathogen Cryptococcus neoformans, stalled spliceosomes on transcripts at transposon-like centromeric DNA promote sRNA biogenesis targeting the transcripts (Dumesic et al., 2013). In C. elegans, the recently identified germline nuclear RNAi factor EMB-4 associates with pre-mRNA and is required for germline nuclear RNAi silencing of intron-containing transgenes (Akay et al., 2017; Tyc et al., 2017). The ∼6 kb Cer3 gag/pol domain contains putative 5′ and 3′ splice sites (data not shown), but its actual splicing pattern and efficiency are unclear. Unlike endo-siRNAs of native genes, which cover almost exclusively exonic sequences, the siRNA coverage in the Cer3 gag/pol region appears to be continuous and does not have clear intron/exon boundaries (Fig. 1B). We speculate that Cer3 transcripts are not optimized for C. elegans RNA processing machinery, and therefore are not effectively processed and licensed for nuclear export.
The feature of nuclear enrichment also raises the possibility that endo-siRNA biogenesis for the endogenous HRDE-1 targets occurs in the nucleus or nuclear peripheral structures. This is supported by the findings that many siRNA metabolic proteins (such as RdRPs and mutator family proteins) are located at or near the perinuclear P-granules (Conine et al., 2010; Ishidate et al., 2018; Phillips et al., 2012; Vought et al., 2005; Wan et al., 2018).
What is the functional significance of early embryonic relaxation?
Our observation of LTR transcripts in C. elegans early embryos has interesting parallels in mammals (Faulkner et al., 2009; Fort et al., 2014; Göke et al., 2015; Grow et al., 2015; Peaston et al., 2004; Svoboda et al., 2004). Retrotransposons are transcribed in human pre-implantation embryos at the onset of embryonic genome activation (Göke et al., 2015; Grow et al., 2015), which leads to the formation of viral particles and upregulation of genes in the viral restriction pathway (Grow et al., 2015). Nuclear enrichment of LTR-derived transcripts is also found in mouse and human stem cells (Faulkner et al., 2009; Fort et al., 2014). The function of Cer3 and Cer8 transcripts in early C. elegans embryo is unclear at this point. The burst of Cer3 expression from the 8-cell to the gastrulation stage coincides with the time window when C. elegans embryo blastomeres are pluripotent (Joshi et al., 2010; Yuzyuk et al., 2009), similar to the findings by the aforementioned studies in mammalian systems. This raises an interesting possibility that the transient embryonic expression of LTR elements provides immunity against homologous retrovirus in C. elegans. Alternatively, the observed burst of transcription is perhaps the consequence of the natural life cycle of Cer3 and Cer8. It is interesting to note that both germline and early embryo are good opportunities for transposons to be vertically transmitted. Transient expression of Cer3 and Cer8 at these two stages may provide two waves of siRNA biogenesis to repress the LTR retrotransposons at these crucial developmental stages. Further studies are needed to test these hypotheses and determine the underlying mechanisms of the two waves of expression.
An outstanding question in the field of nuclear RNAi is how epigenetic silencing signals are transmitted to the next generation via the germline. An important finding of this work is that Cer3 and Cer8 transcripts are absent in the gametes, and therefore cannot be the physical carriers of the transgenerational epigenetic memory. Instead, Cer3 and Cer8 are transiently transcribed in early embryos. A recent study reported that early exposure to exogenous dsRNA during embryogenesis triggers strong nuclear RNAi silencing in adult pharyngeal muscle in the same generation, suggesting that the early embryo stage is a critical period for establishing nuclear RNAi silencing (Shiu and Hunter, 2017). It will be of interest to investigate whether the brief activation of Cer3 and Cer8 at the early embryogenesis stage is required to re-establish corresponding small RNA biogenesis and epigenetic silencing.
Cer3 as a model system to study the complex regulation and function of heterochromatin
We previously found that Cer3 chromatin contains a high level of the repressive histone modification H3K9me3. H3K9me3 at Cer3 is deposited through both HRDE-1-dependent and HRDE-1-independent mechanisms (Kalinava et al., 2017). Intriguingly, the high level of H3K9me3 is completely dispensable for Cer3 repression (Kalinava et al., 2017), indicating that the HRDE-1-dependent Cer3 repression can occur through an H3K9me3-independent mechanism. On the other hand, mutant animals with combined losses of H3K9me3 and hrde-1 exhibit a much higher Cer3 expression than the hrde-1 single mutant. Therefore, H3K9me3, likely HRDE-1-independent, functions synergistically with HRDE-1 to repress Cer3 transcription. Similar to hrde-1 mutant animals, the enhanced Cer3 expression in the germline of H3K9 HMT;hrde-1 compound mutants is largely restricted to the distal region of the adult gonads and the PGCs. Cer3 repression in the proximal regions of adult gonads (e.g. diplotene and diakinesis) is mediated by a mechanism(s) that is independent of HRDE-1 and H3K9me3.
In intestinal cells and early embryos, the H3K9me2 histone methyltransferase MET-2 and the nuclear RNAi factor HRDE-1 synthetically suppress Cer3 transcription. In met-2 hrde-1 mutants, the expression of Cer3 RNA transcripts in early embryos increased by >7-fold compared with WT, followed by ectopic Cer3 RNA expression in the intestinal cells in late embryos that persists to the adult stage. In contrast, the H3K9me3 histone methyltransferase SET-25 and the putative histone methyltransferase SET-32 are not required for Cer3 silencing in somatic lineage, highlighting the differential requirements of chromatin-mediated Cer3 silencing in the germline and soma.
MET-2 belongs to a SynMuv B heterochromatin protein family. Many SynMuv B mutants show enhanced RNAi (eri) and misexpression of germline genes in intestine and hypodermis (Kerr et al., 2014; Wang et al., 2005; Wu et al., 2012). We hypothesize that in met-2 mutants, loss of H3K9me2 in early embryos activates a gene expression program characteristic of the less-differentiated germ cells (for example, PGCs). In this model, both the germline nuclear RNAi pathway and Cer3 LTR retrotransposons gain transcription potential in the intestinal cells of met-2 mutants, but Cer3 is repressed by the ectopically activated germline nuclear RNAi pathway. However, this repressive mechanism is not present the met-2 hrde-1 double mutant, resulting in an ectopic de-repression of Cer3. However, we cannot rule out the possibility that a certain non-cell-autonomous function of hrde-1, as suggested in a recent study (Minkina and Hunter, 2017), in combination with the met-2 mutation, leads to de-repression in somatic cells.
MATERIALS AND METHODS
Worm strains
C. elegans strain N2 was used as the standard WT strain. Alleles used in this study were LG I: set-32(red11), gld-1(q485), LG II: nrde-2(gg091), LG III: hrde-1(tm1200), hrde-1(red3), met-2(n4256), set-25(n5021), glp-1(ar202), ruIs32 [pie-1::GFP::H2B+unc-119(+)], LG IV: oma-1(tm1396), LG V: bcIs39 [lim-7p::ced-1::GFP+lin-15(+)]. JU533 is a C. elegans wild isolate (Finistère, France) (Barriere and Felix, 2005) acquired from Caenorhabditis Genetics Center (CGC). JU533 has Cer3/gag deletion that was confirmed by Sanger sequencing. C. elegans culture was as previously described (Brenner, 1974) in a temperature-controlled incubator.
Single-molecule fluorescence in situ hybridization (smFISH)
smFISH fluorescent probe sets were designed using Stellaris Probe Designer (Biosearch). Sequence information of each probe sets are in Table S1. The Cer3/gag smFISH probes were designed using a 2242-nt protein-coding sequence in the Cer3 gag gene. Cer8 has two full-length copies in C. elegans genome, only one of which has a Tc1 DNA transposon insertion in its internal sequence. The two copies are both endogenous nuclear RNAi targets and have non-homologous sequence around the Tc1 insertion site. We designed Cer8 smFISH probes using 2001-nt non-homologous sequence specific to Cer8 copy that lacks Tc1 insertion.
The embryo fixation protocol was adapted from Tintori et al. (2016). Embryos were isolated from gravid adult worms by bleaching, re-suspended in methanol (−20°C), freeze-cracked in liquid nitrogen, and then stored at −20°C overnight. Larval fixation protocol was adapted from Ji and van Oudenaarden (2012). Developmentally synchronized worms of the desired stages were re-suspended in fixation solution (4% paraformaldehyde in 1× PBS) and rotated at 19°C for 45 min. For both embryo and larval samples, hybridization, washing and mounting were carried out as described by Ji and van Oudenaarden (2012). Briefly, fixed samples were washed in wash buffer (10% formamide in 2× SSC) for 5 min, and then incubated in 100 µl of 125 nM smFISH probe set in hybridization buffer (0.1 g/ml dextran sulfate, 1 mg/ml Escherichia coli tRNA, 2 mM vanadyl ribonucleoside complex, 0.2 mg/ml RNase-free BSA, 10% formamide) at 30°C overnight. After hybridization, samples were washed in wash buffer at 30°C for 30 min, incubated in 50 ng/ml DAPI in wash buffer at 30°C for 30 min, washed once in 2× SSC for 2 min at room temperature, and then stored and mounted in SlowFade (Thermo Fisher Scientific) on glass slides.
Image acquisition and quantification
Single-color smFISH images were acquired using a DeltaVision Image Restoration Microscope system (Applied Precision) using a 100×/1.35 UplanApo objective and a Cool Snap HQ2 camera. Images were deconvoluted with Huygens Essential software (Scientific Volume Imaging). Two-color smFISH images were acquired using Zeiss Axiovert 200M Microscope using a 63×/1.3 objective and a cooled CCD camera (QImaging). Images were captured by MetaMorph software and then were deconvoluted by AutoQuant X3 software. All gonads were imaged with a z-step size of 0.2 μm. Fiji (ImageJ) (Schindelin et al., 2012) was used for viewing and processing deconvoluted image data. To generate figures, ImageJ maximum projection was used to project z-stack images to a single plane. Images were processed with linear contrast enhancement in Fiji (ImageJ) (Schindelin et al., 2012) by setting minimum contrast of 1× mean background signal intensity and a maximum contrast of 3× maximum signal intensity (Ji and van Oudenaarden, 2012).
smFISH signal in embryos was observed as scattered spots seen in both nucleus and cytoplasm. We imaged the entire depth of each embryo and generated maximum intensity z-projections using Fiji (ImageJ) (Schindelin et al., 2012). The number of spots was quantified using smFISH quantification software StarSearch (Arujun Raj, University of Pennsylvania, PA, USA).
The majority of smFISH signals observed in hrde-1, and hrde-1, HMT mutants have irregular shapes, which are unlike typical single smFISH spots. We suspect this non-standard smFISH pattern is due to the high number of RNA transcripts concentrated in some areas of the nucleus. We quantified their smFISH fluorescence intensity using Fiji (ImageJ) (Schindelin et al., 2012). We took the sum intensity of ten z-stacks (2 μm, ∼one nuclear diameter observed at distal gonad) from the surface to the center of each developmental zone, highlighting the smFISH signal using the automated intermode threshold function, and then measured the integrated intensity of the highlighted smFISH fluorescence.
RT-qPCR of dissected gonads
Adult worms were washed in 1× PBS twice and paralyzed in 0.2 mM levamisole in 1× PBS. Around 50 paralyzed worms were transferred to a cavity slide and were cut at the pharynx using two 25-gauge syringe needles. Ten intact gonads (with both proximal and distal ends) were collected and transferred to 500 µl TRIzol Reagent (Thermo Fisher Scientific), and RNA was extracted following the manufacturer's instructions. GlycoBlue (final concentration 50 µg/ml) was used at the precipitation step to aid the recovery of RNA. Total RNA was treated by DNaseI (New England Labs) followed by phenol:chloroform extraction. We usually obtained ∼100 ng total RNA from ten gonads. Random hexamer-mediated cDNA synthesis was performed using SuperScript III reverse transcriptase (Thermo Fisher Scientific) according to the manufacturer's instructions using 100 ng total RNA at input. qPCR reactions were performed using Mastercycler EP Realplex real-time PCR system (Eppendorf). Reactions were performed in a total volume of 20 µl, comprising 10 µl of Powerup SYBR Green Master Mix (Thermo Fisher Scientific), 400 nM (final concentration) of each primer, and 1/40 of the cDNA sample (synthesized using 100 ng total RNA). qPCR reactions were performed in triplicate and Ct values produced by default threshold (Eppendorf Realplex 2.2 software) were used for quantification.
CRISPR/Cas9-mediated genome editing
A worm strain carrying strep II-FLAG tagged HRDE-1 (SF-HRDE-1) was constructed using CRISPR (hrde-1[red3]). The SF tag (DYKDDDDKGSAASWSHPQFEKGGGSGGGSGGGSWSHPQFEK) coding DNA was inserted after the start codon of the native hrde-1. Based on anti-FLAG immunofluorescence analysis, we determined that SF-HRDE-1 is expressed in the germline nuclei (Fig. S1A) as previously reported (Ashe et al., 2012; Buckley et al., 2012; Shirayama et al., 2012). CRISPR/Cas9-mediated genome editing was performed using protocols described by Arribere et al. (2014) and Paix et al. (2015).
Immunofluorescence of SF-HRDE-1
Adult worms were washed in 1× PBS twice and paralyzed in 0.2 mM levamisole in 1× PBS. Around 50 paralyzed worms were transferred to a cavity slide and were cut at the pharynx using two 25-gauge syringe needles such that at least one gonad arm extruded from the cutting site. Dissected gonads were immediately transferred to 1.5% paraformaldehyde solution in 1× PBS and then fixed for 10 min at room temperature. After fixation, gonads were blocked in 1 mg/ml BSA, 0.1% Tween 20, 1× PBS for 45 min, and then washed twice for 15 min each wash in 1× PBS/0.1% Tween 20. For antibody staining, gonads were incubated overnight at 4°C in mouse-anti-FLAG antibody (Sigma, F1804) at 1:100 in 1 mg/ml BSA and 1× PBS/0.1%Tween 20. The next day, gonads were washed three times for 30 min each wash in 1× PBS/0.1% Tween 20, and were then incubated in 1:200 anti-mouse Alexa Fluor 488 (Jackson ImmunoResearch Laboratories, 715-545-150) in 1 mg/ml BSA and 1× PBS/0.1%Tween 20 at 4°C overnight. The next day, after three washes for 30 min each wash in 1× PBS with 0.1% Tween 20, and one wash for 5 min in 100 ng/ml DAPI in 1× PBS with 0.1% Tween 20, gonads were mounted in Slowfade (Invitrogen) onto a freshly made 2% agarose pad for imaging. Gonads were imaged using a DeltaVision Image Restoration Microscope system (Applied Precision) using a 20× objective and a Cool Snap HQ2 camera. Images were processed with Fiji (ImageJ) (Schindelin et al., 2012).
Preparation of worm grinds
Synchronized young adult worms were prepared using the hypochlorite bleaching method described by Stiernagle (2006). Before grinding, synchronized young adult worms were harvested by washing off the plates with M9 buffer. Bacteria were removed by centrifugation of worms at 600 g in a clinical centrifuge for 1 min in a M9 buffer with 10% sucrose cushion. Worms were then pulverized by grinding in liquid nitrogen with a mortar and pestle and were stored at −80°C.
Pull down of SF-HRDE-1-bound endogenous siRNA
SF-HRDE-1 immunoprecipitation was performed by using the FLAG Immunoprecipitation kit (Sigma, FLAGIPT1) and the manufacturer's suggested protocol. Briefly, frozen worm grind (5000-10,000 adult animals) were first lysed in 1 ml of Lysis Buffer, 10 µl of 100× HALT protease inhibitor (Thermo Fisher Scientific) and 10 µl of RNaseOUT (Thermo Fisher Scientific). After incubating at room temperature for 15 min, the lysate was sonicated three times, 8 min each time, at 4°C using Bioruptor (Diagenode) with a ‘high’ setting and a 30 s-on/30 s-off cycle. Then the lysate was centrifuged at 21,130 g for 4 min at 4°C. The supernatant was mixed with 40 µl anti-FLAG M2 affinity gel and was rotated at 4°C overnight. The next day, the sample was centrifuged at 8200 g for 30 s at 4°C and the supernatant was removed. The resin was washed three times with 0.5 ml of 1× Wash Buffer at 4°C. SF-HRDE-1 was eluted from the resin with 15 µg 3× FLAG peptide in 100 µl of 1× Elution Buffer by rotating 30 min at 4°C. WT adult small RNA and SF-HRDE-1-coIP small RNA were purified using the mirVana miRNA Isolation Kit (Life Technologies).
Nuclear and cytoplasmic RNA purification
Nuclear and cytoplasmic factions were obtained using a Nuclei PURE Prep kit (Sigma-Aldrich, NUC-201) according to the manufacturer's instructions. All procedures were performed at 4°C. Briefly, synchronized young adult worm grinds were re-suspended in 10 ml Lysis Solution with 1 mM DTT and 0.1% Triton X-100. The lysate was mixed with 18 ml 1.8 M Sucrose Cushion, loaded on top of 10 ml 1.8 M Sucrose Cushion Solution, and ultracentrifuged at 30,000 g for 45 min at 4°C in a SW28 rotor. The clear sucrose cushion layer was collected as cytoplasm fraction. The pellet of purified nuclei was wash once with Nuclei PURE Storage Buffer. Both fractions were used for RNA extraction immediately. RNA was extracted from the cytoplasm fraction, nuclei, and whole worm grind using Trizol reagent (Life Technologies). Ribosomal RNA (rRNA) was depleted using RNaseH and PAGE-purified DNA oligos that are antisense to rRNA. Briefly, 1 µg of rRNA antisense oligos were mixed with 1 µg of total RNA. The sample was denatured at 95°C for 2 min, and then cooled at a rate of −0.1°C/s to 22°C. rRNA was digested with 10 units of Hybridase Thermostable RNase H (Epicentre) in 1× RNaseH Reaction Buffer at 45°C for 30 min. DNA oligos were removed by DNaseI (NEB). The rRNA-removed RNA was extracted with phenol chloroform, precipitated, and used for RNA-seq library construction.
High-throughput sequencing
RNA-seq and sRNA-seq: RNA libraries were prepared by using a 5′-monophosphate-independent RNA cloning procedure as described previously (Ni et al., 2014). Briefly, the 3′-linker (/5rApp/CTGTAGGCACCATCAAT/3ddC/, IDT) was ligated to RNA using T4 RNA ligase 2 truncated (New England Biolabs). RNA was precipitated by adding 100 µl stop solution (1 M NH4OAC, 10 mM EDTA), 2 µl glycoblue and 250 µl 100% ethanol. The RNA pellet was washed with 100% ethanol and air dried. Mono- and tri-phosphate groups at the 5′ end of RNA were removed by Antarctic Phosphatase (New England Biolabs), followed by kinase reaction using T4 PNK and ATP. The ligation product was purified on an 8% PAGE (7 M urea) gel; 33-54 nt species were selected for small RNA and 50-200 nt for large RNA fragment cloning. 5′ linkers were ligated to sample RNA by using T4 RNA ligase 1 (NEB). The 5′ linker sequence was /5AmMC6/ACGCTCTTCCGATCT r#r#r#r# (# indicates barcode base). A mixture of 4 nt barcode sequences, ‘CTGG’, ‘ACTG’, ‘GAAG’ and ‘TGCC’, were used for each library. cDNA was synthesized by using Superscript III (Invitrogen) and RT oligo (5′-GGAGTTCAGACGTGTGCTCTTCCGATCTATTGATGGTGCCTACAG-3′), and amplified using KAPA HIFI taq DNA polymerase and Illumina primers (5′-AATGATACGGCGACCACCGAGATCTACACTCTTTCCCTACACGACGCTCTTCCGATCT-3′ and 5′-CAAGCAGAAGACGGCATACGAGAT ###### GTGACTGGAGTTCAGACGTGTGCTCTTCC-3′; # indicates multiplexing index base) with 8, 11 or 14 cycles of PCR. PCR products were gel purified and pooled for Illumina sequencing.
Pooled libraries were sequenced on an Illumina HiSeq 2500 platform with the following specifications: rapid run mode, 50-nt single-end run, and index sequencing. De-multiplexed raw data in fastq format were provided by the sequencing service facility. Library information is listed in Table S2. High-throughput sequencing data generated for this study have been deposited in Gene Expression Omnibus (accession number GSE118429). sRNA-seq data used for Fig. S3 (WT, glp-1 and fem-1 strains) were from Gent et al. (2010) (NCBI GEO accession number GSE19414).
High-throughput screening
Sequencing reads were aligned to C. elegans genome (WS190) using Bowtie 0.12.7 (Langmead et al., 2009). For the plots in Fig. 3B, Fig. S1C, Fig. S3 and Fig. S8B, log2 ratio of two samples was plotted as a function of the mean value of the read counts at each 1 kb window from two samples throughout the genome. Only perfect alignments were used. A read that aligned to multiple locations was counted as 1/N (N, number of locations) at each location. The counts were normalized to the total aligned reads (in millions). P-values were calculated using a Bayesian method described by Maniar and Fire (2011). R was used to plot.
Supplementary Material
Acknowledgements
We thank Barth Grant, Anne Norris, Helen Ushakov, Elaine Gavin, Monica Driscoll, Guoqiang Wang and Shobhna Patel for technical assistance and discussions. Some strains were provided by the CGC, which is funded by NIH Office of Research Infrastructure Programs [P40 OD010440].
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: J.Z.N., S.G.G.; Methodology: J.Z.N., S.G.G.; Software: S.G.G.; Validation: J.Z.N., S.G.G.; Formal analysis: J.Z.N., N.K., S.G.M., S.G.G.; Investigation: J.Z.N., N.K., S.G.M., S.G.G.; Writing - original draft: J.Z.N., S.G.G.; Writing - review & editing: J.Z.N., S.G.G.; Visualization: J.Z.N.; Project administration: S.G.G.; Funding acquisition: S.G.G.
Funding
This work was supported by the National Institute of General Medical Sciences [R01GM111752 to S.S.G.]. Deposited in PMC for release after 12 months.
Data availability
High-throughput sequencing data generated for this study have been deposited in Gene Expression Omnibus under accession number GSE118429.
Supplementary information
Supplementary information available online at http://dev.biologists.org/lookup/doi/10.1242/dev.167346.supplemental
References
- Akay A., Di Domenico T., Suen K. M., Nabih A., Parada G. E., Larance M., Medhi R., Berkyurek A. C., Zhang X., Wedeles C. J. et al. (2017). The helicase aquarius/EMB-4 is required to overcome intronic barriers to allow nuclear RNAi pathways to heritably silence transcription. Dev. Cell 42, 241-255.e246. 10.1016/j.devcel.2017.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arribere J. A., Bell R. T., Fu B. X. H., Artiles K. L., Hartman P. S. and Fire A. Z. (2014). Efficient marker-free recovery of custom genetic modifications with CRISPR/Cas9 in Caenorhabditis elegans. Genetics 198, 837-846. 10.1534/genetics.114.169730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashe A., Sapetschnig A., Weick E.-M., Mitchell J., Bagijn M. P., Cording A. C., Doebley A.-L., Goldstein L. D., Lehrbach N. J., Le Pen J. et al. (2012). piRNAs can trigger a multigenerational epigenetic memory in the germline of C. elegans. Cell 150, 88-99. 10.1016/j.cell.2012.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barriere A. and Felix M.-A. (2005). Natural variation and population genetics of Caenorhabditis elegans. In WormBook (ed. The C. elegans Research Community), pp. 1-19. WormBook; 10.1895/wormbook.1.153.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayne E. H., Portoso M., Kagansky A., Kos-Braun I. C., Urano T., Ekwall K., Alves F., Rappsilber J. and Allshire R. C. (2008). Splicing factors facilitate RNAi-directed silencing in fission yeast. Science 322, 602-606. 10.1126/science.1164029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayne E. H., Bijos D. A., White S. A., de Lima Alves F., Rappsilber J. and Allshire R. C. (2014). A systematic genetic screen identifies new factors influencing centromeric heterochromatin integrity in fission yeast. Genome Biol. 15, 481 10.1186/s13059-014-0481-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco-Melo D., Gifford R. J. and Bieniasz P. D. (2017). Co-option of an endogenous retrovirus envelope for host defense in hominid ancestors. eLife 6, e22519 10.7554/eLife.22519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen N. J. and McDonald J. F. (1999). Genomic analysis of Caenorhabditis elegans reveals ancient families of retroviral-like elements. Genome Res. 9, 924-935. 10.1101/gr.9.10.924 [DOI] [PubMed] [Google Scholar]
- Brenner S. (1974). The genetics of Caenorhabditis elegans. Genetics 77, 71-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley B. A., Burkhart K. B., Gu S. G., Spracklin G., Kershner A., Fritz H., Kimble J., Fire A. and Kennedy S. (2012). A nuclear Argonaute promotes multigenerational epigenetic inheritance and germline immortality. Nature 489, 447-451. 10.1038/nature11352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castel S. E. and Martienssen R. A. (2013). RNA interference in the nucleus: roles for small RNAs in transcription, epigenetics and beyond. Nat. Rev. Genet. 14, 100-112. 10.1038/nrg3355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E. S., Zhang K., Nicolas E., Cam H. P., Zofall M. and Grewal S. I. S. (2008). Cell cycle control of centromeric repeat transcription and heterochromatin assembly. Nature 451, 734-737. 10.1038/nature06561 [DOI] [PubMed] [Google Scholar]
- Conine C. C., Batista P. J., Gu W., Claycomb J. M., Chaves D. A., Shirayama M. and Mello C. C. (2010). Argonautes ALG-3 and ALG-4 are required for spermatogenesis-specific 26G-RNAs and thermotolerant sperm in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 107, 3588-3593. 10.1073/pnas.0911685107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crichton J. H., Dunican D. S., Maclennan M., Meehan R. R. and Adams I. R. (2014). Defending the genome from the enemy within: mechanisms of retrotransposon suppression in the mouse germline. Cell. Mol. Life Sci. 71, 1581-1605. 10.1007/s00018-013-1468-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curcio M. J., Lutz S. and Lesage P. (2015). The Ty1 LTR-retrotransposon of budding yeast, Saccharomyces cerevisiae. Microbiol. Spectr. 3, 1-35. 10.1128/microbiolspec.mdna3-0053-2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumesic P. A., Natarajan P., Chen C., Drinnenberg I. A., Schiller B. J., Thompson J., Moresco J. J., Yates J. R. III, Bartel D. P. and Madhani H. D. (2013). Stalled spliceosomes are a signal for RNAi-mediated genome defense. Cell 152, 957-968. 10.1016/j.cell.2013.01.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkner G. J., Kimura Y., Daub C. O., Wani S., Plessy C., Irvine K. M., Schroder K., Cloonan N., Steptoe A. L., Lassmann T. et al. (2009). The regulated retrotransposon transcriptome of mammalian cells. Nat. Genet. 41, 563-571. 10.1038/ng.368 [DOI] [PubMed] [Google Scholar]
- Fort A., Hashimoto K., Yamada D., Salimullah M., Keya C. A., Saxena A., Bonetti A., Voineagu I., Bertin N., Kratz A. et al. (2014). Deep transcriptome profiling of mammalian stem cells supports a regulatory role for retrotransposons in pluripotency maintenance. Nat. Genet. 46, 558-566. 10.1038/ng.2965 [DOI] [PubMed] [Google Scholar]
- Francis R., Barton M. K., Kimble J. and Schedl T. (1995a). gld-1, a tumor suppressor gene required for oocyte development in Caenorhabditis elegans. Genetics 139, 579-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis R., Maine E. and Schedl T. (1995b). Analysis of the multiple roles of gld-1 in germline development: interactions with the sex determination cascade and the glp-1 signaling pathway. Genetics 139, 607-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganko E. W., Fielman K. T. and McDonald J. F. (2001). Evolutionary history of Cer elements and their impact on the C. elegans genome. Genome Res. 11, 2066-2074. 10.1101/gr.196201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gent J. I., Lamm A. T., Pavelec D. M., Maniar J. M., Parameswaran P., Tao L., Kennedy S. and Fire A. Z. (2010). Distinct phases of siRNA synthesis in an endogenous RNAi pathway in C. elegans soma. Mol. Cell 37, 679-689. 10.1016/j.molcel.2010.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göke J., Lu X., Chan Y.-S., Ng H.-H., Ly L.-H., Sachs F. and Szczerbinska I. (2015). Dynamic transcription of distinct classes of endogenous retroviral elements marks specific populations of early human embryonic cells. Cell Stem Cell 16, 135-141. 10.1016/j.stem.2015.01.005 [DOI] [PubMed] [Google Scholar]
- Grow E. J., Flynn R. A., Chavez S. L., Bayless N. L., Wossidlo M., Wesche D. J., Martin L., Ware C. B., Blish C. A., Chang H. Y. et al. (2015). Intrinsic retroviral reactivation in human preimplantation embryos and pluripotent cells. Nature 522, 221-225. 10.1038/nature14308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu S. G., Pak J., Guang S., Maniar J. M., Kennedy S. and Fire A. (2012). Amplification of siRNA in Caenorhabditis elegans generates a transgenerational sequence-targeted histone H3 lysine 9 methylation footprint. Nat. Genet. 44, 157-164. 10.1038/ng.1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guang S., Bochner A. F., Pavelec D. M., Burkhart K. B., Harding S., Lachowiec J. and Kennedy S. (2008). An Argonaute transports siRNAs from the cytoplasm to the nucleus. Science 321, 537-541. 10.1126/science.1157647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guang S., Bochner A. F., Burkhart K. B., Burton N., Pavelec D. M. and Kennedy S. (2010). Small regulatory RNAs inhibit RNA polymerase II during the elongation phase of transcription. Nature 465, 1097-1101. 10.1038/nature09095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumienny T. L., Lambie E., Hartwieg E., Horvitz H. R. and Hengartner M. O. (1999). Genetic control of programmed cell death in the Caenorhabditis elegans hermaphrodite germline. Development 126, 1011-1022. [DOI] [PubMed] [Google Scholar]
- Holoch D. and Moazed D. (2015). RNA-mediated epigenetic regulation of gene expression. Nat. Rev. Genet. 16, 71-84. 10.1038/nrg3863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibarra C. A., Feng X., Schoft V. K., Hsieh T.-F., Uzawa R., Rodrigues J. A., Zemach A., Chumak N., Machlicova A., Nishimura T. et al. (2012). Active DNA demethylation in plant companion cells reinforces transposon methylation in gametes. Science 337, 1360-1364. 10.1126/science.1224839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishidate T., Ozturk A. R., Durning D. J., Sharma R., Shen E. Z., Chen H., Seth M., Shirayama M. and Mello C. C. (2018). ZNFX-1 functions within perinuclear nuage to balance epigenetic signals. Mol. Cell 70, 639-649.e636. 10.1016/j.molcel.2018.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji N. and van Oudenaarden A. (2012). Single molecule fluorescent in situ hybridization (smFISH) of C. elegans worms and embryos. In WormBook (ed. T. C. e. R. Community ), pp. 1-16. WormBook. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi P. M., Riddle M. R., Djabrayan N. J. V. and Rothman J. H. (2010). Caenorhabditis elegans as a model for stem cell biology. Dev. Dyn. 239, 1539-1554. 10.1002/dvdy.22296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinava N., Ni J. Z., Peterman K., Chen E. and Gu S. G. (2017). Decoupling the downstream effects of germline nuclear RNAi reveals that H3K9me3 is dispensable for heritable RNAi and the maintenance of endogenous siRNA-mediated transcriptional silencing in Caenorhabditis elegans. Epigenet. Chromatin 10, 6 10.1186/s13072-017-0114-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinava N., Ni J., Gajic Z., Ushakov H. and Gu S. (2018). Caenorhabditis elegans heterochromatin factor SET-32 plays an essential role in transgenerational establishment of nuclear RNAi-mediated epigenetic silencing. bioRxiv doi:10.1101/255562 10.1101/255562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly W. G., Schaner C. E., Dernburg A. F., Lee M. H., Kim S. K., Villeneuve A. M. and Reinke V. (2002). X-chromosome silencing in the germline of C. elegans. Development 129, 479-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr S. C., Ruppersburg C. C., Francis J. W. and Katz D. J. (2014). SPR-5 and MET-2 function cooperatively to reestablish an epigenetic ground state during passage through the germ line. Proc. Natl. Acad. Sci. USA 111, 9509-9514. 10.1073/pnas.1321843111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimble J. and Crittenden S. L. (2005). Germline proliferation and its control. In WormBook (ed. The C. elegans Research Community ): WormBook; 10.1895/wormbook.1.13.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloc A., Zaratiegui M., Nora E. and Martienssen R. (2008). RNA interference guides histone modification during the S phase of chromosomal replication. Curr. Biol. 18, 490-495. 10.1016/j.cub.2008.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson A. K., Egelhofer T., Rechtsteiner A. and Strome S. (2017). Germ granules prevent accumulation of somatic transcripts in the adult Caenorhabditis elegans germline. Genetics 206, 163-178. 10.1534/genetics.116.198549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B., Trapnell C., Pop M. and Salzberg S. L. (2009). Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 10, R25 10.1186/gb-2009-10-3-r25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik H. S., Henikoff S. and Eickbush T. H. (2000). Poised for contagion: evolutionary origins of the infectious abilities of invertebrate retroviruses. Genome Res. 10, 1307-1318. 10.1101/gr.145000 [DOI] [PubMed] [Google Scholar]
- Maniar J. M. and Fire A. Z. (2011). EGO-1, a C. elegans RdRP, modulates gene expression via production of mRNA-templated short antisense RNAs. Curr. Biol. 21, 449-459. 10.1016/j.cub.2011.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao H., Zhu C., Zong D., Weng C., Yang X., Huang H., Liu D., Feng X. and Guang S. (2015). The Nrde Pathway Mediates Small-RNA-Directed Histone H3 Lysine 27 Trimethylation in Caenorhabditis elegans. Curr. Biol. 25, 2398-2403. 10.1016/j.cub.2015.07.051 [DOI] [PubMed] [Google Scholar]
- Martínez G. and Slotkin R. K. (2012). Developmental relaxation of transposable element silencing in plants: functional or byproduct? Curr. Opin. Plant Biol. 15, 496-502. 10.1016/j.pbi.2012.09.001 [DOI] [PubMed] [Google Scholar]
- McGhee J. D. (2007). The C. elegans intestine. In WormBook (ed. The C. elegans Research Community): WormBook; 10.1895/wormbook.1.133.1 [DOI] [Google Scholar]
- Minkina O. and Hunter C. P. (2017). Stable heritable germline silencing directs somatic silencing at an endogenous locus. Mol. Cell 65, 659-670.e655. 10.1016/j.molcel.2017.01.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muotri A. R., Marchetto M. C. N., Coufal N. G. and Gage F. H. (2007). The necessary junk: new functions for transposable elements. Hum. Mol. Genet. 16, R159-R167. 10.1093/hmg/ddm196 [DOI] [PubMed] [Google Scholar]
- Nabih A., Sobotka J. A., Wu M. Z., Wedeles C. J. and Claycomb J. M. (2017). Examining the intersection between splicing, nuclear export and small RNA pathways. Biochim. Biophys. Acta 1861, 2948-2955. 10.1016/j.bbagen.2017.05.027 [DOI] [PubMed] [Google Scholar]
- Ni J., Chen E. and Gu S. (2014). Complex coding of endogenous siRNA, transcriptional silencing and H3K9 methylation on native targets of germline nuclear RNAi in C. elegans. BMC Genomics 15, 1157 10.1186/1471-2164-15-1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni J. Z., Kalinava N., Chen E., Huang A., Trinh T. and Gu S. G. (2016). A transgenerational role of the germline nuclear RNAi pathway in repressing heat stress-induced transcriptional activation in C. elegans. Epigenet. Chromatin 9, 358 10.1186/s13072-016-0052-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paix A., Folkmann A., Rasoloson D. and Seydoux G. (2015). High efficiency, homology-directed genome editing in Caenorhabditis elegans using CRISPR-Cas9 Ribonucleoprotein complexes. Genetics 201, 47-54. 10.1534/genetics.115.179382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peaston A. E., Evsikov A. V., Graber J. H., de Vries W. N., Holbrook A. E., Solter D. and Knowles B. B. (2004). Retrotransposons regulate host genes in mouse oocytes and preimplantation embryos. Dev. Cell 7, 597-606. 10.1016/j.devcel.2004.09.004 [DOI] [PubMed] [Google Scholar]
- Pepper A. S.-R., Lo T.-W., Killian D. J., Hall D. H. and Hubbard E. J. A. (2003). The establishment of Caenorhabditis elegans germline pattern is controlled by overlapping proximal and distal somatic gonad signals. Dev. Biol. 259, 336-350. 10.1016/S0012-1606(03)00203-3 [DOI] [PubMed] [Google Scholar]
- Phillips C. M., Montgomery T. A., Breen P. C. and Ruvkun G. (2012). MUT-16 promotes formation of perinuclear mutator foci required for RNA silencing in the C. elegans germline. Genes Dev. 26, 1433-1444. 10.1101/gad.193904.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj A., van den Bogaard P., Rifkin S. A., van Oudenaarden A. and Tyagi S. (2008). Imaging individual mRNA molecules using multiple singly labeled probes. Nat. Methods 5, 877-879. 10.1038/nmeth.1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebollo R., Romanish M. T. and Mager D. L. (2012). Transposable elements: an abundant and natural source of regulatory sequences for host genes. Annu. Rev. Genet. 46, 21-42. 10.1146/annurev-genet-110711-155621 [DOI] [PubMed] [Google Scholar]
- Sandmeyer S., Patterson K. and Bilanchone V. (2015). Ty3, a position-specific Retrotransposon in budding yeast. Microbiol. Spectr. 3, MDNA3-0057-2014 10.1128/microbiolspec.MDNA3-0057-2014 [DOI] [PubMed] [Google Scholar]
- Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B. et al. (2012). Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676-682. 10.1038/nmeth.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoft V. K., Chumak N., Mosiolek M., Slusarz L., Komnenovic V., Brownfield L., Twell D., Kakutani T. and Tamaru H. (2009). Induction of RNA-directed DNA methylation upon decondensation of constitutive heterochromatin. EMBO Rep. 10, 1015-1021. 10.1038/embor.2009.152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirayama M., Seth M., Lee H.-C., Gu W., Ishidate T., Conte D. Jr and Mello C. C. (2012). piRNAs initiate an epigenetic memory of nonself RNA in the C. elegans germline. Cell 150, 65-77. 10.1016/j.cell.2012.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiu P. K. and Hunter C. P. (2017). Early developmental exposure to dsRNA is critical for initiating efficient nuclear RNAi in C. elegans. Cell Rep. 18, 2969-2978. 10.1016/j.celrep.2017.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sijen T., Fleenor J., Simmer F., Thijssen K. L., Parrish S., Timmons L., Plasterk R. H. A. and Fire A. (2001). On the role of RNA amplification in dsRNA-triggered gene silencing. Cell 107, 465-476. 10.1016/S0092-8674(01)00576-1 [DOI] [PubMed] [Google Scholar]
- Slotkin R. K., Vaughn M., Borges F., Tanurdžić M., Becker J. D., Feijó J. A. and Martienssen R. A. (2009). Epigenetic reprogramming and small RNA silencing of transposable elements in pollen. Cell 136, 461-472. 10.1016/j.cell.2008.12.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smardon A., Spoerke J. M., Stacey S. C., Klein M. E., Mackin N. and Maine E. M. (2000). EGO-1 is related to RNA-directed RNA polymerase and functions in germ-line development and RNA interference in C. elegans. Curr. Biol. 10, 169-178. 10.1016/S0960-9822(00)00323-7 [DOI] [PubMed] [Google Scholar]
- Spracklin G., Fields B., Wan G., Becker D., Wallig A., Shukla A. and Kennedy S. (2017). The RNAi inheritance machinery of Caenorhabditis elegans. Genetics 206, 1403-1416. 10.1534/genetics.116.198812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starck J. (1977). Radiographic study of RNA synthesis in Caenorhabditis elegans (Bergerac variety) oogenesis. Biol. Cell 30, 181-182. [Google Scholar]
- Stiernagle T. (2006). Maintenance of C. elegans. In WormBook (ed. The C. elegans Research Community): WormBook; 10.1895/wormbook.1.101.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda P., Stein P., Anger M., Bernstein E., Hannon G. J. and Schultz R. M. (2004). RNAi and expression of retrotransposons MuERV-L and IAP in preimplantation mouse embryos. Dev. Biol. 269, 276-285. 10.1016/j.ydbio.2004.01.028 [DOI] [PubMed] [Google Scholar]
- Tintori S. C., Osborne Nishimura E., Golden P., Lieb J. D. and Goldstein B. (2016). A transcriptional lineage of the early C. elegans embryo. Dev. Cell 38, 430-444. 10.1016/j.devcel.2016.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyc K. M., Nabih A., Wu M. Z., Wedeles C. J., Sobotka J. A. and Claycomb J. M. (2017). The conserved intron binding protein EMB-4 plays differential roles in germline small RNA pathways of C. elegans. Dev. Cell 42, 256-270.e256. 10.1016/j.devcel.2017.07.003 [DOI] [PubMed] [Google Scholar]
- Vought V. E., Ohmachi M., Lee M.-H. and Maine E. M. (2005). EGO-1, a putative RNA-directed RNA polymerase, promotes germline proliferation in parallel with GLP-1/notch signaling and regulates the spatial organization of nuclear pore complexes and germline P granules in Caenorhabditis elegans. Genetics 170, 1121-1132. 10.1534/genetics.105.042135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan G., Fields B. D., Spracklin G., Shukla A., Phillips C. M. and Kennedy S. (2018). Spatiotemporal regulation of liquid-like condensates in epigenetic inheritance. Nature 557, 679-683. 10.1038/s41586-018-0132-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Kennedy S., Conte D. Jr, Kim J. K., Gabel H. W., Kamath R. S., Mello C. C. and Ruvkun G. (2005). Somatic misexpression of germline P granules and enhanced RNA interference in retinoblastoma pathway mutants. Nature 436, 593-597. 10.1038/nature04010 [DOI] [PubMed] [Google Scholar]
- Wu X., Shi Z., Cui M., Han M. and Ruvkun G. (2012). Repression of germline RNAi pathways in somatic cells by retinoblastoma pathway chromatin complexes. PLoS Genet. 8, e1002542 10.1371/journal.pgen.1002542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuzyuk T., Fakhouri T. H. I., Kiefer J. and Mango S. E. (2009). The polycomb complex protein mes-2/E(z) promotes the transition from developmental plasticity to differentiation in C. elegans embryos. Dev. Cell 16, 699-710. 10.1016/j.devcel.2009.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z., Hartwieg E. and Horvitz H. R. (2001). CED-1 is a transmembrane receptor that mediates cell corpse engulfment in C. elegans. Cell 104, 43-56. 10.1016/S0092-8674(01)00190-8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.