Abstract
Background
The polymicrobial nature of diabetic foot infection (DFI) and the emergence of antimicrobial resistance have complicated DFI treatment. Current treatment guidelines for deep DFI recommend coverage of methicillin-resistant Staphylococcus aureus (MRSA) and susceptible Enterobacteriaceae. This study aimed to describe the epidemiology of DFI and to identify predictors for DFI associated with multidrug-resistant organisms (MDROs) and pathogens resistant to recommended treatment (PRRT).
Methods
Adult patients admitted to Detroit Medical Center from January 2012 to December 2015 with DFI and positive cultures were included. Demographics, comorbidities, microbiological history, sepsis severity, and antimicrobial use within 3 months before DFI were obtained retrospectively. DFI-PRRT was defined as a DFI associated with a pathogen resistant to both vancomycin and ceftriaxone. DFI-MDRO pathogens included MRSA in addition to PRRT.
Results
Six-hundred forty-eight unique patients were included, with a mean age of 58.4 ± 13.7 years. DFI-MDRO accounted for 364 (56%) of the cohort, and 194 (30%) patients had DFI-PRRT. Independent predictors for DFI-PRRT included history of PRRT in a diabetic foot ulcer, antimicrobial exposure in the prior 90 days, peripheral vascular disease, and chronic kidney disease. Long-term care facility residence was independently associated with DFI due to ceftriaxone-resistant Enterobacteriaceae, and recent hospitalization was an independent predictor of DFI due to vancomycin-resistant Enterococcus.
Conclusions
An unexpectedly high prevalence of DFI-PRRT pathogens was identified. History of the same pathogen in a prior diabetic foot ulcer and recent antimicrobial exposure were independent predictors of DFI-PRRT and should be considered when selecting empiric DFI therapy.
Keywords: diabetic foot infection, empiric therapy, multidrug-resistant organisms, PRRT
The choice of antibiotic agents for diabetic foot infection (DFI) is challenging considering the complex microbiology of DFI, which is often polymicrobial [1]. In addition, patients who have diabetic foot ulcers (DFUs) frequently have conditions that are associated with colonization and/or infection with multidrug-resistant organisms (MDROs), including frequent health care exposures, chronic wound care, and recurrent and prolonged antibiotic treatment courses [2].
The microbiology of DFI has been assessed in several studies outside the United States, and geographic variation of predominant pathogens has been reported [1, 3–5]. In addition, microbiological variation exists as a function of the acuity and depth of DFI. Whereas acute and superficial infections tend to be monomicrobial and the most common pathogens are Gram-positive cocci, chronic and deep infections are more commonly polymicrobial and more frequently involve Gram-negative bacilli [6].
Current national guidelines from the Infectious Diseases Society of America (IDSA) recommend empiric treatment of severe and deep moderate DFI with antimicrobial agents that have activity against Staphylococcus aureus, Streptococci spp., and Enterobacteriaceae, whereas empiric coverage of MDROs is recommended only if MDROs are common in the locale where the infection is being managed or if specific MDRO risk factors are present.
Although early appropriate antibiotic therapy may be associated with favorable outcomes (such as wound healing and limb salvage) [3, 4], overuse of broad-spectrum antibiotics may further contribute to the occurrence of DFI associated with MDROs. As MRSA prevalence is relatively high throughout the United States, guidelines recommend that empiric therapy typically include MRSA coverage. However, routine Pseudomonas aeruginosa coverage is not advocated, and guideline-recommended empiric antimicrobial regimens generally target more susceptible forms of Enterobacteriaceae. Thus, in accordance with IDSA guidelines and based on local susceptibility patterns of S. aureus and Enterobacteriaceae, vancomycin + ceftriaxone is a commonly employed empiric regimen for DFI at the Detroit Medical Center (DMC).
Data related to the overall microbiological epidemiology of DFI in the United States and risk factors for DFI due to MDROs are scarce. Furthermore, risk factors for specific populations with DFI due to pathogens that are resistant to recommended empiric therapeutic regimens, such as vancomycin + ceftriaxone, are unknown.
The primary objectives of this study were to describe the microbiologic and clinical epidemiology of DFI in patients who were admitted to the DMC and to identify risk factors for MDRO pathogens (DFI-MDRO). The secondary objective was to identify risk factors for DFI associated with pathogens resistant to vancomycin + ceftriaxone in order to identify patients who warrant empiric therapy that provides a broader spectrum of antimicrobial coverage.
METHODS
Study Setting and Cohort Description
Multiple nested retrospective case–control studies were performed within a large cohort to determine predictors of DFI associated with resistant pathogens. All adult patients with DFI who were admitted to the DMC (a health system including 4 acute care hospitals and 1 rehabilitation center) between January 2012 and December 2015 with positive cultures from DFI lesions were included. DFI subjects were identified based on International Classification of Diseases, 9th revision (ICD-9), codes for diabetes mellitus and either skin and soft tissue infection (SSTI) and/or osteomyelitis (ICD-9 codes that were used were 249, 250, 680–686, 730). In addition, admission and discharge notes, as well as podiatry and infectious diseases consultant notes, were reviewed. Confirmation of an actual diabetic foot infection was corroborated by documented signs and symptoms of infection (erythema, warmth, pus drainage and/or fetid odor). Patients were excluded from the study if (1) infection status of the ulcer could not be determined from chart review or infection was ruled out by care providers, (2) the SSTI was not related to the foot, (3) infection following a fracture and/or a surgical site infection was present, (4) the only organisms recovered from DFI were coagulase-negative staphylococcus and/or corynebacterium (unless recovered on multiple occasions under sterile conditions [eg, in the operative room and/or from bone]).
This study was approved by the institutional review boards of Wayne State University and the Detroit Medical Center.
Definitions
The date of DFI diagnosis was defined as the day of first positive culture for a DFI episode. An index episode was defined as the following: among subjects who had an episode of DFI associated with an MDRO during the study period, the index episode was the first DFI episode associated with an MDRO (DFI-MDRO). Among subjects who did not have a DFI associated with an MDRO during the study period, the index episode was the first DFI episode during the study period and was considered DFI-Non-MDRO. Prior and recurrent DFI episodes for all subjects were captured throughout the study period. Thus, during the study period, all DFI episodes for study subjects were captured.
For the purposes of this analysis, MDROs included methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant Enterococci (VRE), Enterobacteriaceae resistant to third-generation cephalosporins and/or to a carbapenem, and all antimicrobial susceptibility phenotypes of Pseudomonas aeruginosa, Acinetobacter baumannii, and Stenotrophomonas maltophilia.
Guidelines for empiric treatment of DFI recommended coverage of MRSA and susceptible strains of Enterobacteriaceae. As ceftriaxone offered a significant in vitro activity advantage over earlier-generation cephalosporins at the DMC, ceftriaxone was considered an appropriate empiric regimen for susceptible strains of Enterobacteriaceae [1]. Therefore, for this study, pathogens resistant to recommended empiric treatment (PRRT) were defined as aerobic Gram-negative bacilli resistant to ceftriaxone (including resistant strains of Enterobacteriaceae and all strains of P. aeruginosa, A. baumannii, and S. maltophilia) and Enterococci resistant to vancomycin. MRSA was excluded from the PRRT definition. As coverage for anaerobic organisms is controversial and not routinely recommended in guidelines, these organisms were not considered with regards to determining the appropriateness of therapy.
Study Variables
Data pertaining to demographics, source of admission (home, long-term care facility [LTCF], transfer from another hospital), hospitalization within the past 90 days, comorbidities included in Charlson Comorbidity Index (according to ICD-9 codes and/or physician documentation), insurance type, severity of sepsis at time of DFI episode diagnosis (defined by the most abnormal values of systemic inflammatory response syndrome score and vital signs within 2 days of DFI diagnosis) [7], admission unit, highest HbA1C value within 3 months of DFI episode, diabetes-related end-organ damage, and ankle-brachial index (ABI) values were obtained from the medical record. The depth of involvement of DFI was determined based on providers’ documentation, radiology findings, and pathology findings and was classified as superficial, deep tissue, or bone involvement [8]. Antimicrobial treatment information in the prior 3 months was abstracted from the medical record. Intensive care unit (ICU) admission and mechanical ventilation and acute hemodialysis status were captured within 7 days of the date of the DFI diagnosis. Surgical interventions were recorded for current and prior DFI episodes, including bedside debridement, operating room debridement, and amputations.
Microbiology
Microbiology data for each patient included cultures obtained from DFI lesions and were categorized according to the anatomic depth of the lesion from which the culture was obtained and the type of specimen obtained. Specimen types were categorized as swab cultures obtained at the bedside, tissue cultures obtained at the bedside, swab cultures obtained in the operating room, tissue cultures obtained in the operating room, and bone cultures collected in the operating room. For a given episode, all cultures from the DFI site that were obtained within a period of 14 days of the initial culture (and thus, within 14 days of the index episode) were considered part of the index DFI episode. A polymicrobial episode was defined as an episode during which more than 1 pathogen was recovered. Bloodstream infection associated with DFI was defined as growth of the same pathogen in the blood as was recovered from the DFI lesion within 7 days of DFI diagnosis. A prior recovery of an MDRO in a diabetic foot ulcer was defined as a history of infection or colonization of a DFU with 1 or more MDROs during the study period and, for subjects enrolled during the first year of the study, for 1 year before the subject’s index DFI episode.
Data Analysis
Epidemiology of the cohort and prevalence of MDROs were calculated using means and standard deviations, and medians with interquartile ranges (IQRs). To identify risk factors for DFI associated with MDRO (DFI-MDRO) and for DFI associated with PRRT (DFI-PRRT), patients who had DFI-MDRO were compared with patients who had DFI associated with susceptible pathogens (DFI-Non-MDRO), and patients who had DFI-PRRT were compared with patients who had DFI associated with non-PRRT pathogens (DFI-Non-PRRT), using the t test or Wilcoxon rank-sum test for continuous variables and the Fisher exact test or chi-square test for dichotomous and categorical variables. Risk factor analyses for individual pathogens were performed using the same methodology as was used to determine risk factors for the PRRT group. For example, patients who had DFI associated with P. aeruginosa (DFI-P. aeruginosa) were compared with patients who had DFI associated with all other pathogens, excluding P. aerugniosa (DFI-Non-P. aeruginosa).
Multivariable analysis was performed using logistic regression. Variables with a P value of <.2 in bivariable analysis were included in the candidate multivariable model (“primary model”). Backwards stepwise regression was performed to identify independent predictors for DFI-MDRO. Variables not selected were evaluated for confounding. If a variable impacted the Beta coefficient value of 1 or more of the selected variables by ≥10%, it was considered a confounder and was left in the model. Similar analysis methodology was implemented to identify independent predictors for DFI-PRRT and for each of PRRT pathogens individually. All P values were 2-sided.
RESULTS
Cohort
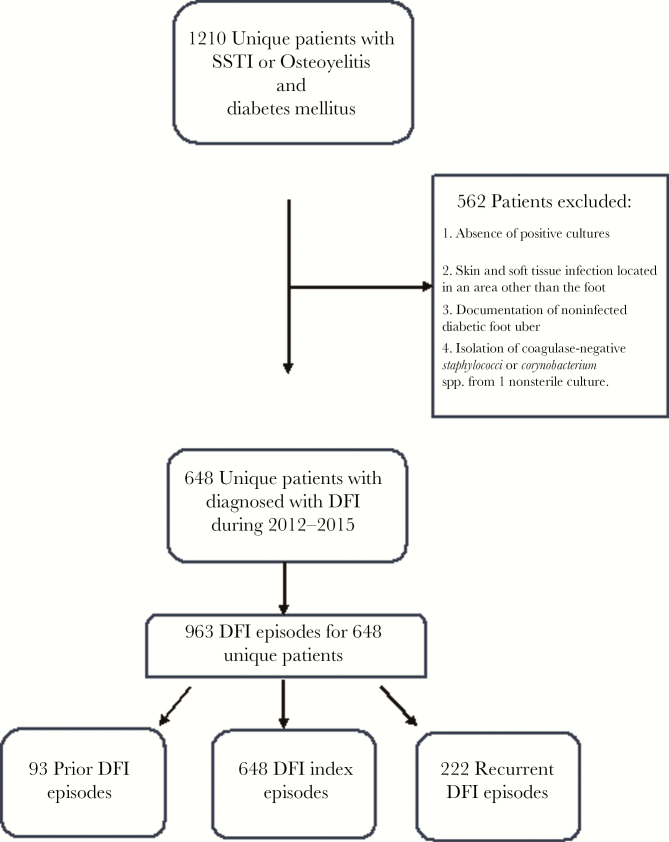
Between 2012 and 2015, 1210 subjects who had diabetes mellitus, SSTI and/or osteomyelitis, and a wound culture obtained were identified. Five hundred sixty-two patients were excluded for the following reasons: absence of positive cultures, SSTI located in an area other than the foot, documentation of noninfected DFU, or isolation of coagulase-negative Staphylococci or Corynobacterium spp. from a single nonsterile culture. Six-hundred forty-eight unique patients were determined to have DFI per the study definition and were included in the study cohort (Figure 1). Overall, there were 963 DFI episodes throughout the entire study period, 648 were categorized as index episodes, 93 were DFI episodes occurring before MDRO-DFI, and 222 were recurrent DFI episodes, following the index episode.
Figure 1.
Flow chart of patients who were included and excluded from the study cohort. Abbreviations: DFI, diabetic foot infection; SSTI, skin and soft tissue infection.
Prevalence of DFI During the Entire Study Period
There were 2450 organisms recovered from DFI lesions of the 963 episodes during the study period (Table 1). Seventy-two percent (n = 691) of the episodes were polymicrobial. The median number of pathogens recovered from DFI (IQR) was 2 (1–3). S. aureus (MSSA or MRSA) accounted for 57% of monomicrobial cultures.
Table 1.
Number of Organisms Recovered From DFI Lesions During the Entire Study Period and During the Index Episode
| Pathogen | Pathogens During Entire Study Period (No. Episodes = 963), No. (%) |
Pathogens During Index Episode (No. Episodes = 648), No. (%) |
|
|---|---|---|---|
| Gram-positivea | 829 (86.0) | 579 (89.4) | |
| MRSA | 299 (31.4) | 224 (34.6) | |
| MSSA | 231 (23.6) | 160 (24.7) | |
| Streptococci | 265 (32.0) | 195 (30.1) | |
| Enterococci (vancomycin-susceptible) | 229 (23.8) | 166 (25.6) | |
| VRE | 68 (7.1) | 52 (8.0) | |
| Coagulase-negative Staphylococci | 129 (13.4) | 115 (17.8) | |
| Gram-negativea | 547 (56.8) | 375 (57.9) | |
| Enterobacteriaceae (ceftriaxone-susceptible) | 394 (40.9) | 275 (42.4) | |
| Enterobacteriaceae (ceftriaxone-resistant) | 74 (7.7) | 51 (7.9) | |
| CRE | 6 (0.6) | 6 (0.6) | |
| P. aeruginosa | 131 (13.6) | 94 (14.5) | |
| A. baumannii | 29 (3.0) | 22 (3.4) | |
| Other GNB | 74 (7.9) | 57 (8.8) | |
| Anaerobes | 197 (28.8) | 160 (24.7) | |
| Othersb | 124 (12.9) | 115 (17.7) | |
Abbreviations: CRE, carbapenem-resistant Enterobacteriaceae; GNB, Gram-negative bacilli; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-susceptible Staphylococcus aureus; VRE, vancomycin-resistant Enterococci.
aThe percentages for each of the pathogens represent the proportion of pathogens of number of episodes. As some of the patients had more than 1 pathogen per episode, the sum of the percentages is >100%.
bOthers: Corynobacterium spp., Candida spp.
Most of the organisms (68%) grew from samples obtained in the operating room (Supplementary Figures 1 and 2): 18% were obtained from the bone, 28% were obtained from deep tissue, and 22% were obtained from swabs. In addition, 9% of the organisms grew from tissue cultures that were obtained at the bedside, and 22% grew in swab cultures that were obtained at the bedside. The distribution of organism source was consistent across all types of bacterial species included in the cohort.
Of 963 episodes (Table 1), DFI was associated with Gram-positive cocci (GPC) in 86% of the episodes, and S. aureus was the most common pathogen (53% of GPC; MRSA represented 58.5% of S. aureus). Gram-negative bacilli were present in 56.8% of DFI episodes, and Enterobacteriaceae were the most common Gram-negative pathogens (80.6%).
More than half of all episodes (n = 495; 51.4%) were associated with at least 1 MDRO. The most common MDRO was MRSA (299 episodes; present in 60% of MDRO episodes), followed by P. aeruginosa (n = 131; 26.4% of MDRO episodes), resistant Enterobacteriaceae (including ceftriaxone-resistant Enterobacteriaceae and carbapenem-resistant Enterobacteriaceae; n = 79; 15.9% of MDRO episodes), and VRE (n = 68; 13.7% of MDRO episodes).
Demographics and Epidemiology of Index Episode and Risk Factors for DFI-MDRO
Six hundred forty-eight unique patients had index DFI episodes during the study period. The mean age of this cohort was 58.4 ± 13.7 years, 64.3% were male, and 72.8% were African American. The majority of patients (86.9%) were admitted from home (Table 1). One hundred ninety-seven patients (30.4%) were hospitalized within the 90 days before the DFI index episode. The median Charlson Comorbidity Index score (IQR) was 5 (3–6). Additional characteristics of the cohort are presented in Table 2. Approximately one-quarter of the patients presented with severe sepsis or septic shock. The majority of patients who were evaluated for depth of infection were categorized as having deep infections (n = 586; 90.4%), and 407 of these (69.5%) involved bone.
Table 2.
Cohort Description and Bivariable and Multivariable Analyses of Risk Factors for DFI Associated With MDRO and PRRT
| Entire Cohort (n = 648) |
DFI-MDRO (n = 364) |
DFI-Non-MDRO (n = 293) |
P Value Unadjusted | Adjusted ORe (95% CI) |
DFI-PRRT (n = 194) |
DFI-Non-PRRT (n = 454) |
P Value Unadjusted | Adjusted ORf (95% CI) |
|
|---|---|---|---|---|---|---|---|---|---|
| Age, mean ± SD, y | 58.4 ± 13.7 | 59.2 ± 13.8 | 57.4 ± 13.6 | .09 | 61.3 ± 13.7 | 57.1 ± 13.6 | <.001 | ||
| Female | 231 (35.7) | 135 (37.1) | 96 (33.8) | .41 | 73 (37.6) | 158 (34.8) | .53 | ||
| Insurance type | .26 | <.001 | |||||||
| Private | 168 (25.9) | 88 (24.2) | 80 (28.3) | 42 (21.7) | 126 (27.8) | ||||
| Medicare | 320 (49.4) | 190 (52.2) | 130 (45.8) | 119 (61.3) | 201 (44.3) | ||||
| Medicaid or no insurance | 160 (24.7) | 86 (23.6) | 77 (26.2) | 33 (17.0) | 127 (28.0) | ||||
| Origin | .27 | .15 | |||||||
| Home | 563 (86.9) | 307 (84.3) | 256 (90.1) | 159 (82.8) | 404 (90.0) | ||||
| LTCF | 58 (8.95) | 40 (11.0) | 18 (6.3) | 27 (13.9) | 31 (6.8) | ||||
| Other | 20 (3.1) | 14 (3.9) | 6 (2.4) | 6 (3.1) | 14 (3.1) | ||||
| LTCF (vs others) | 58 (8.95) | 40 (11.0) | 18 (6.3) | .05 | 1.48 (0.74–2.95) | 20 (14.0) | 38 (7.5) | .006 | 1.47 (0.76–2.85) |
| Dependent status (bedridden) | 110 (17.7) | 74 (21.1) | 36 (13.2) | .01 | 1.32 (0.81–2.16) | 48 (25.8) | 62 (14.2) | <.001 | 1.62 (0.99–2.67) |
| Recent hospitalization | 197 (30.4) | 141 (38.7) | 56 (19.7) | <.001 | 1.53 (0.99–2.38) | 91 (46.9) | 106 (23.4) | <.001 | 1.25 (0.77–2.02) |
| Comorbid conditions | |||||||||
| Charlson Comorbidity Index, median (IQR) | 5 (3–6) | 5 (3–7) | 4 (3–6) | <.001 | 5 (4–7) | 4 (3–6) | <.001 | ||
| CHF | 210 (32.4) | 125 (34.3) | 85 (29.9) | .24 | 74 (38.1) | 136 (30.0) | .04 | ||
| Chronic lung disease | 158 (24.4) | 97 (26.7) | 61 (21.5) | .15 | 61 (31.4) | 97 (21.4) | .007 | ||
| CKD | 183 (28.2) | 118 (32.4) | 65 (22.9) | .008 | 1.48 (1.00–2.18) | 75 (38.7) | 108 (23.8) | <.001 | 1.56 (1.04–2.34) |
| PVD | 442 (68.2) | 270 (74.2) | 172 (60.6) | <.001 | 1.45 (1.00–2.09) | 163 (84.0) | 279 (61.5) | <.001 | 2.38 (1.51–3.77) |
| Malignancy | 61 (9.4) | 30 (8.2) | 31 (10.9) | .29 | 19 (9.8) | 42 (9.3) | .88 | ||
| Dementia | 62 (9.6) | 39 (10.7) | 23 (8.1) | .28 | 23 (11.9) | 39 (8.6) | .24 | ||
| Cerebrovascular disease | 123 (19.0) | 80 (22.0) | 43 (15.1) | .03 | 43 (22.2) | 80 (17.6) | .19 | ||
| Diabetes-associated conditions | |||||||||
| Retinopathy | 117 (18.1) | 68 (18.7) | 49 (17.3) | .68 | 38 (19.6) | 79 (17.4) | .51 | ||
| Neuropathy | 535 (82.6) | 308 (84.6) | 227 (79.9) | .14 | 162 (83.5) | 373 (82.2) | .74 | ||
| HbA1c, mean ± SD | 9 ± 2.6 | 8.8 ± 2.5 | 9.4 ± 2.8 | .01 | 8.6 ± 2.4 | 9.3 ± 2.7 | .004 | ||
| ABI, median (IQR) (n = 243) |
0.99 (0.68–1.19) |
0.99 (0.69–1.20) |
0.99 (0.67–1.17) |
.38 | 0.99 (0.79–1.10) |
0.99 (0.66–1.20) |
.85 | ||
| Max glucose on presentation >300 mg/dL | 249 (38.4) | 134 (36.8) | 115 (40.5) | .37 | 65 (33.5) | 184 (40.5) | .09 | ||
| Min glucose on presentation <70 mg/dL | 130 (20.1) | 80 (22.0) | 50 (17.6) | .20 | 46 (23.7) | 84 (18.5) | .14 | ||
| BMI, median (IQR), kg/m2 | 29.7 (25.7–35.3) | 29.4 (25.4–34.7) | 30.2 (26.0–35.8) | .15 | 29.4 (25.5–34.6) | 30 (25.8–35.5) | .28 | ||
| Severity of illness | |||||||||
| Severe sepsis and septic shockb | 154 (23.8) | 98 (26.9) | 56 (19.7) | .03 | 51 (26.3) | 103 (22.7) | .36 | ||
| BSI | 64 (9.9) | 39 (10.9) | 25 (8.8) | .43 | 18 (9.3) | 46 (10.1) | .78 | ||
| ICU admissionc | 62 (9.6) | 39 (10.7) | 21 (7.4) | .17 | 23 (11.9) | 37 (8.2) | .14 | ||
| Mechanical ventilation | 24 (3.7) | 15 (4.1) | 9 (3.2) | .68 | 13 (6.7) | 11 (2.4) | .01 | ||
| Acute kidney injury | 155 (23.9) | 91 (25.0) | 64 (22.5) | .52 | 47 (24.2) | 108 (23.8) | .92 | ||
| Highest WBC, median (IQR) (n = 289)a |
16.4 (13.9–20.3) | 16.6 (13.9–20.7) | 16.3 (13.7–19.3) | .35 | 17.7 (14.7–21.7) | 16.2 (13.7–19.7) | .12 | ||
| MAP <65 mmHg | 95 (18.3) | 66 (18.1) | 29 (10.2) | .005 | 46 (23.7) | 49 (10.8) | <.001 | ||
| Bone involvement | 407 (62.8) | 243 (66.8) | 164 (57.8) | .02 | 136 (70.1) | 271 (59.7) | .01 | ||
| Osteomyelitis per pathology | 276 (42.6) | 157 (43.1) | 119 (41.9) | .81 | 89 (45.9) | 187 (41.2) | .30 | ||
| Prior DFId | 58 (9.0) | 16 (13.5) | 3 (4.2) | .05 | 10 (16.1) | 9 (7.0) | .07 | ||
| History of diabetic foot ulcer associated with MDRO or PRRT, respectively | 74 (11) | 58 (15.9) | 16 (5.6) | <.001 | 2.62 (1.39–4.96) |
21 (10.8) | 20 (4.4) | .004 | 2.45 (1.20–5.01) |
| Prior debridement of foot ulcer/infection | 180 (27.8) | 125 (34.3) | 55 (19.4) | <.001 | 1.26 (0.83–1.93) |
70 (36.1) | 110 (24.2) | .003 | 0.99 (0.64–1.55) |
| Prior antibiotic use (past 3 mo) | 239 (36.9) | 168 (46.2) | 71 (25.0) | <.001 | 1.81 (1.21–2.72) |
112 (57.7) | 127 (28.0) | <.001 | 2.79 (1.81–4.3) |
| BLBLI | 83 (12.8) | 66 (18.1) | 17 (6.0) | <.001 | 47 (24.2) | 36 (7.9) | <.001 | 2.16 (1.18–3.97) |
|
| Third-generation cephalosporin | 33 (5.0) | 25 (6.9) | 8 (2.8) | .02 | 15 (7.7) | 18 (4.0) | .05 | ||
| Cefepime | 37 (5.7) | 29 (8.2) | 8 (2.8) | .006 | 22 (11.3) | 15 (3.3) | <.001 | 1.85 (0.79–4.35) |
|
| Quinolones | 50 (7.7) | 38 (10.4) | 12 (4.2) | .003 | 23 (11.9) | 27 (6.0) | .02 | 1.31 (0.66–2.58) |
|
| Vancomycin | 117 (18.1) | 94 (25.8) | 23 (8.1) | <.001 | 64 (33.0) | 53 (11.7) | <.001 | 1.44 (0.80–2.57) |
|
| Clindamycin | 41 (6.3) | 31 (8.5) | 10 (3.5) | .009 | 22 (11.3) | 19 (4.2) | .001 | 1.96 (0.94–4.11) |
|
Data are No. (%) unless otherwise presented.
Abbreviations: ABI, ankle-brachial index; BLBLI, beta-lactam/beta-lactamase inhibitor; BMI, body mass index; BSI, bloodstream infection; CHF, congestive heart failure; CKD, chronic kidney disease; DFI, diabetic foot infection; ICU, intensive care unit; LTCF, long-term care facility, including nursing home and long-term facilities; MAP, mean arterial pressure; MI/CAD, myocardial infarction and/or coronary artery diseases with stent inserted; PRRT, pathogens resistant to empiric recommended treatment; TMP/SMZ, trimethoprim, sulfamethoxazole; WBC, white blood cell count.
aHighest WBC was calculated among patients who had WBC >12 000.
bDefined as systemic inflammatory response syndrome accompanied by end organ compromise, with or without the need for vasopressors support.
cWithin 7 days of DFI diagnosis.
dFor the analysis of prior DFI episode, only patients who had DFIs during the first year of the study period were included (n = 190) because patients with DFI-Non-MDRO could only have prior DFI episodes if they were before the study period (per inclusion criteria, among DFI-Non-MDRO, the first episode within the study period was included, whereas for DFI-MDRO, the first DFI-MDRO episode was included).
eDFI-MDRO model was adjusted for prior hospitalization, LTCF residency, dependent status, and prior debridement. Odds ratios of variables that were included in the final model are presented.
fDFI-PRRT model was adjusted for prior hospitalization, LTCF residency, dependent status, prior debridement, prior use of cefepime, quinolones, vancomycin, and clindamycin. Odds ratios of variables that were included in the final model are presented.
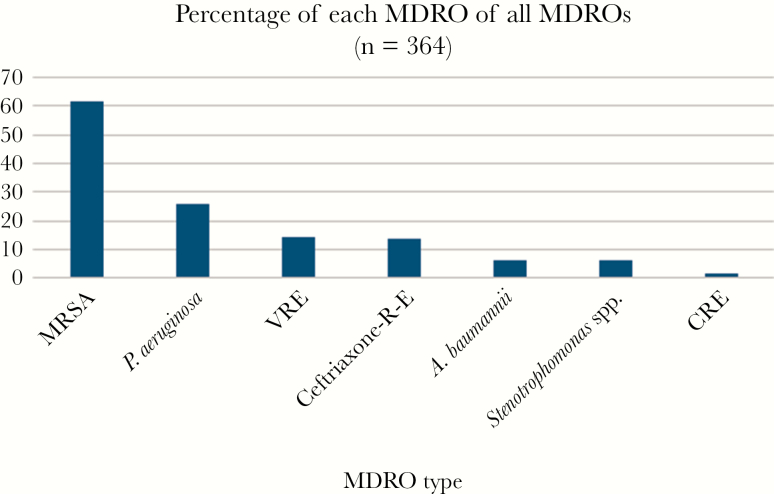
Three-hundred sixty-four patients (56.2%) had at least 1 MDRO (DFI-MDRO). The most common MDRO was MRSA (n = 224 patients; 61.5% of patients who had DFI-MDRO), followed by P. aeruginosa (n = 94; 26% of DFI-MDRO), ceftriaxone-resistant Enterobacteriaceae, and VRE (Figure 2). Most cultures were polymicrobial and were obtained in the operating room or from bone biopsy (70%).
Figure 2.
Index episode, diabetic foot infection– multidrug-resistant organism types. As some patients had >1 multidrug-resistant organism, the sum of the percentages is >100%. Abbreviations: Ceftriaxone-R E, ceftriaxone-resistant Enterobacteriaceae; CRE, carbapenem-resistant Enterobacteriaceae; MDRO, multidrug-resistant organism; MRSA, methicillin-resistant Staphylococcus aureus; VRE, vancomycin-resistant Enterococcus spp.
In bivariable analysis, patients who had DFI-MDRO were more likely to have comorbid conditions and to be hospitalized within 90 days before the index episode than patients who had DFI-Non-MDRO (39% vs 19.8%; P < .001) (Table 2). Severe sepsis and septic shock occurred more frequently upon presentation in patients with DFI-MDRO compared with those with DFI-Non-MDRO (26.9% vs 19.7%, respectively; P = .03). Interestingly, both groups had poor glycemic control (mean HbA1C, 9 ± 2.6 for the entire cohort), but patients who had DFI-MDRO had significantly lower mean HbA1C values than patients who had DFI-Non-MDRO (8.8 vs 9.4; P = .02) (Table 2).
In multivariable analysis, independent predictors for DFI-MDRO included treatment with any antibiotic within 90 days before the index episode (odds ratio [OR], 1.81; 95% confidence interval [CI], 1.21–2.72), history of colonization or infection of a diabetic foot lesion with an MDRO (OR, 2.62; 95% CI, 1.39–4.96), peripheral vascular disease (PVD; OR, 1.45; 95% CI, 1.00–2.09), and chronic kidney disease (CKD; OR, 1.48; 95% CI, 1.00–2.18) (Table 2).
Risk Factors for DFI-PRRT
Patients with DFI-PRRT accounted for 29.9% of DFI index episodes (n = 194). Most DFI-PRRT were due to P. aeruginosa (Figure 2). In bivariable analysis, patients with DFI-PRRT were older and had higher prevalence of comorbid conditions than did patients who had DFI due to being non-PRRT (DFI-Non-PRRT). In addition, patients with DFI-PRRT were more likely to be admitted from an LTCF (14% vs 7.5%; P = .006) and to be hospitalized in the 90 days before the index episode (46.9% vs 23.4%; P < .001) (Table 2). Patients who had DFI-PRRT had significantly lower mean HbA1C values than did patients who had DFI-Non-PRRT (8.6 vs 9.3; P = .004).
In multivariable analysis, independent predictors for DFI-PRRT included PVD (OR, 2.38; 95% CI, 1.51–3.77), CKD (OR, 1.56; 95% CI, 1.04–2.34), history of colonization or infection of a diabetic foot ulcer with a PRRT (OR, 2.45; 95% CI, 1.20–5.01), and prior use of a beta-lactam beta-lactamase inhibitor combination (OR, 2.16; 95% CI, 1.18–3.97) (Table 2).
Risk Factors for DFI Associated With Individual Pathogens Among the PRRT Group
Cohort characteristics and risk factors for each individual pathogen are presented in Supplementary Table 1. There were 94 patients with DFI associated with P. aeruginosa, 51 with ceftriaxone-resistant Enterobacteriaceae, and 52 with VRE. Of note, patients with DFI associated with ceftriaxone-resistant Enterobacteriaceae had the highest rate of severe sepsis and septic shock (40%).
In multivariable analysis, having had a prior diabetic foot culture positive for the same PRRT pathogen was an independent predictor for DFI associated with P. aeruginosa (OR, 3.06; 95% CI, 1.14–8.20) and for DFI associated with VRE (OR, 7.83; 95% CI, 1.82–33.64) (Table 3).
Table 3.
Independent Predictors for Individual DFI-PRRT
| PAb vs Non-PA, OR (95% CI) (n = 94) |
VREc vs Non-VRE, OR (95% CI) (n = 52) |
3GCE-REd vs Non-3GCE-RE, OR (95% CI) (n = 51) |
|
|---|---|---|---|
| Recent hospitalization | 1.50 (0.91–2.46) |
3.37 (1.66–6.84) |
1.00 (0.46–2.21) |
| Residence of LTCF | 1.11 (0.51–2.41) |
a | 3.85 (1.58–9.39) |
| Peripheral vascular disease | 1.68 (0.95–2.96) |
17.51 (2.37–129.21) |
3.45 (1.29–9.25) |
| Congestive heart failure | a | a | 1.31 (0.68–2.52) |
| Dementia | a | a | 1.70 (0.68–4.27) |
| Dependent status (bedridden) | 1.74 (0.98–3.09) |
a | 1.21 (0.51–2.86) |
| Prior debridement | a | 0.97 (0.51–1.86) |
a |
| History of diabetic foot ulcer associated with PA | 3.06 (1.14–8.20) |
a | a |
| History of diabetic foot ulcer associated with VRE | a | 7.83 (1.82–33.64) |
a |
| History of diabetic foot ulcer associated with 3GCE-RE | a | a | 8.95 (0.76–105.60) |
| Prior BLBLI use | a | a | 2.69 (1.18–6.15) |
| Prior cephalosporin, third generation | a | a | 2.95 (1.11–7.88) |
| Prior cefepime use | 2.41 (1.09–5.34) |
a | 3.27 (1.27–8.40) |
| Prior vancomycin use | a | 1.67 (0.83–3.37) |
a |
Abbreviations: 3GCE-RE, third-generation cephalosporin-resistant Enterobacteriaceae; BLBLI, beta-lactam/beta-lactamase inhibitor; DFI, diabetic foot infection; LTCF, long-term care facility; NS, nonsignificant; PA, Pseudomonas aeruginosa; PRRT, pathogens resistant to empiric recommended treatment; VRE, vancomycin-resistant Enterococcus spp.
aVariables were not included in the model.
bControlled for LTCF, dependent status, recent hospitalization, chronic kidney disease, peripheral vascular disease, prior quinolone use, history of PA in a diabetic foot ulcer, and prior use of cefepime.
cControlled for LTCF, chronic kidney disease, prior debridement, and prior vancomycin use.
dControlled for dependent status, recent hospitalization, congestive heart failure, dementia, and history of 3GCE-RE in a diabetic foot ulcer.
Different prior antibiotic exposures were significantly associated with different types of Gram-negative PRRT. Prior use of beta-lactam/beta-lactamase inhibitor (BLBLI), third- and fourth-generation cephalosporins was an independent predictor for DFI associated with ceftriaxone-resistant Enterobacteriaceae (BLBLI: OR, 2.69; 95% CI, 1.18–6.15; third-generation cephalosporins: OR, 2.95; 95% CI, 1.11–7.88; cefepime: OR, 3.27; 95% CI, 1.27–8.40), and prior use of cefepime was an independent predictor for DFI due to P. aeruginosa (OR, 2.41; 95% CI, 1.09–5.34).
With regards to prior health care exposure, residence in an LTCF was an independent predictor only for DFI associated with ceftriaxone-resistant Enterobacteriaceae (OR, 3.85; 95% CI, 1.58–9.39), and recent hospitalization was an independent predictor only for DFI associated with VRE (OR, 3.37; 95% CI, 1.66–6.84) (Table 3).
The only comorbid condition that was a PRRT risk factor was PVD, which was significantly associated with DFI-VRE (OR, 17.51; 95% CI, 2.37–129.21) and DFI-ceftriaxone-resistant Enterobacteriaceae (OR, 3.45; 95% CI, 1.29–9.25).
DISCUSSION
The proportion of DFI associated with pathogens that were not covered by guideline-recommended empiric therapy regimens was higher than expected. Almost one-third of the index episodes exhibited 1 or more pathogens resistant to the recommended empiric therapies, most commonly P. aeruginosa, followed by ceftriaxone-resistant Enterobacteriaceae and VRE (7.7% and 7.1%, respectively). Importantly, P. aeruginosa was an unexpectedly common pathogen, being present in 15% of index episodes in this study. This is notable as P. aeruginosa is typically reported to occur in less than 10% of DFI in the United States [9–12]. In addition, this study adds important and striking data pertaining to the prevalence of DFI-MDRO due to VRE and ceftriaxone-resistant Enterobacteriaceae [2, 13, 14]. Consistent with published data, MRSA, which occurred in almost one-third of DFI cases, was the most common MDRO [2].
In this study, the use of any antibiotic in the preceding 3 months was an independent predictor for DFI-MDRO, as was prior history of an MDRO isolated in diabetic foot ulcers, PVD, and CKD. Previous vancomycin and BLBLI approached statistical significance in the multivariable model for DFI-MDRO (OR, 1.78; 95% CI, 0.92–3.64; OR, 1.77; 95% CI, 0.91–3.64, respectively).
To our knowledge, this is the first study evaluating risk factors for having DFI associated with pathogens not covered by recommended empiric therapy of DFI. Similar to risk factors for DFI-MDRO, prior use of any antibiotic was independently associated with DFI- PRRT (OR, 2.79; 95% CI, 1.81–4.3). Other independent predictors for DFI-PRRT (and for DFI-MDRO) included prior history of recovery of PRRT in a diabetic foot ulcer, as well as PVD and CKD.
In this study, a patient who had a history of recovery of a PRRT in a diabetic foot ulcer had a >2-fold risk for subsequently having DFI-PRRT. This association between prior colonization with resistant organisms and subsequent infection has been reported with other types of infections [15, 16]. Therefore, in addition to previous antimicrobial exposure, prior growth of a PRRT (or MDRO) in a DFU should be considered when choosing empiric treatment for DFI.
Peripheral vascular disease and CKD were relatively common in the study cohort. Having PVD was independently associated with >2-fold increased risk of having DFI-PRRT. Poor vascular supply impairs wound healing and achieves appropriate antibiotic concentrations at the site of infection, both of which have been associated with selection of resistant bacteria [17, 18]. However, because PVD prevalence was also very high among patients with DFI-Non-PRRT (>60%), there was little predictive value for the presence of PVD in determining the likelihood of DFI-PRRT. In addition, having CKD increased the risk of DFI-PRRT by more than 50%. Among patients with CKD, it is plausible that use of lower doses of antibiotics, particularly in the presence of poor vascular supply and tissue penetration, might lead to subtherapeutic concentrations at the site of infection and subsequent development of antimicrobial resistance.
When individual PRRT were evaluated, a variety of different antibiotic risk factors were identified. Consistent with previous reports in infections other than DFI [19, 20], prior third-generation cephalosporin exposure was associated with ceftriaxone-resistant Enterobacteriaceae, and previous cefepime was associated with P. aeruginosa. This association between previous use of cefepime and/or BLBLI and higher prevalence of P. aeruginosa may be a marker for patients with comorbid conditions that place them at increased risk for P. aeruginosa infection. Alternatively, exposure to these antibiotics might have inhibited the growth of competing bacteria, giving P. aeruginosa a selective advantage. Interestingly, residence in LTCF was an independent predictor for DFI-ceftriaxone-resistant Enterobacteriaceae but not for other MDROs. Similarly, recent hospitalization was an independent predictor only for DFI-VRE. Due to the to numerous published reports noting associations between LTCF exposure and recent hospitalization with a variety of MDRO infections, we believe that these health care exposures are an important risk factor to consider when choosing empiric therapy for DFI [21].
This study has limitations. As a result of its retrospective design, data for the duration of diabetes before DFI, outpatient management variables, and wound characteristics could not be fully captured. However, expansive medical chart evaluation allowed for the extraction of clinical information pertaining to depth of infection and sepsis severity, which enabled the classification of >90% of the patients in the index cohort as having DFI severity levels that were moderate or severe according to IDSA criteria [1]. Also, the omission of patients from this study who either had no culture obtained or only negative cultures may have provided an overestimation of the incidence of DFI-MDRO. The results pertaining to DFI-PRRT predictors are most relevant to the practice at the study institution, which is in line with guideline recommendations, but cannot be generalized to institutions that use different regimens that include broader-spectrum empiric antibiotics. Twenty-two percent of the cultures in the study were from bedside swabs. Bedside swab cultures may be less specific for pathogens than other types of cultures in the cohort. In a subgroup analysis that included DFI episodes with deep cultures only (deep tissue and bone), the distribution of MDROs was similar to that of the original cohort, and the independent predictors for DFI-MDRO in this cohort were similar except for PVD, which was no longer a predictor for DFI-MDRO (data not shown).
In conclusion, this study demonstrated that the prevalence of MDROs and PRRT, particularly P. aeruginosa, was unexpectedly high among diabetic patients with culture-positive DFI. History of the same MDRO in a prior diabetic foot ulcer and prior antibiotic exposure were risk factors for most individual types of PRRT. In addition, PVD was an independent predictor of DFI-MDRO, DFI-PRRT, and DFI-P. aeruginosa. Clinicians and antimicrobial stewards can utilize these variables to help guide empiric therapeutic decisions and guidelines.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
Financial support. This study was not funded.
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Lipsky BA, Berendt AR, Cornia PB, et al. . Infectious Diseases Society of America 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis 2012; 54:e132–73. [DOI] [PubMed] [Google Scholar]
- 2. Kandemir O, Akbay E, Sahin E, et al. . Risk factors for infection of the diabetic foot with multi-antibiotic resistant microorganisms. J Infect 2007; 54:439–45. [DOI] [PubMed] [Google Scholar]
- 3. Richard JL, Sotto A, Jourdan N, et al. . Nîmes University Hospital Working Group on the Diabetic Foot (GP30) Risk factors and healing impact of multidrug-resistant bacteria in diabetic foot ulcers. Diabetes Metab 2008; 34:363–9. [DOI] [PubMed] [Google Scholar]
- 4. Hartemann-Heurtier A, Robert J, Jacqueminet S, et al. . Diabetic foot ulcer and multidrug-resistant organisms: risk factors and impact. Diabet Med 2004; 21:710–5. [DOI] [PubMed] [Google Scholar]
- 5. Hatipoglu M, Mutluoglu M, Turhan V, et al. . Turk-Day Study Group Causative pathogens and antibiotic resistance in diabetic foot infections: a prospective multi-center study. J Diabetes Complications 2016; 30:910–6. [DOI] [PubMed] [Google Scholar]
- 6. Charles PG, Uçkay I, Kressmann B, et al. . The role of anaerobes in diabetic foot infections. Anaerobe 2015; 34:8–13. [DOI] [PubMed] [Google Scholar]
- 7. Dellinger RP, Levy MM, Rhodes A, et al. . Surviving Sepsis Campaign Guidelines Committee including The Pediatric Subgroup Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med 2013; 39:165–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stevens DL, Bisno AL, Chambers HF, et al. . Infectious Diseases Society of America Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis 2014; 59:e10–52. [DOI] [PubMed] [Google Scholar]
- 9. Noel GJ, Bush K, Bagchi P, et al. . A randomized, double-blind trial comparing ceftobiprole medocaril with vancomycin plus ceftazidime for the treatment of patients with complicated skin and skin-structure infections. Clin Infect Dis 2008; 46:647–55. [DOI] [PubMed] [Google Scholar]
- 10. Lipsky BA, Armstrong DG, Citron DM, et al. . Ertapenem versus piperacillin/tazobactam for diabetic foot infections (SIDESTEP): prospective, randomised, controlled, double-blinded, multicentre trial. Lancet 2005; 366:1695–703. [DOI] [PubMed] [Google Scholar]
- 11. Young H, Knepper B, Hernandez W, et al. . Pseudomonas aeruginosa: an uncommon cause of diabetic foot infection. J Am Podiatr Med Assoc 2015; 105:125–9. [DOI] [PubMed] [Google Scholar]
- 12. Citron DM, Goldstein EJ, Merriam CV, et al. . Bacteriology of moderate-to-severe diabetic foot infections and in vitro activity of antimicrobial agents. J Clin Microbiol 2007; 45:2819–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Al Benwan K, Al Mulla A, Rotimi VO. A study of the microbiology of diabetic foot infections in a teaching hospital in Kuwait. J Infect Public Health 2012; 5:1–8. [DOI] [PubMed] [Google Scholar]
- 14. Shankar EM, Mohan V, Premalatha G, et al. . Bacterial etiology of diabetic foot infections in South India. Eur J Intern Med 2005; 16:567–70. [DOI] [PubMed] [Google Scholar]
- 15. Gómez-Zorrilla S, Camoez M, Tubau F, et al. . Prospective observational study of prior rectal colonization status as a predictor for subsequent development of Pseudomonas aeruginosa clinical infections. Antimicrob Agents Chemother 2015; 59:5213–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tischendorf J, de Avila RA, Safdar N. Risk of infection following colonization with carbapenem-resistant Enterobactericeae: a systematic review. Am J Infect Control 2016; 44:539–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vella J, Vella M, Cassar K, et al. . Factors affecting penetration of ciprofloxacin in lower extremity ischemic tissues. Int J Low Extrem Wounds 2016; 15:126–31. [DOI] [PubMed] [Google Scholar]
- 18. Sandegren L. Selection of antibiotic resistance at very low antibiotic concentrations. Ups J Med Sci 2014; 119:103–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee SO, Lee ES, Park SY, et al. . Reduced use of third-generation cephalosporins decreases the acquisition of extended-spectrum beta-lactamase-producing Klebsiella pneumoniae. Infect Control Hosp Epidemiol 2004; 25:832–7. [DOI] [PubMed] [Google Scholar]
- 20. Lin MF, Huang ML, Lai SH. Risk factors in the acquisition of extended-spectrum beta-lactamase Klebsiella pneumoniae: a case-control study in a district teaching hospital in Taiwan. J Hosp Infect 2003; 53:39–45. [DOI] [PubMed] [Google Scholar]
- 21. Dumyati G, Stone ND, Nace DA, et al. . Challenges and strategies for prevention of multidrug-resistant organism transmission in nursing homes. Curr Infect Dis Rep 2017; 19:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.