Abstract
Aim of the Study:
The regulation and actions of fibroblast growth factor 21 (FGF21) are responsive to energy status and macronutrient balance, and investigations of FGF21 in normal pregnancy, which could be informative for FGF21 biology, are seldom. The goal of our study was to examine FGF21 levels in a contemporaryhealthy, pregnant population.
Methods:
We phenotyped 43 women with overweight and obesity during pregnancy for weight, body composition, and fasting blood. Serum FGF21 was measuredduring the first and third trimesters. Placentas were collected at delivery.
Results:
Maternal FGF21 concentrations were positively correlated with body mass index and adiposity, but not lean mass or glucose homeostasis. In support of our hypothesis, FGF21 concentrations significantly increased from the first to third trimester of pregnancy (0.105 vs. 0.256 ng/mL, p<0.0001). Changes in FGF21concentrations across pregnancy were not associated with changes in body weight or composition but inversely with the change in fasting glucose. FGF21 mRNA levels in placenta were very low and do not likely contribute to FGF21 in the maternal circulation.
Conclusions:
FGF21 increases throughout pregnancyin our healthy cohort with overweight and obesity, independent of the placenta, and does not appear to be sensing the changes in energy balance (reflected in the change in maternal energy stores), but changes in macronutrient status. Thus, we propose FGF21 may be a potential signal of maternal nutrient status in pregnancy.
Keywords: FGF21, Pregnancy, Obesity, Placenta
INTRODUCTION
Fibroblast growth factor 21 (FGF21) is secreted in response to energy imbalance for the regulation of energy and nutrient metabolism. First discovered as a glucose sensitizer of adipose tissue, FGF21 has since been shown in both animal models and human studies to regulate glucose and lipid metabolism under various states of energy balance1. Metabolically challenged transgenic models overexpressing FGF21 and pharmacological administration of FGF21 to obese and diabetic animals and humans have revealed FGF21 to be capable of reducing body weight, protecting against diet induced obesity, improving glucose tolerance, and improving lipid profiles1–7. However, cross sectional studies of the human population show that FGF21 is elevated in clinical conditions of energy or nutrient excess, such as obesity and type 2 diabetes8–15. FGF21 is also positively correlated with metabolically unfavorable characteristics, such as hyperinsulinemia, insulin resistance, hypertriglyceridemia, and total cholesterol11,14,16–22.
Pregnancy is a state of energy flux with energy and macronutrient demand increasing at variable rates and in response to various cues throughout gestation. Considering the impact of energy and macronutrient balance on FGF21 expression and action, understanding how FGF21 is regulated and/or acting in pregnancy, particularly in the presence of excess adiposity, could be highly informative to FGF21 biology. Conversely, given that adequate maternal nutrition (both over- and under-nutrition) is important for optimal pregnancy outcomes, FGF21 may serve as a novel hormone to indicate patients at risk. Descriptions of FGF21 in the pregnant population are surprisingly limited to cross sectional studies in women with gestational diabetes or preeclampsia, with the majority of these studies showing FGF21 elevated in these conditions22–26. These reports also confirm well established associations between FGF21 and an unfavorable metabolic milieu described in the non-pregnant state. For example, FGF21 in late pregnancy positively correlates with triglycerides and insulin resistance and inversely with adiponectin and HDL-cholesterol22,23,25. The current knowledge of FGF21 biology in pregnancy is in need of more in depth studies throughout pregnancy. The aim of this study was to examine FGF21 levels in pregnant womenin a contemporary cohort with overweight and obesity and to understand the role maternal energy stores and the placenta mayplay on maternal FGF21 secretion. We hypothesized FGF21 concentrations would be higher in women with increased adiposity. Moreover, commensurate with the increase in energy status (fat mass) and glucose intolerance (macronutrient balance) across pregnancy, we hypothesized that maternal FGF21 would increase throughout gestation.
MATERIALS AND METHODS
Study population
One hundred and fourteen pregnant women enrolled in the Expecting Success (n=54, NCT01610752) or the MomEE (n=60, NCT01954342) studies at Pennington Biomedical Research Center (PBRC) in Baton Rouge, Louisiana were potentially eligible for inclusion in this ancillary study. As detailed elsewhere, participants were recruited primarily from obstetrical offices, augmented by print and social media advertisements27. For entry into the parent studies, participants were required to be healthy, overweight or obese women (BMI>25kg/m2), aged 18–40 years with a single, viable, first trimester pregnancy (<14 weeks gestation). Patients were excluded from the parent studies for pregnancy-related conditions (known fetal anomaly, planned termination of pregnancy or adoption of infant, history of ≥3 consecutive miscarriages), pre-existing hypertension or diabetes (diagnosis prior to pregnancy, elevated HbA1c, or first trimester OGTT diagnosis of diabetes), psychological criteria (history or current psychotic disorder, current major depressive episode, bipolar disorder, history of anorexia or bulimia, current eating disorder, actively suicidal), medications (metformin, systemic steroids, antipsychotic agents, anti-seizure medications, or medications for ADHD), HIV, severe anemia, contraindications to exercise28, prior or planned (within one year of expected delivery) bariatric surgery, or recent history of or current smoking, alcohol, or drug use.
Of the 114 participants in the parent studies, 43 satisfied inclusion criteria for this ancillary study, provided consent for future use of biospecimens (i.e. blood and/or placenta), and had the required clinical data available at one or more time points for analysis. Briefly, the Expecting Success Study (NCT01610752) was an interventional study testing the efficacy of a lifestyle intervention designed to help overweight and obese pregnant women gain the recommended amount of weight in pregnancy. The MomEE Study (NCT01954342) was an observational study determining energy requirements of overweight and obese women across pregnancy. Both parent studies and this ancillary study were approved and monitored by the PBRC Institutional Review Board and all participants provided verbal and written consent prior to study initiation.
Clinic Assessments
For both the Expecting Success and MomEE Studies, study visits were performed in the first trimester (<16 weeks) and third trimester (35–36 weeks) in the morning following an overnight fast. Body weight, body composition, and blood measurements were collected in accordance with standard operating procedures of Pennington Biomedical Research Center to ensure scientific rigor and reproducibility.
Maternal Anthropometrics
Body weight was recorded twice with the participant fasting and wearing a hospital gown and undergarments only. The two recorded weights were averaged and the hospital gown weight subtracted. Body mass index was calculated as body weight (kg) divided by the square of study-measured height (m2). Body composition was assessed by air displacement plethysmography using a BOD POD® (COSMED, Chicago, IL) and fat mass and fat free mass were calculated from body volume as determined by the BOD POD® using equations developed by van Raaij et al. as previously validated29,30.
Blood chemistry
Serum glucose and insulin were assayed with the Beckman Coulter DXC 600 Pro (Beckman Coulter Inc., Brea, CA). FGF21 was measured in duplicate by sandwich enzyme-linked immunosorbent assay (ELISA) according to manufacturer instructions (RD191108200R, Fibroblast Growth Factor Human ELISA, Biovendor, Brno, Czech Republic). Serum was diluted 1:2 (125μL of serum in 125μL of dilution buffer) before analysis. The detectable concentration by the assay is 0.03 ng/mL. The intra-assay coefficient of variation was 3.9%.
Placenta collection and quantitative real time PCR
Placenta samples were collected, dissected and frozen within two hours of delivery. Samples were dissected at four separate sites of the placental disc and stored by section (basal plate, villous tissue, and chorionic plate) at −80°C until being thawed for study. Experiments in this study used villous tissue snap frozen within two hours of delivery and pooled from all four collection sites. PlacentalRNA was isolated from flash-frozen tissue with the RNeasy Mini Kit (QIAGEN). Samples were quantified by Nanodrop and all samples had 260:280 and 260:230 ratios greater than 1.75. A 2000ngsample of RNA was reverse transcribed with the High Capacity cDNA Reverse Transcription kit (Applied Biosystems) into cDNA. Quantitative real-time PCR was performed with 20ng of cDNA, 300nM of each forward and reverse primer, and iTaq universal SYBR green mastermix. The PCR protocol was performed on a 7900HT PCR Machine (ThermoFisher Scientific, Waltham, MA) beginning with one cycle at 95⁰C for 10 minutes, then 40 cycles of 95⁰C for 15 seconds and 59⁰C for 1 minute, and ended with a dissociation curve analysis. Primer sequences unique for each target gene were designed with primer BLAST to span exon-exon junctions (Table 1). The geometric mean of SDHA and TBP was used as an endogenous control and data was analyzed by ΔΔCt method.
Table 1:
Primer sequences
| Gene | Forward | Reverse |
|---|---|---|
| FGF21 | TGGATCGCTCCACTTTGACC | GGGCTTCGGACTGGTAAACA |
| FGF21 | GCTTCGGACTGGTAAACATTG | GGAGTCAAGACATCCAGGTT |
| SDHA | CGGTCCATGACTCTGGAGAT | AGCGAAGATCATGGCTGTCT |
| TBP | TGCACAGGAGCCAAGAGTGAA | CACATCACAGCTCCCCACCA |
Statistical analysis
Data are presented as mean ± standard error. Fasting insulin and HOMA-IR were logarithmically transformed. Statistical significance was determined by Spearman’s correlation or paired student’s t-tests when appropriate. Tests were performed with significance level α=0.05, and findings considered significant when P<α.
RESULTS
Participants
The study cohort was 29±5 years old at the time of enrollment and was comprised of participants who self-identified as Caucasian (n=37), black (n=5) and other (n=1) (Table 2). Using first measured study height and weight (<14 weeks gestation), 14 participants were overweight (BMI 25–29.9kg/m2) and 29 participants were obese (BMI ≥ 30 kg/m2) with 16 classified as obeseclass I, 7 obeseclass II, and 6 obeseclass III. All participants were in good health at enrollment. During study participation, four participants were diagnosed with gestational diabetes mellitus (GDM) (three treated with glyburide and one with glipizide), two with preeclampsia (one receiving magnesium sulfate during labor), and one participant with both GDM and PE (treated with diet only and magnesium sulfate during labor respectively)(described in Supplement Table 1).
Table 2:
Participant Characteristics
| Pre-pregnancy | 1st Trimester | 3rd Trimester | p value* | |
|---|---|---|---|---|
| Age at enrollment | 29.1 (5.0) | |||
| Parity (0/1/2/3/4) | 20/20/0/2/1 | |||
| Race | ||||
| Black | 5 (12%) | |||
| White | 37 (86%) | |||
| Other | 1 (2%) | |||
| BMI Class | ||||
| Overweight | 13 | 14 | 2 | |
| Obese- Class I | 17 | 16 | 17 | |
| Obese- Class II | 8 | 7 | 14 | |
| Obese- Class III | 5 | 6 | 10 | |
| BMI (kg/m2) | 32.8 (5.4) | 33.5 (5.3) | 36.6 (4.7) | <0.0001 |
| Weight (kg) | 86.8 (14.5) | 88.6 (14.5) | 96.9 (13.3) | <0.0001 |
| Fat mass (kg) | 40.4 (10.5) | 41.8 (9.7) | 0.04 | |
| Lean mass (kg) | 48.3 (6.1) | 55.3 (6.1) | <0.0001 | |
| Fasting glucose (mg/dL) | 84.6 (8.0) | 81.5 (7.6) | 0.002 | |
| Fasting insulin (mU/L) | 12.4 (7.9) | 16.8 (9.3) | <0.0001 | |
| HOMA-IR | 2.7 (2.0) | 3.4 (2.1) | 0.002 | |
Values reported as mean ± standard deviation
represents significant differences between first and third trimester
Pattern of FGF21 in human pregnancy
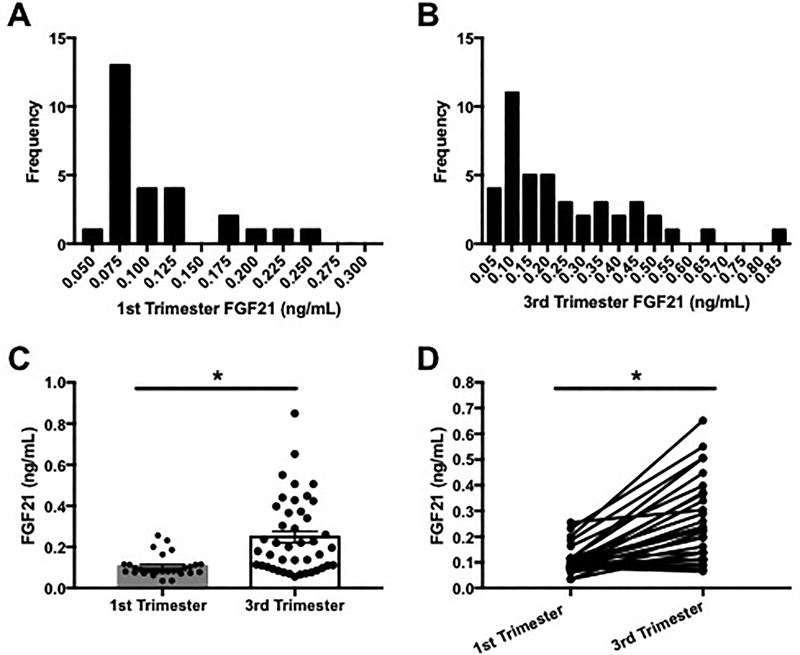
In fasting serum samples collected from healthy women in the first and third trimester of pregnancy, FGF21 concentrations were found to be highly variable. In the first trimester (n=29), FGF21 concentrations ranged from 0.035 to 0.256 ng/mL (Figure 1A) and an even larger degree of variability was observed in the third trimester (n=43, range 0.056–0.850 ng/mL, Figure 1B). First trimester FGF21 concentrations showed ten-fold less variance compared to third trimester concentrations, 0.003 versus 0.033 respectively (p<0.0001). For participants with FGF21 concentrations measured at both the first and third trimesters (n=29), FGF21 concentrations increased across pregnancy and were more than 2-fold higher in the third trimester of pregnancy (35–36 weeks) compared to concentrations measured in the first trimester (<16 weeks), 0.256 vs. 0.105 ng/mL respectively (Figures 1C and 1D).
Figure 1: Serum FGF21 concentrations in the first and third trimesters of our study population.
(A) Distribution of FGF21 in the 1st trimester (<16 weeks), (B) distribution of FGF21 in the 3rd trimester (35–36 weeks), (C) individual and mean ± SEM serum FGF21 measured across pregnancy, (D) individual change in serum FGF21 between first and third trimesters, *p<0.0001.
FGF21 is correlated with maternal body size and adiposity throughout pregnancy
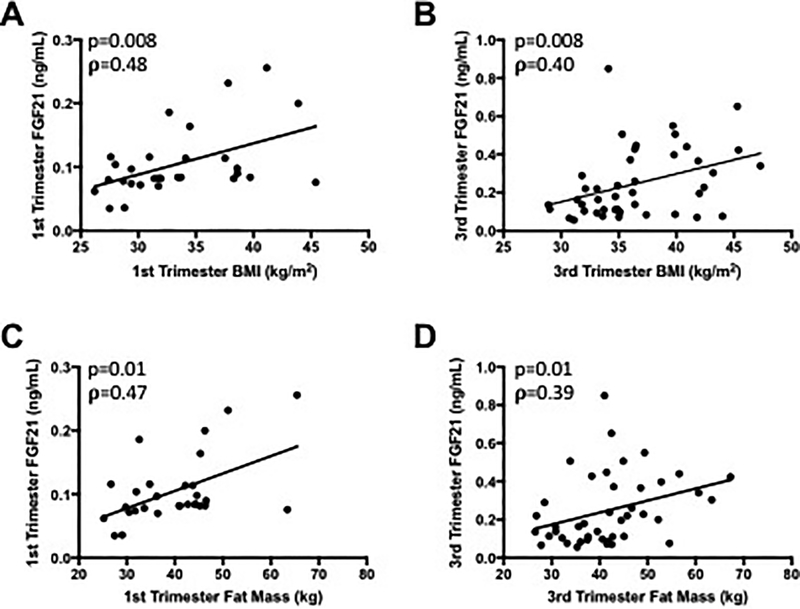
In non-pregnant individuals, FGF21 is elevated with higher BMI andincreased adiposity14. In the pregnant state, we found FGF21 concentrations are significantly and positively correlated with maternal BMI in both the first (ρ=0.48, p=0.008) and third trimesters (ρ=0.40, p=0.008) (Figures 2A and B). However, FGF21 correlated to body weight in the first trimester only (ρ=0.39, p=0.04, data not shown).FGF21 concentrations are also strongly and significantly correlated with maternal adiposity reported as total fat mass (kg) in both the first and third trimesters (1st trimester: ρ=0.47, p=0.01; 3rd trimester: ρ=0.39, p=0.01) (Figures 2Cand 2D). FGF21 concentration was not significantly correlated with maternal fat free mass in either trimester (1st trimester: ρ= 0.21, p=0.26, 3rd trimester: ρ= −0.06, p=0.68) (data not shown).
Figure 2: Serum FGF21 is significantly correlated with maternal body mass index and fat mass throughout pregnancy.
(A) 1st trimester BMI, (B) 3rd trimester BMI, (C) 1st trimester fat mass, (D) 3rd trimester fat mass.
FGF21 is not correlated with glucose homeostasis in normoglycemic pregnancy
FGF21 has been shown to positively correlate with fasting glucose, fasting insulin, and insulin resistance in animal models and cross sectional, non-pregnant human studies8,11,16–18. In our population of pregnant women with majority normal glucose tolerance and maternal overweight or obesity, no significant relationships between FGF21 and fasting glucose (p=0.61 and 0.63 respectively, data not shown), fasting insulin (p=0.23 and 0.54respectively, data not shown), or HOMA-IR (p=0.24 and 0.52 respectively, data not shown) were observed in either the first or third trimester.
Change in glucose, not adiposity, correlates with change in FGF21 across pregnancy
In an effort to understand if alterations in maternal characteristics throughout gestation may explain the increase in FGF21, we tested for relationships between the change in FGF21 (absolute and percent) and changein known contributors of FGF21 in the non-pregnant state, namely adiposity and metabolic status. As expected, body weight, fat mass, fat free mass, and fasting insulin each increased from the first to the third trimester, although fasting glucosemarginally decreased (Table 2). Although we observed significant relationships with body composition, namely adiposity, at single time points, the changes in these parameters across pregnancy were not associated with the changes in FGF21 concentrations measured across the same time interval (Table 3). However, the change in FGF21 concentrations was significantly and inversely correlated with the change in fasting glucose (ρ= −0.44, p=0.02). No relationship was observed between changes in FGF21 and alterations in insulin or HOMA-IR (ρ= −0.16, p=0.40 and ρ= −0.26, p=0.18 respectively).
Table 3:
Spearman correlations between percent change in FGF21 and percent change in body size, composition and macronutrient balance from the first to third trimesters
| ρ | P value | |
|---|---|---|
| Change in body weight | 0.17 | 0.38 |
| Change in BMI | 0.17 | 0.38 |
| Change in fat mass | 0.16 | 0.41 |
| Change in fat free mass | 0.14 | 0.48 |
| Change in glucose | -0.44 | 0.02 |
| Change in insulin | -0.16 | 0.40 |
| Change in HOMA-IR | -0.26 | 0.18 |
The placenta is not a primary source of FGF21 production in pregnancy
Considering the increase in FGF21 is not correlated with changes in maternal energy storesand considering previous studies24,31,32 that suggest FGF21 may be secreted by the placenta, we hypothesized that the increase in FGF21 concentrations across pregnancy may originate from products of conception (i.e. placenta and fetus). Utilizing two different sets of primers including the primers reported in past studies31, we were unable to detect meaningful and replicable quantities of FGF21 transcript in human placenta by qPCR. These observations are not due to poor sample quality or error in qPCR protocol. The RNA extraction protocol used results in a RIN of 7 in human placenta; moreover, we were able to measure over 20 additional genes (including housekeeping genes SDHA and TBP) with resulting CTs as would be expected based on published findings in human placenta.
DISCUSSION
In this study, we report FGF21 levels in a pregnant population with pregravid overweight or obesity. The three novel observations from this body of work are that circulating FGF21 measured in fasting conditions is correlated with maternal body mass indexand adiposity at cross sectional timepoints throughout pregnancy, FGF21 concentrations increase across pregnancy, and this increase in FGF21 does not appear to be coming from the placenta or a change in maternal energy stores but with alterations in maternal glucose concentrations.
The relationship of FGF21, BMI, and adiposity has been well reported in non-pregnant animal models and human populations10,14,15,18. In this study, we demonstrate FGF21 concentrations positively correlate with maternal body mass index and adiposity in pregnant women in both the first and third trimesters. Considering pregnancy is a time for increased energy deposition in preparation for postpartum energy demands, e.g. lactation, these findings show the relationship between FGF21 and adiposity observed in non-pregnant states holds in cross sectional analysis performed at different timepoints across pregnancy. However, it is important to note the individual changes in adiposity across pregnancy did not predict the variations in FGF21 concentrations. These data suggest that the physiological driversfor deposition of maternal energy in fat mass are independent of changes in FGF21.
Notably, we were unable to confirm that high concentrations of FGF21 are present in pregnant individuals with increasing levels of glucose intolerance. Lack of support for this relationship in our population could be due to insufficient statistical power because we excluded participants with pregravid diabetes and impaired glucose tolerance in their first trimester (HbA1c > 6.5% and/or failed 75g oral glucose tolerance test). Although five participants were diagnosed with GDM during study participation, this initial exclusion likely limited the variability in glucose dysfunction within our cohort (fasting glucose ranged 72–109 mg/dL) despite the large range of maternal BMI(25.6 to 45.4 kg/m2). While fasting insulin and HOMA-IR showed moderate distribution among participants across pregnancy (see Table 2), glucose regulation appeared well controlled. Indeed, the percent change in fasting glucose (from the first to third trimester) ranged from −20% to +9%, with fasting glucose decreasing in two-thirds of participants. Therefore, there was limited variability in measures of glucose homeostasis for comparison to FGF21. Future studies need to be conducted in pregnant women with varying degrees of glucose tolerance to further elucidate the role of FGF21 in response to the worsened maternal glucose homeostasis throughout pregnancy.
We observed FGF21 concentrations were less variable and significantly lower in the first trimester compared to the third trimester of pregnancy. Considering FGF21 has been reported to be produced by the human placenta by two separate laboratories (in one study by ELISA of placenta explant media 24 and two other studies by qPCR of flash frozen human placenta samples31,32), it was reasonable to hypothesize that the increase in circulating FGF21 could be attributed to placenta tissue. Although, after extensive qPCR experiments in term human placenta from a subset of our cohort (n=19), we were unable to quantify expression of FGF21 in the samples studieddespite detection of other genes such as SDHA and TBP. We are therefore confident the human placenta is not contributing meaningful amounts of FGF21 into the maternal circulation.Instead, considering evidence of robust elevation in hepatic FGF21 mRNA throughout pregnancy recently reported in wild-type mice33, we posit the elevation in circulating FGF21 observed in our human cohort is likelyresultant from the liver. Moreover, there is extensive evidence showing FGF21 synthesis and secretion by adipocytes in rodents and humans, and it may also be likelythat maternal adipocytesare contributing in part to this FGF21 elevation considering the increase in fat mass across pregnancy14,34.
FGF21 has been regularly shown to correlate with glucose homeostasis in non-pregnant and pregnant populations with glucose dysfunction which could be another possible explanation for the change in FGF21 across pregnancy. Despite the limited variability of glucose homeostasis within our cohort, we observed a significant negative correlation between alterations in FGF21 and changes in fasting glucose. Of note, there was not a significant correlation between change in insulin or HOMA-IR and change in FGF21. The original role elucidated for FGF21 was as a glucose regulator, however the relationship between FGF21 and glucose regulation in pregnancy merits further investigation as we made these observations within a healthy population with little variability.
Longitudinal measurements of FGF21, body composition assessments in pregnancy by BOD POD®, as well as the availability of archived placenta tissue provide strength to our study. However, our study does have limitations. First is our small sample size. In an effort to maximize resources, we leveraged existing data and archived biospecimens from two parent studies (totaling n=114 at the time of analysis) to test our hypothesis. Considering the necessary data and biospecimens to answer our question (i.e. archived third trimester serum, data collected in the first and third trimesters, and consent for future use of biospecimens and data), only 43 of the 114 enrolled participants were eligible for this ancillary.Furthermore, our sample is primarily made up of Caucasian women and exclusively of women with overweight and obesity. Indeed, replication of these results in a larger and more diverse cohort as well as a normal weight cohortwould be advantageous next steps. Second, 21 of 43 subjects participated in a lifestyle intervention aimed toattenuate gestational weight gain during pregnancy.We conductedadditional analysesto investigate the potential influence of the intervention and found no differences across pregnancy for individuals receiving or not receiving the intervention, including change in FGF21,weight, BMI, fat mass, fat free mass, glucose, insulin, or HOMA-IR (Supplement Table 3). There were significant differences in BMI, weight, and fat mass at enrollment between individuals receiving the intervention compared to no intervention (Supplement Table 2). However, this finding is expected as our “no intervention” group is a compilation of a subset of the control group from the Expecting Success Study (n=6) whose inclusion criteria was BMI ≥ 25kg/m2 and participants from the MomEE Study (n=16) which did not have an intervention and whose inclusion criteria BMI ≥ 30kg/m2.
Since the changes in FGF21 were associated with changes in fasting glucose and not with the changes in body energy stores (i.e. weight or fat mass), we hypothesize that variations in acute energy status, such as with the maternal diet that influence both tissue deposition and glucose regulation, might be involved. In summary, we observed maternal FGF21 is positively correlated with maternal body mass index and adiposity before and throughout pregnancy, and FGF21 is likely responsive to short-term changes in macronutrient balance induced by maternal diet rather than long-term changes in energy balance reflected in the maternal energy stores.
Supplementary Material
Acknowledgements:
We graciously thank all study subjects who participated in our studies as well as the clinical research staff, Mrs. Abby Altazan, Ms. Porsha Vallo, Mrs. Loren Cain and Ms. Caitlin Hebert, who worked diligently in the collection of these data. We also thank Dr. Nicholas Broskey, Dr. Jenny Sones, Ms. Anik Boudreaux, and Mr. Hardy Hang for their guidance and technical support. We thank the LIFE-Moms consortium members for their contributions to the development and oversight of the common measures and procedures shared across the trials.
Funding:EFS is supported by F31HD084199. The MomEE trial is supported by the NIDDK (R01DK099175). LIFE-Moms is supported by the NIH through the NIDDK (U01DK094418 (Redman-Expecting Success), U01DK094463, U01DK094416, 5U01DK094466 (RCU)), NHLBI (U01HL114344, U01HL114377), NICHD (U01HD072834), NCCIH, the NIH Office of Research in Women’s Health (ORWH), the Office of Behavioral and Social Science Research (OBSSR), the Indian Health Service, and the Intramural Research Program of the NIDDK.
Footnotes
Declaration of interest: The authors have nothing to disclose.
REFERENCES
- 1.Kharitonenkov A, Shiyanova TL, Koester A, et al. FGF-21 as a novel metabolic regulator. J Clin Invest. 2005;115(6):1627–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coskun T, Bina HA, Schneider MA, et al. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology. 2008;149(12):6018–6027. [DOI] [PubMed] [Google Scholar]
- 3.Xu J, Lloyd DJ, Hale C, et al. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes. 2009;58(1):250–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Camporez JP, Jornayvaz FR, Petersen MC, et al. Cellular mechanisms by which FGF21 improves insulin sensitivity in male mice. Endocrinology. 2013;154(9):3099–3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kharitonenkov A, Wroblewski VJ, Koester A, et al. The metabolic state of diabetic monkeys is regulated by fibroblast growth factor-21. Endocrinology. 2007;148(2):774–781. [DOI] [PubMed] [Google Scholar]
- 6.Gaich G, Chien JY, Fu H, et al. The effects of LY2405319, an FGF21 analog, in obese human subjects with type 2 diabetes. Cell Metab. 2013;18(3):333–340. [DOI] [PubMed] [Google Scholar]
- 7.Wente W, Efanov AM, Brenner M, et al. Fibroblast growth factor-21 improves pancreatic beta-cell function and survival by activation of extracellular signal-regulated kinase 1/2 and Akt signaling pathways. Diabetes. 2006;55(9):2470–2478. [DOI] [PubMed] [Google Scholar]
- 8.Chavez AO, Molina-Carrion M, Abdul-Ghani MA, Folli F, Defronzo RA, Tripathy D. Circulating fibroblast growth factor-21 is elevated in impaired glucose tolerance and type 2 diabetes and correlates with muscle and hepatic insulin resistance. Diabetes Care. 2009;32(8):1542–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen WW, Li L, Yang GY, et al. Circulating FGF-21 levels in normal subjects and in newly diagnose patients with Type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes. 2008;116(1):65–68. [DOI] [PubMed] [Google Scholar]
- 10.Mraz M, Bartlova M, Lacinova Z, et al. Serum concentrations and tissue expression of a novel endocrine regulator fibroblast growth factor-21 in patients with type 2 diabetes and obesity. Clin Endocrinol (Oxf). 2009;71(3):369–375. [DOI] [PubMed] [Google Scholar]
- 11.Lin Z, Wu Z, Yin X, et al. Serum levels of FGF-21 are increased in coronary heart disease patients and are independently associated with adverse lipid profile. PLoS One. 2010;5(12):e15534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen C, Cheung BM, Tso AW, et al. High plasma level of fibroblast growth factor 21 is an Independent predictor of type 2 diabetes: a 5.4-year population-based prospective study in Chinese subjects. Diabetes Care. 2011;34(9):2113–2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Y, Xie Y, Berglund ED, et al. The starvation hormone, fibroblast growth factor-21, extends lifespan in mice. Elife. 2012;1:e00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang X, Yeung DC, Karpisek M, et al. Serum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes. 2008;57(5):1246–1253. [DOI] [PubMed] [Google Scholar]
- 15.Dushay J, Chui PC, Gopalakrishnan GS, et al. Increased fibroblast growth factor 21 in obesity and nonalcoholic fatty liver disease. Gastroenterology. 2010;139(2):456–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li L, Yang G, Ning H, Yang M, Liu H, Chen W. Plasma FGF-21 levels in type 2 diabetic patients with ketosis. Diabetes Res Clin Pract. 2008;82(2):209–213. [DOI] [PubMed] [Google Scholar]
- 17.Li K, Li L, Yang M, Zong H, Liu H, Yang G. Effects of rosiglitazone on fasting plasma fibroblast growth factor-21 levels in patients with type 2 diabetes mellitus. Eur J Endocrinol. 2009;161(3):391–395. [DOI] [PubMed] [Google Scholar]
- 18.Tan BK, Hallschmid M, Adya R, Kern W, Lehnert H, Randeva HS. Fibroblast growth factor 21 (FGF21) in human cerebrospinal fluid: relationship with plasma FGF21 and body adiposity. Diabetes. 2011;60(11):2758–2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stein S, Stepan H, Kratzsch J, et al. Serum fibroblast growth factor 21 levels in gestational diabetes mellitus in relation to insulin resistance and dyslipidemia. Metabolism. 2010;59(1):33–37. [DOI] [PubMed] [Google Scholar]
- 20.Gallego-Escuredo JM, Gomez-Ambrosi J, Catalan V, et al. Opposite alterations in FGF21 and FGF19 levels and disturbed expression of the receptor machinery for endocrine FGFs in obese patients. Int J Obes (Lond). 2015;39(1):121–129. [DOI] [PubMed] [Google Scholar]
- 21.Li H, Bao Y, Xu A, et al. Serum fibroblast growth factor 21 is associated with adverse lipid profiles and gamma-glutamyltransferase but not insulin sensitivity in Chinese subjects. J Clin Endocrinol Metab. 2009;94(6):2151–2156. [DOI] [PubMed] [Google Scholar]
- 22.Wang D, Zhu W, Li J, An C, Wang Z. Serum concentrations of fibroblast growth factors 19 and 21 in women with gestational diabetes mellitus: association with insulin resistance, adiponectin, and polycystic ovary syndrome history. PLoS One. 2013;8(11):e81190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stepan H, Kley K, Hindricks J, et al. Serum levels of the adipokine fibroblast growth factor-21 are increased in preeclampsia. Cytokine. 2013;62(2):322–326. [DOI] [PubMed] [Google Scholar]
- 24.Tan BK, Sivakumar K, Bari MF, Vatish M, Randeva HS. Lower cerebrospinal fluid/plasma fibroblast growth factor 21 (FGF21) ratios and placental FGF21 production in gestational diabetes. PLoS One. 2013;8(6):e65254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li SM, Wang WF, Zhou LH, et al. Fibroblast growth factor 21 expressions in white blood cells and sera of patients with gestational diabetes mellitus during gestation and postpartum. Endocrine. 2015;48(2):519–527. [DOI] [PubMed] [Google Scholar]
- 26.Megia A, Gil-Lluis P, Naf S, et al. Cord blood FGF21 in gestational diabetes and its relationship with postnatal growth. Acta Diabetol. 2015;52(4):693–700. [DOI] [PubMed] [Google Scholar]
- 27.Sutton EF, Cain LE, Vallo PM, Redman LM. Strategies for Successful Recruitment of Pregnant Patients Into Clinical Trials. Obstet Gynecol. 2017;129(3):554–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.ACOG Committee opinion. Number 267, January 2002: exercise during pregnancy and the postpartum period. Obstet Gynecol. 2002;99(1):171–173. [DOI] [PubMed] [Google Scholar]
- 29.Marshall NE, Murphy EJ, King JC, et al. Comparison of multiple methods to measure maternal fat mass in late gestation. Am J Clin Nutr. 2016;103(4):1055–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Raaij JM, Peek ME, Vermaat-Miedema SH, Schonk CM, Hautvast JG. New equations for estimating body fat mass in pregnancy from body density or total body water. Am J Clin Nutr. 1988;48(1):24–29. [DOI] [PubMed] [Google Scholar]
- 31.Dekker Nitert M, Barrett HL, Kubala MH, et al. Increased placental expression of fibroblast growth factor 21 in gestational diabetes mellitus. J Clin Endocrinol Metab. 2014;99(4):E591–598. [DOI] [PubMed] [Google Scholar]
- 32.Dekker Nitert M, Scholz-Romero K, Kubala MH, McIntyre HD, Callaway LK, Barrett HL. Placental fibroblast growth factor 21 is not altered in late-onset preeclampsia. Reprod Biol Endocrinol. 2015;13:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cui Y, Giesy SL, Hassan M, Davis K, Zhao S, Boisclair YR. Hepatic FGF21 production is increased in late pregnancy in the mouse. Am J Physiol Regul Integr Comp Physiol. 2014;307(3):R290–298. [DOI] [PubMed] [Google Scholar]
- 34.Dutchak PA, Katafuchi T, Bookout AL, et al. Fibroblast growth factor-21 regulates PPARgamma activity and the antidiabetic actions of thiazolidinediones. Cell. 2012;148(3):556–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.