Abstract
Early cardiology involvement after atrial fibrillation (AF) diagnosis is associated with increased oral anticoagulant prescription fills and reduced stroke risk. It is unknown if this association varies by race, sex, or education. We examined anticoagulant fills in 223,891 patients with incident non-valvular AF (mean age=71 years; 44% female; 84% white; 9% black; 5% Hispanic; 2% Asian) from the Optum Clinformatics database (2009–2014). Provider specialty and filled anticoagulant prescriptions 3 months prior to and 6 months after AF diagnosis were obtained. Poisson regression was used to compute the probability of oral anticoagulant prescription fill and Cox regression was used to estimate the risk of stroke and major bleeding. Cardiology involvement was less likely among non-whites (white=Referent; black=relative risk=0.96, 95% confidence interval (0.95–0.97); Hispanic=0.99(0.98–1.00); Asian=0.95(0.93–0.97)) and women (0.92(0.91–0.93)), but more likely with higher education level (high school or less=Referent; some college=1.03(1.02–1.04); college or more=1.08(1.07–1.09)). Patients seen by cardiology providers were more likely to fill anticoagulant prescriptions (Any=1.67(1.64–1.69); direct oral anticoagulants=2.59(2.49–2.68); warfarin=1.38(1.35–1.41)) compared to patients not seen by a cardiology provider. Patients seen by a cardiologist had a reduced stroke risk (hazard ratio=0.84(0.79–0.88)) and similar bleeding risk (1.01(0.96–1.06)). Outcomes did not vary by race, sex, or education level. In conclusion, although race, sex, and education differences exist in early cardiology involvement after AF diagnosis, the influence of cardiology involvement on anticoagulant prescription fills and AF-related outcomes does not vary by these factors. Initiatives to improve early cardiology referral in non-whites, women, and those with lower educational attainment may improve AF outcomes.
Keywords: provider specialty, anticoagulation, stroke, bleeding, race, sex, education
Recent reports have suggested that use of oral anticoagulants is more common among atrial fibrillation patients (AF) who see a cardiologist shortly after their diagnosis.1,2 A lower risk of stroke also been reported when patients with AF are seen by an outpatient cardiology provider within 3 months of their diagnosis.2 The reduced risk of stroke has been attributed to early oral anticoagulant prescription. Although AF occurs more frequently in men and whites,3 women and black patients with AF have higher rates of stroke.4–6 Due to the fact that early cardiology involvement is associated with greater use of oral anticoagulant therapy, and oral anticoagulants reduce the risk of stroke in patients with AF, it is possible that sex or race differences exist in the association between provider specialty and AF-related outcomes. Additionally, the benefit of outpatient cardiology providers may be related to differences in education level, as education has a direct influence on oral anticoagulation management in AF.7 Therefore, the purpose of this study was to examine if the association between provider specialty and AF-related outcomes varies by race, sex, or education level.
METHODS
This study used administrative claims data from the Optum’s Clinformatics® Datamart database between January 1, 2009 and December 31, 2014.8 The database consists of commercial and Medicare Advantage enrollees from geographically diverse regions of the United States. Individual patient enrollment data are linked to medical and pharmacy claims. This analysis included health plan enrollees with ≥6 months of enrollment prior to the first non-valvular AF diagnosis. A minimum of 6 months between enrollment and first AF diagnosis was selected to identify incident cases of AF. AF was defined by the presence of International Classification of Diseases Ninth Revision, Clinical Modification (ICD-9-CM) codes 427.31 or 427.32 in any position on an inpatient claim or on two outpatient claims at least 7 days but less than 1 year apart, and without any inpatient diagnosis of mitral stenosis (ICD-9-CM 394.0) or mitral valve disorder (ICD-9-CM 424.0). The AF diagnosis date was defined as the earliest of the following: 1) the discharge date for the qualifying inpatient claim; or 2) the service date of the second qualifying outpatient claim.9 The final analytic sample was further restricted to patients who had at least one claim with an outpatient medical provider in a window 3 months prior to AF diagnosis and 6 months after AF diagnosis. The period prior to AF diagnosis was considered to account for imprecisions in the date of AF diagnosis. Participants with oral anticoagulant prescriptions >3 months prior to AF diagnosis were presumed to use these agents for other conditions. This study was approved by the Institutional Review Board at Emory University.
Cardiology and primary care outpatient claims were used to identify outpatient visits. Providers seen by patients in the hospital were not included, as the main purpose of the analysis was to determine the influence of outpatient specialty involvement on oral anticoagulant use. Patients who saw a cardiology provider (i.e., general cardiology or electrophysiology) within the predetermined period (3 months prior to and 6 months after AF diagnosis) were classified as the cardiology group, while patients seen exclusively by internal medicine or family practice providers were classified as primary care. Patients seen by a cardiologist were included in the cardiology provider group, regardless of primary care visit.
Filled pharmacy claims are included in the Optum Clinformatics database. Each pharmacy claim includes the National Drug Code, prescription fill date, and the number of days supplied. Oral anticoagulant prescription fills during the study period were identified for warfarin and direct oral anticoagulants (DOAC: dabigatran, rivaroxaban and apixaban). Oral anticoagulant prescriptions were limited to the 3 months prior to and 6 months after AF diagnosis.
Age and sex were recorded at the time of health plan enrollment. We considered 4 race/ethnicity groups: non-Hispanic white, black, Hispanic, and Asian. Approximately 30% of the race/ethnicity data were collected directly from public records (e.g., driver’s license records), while the remaining data were imputed using commercial software (E-Tech by Ethnic Technologies). This imputation method has been validated and demonstrates 97% specificity and 48% sensitivity for estimating the race of black individuals.10 Education level was obtained at the time of health plan enrollment and categorized as follows: high school or less, some college, college or more, or unknown. ICD-9-CM diagnosis codes were used to detect the presence of the following comorbid conditions: heart failure, hypertension, diabetes, stroke, myocardial infarction, peripheral artery disease, kidney disease, liver disease, alcohol use, and bleeding history. The codes used to define each condition are shown in Supplemental Table 1. At least one ICD-9-CM diagnosis code present prior to or at the time of AF diagnosis, in any position, was considered evidence of the condition. Similarly, pharmacy claims were used to identify the following medications: angiotensin-converting-enzyme (ACE) inhibitors, angiotensin II receptor blockers (ARB), beta blockers, calcium channel blockers, diuretics, antiarrhythmic agents, and digoxin. These medications were considered if filled prior to or at the time of AF diagnosis. CHA2DS2-VASc and HAS-BLED scores were computed at the time of AF diagnosis.4,11
The main outcome variable was hospitalization for ischemic stroke. ICD-9-CM codes in the primary position of an inpatient claim that occurred after the diagnosis of AF were used to identify events. We also examined the risk of major bleeding events (intracranial, gastrointestinal, or other) associated with cardiology involvement. ICD-9-CM codes in the primary position were used to identify these events in a similar manner to ischemic stroke events, and the algorithms to ascertain these events have been previously described.12 The ICD-9-CM codes used to identify stroke and bleeding events are shown in Supplemental Table 1.
We compared the anticoagulant prescription fill patterns between AF patients who had cardiology involvement with those who did not. Categorical data were compared using the chi-square test and continuous data using the Student’s t-test. Poisson regression models with robust variance estimates were used to compute relative risk (RR) and 95% confidence intervals (CI) of anticoagulant prescription fills for patients managed by cardiology vs. primary care providers.13 Models were adjusted for age, sex, race, education, heart failure, hypertension, diabetes, stroke, myocardial infarction, kidney disease, liver disease, bleeding history, alcohol use, antiplatelet agents, angiotensin converting enzyme inhibitors, angiotensin II receptor blockers, calcium channel blockers, diuretics, antiarrhythmic agents, digoxin, CHA2DS2-VASc, and HAS-BLED. We also performed a greedy propensity score-matched analysis using multivariable logistic regression to predict the probability of being seen in cardiology versus primary care using the same covariates in the primary analysis, and a 1:1 matching was performed where the absolute difference between propensity scores was ± 0.01.14 The original Poisson regression analysis was repeated in the propensity score-matched cohort. An additional analysis was performed limited to AF patients who would qualify for oral anticoagulants (CHA2DS2-VASc scores ≥2). Cox regression was used to estimate hazard ratios (HR) and 95% confidence intervals (CI) of the future risk of ischemic stroke and major bleeding events associated with cardiology compared with primary care providers, adjusting for the same covariates as the oral anticoagulant analysis. We also examined the risk of each outcome in the propensity score-matched cohort described above, with adjustment for the same covariates. All analyses were stratified by race, sex, and education level and we tested for multiplicative interactions. We also examined if early cardiology involvement varied by race, sex, and education level using Poisson regression with adjustment for the same covariates as the main analysis. SAS Version 9.4 (Cary, NC) was used for all analyses.
RESULTS
A total of 223,891 AF patients (mean age=71±12 years; 44% female; 84% white; 9% black; 5% Hispanic; 2% Asian) were included in the final sample. There were 153,822 (69%) patients who were seen by a cardiology provider shortly after AF diagnosis, while 70,069 (31%) were exclusively managed by primary care. The baseline characteristics are shown in Table 1. The characteristics for the propensity-matched cohort are shown in Supplemental Table 2.
Table 1.
Patient Characteristics at Time of Nonvalvular Atrial Fibrillation Diagnosis, 2009–2014 (N=223,891)*
| Characteristic | Cardiology 153,822 (69%) |
Primary Care 70,069 (31%) |
|---|---|---|
| Age, mean ± SD (years) | 70 ± 12 | 74 ± 12 |
| Women | 41% | 50% |
| White | 85% | 83% |
| Black | 8% | 10% |
| Hispanic | 5% | 5% |
| Asian | 2% | 2% |
| Education | ||
| High school or less | 30% | 33% |
| Some college | 55% | 55% |
| College or more | 14% | 11% |
| Unknown | <1% | <1% |
| Heart failure | 32% | 30% |
| Hypertension | 82% | 83% |
| Diabetes mellitus | 33% | 35% |
| Stroke | 24% | 27% |
| Myocardial infarction | 14% | 12% |
| Peripheral artery disease | 3% | 3% |
| CHA2DS2-VASc, mean ± SD | 3.6 ± 2.0 | 3.9 ± 1.9 |
| Kidney disease | 16% | 20% |
| Liver disease | 7% | 9% |
| Alcohol use | 2% | 4% |
| Bleeding history | 21% | 25% |
| Antiplatelet agents | 14% | 9% |
| HAS-BLED, mean ± SD | 2.5 ± 1.4 | 2.7 ± 1.3 |
| ACE inhibitors | 35% | 31% |
| Angiotensin II receptor blocker | 17% | 14% |
| Beta blockers | 57% | 44% |
| Calcium channel blockers | 32% | 31% |
| Diuretics | 34% | 34% |
| Antiarrhythmic agents | 15% | 6% |
| Digoxin | 9% | 8% |
Statistical significance for continuous data was tested using the Student’s t-test and categorical data was tested using the Chi-square test. All p-values were significant at p<0.001 except peripheral artery disease (p=0.40) and diuretics (p=0.33).
ACE=angiotensin-converting-enzyme; CHA2DS2-VASc=congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, stroke/transient ischemic attack, vascular disease, age 65–75 years, and sex category; HAS-BLED=hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalized ratio, elderly (age >65 years), drugs/alcohol concomitantly; SD=standard deviation.
Cardiology involvement was less likely to occur among non-whites compared with whites, and among women compared with men (Table 2). Differences also were observed by education level, with patients who had a college degree or higher more likely to see a cardiology provider than those with lower educational attainment.
Table 2.
Cardiology Involvement in Patients with Nonvalvular Atrial Fibrillation by Race, Sex, and Education Level, 2009–2014 (N=223,891)
| 2009–2014 | Cardiology | Primary Care | RR* (95%CI) |
|---|---|---|---|
| Race | |||
| White | 85% | 83% | Ref |
| Black | 8% | 10% | 0.96 (0.95, 0.97) |
| Hispanic | 5% | 5% | 0.99 (0.98, 1.00) |
| Asian | 2% | 2% | 0.95 (0.93, 0.97) |
|
| |||
| Sex | |||
| Male | 59% | 50% | Ref |
| Female | 41% | 50% | 0.92 (0.91, 0.93) |
|
| |||
| Education | |||
| High school or less | 30% | 33% | Ref |
| Some college | 55% | 55% | 1.03 (1.02, 1.04) |
| College or more | 14% | 11% | 1.08 (1.07, 1.09) |
Relative risk of cardiology involvement. Adjusted for age, sex, race, education, heart failure, hypertension, diabetes, stroke, myocardial infarction, kidney disease, liver disease, bleeding history, alcohol use, antiplatelet agents, angiotensin converting enzyme inhibitors, angiotensin II receptor blockers, calcium channel blockers, diuretics, antiarrhythmic agents, digoxin, CHA2DS2-VASc, and HAS-BLED.
CHA2DS2-VASc=congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, stroke/transient ischemic attack, vascular disease, age 65–75 years, and sex category; CI=confidence interval; DOAC=direct oral anticoagulant; HAS-BLED=hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalized ratio, elderly (age >65 years), drugs/alcohol concomitantly; RR=relative risk.
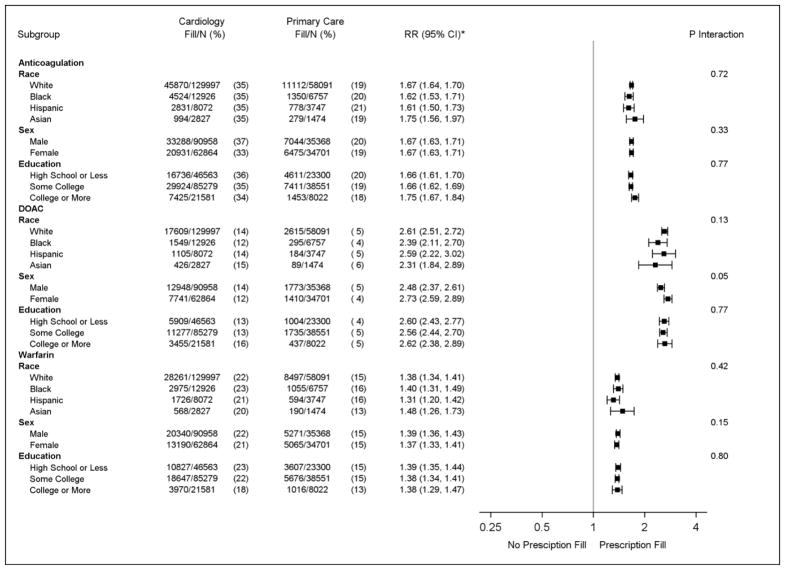
Patients seen by cardiology providers were more likely to fill anticoagulant prescriptions than those seen exclusively by primary care (Table 3). Differences were observed for both DOACs and warfarin. Similar results were obtained in patients with CHA2DS2-VASc scores ≥2 and in a propensity score-matched cohort (Table 3). The relationship between provider specialty and prescription fills for oral anticoagulants, DOACs, and warfarin was similar across race, sex, and education (Figure 1). Similar results were observed in the propensity score-matched cohort (data not shown).
Table 3.
Anticoagulation Fill Patterns of Patients with Nonvalvular Atrial Fibrillation, 2009–2014
| 2009–2014 | Total | Cardiology | Primary Care | RR*† (95%CI) | RR*‡ (95%CI) |
|---|---|---|---|---|---|
| All (N=223,891) | 69% | 31% | |||
| Anticoagulant | 31% | 35% | 19% | 1.67 (1.64, 1.69) | 1.66 (1.63, 1.69) |
| DOAC | 11% | 13% | 4% | 2.59 (2.49, 2.68) | 2.57 (2.48, 2.68) |
| Warfarin | 20% | 22% | 15% | 1.38 (1.35, 1.41) | 1.38 (1.35, 1.41) |
| CHA2DS2-VASc ≥ 2 (N=191,898) | 67% | 33% | |||
| Anticoagulant | 31% | 36% | 20% | 1.65 (1.63, 1.68) | 1.65 (1.62, 1.68) |
| DOAC | 11% | 13% | 5% | 2.55 (2.45, 2.65) | 2.53 (2.43, 2.64) |
| Warfarin | 20% | 23% | 15% | 1.38 (1.35, 1.41) | 1.39 (1.35, 1.42) |
Comparison between cardiology and primary care.
Relative risk of anticoagulant, DOAC, and warfarin prescription fills for patients seen by cardiology vs. primary care providers. Adjusted for age, sex, race, education, heart failure, hypertension, diabetes, stroke, myocardial infarction, kidney disease, liver disease, bleeding history, alcohol use, antiplatelet agents, angiotensin converting enzyme inhibitors, angiotensin II receptor blockers, calcium channel blockers, diuretics, antiarrhythmic agents, digoxin, CHA2DS2-VASc, and HAS-BLED.
Results of 1:1 propensity score matched analysis. Propensity score was computed using multivariable logistic regression with the following variables: age, sex, race, education, heart failure, hypertension, diabetes, stroke, myocardial infarction, kidney disease, liver disease, bleeding history, alcohol use, antiplatelet agents, angiotensin converting enzyme inhibitors, angiotensin II receptor blockers, calcium channel blockers, diuretics, antiarrhythmic agents, digoxin, CHA2DS2-VASc, and HAS-BLED. Adjusted for all covariates used to derive the propensity-matched cohort (N=138,170).
CHA2DS2-VASc=congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, stroke/transient ischemic attack, vascular disease, age 65–75 years, and sex category; CI=confidence interval; DOAC=direct oral anticoagulant; HAS-BLED=hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalized ratio, elderly (age >65 years), drugs/alcohol concomitantly; RR=relative risk.
Figure 1. Anticoagulation Patterns of Patients with Nonvalvular Atrial Fibrillation by Sociodemographic Factors, 2009–2014 (N=223,891).
* Relative risk of anticoagulant, DOAC, and warfarin prescription fills for patients seen by cardiology vs. primary care providers. Adjusted for age, sex, race, education, heart failure, hypertension, diabetes, stroke, myocardial infarction, kidney disease, liver disease, bleeding history, alcohol use, antiplatelet agents, angiotensin converting enzyme inhibitors, angiotensin II receptor blockers, calcium channel blockers, diuretics, antiarrhythmic agents, digoxin, CHA2DS2-VASc, and HAS-BLED.
CHA2DS2-VASc=congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, stroke/transient ischemic attack, vascular disease, age 65–75 years, and sex category; CI=confidence interval; DOAC=direct oral anticoagulant; HAS-BLED=hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalized ratio, elderly (age >65 years), drugs/alcohol concomitantly; RR=relative risk.
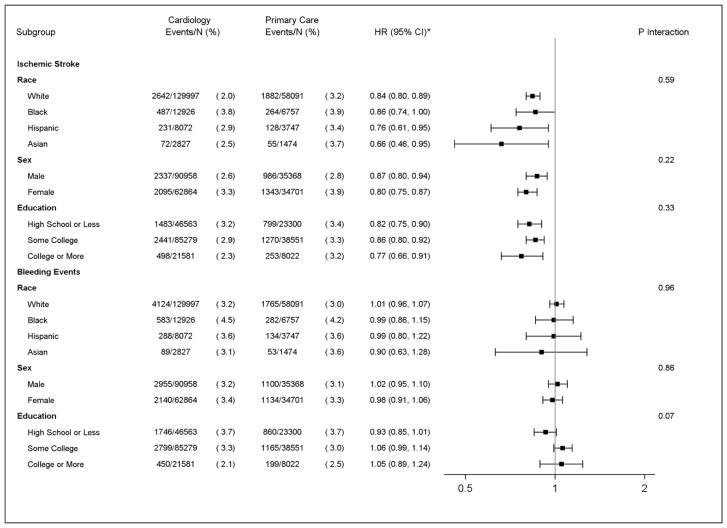
A reduced risk of stroke was observed among those seen by cardiology providers (HR=0.84, 95%CI=0.79, 0.88) compared with primary care, without an increased bleeding risk (HR=1.01, 95%CI=0.96, 1.06) (Supplemental Table 3). Differences were not observed when the risk of stroke or bleeding was stratified by race, sex, or education level (Figure 2), and similar results were observed in the propensity score-matched cohort (data not shown).
Figure 2. Association of Provider Specialty with Ischemic Stroke and Bleeding in Patients with Nonvalvular Atrial Fibrillation by Sociodemographic Factors, 2009–2014 (N=223,891).
* Results of multivariable Cox regression analysis adjusted for age, sex, race, education, heart failure, hypertension, diabetes, stroke, myocardial infarction, kidney disease, liver disease, bleeding history, alcohol use, antiplatelet agents, angiotensin converting enzyme inhibitors, angiotensin II receptor blockers, calcium channel blockers, diuretics, amiodarone, digoxin, CHA2DS2-VASc, and HAS-BLED.
CHA2DS2-VASc=congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, stroke/transient ischemic attack, vascular disease, age 65–75 years, and sex category; CI=confidence interval; DOAC=direct oral anticoagulant; HAS-BLED=hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalized ratio, elderly (age >65 years), drugs/alcohol concomitantly; HR=hazard ratio.
DISCUSSION
In this analysis from a large administrative claims database representative of AF care in the United States, early cardiology involvement after AF diagnosis varied by race, sex, and educational attainment. Patients with early cardiology involvement were more likely to fill prescriptions for oral anticoagulants and were at reduced risk of stroke, independent of the aforementioned subgroups. Overall, our data suggest that initiatives to increase early cardiology involvement in non-whites, women, and those with lower educational attainment may improve the proportion of patients who receive oral anticoagulants, and reduce the risk of future stroke events.
The findings in this analysis confirm prior reports that have demonstrated a beneficial association of early cardiology involvement with a greater likelihood of oral anticoagulant prescription fill,1,2 and a reduced risk of future stroke events.2 However, these reports were limited to a population of >98% male veterans of white (~88%) race/ethnicity. They were unable to explore if the positive influence of early cardiology involvement shortly after AF diagnosis was similar in a racially diverse cohort of men and women. Additionally, data on educational attainment were not provided. Our findings show that the positive influence of early cardiology involvement after AF diagnosis does not vary by race, sex, or education.
The likelihood of patients being seen by a cardiology provider after AF diagnosis was found to vary by race, sex, and education. Non-whites and females with AF have higher rates of adverse events than whites and men.5,6 Therefore, differences in AF-related outcomes may be related to the lower likelihood for these high-risk groups to see a cardiology provider. Although the impact of education level on outcomes in AF has not been explored, poorer outcomes are expected similar to other cardiovascular diseases.15 Education has been shown to influence knowledge of anticoagulation management in AF,7 and this possibly impacts the decision to follow-up with a cardiology provider. Accordingly, efforts to increase early cardiology involvement among at-risk groups may reduce the higher burden of adverse events in AF patients who are non-white, female, or who have lower levels of educational attainment. However, the hypothesis is speculative and further research is needed before changes in clinical practice are recommended.
Our data also demonstrated that oral anticoagulants are underfilled in patients who have new-onset AF. Data from a cohort of veterans demonstrated that filled warfarin prescriptions were present in 54% of patients.1 A higher percentage was reported from the provider-tabulated Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) (73.6% for internal medicine/primary care providers and 76.7% for cardiology providers), which included prevalent and incident AF cases.16 The data from this report are closer to the number reported in the veteran population, as these represent filled prescriptions. Therefore, it is likely that barriers or a period of contemplation exist prior to filling oral anticoagulant prescriptions. The higher percentage from ORBIT-AF may reflect a population of prevalent AF in which patients have already decided to begin oral anticoagulant therapies. Nonetheless, our data demonstrated that early outpatient cardiology involvement results in more AF patients initiating oral anticoagulants, and this association is independent of race, sex, and education level. Future work is needed to explore the potential benefit of early referral to cardiology specialists shortly after AF diagnosis to improve the initiation of guideline-recommended oral anticoagulation.
The current analysis should be interpreted in the context of several limitations. The misclassification of AF cases, comorbid conditions, and outcomes was possible. We were unable to determine how many patients received prescriptions from their provider, as only filled prescriptions were available. Race/ethnicity data were imputed in approximately two-thirds of patients. However, this method has a 97% specificity and 71% positive predictive value for estimating black race.10 We considered cardiology involvement if a patient had a specialty outpatient claim within 3 months prior to or 6 months after AF diagnosis, and did not consider cardiology involvement if it occurred outside this time period. Furthermore, we acknowledge that unmeasured patient characteristics possibly influenced our findings.
In conclusion, early cardiology involvement after AF diagnosis was found to be lower among AF patients who were non-white, female, and among those with lower educational attainment. The advantage of early cardiology involvement with oral anticoagulant prescription fills and reduced stroke risk, without higher bleeding risk, did not vary by race, sex, or education level. Initiatives to increase early cardiology involvement among the aforementioned subgroups may improve well-known disparities in AF outcomes.
Supplementary Material
Acknowledgments
FUNDING
Research reported in this publication was supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health under award numbers R01-HL122200 and F32-HL134290, and from the American Heart Association under award number 16EIA26410001. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the American Heart Association.
Footnotes
DISCLOSURES
Dr. Bengtson is an employee of Optum. All other authors have no disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Turakhia MP, Hoang DD, Xu X, Frayne S, Schmitt S, Yang F, Phibbs CS, Than CT, Wang PJ, Heidenreich PA. Differences and trends in stroke prevention anticoagulation in primary care vs cardiology specialty management of new atrial fibrillation: The Retrospective Evaluation and Assessment of Therapies in AF (TREAT-AF) study. Am Heart J. 2013;165:93–101. e101. doi: 10.1016/j.ahj.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 2.Perino AC, Fan J, Schmitt SK, Askari M, Kaiser DW, Deshmukh A, Heidenreich PA, Swan C, Narayan SM, Wang PJ, Turakhia MP. Treating Specialty and Outcomes in Newly Diagnosed Atrial Fibrillation: From the TREAT-AF Study. J Am Coll Cardiol. 2017;70:78–86. doi: 10.1016/j.jacc.2017.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 4.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137:263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 5.Magnani JW, Norby FL, Agarwal SK, Soliman EZ, Chen LY, Loehr LR, Alonso A. Racial Differences in Atrial Fibrillation-Related Cardiovascular Disease and Mortality: The Atherosclerosis Risk in Communities (ARIC) Study. JAMA Cardiol. 2016;1:433–441. doi: 10.1001/jamacardio.2016.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piccini JP, Simon DN, Steinberg BA, Thomas L, Allen LA, Fonarow GC, Gersh B, Hylek E, Kowey PR, Reiffel JA, Naccarelli GV, Chan PS, Spertus JA, Peterson ED Outcomes Registry for Better Informed Treatment of Atrial Fibrillation I Patients. Differences in Clinical and Functional Outcomes of Atrial Fibrillation in Women and Men: Two-Year Results From the ORBIT-AF Registry. JAMA Cardiol. 2016;1:282–291. doi: 10.1001/jamacardio.2016.0529. [DOI] [PubMed] [Google Scholar]
- 7.Hernandez Madrid A, Potpara TS, Dagres N, Chen J, Larsen TB, Estner H, Todd D, Bongiorni MG, Sciaraffia E, Proclemer A, Cheggour S, Amara W, Blomstrom-Lundqvist C. Differences in attitude, education, and knowledge about oral anticoagulation therapy among patients with atrial fibrillation in Europe: result of a self-assessment patient survey conducted by the European Heart Rhythm Association. Europace. 2016;18:463–467. doi: 10.1093/europace/euv448. [DOI] [PubMed] [Google Scholar]
- 8.Wallace PJ, Shah ND, Dennen T, Bleicher PA, Crown WH. Optum Labs: building a novel node in the learning health care system. Health Aff (Millwood) 2014;33:1187–1194. doi: 10.1377/hlthaff.2014.0038. [DOI] [PubMed] [Google Scholar]
- 9.Piccini JP, Hammill BG, Sinner MF, Jensen PN, Hernandez AF, Heckbert SR, Benjamin EJ, Curtis LH. Incidence and prevalence of atrial fibrillation and associated mortality among Medicare beneficiaries, 1993–2007. Circ Cardiovasc Qual Outcomes. 2012;5:85–93. doi: 10.1161/CIRCOUTCOMES.111.962688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeFrank JT, Bowling JM, Rimer BK, Gierisch JM, Skinner CS. Triangulating differential nonresponse by race in a telephone survey. Prev Chronic Dis. 2007;4:A60. [PMC free article] [PubMed] [Google Scholar]
- 11.Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138:1093–1100. doi: 10.1378/chest.10-0134. [DOI] [PubMed] [Google Scholar]
- 12.Cunningham A, Stein CM, Chung CP, Daugherty JR, Smalley WE, Ray WA. An automated database case definition for serious bleeding related to oral anticoagulant use. Pharmacoepidemiol Drug Saf. 2011;20:560–566. doi: 10.1002/pds.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 14.Austin PC. A comparison of 12 algorithms for matching on the propensity score. Stat Med. 2014;33:1057–1069. doi: 10.1002/sim.6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Havranek EP, Mujahid MS, Barr DA, Blair IV, Cohen MS, Cruz-Flores S, Davey-Smith G, Dennison-Himmelfarb CR, Lauer MS, Lockwood DW, Rosal M, Yancy CW American Heart Association Council on Quality of C, Outcomes Research CoE, Prevention CoC, Stroke Nursing CoL, Cardiometabolic H Stroke C. Social Determinants of Risk and Outcomes for Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation. 2015;132:873–898. doi: 10.1161/CIR.0000000000000228. [DOI] [PubMed] [Google Scholar]
- 16.Fosbol EL, Holmes DN, Piccini JP, Thomas L, Reiffel JA, Mills RM, Kowey P, Mahaffey K, Gersh BJ, Peterson ED Investigators O-A, Patients. Provider specialty and atrial fibrillation treatment strategies in United States community practice: findings from the ORBIT-AF registry. J Am Heart Assoc. 2013;2:e000110. doi: 10.1161/JAHA.113.000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.