ABSTRACT
Miniature pigs have advantages over rodents in modeling atherosclerosis because their cardiovascular system and physiology are similar to that of humans. Apolipoprotein E (ApoE) deficiency has long been implicated in cardiovascular disease in humans. To establish an improved large animal model of familial hypercholesterolemia and atherosclerosis, the clustered regularly interspaced short palindromic repeats (CRISPR)-associated protein 9 system (CRISPR/Cas9) was used to disrupt the ApoE gene in Bama miniature pigs. Biallelic-modified ApoE pigs with in-frame mutations (ApoEm/m) and frameshift mutations (ApoE−/−) were simultaneously produced. ApoE−/− pigs exhibited moderately increased plasma cholesterol levels when fed with a regular chow diet, but displayed severe hypercholesterolemia and spontaneously developed human-like atherosclerotic lesions in the aorta and coronary arteries after feeding on a high-fat and high-cholesterol (HFHC) diet for 6 months. Thus, these ApoE−/− pigs could be valuable large animal models for providing further insight into translational studies of atherosclerosis.
KEY WORDS: Atherosclerosis, ApoE, Bama miniature pigs, CRISPR/Cas9
Editor's choice: ApoE knockout pigs displayed severe hypercholesterolemia and spontaneously developed human-like atherosclerotic lesions in the aorta and coronary arteries within 6 months of feeding on a high-fat and high-cholesterol diet.
INTRODUCTION
Atherosclerosis is a chronic inflammatory disease characterized by the accumulation of proliferative smooth muscle cells, macrophages, lipids, cholesterol, calcium deposits and cellular debris in arterial walls (Libby et al., 2011; Mastenbroek et al., 2015). The most common clinical manifestations of this disease are myocardial infarction, ischemic stroke and ischemic damage to kidneys and intestines, due to occlusive thrombosis and severe arterial stenosis (Libby et al., 2013; Murray et al., 2012). Although the pathogenesis of atherosclerosis remains elusive, plasma lipid levels, particularly those of low-density lipoprotein (LDL) cholesterol, correlate with the risk of atherogenesis.
Apolipoprotein E (ApoE) is a major component of very-low-density lipoprotein (VLDL) and chylomicrons, playing a critical role in the metabolism of cholesterol and clearance of lipoprotein remnants (Mahley, 1988). ApoE was initially recognized for its importance in lipoprotein metabolism and cardiovascular disease because dysfunction of ApoE protein results in familial dysbetalipoproteinemia (type III hyperlipoproteinemia), which often leads to atherosclerosis (Eichner et al., 2002; Georgiadou et al., 2011; Ghiselli et al., 1981). Thus, ApoE knockout (KO) rodent models have been favored in atherosclerosis studies and have provided valuable insights into mechanisms underlying this disease (Chistiakov et al., 2017; Iqbal et al., 2016; Niimi et al., 2016; Piedrahita et al., 1992; Plump et al., 1992). However, rodent models have limitations because many important human atherosclerosis features, including plaque rupture and thrombosis, rarely occur in rodents (Al-Mashhadi et al., 2013; Bentzon and Falk, 2010). In addition, rodents are dissimilar to humans in vascular size and lipoprotein profiles and metabolism, which have limited studies on intravascular devices and hampered the translation of experimental findings into clinic applications (Al-Mashhadi et al., 2013; Langheinrich et al., 2007). Hence, suitable large animal models that can adequately mimic human atherosclerosis are needed.
Currently, pigs are broadly used as large animal models in biomedical research because they share many similarities with humans with respect to physiology, pathology, immunology and the cardiovascular system (Aigner et al., 2010; Reyes et al., 2014; Xi et al., 2004). Moreover, compared to other large animals such as non-human primates, pigs are more ethically and economically acceptable, making them preferable models for cardiovascular disease research. However, it is very difficult to develop advanced atherosclerotic lesions in normal pigs, even with a high-fat and high-cholesterol (HFHC)-diet induction. These considerations suggest the need for the development of genetically modified miniature pigs.
The clustered regularly interspaced short palindromic repeats (CRISPR)-associated protein 9 (CRISPR/Cas9) system has emerged as a powerful and efficient tool for gene editing (Hsu et al., 2014; Mali et al., 2013; Niu et al., 2014), and has significantly facilitated the generation of genetically modified pig models (Chen et al., 2015; Estrada et al., 2015; Hai et al., 2014; Zhang et al., 2017). Recently, there was a report of ApoE/LDL receptor (LDLR) double-KO minipigs produced by CRISPR/Cas9-mediated gene targeting (Huang et al., 2017). Although slightly increased plasma lipid levels were observed, an atherosclerosis phenotype was not ever described in those pigs, possibly because in-frame (IF) mutations in both the ApoE and LDLR alleles may give rise to truncated ApoE and LDLR proteins with partially retained function (Huang et al., 2017).
In the present study, we used the CRISPR/Cas9 system to disrupt the ApoE gene, and established ApoE KO Bama miniature pigs by somatic cell nuclear transfer (SCNT) technology. We have found that ApoE KO pigs fed an HFHC diet had severe hypercholesterolemia and spontaneously developed human-like atherosclerotic lesions in the aorta and coronary arteries. These results indicated that the ApoE KO pigs are an ideal model for the study of human atherosclerosis.
RESULTS
sgRNA-directed site-specific indels in primary pig fetal fibroblasts (PFFs)
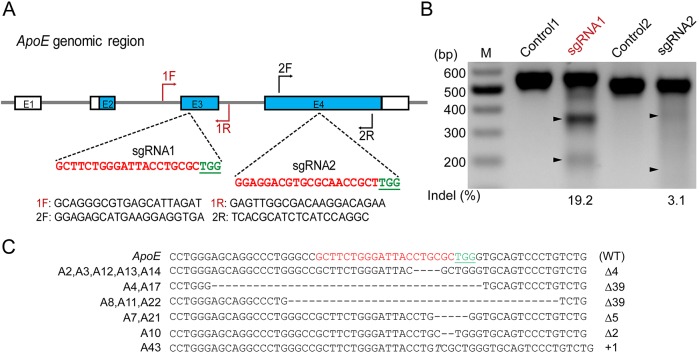
To disrupt the ApoE gene in Bama miniature pigs, two single-guide RNAs (sgRNAs) were designed based on exon 2 and 3 sequences determined by PCR sequencing. The precise target sites are shown in Fig. 1A. To test their cleavage efficiency, these sgRNAs were cloned into a Cas9 targeting vector, pX330 (#42230, Addgene, Cambridge, MA, USA) and introduced into PFFs. At 48 h post-transfection, primary PFFs were harvested for genomic DNA extraction. PCR amplicons spanning the ApoE target region were subjected to a T7 endonuclease 1 digestion assay followed by gel electrophoresis. The cleavage fragments shown in Fig. 1B indicated that both sgRNAs could induce insertions/deletions (indels) in the target region of the ApoE gene, although sgRNA1 (19.2%) had higher cleavage efficiency than sgRNA2 (3.1%). To establish ApoE KO cell lines, the Cas9-sgRNA1 targeting vector was co-transfected with a neomycin expression plasmid into early passages of PFFs derived from a 35-day-old male Bama mini fetus. After 10 days of selection with G418, resistant cell colonies were harvested. PCR amplification was performed on each colony and the resultant fragments were subjected to TA cloning for Sanger sequencing. Of the 44 colonies analyzed, 27 were identified as having biallelic modifications, 14 of which were homozygous, with 6 indel types ranging from 1 to 39 base pairs (bp) in the ApoE gene target region (Fig. 1C).
Fig. 1.
CRISPR/Cas9 mediates ApoE gene targeting in PFFs. (A) Schematic diagram of Cas9-sgRNA targeting sites of the pig ApoE locus. The sgRNA targeting sequences are shown in red, and the protospacer-adjacent motif (PAM) sequences are shown in green and underlined. (B) T7E1 assay for Cas9-mediated cleavage at ApoE targeting sites in PFFs. M: DNA marker; Controls: PCR products of untransfected PFFs treated with T7E1; sgRNA1 and sgRNA2: PCR products of PFFs transfected with Cas9-sgRNA1 and Cas9-sgRNA2 treated with T7E1, respectively. (C) Genotypes of homozygous ApoE biallelic-modified colonies. The WT sequence is shown at the top. Deletion (Δ); insertion (+); italic letter denotes the inserted base pair.
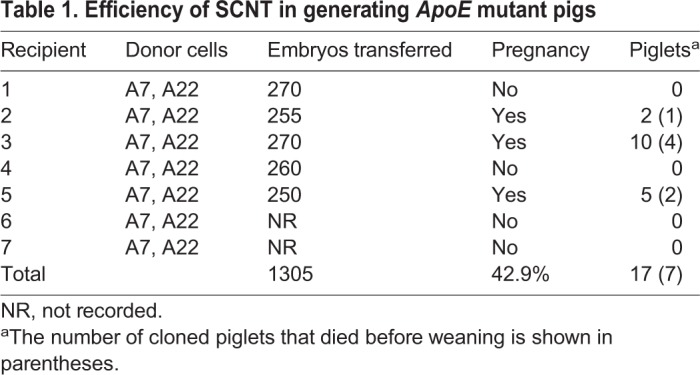
Production of ApoE KO pigs by SCNT
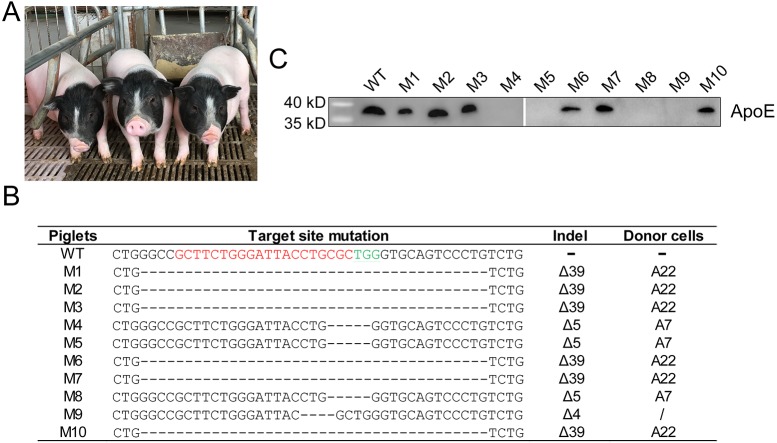
ApoE biallelic-modified colonies (A7 and A22) were pooled and used as nuclear donors for SCNT to produce ApoE KO piglets. Morula- and blastula-stage reconstructed embryos were transferred to 7 surrogates, yielding 3 full-term pregnancies, from which 17 live-born male piglets were delivered (Fig. 2A). Seven of them died before weaning (Table 1). Genomic DNA from the ear tissues of the 10 remaining piglets (designated M1 to M10) was isolated, and mutations were detected via PCR and Sanger sequencing. Piglets M1, M2, M3, M6, M7 and M10 carried a 39 bp deletion corresponding to A22 donor cells; piglets M4, M5 and M8 harbored a 5 bp deletion mutation derived from A7 donor cells; and piglet M9 bore a 4 bp deletion in the ApoE target region that did not originate from the A7 and A22 colonies, indicating that a small portion of cells with a 4 bp deletion in the ApoE region was in the donor-cell mixture (Fig. 2B).
Fig. 2.
Generation of ApoE-gene-targeted piglets via SCNT. (A) Litter of ApoE targeted male founder piglets. (B) Genotypes of cloned piglets. The WT sequence is shown at the top, in which the sgRNA sequences are shown in red, and the PAM sequences are shown in green. Deletion (Δ). The genotype of piglet M9 was different from A7 and A22 donor cells. (C) Representative western blot of plasma ApoE protein of cloned piglets and WT control.
Table 1.
Efficiency of SCNT in generating ApoE mutant pigs
Because the undesired off-target effects of the CRISPR/Cas9 system are still a concern, we computationally predicted off-target sites by screening the pig genome using an online tool (http://www.rgenome.net/cas-offinder/) based on sequence homology to the ApoE sgRNA1 sequence, allowing for un-gapped alignments with up to 4 mismatches. In this way, a total of 81 potential off-target sites (OTSs) were identified. To verify whether off-target cleavage occurred in these genetically modified piglets, 25 OTSs (Table S1) were randomly selected for PCR amplification and subjected to Sanger sequencing. The primers for amplifying the off-target sites are listed in Table S2. The results indicated that none of the sequencing reads exhibited indel mutations, suggesting that there were no off-target effects at these sites.
Elevated serum cholesterol levels in ApoE−/− pigs
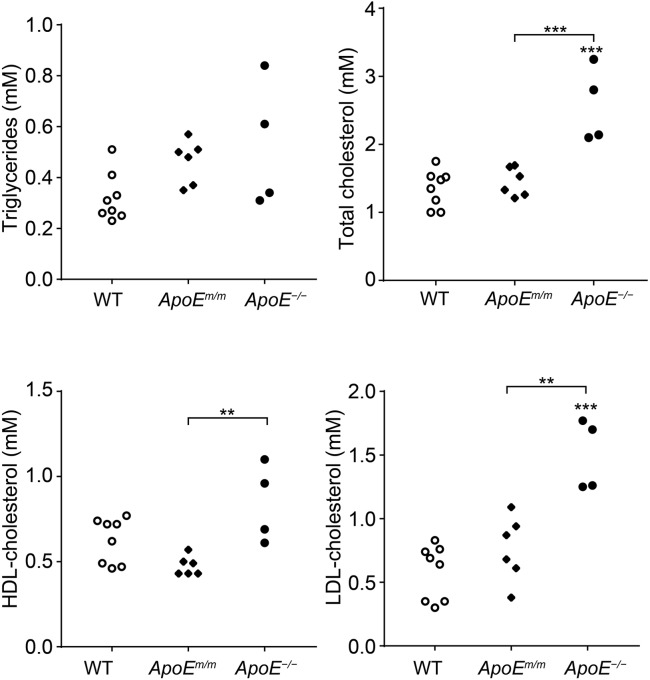
Body weights and appetites of the ApoE targeted piglets, and their age-matched wild-type (WT) Bama minipigs, were comparable at birth and after weaning (data not shown). Plasma ApoE in all piglets was determined by western blot analysis. The ApoE protein was absent in mutant piglets harboring a 4 or 5 bp deletion (M4, M5, M8 and M9), whereas the mutant piglets bearing a 39 bp deletion showed obvious ApoE expression (Fig. 2C). We speculated that a 4 or 5 bp deletion in the ApoE gene region would give rise to a frameshift (FS) mutation, whereas a 39 bp deletion would lead to a 13 amino acids (aa) IF deletion in the ApoE protein. To assess the potential effects of different ApoE gene mutations on serum lipids, these 10 mutant piglets were categorized into ApoEm/m (13 aa deletion) and ApoE−/− (FS mutation) groups. Plasma cholesterol and triglyceride levels were measured in mutant and WT animals 4 weeks after weaning. No significant difference in fasting serum triglyceride concentrations was observed among WT, ApoEm/m and ApoE−/− piglets. The ApoEm/m founders had comparable total cholesterol level as the controls. However, a significantly increased serum cholesterol was observed in ApoE−/− piglets [2.57±0.55 mM (mean±s.d.)] compared to WT (1.35±0.27 mM) and ApoEm/m pigs (1.45±0.21 mM), which was mostly contributed by LDL cholesterol (Fig. 3).
Fig. 3.
Plasma triglycerides and cholesterol of ApoE targeted piglets and WT controls fed a low-fat diet. Data represent fasting plasma measurements at 8 weeks of age in ApoEm/m piglets (n=6), ApoE−/− piglets (n=4) and age-matched WT pigs (n=8). **P<0.01, ***P<0.0001 versus the control group (ANOVA). All values represent the means±s.d.
Severe hypercholesterolemia during HFHC feeding
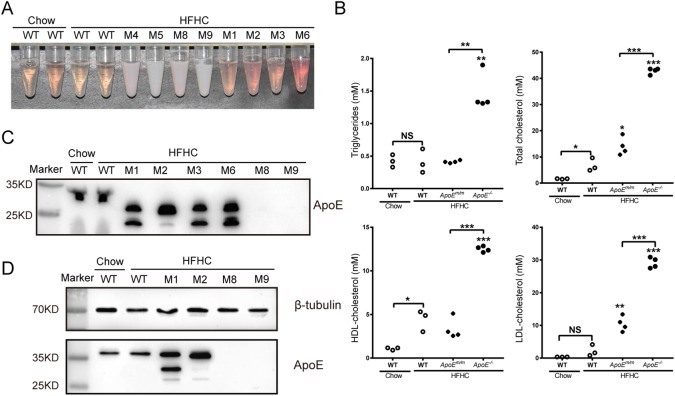
Three-month-old ApoE targeted piglets and age-matched WT pigs were challenged with a HFHC diet containing 30% saturated fat and 1.5% cholesterol for 3 consecutive months. WT pigs fed with chow diet were used as controls. Fasting blood was collected monthly for the assessment of plasma cholesterol and triglyceride levels. During this period, two ApoEm/m piglets (M7 and M10) died because of infection. Unlike WT and ApoEm/m piglets, ApoE−/− piglets maintained on a HFHC diet exhibited chylemia, as indicated by the turbid appearance of their sera (Fig. 4A). Compared to WT pigs on normal chow, increased total cholesterol levels, mostly contributed by high-density lipoprotein (HDL) cholesterol, were observed in WT pigs fed an HFHC diet. However, triglyceride levels did not significantly differ between WT pigs fed an HFHC or normal diet (Fig. 4B). Among all groups, the ApoE−/− group had the highest levels of triglycerides and total cholesterol after 3 months of HFHC feeding, with mean levels of 1.47 and 42.86 mM, respectively (Fig. 4B). ApoEm/m piglets showed less severe hypercholesterolemia, as their total cholesterol levels were about 2-fold that of WT groups on HFHC feeding (14.96 vs 6.76 mM), and their plasma triglyceride levels were comparable to that of WT controls (0.52 vs 0.40 mM) (Fig. 4B). LDL but not HDL cholesterol levels were significantly increased in HFHC-fed ApoEm/m pigs compared to HFHC-fed WT pigs. Interestingly, both LDL and HDL cholesterol levels were dramatically elevated in the HFHC-fed ApoE−/− group compared to the other groups.
Fig. 4.
Phenotype of ApoE targeted piglets during HFHC feeding. (A) Representative image of fasting serum of ApoE targeted piglets fed a HFHC diet, and age-matched WT animals fed a HFHC diet and normal chow for 3 months. ApoE−/− piglet serum had a turbid appearance. (B) Plasma triglycerides and cholesterol of ApoE targeted and WT piglets fed a HFHC diet for 3 months (NS, not significant; *P<0.05, **P<0.01, ***P<0.0001 versus the control group, ANOVA). HFHC-fed WT pigs were also compared with chow-fed WT pigs (Student's t-test). All values represent the means±s.d. (C) Representative western blot of plasma ApoE protein of cloned piglets and WT control after 6 months feeding of HFHC diet. (D) Representative western blot of liver ApoE protein of cloned piglets and WT control after 6 months feeding with a HFHC diet.
Progressive atherosclerosis in ApoE targeted pigs
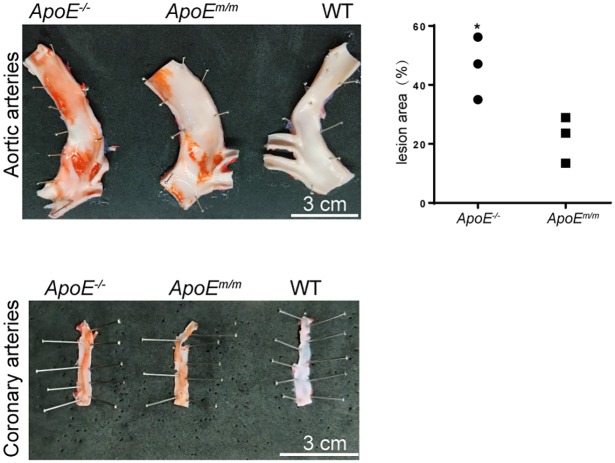
To facilitate development of atherosclerosis plaques in these pigs, HFHC diet feeding was prolonged to 6 months. Serum ApoE protein in the WT, ApoEm/m and ApoE−/− groups was examined by western blot. As expected, ApoE protein was absent in ApoE−/− piglets. An upregulated expression of ApoE was observed in ApoEm/m piglets compared to WT controls (Fig. 4C). Moreover, the molecular mass of ApoE protein in ApoEm/m piglets was smaller than that in WT animals, due to the deletion of 13 aa in the ApoE protein or degradation of the truncated ApoE protein. To test whether disruption of ApoE accelerated atherosclerosis development, 2 pigs from each group (WT, ApoEm/m and ApoE−/−) were euthanized after 6 months of HFHC feeding. ApoE expression in these sacrificed pig livers was also examined by western blot with β-tubulin as an internal control. Consistent with serum ApoE levels, ApoE expression was elevated in the ApoEm/m group and absent in the ApoE−/− group compared to WT pigs (Fig. 4D). The ApoE−/− pigs displayed a significantly higher percentage of aortic surface area covered with lesions than ApoEm/m pigs, namely a 2.1-fold increase compared with the ApoEm/m pigs (Fig. 5). However, atherosclerotic lesions were barely seen in the aorta and coronary arteries of WT pigs even when fed a HFHC diet. Interestingly, coronary area covered with atherosclerotic lesion was comparable between ApoEm/m and ApoE−/− pigs fed a HFHC diet (Fig. 5). The aorta from WT, ApoEm/m and ApoE−/− pigs were further stained to assess the presence of atherosclerosis. WT pigs on HFHC feeding exhibited no significant pathological alterations, whereas ApoEm/m pigs had appreciable aorta areas of fatty streaks, intimal thickening as well as apparent foam cell accumulation as indicated by CD68 staining. The ApoE−/− pigs showed progressive atherosclerotic lesions of fibrous plaque, which was symbolic of fibrosis as well as hyaline degeneration in aorta (Fig. 6).
Fig. 5.
Aortic and coronary atherosclerosis in ApoE targeted piglets fed a HFHC diet. Left: photographs of Sudan-IV-stained aorta (top) and coronary arteries (bottom) of ApoEm/m and ApoE−/− pigs showed atherosclerotic lesions. Right: the percentage of Sudan-IV-stained area was measured by ImageJ software. *P<0.05.
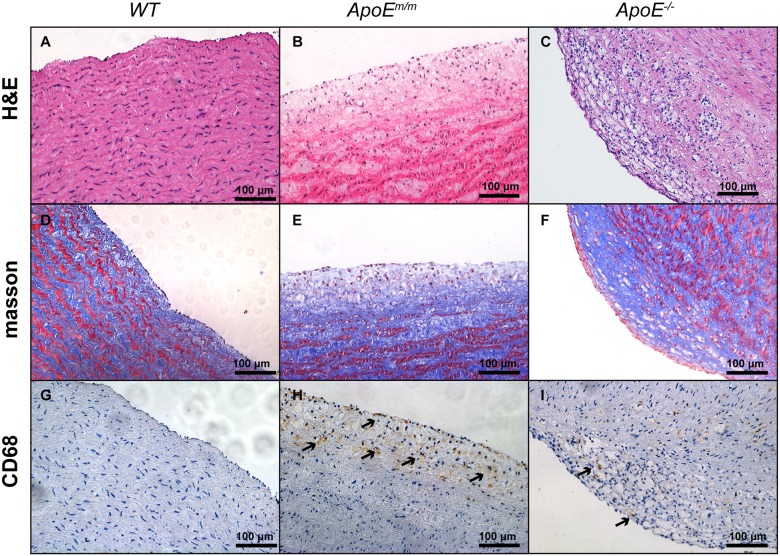
Fig. 6.
Representative histological images of aorta from WT, ApoEm/m and ApoE−/− pigs. No visible atherosclerosis was seen in the aorta from WT pigs (A,D,G). ApoEm/m pigs had pathological fatty streak of the aorta (B,E,H). ApoE−/− pigs showed progressive atherosclerotic lesions in the luminal part of the arterial intima (C,F,I).
DISCUSSION
Pigs have advantages over rodent models in recapitulating the human pathophysiology of atherosclerosis because they are similar to humans with regard to vascular size and physiology. Modeling atherosclerosis in minipigs has been hampered by a low propensity to atherosclerosis even with long-term feeding with atherogenic diets. Genetically modified pig models have been considered promising in solving the problem. However, utilization of genetically modified pigs as atherosclerosis models is still in its infancy, with the exception of PSCK9 transgenic pigs created by transposon integration and LDLR KO pigs generated by homology-directed recombination (HDR)-based methods (Al-Mashhadi et al., 2013; Davis et al., 2014; Li et al., 2016). Here, we successfully established novel porcine models of accelerated atherosclerosis in Bama miniature pigs by disrupting the endogenous ApoE gene using the CRISPR/Cas9 system. These pigs exhibited moderate, yet consistently, elevated serum cholesterol levels when fed a standard chow diet, and showed severe hypercholesterolemia and developed aortic and coronary atherosclerotic lesions when fed a HFHC diet.
With high mutagenesis efficiency, CRISPR/Cas9 technology has been extensively used for gene targeting in pigs (Chen et al., 2015; Estrada et al., 2015; Zhang et al., 2017). Consistent with previous studies, high gene-targeting efficiency of the CRISPR/Cas9 system was achieved in PFFs: 11 and 28 of 48 colonies were identified as monoallelic and biallelic ApoE modified, respectively, which was much higher than that garnered with HDR-based ApoE targeting in rodents (Piedrahita et al., 1992; Plump et al., 1992). A total of 17 homozygous ApoE KO piglets were produced by a single round of SCNT and no off-target mutagenesis was detected in them, supporting that the high efficiency and relative simplicity of the CRISPR/Cas9 technology can greatly facilitate the efficient production of gene KO pigs. Due to its preference for the error-prone non-homologous end joining (NHEJ) pathway, the CRISPR/Cas9 system has the advantage of being able to induce multiple gene modifications. Founder pigs with −4 or −5 bp deletions in the ApoE targeting site were generated and showed identical serum lipid profiles. Thus, we suspected that epigenetic status had little impact on the phenotype of the cloned founder pigs.
Hypercholesterolemia has long been implicated in atherosclerosis. Similar to PCSK9 transgenic pigs and LDLR KO pigs, the ApoE−/− pigs showed increased plasma cholesterol levels on a low-fat diet, resembling the human familial hypercholesterolemia phenotype. Interestingly, when fed a HFHC diet for 3 months, total plasma cholesterol levels plateaued to ∼40 mM in ApoE−/− pigs, but only reached ∼20-25 mM in PCSK9 transgenic and LDLR KO pigs. In addition, HDL cholesterol levels were dramatically increased in ApoE−/− pigs, whereas the PCSK9 transgene and LDLR KO did not exert statistically significant effects on HDL cholesterol levels as compared to WT controls. Contrary to relatively low triglyceride levels in PCSK9 and LDLR KO pigs, hypertriglyceridemia was observed in ApoE−/− pigs by HFHC feeding. Thus, the ApoE−/− pigs could be beneficial for delineating the underlying pathophysiology of hypercholesterolemia and atherosclerosis because their serum lipid profiles differ from existing pig models, which cannot sufficiently recapitulate human disease. More recently, a line of ApoE/LDLR KO pigs generated by CRISPR/Cas9-mediated gene targeting has been reported (Huang et al., 2017). Surprisingly, cholesterol levels in those ApoE/LDLR KO pigs were lower than those of LDLR KO pigs reported previously (Davis et al., 2014; Li et al., 2016) or ApoE KO pigs in this study. Moreover, ApoE protein was still present in the ApoE/LDLR KO pigs and no atherosclerosis phenotype was reported. Considering that those pigs harbored a −18 bp deletion in the ApoE gene and +1/−15 bp indels in the LDLR gene, we speculated that IF-mutated ApoE and LDLR proteins did not completely lose their functions. Thus, the ApoE−/− pigs established in this study are the first ApoE KO pig model with severe atherosclerosis phenotypes.
Although the results presented here are encouraging, there were some limitations to this study. First, we did not investigate the advanced stages of atherosclerosis, such as plaque rupture or thrombosis. Thus, a study with a longer duration is needed to establish the full spectrum of atherosclerotic plaque progression from onset to rupture. Second, this study also did not take into account the effects of gender and puberty on phenotype because only male ApoE−/− pigs were investigated and development of coronary atherosclerosis was observed in the ApoE−/− pigs on HFHC feeding for 6 months, when they have reached puberty. Finally, in addition to its implication in lipoprotein metabolism and cardiovascular disease, ApoE exerts pleiotropic roles in Alzheimer's disease (Shi et al., 2017; Yu et al., 2014), immunoregulation (Braesch-Andersen et al., 2013) and cognition (Davies et al., 2014). However, the developmental consequences of ApoE depletion in other tissues/organs were not evaluated, being beyond the scope of this study.
In conclusion, ApoE−/− pigs fed a HFHC diet showed severe hypercholesterolemia and developed progressive atherosclerotic lesions. These pigs could serve as ideal large animal models for the elucidation of ApoE gene functions and translational studies of atherosclerosis.
MATERIALS AND METHODS
Ethics statement
WT mature Landrace gilts and WT Bama minipigs were purchased from Chia Tai Co., Ltd (Huaiyin, China) and housed in a large animal facility affiliated with Nanjing Medical University, Nanjing, China. Standard chow diets were purchased from Chia Tai Co., Ltd and the HFHC diet was purchased from Xietong Organism Co., Ltd (Nanjing, China). Standard pig husbandry procedures were applied to all animals. Embryo transplantation and sacrifice procedures were conducted under isoflurane anesthesia to minimize animal suffering. All animal experiments were carried out in accordance with the guidelines approved by the Institutional Animal Care and Use Committee (IACUC) of the Nanjing Medical University.
CRISPR/Cas9 plasmid constructs
To target the porcine ApoE gene, sgRNAs were designed using online tools (http://crispr.mit.edu/). The DNA oligos of sgRNAs were purchased from Genscript (Nanjing, China) (ApoE sgRNA1: 5′-CACCGCTTCTGGGATTACCTGCGC-3′, 5′-AAACGCGCAGGTAATCCCAGAAGC-3′; and ApoE sgRNA2: 5′-CACCGGAGGACGTGCGCAACCGCT-3′, 5′-AAACAGCGGTTGCGCACGTCCTCC-3′). These complementary DNA oligos were annealed and ligated into BbsI (Thermo Fisher Scientific, Waltham, MA, USA)-digested pX330 (Addgene plasmid 423230) using thermal conditions 37°C for 30 min, 95°C for 5 min, and annealed by decreasing by 5°C/min to 25°C to generate the Cas9-sgRNA targeting plasmids for ApoE.
T7E1 cleavage assay
PFFs were isolated from Chinese Landrace fetuses at day 35 of gestation using 200 U/ml collagenase (Invitrogen) and 25 kU/ml DNaseI (Invitrogen), and cultured as previously described (Chen et al., 2015). PFFs transfected with or without Cas9-sgRNA targeting plasmids (as described above) were cultured for 48 h. Genomic DNA was extracted using a DNA extraction kit (TianGen, Beijing, China) and the genomic regions surrounding the CRISPR/Cas9 target site were PCR amplified. PCR primers used are as follows: 5′-CCTCAGGTGGTCTAGGTTGG-3′ and 5′-TTTTGCAATGGAGGAGTGGC-3′ for sgRNA1; 5′-CGCCTCTCTCTGTTCATTGC-3′ and 5′-TTTTGCAATGGAGGAGTGGC-3′ for sgRNA2. The PCR conditions were as follows: 95°C for 5 min, followed by 30 cycles of 95°C for 10 s, 60°C for 30 s, and 72°C for 40 s, and a final 72°C for 7 min. A total of 250 ng of the purified PCR product was mixed with NEB Buffer 2, denatured, and annealed to allow formation of heteroduplexes using the following conditions: 95°C for 5 min, 95°C to 85°C ramping at −2°C/s, 85°C to 25°C at −0.1°C/s, and 4°C hold. After reannealing, the products were digested with 1 μl of T7 endonuclease I (NEB, Beverly, MA, USA) at 37°C for 15 min and then run on a 2% agarose gel stained with ethidium bromide.
PFF transfection and selection
Approximately 5 μg of the ApoE Cas9-sgRNA targeting plasmid was co-transfected with 1 μg of the neomycin expression plasmid (pCMV-tdTomato) into 1×106 early passage of PFFs using the Basic Fibroblast Nucleofection Kit (Amaxa Biosystems/Lonza, Cologne, Germany), according to the manufacturer's instructions. A total of 800 μg/ml of G418 (Gibco, Grand Island, NY, USA) was applied to the post-transfection cells 48 h later and maintained for 10-14 days thereafter. The G418-resistant colonies were seeded in 24-well plates and then passaged to 12-well plates. Approximately 1/5 of the cells of a single colony were lysed with NP-40 (55°C for 30 min, 95°C for 10 min) for PCR screening and 4/5 of the remaining cells were used for SCNT. The primers used in amplifying the target regions were as follows: forward: 5′-CCTCAGGTGGTCTAGGTTGG-3′, reverse: 5′-TTTTGCAATGGAGGAGTGGC-3′. The PCR conditions were as follows: 95°C for 5 min, followed by 35 cycles of 95°C for 10 s, 60°C for 30 s, and 72°C for 50 s, and a final 72°C for 4 min. The PCR products were subcloned into a pMD18-T vector (Takara Clontech, Tokyo, Japan) according to the manufacturer's instructions. Fifteen to 20 individual clones were picked and sequenced.
SCNT and embryo transfer
The oocytes for SCNT were collected from ovaries purchased from a local slaughterhouse and cultured for 42-44 h in vitro. The mature oocytes were enucleated as described elsewhere (Li et al., 2017). A single targeted PFF cell was transferred into the perivitelline space of the enucleated oocyte. After electrofusion of membranes between the donor cell and recipient cytoplast, the reconstructed embryos were cultured in embryo development medium at 38.5°C for 24 h. About 250 reconstructed oocytes were transferred into the uterus of an estrus-synchronized recipient gilt. Pregnancy was confirmed by ultrasound 30 days after transplantation and monitored until the perinatal period. All cloned piglets were born by normal delivery.
Serum lipid chemistry
Whole blood was drawn from the jugular veins of overnight-fasted pigs at the indicated time points into serum-separating tubes (BD Diagnostics, Franklin Lakes, NJ, USA). Sera were prepared by centrifugation at 500 rcf for 15 min at room temperature. The levels of total cholesterol, triglycerides, HDL cholesterol and LDL cholesterol were determined by Dian Diagnostics (Nanjing, China).
Western blot analysis
Western blotting was performed with 10-fold diluted plasma samples or 20-50 μg of liver tissue extract. Proteins were separated on 15% SDS-PAGE gels and transferred to polyvinylidene fluoride membranes (Bio-Rad, Hercules, CA, USA). The membranes were incubated overnight at 4°C with anti-ApoE primary antibody (1:2000; Novus Biological, Littleton, CO, USA). The membranes were incubated with goat anti-rabbit IgG horseradish peroxidase (HRP)-conjugated secondary antibody (1:2000; CWBiotech, Shanghai, China) and proteins were detected with the SuperSignal West Pico Chemiluminescent Substrate (Thermo Fisher Scientific) with a ChemiDoc Touch Imaging System (Bio-Rad). β-tubulin was used as an internal control with anti β-tubulin primary antibody (1:10,000; Proteintech, Rosemont, IL, USA) and goat anti-mouse HRP-conjugated secondary antibody (1:2000; Santa Cruz Biotechnology, Santa Cruz, CA, USA).
Histology analysis
The aorta and coronary arteries were isolated from overnight-fasted pigs and fixed in 10% buffered formalin. Sudan IV was used to stain the atherosclerotic lesions. Images were collected on an Olympus FSX100 microscope (Olympus, Tokyo, Japan). The stained area was quantified using ImageJ software (NIH, Bethesda, MD, USA) and expressed as a percentage of the total image area. The aorta was divided into 1-cm segments taken perpendicular to the direction of blood flow and embedded in paraffin. H&E, Masson's trichrome and immunohistochemistry staining were conducted on 5 μm sections to confirm lesion details as described previously (Chen et al., 2018). CD68 monoclonal antibody (KP1; 1:100; Thermo Fisher Scientific) and HRP-conjugated goat anti-mouse secondary antibody (1:500; Proteintech, Rosemont, IL, USA) were used for immunostaining.
Statistical methods
All data are expressed as the means±s.d. P-values were determined using a 2-tailed Student's t-test or 1-way analysis of variance (ANOVA) with a Tukey's post hoc correction for multiple group comparisons. A P-value of less than 0.05 was considered statistically significant.
Supplementary Material
Acknowledgements
We are grateful to Dr Tao Fu for his critical reading of this manuscript.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: B.F., Y.D.; Methodology: B.F., Y.W., L. Zhao, C.L., Z.Z., L.C., J.L., X. Liu, L.L., H.W., H.Y., R.L., Y.D.; Software: B.F., M.Z., Z.Z., L.C., X. Li, L.L., H.Y.; Validation: B.F., Z.L., H.Y., R.L.; Formal analysis: B.F., X.R., Z.L., L. Zhao, X. Li, X. Liu, H.Y., R.L.; Investigation: L.C., R.L.; Resources: B.F., X.R., Z.L., C.L., J.L., Q.X., L. Zhang, Y.J., X. Liu, L.L., H.Y., R.L.; Data curation: B.F., X.R., Y.W., L. Zhao, M.Z., C.L., Z.Z., X. Li, Q.X., L. Zhang, Y.J., H.Y., Y.D.; Writing - original draft: B.F., H.Y.; Writing - review & editing: B.F., H.Y.; Supervision: B.F., J.L., H.W., H.Y., Y.D.; Project administration: B.F., Y.W., H.Y., Y.D.; Funding acquisition: Y.D.
Funding
This work was supported by grants from the National Natural Science Foundation of China (81570402 and 31701283), the National Key R&D Program of China (2017YFC1103701 & 2017YFC1103702), the Jiangsu Key Laboratory of Xenotransplantation (BM2012116), the Sanming Project of Medicine in Shenzhen (SZSM201412020), the Fund for High-Level Medical Discipline Construction of Shenzhen (2016031638), and the Shenzhen Foundation of Science and Technology (JCYJ20160229204849975 and GCZX2015043017281705).
Supplementary information
Supplementary information available online at http://dmm.biologists.org/lookup/doi/10.1242/dmm.036632.supplemental
References
- Aigner B., Renner S., Kessler B., Klymiuk N., Kurome M., Wünsch A. and Wolf E. (2010). Transgenic pigs as models for translational biomedical research. J. Mol. Med. 88, 653-664. 10.1007/s00109-010-0610-9 [DOI] [PubMed] [Google Scholar]
- Al-Mashhadi R. H., Sorensen C. B., Kragh P. M., Christoffersen C., Mortensen M. B., Tolbod L. P., Thim T., Du Y., Li J., Liu Y. et al. (2013). Familial hypercholesterolemia and atherosclerosis in cloned minipigs created by DNA transposition of a human PCSK9 gain-of-function mutant. Sci. Transl. Med. 5, 166ra1 10.1126/scitranslmed.3004853 [DOI] [PubMed] [Google Scholar]
- Bentzon J. F. and Falk E. (2010). Atherosclerotic lesions in mouse and man: is it the same disease? Curr. Opin. Lipidol. 21, 434-440. 10.1097/MOL.0b013e32833ded6a [DOI] [PubMed] [Google Scholar]
- Braesch-Andersen S., Paulie S., Smedman C., Mia S. and Kumagai-Braesch M. (2013). ApoE production in human monocytes and its regulation by inflammatory cytokines. PLoS ONE 8, e79908 10.1371/journal.pone.0079908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F., Wang Y., Yuan Y., Zhang W., Ren Z., Jin Y., Liu X., Xiong Q., Chen Q., Zhang M. et al. (2015). Generation of B cell-deficient pigs by highly efficient CRISPR/Cas9-mediated gene targeting. J. Genet. Genomics 42, 437-444. 10.1016/j.jgg.2015.05.002 [DOI] [PubMed] [Google Scholar]
- Chen Q., Fang B., Wang Y., Li C., Li X., Wang R., Xiong Q., Zhang L., Jin Y., Zhang M. et al. (2018). Overexpressing dominant-negative FGFR2-IIIb impedes lung branching morphogenesis in pigs. J. Genet. Genomics 45, 147-154. 10.1016/j.jgg.2018.02.002 [DOI] [PubMed] [Google Scholar]
- Chistiakov D. A., Myasoedova V. A., Revin V. V., Orekhov A. N. and Bobryshev Y. V. (2017). The phenomenon of atherosclerosis reversal and regression: lessons from animal models. Exp. Mol. Pathol. 102, 138-145. 10.1016/j.yexmp.2017.01.013 [DOI] [PubMed] [Google Scholar]
- Davies G., Harris S. E., Reynolds C. A., Payton A., Knight H. M., Liewald D. C., Lopez L. M., Luciano M., Gow A. J., Corley J. et al. (2014). A genome-wide association study implicates the APOE locus in nonpathological cognitive ageing. Mol. Psychiatry 19, 76-87. 10.1038/mp.2012.159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis B. T., Wang X.-J., Rohret J. A., Struzynski J. T., Merricks E. P., Bellinger D. A., Rohret F. A., Nichols T. C. and Rogers C. S. (2014). Targeted disruption of LDLR causes hypercholesterolemia and atherosclerosis in Yucatan miniature pigs. PLoS ONE 9, e93457 10.1371/journal.pone.0093457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichner J. E., Dunn S. T., Perveen G., Thompson D. M., Stewart K. E. and Stroehla B. C. (2002). Apolipoprotein E polymorphism and cardiovascular disease: a HuGE review. Am. J. Epidemiol. 155, 487-495. 10.1093/aje/155.6.487 [DOI] [PubMed] [Google Scholar]
- Estrada J. L., Martens G., Li P., Adams A., Newell K. A., Ford M. L., Butler J. R., Sidner R., Tector M. and Tector J. (2015). Evaluation of human and non-human primate antibody binding to pig cells lacking GGTA1/CMAH/beta4GalNT2 genes. Xenotransplantation 22, 194-202. 10.1111/xen.12161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiadou D., Chroni A., Vezeridis A., Zannis V. I. and Stratikos E. (2011). Biophysical analysis of apolipoprotein E3 variants linked with development of type III hyperlipoproteinemia. PLoS ONE 6, e27037 10.1371/journal.pone.0027037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiselli G., Schaefer E. J., Gascon P. and Breser H. B. Jr. (1981). Type III hyperlipoproteinemia associated with apolipoprotein E deficiency. Science 214, 1239-1241. 10.1126/science.6795720 [DOI] [PubMed] [Google Scholar]
- Hai T., Teng F., Guo R., Li W. and Zhou Q. (2014). One-step generation of knockout pigs by zygote injection of CRISPR/Cas system. Cell Res. 24, 372-375. 10.1038/cr.2014.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu P. D., Lander E. S. and Zhang F. (2014). Development and applications of CRISPR-Cas9 for genome engineering. Cell 157, 1262-1278. 10.1016/j.cell.2014.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L., Hua Z., Xiao H., Cheng Y., Xu K., Gao Q., Xia Y., Liu Y., Zhang X., Zheng X. et al. (2017). CRISPR/Cas9-mediated ApoE−/− and LDLR−/− double gene knockout in pigs elevates serum LDL-C and TC levels. Oncotarget 8, 37751-37760. 10.18632/oncotarget.17154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal J., Chamberlain J., Francis S. E. and Gunn J. (2016). Role of animal models in coronary stenting. Ann. Biomed. Eng. 44, 453-465. 10.1007/s10439-015-1414-4 [DOI] [PubMed] [Google Scholar]
- Langheinrich A. C., Michniewicz A., Bohle R. M. and Ritman E. L. (2007). Vasa vasorum neovascularization and lesion distribution among different vascular beds in ApoE−/−/LDL−/− double knockout mice. Atherosclerosis 191, 73-81. 10.1016/j.atherosclerosis.2006.05.021 [DOI] [PubMed] [Google Scholar]
- Li Y., Fuchimoto D., Sudo M., Haruta H., Lin Q. F., Takayama T., Morita S., Nochi T., Suzuki S., Sembon S. et al. (2016). Development of human-like advanced coronary plaques in low-density lipoprotein receptor knockout pigs and justification for statin treatment before formation of atherosclerotic plaques. J. Am. Heart Assoc. 5, e002779 10.1161/JAHA.115.002779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Yang H., Wang Y., Zhang M., Liu X., Xiong Q., Zhang L., Jin Y., Mou L., Liu Y. et al. (2017). Generation of tryptophan hydroxylase 2 gene knockout pigs by CRISPR/Cas9-mediated gene targeting. J. Biomed. Res. 31, 445-452. 10.7555/JBR.31.20170026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P., Ridker P. M. and Hansson G. K. (2011). Progress and challenges in translating the biology of atherosclerosis. Nature 473, 317-325. 10.1038/nature10146 [DOI] [PubMed] [Google Scholar]
- Libby P., Lichtman A. H. and Hansson G. K. (2013). Immune effector mechanisms implicated in atherosclerosis: from mice to humans. Immunity 38, 1092-1104. 10.1016/j.immuni.2013.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahley R. W. (1988). Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science 240, 622-630. 10.1126/science.3283935 [DOI] [PubMed] [Google Scholar]
- Mali P., Yang L., Esvelt K. M., Aach J., Guell M., DiCarlo J. E., Norville J. E. and Church G. M. (2013). RNA-guided human genome engineering via Cas9. Science 339, 823-826. 10.1126/science.1232033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastenbroek T. G., van Geffen J. P., Heemskerk J. W. M. and Cosemans J. M. E. M. (2015). Acute and persistent platelet and coagulant activities in atherothrombosis. J. Thromb. Haemost. 13 Suppl. 1, S272-S280. 10.1111/jth.12972 [DOI] [PubMed] [Google Scholar]
- Murray C. J. L., Vos T., Lozano R., Naghavi M., Flaxman A. D., Michaud C., Ezzati M., Shibuya K., Salomon J. A., Abdalla S. et al. (2012). Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380, 2197-2223. 10.1016/S0140-6736(12)61689-4 [DOI] [PubMed] [Google Scholar]
- Niimi M., Yang D., Kitajima S., Ning B., Wang C., Li S., Liu E., Zhang J., Eugene Chen Y. and Fan J. (2016). ApoE knockout rabbits: a novel model for the study of human hyperlipidemia. Atherosclerosis 245, 187-193. 10.1016/j.atherosclerosis.2015.12.002 [DOI] [PubMed] [Google Scholar]
- Niu Y., Shen B., Cui Y., Chen Y., Wang J., Wang L., Kang Y., Zhao X., Si W., Li W. et al. (2014). Generation of gene-modified cynomolgus monkey via Cas9/RNA-mediated gene targeting in one-cell embryos. Cell 156, 836-843. 10.1016/j.cell.2014.01.027 [DOI] [PubMed] [Google Scholar]
- Piedrahita J. A., Zhang S. H., Hagaman J. R., Oliver P. M. and Maeda N. (1992). Generation of mice carrying a mutant apolipoprotein E gene inactivated by gene targeting in embryonic stem cells. Proc. Natl. Acad. Sci. USA 89, 4471-4475. 10.1073/pnas.89.10.4471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plump A. S., Smith J. D., Hayek T., Aalto-Setälä K., Walsh A., Verstuyft J. G., Rubin E. M. and Breslow J. L. (1992). Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell 71, 343-353. 10.1016/0092-8674(92)90362-G [DOI] [PubMed] [Google Scholar]
- Reyes L. M., Estrada J. L., Wang Z. Y., Blosser R. J., Smith R. F., Sidner R. A., Paris L. L., Blankenship R. L., Ray C. N., Miner A. C. et al. (2014). Creating class I MHC-null pigs using guide RNA and the Cas9 endonuclease. J. Immunol. 193, 5751-5757. 10.4049/jimmunol.1402059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y., Yamada K., Liddelow S. A., Smith S. T., Zhao L., Luo W., Tsai R. M., Spina S., Grinberg L. T., Rojas J. C. et al. (2017). ApoE4 markedly exacerbates tau-mediated neurodegeneration in a mouse model of tauopathy. Nature 549, 523-527. 10.1038/nature24016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi S., Yin W., Wang Z., Kusunoki M., Lian X., Koike T., Fan J. and Zhang Q. (2004). A minipig model of high-fat/high-sucrose diet-induced diabetes and atherosclerosis. Int. J. Exp. Pathol. 85, 223-231. 10.1111/j.0959-9673.2004.00394.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J.-T., Tan L. and Hardy J. (2014). Apolipoprotein E in Alzheimer's disease: an update. Annu. Rev. Neurosci. 37, 79-100. 10.1146/annurev-neuro-071013-014300 [DOI] [PubMed] [Google Scholar]
- Zhang W., Wang G., Wang Y., Jin Y., Zhao L., Xiong Q., Zhang L., Mou L., Li R., Yang H. et al. (2017). Generation of complement protein C3 deficient pigs by CRISPR/Cas9-mediated gene targeting. Sci. Rep. 7, 5009 10.1038/s41598-017-05400-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.