Abstract
Background and Objectives
According to the strength and vulnerability integration (SAVI) model, older people are better able to avoid negative social interactions than younger people, but when they do experience negative interactions, they are equally or more emotionally and physiologically reactive than younger people. Less is known about the links between daily negative and positive social encounters and the sympathetic adrenal medullary system (a key stress pathway) and whether there are age differences in these links. This study considers whether negative and positive social interactions are associated with diurnal alpha-amylase (a measure of the sympathetic adrenal medullary system) and whether there are differences in these links by age.
Research Design and Methods
Participants were from the Daily Health, Stress, and Relationship Study, which includes a random sample of 89 individuals (aged 40–95) who completed 14 days of daily diary interviews and provided saliva samples four times a day (wake, 30 min after wake, lunch, and bedtime) for four of those days that were assayed for alpha-amylase.
Results
Days in which people reported more negative interactions were associated with flatter morning declines in alpha-amylase, indicating greater stress. Links between positive interactions and diurnal alpha-amylase varied by age group.
Discussion and Implications
Findings are consistent with the SAVI model indicating that older adults respond differently to social stimuli than younger people.
Keywords: Daily social interactions, Biological stress response, Alpha-amylase, Age differences
Negative social interactions (e.g., irritations, tensions) are the most frequently reported daily stressors, and they are more highly associated with poor well-being compared to noninterpersonal stressors (Almeida, 2005). A burgeoning literature shows that older people experience negative social interactions differently than younger people. Older people tend to report fewer negative interactions (Birditt, Fingerman, & Almeida, 2005), and they are more likely to use avoidant coping strategies in response to negative interactions, such as ignoring the problem or doing nothing. When older people do experience negative interactions, their well-being tends to be less detrimentally affected by these interactions than younger individuals’ well-being (Charles, Piazza, Luong, & Almeida, 2009), particularly when they are able to use avoidant strategies (Birditt, 2014).
Several gaps remain in our understanding of age differences in social interactions in daily life. There is little research on daily positive interactions (e.g., pleasant interactions, interactions that bring happiness) although they occur more frequently than negative interactions and may have distinct effects on stress physiology (Birditt et al., 2017). Furthermore, there is little research on daily social interactions among oldest-old adults (i.e., aged 80 and older) who may have distinct relationship experiences compared with other age groups due to increased resilience and social expertise, greater use of avoidance, and loss of social roles (e.g., widowhood; Poon & Cohen-Mansfield, 2011). There is also some controversy in the literature regarding whether oldest-old adults show continued trends of increased well-being and emotion regulation or whether there are decreases in well-being and emotion regulation among this age group (Davey, Halverson, Zonderman, & Costa, 2004; Stone, Schwartz, Broderick, & Deaton, 2010). In addition, very little is known about daily social interactions and their links with the sympathetic adrenal medullary (SAM) system; a biological stress pathway that has received less attention in the daily stress literature than the hypothalamic–pituitary–adrenal (HPA) axis (i.e., cortisol). It is particularly important to examine the SAM system as it is considered the precursor to the activation of the HPA axis and may provide additional insights into how daily experiences affect health (Cannon, 1914; Granger et al., 2006). Furthermore, research indicates that alpha-amylase may be responsive to arousal or emotional intensity, regardless of whether it reflects negative or positive events (Adam, Till Hoyt, & Granger, 2011; Liu, Almeida, Rovine, & Zarit, 2016). The present study examines links between daily negative and positive social interactions and diurnal alpha-amylase (an indicator of SAM) and potential age group differences in those associations among middle-aged, young-old, and oldest-old individuals.
Theoretical Framework
The strength and vulnerability integration (SAVI) model provides a useful framework for understanding age differences in the experience and implications of negative and positive social interactions (Charles, 2010). The SAVI model emerged from socioemotional selectivity theory and suggested that as people age they become increasingly motivated to achieve emotion-focused goals that often involve maintaining emotionally meaningful social ties (Charles & Carstensen, 2010). Thus, older adults are increasingly likely to avoid or decrease their exposure to negative stimuli, particularly with regards to their social ties (Birditt et al., 2005), and thus they report better well-being than younger people. However, when older adults are not able to avoid negative interactions, they do not experience improved well-being and respond similarly to younger people. For example, under circumstances in which older adults experience increased and sustained negative emotions, they have reduced physiological ability to recover and thus decreased well-being (Charles, 2010). The present study focuses on whether there are age differences in links between daily social interactions and alpha-amylase, a key indicator of the SAM system.
Activation of the SAM system is often referred to as the “fight or flight” response (Cannon, 1914). Activation of this system leads to the release of catecholamines, including epinephrine and norepinephrine, into the blood stream. SAM activity can be measured with levels of epinephrine, norepinephrine, and more recently, alpha-amylase. Alpha-amylase is an enzyme produced by the salivary glands primarily to help with the digestion of carbohydrates and to reduce bacteria (Granger et al., 2006). Alpha-amylase has a diurnal rhythm with a decrease in the first 60 min after awakening (awakening response [AR]) and a steady increase in activity over the day (wake-evening slope [WES]; MacKinnon, Lockwood, Hoffman, West, & Sheets, 2002; Nater, Rohleder, Schlotz, Ehlert, & Kirschbaum, 2007). Studies examining age differences in diurnal alpha-amylase reveal that older people have higher daily alpha-amylase levels (Nater, Hoppmann, & Scott, 2013). Emerging research shows that alpha-amylase may be responsive to emotional intensity rather than valence of emotion (i.e., as positive or negative; Adam et al., 2011). Thus, this study considers both negative and positive interactions as they may have similar effects on the SAM and there may be age differences in those associations.
Daily Social Interactions and the Stress Response
Negative Interactions
Recent studies reveal that negative interactions can be nuanced and associated with increased as well as decreased physiological stress. Studies of diurnal cortisol have shown that negative interactions are associated with steeper increases in the morning (Birditt, 2014), as well as steeper cortisol declines (Birditt, Kim, Zarit, Fingerman, & Loving, 2016; Birditt, Manalel, Kim, Zarit, & Fingerman, 2017), implying that individuals may anticipate negative interactions and may be soothed by interactions even if they are negative. To date, there is little research examining links between daily negative social interactions and diurnal alpha-amylase and research findings are inconsistent, in some cases showing that negative interactions are associated with increased physiological stress and in others showing that the interactions are associated with reduced stress. For spouses of persons with mild cognitive impairment, those who reported negative marital interactions showed higher alpha-amylase and flatter rhythms on the day of the interactions (Savla et al., 2013). Wingenfeld and colleagues (2010) found a link between social difficulties (e.g., social isolation or social pressure of caring for another) and an increase in diurnal alpha-amylase over the course of the day among nurses. By contrast, caregivers’ daily stressors are associated with lower alpha-amylase levels and flatter as well as steeper rhythms (Liu et al., 2016; Rohleder, Marin, Ma, & Miller, 2009), suggesting that daily stressors may be associated with lower as well as greater physiological stress among people under chronic stress.
Positive Interactions
Likewise, research on daily positive interactions shows that they can be associated with less, as well as greater, physiological stress. It is possible that positive interactions and emotions can be calming but in some cases they are associated with increased arousal or anticipation. For example, research on cortisol shows that greater intimacy is associated with lower overall cortisol levels among couples (Ditzen, Hoppmann, & Klumb, 2008) but that increases in cortisol are associated with increased likelihood of positive emotional states (Hoyt, Zeiders, Ehrlich, & Adam, 2016). Daily positive events are also associated with greater waking cortisol but steeper declines in cortisol over the day (Sin, Ong, Stawski, & Almeida, 2017). There is little research on links between positive interactions and diurnal alpha-amylase. Liu and colleagues (2016) found that daily positive events (e.g., positive social interactions, positive work events) experienced by caregivers of older adults with dementia were associated with steeper rhythms in the afternoon, which may indicate that positive events are soothing. Other studies suggest that alpha-amylase reflects arousal (i.e., the intensity of emotion) rather than the specific valence (i.e., as positive or negative) of the emotion. Adam and colleagues (2011) found that emotions associated with the greatest arousal, in terms of extreme positive or negative emotions, were associated with greater increases in alpha-amylase. The mixed findings in the literature suggest the need for further research on how negative and positive daily social interactions are associated with diurnal alpha-amylase.
Age Differences in Links Between Social Interactions and Stress Response
Research on physiological reactions to stress shows that the links between daily events and physiology vary by age; as people age, the HPA axis becomes less flexible. Thus, older people may be slower to recover from stress and show a greater cortisol reaction to stress (Giordano et al., 2005). They also have greater cortisol levels in response to daily negative affect (Piazza, Charles, Stawski, & Almeida, 2013). SAVI suggests that when older adults are not able to avoid stress, they show less ability to return back to homeostasis (Charles & Luong, 2013). Charles and colleagues (2009) found that older adults are less emotionally reactive to conflict when it is avoided but equally as emotionally reactive to conflict when it is not avoided (i.e., to arguments). Furthermore, Birditt (2014) found that middle-aged and oldest-old adults reported greater negative affect on days in which they had more negative interactions compared with young-old individuals. Although to our knowledge there have not been any diary studies examining how alpha-amylase and daily interactions vary by age, researchers have examined diurnal alpha-amylase rhythms among ballroom dancers, aged 15–75 (Strahler, Berndt, Kirschbaum, & Rohleder, 2010). They found a flattened rhythm among younger male dancers compared with controls, which may be a sign of repeated stress. They also found that older adults had higher overall levels of alpha-amylase (both dancers and controls). There are no studies to our knowledge that address age differences in the links between positive interactions and alpha-amylase. Older people may be more reactive to both positive and negative interactions due to their greater investment in social relationships.
The Present Study
Examining links between daily social interactions and daily alpha-amylase may provide insights into how social relationships affect physical health. In addition, examining age differences in these links provides valuable information regarding potential age differences in emotion regulation in social relationships. Older individuals may react differently due to variations in investment in maintaining emotionally meaningful relations, age-related physiological changes, and greater efforts to regulate their emotional reactions. There have been few studies examining whether links between interpersonal interactions and biological stress reactions vary by among middle-aged, young-old, and oldest-old individuals. The present study examined the implications of negative and positive daily social interactions for diurnal alpha-amylase and whether those links varied by age group among middle-aged (40–59), young-old (60–79), and oldest-old adults (80 and older). We consider these age groups as they represent distinct periods of adult development that are characterized by nonlinear age differences in emotional experiences (Birditt, 2014). Social interactions can often have same day associations as well as lingering or delayed associations with biological systems on the next day (Birditt, Cichy, & Almeida, 2011; Birditt et al., 2017). Thus, in order to understand potential links between daily social interactions and alpha-amylase, we considered associations between daily social interactions and same day as well as next day alpha-amylase. We examined two research questions in particular:
1. Are negative and positive daily interpersonal interactions associated with diurnal rhythms of alpha-amylase on the same and next day?
Based on previous research, we predicted that negative and positive interactions would be associated with diurnal alpha-amylase rhythms indicating greater stress (i.e., higher overall levels of alpha-amylase and flatter rhythms).
2. Do the links between negative and positive daily interpersonal interactions diurnal rhythms of alpha-amylase on the same and next day vary by age group?
Consistent with the SAVI model, we predicted that oldest-old adults would show greater biological responses to both positive and negative interactions compared with young-old and middle-aged adults.
Methods
Participants and Procedure
Participants were from the Daily Health, Stress, and Relationships study (Birditt, 2014), which included a total of 110 participants (59% women) who completed a baseline phone interview and daily phone interviews for 14 days. Data were collected by the Survey Research Operations at the Institute for Social Research, University of Michigan. Participants were randomly selected from a list sample of individuals in the metro Detroit Wayne County purchased from Marketing Systems Group/GENESYS Sampling Systems. The list was composed of households that have agreed to be included (or “listed”) in published or electronic telephone directories. Participants ranged from age 40 to 95 years old. The sample was stratified by age and gender.
Participants completed an average of 13.51 (SD = 1.07) days of daily interviews (response rate = 96%); 75% (n = 70) of participants completed all 14 days. Participants completed all interviews over the phone. After each daily interview participants scheduled a time for the next interview. Thus, interview times varied somewhat across days, which is typical for daily diary studies (Almeida, 2005). Participants received a total of $190 for completing all of the interviews ($50 for baseline; $10 per daily). The baseline interviews lasted about an hour and the daily interviews lasted 20 min each, on average. During the diary calls, participants were also invited to participate in saliva collection. The study received approval from the institutional review board of the University of Michigan.
All participants were invited to provide saliva and 98 participants (89%) agreed. Participants provided saliva via passive drool into Sarstedt 5-ml tubes. Samples were collected on four consecutive days on Days 6 through 9 of the diary calls. Participants provided samples when they woke and before they got out of bed, 30 min after waking, at lunch, and before going to bed. A selection analysis was conducted to compare the 89 participants in the final analysis to the 9 participants who provided saliva but were removed for the reasons listed in Supplementary Table 1. We examined differences in demographics and daily interactions. Participants in the final analysis reported more daily positive interactions (M = 1.45, SD = 1.09) compared with those excluded from the analysis (M = 0.82, SD = 0.62).
Participants were evenly distributed in the age ranges of 40–59 (n = 29), 60–79 (n = 31), and 80–95 (n = 29; see Table 1 for sample description). Participants reported average to very good health, a total of 45% were married, and they reported an average of some college education.
Table 1.
Description of the Daily Health, Stress, and Relationships Study Sample (N = 89)
| Overall | Middle-age | Young-old | Oldest-old | |||||
|---|---|---|---|---|---|---|---|---|
| Variable | M (SD) | % | M (SD) | % | M (SD) | % | M (SD) | % |
| Age (M, SD) | 67.72 (14.43) | 51.24 (5.56) | 67.45 (5.57) | 84.48 (4.21) | ||||
| Education (M, SD) | 7.08 (2.56) | 7.45 (2.16) | 6.74 (2.65) | 7.07 (2.85) | ||||
| Self-rated health | 3.37 (0.96) | 3.31 (0.85) | 3.58 (0.96) | 3.21 (1.05) | ||||
| Female | 55 | 59 | 61 | 45 | ||||
| White | 71 | 62 | 77 | 72 | ||||
| Black | 26 | 35 | 19 | 24 | ||||
| Number of negative interactions | 0.51 (0.47) | 0.69 (0.57) | 0.50 (0.44) | 0.33 (0.31) | ||||
| Number of positive interactions | 1.45 (1.09) | 1.57 (1.24) | 1.64 (1.10) | 1.14 (0.85) | ||||
Note: Education included 12 responses ranging from 1 (no school/some grade school), 6 (graduated from high school), to 12 (PH.D., ED.D., MD, DDS, LLD, JD, or other professional degree). Because higher scores signified greater education, this scale is treated as a continuous variable in analyses. Self-rated health was rated from 1 (poor) to 5 (excellent).
Measures
Diurnal Alpha-Amylase
Saliva was assayed by the Johns Hopkins Center for Interdisciplinary Salivary Bioscience using a kinetic reaction assay (Granger et al., 2007). See Supplementary Appendix A for collection and exclusion details and Supplemental Table 1 for a description of alpha-amylase data. The skewness of the raw alpha-amylase samples ranged from 1.79 to 3.26, and so samples were log transformed. Skewness and kurtosis values were all acceptable after the transformation with the exception of the skewness value for waking cortisol (see Supplementary Appendix A for assay details).
Negative Social Interactions
Participants were asked: “Since (this time/we spoke) yesterday, did you have any social interactions (in person, over the phone, or electronically) that made you feel irritated, hurt or annoyed?” and “Sometimes people do irritating or annoying things but we avoid feeling irritated or annoyed with them. Since (this time/we spoke) yesterday, did you have social interactions (in person, over the phone, or electronically) in which you could have felt irritated, hurt or annoyed but decided not to?” Participants could list up to five individuals a day for each negative interaction item, which were added together (range = 0–10). To confirm that negative interactions were stressful, we examined the average rating of stress for each type of interaction and it was 2.95 (SD = 0.87) for irritating interactions and 2.70 (SD = 0.86) for avoiding irritations on a scale from 1 to 4 with 4 being most stressful. Negative interactions were also correlated with daily negative affect (r = .36, p < .001).
Positive Social Interactions
Participants were asked: “Since (this time, we spoke) yesterday, can you think of any social interactions (in person, over the phone, or electronically) that you had that made you feel good or happy?” Next, they were asked to list up to five people with whom they had those interactions. We summed the number of people listed to create a sum of positive interactions each day (range = 0–5). Although we did not ask participants to rate the stressfulness of these interactions, daily positive interactions were positively correlated with daily positive affect (r = .25, p < .01).
Age Group
Participants reported their birthdate, and we included age as a categorical variable: 1 = middle-aged (40–59), 2 = young-old (60–79), and 3 = oldest-old (80 and older). These groupings are commonly used and represent distinct periods of adult development (Birditt, 2014; Birditt, Jackey, & Antonucci, 2009).
Covariates
Factors associated with social relationships and biological indicators of stress, including gender, race, self-rated health, education, neuroticism, medication use, smoking, and weekend day, were included as covariates (Almeida, 2005). Gender was coded as 0 (men) or 1 (women). Race was coded as 0 (Not White) or 1 (White), with the majority of not White participants being Black. Participants rated the quality of their physical health from 1 (excellent) to 5 (poor), and scores were reverse coded so that higher scores reflected better health. Education included 12 responses ranging from 1 (no school/some grade school), 6 (graduated from high school), to 12 (professional or doctoral degree). Neuroticism was assessed with 12 items (Eysenck, Eysenck, & Barrett, 1985) in which participants were asked the extent to which different experiences described them such as “Does your mood often go up and down?” and “Do you ever feel miserable for no reason?” Participants responded to each item with a yes (1) or a no (0). A sum score of the items was created, and higher scores indicate more neuroticism (α = .80).
Weekend day was a time-varying variable, and days were coded as 1 (weekend day) or 0 (weekday). Medication use was derived from a list of medications including over the counter or prescription allergy medication (e.g., Flonase, Benadryl); a steroid inhaler; other steroid medication (e.g., Prednisone); medications or creams containing cortisone (e.g., Cortaid or anti-itch creams); birth control pills; other hormonal medication; and antidepressants or antianxiety medication. Participants indicated daily whether they had used each type of medication (1 = yes, 0 = no), medications were summed, and days were coded as 0 (no medication used) and 1 (at least one medication used). Participants also indicated whether they smoked cigarettes each day (0 = did not smoke, 1 = smoked), and because there was no variance in smoking across days, smoking was a person-level variable.
Analysis Strategy
Similar to previous studies of diurnal biomeasure rhythms, three-level piecewise multilevel models were estimated to assess whether diurnal alpha-amylase rhythms varied by daily social interactions (Stawski et al., 2011). Analyses were conducted using Stata 14.2. The lowest level referred to the alpha-amylase assessment within day, the second level referred to the day, and the upper level referred to the participant (Stawski et al., 2011). These models allow for examination of variability in biomeasures within day, between days, and between individuals. Piecewise models captured the within day patterns of alpha-amylase with two predictors (i.e., pieces) that represented the AR (time difference between waking and 30-min collection) and the WES (time difference between 30-min collection and bedtime collection) centered on the 30-min collection. This model allowed us to examine whether the overall level of alpha-amylase over the course of the day, as well as the diurnal pattern of alpha-amylase (AR, WES), varied by daily social interactions on the same day and the previous day.
Several models, including random intercepts and pieces, were estimated to determine which model had the best fit. The best fitting model for alpha-amylase included a random intercept, AR, and WES between participants and a random intercept within participants. In these models, the alpha-amylase levels of person i on day d at occasion o are regressed on AR (Piece 1), WES (Piece 2), the daily social encounters on the same and previous day, and the interactions between the pieces (AR, WES) and the daily encounters. We estimated a series of two models: Model 1 included covariates, age group, daily encounters; Model 2 included covariates, daily encounters, age group, and interactions between daily encounters and age group (Tables 2 and 3). Models were estimated in two steps: Step 1 allowed for an examination of whether the overall level of alpha-amylase varied by daily social encounters; Step 2 allowed for an assessment of whether the pattern of alpha-amylase over the course of the day (AR, WES) varied by daily social encounters. The pieces were assessed in the same models, and only linear associations with pieces were considered. All continuous variables were centered, and all dichotomous categorical variables were effect coded (1, −1) before entering them in the models. Daily continuous variables were group mean centered, and between person continuous variables were grand mean centered. The age groups were included in all models and entered as dummy variables for middle-aged and young-old with oldest-old as the comparison group. All models controlled for gender, race, self-rated health, education, neuroticism, medication use, smoking, and weekend sample. Covariates in the model were included as correlates of the intercepts and not the slopes because including covariates linked with pieces would have involved testing a large number of statistical interactions, which is beyond the scope of this sample (see Supplementary Appendix B for a piecewise model equation example).
Table 2.
Multilevel Models Examining Diurnal Alpha-Amylase Rhythms by Negative Daily Interactions and Age
| Model 1 | Model 2 | |||||||
|---|---|---|---|---|---|---|---|---|
| Step 1 | Step 2 | Step 1 | Step 2 | |||||
| INT | INT | AR | WES | INT | INT | AR | WES | |
| b (SE) | b (SE) | b (SE) | b (SE) | b (SE) | b (SE) | b (SE) | b (SE) | |
| Sample average | 4.592 (0.18)** | 4.594 (0.18)** | 4.593 (0.18)** | 4.626 (0.19)** | ||||
| Same day interactions | −0.015 (0.03) | 0.036 (0.04) | −0.204 (0.10)* | −0.005 (0.00) | −0.077 (0.06) | −0.019 (0.08) | −0.003 (0.21) | −0.012 (0.01) |
| Previous day interaction | 0.026 (0.03) | 0.014 (0.04) | 0.074 (0.10) | 0.001 (0.00) | 0.020 (0.07) | 0.047 (0.10) | 0.155 (0.28) | −0.008 (0.01) |
| Same day interaction × age | ||||||||
| Middle-aged | 0.095 (0.07) | 0.041 (0.10) | −0.035 (0.26) | 0.012 (0.01) | ||||
| Young-old | 0.056 (0.07) | 0.092 (0.10) | −0.456 (0.26) | 0.005 (0.01) | ||||
| Oldest-old | – | – | – | – | ||||
| Previous day interaction × age | ||||||||
| Middle-aged | 0.008 (0.08) | −0.053 (0.11) | 0.016 (0.32) | 0.011 (0.01) | ||||
| Young-old | 0.006 (0.08) | −0.046 (0.12) | −0.131 (0.34) | 0.013 (0.01) | ||||
| Oldest-old | – | – | – | – | ||||
| −2 log likelihood | 2,268.9 | 2,263.6 | 2,267.0 | 2,248.5 | ||||
| Change in likelihood | 5.3 | 18.5 | ||||||
Note: AR = awakening response; INT = intercept; WES = wake-evening slope. Models include the quadratic decline and controlled for age group, gender, race, self-rated health, education, neuroticism, medication use, smoking, and weekend sample. Change in likelihood for Step 2 is change from Step 1.
*p ≤ .05. **p ≤ .01.
Table 3.
Multilevel Models Examining Diurnal Alpha-Amylase Rhythms by Positive Daily Interactions and Age
| Model 1 | Model 2 | |||||||
|---|---|---|---|---|---|---|---|---|
| Step 1 | Step 2 | Step 1 | Step 2 | |||||
| INT | INT | AR | WES | INT | INT | AR | WES | |
| b (SE) | b (SE) | b (SE) | b (SE) | b (SE) | b (SE) | b (SE) | b (SE) | |
| Sample average | 4.597 (0.18)** | 4.596 (0.18)** | 4.599 (0.18)** | 4.629 (0.19)** | ||||
| Same day interactions | 0.001 (0.02) | −0.006 (0.03) | 0.096 (0.08) | −0.001 (0.00) | −0.055 (0.03) | 0.014 (0.05) | −0.127 (0.14) | −0.011 (0.01)* |
| Previous day interaction | 0.004 (0.02) | −0.016 (0.03) | 0.073 (0.08) | 0.002 (0.00) | −0.034 (0.03) | −0.036 (0.05) | −0.055 (0.14) | 0.002 (0.00) |
| Same day interaction × age | ||||||||
| Middle-aged | 0.065 (0.05) | −0.014 (0.07) | 0.159 (0.21) | 0.012 (0.01) | ||||
| Young-old | 0.080 (0.04) | −0.035 (0.06) | 0.365 (0.18)* | 0.014 (0.01)* | ||||
| Oldest-old | – | – | – | – | ||||
| Previous day interaction × age | ||||||||
| Middle-aged | −0.054 (0.05) | 0.008 (0.08) | −0.330 (0.20) | −0.002 (0.01) | ||||
| Young-old | 0.099 (0.04)* | 0.045 (0.06) | 0.462 (0.18)** | −0.000 (0.01) | ||||
| Oldest-old | – | – | – | – | ||||
| −2 Log likelihood | 2,270.4 | 2,266.8 | 2,254.0 | 2,216.4 | ||||
| Change in likelihood | 3.6 | 37.6* | ||||||
Note: AR = awakening response; INT = intercept; WES = wake-evening slope. Models include the quadratic decline and controlled for age group, gender, race, self-rated health, education, neuroticism, medication use, smoking, and weekend sample. Change in likelihood for Step 2 is change from Step 1.
*p ≤ .05. **p ≤ .01.
To show the alpha-amylase values descriptively, and by occasion and age group, we plotted the estimated mean values by occasion for each age group. To assess the interactions, we examined whether there were significant differences between the simple slopes using Stata.
We assessed whether there was a significant difference between the fit of the models by subtracting the −2 log likelihood estimations of models and examining differences in a chi-square distribution with degrees of freedom equaling the change in number of parameters.
Results
Description of the Variables
Participants reported an average of 0.54 (SD = 1.01; range = 0–7) negative interactions and an average of 1.38 (SD = 1.57; range = 0–5) positive interactions on each day. Positive and negative interactions were positively correlated (r = .16; p < .01).
Unconditional models were estimated to examine the proportion of variance that was between and within participants. A total of 81% of the variance was between person, and 19% of the variance was within person.
We estimated models to examine the daily pattern of alpha-amylase, which includes the AR and WES. Because the models were centered on the 30-min sample, a positive AR is interpreted as a decline. Consistent with previous research, alpha-amylase showed a decline in the morning (b = 0.61, SE = 0.12, p < .001) and an increase over the course of the day (b = 0.16, SE = 0.01, p < .001).
Age group was associated with the overall level of alpha-amylase. Middle-aged individuals had lower levels of alpha-amylase than did oldest-old individuals (b = −0.57, SE = 0.18, p = .01). The diurnal rhythm of alpha-amylase did not vary by age group.
Are Negative and Positive Interactions Associated with Alpha-Amylase?
Multilevel models predicting alpha-amylase are presented in Tables 2 and 3. Models revealed associations between negative interpersonal interactions and same day alpha-amylase but not next day alpha-amylase. No main effect associations were found between positive interpersonal interactions and diurnal alpha-amylase. More frequent negative interactions were associated with a flatter decline in alpha-amylase in the morning (AR), which indicates higher sustained levels of alpha-amylase (Table 2). These higher sustained levels of alpha-amylase in the morning may be indicative of anticipatory stress. The change in log likelihood from Step 1 to Step 2 for negative interactions was not significant. An analysis of only the effect of negative interactions revealed a marginally significant increase in the model fit (Δ −2 log likelihood = 3.2, df = 1, p < .10) compared to Model 1 Step 1.
Do the Links Between Positive and Negative Daily Interpersonal Interactions and Diurnal Rhythms of Alpha-Amylase Vary by Age Group?
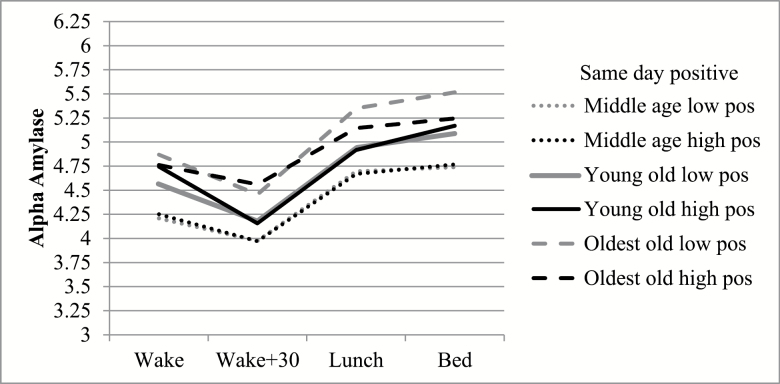
Models revealed no significant interactions between negative interpersonal interactions and age, but interactions between positive interpersonal interactions and age group predicted same and next day alpha-amylase. Although oldest-old respondents had higher alpha-amylase overall, the link between positive interactions and same day overall level, AR, and WES varied by age group (Table 3; Figure 1). Positive interactions were associated with lower overall alpha-amylase levels among oldest-old respondents, whereas there was no significant association among positive interactions and overall alpha-amylase levels among the young-old or middle-aged respondents. Comparisons of simple slopes revealed that the link between positive interactions and overall alpha-amylase was significantly different between oldest-old respondents and young-old respondents (contrast = −0.08, SE = 0.04, p = .05). The interaction between age group and positive interactions predicting AR revealed oldest-old respondents who reported high numbers of positive interactions had a flatter alpha-amylase decline in the morning, whereas young-old respondents who reported more positive interactions had a steeper decline in alpha-amylase in the morning (contrast = −0.80, SE = 0.35, p < .05). The interaction between age group and positive interactions predicting WES revealed that oldest-old participants who reported more positive interactions showed a flatter increase in alpha-amylase over the course of the day (WES) than young-old respondents who reported high numbers of positive interactions (contrast = −0.02, SE = 0.01, p < .05). Thus, positive interactions appear to be associated with lower overall levels and flatter diurnal rhythms of alpha-amylase among oldest-old respondents, which may be a sign that positive interactions are simultaneously stressful (i.e., flatter AR and WES) as well as rewarding (i.e., lower levels of alpha-amylase).
Figure 1.
Estimated means of alpha-amylase for each time point by age group and same day positive interactions. Low and high positive interactions represent 1 SD above and below the mean.
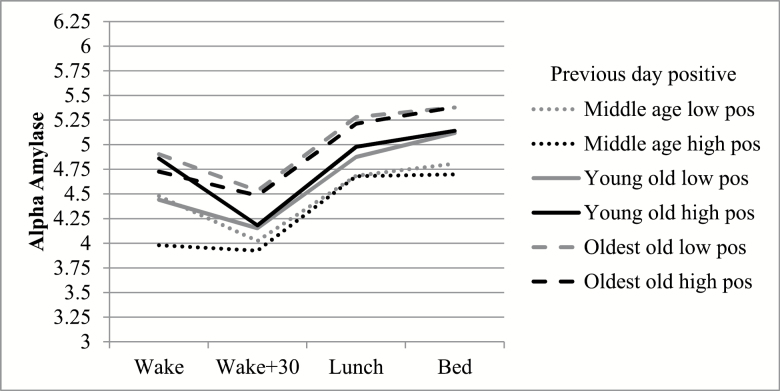
Models also revealed that the link between positive interpersonal interactions and next day diurnal rhythms of alpha-amylase (overall levels, AR) varied by age group (Table 3). The interaction between age group and positive interactions predicting next day overall levels of alpha-amylase showed that oldest-old (contrast = 0.10, SE = 0.04, p < .05) and middle-age respondents (contrast = 0.15, SE = 0.04, p < .01; Figure 2) had lower alpha-amylase levels on days after they had more positive interactions than young-old respondents. The interaction between age group and positive interactions predicting next day AR revealed oldest-old and middle-aged respondents who reported high numbers of positive interactions showed a flatter decline in alpha-amylase in the morning on the next day compared with young-old respondents (contrast = −0.91, SE = 0.36, p < .05; contrast = −1.33, SE = 0.37, p < .001; Figure 2). In this case, it appears that oldest-old and middle-aged respondents experienced positive interactions as simultaneously soothing (i.e., with lower levels of alpha-amylase) and stressful (i.e., with flatter AR).
Figure 2.
Estimated means of alpha-amylase for each time point by age group and previous day positive interactions. Low and high positive interactions represent 1 SD above and below the mean.
Post Hoc Analyses
In post hoc analyses, we considered the effects of types of medication, depressive symptoms, and chronic illnesses in terms of whether the results remained the same after each were included. Findings remained the same after considering these variables.
We estimated two multilevel models with age group as a predictor of number of positive interactions and number of negative interactions as the outcomes. Age group was not a significant predictor of either positive or negative interactions (controlling for neuroticism, gender, race, self-rated health, and education).
Discussion
Daily social interactions are linked with diurnal alpha-amylase, an indicator of the SAM system, and these links vary by age. This study contributes to the literature by demonstrating that (a) daily social interactions are indeed linked with alpha-amylase, a stress hormone less typically studied than other biomeasures such as cortisol; (b) positive interactions, which have often been overlooked, are important for individuals’ daily biological stress responses; and (c) links between daily positive social interactions and alpha-amylase vary by age group. The findings reveal the complexity of social interactions and their critical contribution to key indicators of physiological stress.
Negative Interactions and Diurnal Alpha-Amylase
Negative interactions were associated with diurnal alpha-amylase. Days in which participants had more negative interactions were characterized by a flatter AR decrease in alpha-amylase. This finding shows that negative interactions may be associated with greater anticipatory SAM activity. Similarly, previous research of spousal caregivers demonstrated that negative marital interactions were associated with higher morning alpha-amylase with flatter alpha-amylase slopes in the afternoon and evening (Savla et al., 2013; Wingenfeld et al., 2010). The finding that there are variations in diurnal rhythms in the morning of the social interaction (indicating that the biological response is happening before the social interaction) is similar to previous research showing that individuals have higher cortisol levels on days in which they anticipate having more stressful social interactions (i.e., with adult children who suffer problems; Birditt et al., 2016; Birditt, Nevitt, & Almeida, 2015) or events (i.e., competitive sports event; Rohleder, Beulen, Chen, Wolf, & Kirschbaum, 2007). The current study moves beyond the previous literature by demonstrating links between alpha-amylase and daily negative interactions among individuals more generally. In addition, the finding is consistent with the SAVI model, which postulates that older adults’ emotional reactions look similar to that of younger people when older adults are unable to avoid conflict.
Positive Interactions and Diurnal Alpha-Amylase
The present study revealed that the links between positive interactions and alpha-amylase varied by age group. Overall, it appears that oldest-old individuals were more affected by positive interactions on the day in which the interactions occurred than young-old individuals. Oldest-old adults appear to benefit from positive interactions but also show signs of physiological stress. On days in which oldest-old individuals reported more positive interactions, they showed lower alpha-amylase and flatter alpha-amylase rhythms (i.e., less of a decline in alpha-amylase in the morning and less of an increase in alpha-amylase over the day) compared with young-old respondents. These findings may indicate that anticipating positive interactions is soothing but that positive interactions are also associated greater stress throughout the day among oldest-old respondents compared with young-old respondents. Indeed, previous literature indicates that positive emotions and events can be associated with increased physiological stress (e.g., Birditt et al., 2017; Hoyt et al., 2016; Sin et al., 2017). These results support the prediction that there would be stronger links between daily social interactions and diurnal alpha-amylase rhythms among oldest-old adults. Oldest-old individuals may be more physiologically affected by positive interactions because they are less able to return to homeostasis due to changes in physiology and they more invested in achieving emotion-focused goals which often involve maintaining emotionally meaningful social ties (Charles & Carstensen, 2010).
When examining positive interactions and next day alpha-amylase values the findings were less clear with regard to age differences. Positive interactions appeared to be associated with greater as well as lower physiological stress, but the specific associations varied by age group. In particular, positive interactions were associated with higher overall alpha-amylase levels and steeper morning declines on the next day among young-old adults, whereas for middle-aged and oldest-old adults, positive interactions were associated with lower levels and flatter rhythms. Other research on positive events and alpha-amylase has also revealed inconsistent findings. For example, Liu and colleagues (2016) found that positive events were associated with steeper alpha-amylase rhythms among caregivers of individuals with dementia, which may indicate lower stress levels. However, Adam and colleagues (2011) found that intense positive or negative emotions were associated with greater increases in alpha-amylase. Thus, positive interactions appear to be associated with both decreased and increased physiological stress, and age differences in these experiences may be due to variations in emotion-focused goals as well as physiological changes.
These findings both reflect and inform the SAVI model. Just as the SAVI model has suggested, negative interactions had similar implications by age group. When older people are confronted with negative experiences that they cannot avoid, they experience similar emotional reactions to younger individuals. In addition, whereas most studies have examined indicators of the HPA axis (e.g., cortisol), this study extends the SAVI model by examining alpha-amylase, a key indicator of the SAM system. We further extend the theory by showing that links between positive interactions and alpha-amylase vary by age group, showing that oldest-old appear to be more reactive to positive interactions on the same day, but young-old adults are more reactive to positive interactions on the next day. This may be due to the fact that aging adults are more invested in achieving emotion-focused goals and may feel there is more a stake when engaging in interactions. Older people may also be more likely to experience mixed or poignant emotions in relationships due to the perception of time as more limited (Ersner-Hershfield, Mikels, Sullivan, & Carstensen, 2008; Schneider & Stone, 2015).
Limitations and Future Directions
There are some limitations to the present study. First, the saliva collection included self-reported time rather than computerized time stamps and the collection of four samples rather than a more frequent within day collection. Newly published guidelines (Stadler et al., 2016) indicate that the use of objective time collections and more frequent collection is important and future studies should attempt to follow these guidelines. However, it is important to note that previous studies have successfully used similar data collection techniques as the present study (Birditt et al., 2015, 2016; Zarit et al., 2014) and that the self-reported time and objective measurements are highly correlated (Almeida, McGonagle, & King, 2009). The sample was relatively small. Larger national samples would provide more representative results, as types of stressors and social interactions may differ by population and region. Replication of findings is also necessary to assure validity, reliability, and applicability to diverse populations. The cross-sectional nature of this study is another limitation. A longitudinal study could assess whether reactivity to daily interpersonal events changes as people age and better control for intrapersonal differences in reactivity. Further research could analyze how daily social interactions affect cardiovascular, immune, and other body systems. This research could also be expanded with an examination of the coping mechanisms and specific emotional reactions involved in these interactions. For instance, the level of emotional reactivity to events (e.g., intensity of stress), types of emotions experienced (e.g., anger vs. sadness), and the types of behavioral reactions (e.g., yelling vs. remaining quiet) may affect the biological stress response.
In conclusion, this study contributes to the literature by demonstrating the complex nature of social interactions and their interface with biology. Positive social interactions are linked with diurnal alpha-amylase, and these links vary in important ways by age. These findings provide insight into the specific vulnerabilities as well as protective factors associated with advanced age.
Supplementary Material
Supplementary data is available at The Gerontologist online.
Funding
This study was funded by Promoting Well Being Across Adulthood: The Role of Conflict Avoidance, K99/ROO (AG029879; K. S. Birditt, PI) from the National Institute of Aging.
Conflict of Interest
None reported.
Supplementary Material
Acknowledgments
We would like to thank Angela Turkelson for her assistance with the analyses.
References
- Adam E. K., Till Hoyt L., & Granger D. A (2011). Diurnal alpha amylase patterns in adolescents: Associations with puberty and momentary mood states. Biological Psychology, 88, 170–173. doi:10.1016/j.biopsycho.2011.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida D. M. (2005). Resilience and vulnerability to daily stressors assessed via diary methods. Current Directions in Psychological Science, 14, 64–68. doi:10.1111/j.0963-7214.2005.00336.x [Google Scholar]
- Almeida D. M., McGonagle K., & King H (2009). Assessing daily stress processes in social surveys by combining stressor exposure and salivary cortisol. Biodemography and Social Biology, 55, 219–237. doi:10.1080/19485560903382338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birditt K. S. (2014). Age differences in emotional reactions to daily negative social encounters. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 69, 557–566. doi:10.1093/geronb/gbt045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birditt K. S., Cichy K. E., & Almeida D (2011). Age differences in exposure and reactivity to interpersonal tensions among black and white individuals across adulthood. Race and Social Problems, 3, 225–239. doi:10.1007/s12552-011-9058-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birditt K. S., Fingerman K. L., & Almeida D. M (2005). Age differences in exposure and reactions to interpersonal tensions: A daily diary study. Psychology and Aging, 20, 330–340. doi:10.1037/0882-7974.20.2.330 [DOI] [PubMed] [Google Scholar]
- Birditt K. S., Jackey L. M., & Antonucci T. C (2009). Longitudinal patterns of negative relationship quality across adulthood. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 64, 55–64. doi:10.1093/geronb/gbn031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birditt K. S., Kim K., Zarit S. H., Fingerman K. L., & Loving T. J (2016). Daily interactions in the parent-adult child tie: Links between children’s problems and parents’ diurnal cortisol rhythms. Psychoneuroendocrinology, 63, 208–216. doi:10.1016/j.psyneuen.2015.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birditt K. S., Manalel J. A., Kim K., Zarit S. H., & Fingerman K. L (2017). Daily interactions with aging parents and adult children: Associations with negative affect and diurnal cortisol. Journal of Family Psychology, 31, 699–709. doi:10.1037/fam0000317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birditt K. S., Nevitt M. R., & Almeida D. M (2015). Daily interpersonal coping strategies: Implications for self-reported well-being and cortisol. Journal of Social and Personal Relationships, 32, 687–706. doi:10.1177/0265407514542726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon W. B. (1914). The emergency function of the adrenal medulla in pain and the major emotions. American Journal of Physiology Legacy Content, 33, 356–372. [Google Scholar]
- Charles S. T. (2010). Strength and vulnerability integration: A model of emotional well-being across adulthood. Psychological Bulletin, 136, 1068–1091. doi:10.1037/a0021232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles S. T., & Carstensen L. L (2010). Social and emotional aging. Annual Review of Psychology, 61, 383–409. doi:10.1146/annurev.psych.093008.100448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles S. T., & Luong G (2013). Emotional experience across adulthood the theoretical model of strength and vulnerability integration. Current Directions in Psychological Science, 22, 443–448. doi:10.1177/0963721413497013 [Google Scholar]
- Charles S. T., Piazza J. R., Luong G., & Almeida D. M (2009). Now you see it, now you don’t: Age differences in affective reactivity to social tensions. Psychology and Aging, 24, 645–653. doi:10.1037/a0016673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey A., Halverson C. F. Jr., Zonderman A. B., & Costa P. T. Jr (2004). Change in depressive symptoms in the Baltimore longitudinal study of aging. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 59, P270–P277. doi:10.1093/geronb/59.6.p270 [DOI] [PubMed] [Google Scholar]
- Ditzen B., Hoppmann C., & Klumb P (2008). Positive couple interactions and daily cortisol: On the stress-protecting role of intimacy. Psychosomatic Medicine, 70, 883–889. doi:10.1097/PSY.0b013e318185c4fc [DOI] [PubMed] [Google Scholar]
- Ersner-Hershfield H., Mikels J. A., Sullivan S. J., & Carstensen L. L (2008). Poignancy: Mixed emotional experience in the face of meaningful endings. Journal of Personality and Social Psychology, 94, 158–167. doi:10.1037/0022-3514.94.1.158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eysenck S. B., Eysenck H. J., & Barrett P (1985). A revised version of the Psychoticism scale. Personality and Individual Differences, 6, 21–29. [Google Scholar]
- Giordano R., Bo M., Pellegrino M., Vezzari M., Baldi M., Picu A.,…Arvat E (2005). Hypothalamus-pituitary-adrenal hyperactivity in human aging is partially refractory to stimulation by mineralocorticoid receptor blockade. The Journal of Clinical Endocrinology and Metabolism, 90, 5656–5662. doi:10.1210/jc.2005-0105 [DOI] [PubMed] [Google Scholar]
- Granger D. A., Blair C., Willoughby M., Kivlighan K. T., Hibel L. C., Fortunato C. K.,…Wiegand L. E; Family Life Project Investigators (2007). Individual differences in salivary cortisol and alpha-amylase in mothers and their infants: Relation to tobacco smoke exposure. Developmental Psychobiology, 49, 692–701. doi:10.1002/dev.20247 [DOI] [PubMed] [Google Scholar]
- Granger D. A., Kivlighan K. T., Blair C., El-Sheikh M., Mize J., Lisonbee J. A., & Schwartz E. B (2006). Integrating the measurement of salivary α-amylase into studies of child health, development, and social relationships. Journal of Social and Personal Relationships, 23, 267–290. doi:10.1177/0265407506062479 [Google Scholar]
- Hoyt L. T., Zeiders K. H., Ehrlich K. B., & Adam E. K (2016). Positive upshots of cortisol in everyday life. Emotion, 16, 431–435. doi:10.1037/emo0000174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Almeida D. M., Rovine M. J., & Zarit S. H (2016). Modeling cortisol daily rhythms of family caregivers of individuals with dementia: Daily stressors and adult day services use. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences. doi:10.1093/geronb/gbw140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon D. P., Lockwood C. M., Hoffman J. M., West S. G., & Sheets V (2002). A comparison of methods to test mediation and other intervening variable effects. Psychological Methods, 7, 83–104. doi: 10.1037/1082-989x.7.1.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nater U. M., Hoppmann C. A., & Scott S. B (2013). Diurnal profiles of salivary cortisol and alpha-amylase change across the adult lifespan: Evidence from repeated daily life assessments. Psychoneuroendocrinology, 38, 3167–3171. doi:10.1016/j.psyneuen.2013.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nater U. M., Rohleder N., Schlotz W., Ehlert U., & Kirschbaum C (2007). Determinants of the diurnal course of salivary alpha-amylase. Psychoneuroendocrinology, 32, 392–401. doi:10.1016/j.psyneuen.2007.02.007 [DOI] [PubMed] [Google Scholar]
- Piazza J. R., Charles S. T., Stawski R. S., & Almeida D. M (2013). Age and the association between negative affective states and diurnal cortisol. Psychology and Aging, 28, 47–56. doi:10.1037/a0029983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon L. W., & Cohen-Mansfield J (2011). Understanding well-being in the oldest old. New York: Cambridge University Press. doi: 10.1017/cbo978051192097 [Google Scholar]
- Rohleder N., Beulen S. E., Chen E., Wolf J. M., & Kirschbaum C (2007). Stress on the dance floor: The cortisol stress response to social-evaluative threat in competitive ballroom dancers. Personality & Social Psychology Bulletin, 33, 69–84. doi:10.1177/0146167206293986 [DOI] [PubMed] [Google Scholar]
- Rohleder N., Marin T. J., Ma R., & Miller G. E (2009). Biologic cost of caring for a cancer patient: Dysregulation of pro- and anti-inflammatory signaling pathways. Journal of Clinical Oncology, 27, 2909–2915. doi:10.1200/JCO.2008.18.7435 [DOI] [PubMed] [Google Scholar]
- Savla J., Granger D. A., Roberto K. A., Davey A., Blieszner R., & Gwazdauskas F (2013). Cortisol, alpha amylase, and daily stressors in spouses of persons with mild cognitive impairment. Psychology and Aging, 28, 666–679. doi:10.1037/a0032654 [DOI] [PubMed] [Google Scholar]
- Schneider S., & Stone A. A (2015). Mixed emotions across the adult life span in the United States. Psychology and Aging, 30, 369–382. doi:10.1037/pag0000018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sin N. L., Ong A. D., Stawski R. S., & Almeida D. M (2017). Daily positive events and diurnal cortisol rhythms: Examination of between-person differences and within-person variation. Psychoneuroendocrinology, 83, 91–100. doi:10.1016/j.psyneuen.2017.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler T., Kirschbaum C., Kudielka B. M., Adam E. K., Pruessner J. C., Wust S., & Clow A (2016). Assessment of cortisol awakening response: Expert consensus guidelines. Psychoneuroendocrinology, 63, 414–432. doi:10.1016/j.psyneuen.2015.10.010 [DOI] [PubMed] [Google Scholar]
- Stawski R. S., Almeida D. M., Lachman M. E., Tun P. A., Rosnick C. B., & Seeman T (2011). Associations between cognitive function and naturally occurring daily cortisol during middle adulthood: Timing is everything. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 66(Suppl 1), i71–i81. doi:10.1093/geronb/gbq094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone A. A., Schwartz J. E., Broderick J. E., & Deaton A (2010). A snapshot of the age distribution of psychological well-being in the United States. Proceedings of the National Academy of Sciences of the United States of America, 107, 9985–9990. doi:10.1073/pnas.1003744107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strahler J., Berndt C., Kirschbaum C., & Rohleder N (2010). Aging diurnal rhythms and chronic stress: Distinct alteration of diurnal rhythmicity of salivary alpha-amylase and cortisol. Biological Psychology, 84, 248–256. doi:10.1016/j.biopsycho.2010.01.019 [DOI] [PubMed] [Google Scholar]
- Wingenfeld K., Schulz M., Damkroeger A., Philippsen C., Rose M., & Driessen M (2010). The diurnal course of salivary alpha-amylase in nurses: An investigation of potential confounders and associations with stress. Biological Psychology, 85, 179–181. doi:10.1016/j.biopsycho.2010.04.005 [DOI] [PubMed] [Google Scholar]
- Zarit S. H., Whetzel C. A., Kim K., Femia E. E., Almeida D. M., Rovine M. J., & Klein L. C (2014). Daily stressors and adult day service use by family caregivers: Effects on depressive symptoms, positive mood, and dehydroepiandrosterone-sulfate. The American Journal of Geriatric Psychiatry, 22, 1592–1602. doi:10.1016/j.jagp.2014.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.