Abstract
Background:
Traditional risk factors associated with idiopathic intracranial hypertension (IIH) include obesity, weight gain, and female sex. The incidence of IIH is increasing and yet the underlying trigger and the fueling pathological mechanisms are still poorly understood.
Evidence Acquisition:
Review of ophthalmology, neurology, general surgery, obesity, endocrinology, nutrition, and neurosurgery literature was made.
Results:
The facts that implicate sex and obesity in IIH and headache are examined. The role of fat distribution in IIH is questioned, and the concept of adipose tissue functioning as an endocrine organ driving IIH is discussed. The impact of androgen metabolism in IIH is reviewed as is the emerging role of glucagon-like-peptide-1 analogues in modulating intracranial pressure. This introduces the concept of developing targeted disease-modifying therapeutic strategies for IIH.
Conclusions:
This review will discuss the possible role of the adipose/gut/brain metabolism axis in IIH and speculate how this may impact the pathogenesis of IIH and therapeutic opportunities.
Idiopathic intracranial hypertension (IIH) is a condition of increased intracranial pressure (ICP) of unknown etiology (1). Clinicians often debate the principal pathogenic mechanisms, but no unifying theory has yet emerged. Cerebrospinal fluid (CSF) dynamics (overproduction or underdrainage) is likely a result of an initial trigger or ongoing insult. Cerebral venous sinus stenosis may occur in IIH, although its role in the disease pathogenesis is debated. Exploration of the well-known associations of female sex, obesity, weight gain, and polycystic ovary syndrome (PCOS) has led to the active investigation of the role of adipose tissue, androgens, and gut peptides in modulating ICP in IIH. A pathological basis for IIH, which may also contribute to headache in IIH, could potentially involve how the gut/brain metabolism axis interacts. For IIH, these newly described pathways could lead to targeted weight loss therapies, such as customized bariatric surgery, or other novel therapeutics such as glucagon-like-peptide-1 (GLP-1) receptor agonists. This review will discuss the possible role of the adipose/gut/brain metabolism axis in IIH and speculate how this may impact pathogenesis and therapeutic opportunities.
THE EVIDENCE IMPLICATING SEX AND OBESITY
IIH predominately affects women, with less than 10% of affected patients being men (1–4). There also is an association between IIH and obesity, as over 90% of patients are obese (5). The incidence of IIH is rising and seems to be increasing with the general trend of rising obesity rates (6), with the age- and sex-adjusted annual incidence more than doubling in the period between 2002 and 2014 compared with 1990–2001 in 1 study in the United States increasing from 1.0 to 2.8 per 100,000 (7). When stratified for reproductive age, female sex, and weight, the incidence has been reported between 12 and 28/100,000 per year (8). The risk of IIH also increases with increasing body mass index (BMI) (8). The greater degree of weight gain the year before symptom onset results in a greater risk of IIH. Weight loss has been shown to result in clinical improvement in several parameters, including reduction in ICP, papilledema, and headache (9). However, for an individual patient, the amount of weight modification needed to put the disease into remission varies, which raises a question of whether it is the type of adipose tissue or its location that is more relevant than the absolute amount of adipose tissue that is lost.
QUESTIONING THE ROLE OF FAT DISTRIBUTION
There is a strong association between abdominal fat deposition and health outcomes, including an increased risk of type 2 diabetes, adverse cardiovascular effects, insulin resistance, and dyslipidemia (10–12). The pattern of fat distribution in patients with IIH may be of relevance. One long-standing theory is that the mechanical effects of excessive abdominal fat elevate intra-abdominal pressure, which increases intrathoracic pressure and, thereby, increases cerebral venous pressure, and, consequently ICP (13). However, this hypothesis would not explain why only a proportion of all individuals with obesity develop IIH. Some studies measuring waist–hip ratios have reported increased lower-body fat in IIH as opposed to centripetal obesity (4,14). The use of dual-energy x-ray absorptiometry scanning, a validated accurate method to define and quantify body fat deposition, revealed that patients with IIH have a similar, centripetal fat distribution compared with simple obesity (15). Interestingly, in IIH, truncal fat mass correlates with opening pressure on lumbar puncture although BMI does not. However, we cannot exclude a false negative error affecting these results (15). It has also been noted that fat is preferentially lost from the trunk compared with the limbs in patients with IIH undergoing therapeutic weight loss resulting in significant reduction of ICP. This potentially implies that truncal fat mass may be pathogenic in IIH. It is also interesting to note that although BMI does not correlate with LP pressure in patients with IIH, there is a significant correlation between BMI and LP pressure in patients who do not have increased ICP disorders (16).
EARLY INDICATIONS THAT ADIPOSE TISSUE IS THE ENDOCRINE ORGAN DRIVING INTRACRANIAL HYPERTENSION
Adipose tissue functions as an endocrine organ, secreting a myriad of factors including proinflammatory cytokines, chemokines, adipokines, and hormones (17) that may be involved in IIH pathogenesis. Of note, some reports have indicated that the adipokine, leptin, is elevated in the serum (18) and CSF of patients with IIH, independent of BMI, compared with BMI-matched control subjects (19). There was a correlation of leptin levels between CSF and serum in patients with IIH. In addition, the increased CSF–serum leptin ratios in patients with IIH compared with controls suggest that transfer of leptin over the blood–brain barrier is not impaired in IIH, in contrast to obesity. Leptin is involved in hypothalamic control of satiety and weight regulation (20), and typically low CSF leptin levels are found in patients with obesity. It is, therefore, curious that CSF leptin levels are high in IIH (consequently driving hypothalamic satiety), yet those patients remain obese. This has increased the possibility of hypothalamic leptin resistance in IIH. Leptin receptors are localized to the choroid plexus and contribute to leptin transport in to the CSF (21). In addition, choroid plexus is the principle site for CSF secretion. However, the inconsistent findings from research in this area diminish the likelihood that leptin has a dominant role in pathogenesis, but aberrant leptin signaling may be a contributing factor for obesity in IIH (19).
Obesity is a chronic inflammatory condition and, consequently, another theory is the possibility of pathogenic inflammation causing IIH (22–24). Investigation of the cytokine and chemokine profiles in both the serum and CSF of patients with IIH has shown a significantly increased level of chemokine profiles in both the serum and CSF of patients with IIH has shown a significantly increased level of the chemokine CCL2 in the CSF of patients with IIH compared with controls, whereas CCL8, CCL7, and IL-1α were increased in the plasma in IIH, but not significantly (25). However, this study did not use a control group that was well matched to the patients with IIH as BMI of the IIH cohort was significantly higher than that of controls. Therefore, these findings could be representative of the inflammatory profile in obesity rather than in IIH. In addition, there were no significant differences in the levels of serum and CSF interleukin-8 (IL-8), monocyte chemoattractant protein-1 (MCP-1), resistin plasminogen activator inhibitor-1 (PAI-1), tissue necrosis factor-α (TNF-α), and hepatocyte growth factor (25). CSF IL-6 was also not significantly different in patients with IIH nor were CSF:serum cytokine ratios. However, others have found a proinflammatory cytokine profile in patients with IIH, with significant elevations in IL-2 and IL-17 in the CSF of patients with IIH compared with controls with other neurological conditions. There was no difference in serum cytokine levels, but a potential confounder was that the controls were not BMI matched with the patients with IIH (26).
Obesity occurs in conditions with excess secretion of glucocorticoids such as Cushing syndrome. Systemic levels of cortisol are regulated by the hypothalamic–pituitary–adrenal axis. At a tissue level, cortisol is regulated by the enzyme 11β-hydroxysteroid dehydrogenase (11β-HSD). 11β-HSD type 1 (11β-HSD1) which converts inactive cortisone into cortisol and is found in high levels in the liver and adipose tissue (27). Mice overexpressing 11β-HSD1 globally, or specifically in adipose tissue, have a metabolic syndrome phenotype of obesity, dyslipidemia, and glucose intolerance (28). In humans, obesity is associated with normal systemic cortisol levels but dysregulated 11β-HSD1 activity in adipose tissue (29). The relationship between glucocorticoids and IIH is somewhat more complicated, as IIH may occur during withdrawal or replacement of glucocorticoid therapy (30). Activity and expression of 11β-HSD1 is especially high in fat and, interestingly, 11β-HSD1 has been localized to the choroid plexus, where it drives local cortisol production (31). Cortisol generation in the choroid plexus is believed to drive epithelial sodium transporters on the apical membrane of the choroid plexus epithelial cells and establish an ionic gradient, promoting CSF production. 11β-HSD1 may, therefore, have a key role in influencing the regulation of CSF secretion, and thus ICP. Preliminary studies in patients with IIH have demonstrated that therapeutic weight loss causes a reduction in global 11β-HSD1 activity, which correlates with the reduction in ICP (32). The effects of 11 β-HSD1 inhibition in reducing ICP in patients with IIH is currently being assessed in a randomized clinical trial (33).
POSTULATING THE ROLE OF ANDROGEN METABOLISM IN INTRACRANIAL HYPERTENSION
Polycystic ovary syndrome (PCOS) and IIH share many overlapping clinical features. PCOS is the most common endocrine condition in women of reproductive age, and androgen excess is a cardinal defining feature in most diagnostic consensus criteria (34,35). The most severe clinical and biochemical features of androgen excess in PCOS are observed in the context of comorbid obesity (36). A number of reports have found that the prevalence of PCOS is significantly higher in women with IIH women compared with the general female population (up to 57% compared with 5%–10%). However, these studies did not compare cohorts matched for age and BMI and, consequently, this could represent a chance association (37). It is possible that obesity, often noted in women with PCOS, could represent the pathogenic driver in those individuals with coexisting IIH and PCOS. However, although PCOS is more common in women with obesity, it can also occur in women of normal body weight, so the association of IIH and PCOS may not be due to obesity alone. Of interest, the prevalence of PCOS is reported as significantly lower in female cohorts with simple obesity (28.3% in 1 study) compared with the incidence reported in patients with IIH (57%) (38).
It has been proposed that androgen excess may be a risk factor in women with IIH, independent of obesity, and that hyperandrogenism may play a pathophysiological role in disease onset. One study found that androgen excess was associated with a younger age at onset of IIH, but did not correlate with BMI, waist-to-hip ratios, or duration of IIH (39). Patients with IIH have increased adipose tissue, and androgen activation in adipose tissue may be relevant to the pathophysiology of IIH. Adipose tissue plays a major role in peripheral androgen generation (40), and increased intra-adipose androgen activation is observed even in women with simple obesity (41).
Case reports of transgender patients undergoing female to male gender reassignment provide important insights into the role of androgens in IIH. Five case reports detail that patients receiving testosterone injections subsequently developed IIH, 4 of whom showed a temporal relationship between commencement of testosterone and symptoms of IIH (Table 1) (42–46). There needs to be caution when interpreting these reports because confounders such as weight gain may have influenced the development of increased ICP. Further evaluation of the role of testosterone in ICP regulation would be of great interest.
TABLE 1.
Reports of idiopathic intracranial hypertension in patients with sex reassignment
IIH in men, although uncommon, also provides insights into the complex and potentially sexually dimorphic role that androgens may play in this disorder. Men with androgen deficiency may be at increased risk of IIH; case reports of developing IIH after induction of hypogonadism with androgen deprivation therapy for prostate cancer have been reported (47). It also has been documented that men with IIH are more likely to have symptoms of testosterone deficiency, such as erectile dysfunction and reduced libido (48).
We suggest that a pathophysiological window of abnormal circulating androgen levels, with serum testosterone concentrations at a level shared by men with androgen deficiency and women with androgen excess, could increase the risk of IIH. Sexually dimorphic associations of androgens are well recognized. Metabolic diseases such as nonalcoholic fatty liver disease and diabetes are noted to have a sexually dimorphic relationship to androgens (49). In these metabolic conditions, women with androgen excess and men with androgen deficiency have an overlapping constellation of adverse cardio-metabolic risk factors (50). Circulating testosterone concentrations in women with androgen excess may overlap with those of men with androgen deficiency; at these serum concentrations, there is preferential accumulation of visceral adiposity in both sexes and metabolically deleterious loss of skeletal muscle bulk in men (51).
Therefore, it is conceivable that IIH represents a distinct neurological manifestation of this adverse metabolic phenotype with female androgen excess and male androgen deficiency conferring a risk factor for the development of IIH. Although female androgen excess and male hypogonadism are associated with abdominal obesity, which could be the trigger for IIH in these cases, it is also important to note that severe hypogonadism, with low androgen levels, can occur independently of obesity in men and remains associated with increased cardiovascular and all causes of mortality (52). Equally, women with PCOS and androgen excess are at increased risk of metabolic complications, such as nonalcoholic fatty liver disease, independent of obesity (53). Further studies in men and women are needed to understand these complex sexually dimorphic associations and their relationship to IIH and ICP regulation in the context of obesity.
There also is very limited understanding of the roles of estrogen and progesterone in IIH (54,55). Studies to date describing estrogen (estrone) and progesterone levels in the CSF have been small (n = 7 and n = 15), lacking matched control groups, and hampered through the use radioimmunoassays with a poor lower limit of detection. This has led to levels of estrogens being inconsistently detected in the CSF. Estrogen receptors are expressed in the choroid plexus epithelial cells, however, and their potential involvement in CSF secretion is an interesting avenue for future studies (56,57).
The Emerging Role of Gut Peptides in Modulating Intracranial Pressure
Glucagon-like peptide-1 (GLP-1) is a gut peptide secreted by the distal small intestine in response to food intake and synthesized in neurons of the solitary tract nucleus. GLP-1 stimulates glucose-dependent insulin secretion and inhibits glucagon release, lowering blood glucose (58) and promoting satiety and weight loss (59). GLP-1 mimetics are currently being used clinically to treat obesity and diabetes. In addition, GLP-1 has effects on renal proximal tubule sodium ion (Na+) secretion by reducing Na+ resorption into the blood stream and increasing diuresis (60). In the brain, CSF secretion by the choroid plexus epithelial cells is also driven by the net movement of Na+ from the blood into the cerebral ventricles. The localization of the receptor to the choroid plexus (61,62) suggests that GLP-1 may be able to modulate Na+ transport in the choroid plexus and, thereby, CSF production. Recently, it was demonstrated that exendin-4, which is a GLP-1 receptor agonist, also known as exenatide, currently prescribed to treat obesity and diabetes, reduces Na+/K+ATPase activity a marker of CSF secretion. Of note, exendin-4 dramatically reduced ICP in rodents with increased ICP. Exendin-4 reduced ICP by 44% within 10 minutes of dosing. Not only did the treatment effects last for at least 24 hours, cumulatively, the doses lowered the baseline ICP (61). Although there is no current evidence that gut neuropeptides or GLP-1 are implicated in the pathogenesis of IIH, these studies do highlight the therapeutic potential for GLP-1 receptor agonist in the management of IIH.
DEVELOPING TARGETED DISEASE-MODIFYING STRATEGIES FOR INTRACRANIAL HYPERTENSION
Weight loss typically results in clinical resolution of IIH (9,63,64). A large number of case reports discuss the use of bariatric surgery as a treatment for IIH, reporting significant improvement of symptoms and clinical signs in most patients (65–71). The IIH weight study (IIH:WT) (72) finished recruitment in April 2017 and will test the hypothesis that bariatric surgery will be more beneficial in the long term for treating IIH compared with community weight loss programs.
There are several bariatric surgical approaches, including Roux-en-Y gastric bypass, laparoscopic adjustable gastric banding, and laparoscopic sleeve gastrectomy. All 3 surgical methods result in a greater degree of weight loss compared with nonsurgical interventions (73). Reports of the effect of bariatric surgery on outcomes such as diabetes, lipid profile, and blood pressure are variable. One study found remission of type 2 diabetes in 28.6%–67.5% of patients after surgery (depending on the procedure) (74). What is relevant is that gastric bypass, in particular Roux-en-Y bypass, enhances GLP-1 levels contributing to the beneficial effects of bariatric surgery in diabetes (75). Given the emerging role of gut peptides in modifying ICP, it is interesting to speculate that bariatric surgery techniques that modify GLP-1 to the greatest degree (Roux-en-Y bypass), may be most effective in inducing IIH remission, not only by virtue of weight lost but also because of the effect on gut peptides and potentially altering in CSF secretion.
Future therapies would ideally modify CSF secretion to control ICP but also induce weight loss. The novel data on the properties of GLP-1 receptor agonist to reduce ICP (61) may represent an important advance that would have these properties. Exenatide, a synthetic version of exendin-4, a GLP-1 receptor agonist, is widely used to treat diabetes and obesity and has the potential to be repurposed for the treatment of increased ICP (Fig. 1). Furthermore, exenatide has received orphan drug designation for the treatment of IIH from the European Medicines Agency and the US Food and Drug Administration. A clinical trial is currently underway exploring the physiological effects of exenatide in reducing ICP in a small cohort of active patients with IIH.
FIG. 1.
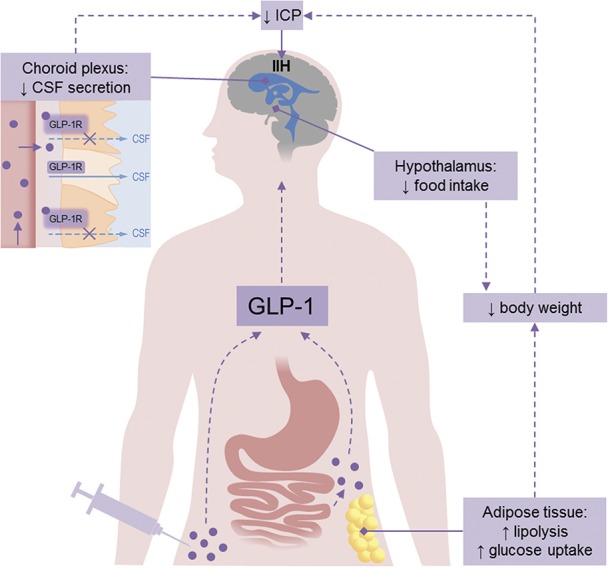
Glucagon-like peptide 1 (GLP-1) mechanism of action in IIH. Normally GLP-1 (purple circles) is produced by L-cells in the small intestine in response to food. GLP-1 mimetics are used to treat diabetes and obesity and are administered by subcutaneous injection. GLP-1 mimetics could be beneficial for patients with IIH as they act on the hypothalamus to reduce food intake and on adipose tissue to increase lipolysis resulting in weight loss. In addition, GLP-1 mimetics bind to GLP-1 receptors (GLP-1R) on the choroid plexus, leading to a reduction in CSF secretion and ICP. CSF, cerebrospinal fluid; ICP, intracranial pressure; IIH, idiopathic intracranial hypertension.
CONCLUSIONS
The emerging link between IIH and metabolism is potentially important. The areas outlined in this review start to establish a link between IIH and the concept of metabolic dysregulation (Fig. 2).
FIG. 2.
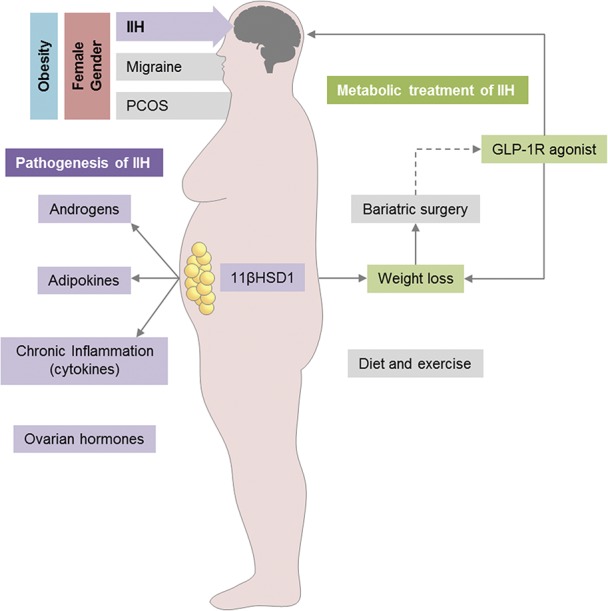
Metabolic pathogenesis and treatment in IIH. IIH is most prevalent in obese women of childbearing age, similar to migraine and polycystic ovary syndrome. A combination of dysregulated adipose tissue and hormones may be involved in IIH pathogenesis. Weight loss through bariatric surgery and diet has been shown to improve IIH symptoms. GLP-1R agonists may have additional benefits for patients with IIH by directly affecting ICP and causing weight loss. GLP-1R, glucagon-like-peptide-1 receptor; ICP, intracranial pressure; IIH, idiopathic intracranial hypertension.
The underlying pathophysiological mechanism of IIH has yet to be established. However, the answer may lie in the location and metabolic activity of the excessive adipose tissue in these patients. Ongoing randomized controlled trials will potentially provide data that will help uncover the relationship between obesity, weight loss, and IIH (72). Novel, in-vivo and in vitro data suggest that exenatide, a GLP-1 receptor agonist, may represent an ideal therapy for IIH through rapid reduction of CSF secretion at the choroid plexus, lowering ICP in addition to its weight loss properties. Expanding our knowledge of the metabolic pathways involved in ICP regulation and IIH maybe an important step for future research, which could lead to the identification of novel targeted therapies for patients with IIH.
STATEMENT OF AUTHORSHIP
Category 1: a. Conception and design: C. Hornby, S. P. Mollan, and A. J. Sinclair; b. Acquisition of data: C. Hornby, S. P. Mollan, H. Botfield, M. W. O'Reilly, and A. J. Sinclair; c. Analysis and interpretation of data: C. Hornby, S. P. Mollan, H. Botfield, M. W. O'Reilly, and A. J. Sinclair. Category 2: a. Drafting the manuscript: C. Hornby, S. P. Mollan, H. Botfield, M. W. O'Reilly, and A. J. Sinclair; b. Revising it for intellectual content: C. Hornby, S. P. Mollan, H. Botfield, M. W. O'Reilly, and A. J. Sinclair. Category 3: a. Final approval of the completed manuscript: C. Hornby, S. P. Mollan, H. Botfield, M. W. O'Reilly, and A. J. Sinclair.
Footnotes
A. J. Sinclair is funded by an NIHR Clinician Scientist Fellowship (NIHR-CS-011-028) and by the Medical Research Council, United Kingdom.
The authors report no conflicts of interest.
Joint first authors are C. Hornby and S. P. Mollan.
REFERENCES
- 1.Markey KA, Mollan SP, Jensen RH, Sinclair AJ. Understanding idiopathic intracranial hypertension: mechanisms, management, and future directions. Lancet Neurol. 2016;15:78–91. [DOI] [PubMed] [Google Scholar]
- 2.Digre KB, Corbett JJ. Pseudotumor cerebri in men. Arch Neurol. 1988;45:866–872. [DOI] [PubMed] [Google Scholar]
- 3.Bruce BB, Kedar S, Van Stavern GP, Monaghan D, Acierno MD, Braswell RA, Preechawat P, Corbett JJ, Newman NJ, Biousse V. Idiopathic intracranial hypertension in men. Neurology. 2009;72:304–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwartz R, Kliper E, Stern N, Dotan G, Berliner S, Kesler A. The obesity pattern of idiopathic intracranial hypertension in men. Graefes Arch Clin Exp Ophthalmol. 2013;251:2643–2646. [DOI] [PubMed] [Google Scholar]
- 5.Mollan SP, Ali F, Hassan-Smith G, Botfield H, Friedman DI, Sinclair AJ. Evolving evidence in adult idiopathic intracranial hypertension: pathophysiology and management. J Neurol Neurosurg Psychiatry. 2016;87:982–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kilgore KP, Lee MS, Leavitt JA, Mokri B, Hodge DO, Frank RD, Chen JJ. Re-evaluating the incidence of idiopathic intracranial hypertension in an era of increasing obesity. Ophthalmology. 2017;124:697–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daniels AB, Liu GT, Volpe NJ, Galetta SL, Moster ML, Newman NJ, Biousse V, Lee AG, Wall M, Kardon R, Acierno MD, Corbett JJ, Maguire MG, Balcer LJ. Profiles of obesity, weight gain, and quality of life in idiopathic intracranial hypertension (pseudotumor cerebri). Am J Ophthalmol. 2007;143:635–641. [DOI] [PubMed] [Google Scholar]
- 8.Andrews LE, Liu GT, Ko MW. Idiopathic intracranial hypertension and obesity. Horm Res Paediatr. 2014;81:217–225. [DOI] [PubMed] [Google Scholar]
- 9.Sinclair AJ, Burdon MA, Nightingale PG, Ball AK, Good P, Matthews TD, Jacks A, Lawden M, Clarke CE, Stewart PM, Walker EA, Tomlinson JW, Rauz S. Low energy diet and intracranial pressure in women with idiopathic intracranial hypertension: prospective cohort study. BMJ. 2010;341:c2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Canoy D, Luben R, Welch A, Bingham S, Wareham N, Day N, Khaw KT. Fat distribution, body mass index and blood pressure in 22,090 men and women in the Norfolk cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC-Norfolk) study. J Hypertens. 2004;22:2067–2074. [DOI] [PubMed] [Google Scholar]
- 11.Grundy SM, Adams-Huet B, Vega GL. Variable contributions of fat content and distribution to metabolic syndrome risk factors. Metab Syndr Relat Disord. 2008;6:281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yusuf S, Hawken S, Ounpuu S, Bautista L, Franzosi MG, Commerford P, Lang CC, Rumboldt Z, Onen CL, Lisheng L, Tanomsup S, Wangai P, Jr, Razak F, Sharma AM, Anand SS; INTERHEART Study Investigators. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case-control study. Lancet. 2005;366:1640–1649. [DOI] [PubMed] [Google Scholar]
- 13.Sugerman HJ, DeMaria EJ, Felton WL, III, Nakatsuka M, Sismanis A. Increased intra-abdominal pressure and cardiac filling pressures in obesity-associated pseudotumor cerebri. Neurology. 1997;49:507–511. [DOI] [PubMed] [Google Scholar]
- 14.Kesler A, Kliper E, Shenkerman G, Stern N. Idiopathic intracranial hypertension is associated with lower body adiposity. Ophthalmology. 2010;117:169–174. [DOI] [PubMed] [Google Scholar]
- 15.Hornby C, Botfield H, O'Reilly M, Westgate C, Mitchell J, Mollan S, Manolopoulos K, Tomlinson J. Sinclair A. Evaluating the fat distribution in idiopathic intracranial hypertension using dual-energy x-ray absorptiometry scanning. Neuroophthalmology. 2017;42:99–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berdahl JP, Fleischman D, Zaydlarova J, Stinnett S, Allingham RR, Fautsch MP. Body mass index has a linear relationship with cerebrospinal fluid pressure. Invest Ophthalmol Vis Sci. 2012;53:1422–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fain JN. Release of interleukins and other inflammatory cytokines by human adipose tissue is enhanced in obesity and primarily due to the nonfat cells. Vitam Horm. 2006;74:443–477. [DOI] [PubMed] [Google Scholar]
- 18.Lampl Y, Eshel Y, Kessler A, Fux A, Gilad R, Boaz M, Matas Z, Sadeh M. Serum leptin level in women with idiopathic intracranial hypertension. J Neurol Neurosurg Psychiatry. 2002;72:642–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ball AK, Sinclair AJ, Curnow SJ, Tomlinson JW, Burdon MA, Walker EA, Stewart PM, Nightingale PG, Clarke CE, Rauz S. Elevated cerebrospinal fluid (CSF) leptin in idiopathic intracranial hypertension (IIH): evidence for hypothalamic leptin resistance? Clin Endocrinol (Oxf). 2009;70:863–869. [DOI] [PubMed] [Google Scholar]
- 20.Klok MD, Jakobsdottir S, Drent ML. The role of leptin and ghrelin in the regulation of food intake and body weight in humans: a review. Obes Rev. 2007;8:21–34. [DOI] [PubMed] [Google Scholar]
- 21.Merino B, Diez-Fernandez C, Ruiz-Gayo M, Somoza B. Choroid plexus epithelial cells co-express the long and short form of the leptin receptor. Neurosci Lett. 2006;393:269–272. [DOI] [PubMed] [Google Scholar]
- 22.Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest. 1995;95:2409–2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. [DOI] [PubMed] [Google Scholar]
- 24.Sinclair AJ, Ball AK, Burdon MA, Clarke CE, Stewart PM, Curnow SJ, Rauz S. Exploring the pathogenesis of IIH: an inflammatory perspective. J Neuroimmunol. 2008;201–202:212–220. [DOI] [PubMed] [Google Scholar]
- 25.Dhungana S, Sharrack B, Woodroofe N. Cytokines and chemokines in idiopathic intracranial hypertension. Headache. 2009;49:282–285. [DOI] [PubMed] [Google Scholar]
- 26.Edwards LJ, Sharrack B, Ismail A, Tench CR, Gran B, Dhungana S, Brettschneider J, Tumani H. Increased levels of interleukins 2 and 17 in the cerebrospinal fluid of patients with idiopathic intracranial hypertension. Am J Clin Exp Immunol. 2013;2:234–244. [PMC free article] [PubMed] [Google Scholar]
- 27.Chapman K, Holmes M, Seckl J. 11beta-hydroxysteroid dehydrogenases: intracellular gate-keepers of tissue glucocorticoid action. Physiol Rev. 2013;93:1139–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Masuzaki H, Paterson J, Shinyama H, Morton NM, Mullins JJ, Seckl JR, Flier JS. A transgenic model of visceral obesity and the metabolic syndrome. Science. 2001;294:2166–2170. [DOI] [PubMed] [Google Scholar]
- 29.Sandeep TC, Andrew R, Homer NZ, Andrews RC, Smith K, Walker BR. Increased in vivo regeneration of cortisol in adipose tissue in human obesity and effects of the 11beta-hydroxysteroid dehydrogenase type 1 inhibitor carbenoxolone. Diabetes. 2005;54:872–879. [DOI] [PubMed] [Google Scholar]
- 30.Zada G, Tirosh A, Kaiser UB, Laws ER, Woodmansee WW. Cushing's disease and idiopathic intracranial hypertension: case report and review of underlying pathophysiological mechanisms. J Clin Endocrinol Metab. 2010;95:4850–4854. [DOI] [PubMed] [Google Scholar]
- 31.Sinclair AJ, Onyimba CU, Khosla P, Vijapurapu N, Tomlinson JW, Burdon MA, Stewart PM, Murray PI, Walker EA, Rauz S. Corticosteroids, 11beta-hydroxysteroid dehydrogenase isozymes and the rabbit choroid plexus. J Neuroendocrinol. 2007;19:614–620. [DOI] [PubMed] [Google Scholar]
- 32.Sinclair AJ, Walker EA, Burdon MA, van Beek AP, Kema IP, Hughes BA, Murray PI, Nightingale PG, Stewart PM, Rauz S, Tomlinson JW. Cerebrospinal fluid corticosteroid levels and cortisol metabolism in patients with idiopathic intracranial hypertension: a link between 11beta-HSD1 and intracranial pressure regulation? J Clin Endocrinol Metab. 2010;95:5348–5356. [DOI] [PubMed] [Google Scholar]
- 33.Markey KA, Ottridge R, Mitchell JL, Rick C, Woolley R, Ives N, Nightingale P, Sinclair AJ. Assessing the efficacy and safety of an 11beta-hydroxysteroid dehydrogenase type 1 inhibitor (AZD4017) in the idiopathic intracranial hypertension drug trial, IIH: DT: clinical methods and design for a phase II randomized controlled trial. JMIR Res Protoc. 2017;6:e181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Randeva HS, Tan BK, Weickert MO, Lois K, Nestler JE, Sattar N, Lehnert H. Cardiometabolic aspects of the polycystic ovary syndrome. Endocr Rev. 2012;33:812–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, Janssen OE, Legro RS, Norman RJ, Taylor AE, Witchel SF. Androgen Excess Society. Positions statement: criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: an Androgen Excess Society guideline. J Clin Endocrinol Metab. 2006;91:4237–4245. [DOI] [PubMed] [Google Scholar]
- 36.O'Reilly MW, Taylor AE, Crabtree NJ, Hughes BA, Capper F, Crowley RK, Stewart PM, Tomlinson JW, Arlt W. Hyperandrogenemia predicts metabolic phenotype in polycystic ovary syndrome: the utility of serum androstenedione. J Clin Endocrinol Metab. 2014;99:1027–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Glueck CJ, Aregawi D, Goldenberg N, Golnik KC, Sieve L, Wang P. Idiopathic intracranial hypertension, polycystic-ovary syndrome, and thrombophilia. J Lab Clin Med. 2005;145:72–82. [DOI] [PubMed] [Google Scholar]
- 38.Alvarez-Blasco F, Botella-Carretero JI, San Millan JL, Escobar-Morreale HF. Prevalence and characteristics of the polycystic ovary syndrome in overweight and obese women. Arch Intern Med. 2006;166:2081–2086. [DOI] [PubMed] [Google Scholar]
- 39.Klein A, Stern N, Osher E, Kliper E, Kesler A. Hyperandrogenism is associated with earlier age of onset of idiopathic intracranial hypertension in women. Curr Eye Res. 2013;38:972–976. [DOI] [PubMed] [Google Scholar]
- 40.O'Reilly MW, House PJ, Tomlinson JW. Understanding androgen action in adipose tissue. J Steroid Biochem Mol Biol. 2014;143:277–284. [DOI] [PubMed] [Google Scholar]
- 41.Quinkler M, Sinha B, Tomlinson JW, Bujalska IJ, Stewart PM, Arlt W. Androgen generation in adipose tissue in women with simple obesity—a site-specific role for 17beta-hydroxysteroid dehydrogenase type 5. J Endocrinol. 2004;183:331–342. [DOI] [PubMed] [Google Scholar]
- 42.Buchanan I, Hansen K, Bedolla J. Idiopathic intracranial hypertension in a transgender male on hormone therapy. Arch Emerg Med Crit Care. 2017;2:1019. [Google Scholar]
- 43.Park S, Cheng CP, Lim LT, Gerber D. Secondary intracranial hypertension from testosterone therapy in a transgender patient. Semin Ophthalmol. 2014;29:156–158. [DOI] [PubMed] [Google Scholar]
- 44.Mowl AD, Grogg JA, Klein J. Secondary pseudotumour cerebri in a patient undergoing sexual reassignment therapy. Clin Exp Optom. 2009;92:449–453. [DOI] [PubMed] [Google Scholar]
- 45.Sheets C, Peden M, Guy J. Idiopathic intracranial hypertension in a transgender man. J Neuroophthalmol. 2007;27:313–315. [DOI] [PubMed] [Google Scholar]
- 46.Hornby C, Mollan SP, Mitchell J, Markey KA, Yangou A, Wright BLC, O'Reilly MW, Sinclair AJ. What do transgender patients teach us about idiopathic intracranial hypertension? Neuroophthalmology. 2017;41:326–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Valcamonico F, Arcangeli G, Consoli F, Nonnis D, Grisanti S, Gatti E, Berruti A, Ferrari V. Idiopathic intracranial hypertension: a possible complication in the natural history of advanced prostate cancer. Int J Urol. 2014;21:335–337. [DOI] [PubMed] [Google Scholar]
- 48.Fraser JA, Bruce BB, Rucker J, Fraser LA, Atkins EJ, Newman NJ, Biousse V. Risk factors for idiopathic intracranial hypertension in men: a case-control study. J Neurol Sci. 2010;290:86–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mongraw-Chaffin ML, Anderson CA, Allison MA, Ouyang P, Szklo M, Vaidya D, Woodward M, Golden SH. Association between sex hormones and adiposity: qualitative differences in women and men in the multi-ethnic study of atherosclerosis. J Clin Endocrinol Metab. 2015;100:E596–E600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schiffer L, Kempegowda P, Arlt W, O'Reilly MW. Mechanisms in endocrinology: the sexually dimorphic role of androgens in human metabolic disease. Eur J Endocrinol. 2017;177:R125–R143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Escobar-Morreale HF, Alvarez-Blasco F, Botella-Carretero JI, Luque-Ramirez M. The striking similarities in the metabolic associations of female androgen excess and male androgen deficiency. Hum Reprod. 2014;29:208320–208391. [DOI] [PubMed] [Google Scholar]
- 52.Pye SR, Huhtaniemi IT, Finn JD, Lee DM, O'Neill TW, Tajar A, Bartfai G, Boonen S, Casanueva FF, Forti G, Giwercman A, Han TS, Kula K, Lean ME, Pendleton N, Punab M, Rutter MK, Vanderschueren D, Wu FC; EMAS Study Group. Late-onset hypogonadism and mortality in aging men. J Clin Endocrinol Metab. 2014;99:1357–1366. [DOI] [PubMed] [Google Scholar]
- 53.Jones H, Sprung VS, Pugh CJ, Daousi C, Irwin A, Aziz N, Adams VL, Thomas EL, Bell JD, Kemp GJ, Cuthbertson DJ. Polycystic ovary syndrome with hyperandrogenism is characterized by an increased risk of hepatic steatosis compared to nonhyperandrogenic PCOS phenotypes and healthy controls, independent of obesity and insulin resistance. J Clin Endocrinol Metab. 2012;97:3709–3716. [DOI] [PubMed] [Google Scholar]
- 54.Donaldson JO, Horak E. Cerebrospinal fluid oestrone in pseudotumour cerebri. J Neurol Neurosurg Psychiatry. 1982;45:734–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Soelberg Sorensen P, Gjerris F, Svenstrup B. Endocrine studies in patients with pseudotumor cerebri. Estrogen levels in blood and cerebrospinal fluid. Arch Neurol. 1986;43:902–906. [DOI] [PubMed] [Google Scholar]
- 56.Santos CR, Duarte AC, Quintela T, Tomas J, Albuquerque T, Marques F, Palha JA, Gonçalves I. The choroid plexus as a sex hormone target: functional implications. Front Neuroendocrinol. 2017;44:103–121. [DOI] [PubMed] [Google Scholar]
- 57.Iwabuchi J, Koshimizu K, Nakagawa T. Expression profile of the aromatase enzyme in the Xenopus brain and localization of estradiol and estrogen receptors in each tissue. Gen Comp Endocrinol. 2013;194:286–294. [DOI] [PubMed] [Google Scholar]
- 58.Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131–2157. [DOI] [PubMed] [Google Scholar]
- 59.Flint A, Raben A, Astrup A, Holst JJ. Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J Clin Invest. 1998;101:515–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Carraro-Lacroix LR, Malnic G, Girardi AC. Regulation of Na+/H+ exchanger NHE3 by glucagon-like peptide 1 receptor agonist exendin-4 in renal proximal tubule cells. Am J Physiol Renal Physiol. 2009;297:F1647–F1655. [DOI] [PubMed] [Google Scholar]
- 61.Botfield HF, Uldall MS, Westgate CSJ, Mitchell JL, Hagen SM, Gonzalez AM, Hodson DJ, Jensen RH, Sinclair AJ. A glucagon-like peptide-1 receptor agonist reduces intracranial pressure in a rat model of hydrocephalus. Sci Transl Med. 2017;9:eaan0972. [DOI] [PubMed] [Google Scholar]
- 62.Alvarez E, Roncero I, Chowen JA, Thorens B, Blazquez E. Expression of the glucagon-like peptide-1 receptor gene in rat brain. J Neurochem. 1996;66:920–927. [DOI] [PubMed] [Google Scholar]
- 63.Subramaniam S, Fletcher WA. Obesity and weight loss in idiopathic intracranial hypertension: a narrative review. J Neuroophthalmol. 2017;37:197–205. [DOI] [PubMed] [Google Scholar]
- 64.Committee NIIHSGW, Wall M, McDermott MP, Kieburtz KD, Corbett JJ, Feldon SE, Friedman DI, Katz DM, Keltner JL, Schron EB, Kupersmith MJ. Effect of acetazolamide on visual function in patients with idiopathic intracranial hypertension and mild visual loss: the idiopathic intracranial hypertension treatment trial. JAMA. 2014;311:1641–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Roth J, Constantini S, Kesler A. Over-drainage and persistent shunt-dependency in patients with idiopathic intracranial hypertension treated with shunts and bariatric surgery. Surg Neurol Int. 2015;6(suppl 27):S655–S5660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Levin AA, Hess D, Hohler AD. Treatment of idiopathic intracranial hypertension with gastric bypass surgery. Int J Neurosci. 2015;125:78–80. [DOI] [PubMed] [Google Scholar]
- 67.Egan RJ, Meredith HE, Coulston JE, Bennetto L, Morgan JD, Norton SA. The effects of laparoscopic adjustable gastric banding on idiopathic intracranial hypertension. Obes Surg. 2011;21:161–166. [DOI] [PubMed] [Google Scholar]
- 68.Fridley J, Foroozan R, Sherman V, Brandt ML, Yoshor D. Bariatric surgery for the treatment of idiopathic intracranial hypertension. J Neurosurg. 2011;114:34–39. [DOI] [PubMed] [Google Scholar]
- 69.Williams A, Morgan J, Johnson A, Bates S, Pople I, Norton S. Resolution of pseudotumor cerebri following surgery for morbid obesity. J Surg Case Rep. 2010;2010:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nadkarni T, Rekate HL, Wallace D. Resolution of pseudotumor cerebri after bariatric surgery for related obesity. Case report. J Neurosurg. 2004;101:878–880. [DOI] [PubMed] [Google Scholar]
- 71.Sugerman HJ, Felton WL, III, Sismanis A, Kellum JM, DeMaria EJ, Sugerman EL. Gastric surgery for pseudotumor cerebri associated with severe obesity. Ann Surg. 1999;229:634–640; discussion 40–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ottridge R, Mollan SP, Botfield H, Frew E, Ives NJ, Matthews T, Mitchell J, Rick C, Singhal R, Woolley R, Sinclair AJ. Randomised controlled trial of bariatric surgery versus a community weight loss programme for the sustained treatment of idiopathic intracranial hypertension: the idiopathic intracranial hypertension weight trial (IIH: WT) protocol. BMJ Open. 2017;7:e017426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Colquitt JL, Pickett K, Loveman E, Frampton GK. Surgery for weight loss in adults. Cochrane Database Syst Rev. 2014:CD003641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Courcoulas AP, Christian NJ, Belle SH, Berk PD, Flum DR, Garcia L, Horlick M, Kalarchian MA, King WC, Mitchell JE, Patterson EJ, Pender JR, Pomp A, Pories WJ, Thirlby RC, Yanovski SZ, Wolfe BM; Longitudinal Assessment of Bariatric Surgery (LABS) Consortium. Weight change and health outcomes at 3 years after bariatric surgery among individuals with severe obesity. JAMA. 2013;310:2416–2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jirapinyo P, Jin DX, Qazi T, Mishra N, Thompson CC. A meta-analysis of GLP-1 after Roux-en-Y gastric bypass: impact of surgical technique and measurement strategy. Obes Surg. 2018;28:615–626. [DOI] [PubMed] [Google Scholar]