Abstract
Negative cognitions are central to the perpetuation of chronic pain and sleep disturbances. Patients with temporomandibular joint disorder (TMJD), a chronic pain condition characterized by pain and limitation in the jaw area, have a high comorbidity of sleep disturbances that possibly exacerbate their condition. Ethnic group differences are documented in pain, sleep and coping, yet the mechanisms driving these differences are still unclear, especially in clinical pain populations. 156 women (79% white, 21% African American (AA)) diagnosed with TMJD were recruited as part of a randomized controlled trial evaluating the effectiveness of interventions targeting sleep and pain catastrophizing on pain in TMJD. Analysis of baseline data demonstrated that relative to white participants, AA exhibited higher levels of clinical pain, insomnia severity and pain catastrophizing, yet there was no ethnic group difference in negative sleep-related cognitions. Mediation models revealed pain catastrophizing, but not sleep-related cognitions or insomnia severity, to be a significant single mediator of the relationship between ethnicity and clinical pain. Only the helplessness component of catastrophizing together with insomnia severity sequentially mediated the ethnicity-pain relationship. These findings identify pain catastrophizing as a potentially important link between ethnicity and clinical pain and suggest that interventions targeting pain-related helplessness could improve both sleep and pain especially for AA patients.
Keywords: Temporomandibular joint disorder, Ethnicity, Insomnia, Catastrophizing, Negative cognitions
Introduction
Temporomandibular Joint Disorder (TMJD) consists of a cluster of chronic pain conditions characterized by pain in the jaw or surrounding muscles, which can lead to significant distress and functional limitation 5, 26. While there are documented ethnic differences in acute and chronic pain31, the role of ethnicity in TMJD is somewhat inconsistent. The OPPERA study has recently reported higher pain sensitivity in African American (AA) individuals relative to Caucasian individuals42 and a greater new-onset rate of TMJD for AA48. However, earlier studies found a higher prevalence of TMJD in Caucasians relative to AA37, 43, 44, suggesting that ethnic differences in TMJD might be influenced by multiple factors.
One such factor is sleep disturbances, a common comorbidity of TMJD 2, 51, with patients reporting nightly awakenings due to pain7. Longitudinal data provide evidence for a strong relationship between sleep and pain in TMJD, indicating that insomnia symptom severity predicts subsequent pain a month later46 and decreased sleep quality predicts TMJD onset48, 49, 53. Ethnic differences in sleep have also been documented in the insomnia literature; AA report poorer sleep quality, longer sleep onset latency and shorter sleep duration compared to Caucasians15, 30, 61 yet paradoxically, AA women tend to report less trouble sleeping3. These disparities in sleep may in part account for the ethnic differences found in pain. Similarly, ethnic group differences are also found in stress and in the use of coping strategies39, 52, which may be a factor that contributes to the effects of ethnicity on sleep.
Negative cognitions play a critical role in the experience and maintenance of both chronic pain13, 21 and poor sleep4, 22, and may contribute to the ethnic differences frequently observed in laboratory33 and clinical pain27 including TMJD42. The most widely studied negative pain-related cognition is pain catastrophizing, a dysfunctional and exaggerated mental reaction to actual or anticipated pain57. It is comprised of three elements: (1) rumination and negative emotions, (2) magnification of, and directing excessive attention to, pain and (3) beliefs of helplessness57 and is one of the most robust predictors of pain and adjustment20. While AA with clinical pain report higher levels of dispositional (general trait-like) pain catastrophizing 12, 39, it is not clear that pain catastrophizing mediates ethnic differences in clinical pain. There is evidence that the three components of catastrophizing might not contribute to pain and sleep in the same manner. For example, laboratory studies find that situation specific pain catastrophizing, mainly rumination, mediates the relationship between ethnicity and pain response 25, 40. In TMJD, we found that the relationship between rumination and clinical pain was mediated by sleep disturbance9. Thus, investigating the separate and combined dimensions of pain catastrophizing may yield important information about the cognitive mechanisms that contribute to ethnic differences in clinical pain.
Negative sleep-related cognitions contribute to the perpetuation of poor sleep and include dysfunctional thoughts about sleep, worry and pre-sleep arousal22. In populations with comorbid chronic pain and insomnia, pain-related cognitions can also interfere with sleep55. There is scant literature on ethnic differences in the negative cognitions related to poor sleep or sleep disorders such as insomnia. However, one study by Grandner and colleagues29 found that AA tend to have a smaller appreciation for the impact of sleep on their health and have less motivation to make time for sleep.
While the individual relationships between ethnicity, negative cognitions, sleep and pain, have been investigated, to the best of our knowledge no study has tested all of these factors as a whole in clinical pain populations. In an attempt to explain the mechanism by which ethnicity affects clinical pain, we built upon our previous findings, that sleep disturbance mediates the relationship between pain catastrophizing and pain in TMJD (catastrophizing -> sleep -> pain9) and have added ethnicity to this mediation model. This has resulted in a series of models which test whether ethnic differences in clinical pain are sequentially mediated by negative cognitions (related to sleep or to pain) and sleep disturbance (ethnicity -> negative cognitions -> sleep -> pain).
Methods
Procedure
Approval for this study was granted by the Institutional Review Board. Data were collected as part of the baseline assessment for an ongoing randomized clinical trial testing the effectiveness of different psychological interventions on sleep and pain in women with TMJD (ClinicalTrials.gov Identifier: NCT01794624). Participants were recruited through fliers, radio advertisements, mass mailing and referrals from dentists. Participants were first screened for eligibility through a telephone screening and scheduled for a baseline visit. Those who agreed to participate in the study signed an informed consent form approved by the university IRB and proceeded to complete self-report questionnaires. A comprehensive dental evaluation was performed and those who met the Research Diagnostic Criteria/Temporomandibular Disorders (RDC/TMD) Axis I TMD diagnosis16, 50 were offered enrollment in the study.
Inclusion criteria included female participants between 18 and 60 years of age, reporting facial pain for at least 3 months, having pain for at least 10 out of the last 30 days and who also reported trouble initiating and/or maintaining sleep regularly (≥ 3 days/week) for at least 1 month.
Exclusion criteria included BMI of above 40, history of any type of Temporomandibular joint (TMJ) surgery or TMJ growth disturbances, neoplasm, or injury to the TMJ area within the past six months, scheduled surgery for TMJ during study participation period, history of major medical disease known to impact sleep, the CNS, or peripheral neuropathy, diagnosis of Raynaud’s Syndrome, unstable major psychiatric disorder or self-reported suicidal ideation, active [within 6 months] substance or alcohol abuse, regular (≥ 3x/week) use of opioids, benzodiazepines/benzodiazepine receptor agonists, or sedating tricyclic antidepressants, stable preferred sleep phase between 10pm and 10am or self-reported variability in sleep due to changes in work shifts.
Self-report questionnaires
Clinical pain severity was assessed with the Brief Pain Inventory (BPI). This is an 11 item selfreport questionnaire that measures pain severity and pain-related interference in patients diagnosed with a variety of chronic pain conditions14, 59. Higher scores correspond to greater pain severity. Only the pain severity scale was used for the analyses. Internal consistency was Cronbach’s α= .87.
Insomnia severity was assessed with the Insomnia Severity Index (ISI)6. This 7-item measure identifies severity of sleep continuity disturbance, which is defined as difficulty in initiating sleep, staying asleep and early morning waking, rated on 5–point Likert scale. Higher scores correspond to greater sleep disturbance. Internal consistency was Cronbach’s α= .81.
Catastrophizing was assessed with the Pain Catastrophizing Scale (PCS)56. This questionnaire consists of 13 items rated on a 5-point scale ranging from 0 = “not at all” to 4 = “all the time”. Subjects indicate the degree to which they have experienced thoughts and feelings when experiencing pain. This instrument assesses 3 domains of catastrophizing: rumination, magnification, and helplessness. Internal consistency for the full scale was Cronbach’s α= .90, for rumination subscale Cronbach’s α= .87, magnification subscale Cronbach’s α= .75 and helplessness subscale Cronbach’s α= .82.
Negative sleep cognitions were assessed with the Dysfunctional Beliefs and Attitudes about Sleep (DBAS)18, 23. The DBAS evaluates sleep-disruptive cognitions including misattributions about the consequences of insomnia as well as unpredictability and lack of control over sleep. It contains 10-items and ratings are based on a 10 point Likert Scale where 0= “strongly disagree” and 10= “strongly agree.” Example items: “I am concerned that chronic insomnia may have serious consequences on my physical health”; “I am worried that I may lose control over my abilities to sleep”; “When I sleep poorly on one night, I know it will disturb my sleep schedule for the whole week”. Internal consistency was Cronbach’s α= .79.
Depressive symptoms were assessed with the Center for Epidemiologic Studies Depression Scale (CES-D)47. This is a 20-item self-report questionnaire in which participants indicate how often they experienced depressive symptoms in the past week using a 4-point scale (0–3). The CES-D appears to discriminate well between pain patients with and without major depression 28. Internal consistency was Cronbach’s α= .88.
Statistical Analysis
Only participants who self-identified as AA or Caucasian where included in the analyses; 13 participants of other ethnicities were excluded from these analyses. In order to examine ethnic differences in clinical pain, insomnia severity, catastrophizing and sleep cognitions, independent sample t-tests were used. Hedges’ g was used as a measure of effect size since the groups have different sample sizes34. The interpretation of Hedges’ g is similar to that of Cohen’s d, yet it is calculated by using pooled weighted standard deviations (instead of pooled standard deviations), thereby, taking into account the differences in group sizes that may impact the calculation of the mean. Zero order correlations were calculated to examine the relationship between the outcome variable (i.e. clinical pain) and the other study variables.
Sequential multiple mediation analyses were conducted in order to assess the role of negative cognitions (about pain and sleep) and insomnia severity on the relationship between ethnicity and clinical pain severity. In a sequential multiple mediation model two mediators (B and C) can be tested simultaneously (A->B->C->D), which can be done in parallel to testing the single or separate indirect effects of each mediator (A->B->D and A->C->D). Mediation was tested using the SPSS macro PROCESS32 (Model 6), which employs a bootstrapping technique, a non-parametric approach in which repeated random resampling is used to estimate the indirect effects and construct confidence intervals (CI). One thousand bootstrapping samples were used with a confidence interval (CI) of 95%. The indirect effect is interpreted as statistically significant if the bootstrapped CI does not include zero. This approach allows for no assumptions regarding distribution shape and there is a decreased chance of Type I and II errors45.
Five mediation models were tested, all testing mediators of the relationship between ethnicity and clinical pain severity. The first model tested if negative cognitions about sleep and insomnia severity sequentially mediated the relationship between ethnicity and clinical pain (ethnicity -> sleep cognitions -> insomnia -> pain). The second model tested if catastrophizing (PCS total score) and insomnia severity sequentially mediated the relationship between ethnicity and clinical pain (ethnicity -> catastrophizing -> insomnia -> pain). The last three models separately tested each one of the catastrophizing subscales (rumination, magnification and hopelessness) along with insomnia severity as sequential mediators of the ethnicity – clinical pain association. The confounding effects of education, depression and age, were controlled for in all models. Demographic variables that did not correlate with the outcome variables were not included in the models.
Results
Patient characteristics and univariate analyses
The sample included 156 women with TMJD with a mean age of 37 years (SD=11.8). Seventy-nine percent (n=123) were white and the rest African American (n=33; 21%). Sixty-two percent had a college degree, 46% had an annual household income of above $50,000. Forty-five percent were single, 40% percent married, 9% divorced or separated, 5% living with a partner and 1% was widowed. Demographic characteristics by ethnic group can be found in Table 1. Significant ethnic group differences were observed in age (t(154) = 2.94, p < .01 Hedges’ g = .58), BMI (t(154) = 2.11, p < .05, Hedges’ g = .41), TMJD duration (t(149) = −2.40, p < .05, Hedges’ g = .48) and education (χ2=21.6, p<.001).
Table 1:
Demographic and clinical characteristics by ethnic group
| Variable | Values = mean (SD) or N | ||
|---|---|---|---|
| AA N=33 | White N=123 | Total Sample N=156 |
|
| Age* | 42.24 (11.42) | 35.63 (11.49) | 37.03 (11.76) |
| BMI* | 28.71 (5.21) | 26.45 (5.52) | 26.93 (5.52) |
| TMJD duration (in months)* | 70.28 (80.58) | 120.92 (111.45) | 110.19 (107.44) |
| Apnea Hypopnea Index (AHI) | 2.10 (2.52) | 1.40 (2.06) | 1.52 (2.18) |
| Medication | |||
| OTC pain (Motrin, Tylenol) | 13 | 56 | 69 |
| OTC sleep (Benadryl) | 0 | 5 | 5 |
| Hispanic heritage | |||
| Hispanic | 0 | 7 | 7 |
| Non-Hispanic | 30 | 109 | 139 |
| Not reported | 3 | 7 | 10 |
| Education* | |||
| No college degree | 24 | 35 | 59 |
| College degree | 9 | 88 | 97 |
| Annual income | |||
| < $50,000 | 17 | 46 | 63 |
| > $50,000 | 10 | 65 | 75 |
| Not reported | 6 | 12 | 18 |
| Marital status | |||
| Single | 17 | 51 | 68 |
| Cohabitated or married | 9 | 55 | 64 |
| Living alone (widowed/divorced/separated) |
3 | 10 | 13 |
| Not reported | 4 | 7 | 11 |
BMI = Body Mass Index; TMJD = Temporomandibular Joint Disorder
Statistically significant difference between AA and white groups, p < .05
As seen in Table 2, ethnic group differences were observed on the Brief Pain Inventory (severity subscale), such that AA reported higher levels of pain compared to whites t(153) = 4.66, p < .001; Hedges’ g = .83). Similarly, AA reported higher levels of catastrophizing (total scale: t(154) = 2.58, p = .01; Hedges’ g = .50), which was due to group differences in rumination (t(154) = 2.36, p < .05; Hedges’ g = .46) and magnification (t(154) = 2.93, p < .01; Hedges’ g = .57), but not helplessness (t(154) = 1.65, p = .10). AA reported poorer sleep, reporting greater insomnia severity as compared to whites (t(154) = 1.95, p = .05; Hedges’ g = .38); there were no ethnic group differences in dysfunctional thoughts about sleep (t(149) = 0.42, p = .68).
Table 2:
Ethnic group differences in the study variables
| Variable | AA Mean (SD) | White Mean (SD) | Total sample Mean (SD) | t value | Effect size |
|---|---|---|---|---|---|
| Pain | 5.56 (1.82) | 4.10 (1.52) | 4.41 (1.69) | 4.66** | .83 |
| Insomnia | 17.85 (4.97) | 16.02 (4.74) | 16.40 (4.38) | 1.95* | .38 |
| PCS total | 25.18 (10.90) | 20.34 (9.21) | 21.37 (9.76) | 2.58* | .50 |
| PCS rumination | 9.27 (4.15) | 7.53 (3.66) | 7.90 (3.82) | 2.36* | .46 |
| PCS magnification | 5.51 (3.29) | 3.90 (2.66) | 4.24 (2.87) | 2.93** | .57 |
| PCS helplessness | 10.39 (4.71) | 8.91 (4.56) | 9.22 (4.62) | 1.65 | .32 |
| Sleep cognitions | 6.55 (2.01) | 6.41 (1.51) | 6.44 (1.62) | 0.42 | .09 |
| Depression | 18.97 (9.48) | 19.15 (8.72) | 19.11 (8.86) | −0.1 | .02 |
p ≤ .05
p ≤ .01
χ2 value
PCS = Pain Catastrophizing Scale; AA = African American
Zero-order correlations among clinical measures of catastrophizing, sleep, and pain as well as demographic variables are presented in Table 3. As expected, correlates of pain included catastrophizing (total scale: r = .47; rumination: r = .40; magnification: r = .36; helplessness: r = .45), insomnia severity (r = .50), and dysfunctional sleep thoughts (r = .21). Since correlates of pain also included depression (r = .24) it was carried forward as a covariate in the multivariate models. BMI and TMJD duration did not correlate with pain and thus were not controlled for in the models.
Table 3:
Correlations among pain, sleep and negative cognitions
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. Pain | 1 | |||||||||
| 2. Insomnia | .50** | 1 | ||||||||
| 3. PCS | .47** | .31** | 1 | |||||||
| 4. PCS Rumination | .40** | .25** | .90** | 1 | ||||||
| 5. PCS Magnification | .36** | .17* | .75** | .54** | 1 | |||||
| 6. PCS Helplessness | .45** | .35** | .91** | .72** | .51** | 1 | ||||
| 7. Sleep cognitions | .17* | .56** | .26** | .20* | .19* | .26** | 1 | |||
| 8. Depression | .24** | .40** | .47** | .41** | .27** | .50** | .40** | 1 | ||
| 9. Age | .13 | .18* | 0.03 | 0.02 | 0.05 | −0.04 | 0.07 | −0.01 | 1 | |
| 10. BMI | .15 | .08 | .01 | −.01 | .13 | −.07 | −.06 | −.10 | .25** | 1 |
| 11. TMJD duration | −.02 | .08 | −.11 | −.09 | −.10 | −.10 | −.12 | .04 | .28** | .10 |
p < .05
p < .01
PCS = Pain Catastrophizing Scale; BMI = Body Mass Index; TMJD = Temporomandibular Joint Disorder
Negative Pain and Sleep related Cognitions and Insomnia as Mediators of Ethnic Group Differences in Pain
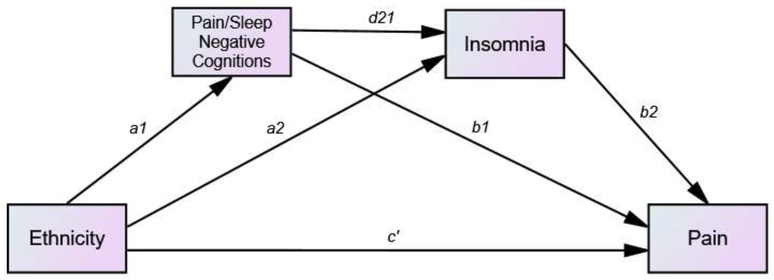
We conducted a series of analyses testing the role of negative pain related cognitions (i.e. catastrophizing and the PCS subscales) and negative sleep-related cognitions (DBAS) as well as insomnia severity as mediators of the relationship between ethnicity and clinical pain using sequential multiple mediation models controlling for age, education, and depression (see Figure 1). Due to the high correlation between education and ethnicity, all models were also tested without education as a covariate and the same pattern of results was maintained. Path coefficients, standard error, confidence intervals for each path of the mediation models are found in Table 4.
Figure 1: Sequential mediation model for the effects of ethnicity on pain.
Indirect effect of ethnicity on pain through negative cognitions (about sleep or pain) = a1b1
Indirect effect of ethnicity on pain through insomnia = a2b2
Indirect effect of ethnicity on pain through sequential negative cognitions and insomnia = a1 d21 b2
Direct effect of ethnicity on pain = c’
Table 4: Results of the mediation analyses.
Path coefficients, standard error, confidence intervals for each path of the model. Effect size is presented for the indirect effects. Each of the paths are represented in Figure 1. Mediation can be assumed when the indirect effect is significant, represented by CI that do not cross zero.
| Model | Path coefficienta | SE | t Test statistic | 95% CI | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Model 1: SLP-COG & Insomnia | |||||
| Direct effect: Ethnicity -> SLP-COG (a1) | .08 | .33 | .24 | −.57 | .73 |
| Direct effect: Ethnicity -> insomnia (a2) | −.55 | .83 | −.67 | −2.18 | 1.08 |
| Direct effect: SLP-COG ->Pain (b1) | −.18 | .09 | −2.08* | −.35 | −.01 |
| Direct effect: insomnia ->Pain (b2) | .17 | .03 | 5.69** | .11 | .23 |
| Direct effect: SLP-COG ->insomnia (d21) | 1.33 | .21 | 6.41** | .92 | 1.75 |
| Direct effect: Ethnicity ->Pain (c’) | −.92 | .30 | −3.07* | −1.51 | −.33 |
| Indirect effect: Ethnicity->SLP-COG->Pain (a1b1) | [−.01] | .07 | −.19 | .12 | |
| Indirect effect: Ethnicity->Insomnia->Pain (a2b2) | [−.09] | .15 | −.46 | .20 | |
| Indirect effect: Ethnicity->SLP-COG->Insomnia ->Pain (a2 d21 b2) | [.02] | .09 | −.16 | .19 | |
| Model 2: PCS & Insomnia | |||||
| Direct effect: Ethnicity -> PCS (a1) | −4.78 | 1.82 | −2.63** | −8.37 | −1.18 |
| Direct effect: Ethnicity -> insomnia (a2) | −.28 | .94 | −.30 | −2.14 | 1.58 |
| Direct effect: PCS ->Pain (b1) | .06 | .01 | 4.76** | .04 | .08 |
| Direct effect: insomnia ->Pain (b2) | .12 | .02 | 5.01** | .08 | .17 |
| Direct effect: PCS ->insomnia (d21) | .05 | .04 | 1.29 | −.03 | .13 |
| Direct effect: Ethnicity ->Pain (c’) | −.64 | .28 | −2.26* | −1.21 | −.08 |
| Indirect effect: Ethnicity->PCS->Pain (a1b1) | [−.28]* | .14 | −.65 | −.05 | |
| Indirect effect: Ethnicity->Insomnia->Pain (a2b2) | [−.03] | .13 | −.34 | .18 | |
| Indirect effect: Ethnicity->PCS->Insomnia->Pain (a2 d21 b2) | [−.03] | .03 | −.14 | .01 | |
| Model 3: PCS-R & Insomnia | |||||
| Direct effect: Ethnicity -> PCS-R (a1) | −1.74 | .74 | −2.34* | −3.21 | −.27 |
| Direct effect: Ethnicity -> insomnia (a2) | −.41 | .94 | −.43 | −2.26 | 1.45 |
| Direct effect: PCS-R ->Pain (b1) | .12 | .03 | 3.75** | .06 | .18 |
| Direct effect: insomnia ->Pain (b2) | .13 | .03 | 5.17** | .08 | .18 |
| Direct effect: PCS-R ->insomnia (d21) | .07 | .10 | .72 | −.12 | .27 |
| Direct effect: Ethnicity ->Pain (c’) | −.72 | .29 | −2.48* | −1.29 | −.15 |
| Indirect effect: Ethnicity->PCS-R->Pain (a1b1) | [−.21]* | .10 | −.45 | −.05 | |
| Indirect effect: Ethnicity->Insomnia->Pain (a2b2) | [−.03] | .09 | −.22 | .11 | |
| Indirect effect: Ethnicity->PCS-R->Insomnia->Pain (a2 d21 b2) | [−.01] | .02 | −.06 | .01 | |
| Model 4: PCS-M & Insomnia | |||||
| Direct effect: Ethnicity -> PCS-M (a1) | −1.33 | .59 | −2.27* | −2.49 | −.17 |
| Direct effect: Ethnicity -> insomnia (a2) | −.55 | .94 | −.59 | −2.41 | 1.31 |
| Direct effect: PCS-M ->Pain (b1) | .13 | .04 | 3.14** | .05 | .21 |
| Direct effect: insomnia ->Pain (b2) | .14 | .03 | 5.36** | .09 | .19 |
| Direct effect: PCS-M ->insomnia (d21) | −.01 | .13 | −.11 | −27 | .24 |
| Direct effect: Ethnicity ->Pain (c’) | −.75 | .29 | −2.56* | −1.33 | −.17 |
| Indirect effect: Ethnicity->PCS-M->Pain (a1b1) | [−.17*] | .10 | −.44 | −.02 | |
| Indirect effect: Ethnicity->Insomnia->Pain (a2b2) | [−.08] | .14 | −.37 | .15 | |
| Indirect effect: Ethnicity->PCS-M->Insomnia ->Pain (a2 d21 b2) | [.01] | .03 | −.06 | .06 | |
| Model 5: PCS-H & Insomnia | |||||
| Direct effect: Ethnicity -> PCS-H (a1) | −1.70 | .86 | −1.97* | −3.41 | .01 |
| Direct effect: Ethnicity -> insomnia (a2) | −.21 | .92 | −.23 | −2.04 | 1.61 |
| Direct effect: PCS-H ->Pain (b1) | .12 | .03 | 4.46** | .07 | .17 |
| Direct effect: insomnia ->Pain (b2) | .12 | .03 | 4.62** | .07 | .17 |
| Direct effect: PCS-H ->insomnia (d21) | .19 | .08 | 2.20* | .02 | .36 |
| Direct effect: Ethnicity ->Pain (c’) | −.73 | .28 | −2.57* | −1.29 | −.17 |
| Indirect effect: Ethnicity->PCS-H->Pain (a1b1) | [−.21]* | .13 | −.53 | −.01 | |
| Indirect effect: Ethnicity->Insomnia->Pain (a2b2) | [−.03] | .12 | −.56 | .12 | |
| Indirect effect: Ethnicity->PCS-H->Insomnia->Pain (a2 d21 b2) | [−.04]* | .03 | −.14 | −.01 | |
SE – Standard error; CI – Confidence interval; IV – Independent variable; SLP-COG = Negative sleep cognitions; PCS – Pain Catastrophizing Scale
For the indirect effect this represents effect size in brackets [] and not a path coefficient
p < .05
p < .001.
The first model included the two mediators, negative sleep-related cognitions (DBAS) and insomnia severity, and accounted for 39% of the variance in pain [F(6,144)=15.35, p<.001]. Only the direct effect of ethnicity on pain was significant in this model (effect size = −.92; 95% CI: −1.51 to −.32). None of the indirect effects were significant.
The second model included total pain catastrophizing score and insomnia severity and accounted for 50% of the variance in pain [F(6,147)=24.64, p<.001]. While the direct effect of ethnicity on pain remained (effect size = −.57; 95% CI: −1.13 to −.01), we found a significant indirect effect of catastrophizing mediating the relationship between ethnicity and pain (effect size = −.32; 95% CI: −.69 to −.05), but no sequential effect of insomnia severity or indirect mediating effect of insomnia severity.
The next set of models tested the sequential mediation of each of the three subscales of the pain catastrophizing scale. The third model, including rumination and insomnia severity, accounted for 42% of the variance in pain [F(6,147)=17.82, p<.001]. While the direct effect of ethnicity on pain remained (effect size = −.72; 95% CI: −1.29 to −.15), we found a significant indirect effect of rumination mediating the relationship between ethnicity and pain (effect size = −.21; 95% CI: −.45 to −.05), with no mediating or sequential effect of insomnia severity. Similarly, the fourth model, testing magnification and insomnia severity, accounted for 41% of the variance in pain [F(6,147)=16.71, p<.001]. Again, the direct effect of ethnicity on pain remained significant (effect size = −.75; 95% CI: −1.33 to −.17), and we found a significant indirect effect of magnification mediating this relationship (effect size = −.17; 95% CI: −.44 to .03). There was no mediating or sequential effect of insomnia severity. Finally, a fifth model included helplessness and insomnia severity and accounted for 44% of the variance in pain [F(6,147)=19.36, p<.001]. Again, the direct effect of ethnicity on pain remained significant (effect size = −.73; 95% CI: −1.29 to −.17); however, in this model the indirect sequential mediation by helplessness and insomnia severity was significant (effect size = −.04; 95% CI: −.15 to −.01).
Discussion
In this sample of women with TMJD selected for elevated insomnia and pain catastrophizing scores, we find ethnic differences in clinical pain, insomnia severity, and pain catastrophizing, yet no ethnic differences in negative sleep-related cognitions. Single mediator models revealed pain catastrophizing, but not insomnia severity, to be a significant mediator of the relationship between ethnicity and clinical pain, even after controlling for other pertinent clinical and demographic variables. Further analyses revealed that each of the three pain catastrophizing elements played a role in the single mediator models.
Our primary goal was to explore whether both negative sleep or pain cognitions and insomnia severity sequentially mediated the ethnicity-pain relationship. This sequential model derived from our previous work in TMJD that found sleep disturbances to mediate the relationship between pain catastrophizing and clinical pain (i.e., pain catastrophizing -> sleep disturbance -> clinical pain9), after statistically controlling for ethnic differences. In our current models, we did not find an indirect or sequential effect of insomnia on clinical pain. When we investigated the individual contributions of the three pain catastrophizing components, only the helplessness component produced a sequential mediation effect. Namely, ethnic differences contributed to elevated pain-helplessness, in turn contributing to elevated insomnia severity, which finally resulted in elevated clinical pain. As the helplessness subscale was the only subscale that did not show any ethnic group differences, this pattern of findings suggests a complex relationship between helplessness and insomnia severity. Feelings of helplessness are central to the maintenance of primary insomnia11 and individuals with facial pain can feel estranged and misunderstood due to their condition35. Combined with the finding that AA express helplessness in response to pain that is associated with racial disparities in medical treatment31, 38, 41, it is possible that AA may be more sensitive to the effects of pain-related helplessness on sleep in the context of chronic pain. The significant, yet small, effect must be interpreted with caution and requires future replication. Still, we believe this to be an interesting finding especially since this relationship involving helplessness is independent of depressive symptoms.
Contrary to our current findings, previous work found the rumination component of catastrophizing to be the strongest predictor of pain. Our group has reported that dispositional rumination-catastrophizing had an indirect effect on clinical pain through its association with sleep disturbance in TMJD 9. Laboratory studies have also reported that situational ruminationcatastrophizing mediated the relationship between ethnicity and laboratory pain responses in healthy volunteers25, 40. Rumination is the tendency to think and worry about pain which may maintain one’s vigilance to pain and other threats17. The higher levels of rumination in AA women with TMJD we report here and previously9, may be related to differences in the treatment and management of both acute and chronic pain between ethnic groups. AA and other minorities tend to have reduced access to healthcare and receive less treatment31 which has been associated with elevated worry and rumination58. This is the first study to explore sequential mediation with rumination and insomnia severity in a clinical pain population and to test direct effects of ethnic differences rather than independent effects using ethnicity as a covariate. These inconsistencies indicate that more work is needed to investigate the role of both the helplessness and the rumination components of pain catastrophizing as mediators of ethnic differences in clinical pain.
AA women in our study reported greater insomnia severity, a finding that is consistent with the large body of literature that generally finds AA to have poorer sleep than Caucasians15, 30, 61. Interestingly, we did not find ethnic differences in the severity of negative sleep-related cognitions as measured by the DBAS, regardless of their known associations with the perpetuation of sleep disturbances22. This suggests that a mechanism not related to sleepcognitions may be driving the ethnic differences we found in insomnia severity. Literature on ethnic differences in sleep-related cognitions is very scarce with only one study showing that AA and Caucasian women tended to endorse different dysfunctional thoughts about sleep29. It is possible that other types of negative cognitions may be relevant in understanding why AA exhibit poorer sleep compared to Caucasians. For example, feelings of ethnic discrimination have been associated with sleep disparities between ethnic groups, possibly since these feelings may increase psychological and physiological stress and chronic sympathetic nervous system activation that may impede sleep54. Ethnic discrimination has also been linked to worse pain management24 and is a strong predictor of the report of back pain in AA women19. Future studies should test the contribution and role for other types of negative cognitions, including ethnic discrimination, in TMJD patients.
Our results have important clinical implications, especially since higher levels of catastrophizing have been associated with poorer treatment response in TMJD36. In treating pain in AA patients, focusing on catastrophizing in general and specifically the helplessness component, appears to be important in the context of sleep disturbances. Focusing on maladaptive sleep-related cognitions can be beneficial regardless of ethnic background; however, a greater sensitivity should be used to assess negative pain-related cognitions and feelings of discrimination which may be more pronounced in AA patients. This is consistent with at least one prior study demonstrating that negative thoughts about pain were more robustly associated with poor sleep than negative thoughts about sleep in a heterogeneous sample of chronic pain patients55. Adapting specific interventions and communication styles to be more appropriate to patient’s social context and ethnic background can increase treatment effectiveness for AA and other ethnic minorities8, as well as for low-SES and low literacy individuals with chronic pain 60. This form of adaptation may be particularly important when addressing pain and sleep in different ethnic groups.
A number of limitations should be mentioned. No men were included in this study, and the women who participated were selected to demonstrate at least moderate levels of insomnia severity and catastrophizing in addition to meeting other inclusion criteria as described in the methods section. Thus, generalization of the findings to men and women without sleep disturbances or low catastrophizing should be done with caution. We did not oversample AA in our study, leading to a relatively small percent (21%) of AA; however, our sampling is in line with lower rates of TMJD found in AA in population based studies 43. Given this small percentage, replication in future studies is required. These findings are just one step in understanding the contribution of factors such as negative cognition and insomnia to the complex relationship between ethnicity and clinical pain. We believe that despite this limitation, these findings provide important information on the unique contribution of different aspects of pain related cognitions and have important clinical implications. It is possible that the self-report measures for insomnia and sleep-related cognitions used in this study did not capture the full magnitude or subtle differences of the manifestation of insomnia in AA, possibly since AA tend to under report trouble sleeping despite having shorter sleep durations3. This might have contributed to insomnia not emerging as a mediator of the relationship between ethnicity and clinical pain in our analyses. It may be that a more specific scale for assessing pain-related cognitions about sleep1 could provide better information than the DBAS in TMJD populations and might help explain the complex relationship between ethnicity, sleep and pain in this group. Due to the cross-sectional nature of this study, the temporal relationship assumed in the mediation analyses should be interpreted with caution. Finally, the effect size for the sequential mediation effect involving helplessness and insomnia was small and thus should be replicated in future studies.
It is important to note that in our analyses, controlling for education level did not change the pattern of the results. Yet, there is evidence that both ethnicity and education have a significant impact on the relationship between coping and clinical pain10. Many studies that demonstrate ethnic differences do not control for education level or use homogenous samples of college students25, 40, which does not allow for the assessment of the impact of education on their results. In our sample there was substantial overlap between indicators of socioeconomic status namely ethnicity, education and income which did not allow us to test their individual contribution. Future studies should include socioeconomically diverse populations in order to allow a more in depth understanding of the complex relationship between ethnicity and other socioeconomic factors.
Conclusions
Despite our fairly small sample, the higher levels of pain catastrophizing, sleep disturbances and pain found in the AA women with TMJD suggest that more effort should be made to assess and treat this group, addressing their distress and multiple symptoms. Our results suggest that pain catastrophizing is a potentially important mechanism accounting for some of the ethnic differences in clinical pain, with pain-specific helplessness linking ethnicity, sleep and clinical pain. AA are underrepresented in TMJD research probably due to the smaller prevalence in this population; however, the recent finding that AA have a greater rate of new diagnoses of TMJD53 along with the current findings suggest that more resources should be allocated for studying AA with TMJD.
Highlights.
Ethnic differences were found in clinical pain, insomnia and pain catastrophizing
No ethnic group difference were found in negative sleep-related cognitions
Pain catastrophizing mediated the relationship between ethnicity and clinical pain
Helplessness and insomnia sequentially mediated the ethnicity-pain relationship
Perspective.
Pain related helplessness and insomnia severity contribute to ethnic differences found in clinical pain among woman with temporomandibular joint disorder. Findings can potentially inform interventions that target insomnia and catastrophizing to assist in reducing ethnic disparities in clinical pain.
Footnotes
Disclosures
The authors have no conflict of interest to declare. The present work was supported by NIH Grant R01 DE019731 (Haythornthwaite, JA and Smith, MT)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Afolalu EF, Moore C, Ramlee F, Goodchild CE, Tang NK. Development of the PainRelated Beliefs and Attitudes about Sleep (PBAS) Scale for the Assessment and Treatment of Insomnia Comorbid with Chronic Pain. J. Clin. Sleep Med 12:1269–1277,2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Almoznino G, Benoliel R, Sharav Y, Haviv Y. Sleep disorders and chronic craniofacial pain: Characteristics and management possibilities. Sleep Med. Rev [DOI] [PubMed] [Google Scholar]
- 3.Amyx M, Xiong X, Xie Y, Buekens P. Racial/Ethnic Differences in Sleep Disorders and Reporting of Trouble Sleeping Among Women of Childbearing Age in the United States. Maternal and child health journal. 21:306–314, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ashworth PC, Davidson KM, Espie CA. Cognitive-behavioral factors associated with sleep quality in chronic pain patients. Behav. Sleep Med 8:28–39, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Auvenshine RC. Temporomandibular disorders: associated features. Dent. Clin. North Am 51:105–127, vi, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Bastien CH, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2:297–307, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Benoliel R, Eliav E, Sharav Y. Self-reports of pain-related awakenings in persistent orofacial pain patients. J. Orofac. Pain 23:330–338, 2009 [PubMed] [Google Scholar]
- 8.Bhui KS, Aslam RW, Palinski A, McCabe R, Johnson MR, Weich S, Singh SP, Knapp M, Ardino V, Szczepura A. Interventions to improve therapeutic communications between Black and minority ethnic patients and professionals in psychiatric services:systematic review. Br. J. Psychiatry 207:95–103, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buenaver LF, Quartana PJ, Grace EG, Sarlani E, Simango M, Edwards RR, Haythornthwaite JA, Smith MT. Evidence for indirect effects of pain catastrophizing on clinical pain among myofascial temporomandibular disorder participants: the mediating role of sleep disturbance. Pain. 153:1159–1166, 2012 [DOI] [PubMed] [Google Scholar]
- 10.Cano A, Mayo A, Ventimiglia M. Coping, pain severity, interference, and disability: the potential mediating and moderating roles of race and education. J. Pain 7:459–468, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carney CE, Edinger JD. Identifying critical beliefs about sleep in primary insomnia. Sleep. 29:444–453, 2006 [PubMed] [Google Scholar]
- 12.Chibnall JT, Tait RC. Confirmatory factor analysis of the Pain Catastrophizing Scale in African American and Caucasian Workers’ Compensation claimants with low back injuries. Pain. 113:369–375, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Chou R, Shekelle P. Will this patient develop persistent disabling low back pain? JAMA. 303:1295–1302, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann. Acad. Med. Singapore 23:129–138, 1994 [PubMed] [Google Scholar]
- 15.Durrence HH, Lichstein KL. The Sleep of African Americans: A Comparative Review. Behav. Sleep Med. 4:29–44, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Dworkin SF, LeResche L. Research diagnostic criteria for temporomandibular disorders: review, criteria, examinations and specifications, critique. J. Craniomandib. Disord. 6:301–355, 1992 [PubMed] [Google Scholar]
- 17.Eccleston C, Crombez G, Aldrich S, Stannard C. Worry and chronic pain patients: a description and analysis of individual differences. European Journal of Pain. 5:309–318, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Edinger JD, Wohlgemuth WK. Psychometric comparisons of the standard and abbreviated DBAS-10 versions of the dysfunctional beliefs and attitudes about sleep questionnaire. Sleep Med. 2:493–500, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Edwards RR. The association of perceived discrimination with low back pain. J. Behav.Med. 31:379–389, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Edwards RR, Cahalan C, Mensing G, Smith M, Haythornthwaite JA. Pain, catastrophizing, and depression in the rheumatic diseases. Nat. Rev. Rheumatol 7:216–224, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Edwards RR, Dworkin RH, Sullivan MD, Turk DC, Wasan AD. The Role of Psychosocial Processes in the Development and Maintenance of Chronic Pain. J. Pain 17:T70–92, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Espie CA. Understanding insomnia through cognitive modelling. Sleep Med 8 Suppl 4:S3–8, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Espie CA, Inglis SJ, Harvey L, Tessier S. Insomniacs’ attributions. psychometric properties of the Dysfunctional Beliefs and Attitudes about Sleep Scale and the Sleep Disturbance Questionnaire. J. Psychosom. Res 48:141–148, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Ezenwa MO, Fleming MF. Racial Disparities in Pain Management in Primary Care. Journal of health disparities research and practice. 5:12–26, 2012 [PMC free article] [PubMed] [Google Scholar]
- 25.Fabian LA, McGuire L, Goodin BR, Edwards RR. Ethnicity, catastrophizing, and qualities of the pain experience. Pain Med. 12:314–321, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fillingim RB, Ohrbach R, Greenspan JD, Knott C, Dubner R, Bair E, Baraian C, Slade GD, Maixner W. Potential psychosocial risk factors for chronic TMD: descriptive data and empirically identified domains from the OPPERA case-control study. J. Pain 12:T46–60, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gansky SA, Plesh O. Widespread pain and fibromyalgia in a biracial cohort of young women. J. Rheumatol 34:810–817, 2007 [PubMed] [Google Scholar]
- 28.Geisser ME, Roth RS, Robinson ME. Assessing depression among persons with chronic pain using the Center for Epidemiological Studies-Depression Scale and the Beck Depression Inventory: a comparative analysis. Clin. J. Pain 13:163–170, 1997 [DOI] [PubMed] [Google Scholar]
- 29.Grandner MA, Patel NP, Jean-Louis G, Jackson N, Gehrman PR, Perlis ML, Gooneratne NS. Sleep-related behaviors and beliefs associated with race/ethnicity in women. J. Natl. Med. Assoc 105:4–15, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grandner MA, Williams NJ, Knutson KL, Roberts D, Jean-Louis G. Sleep disparity, race/ethnicity, and socioeconomic position. Sleep Med. 18:7–18, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Green CR, Anderson KO, Baker TA, Campbell LC, Decker S, Fillingim RB, Kalauokalani DA, Lasch KE, Myers C, Tait RC, Todd KH, Vallerand AH. The unequal burden of pain: confronting racial and ethnic disparities in pain. Pain Med. 4:277–294, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Hayes AF: PROCESS: A versatile computational tool for observed variable mediation, moderation, and conditional process modeling Retrieved from http://www.afhayes.com/public/process2012.pdf, 2012.
- 33.Kim HJ, Yang GS, Greenspan JD, Downton KD, Griffith KA, Renn CL, Johantgen M, Dorsey SG. Racial and ethnic differences in experimental pain sensitivity: systematic review and meta-analysis. Pain. 158:194–211, 2017 [DOI] [PubMed] [Google Scholar]
- 34.Calculating Lakens D. and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front. Psychol 4:863, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lennon MC, Link BG, Marbach JJ, Dohrenwend BP. The Stigma of Chronic Facial Pain and Its Impact on Social Relationships*. Soc. Probl 36:117–134, 1989 [Google Scholar]
- 36.Litt MD, Porto FB. Determinants of pain treatment response and nonresponse: identification of TMD patient subgroups. J. Pain 14:1502–1513, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maixner W, Fillingim RB, Williams DA, Smith SB, Slade GD. Overlapping Chronic Pain Conditions: Implications for Diagnosis and Classification. J. Pain 17:T93–t107, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mayberry RM, Mili F, Ofili E. Racial and ethnic differences in access to medical care.Med. Care Res. Rev 57 Suppl 1:108–145, 2000 [DOI] [PubMed] [Google Scholar]
- 39.Meints SM, Miller MM, Hirsh AT. Differences in Pain Coping Between Black and WhiteAmericans: A Meta-Analysis. J. Pain 17:642–653, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meints SM, Stout M, Abplanalp S, Hirsh AT. Pain-Related Rumination, But NotMagnification or Helplessness, Mediates Race and Sex Differences in Experimental Pain.J. Pain 18:332–339, 2017 [DOI] [PubMed] [Google Scholar]
- 41.Mossey JM. Defining Racial and Ethnic Disparities in Pain Management. Clin. Orthop.Relat. Res 469:1859–1870, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ostrom C, Bair E, Maixner W, Dubner R, Fillingim RB, Ohrbach R, Slade GD, Greenspan JD. Demographic Predictors of Pain Sensitivity: Results From the OPPERAStudy. J. Pain 18:295–307, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Plesh O, Adams SH, Gansky SA. Racial/Ethnic and Gender Prevalences in Reported Common Pains in a National Sample. J. Orofac. Pain 25:25–31, 2011 [PMC free article] [PubMed] [Google Scholar]
- 44.Plesh O, Crawford PB, Gansky SA. Chronic pain in a biracial population of young women. Pain. 99:515–523, 2002 [DOI] [PubMed] [Google Scholar]
- 45.Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav. Res. Methods Instrum. Comput 36:717–731, 2004 [DOI] [PubMed] [Google Scholar]
- 46.Quartana PJ, Wickwire EM, Klick B, Grace E, Smith MT. Naturalistic changes in insomnia symptoms and pain in temporomandibular joint disorder: a cross-lagged panel analysis. Pain. 149:325–331, 2010 [DOI] [PubMed] [Google Scholar]
- 47.Radloff LS. A self-reported depression scale for research in the general population. Applied Psychological Measures 1:385–401, 1977 [Google Scholar]
- 48.Sanders AE, Akinkugbe AA, Bair E, Fillingim RB, Greenspan JD, Ohrbach R, Dubner R, Maixner W, Slade GD. Subjective Sleep Quality Deteriorates Before Development of Painful Temporomandibular Disorder. J. Pain 17:669–677, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sanders AE, Slade GD, Bair E, Fillingim RB, Knott C, Dubner R, Greenspan JD, Maixner W, Ohrbach R. General health status and incidence of first-onset temporomandibular disorder: the OPPERA prospective cohort study. J. Pain 14:T51–62, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schiffman EL, Ohrbach R, Truelove EL, Tai F, Anderson GC, Pan W, Gonzalez YM, John MT, Sommers E, List T, Velly AM, Kang W, Look JO. The Research Diagnostic Criteria for Temporomandibular Disorders. V: methods used to establish and validate revised Axis I diagnostic algorithms. J. Orofac. Pain 24:63–78, 2010 [PMC free article] [PubMed] [Google Scholar]
- 51.Schmitter M, Kares-Vrincianu A, Kares H, Bermejo JL, Schindler HJ. Sleep-associated aspects of myofascial pain in the orofacial area among Temporomandibular Disorder patients and controls. Sleep Med 16:1056–1061, 2015 [DOI] [PubMed] [Google Scholar]
- 52.Schulz A, Israel B, Williams D, Parker E, Becker A, James S. Social inequalities, stressors and self reported health status among African American and white women in theDetroit metropolitan area. Soc. Sci. Med 51:1639–1653, 2000 [DOI] [PubMed] [Google Scholar]
- 53.Slade GD, Ohrbach R, Greenspan JD, Fillingim RB, Bair E, Sanders AE, Dubner R, Diatchenko L, Meloto CB, Smith S, Maixner W. Painful Temporomandibular Disorder: Decade of Discovery from OPPERA Studies. J. Dent. Res 95:1084–1092, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Slopen N, Lewis TT, Williams DR. Discrimination and sleep: a systematic review. Sleep Med 18:88–95, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smith MT, Perlis ML, Carmody TP, Smith MS, Giles DE. Presleep cognitions in patients with insomnia secondary to chronic pain. J. Behav. Med 24:93–114, 2001 [DOI] [PubMed] [Google Scholar]
- 56.Sullivan MJ, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychol. Assess:524-532, 1995 [Google Scholar]
- 57.Sullivan MJ, Thorn B, Haythornthwaite JA, Keefe F, Martin M, Bradley LA, Lefebvre JC. Theoretical perspectives on the relation between catastrophizing and pain. Clin. J. Pain 17:52–64, 2001 [DOI] [PubMed] [Google Scholar]
- 58.Tan G, Jensen MP, Thornby J, Anderson KO. Ethnicity, control appraisal, coping, and adjustment to chronic pain among black and white Americans. Pain Med. 6:18–28, 2005 [DOI] [PubMed] [Google Scholar]
- 59.Tan G, Jensen MP, Thornby JI, Shanti BF. Validation of the Brief Pain Inventory for chronic nonmalignant pain. J. Pain 5:133–137, 2004 [DOI] [PubMed] [Google Scholar]
- 60.Thorn BE, Day MA, Burns J, Kuhajda MC, Gaskins SW, Sweeney K, McConley R, Ward LC, Cabbil C. Randomized trial of group cognitive behavioral therapy compared with a pain education control for low-literacy rural people with chronic pain. Pain. 152:2710–2720, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Whinnery J, Jackson N, Rattanaumpawan P, Grandner MA. Short and long sleep duration associated with race/ethnicity, sociodemographics, and socioeconomic position. Sleep. 37:601–611, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]