Abstract
Purpose of review
Lysosomal acid lipase (LAL), encoded by the LIPA gene, is an essential lysosomal enzyme that hydrolyzes cholesteryl ester and triglyceride delivered to the lysosome. This review highlights the novel pathophysiological role of LAL, the functional genomic discoveries of LIPA as a risk locus for coronary heart diseases (CHD), and the clinical advance in therapies for LAL deficiency.
Recent findings
The essential role of LAL in lipid metabolism has been confirmed in human and mice with LAL deficiency. In humans, loss-of-function mutations of LIPA cause rare lysosomal disorders, Wolman disease, and cholesteryl ester storage disease, in which LAL enzyme replacement therapy has shown significant benefits in a phase 3 clinical trial. Recent studies have revealed the role of LAL-mediated lysosomal lipolysis in regulating macrophage M2 polarization, lipid mediator production, VLDL secretion, lysosomal function and autophagy, extracellular degradation of aggregated-LDL, and adipose tissue lipolysis. Genome-wide association studies and functional genomic studies have identified LIPA as a risk locus for CHD, but the causal variants and mechanisms remain to be determined.
Summary
Despite years of research, our understanding of LAL is incomplete. Future studies will continue to focus on the key pathophysiological functions of LAL in health and diseases including CHD.
Keywords: cardiovascular diseases, lipase, lipid, lysosome
INTRODUCTION
Lysosomal acid lipase (LAL), encoded by the lipase A (LIPA) gene, is the only known intracellular lipase active at an acidic pH that hydrolyzes cholesteryl ester and triglyceride in the lysosome [1].
The essential role of LAL in lipid catabolism is evident from the phenotypes of LAL-deficient mice and humans [2,3]. Loss-of-function (LOF) mutations of human LIPA are the causes of rare autosomal recessive lysosomal disorders, Wolman disease, and cholesteryl ester storage disease (CESD). Wolman disease is an infantile-onset lethal disease due to nearly complete loss of LAL activity. CESD is a childhood/adult-onset subtype with a broad spectrum of clinical manifestations depending on the residual LAL activity. CESD patients are characterized by an accumulation of cholesteryl ester and triglyceride predominantly in the liver, spleen, gastrointestinal tract, and in macrophages throughout the body [3]. Premature death of individuals with CESD is due to liver failure and/or accelerated atherosclerosis, presumably secondary to the chronic hyperlipidemia [4]. A Lipa knockout mouse line (Lipa−/−) was generated in 1998 [2]. The phenotypes of Lipa−/− mice resemble human CESD and have been widely used to study the mechanistic and pathophysiological roles of LAL.
Despite decades of studies, our understanding of LAL in atherosclerosis and coronary heart diseases (CHDs) is incomplete [1]. Instead of providing a comprehensive review of the past literature [1], this article highlights the most recent discoveries on: the novel regulatory role of lipolytic products derived from LAL-mediated lipolysis; the role of LAL in intracellular and extracellular lipid catabolism; LAL and macrophage lysosome biogenesis in obesity and atherosclerosis; the genome-wide association studies (GWAS) and functional genomic discoveries of LIPA as a risk locus for CHD; and the clinical advance in human recombinant LAL as an enzyme replacement therapy in patients with LAL deficiency.
THE REGULATORY ROLE OF LYSOSOMAL ACID LIPASE-DERIVED CHOLESTEROL AND FATTY ACIDS
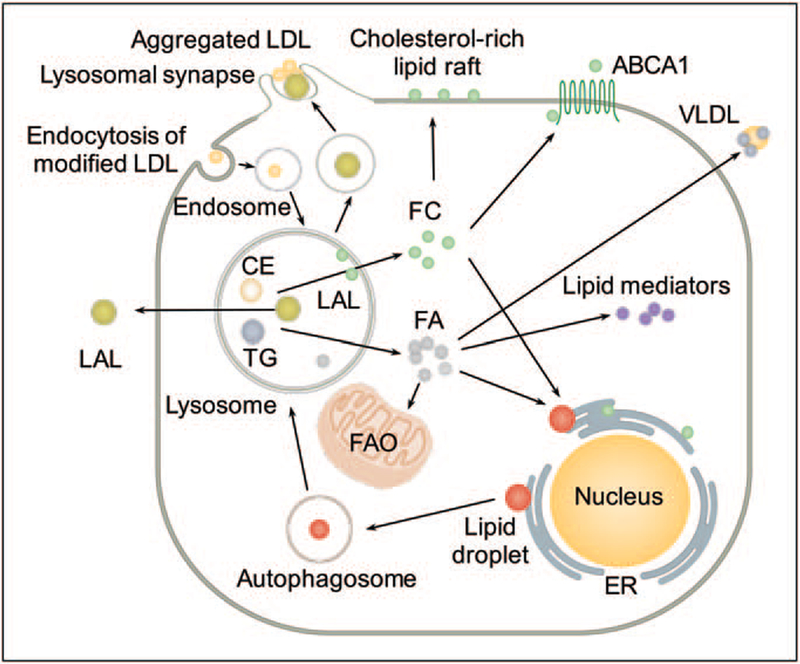
LAL-mediated lipid catabolism releases free cholesterol and fatty acids. Lysosomal hydrolysis of cholesteryl ester and the transfer of free cholesterol for cytosolic storage as lipid droplets play a major role in the formation of foam cells [1]. In addition, hydro-lysis of cholesteryl ester and triglyceride also releases fatty acids for membrane assembly and energy production [1]. Recent work has revealed that fatty acids derived from LAL-mediated lipolysis has important functional impact on macrophage alternative activation [5], metabolic reprogramming of CD8+ memory T cells [6], lipid mediator synthesis [7], and hepatocyte very-low-density lipoprotein (VLDL) assembly [8]. (Fig. 1)
FIGURE 1.
Novel pathophysiological roles of lysosomal acid lipase. Lysosomal acid lipase (LAL), encoded by the LIPA gene, is an essential lysosomal enzyme that hydrolyzes cholesteryl ester (CE) and triglyceride (TG) delivered to the lysosome. LAL-mediated lipid catabolism leads to the release of free cholesterol (FC) and fatty acids (FA). Hydrolysis of CE and the transfer of FC for cytosolic storage as lipid droplets (LD) contribute to the formation of foam cells. Hydrolysis of CE and TG also releases FC and FA for membrane assembly and energy production. LAL-derived lipolytic products mediate macrophage M2 activation fueled by fatty acid oxidation (FAO), lipid mediator production and hepatic very low-density lipoprotein (VLDL) secretion. In hepatocytes and macrophage foam cells, LAL mediates degradation of lipid droplets through an autophagic process. LAL hydrolyzes aggregates of low-density lipoprotein (LDL) in extracellular, acidic and lytic compartment independent of endocytic mechanisms. Human macrophage secretes LAL that is catalytically active. ABCA1, ATP-binding cassette transporter A1; ER, endoplasmic reticulum.
Alternative activation (M2) of macrophages by interleukin (IL)-4 mediates immunity for parasites, wound healing, and metabolic homeostasis [5]. LAL expression and activity is higher in M2 macrophages [5]. LAL-mediated lipolysis of exogenous triglyceride, and to a lesser extent, endogenously synthesized triglyceride, generates fatty acids to fuel fatty acid oxidation (FAO) that is essential for M2 activation characterized by elevated oxidative phosphor-ylation, enhanced spare respiratory capacity, prolonged survival, and expression of M2 markers [5]. Similarly, LAL-mediated lipolysis provides fatty acids for FAO in CD8+ T cells to support its metabolic programming toward the CD8+ memory T cells upon infection [6]. LAL-driven lipolysis also provides a source of lipid mediator precursors. Peritoneal macrophages of Lipa−/− mice show an accumulation of 18 : 2, and 20 : 4 fatty acids, important precursors for lipid mediator generation, in cholesteryl ester fraction, but not in phospholipids or triglyceride fractions. LAL inhibition in wild-type macrophages reduces the release of lipid mediators from all three major enzymatic pathways including cyclooxygenase, lipoxygenase, and cytochrome P450 pathways [7]. LAL also regulates hepatocyte VLDL secretion. Defective lysosomal hydrolysis of cholesteryl ester and triglyceride decreases the availability of fatty acids for hepatic acyl-CoA formation. Consequently, this abolished nuclear expression of hepatocyte nuclear factor 4 alpha and forkhead box protein A2 reduces the expression of microsomal triglyceride transfer protein and attenuats VLDL secretion. The decreased energy supply triggers an increase in glucose tolerance and insulin sensitivity in Lipa−/− mice as metabolic adaptations [8].
LYSOSOMAL ACID LIPASE IN INTRACELLULAR AND EXTRACELLULAR LIPID CATABOLISM
Lysosome-mediated intracellular lipoprotein trafficking and catabolism are dependent upon modification of the lipoprotein, and demonstrate tissue and cell type-specific regulation. Cholesteryl ester in acetylated-LDL is processed rapidly in the lysosome and stored primarily as cytoplasmic lipid droplets, whereas oxidized-LDL causes lysosomal retention of cholesteryl ester and free cholesterol [9,10]. The work of Jerome et al. shows that in human macrophages, lysosomal hydrolysis of mildly oxidized LDL increases free cholesterol levels in the lysosome. This progressive accumulation of free cholesterol inhibits lysosome acidification and subsequent hydrolysis of cholesteryl ester, causing an accumulation of both free cholesterol and cholesteryl ester[10]. In addition to hydrolyzing lipids delivered through receptor-mediated endocytosis, LAL regulates autophagy-dependent intracellular lipid catabolism in hepatocytes [11] and macrophage [9] by hydrolyzing lipid droplet-stored triglyceride and cholesteryl ester delivered to the lysosome from cytoplasmic droplets via autophagy. (Fig. 1) In contrast, in adipose tissue macrophage (ATM)-like cells generated by coculturing murine bone marrow cells with whole adipose tissue, the lack of Atg7, which is necessary for normal autophagy, did not cause an increase in cellular lipid content, suggesting that in ATM, delivery of lipid to the lysosome is not autophagy-dependent [12] These results highlight that intracellular lipid delivery to and metabolism in the lysosome is a tissue- and cell-specific process.
Lysosomal acid lipase also mediates extracellular lipid catabolism. Haka et al. have found that monocyte-derived macrophages [13] and dendritic cells [14] form an extracellular, acidic, lytic compartment, known as lysosomal synapse [15∎∎], to digest aggregated-LDL that are often bound to the subendothelial matrix in the atherosclerotic plaque and cannot be internalized through endocytic mechanisms. The formation of the lysosomal synapse is dependent on local actin polymerization and the pH maintained by vacuolar ATPase for extracellular hydrolysis of aggregated-LDL. Whether this mechanism accelerates the conversion of a macrophage into a foam cell in atherogenesis remains unclear [15∎∎]. (Fig. 1) Moreover, during atherogenesis, the extracellular pH of atherosclerotic lesions decreases [16]. Macrophages acidify their pericellular environment through action of proton pumps and secretion of lactic acid [17]. Indeed, increased acidity has been documented in lipid-rich, macrophage-rich areas of human and rabbit atherosclerotic plaques [18]. Human macrophage-conditioned medium contains catalytically active LAL, and this medium hydro-lyzes LDL, causing LDL fusion and modified LDL-induced lipid droplet formation in cultured vascular smooth muscle cells [19]. Remarkably, these effects can be abolished by LAL immunodepletion [19]. Thus, in the macrophage-rich acidic microenvironments observed in human atherosclerotic lesions, macrophage-secreted LAL may contribute to the modification of retained LDL, and this LAL-modified LDL may promote vascular dysfunction and atherogenesis.
LYSOSOMAL ACID LIPASE AND MACROPHAGE LYSOSOME BIOGENESIS IN OBESITY AND ATHEROSCLEROSIS
LAL-mediated lipid catabolism is important in adi-pose tissue biology. Lipa−/− mice have reduced body weight compared with wild-type controls, and also progressive loss of both white and brown adipose tissue [20]. Conversely, obesity induced lysosome biogenesis in ATM, characterized by an increased expression of lysosome structural genes (Lamp2), hydrolases (Lipa), and subunits of vacuolar proton pumps (Atp6v1b2), but not genes representing classical inflammatory activation. Consistently, chloroquine-treated ATM [20] and Lipa−/− human induced pluripotent stem cell (iPSC)-derived macrophages [21∎∎] exhibit an accumulation of lipid, but not a gene expression pattern of inflammatory activation. Thus, LAL deficiency-induced lipid accumulation does not appear to be associated with an inherent inflammatory effect in macrophages [20,21∎∎]. Instead, chloroquine-induced suppression of lipolysis was abolished in adipose tissue depleted of ATM, supporting the role of macrophage LAL in whole adipose tissue lipolysis [20].
Macrophages in atherosclerotic plaques have lysosomal accumulation of cholesteryl ester and free cholesterol [22], implicating impaired lysosomal lipolysis. However, several studies have reported higher LAL activity in atherosclerotic tissue and lipid-laden lysosomes isolated from atherosclerotic tissue homogenates [23,24], although it is not yet clear whether this increased LAL activity is a result of a higher number of LAL-expressing cells in the lesion and/or an up-regulation of LAL within cells. Furthermore, the mRNA expression levels or the activity measured in vitro may not be correlated to the lipolytic capacity of LAL in an atherosclerotic environment when lysosomal acidification is compromised. Indeed, CD45+/CD64+/merTK+ resident aortic macrophages of Apoe−/− mice fed with Western diet for 2 months showed reduced signal intensity of lysotracker, a fluorescent weak amine that accumulates in late endosomes and lysosomes, indicating dysfunctional lysosome [25]. Lysosomal stress induced by chloroquine or atherogenic lipids, including oxidized-LDL and cholesterol crystal, led to lysosomal dysfunction in murine macrophages characterized by disruptions in lysosomal pH, proteolytic capacity, and membrane integrity [25]. The lysosomal dysfunction is also associated with transcription factor EB (TFEB) nuclear translocation and an increased expression of lysosome (Lipa, Atp6v0d2, Lamp1) and autophagy-related genes (Becn1 and p62/Sqstm1) [25]. TFEB overexpression in macrophages augmented lysosome-autophagy biogenesis, and rescued the proapoptotic and proinflammatory effects of atherogenic lipid loading, without affecting lipid accumulation despite increased LAL activity [26∎∎]. Therefore, enhancing the degradative capacity of macrophages may serve as a therapeutic strategy for atherosclerosis [26∎∎], but the impact of enhancing macrophage Lipa expression and LAL-mediated lipolysis on athero-genesis remains to be fully defined [26∎∎].
FUNCTIONAL GENOMIC DISCOVERIS OF LIPA AS A RISK LOCUS FOR CORONARY HEART DISEASE
Multiple GWAS [27–29] have identified LIPA as a novel locus for CHD. Despite the well known role of LIPA in lipoprotein metabolism, the common LIPA CHD risk alleles, rs1412444 (T) and rs2246833 (T), clustered in introns 2/3 in high linkage disequilibrium, are not associated with altered plasma lipids [30], liver traits [30], and are not associated with LIPA expression [i.e. are not expression quantitative trait locus (eQTL)] in liver tissue [31]. In contrast, hyperlipidemia and hepatomegaly are the major clinical manifestations in CESD patients caused by rare mutations in LIPA, whereas some patients also develop premature atherosclerosis [3]. Surprisingly, multiple eQTL datasets have shown that CHD risk allele carriers are associated with increased LIPA expressions in monocytes [27] and monocyte-derived macrophages [32∎∎] (i.e. are myeloid cell eQTLs), supporting a potential gain-of-function (GOF) effect of the LIPA common alleles in CHD. These paradoxical data raise important questions on the directionality of CHD causal variants at the LIPA GWAS locus, that is, whether the LIPA CHD alleles are LOF as might be anticipated based on the effects of rare Mendelian variants that cause CESD and atherosclerosis, or the alleles may be unexpectedly GOF variants that increase LIPA expression and function in monocytes/macrophages, as suggested by the eQTL studies.
A recent study by Morris et al. [33∎∎] with editorial comments [34] has begun to tackle these intriguing questions. Overexpression of LAL in COS7 cell lines showed that the risk allele (C) at rs1051338, a coding variant in high linkage disequilibrium with the GWAS single-nucleotide polymorphisms (SNPs), encoding a nonsynonymous threonine to proline change (Thr16Pro) within the signal peptide of LAL, may impair LAL protein translocation from the endoplasmic reticulum resulting in proteosomal degradation and reduced LAL protein and activity [33∎∎]. The results in monocytes-derived macrophages from a small subset of participants (n = 4) also showed that the lysosomal LAL activity in the risk allele carriers was lower than that in the nonrisk allele carriers, suggesting potential LOF effects of rs1051338 [33∎∎].
These data are highly suggestive, but not yet definitive for rs1051338 being ‘the’ causal variant at the LIPA GWAS locus [34]. First, this study did not address the published and surprising eQTL data of higher LIPA mRNA levels in risk allele carriers if the rs1051338 coding variant is indeed causal for CHD and associated with lower LAL activity; second, a relative small sample size in this study may not provide sufficient statistical evidence for the detection of the modest effects as expected for a common variant associated with complex trait. Last, the data also do not address other linked SNP at the LIPA locus, including those noncoding SNPs of similar allele frequency yet showing stronger association with increased risk of CHD and also with higher mRNA expression in eQTL studies [34].
Thus, the functional impact and mechanisms of the causal variants at the LIPA locus remain to be fully defined. It is imperative to confirm the directionality of the effects of GWAS variants on LAL activity in a cohort of much larger numbers of risk and nonrisk allele carriers. To identify the causal variants, unbiased clustered regularly interspaced short palindromic repeats (CRISPR) screen [35] represents a novel and powerful approach to characterize the functional regulatory element that mediates LIPA expression. To establish causality, CRISPR/Cas9 gene editing technologies can be adapted to introduce disease-associated variants or correct the risk alleles in human iPSC lines on a specific isogenic background [21∎∎]. With a published highly efficient protocol for differentiation of iPSC to macrophages [36,37∎∎] and validated applications in modeling macrophage-specific LOF phenotypes of LIPA [21∎∎], iPSC-derived macrophages with knock-in of individual or a combination of SNP variants will reveal the functional impact of the GWAS alleles, for example, on macrophage M2 activation, lipid mediator synthesis, extracellular LAL-mediated lipid degradation, and contribution to foam cell formation, lysosomal free cholesterol accumulation, and lysosomal dysfunction. Last, targeted mouse models with knock-in of human LIPA CHD alleles and/or tissue-specific overexpression will reveal the in-vivo effects of specific variants at this GWAS locus and address the impact of potential GOF of LAL on atherosclerosis, thus moving from association to mechanistic insights and advancing therapeutic translation.
CLINICAL STUDIES ON LYSOSOMAL ACID LIPASE REPLACEMENT THERAPIES
Sebelipase alfa (Kanuma) is a recombinant human LAL protein and the first enzyme replacement therapy for the treatment of LAL deficiency. In a phase 3 clinical trial in children and adults, 20 weeks of intravenous administration of sebelipase alfa resulted in normalization of alanine transaminase levels and improvement in abnormal lipid profile and hepatomegaly [38]. In infants with a rapidly progressive form of the disease, sebelipase alfa treatment has improved survival beyond the age of 1 year with improved hepatic and gastrointestinal symptoms [39].
The efficacy and safety of sebelipase alfa in both Wolman disease and CESD patients so far are certainly promising. While the diagnosis in infantile-onset Wolman disease is usually established early on with the more uniform and severe clinical manifestations, the accurate diagnosis of childhood/adult-onset CESD is challenging due to the unspecific clinical presentation with dyslipidemia and steato-hepatitis. The awareness of this condition and powerful diagnostic approach are important to facilitate the incorporation of sebelipase alfa in the treatment strategy for patients. It is also imperative to assess the effects of sebelipase alfa on the long-term progression of liver disease and prevention of cirrhosis in a larger number of patients. Studies in mice have shown that infusion of recombinant human LAL reduced hyperlipidemia and atherosclerosis in Ldlr−/− mice, where the atheroprotection was most likely attributable to the reduction in plasma lipids [40]. Accordingly, it will also be intriguing to determine whether treatment with sebelipase alfa may reduce cardiovascular events and mortality among patients with LAL deficiency.
CONCLUSION
Recent studies have highlighted that LAL-derived lipolytic products play novel regulatory roles in macrophage M2 activation, T-cell reprogramming, lipid mediator synthesis, and hepatocyte VLDL secretion. LAL regulates intracellular and extracellular lipid catabolism in a tissue and cell-specific manner. Macrophage LAL and lysosome biogenesis play important roles in obesity and atherosclerosis. Future functional genomic studies on the role of LIPA in CHD risk should use a combination of isogenic iPSC-derived macrophage, murine models of cell-specific knock-out, knock-in or overexpression. These studies will shed light on the potential for benefit and risk in therapeutic targeting of LIPA in CHD, particularly in the context of the availability of LAL replacement therapy currently approved for use in patients with CESD. The significance of LAL in a broad spectrum of pathophysiological conditions will continue to expand.
KEY POINTS.
Lysosomal acid lipase (LAL)-derived lipolytic products mediate macrophage M2 activation, T-cell reprogramming, lipid mediator production, and VLDL secretion.
LAL mediates intracellular and extracellular lipid catabolism in a tissue- and cell-specific manner.
Genome-wide association studies and functional genomic discoveries have identified LIPA as a risk locus for coronary heart diseases (CHDs), but the functional impact and mechanisms of the causal variants at the LIPA locus remain to be fully defined.
LAL enzyme replacement therapy is approved for clinical use in LAL deficiency, but its effects on cardiovascular outcomes are yet to be determined.
Acknowledgements
Financial support and sponsorship
This work is supported by an American Heart Association Postdoctoral Fellowship 15POST25620017 and the National Institutes of Health (NIH) grants K99-HL-130574 to HZ.
Footnotes
Conflicts of interest
None.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
∎ of special interest
∎∎ of outstanding interest
- 1.Dubland JA, Francis GA. Lysosomal acid lipase: at the crossroads of normal and atherogenic cholesterol metabolism. Frontiers in cell and developmental biology 2015; 3:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Du H, Duanmu M, Witte D, Grabowski GA. Targeted disruption of the mouse lysosomal acid lipase gene: long-term survival with massive cholesteryl ester and triglyceride storage. Hum Mol Genet 1998; 7:1347–1354. [DOI] [PubMed] [Google Scholar]
- 3.Bernstein DL, Hulkova H, Bialer MG, Desnick RJ. Cholesteryl ester storage disease: review of the findings in 135 reported patients with an under-diagnosed disease. J Hepatol 2013; 58:1230–1243. [DOI] [PubMed] [Google Scholar]
- 4.Valayannopoulos V, Mengel E, Brassier A, Grabowski G. Lysosomal acid lipase deficiency: expanding differential diagnosis. Mol Genet Metab 2017; 120:62–66. [DOI] [PubMed] [Google Scholar]
- 5.Huang SC, Everts B, Ivanova Y, et al. Cell-intrinsic lysosomal lipolysis is essential for alternative activation of macrophages. Nat Immunol 2014; 15:846–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Sullivan D, van der Windt GJ, Huang SC, et al. Memory CD8(+) T cells use cell-intrinsic lipolysis to support the metabolic programming necessary for development. Immunity 2014; 41:75–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schlager S, Vujic N, Korbelius M, et al. Lysosomal lipid hydrolysis provides substrates for lipid mediator synthesis in murine macrophages. Oncotarget 2017; 8:40037–40051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Radovic B, Vujic N, Leopold C, et al. Lysosomal acid lipase regulates VLDL synthesis and insulin sensitivity in mice. Diabetologia 2016; 59:1743–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ouimet M, Franklin V, Mak E, et al. Autophagy regulates cholesterol efflux from macrophage foam cells via lysosomal acid lipase. Cell Metab 2011; 13:655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jerome WG, Cox BE, Griffin EE, Ullery JC. Lysosomal cholesterol accumulation inhibits subsequent hydrolysis of lipoprotein cholesteryl ester. Microsc Microanal 2008; 14:138–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh R, Kaushik S, Wang Y, et al. Autophagy regulates lipid metabolism. Nature 2009; 458:1131–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grijalva A, Xu X, Ferrante AW Jr. Autophagy is dispensable for macrophage-mediated lipid homeostasis in adipose tissue. Diabetes 2016; 65:967–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haka AS, Grosheva I, Chiang E, et al. Macrophages create an acidic extracellular hydrolytic compartment to digest aggregated lipoproteins. Molecular biology of the cell 2009; 20:4932–4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haka AS, Singh RK, Grosheva I, et al. Monocyte-derived dendritic cells upregulate extracellular catabolism of aggregated low-density lipoprotein on maturation, leading to foam cell formation. Arterioscler Thromb Vasc Biol 2015; 35:2092–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ∎∎ 15.Singh RK, Barbosa-Lorenzi VC, Lund FW, et al. Degradation of aggregated LDL occurs in complex extracellular sub-compartments of the lysosomal synapse. J Cell Sci 2016; 129:1072–1082.This study highlights the role of LAL in extracellular lipid catabolism.
- 16.Leake DS. Does an acidic pH explain why low density lipoprotein is oxidised in atherosclerotic lesions? Atherosclerosis 1997; 129:149–157. [DOI] [PubMed] [Google Scholar]
- 17.Silver IA, Murrills RJ, Etherington DJ. Microelectrode studies on the acid microenvironment beneath adherent macrophages and osteoclasts. Exp Cell Res 1988; 175:266–276. [DOI] [PubMed] [Google Scholar]
- 18.Naghavi M, John R, Naguib S, et al. pH Heterogeneity of human and rabbit atherosclerotic plaques; a new insight into detection of vulnerable plaque. Atherosclerosis 2002; 164:27–35. [DOI] [PubMed] [Google Scholar]
- 19.Hakala JK, Oksjoki R, Laine P, et al. Lysosomal enzymes are released from cultured human macrophages, hydrolyze LDL in vitro, and are present extra-cellularly in human atherosclerotic lesions. Arterioscler Thromb Vasc Biol 2003; 23:1430–1436. [DOI] [PubMed] [Google Scholar]
- 20.Du H, Heur M, Duanmu M, et al. Lysosomal acid lipase-deficient mice: depletion of white and brown fat, severe hepatosplenomegaly, and shortened life span. J Lipid Res 2001; 42:489–500. [PubMed] [Google Scholar]
- ∎∎ 21.Zhang H, Shi J, Hachet MA, et al. CRISPR/Cas9-mediated gene editing in human iPSC-derived macrophage reveals lysosomal acid lipase function in human macrophages: brief report. Arterioscler Thromb Vasc Biol 2017; 37:2156–2160.This study uses CRISPR/Cas9 technology to knock out LIPA in human induced pluripotent stem cells and then differentiate to macrophages to explore the human macrophage LIPA loss-of-function phenotypes.
- 22.Jerome WG. Advanced atherosclerotic foam cell formation has features of an acquired lysosomal storage disorder. Rejuvenation Res 2006; 9:245–255. [DOI] [PubMed] [Google Scholar]
- 23.Haley NJ, Fowler S, de Duve C. Lysosomal acid cholesteryl esterase activity in normal and lipid-laden aortic cells. J Lipid Res 1980; 21:961–969. [PubMed] [Google Scholar]
- 24.Davis HR, Glagov S, Zarins CK. Role of acid lipase in cholesteryl ester accumulation during atherogenesis. Correlation of enzyme activity with acid lipase-containing macrophages in rabbit and human lesions. Atherosclerosis 1985; 55:205–215. [DOI] [PubMed] [Google Scholar]
- 25.Emanuel R, Sergin I, Bhattacharya S, et al. Induction of lysosomal biogenesis in atherosclerotic macrophages can rescue lipid-induced lysosomal dysfunction and downstream sequelae. Arterioscler Thromb Vasc Biol 2014; 34:1942–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ∎∎ 26.Sergin I, Evans TD, Zhang X, et al. Exploiting macrophage autophagy-lysosomal biogenesis as a therapy for atherosclerosis. Nat Commun 2017; 8:15750.This study suggests that enhancing the degradative capacity of macrophages might serve as a therapeutic strategy for atherosclerosis.
- 27.Wild PS, Zeller T, Schillert A, et al. A genome-wide association study identifies LIPA as a susceptibility gene for coronary artery disease. Circulation. Cardiovasc Genet 2011; 4:403–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Artery Disease (C4D) Genetics Consortium. A genome-wide association study in Europeans and South Asians identifies five new loci for coronary artery disease. Nat Genet 2011; 43:339–344. [DOI] [PubMed] [Google Scholar]
- 29.Consortium IKC. Large-scale gene-centric analysis identifies novel variants for coronary artery disease. PLoS Genet 2011; 7:e1002260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Willer CJ, Schmidt EM, Sengupta S, et al. Discovery and refinement of loci associated with lipid levels. Nat Genet 2013; 45:1274–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Consortium GT, Laboratory DA, Coordinating Center-Analysis Working G, et al. Genetic effects on gene expression across human tissues. Nature 2017; 550:204–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ∎∎ 32.Nedelec Y, Sanz J, Baharian G, et al. Genetic ancestry and natural selection drive population differences in immune responses to pathogens. Cell 2016; 167:657–669; e621.This study reported an RNA-sequencing based immune response eQTL study to test for the effects of African versus European ancestry on the transcriptional response to several live bacterial pathogens.
- ∎∎ 33.Morris GE, Braund PS, Moore JS, et al. Coronary artery disease-associated LIPA coding variant rs1051338 reduces lysosomal acid lipase levels and activity in lysosomes. Arterioscler Thromb Vasc Biol 2017; 37:1050–1057.This study suggests that coronary artery disease-associated coding variant rs1051338 causes reduced lysosomal LAL protein and activity because of increased LAL degradation, providing a plausible causal mechanism of increased coronary artery disease risk.
- 34.Zhang H, Reilly MP. LIPA variants in genome-wide association studies of coronary artery diseases: loss-of-function or gain-of-function? Arterioscler Thromb Vasc Biol 2017; 37:1015–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sanjana NE. Genome-scale CRISPR pooled screens. Anal Biochem 2017; 532:95–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang H, Xue C, Shah R, et al. Functional analysis and transcriptomic profiling of iPSC-derived macrophages and their application in modeling Mendelian disease. Circ Res 2015; 117:17–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ∎∎ 37.Zhang H, Reilly MP. Human induced pluripotent stem cell-derived macrophages for unraveling human macrophage biology. Arterioscler Thromb Vasc Biol 2017; 37:2000–2006.This article reviews the application of human-induced pluripotent stem cell-derived macrophages (IPSDM) in modeling both Mendelian genetic disorders and host–pathogen interactions, and highlighted the potential areas of research using IPSDM in functional genomic analyses, drug testing, and cell therapeutics.
- 38.Burton BK, Balwani M, Feillet F, et al. A phase 3 trial of sebelipase alfa in lysosomal acid lipase deficiency. N Engl J Med 2015; 373:1010–1020. [DOI] [PubMed] [Google Scholar]
- 39.Jones SA, Rojas-Caro S, Quinn AG, et al. Survival in infants treated with sebelipase Alfa for lysosomal acid lipase deficiency: an open-label, multi-center, dose-escalation study. Orphanet J Rare Dis 2017; 12:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Du H, Schiavi S, Wan N, et al. Reduction of atherosclerotic plaques by lysosomal acid lipase supplementation. Arterioscler Thromb Vasc Biol 2004; 24:147–154. [DOI] [PubMed] [Google Scholar]