Abstract
Intraventricular hemorrhage in the setting of prematurity remains the most common cause of acquired hydrocephalus. Neonates with progressive post-hemorrhagic hydrocephalus are at risk for adverse neurodevelopmental outcomes. The goal of this review is to describe the distinct and often overlapping types of brain injury in the preterm neonate, with a focus on neonatal hydrocephalus, and to connect injury on imaging to neurodevelopmental outcome risk. Head ultrasound and magnetic resonance imaging findings are described separately. The current state of the literature is imprecise and we end the review with recommendations for future radiologic and neurodevelopmental research.
Introduction
The goal of this review is to describe the distinct and often overlapping types of brain injury in the preterm neonate, with a focus on neonatal hydrocephalus, and to connect injury on imaging to neurodevelopmental outcomes. The current state of the literature is imprecise. Disparate types of brain injury are often conflated and neuroimaging prediction models often group brain injury by severity, not specific pathology. This complicates neurodevelopmental risk assessment for both babies with isolated injuries and multiple concurrent injury patterns. Types of preterm brain injury include: periventricular leukomalacia (PVL), punctate white matter lesions, and germinal matrix hemorrhage/intraventricular hemorrhage (GMH/IVH) [1], periventricular hemorrhagic infarction (PVHI) or subsequent posthemorrhagic hydrocephalus. Brain injury in the neonate can be assessed through multiple modalities, including head ultrasound (HUS) and magnetic resonance imaging (MRI), each with differing sensitivity and specificity for detection of injury and prediction of outcome.
Despite advances in perinatal and neonatal care, including prenatal corticosteroids and a focus on neuroprotective measures in the first 72 hours of life, IVH in the setting of prematurity remains the most common cause of acquired hydrocephalus. The original IVH classification system was developed by Papile in 1978 based on computed tomography scans of 46 very low birth weight (VLBW, <1500 g) infants (Table 1) [2]. “Grade IV” hemorrhage is now known to be caused by obstruction of venous drainage with subsequent venous infarction and hemorrhage into the surrounding tissue (PVHI), not extension of the original hemorrhage. It is important that PVHI be described separately from other forms of IVH as it has independent risk for outcome. Current radiology practice for HUS assessment is to describe germinal matrix and intraventricular hemorrhage as seen in Table 1 [3–7].
Table 1.
| IVH classification systems | |||
|---|---|---|---|
| Papile system | Current system | ||
| Grade I | Subependymal hemorrhage | Grade 1 | Confined to germinal matrix |
| Grade II | IVH without ventricular dilation | Grade 2 | IVH without ventricular dilation |
| Grade III | IVH with ventricular dilation | Grade 3a | IVH acutely distending the ventricles |
| Grade IV | Extensive IVH with parenchymal involvement | Periventricular hemorrhagic Infarctiona (PVHI) | Venous infarction in area ipsilateral to IVH |
Grade 3 and PVHI considered “severe” IVH
Hydrocephalus is a “mechanical complication of many different conditions as well as a disease itself. It results from any hydrodynamic process by which the cerebrospinal fluid (CSF) compartment is actively enlarged, with resulting damage to the brain tissue” [6]. Grading of ventriculomegaly can be done by subjective assessment (mild, moderate, severe), quantification of largest lateral ventricular dimension (Table 2) [8, 9], or measurement of individual ventricular components in multiple dimensions (Table 3) [10–15].
Table 2.
Ventriculomegaly size classification and corresponding centimeter dilation of lateral ventricle [8, 9]
| Degree of ventriculomegaly | Dilation (cm) of lateral ventricle |
|---|---|
| Mild (Grade I) | 0.5–1 |
| Moderate (Grade II) | 1–1.5 |
| Severe (Grade III) | >1.5 |
Table 3.
| Measurements of ventricle size, resistive index on HUS | |
|---|---|
| Ventricular index (VI) | Distance from lateral wall of the lateral ventricle body to the falx |
| Anterior horn width (AHW) | Distance from medial wall to floor of lateral ventricle at the level of the interventricular foramina of Monro |
| Thalamooccipital distance (TOD) | Depth of the occipital horn, or distance from outermost point of the thalamus at its junction with the choroid plexus to the outermost part of the occipital horn posteriorly in the oblique parasagittal view |
| Ventricle/brain (V/B) ratio | (Vl-left + VI-right)/biparietal diameter |
| Resistive index (RI)a | (Systolic pressure—diastolic pressure)/systolic pressure |
Preterm infants have statistically significant higher baseline RI values in comparison to term infants (0.7 in preterm, 0.66 in term). With compression these numbers remain stable (0.71 and 0.68, respectively), indicating effective autoregulation regardless of gestational age
Post-hemorrhagic hydrocephalus of prematurity (PHHP) is distinct from post-hemorrhagic ventricular dilation (PHVD). PHHP implies CSF is under pressure, as shown by progressive macrocephaly and other symptoms of elevated intracranial pressure, such as bradycardia, and typically requires intervention [16]. The pathophysiology of PHHP and PHVD are active areas of investigation. It is estimated that one-third to half of infants with severe IVH develop PHVD [17, 18]. Approximately 10% of all neonates with IVH and 20% of infants with severe IVH will need surgical intervention [17, 18].
Early recognition of PHHP is important as progressive ventricular dilation associated with increased intracranial pressure (ICP) can alter cerebral hemodynamics and delay myelination [19], effects that are somewhat reversible by CSF drainage [20]. A progressive ICP increase is what distinguishes hydrocephalus from PHVD, and from ex-vacuo ventriculomegaly, compensatory enlargement of the ventricles due to cerebral or cerebellar tissue loss.
Infants with PHHP requiring shunt placement when <6 months of age are at highest risk for shunt failure [21], although in the context of regionalized differences in the United States. Earlier shunt placement is associated with more subsequent procedures [22], each associated with a likely risk of further damaging neural progenitors. Neonates with progressive PHHP requiring neurosurgical intervention are generally at greatest risk for adverse neurodevelop-mental outcomes, compared to those with stabilized PHVD [23, 24], persisting to school age [25]. Abnormal motor outcomes with PHHP and PHVD include a spectrum from cerebral palsy (CP) to minor neuromotor dysfunction/developmental coordination disorder. Other areas of neurodevelopment impacted include intellectual disability, fine motor coordination problems, memory and executive deficits, chronic pain, behavior problems, depression and anxiety, and attention deficit/hyperactivity disorder. Additionally, cortical visual impairment, or visual impairment caused by disturbance of posterior visual pathways, likely though impaired development of the dorsal visual stream [26], is an underappreciated disability in preterms, with an increased risk of those who have PVHI [27]. Studies are mixed on the magnitude and type of these deficits [28–32].
We aim to describe the state of the literature on neonatal PHHP and PHVD, focusing on imaging findings correlating to later neurodevelopmental outcomes. Although there is a lack of precision in the literature, specific terminology has been used when possible.
Methods
For this review, we searched Pubmed and the Cochrane library for English-language articles from January 1, 2007 to 2017. A four concept search was performed with the included concepts of hydrocephalus/ventriculomegaly, neuroimaging, neonate/infant, and prognosis/outcome. Relevant MESH/keywords were also added for each concept. The initial search yielded ~550 articles. Additional relevant articles were identified via hand-search from references found through the initial search. Articles were evaluated, summarized, and grouped according to imaging modality.
Results
Head ultrasound
HUS is a non-invasive bedside technique that was first used in the 1970s [9, 10] and remains the standard in neonatology for detection and monitoring of brain injury. HUS is most commonly used to screen for and monitor progression of GMH/IVH in the preterm infant, with highest risk groups including VLBW and extremely low birth weight (<1000 g) infants, particularly those <32 weeks gestational age at birth. Up to 50% of GMH/IVH occur in the first day of life and up to 90% in the first 4 days [4]. HUS is able to show larger cerebellar hemorrhages, particularly when mastoid views are employed, however MRI has higher sensitivity to detect smaller cerebellar hemorrhages [33].
Both high grade IVH and white matter injury increase risk for later ventriculomegaly and poor brain growth [32, 34–36]. Following IVH, HUS is more reliable to detect ventriculomegaly than clinical assessments of fontanelle exam or serial head circumferences [37]. Measuring head circumferences at regular intervals is common practice; however, the criterion of a 1.5–2 cm/week increase in head circumference as an indicator of PHHP has been shown to be unreliable across multiple studies when compared with HUS imaging [38, 39]. After all, preferential head growth after growth restriction and chronic illness can be difficult to distinguish from head growth from ventriculomegaly without imaging confirmation.
White matter injury characterization on HUS
HUS is used to follow white matter injury and the sequelae of PVL in preterm infants. PHVD is associated with an increase in PVL and PVHI 29-fold in premature infants, compared to ninefold with Grade I–II IVH without dilation [40].
Intraparenchymal white matter lesions in the form of cystic lesions and hyperechogenic areas are detectable on HUS; in neonates with ventriculomegaly they are often located at the level of the frontal horns or along the body of the lateral ventricles/trigone region [40, 41]. These can be the result of axonal damage and demyelination due to compression and stretching of white matter tracts [35]. White matter echogenicity is less reliably detected on HUS than MRI [1, 42].
PVL has two neuropathologic components, both a focal periventricular necrotic component and diffuse gliosis in the surrounding white matter. Cystic PVL develops secondary to focal necrosis in the white matter [1, 43]. Confident HUS diagnosis of PVL is only possible once cystic lesions develop [44], as it does not reliably detect diffuse white matter gliosis [1]. See Table 4 for the HUS classification system of PVL.
Table 4.
Grading of periventricular leukomalacia [44]
| PVL classification system | |
|---|---|
| Grade I | Periventricular echodensities (7 days or more) |
| Grade II | Periventricular echodensities evolving into localized frontoparietal cystic lesions |
| Grade III | Extensive periventricular cystic lesions |
| Grade IV | Cystic lesions extend into deep white matter, subcortical cystic lesions |
HUS can accurately detect PVHI but is not as sensitive to characterize diffuse white and gray matter abnormalities or white matter petechial hemorrhages [42].
Measurements of ventricle size on HUS
HUS ventricle size measurements are reliable and correlate with MRI volumes [45]. Premature neonates may have multiple factors contributing to ventriculomegaly: diffuse white matter injury resulting in parenchymal volume loss and ex-vacuo ventriculomegaly, in addition to, or compounded by, pressure-driven dilation following IVH. The magnitude and pattern of dilation, combined with clinical examination, can help differentiate etiology and guide intervention.
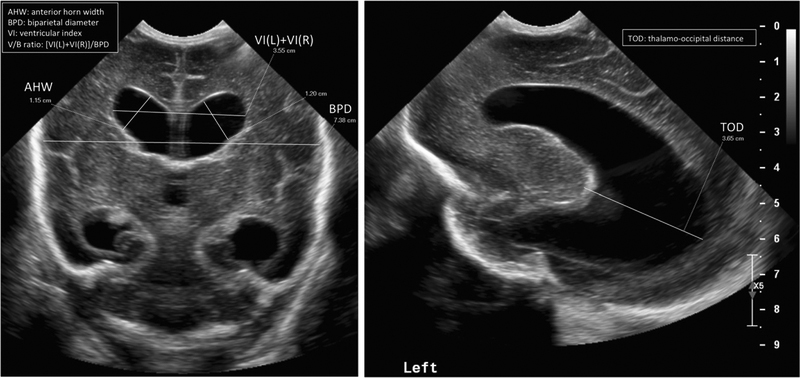
Classification of ventriculomegaly considers mostly overall dilation of the lateral ventricles (Table 3), but individual reference ranges have been established for multiple dimensions. Measurements for the ventricular index (VI), anterior horn width (AHW), and thalamooccipital distance (TOD) have been published and are detailed in Table 3 [10–15, 34]. Intra- and interobserver agreement for degree of ventriculomegaly and these measurements is good across studies [46–48].
PHVD is typically defined by a VI>97th% (2 SD) for gestational age, AHW > 3–6 mm, or TOD > 24.7 mm [21, 49]. VI and AHW values greater than normal (VI >2 + SD and AHW > 6) have been used as cutoffs for surgical intervention [49]. Other groups have compared this typical PHVD cutoff to a higher one (VI > 97th percentile + 4 mm and AHW > 10 mm) [22]. An increase in AHW may be a more sensitive marker for early enlargement; it is seen clinically as the rounding of the frontal horns. The TOD may also be a subtle initial marker of progressing ventricular size in preterm children; the occipital horn is often the first to enlarge in PHVD and may also dilate first in cases of congenital hydrocephalus. Baseline TOD ranges vary across studies [11–13] and isolated occipital horn dilation may also occur in extremely low birth weight infants without IVH [14]. Abnormal ventricle/brain ratios > 0.35 on ultrasound are associated with dilated horns of the lateral ventricles, larger ventricular volumes, and smaller volumes of total brain tissue, cerebrum, and cerebellum on term MRI [50]. See Table 3 and Fig. 1. PHVD is associated with “ballooning” of the ventricles and dilatation of the anterior as well as the posterior part of the ventricles, in contrast to the irregular ventricular margins and predominant dilatation of the occipital horns that serves as a hallmark of ex-vacuo ventriculomegaly [34].
Fig. 1.
Head ultrasound ventricular size measurements
Assessments of third and fourth ventricle sizes can also be performed with HUS, although exact dimensions are challenging to reliably measure between observers [12–14]. Isolated dilatation of the third or fourth ventricle can be associated with posterior fossa hemorrhage [12]. Normative reference values per gestational age for both the third and fourth ventricles are scarce.
It is important to note that preterm infants may develop a larger ventricular size at term-corrected age compared with term infants, even without structural brain injury, probably due to parenchymal volume loss from periventricular white matter and subcortical gray matter injury [12, 34, 43].
Cerebral hemodynamics on HUS: Doppler parameters
Doppler parameters are a tool to assess compliance and intracranial pressure in a non-invasive way. The resistive index (RI) can be measured with and without pressure exerted on the transducer over the fontanelle, effectively transiently increasing the intracerebral volume (Table 3) [51]. In neurologically normal term and preterm infants, RIs with and without compression should have insignificant differences [51].
In post-hemorrhagic hydrocephalus there is an increase in RI caused by an increase in the maximum systolic flow rate and decrease in the end-diastolic flow rates [52, 53]. An increase in RI is also seen with pressure on the transducer; the poor compliance of the subarachnoid space leads to an impaired autoregulatory response and failure to accommodate to the increase in ICP [51].
RI values in the anterior cerebral artery have been positively correlated with increased ICP in hydrocephalic infants [52–55]. Studies have demonstrated both elevated intracranial RIs in infants with hydrocephalus, and subsequent decreases in RI after ventricular tap or shunt [56, 57].
Neurodevelopmental correlates of HUS parameters
Ventriculomegaly is associated with an increased risk for both cognitive and motor sequelae due to associated acute ischemia, hypoxia, and inflammation of both important local periventricular as well as distally related white matter structures. When chronic, ventriculomegaly is associated with gliosis, demyelination, and axonal degeneration [58]. Periventricular brain structures, such as corticospinal tracts, are involved in perceptual-motor integration, sensory integration, and quality of movement. Periventricular white matter damage reduces cortical volume and can damage areas involved in memory, executive function, and language [59].
Motor sequelae
Larger lateral ventricles are independently related to decreased motor score at 2 years (fourfold increased risk with moderate/severe ventriculomegaly per visual template) [59], and to progressive increases in risk per each additional 1 mm in lateral ventricle parameters such as AHW and midbody height [60]. In PHVD, higher rates of gross motor abnormalities like CP as well as decreased fine motor manipulative abilities are even more frequent [28, 61], and likely secondary to cumulative risks from ventriculomegaly, IVH, and additional white matter injury.
Cystic white matter injury, ventriculomegaly, and IVH are strongly and significantly associated with quadriplegia (risk ratio: 24, 17, 5.1), hemiplegia, (risk ratio: 29, 17, and 5.8), and diplegia (risk ratio: 5, 5.7, and 2.3), respectively [41]. Importantly for children with PHVD and white matter injury, these risks seem to be additive; preterm infants with IVH both with larger extent of hemorrhage (two lobes involved on the worst side) and PHHP requiring shunt placement [31, 32], have higher rates of CP. Preterm infants with PHVD and a higher burden of periventricular lesions, especially bilateral, are at further increased risk for CP/severe motor impairment [29, 31, 40, 41].
Parenchymal involvement may be the main factor in determining motor risk for infants with PHVD. In one study, there was an 80% prevalence of CP in a group of shunted preterm infants with PVHI, but no cases of CP in those shunted for grade III IVH without PVHI [31]. Extensive PVHI is independently associated with CP, and risk for severe CP increases with concomitant GMH and cystic PVL [31]. The risk of severe CP also increases with grade of PVL; 10% of children with grade I PVL develop spastic diplegia by school age, increasing to almost 50% of children with cystic PVL [44]. The location and size of the cysts are important; cystic lesions of ≥3 mm in the parietooccipital periventricular white matter are highest risk for motor delay [62, 63].
Cognitive sequelae
IVH, ventriculomegaly, and white matter disease are also important risk factors for cognitive delay in preterm infants. Although some studies analyze these pathologies separately, many combine them to assess risk, making prediction for individual infants difficult.
Moderate/severe ventriculomegaly is independently associated with a significant increase in risk for cognitive delay and later decrease in intelligence quotient [60, 62–64]. Infants with ventriculomegaly (without IVH) have a two- to threefold higher risk of both neurodevelopmental and cognitive impairment and the composite outcome of death or neuro-developmental impairment [65].
Presence of both ventriculomegaly and white matter disease on HUS (PVL, PVHI) multiplies risks for cognitive disabilities. Large proportions (50–80%) of children with PVHI are diagnosed with intellectual disability at school age [66] and are at higher risk for clinical or subclinical behavior problems [28]. Bilateral (versus unilateral) PVHI on HUS may portend worse cognitive outcomes [69], although some found no difference [28, 31]. Visual challenges including cortical visual impairment are also associated with severe cystic PVL [27, 67].
Severe IVH (grade III and PVHI) has been associated with increased needs for special education support in reading, writing, and mathematics [36]. But there is conflicting data regarding cognitive outcomes of preterm infants with PHHP; studies report varying rates of cognitive impairment [28–30].
Some studies suggest that early treatment of PHVD, before development of symptoms of PHHP, may result in improved developmental outcomes [49]; however, others have shown no significant difference in later outcome in early versus late intervention. Other studies have compared different magnitudes of early treatment thresholds and found no long-term developmental outcome differences [22].
Limitations of neurodevelopmental outcome prediction by HUS
The positive predictive value of HUS for CP is lower than that of MRI [68]; comparative sensitivities and specificities vary by study.
Most of the studies cited above, including the ELGAN [36] and Indomethacin Intraventricular Hemorrhage Prevention Trial [36], characterize ventriculomegaly as mild, moderate, and severe without specific measurements of ventricle size. Although visual representations are reliable across examiners, it is difficult to determine if ventriculomegaly for these infants was predominantly ex-vacuo or PHHP without the combination of quantified radiographic measurements and clinical findings. Objective measurements should be used for prognostication in future studies. Children with severe IVH and ventriculomegaly often have multiple HUS abnormalities; in the ELGAN study of infants <28 weeks, only 32% of infants with IVH had no other ultrasound abnormality, emphasizing the difficulty inherent in attempting to analyze only PHVD in the setting of other compounding injuries [59]. Additionally, in many of the above studies ventriculomegaly is grouped in a category of “severe brain injury” with other anomalies such as cystic white matter injury, limiting prognostication for individual patients with isolated PHVD.
Finally, HUS imaging does not fully capture risk; in one study of preterms with normal HUS, 23% had cognitive delay and 26% had motor delays at age 2 [59].
Magnetic resonance imaging
Neonatal MRI sequences include T1- and T2-weighted sequences, diffusion-weighted imaging, and inversion recovery sequences or FLAIR. The most common current clinical uses for these sequences in pediatric hydrocephalus are in “qualitative MRI,” delineating white and gray matter abnormalities. Of note, ultrafast/Haste MRI sequences used to monitor progression of hydrocephalus and verify shunt placement do not obtain well-defined pictures of white matter injury. Other MRI modalities are referred to as “quantitative MRI”. Quantitative MRI involves numerical measurements and the construction of quantitative maps and complex statistical images of the brain [1].
Qualitative MRI
Measurements of ventricle size on MRI
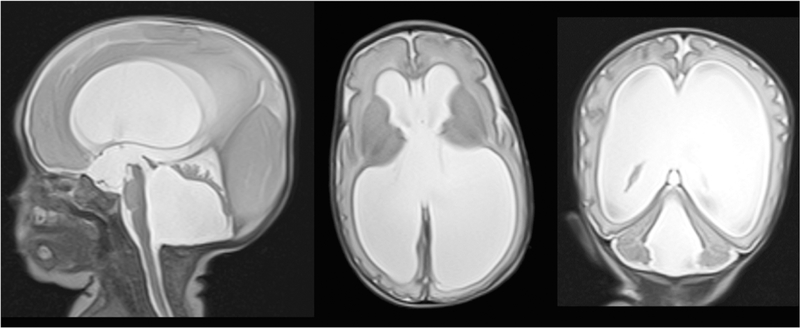
MRI can assess lateral ventricular size in a similar fashion as HUS, with measures such as VI and V/B ratios. When ventriculomegaly is qualitatively described, like on HUS, it is described as mild, moderate, or severe. MRI is superior to HUS when assessing the total burden of ventriculomegaly as it can better visualize the 3rd and 4th ventricles, assessing posterior fossa crowding and 4th ventricle outlet obstruction and dilation, which can be seen in PHVD. Intraventricular obstruction may happen secondary to altered CSF flow dynamics after shunting or anatomical changes after IVH [70], unfortunately there is not sufficient literature on the neurodevelopmental importance of 3rd or 4th ventricle dilation. See Fig. 2 for an example of a T2-weighted MR image of a neonate with post-hemorrhagic hydrocephalus.
Fig. 2.
T2-weighted MR images of premature infant with posthemorrhagic hydrocephalus. There is marked enlargement of all four ventricles. The sagittal image shows marked compression of the cerebellum secondary to fourth ventricular dilatation. The axial and coronal images show thinning of the cerebral mantle and fenestration of the septum pellucidum due to marked dilatation of the lateral ventricles
White matter characterization and classification on MRI
MRI is often used to qualitatively describe white matter lesions associated with ventriculomegaly, including punctate lesions, white matter signal abnormalities, PVL, and PVHI. MRI can also describe the integrity of white matter tracts such as the corpus callosum (CC) [1].
These individual white matter components can be scaled and entered into scoring systems with multiple variables to give overall brain injury scores such as the Kidokoro system [71]. Although these systems include magnitude of ventricle dilation and other important white matter descriptors, they can be difficult to complete in severe hydrocephalus due to the distortion of white matter.
Neurodevelopmental correlates of white matter assessment
Qualitative MRI at term-corrected age for former preterm infants has good prognostic value for predicting CP as mentioned earlier [69, 70]. Moderate to severe white matter abnormalities including PVL are most indicative of later motor disability.
Lower developmental quotient and intellectual quotient scores seen in children with PHVD [28, 59] may be due to concomitant white matter injury. Very preterm and VLBW infants with moderate to severe white matter injury have, in addition to higher risk for global cognitive disability, a specifically increased risk of challenges in executive function [72] and language [73]. White matter injury in very preterm infants is associated with widespread alterations to CC microstructural development that affect later neurodevelopment [74], reviewed in volumetric and diffusion tensor imaging (DTI) sections below.
Quantitative MRI
Volumetric assessment
Three-dimensional precise brain volumes can be obtained using MRI, using an algorithm that classifies given brain areas into myelinated and unmyelinated white matter, cortical and deep gray matter, brainstem, cerebellum, and CSF based on T1- and T2-weighted sequences. Volume is recorded in voxels (volumetric pixels) [75].
VLBW infants with PHVD requiring ventriculoperitoneal shunts have both smaller total brain volumes and larger ventricular volumes than VLBW comparisons [77, 78]. Premature infants with neurodevelopmental impairment have significantly lower baseline MRI volumes of total brain tissue, cerebrum, frontal lobes, basal ganglia and thalami, and cerebellum, and higher ventricular volumes compared to those without impairment [79, 80].
Severe IVH is associated with decreased cross-sectional areas of white matter tracts such as the CC. The CC is commonly damaged in PHVD as it forms the roof of the anterior horns, the body, and part of the posterior horns of the lateral ventricles. The most anterior and posterior parts of the CC seem to be most vulnerable, possibly because they are locations of active organization and premyelination. Children with a history of large IVH have thinning and decreased total area of the CC. Preterm children with white matter injury also have alterations in volume distribution in the CC [76].
Gray matter volume on MRI is decreased in the setting of IVH without ventriculomegaly [75] and in PHVD [77]. Deep gray matter growth failure in preterm infants, predominantly affecting lentiform nuclei and thalamic volumes, is more pronounced with white matter lesions [80]. Deep gray matter volume reduction in hydrocephalus may be secondary to the hemodynamic effects of elevated ICP. PHVD may also interrupt the ventral ganglionic supply of neuron precursors to the thalamus and injure the cortical subplate, disturbing further thalamocortical tract development [4, 43]. There may be important disparities in gray matter by location [79]; one study demonstrated an increase in gray matter frontal lobe volume and a decrease in most temporal foci in former very preterm infants [80].
PHVD is independently associated with decreased volumes of the cerebellum on term equivalent MRI, despite early intervention with shunting [77]. More severe degrees of supratentorial IVH are associated with slower cerebellar growth [33]. Different mechanisms for impaired cerebellar growth with IVH include diaschisis after parenchymal brain injury (loss of function due to input deprivation and output isolation due to damage to brain area neuronally connected to cerebellum) or injury from free radical damage and inflammation due to degrading blood products in the CSF [33].
Neurodevelopmental correlates of conventional MRI volumetric assessment
Larger total brain tissue volumes are associated with higher cognitive and language scores, and higher volumes of white matter volume are positively associated with motor outcome and processing speed [79–81]. Volume reduction of the dorsolateral prefrontal cortex, sensorimotor, parietooccipital, and premotor cortices have been associated with poorer working memory performance, even when other brain injury is accounted for [83]. Higher ventricle volumes are associated with decreased cognitive performance in preterm children with IVH and other brain injury [72, 81]. After adjusting for intracranial cavity volume, very preterm children with persistent attention/hyperactivity problems had 4% less total cerebral tissue and 36% more CSF than preterm comparisons, and 8% less total tissue and 144% more CSF than full term children without attentional issues [84].
Both IVH and white matter injury are associated with decreased posterior CC area. Decreased posterior white matter volume may be particularly important for the neurodevelopmental outcomes for children with hydrocephalus. Deficits in visuospatial and motor function and other nonlanguage skills are well-described deficits in hydrocephalus [82]. Posterior cortical mantle thinning, decreased CC and white matter tract area, and greater posterior CSF percentages are associated with poorer visuospatial skills and nonverbal intelligence [76, 85–87]. The posterior cortex mediates visuospatial activity and the posterior splenium of the CC connects the primary and secondary visual cortex [86, 88]. Damage to these structures may lead to decreased connectivity between the posterior parietal, temporal, and occipital cortical regions associated with sensory processing, language, memory, hearing, and vision [76].
PHVD is associated with altered gray matter volumes, but research on the associated neurodevelopmental consequences is mixed. In one study, premature infants with significantly reduced cortical and deep nuclear gray matter volumes exhibited moderate to severe neurodevelopmental disability at 1 year [89]. Keunen alternatively demonstrated that higher cortical gray matter volumes were associated with worse motor performance and cognition at 24 months and with developmental quotients at age 3.5 years [79].
Decreased cerebellar volumes in PHVD have important neurodevelopmental effects as the cerebellum is involved in motor planning, language, and cognition; higher volumes are associated with improved scores in these areas, even after adjustment for perinatal factors [79–81]. In VLBW infants, smaller cerebellar volumes are associated with poor executive function and motor skills [90]. Smaller cerebellar volumes in moderate to late preterm infants are correlated with lower cognitive and language scores at 2 years [81]. Deformation of the cerebellum in conditions such as hydrocephalus may also disturb the kinestheticproprioceptive basis of hand control, and stretching of the CC may impair bimanual motor function [85–88].
Limitations of neurodevelopmental outcome prediction by MRI
Premature infants have multiple processes that lead to large ventricles: ex-vacuo ventriculomegaly, PHVD, and PHHP. These processes often coexist, but most MRI studies group preterm infants with large ventricles together. For example, in one study preterm brain injury on MRI was graded from 0 to 3 for five criteria: the nature and extent of WM signal abnormality; periventricular WM volume loss; the presence of any cystic abnormalities; ventricular dilatation; and thinning of the CC [71]. All of these types of white matter injury are common and relevant to the infant with PHVD; however, there is a lack of subset analysis to give us specific information about neurodevelopmental outcomes following PHVD.
ADC mapping
Diffusion-weighted imaging can describe white matter microstructure. White matter apparent diffusion coefficients (ADCs) are measured at selected regions of interest. ADCs should decrease with postmenstrual age, likely due to the increase in restricted diffusion from an increase in size and cell density of the brain. There are increased white matter ADC values confined to posterior white matter in cases of PHVD, specifically bilateral occipital and parietooccipital regions and the left parietal region. This may correlate with the particular vulnerability of the parieto-occipital region for periventricular white matter injury. Notably, Grades II-III IVH were not associated with increased ADC values in these areas [77].
Neurodevelopmental correlates of ADC mapping
Increased frontal and occipital white matter ADCs have been described for infants with progressive, symptomatic hydrocephalus [57]. Increased white matter ADC values in infants with PHVD may reflect an increase of the extracellular water compartment due to impaired interstitial fluid absorption. ADC values can decrease following shunting [91]. Cerebellar ADC values on term diffusion-weighted imaging are associated with gross motor outcome at 2 years corrected age [92]. The combination of ADC mapping with other known predictive factors such as term-corrected HUS can combine to improve prognostication for motor outcome in preterm infants [92].
Diffusion tensor imaging
DTI uses the directional preference of molecular water diffusion to describe underlying fiber density and myeli-nation. It increases the predictive value for later neurodevelopmental outcomes by quantifying white matter integrity. As the water content of the preterm brain decreases with maturation, diffusion becomes increasingly restricted to the axes of developed fiber tracts [93]. Some of the recorded diffusivity measures are: fractional anisotropy (FA), axial diffusivity (AD), radial diffusivity (RD, and mean diffusivity (MD). FA describes the magnitude of asymmetry in water diffusion. FA values are higher in tissues with structures organized in a common direction, such as white-matter tracts [93]. AD and RD describe the diffusivity of water parallel and perpendicular to fiber bundles, respectively. MD reflects the amplitude of water diffusion and is the average of axial and radial diffusivity. Increasing AD reflects increases in axonal number, size, or reduction in interaxonal space [94–96]. Increases in RD may indicate changes in myelin that reduce permeability [93, 96, 97].
Brain development is associated with increasing anisotropy in white matter tracts. Premature newborns should have decreasing MD (with RD decreasing more rapidly than AD) and increasing FA as they move towards term-corrected age. In the first 2 years of life FA should increase with postnatal myelination as white matter progenitors mature. VLBW infants at term-corrected age have temporal-spatial differences in white matter development; they have lower RD and higher FA values in posterior vs. anterior and central vs. peripheral regions within the corona radiata, CC, and internal capsule. This is suggestive of more advanced development in posterior and central areas at term [98, 99]. There is also a male-female DTI difference in preterms; males have a higher CC splenium FA and genu MD [100]. Preterm children with ventriculomegaly (as well as moderate to severe white matter injury) tend to have a slower increase of FA values and higher MDs than preterm comparisons [101].
In term infants with hydrocephalus, the CC has significant decreases in FA and increases in MD, AD, and RD [102–106]. These changes reflect impairment of the oligodendrocyte lineage, decreased fiber coherence and/or axonal damage [105]. Compression of the white fibers by fluid-filled ventricles results in increased parallel diffusion along the fibers (AD). Elevated RDs may reflect abnormal oligodendrocyte development, reducing barriers to perpendicular diffusion [106, 107].
Ventricular dilation causes compression of the posterior limb of the internal capsule (PLIC), resulting in a decrease in the extra-cellular space and increase in fiber crowding, coherence, and alignment among the PLIC [100, 102]. Multiple studies show that in term children with hydrocephalus, PLIC FA values are significantly lower than comparisons [103, 104, 106]. PLIC AD values of the hydrocephalus group have been described as higher [103, 104, 106] than non-hydrocephalic cohorts.
Tract-specific diffusion measurements in premature newborns without hydrocephalus have been published [93, 108, 109]. There are many studies assessing DTI changes in term infants with congenital hydrocephalus but a lack of DTI studies in preterm infants with hydrocephalus, particularly those with post-hemorrhagic hydrocephalus.
Neurodevelopmental correlates of DTI
Higher diffusivity values in the splenium of the CC (sCC) are associated with lower motor scores at 2 years in preterm infants [76]. Motor coordination relies upon efficient visual processing, and decreased integrity of callosal tracts involved in the lateralization of vision might play an important role [76]. Importantly, months after ventricular shunting one can see normalization of the FA in the genu of the CC (gCC) [104, 107] and RD in the gCC and sCC [107].
Elevated MD and RD values in the PLIC correlate with motor outcomes [106]. ELBW neonates without hydrocephalus with higher MD PLIC scores had lower motor scores at 18–22 months [112]. In preterm infants without hydrocephalus, there is a strong negative correlation between PLIC FA and Gross Motor Function Classification System score at 4 years of age [100]. Alternatively, Arzoumanian et al. showed a reduction in the FA of the posterior limb of the internal capsule (PLIC) in neurologically abnormal preterm infants compared with controls [110]. ELBWs with higher MD PLIC scores had lower cognitive scores [112].
Serial DTI evaluation can also be diagnostic over time as well as prognostic: DTI changes normalize with time in benign external hydrocephalus, distinguishing it from progressive hydrocephalus [111].
Future directions/techniques
There are numerous new techniques in development for the assessment of children with hydrocephalus. Elastography examines brain tissue stiffness; it may helpful to assess relative compliance in cases of worsening hydrocephalus and increasing ICP. Functional MRI, graph theory, and 3D MRI are techniques that have been used to examine pre- and post-shunt differences in connectivity and changes in white and gray matter.
Conclusions
Both HUS and MRI are helpful with assessment of neuro-developmental risk in the setting of preterm hydrocephalus, but there are limitations of this literature. Many studies do not use specific measurements of ventricle size and do not include clinical information on individual patients, both of which would help delineate PHHP from PHVD, for instance. Patterns of injury that are associated with ventricular enlargement (i.e., IVH, PVL) should be described separately in future research in light of disparate pathophysiologic basis. More outcome studies are needed for preterm infants with PHVD; most existing literature involves large groups of low birth weight or gestational age cohorts with PHVD children included but not separately analyzed. There are far more focused imaging studies on congenital hydrocephalus in the term infant. Lastly, neurodevelopmental outcome measures are most commonly done at 18 months or 2 years of age, prior to school-age when demands increase and a full understanding of cognitive burden can be assessed.
Our recommendations include:
Use precise radiologic terms when describing brain injury (e.g., hyperechogenic white matter lesion, not echodensity) and classify distinct patterns of brain injury into categories according to etiology, whenever possible, and severity.
Use more precise definitions when dealing with overlapping diagnoses: ventriculomegaly, PHVD, PHHP/hydrocephalus are not interchangeable.
Use measurements of lateral ventricle size as VI, AHW, TOD, and V/B ratio to classify ventricular enlargement.
Standardized neonatal neurologic exams would be an important addition to the neurosurgical literature to monitor clinical progression of PHVD.
More longitudinal outcomes are needed: 2 year outcomes are appropriate for most motor impairments, but are not sufficient for cognitive assessment, as individuals have not yet experienced the demands of school-age learning. Measuring components of cognition, especially subdomains of memory and executive function have a profound impact on quality of life and should be a priority in outcome studies in infants with PHVD and associated brain injury.
Acknowledgements
This project was supported by the Thomas Wilson Sanitarium For Children of Baltimore City awarded to Rebecca Dorner and Marilee Allen and the NIH T32 Training Grant (5T32HL125239-03) awarded to Rebecca Dorner.
Footnotes
Compliance with ethical standards
Conflict of interest The authors declare no conflict of interest.
References
- 1.Hinojosa-Rodríguez M, Harmony T, Carrillo-Prado C, Van Horn JD, Irimia A, Torgerson C, et al. Clinical neuroimaging in the preterm infant: diagnosis and prognosis. Neuroimage Clin. 2017;16:355–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Papile L, Burstein J, Burstein R, Koffier A. Incidence and evolution of subependymal and intraventricular hemorrhage in premature infants: a study of infants <1500 g. J Pediatr. 1978;92:529–34. [DOI] [PubMed] [Google Scholar]
- 3.El-Dib M, Massaro AN, Bulas D, Aly H. Neuroimaging and neurodevelopmental outcome of premature infants. Am J Perinatol. 2010;27:803–18. [DOI] [PubMed] [Google Scholar]
- 4.Volpe JJ. Neurology of the newborn. 5th ed. Philadelphia: Saunders/Elsevier; 2008. [Google Scholar]
- 5.Whitelaw A Intraventricular haemorrhage and posthaemorrhagic hydrocephalus: pathogenesis, prevention and future interventions. Semin Neonatol. 2001;6:135–46. [DOI] [PubMed] [Google Scholar]
- 6.Raybaud C MR assessment of pediatric hydrocephalus: a road map. Child’s Nerv Syst. 2016;32:19–41. [DOI] [PubMed] [Google Scholar]
- 7.Gould SJ, Howard S, Hope PL, Reynolds EOR. Periventricular intraparenchymal cerebral hemorrhage in preterm infants: the role of venous infarction. J Pathol. 1987;151: 197–202. [DOI] [PubMed] [Google Scholar]
- 8.Ment LR, Bada HS, Barnes P, Grant PE, Hirtz D, Papile LA, et al. Practice parameter: neuroimaging of the neonate: report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2002;58:1726–38. [DOI] [PubMed] [Google Scholar]
- 9.Pape KE, Blackwell RJ, Cusick G, Sherwood A, Houang MT, Thorburn RJ, et al. Ultrasound detection of brain damage in preterm infants. Lancet. 1979;1:1261–4. [DOI] [PubMed] [Google Scholar]
- 10.Levene MI. Measurement of the growth of the lateral ventricles in preterm infants with real-time ultrasound. Arch Dis Child. 1981;56:900–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brouwer MJ, de Vries LS, Groenendaal F, Koopman C, Pistorius LR, Mulder EJH, et al. New reference values for the neonatal cerebral ventricles. Radiology. 2012;262:224–33. [DOI] [PubMed] [Google Scholar]
- 12.Sondhi V, Gupta G, Gupta PK, Patnaik SK, Tshering K. Establishment of nomograms and reference ranges for intracranial ventricular dimensions and ventriculo-hemispheric ratio in newborns by ultrasonography. Acta Paediatr. 2008;97: 738–44. [DOI] [PubMed] [Google Scholar]
- 13.Davies MW, Swaminathan M, Chuang SL, Betheras FR. Reference ranges for the linear dimensions of the intracranial ventricles in preterm neonates. Arch Dis Child Fetal Neonatal Ed. 2000;82:218–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brouwer MJ, De Vries LS, Pistorius L, Rademaker KJ, Groenendaal F, Benders MJ. Ultrasound measurements of the lateral ventricles in neonates: why, how and when? A systematic review. Acta Paediatr. 2010;99:1298–306. [DOI] [PubMed] [Google Scholar]
- 15.Hartenstein S, Bamberg C, Proquitte H, Metze B, Buhrer C, Schmitz T. Birth weight-related percentiles of brain ventricular system as a tool for assessment of posthemorrhagic hydrocephalus and ventricular enlargement. J Perinat Med. 2016;44:179–85. [DOI] [PubMed] [Google Scholar]
- 16.Wellons JC, Shannon CN, Kulkarni AV, Simon TD, Riva-Cambrin J, Whitehead WD, et al. A multicenter retrospective comparison of conversion from temporary to permanent cerebrospinal fluid diversion in very low birth weight infants with posthemorrhagic hydrocephalus. J Neurosurg Pediatr. 2009;4:50–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robinson S Neonatal posthemorrhagic hydrocephalus from prematurity: pathophysiology and current treatment concepts. J Neurosurg Pediatr. 2012;9:242–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alan N, Manjila S, Minich N, Bass N, Cohen AR, Walsh M, et al. Reduced ventricular shunt rate in very preterm infants with severe intraventricular hemorrhage: an institutional experience. J Neurosurg Pediatr. 2009;10:357–64. [DOI] [PubMed] [Google Scholar]
- 19.Ayannuga OA, Shokunbi MT, Naicker TA. Myelin sheath injury in Kaolin-induced hydrocephalus: a light and electron microscopy study. Pediatr Neurosurg. 2016;51:61–8. [DOI] [PubMed] [Google Scholar]
- 20.Hanlo PW, Gooskens RJ, van Schooneveld M, Tulleken CA, van der Knaap MS, Faber JA, et al. The effect of intracranial pressure on myelination and the relationship with neurodevelopment in infantile hydrocephalus. Dev Med Child Neurol. 1997;39:286–91. [DOI] [PubMed] [Google Scholar]
- 21.Riva-Cambrin J, Shannon CN, Holubkov R, Whitehead WE, Kulkarni AV, Drake J, Hydrocephalus Clinical Research Network. et al. Center effect and other factors influencing temporization and shunting of cerebrospinal fluid in preterm infants with intraventricular hemorrhage. J Neurosurg Pediatr. 2012;9: 473–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Vries LS, Groenendaal F, Liem KD, Heep A, Brouwer AJ, van’t Verlaat E, et al. Treatment thresholds for intervention in posthaemorrhagic ventricular dilation: a randomised controlled trial. Arch Dis Child - Fetal Neonatal Ed. 2018. 10.1136/archdischild-2017-314206 [DOI] [PubMed] [Google Scholar]
- 23.Srinivasakumar P, Limbrick D, Munro R, Mercer D, Rao R, Inder T, et al. Posthemorrhagic ventricular dilatation—impact on early neurodevelopmental outcome. Am J Perinatol. 2013;30: 207–14. [DOI] [PubMed] [Google Scholar]
- 24.Adams-Chapman I, Hansen NI, Stoll BJ, Higgins R, NICHD Research Network. Neurodevelopmental outcome of extremely low birth weight infants with posthemorrhagic hydrocephalus requiring shunt insertion for the NICHD Research Network what’s known on this subject. Pediatrics. 2008;121:e1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holwerda JC, Van Braeckel KNJA, Roze E, Hoving EW, Maathuis CGB, Brouwer OF, et al. Functional outcome at school age of neonatal post-hemorrhagic ventricular dilatation. Early Hum Dev. 2016;96:15–20. [DOI] [PubMed] [Google Scholar]
- 26.Leung MP, Thompson B, Black J, Dai S, Alsweiler JM. The effects of preterm birth on visual development. Clin Exp Optom. 2018;101:4–12. [DOI] [PubMed] [Google Scholar]
- 27.Guzzetta A, Fiori S, Scelfo D, Conti E, Bancale A. Reorganization of visual fields after periventricular haemorrhagic infarction: potentials and limitations. Dev Med Child Neurol. 2013;55 (Suppl 4):23–6. [DOI] [PubMed] [Google Scholar]
- 28.Roze E, Van Braeckel KN, van der Veere CN, Maathuis CG, Martijn A, Bos AF. Functional outcome at school age of preterm infants with periventricular hemorrhagic infarction. Pediatrics. 2009;123:1493–500. [DOI] [PubMed] [Google Scholar]
- 29.Brouwer A, Groenendaal F, van Haastert I-L, Rademaker K, Hanlo P, de Vries L. Neurodevelopmental outcome of preterm infants with severe intraventricular hemorrhage and therapy for post-hemorrhagic ventricular dilatation. J Pediatr. 2008;152: 648–54. [DOI] [PubMed] [Google Scholar]
- 30.Goldstein RF, Cotten CM, Shankaran S, Gantz MG, Poole WK. Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Influence of gestational age on death and neurodevelopmental outcome in premature infants with severe intracranial hemorrhage. J Perinatol. 2013;33:25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsai A, Lasky R, John S, Evans P, Kennedy K. Predictors of neurodevelopmental outcomes in preterm infants with intraparenchymal hemorrhage. J Perinatal. 2014;34:399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ment LR, Vohr B, Allan W, Westerveld M, Katz KH, Schneider KC, et al. The etiology and outcome of cerebral ventriculomegaly at term in very low birth weight preterm infants. Pediatrics. 1999;104:243–8. [DOI] [PubMed] [Google Scholar]
- 33.Tam EW, Rosenbluth G, Rogers EE, Ferriero DM, Glidden D, Goldstein RB, et al. Cerebellar hemorrhage on magnetic resonance imaging in preterm newborns associated with abnormal neurologic outcome. J Pediatr. 2011;158:245–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brouwer MJ, de Vries LS, Pistorius L, Rademaker KJ, Groenendaal F, Benders MJNL. A systematic review. Ultrasound measurements of the lateral ventricles in neonates: why, how and when? Acta Paediatr. 2010;99:1298–306. [DOI] [PubMed] [Google Scholar]
- 35.Vollmer B, Roth S, Baudin J, Stewart AL, Neville BGR, Wyatt JS. Predictors of long-term outcome in very preterm infants: gestational age versus neonatal cranial ultrasound. Pediatrics. 2003;112:1108–14. [DOI] [PubMed] [Google Scholar]
- 36.Luu TM, Ment LR, Schneider KC, Katz KH, Allan WC, Vohr BR. Lasting effects of preterm birth and neonatal brain hemorrhage at 12 years of age what’s known on this subject. Pediatrics. 2009;123:1037–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muller WD, Urlesberger B. Correlation of ventricular size and head circumference after severe intra-periventricular hemorrhage in preterm infants. Childs Nerv Syst. 1992;8:33–5. [DOI] [PubMed] [Google Scholar]
- 38.Riva-Cambrin J, Shannon CN, Holubkov R, Whitehead WE, Kulkarni AV, Drake J, et al. Center effect and other factors influencing temporization and shunting of cerebrospinal fluid in preterm infants with intraventricular hemorrhage. J Neurosurg Pediatr. 2012;9:473–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ingram M-CE, Huguenard AL, Miller BA, Chern JJ. Poor correlation between head circumference and cranial ultrasound findings in premature infants with intraventricular hemorrhage. J Neurosurg Pediatr. 2014;14:184–9. [DOI] [PubMed] [Google Scholar]
- 40.Kuban K, Sanocka U, Leviton A, Allred EN, Pagano M, Dam-mann O, et al. White matter disorders of prematurity: association with intraventricular hemorrhage and ventriculomegaly. J Pediatr. 1999;134:539–46. [DOI] [PubMed] [Google Scholar]
- 41.Kuban KCK, Allred EN, O’Shea TM, Paneth N, Pagano M, Dammann O, et al. Cranial ultrasound lesions in the NICU predict cerebral palsy at age 2 years in children born at extremely low gestational age. J Child Neurol. 2009;24:63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maalouf EF, Duggan PJ, Counsell SJ, Rutherford MA, Cowan F, Azzopardi D, et al. Comparison of findings on cranial ultrasound and magnetic resonance imaging in preterm infants. Pediatrics. 2001;107:719–27. [DOI] [PubMed] [Google Scholar]
- 43.Volpe JJ. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009;8:110–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Vries LS, Eken F’, Dubowitz LS. The spectrum of leukomalacia using cranial ultra- sound. Behav Brain Res. 1992;49: 1–6. [DOI] [PubMed] [Google Scholar]
- 45.Horsch S, Bengtsson J, Nordell A, Lagercrantz H, Aden U, Blennow M. Lateral ventricular size in extremely premature infants: 3D MRI confirms 2D ultrasound measurements. Ultrasound Med Biol. 2009;35:360–6. [DOI] [PubMed] [Google Scholar]
- 46.Hintz SR, Slovis T, Bulas D, Van Meurs KP, Perritt R, Stevenson DK, et al. Interobserver reliability and accuracy of cranial ultrasound scanning interpretation in premature infants. J Pediatr. 2007;150:592–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Inder TE, Anderson NJ, Spencer C, Wells S, Volpe JJ. White matter injury in the premature infant: a comparison between serial cranial sonographic and MR findings at term. AJNR Am J Neuroradiol. 2003;24:805–9. [PMC free article] [PubMed] [Google Scholar]
- 48.Kuban K, Adler I, Allred EN, Batton D, Bezinque S, Betz BW, et al. Observer variability assessing US scans of the preterm brain: the ELGAN study. Pediatr Radiol. 2007;37:1201–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leijser LM, Miller SP, van Wezel-Meijler G, Brouwer AJ, Traubici J, van Haastert IC, et al. Posthemorrhagic ventricular dilatation in preterm infants: when best to intervene? Neurology. 2018;90:e698–e706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Govaert P, de Vries LS An atlas of neonatal brain sonography London: Mac Keith Press; 1997. [Google Scholar]
- 51.Zamora C, Tekes A, Alqahtani E, Kalayci OT, Northington F, Huisman TaGM. Variability of resistive indices in the anterior cerebral artery during fontanel compression in preterm and term neonates measured by transcranial duplex sonography. J Perinatol. 2014;34:306–10. [DOI] [PubMed] [Google Scholar]
- 52.Taylor GA, Madsen JR. Neonatal hydrocephalus: hemodynamic response to fontanelle compression correlation with intracranial pressure and need for shunt placement: pediatric. Radiol Radiol. 1996;201:685–9. [DOI] [PubMed] [Google Scholar]
- 53.Taylor GA, Phillips MD, Ichord RN, Carson BS, Gates JA, James CS. Intracranial compliance in infants: evaluation with doppler US. Radiology. 1994;191:787–91. [DOI] [PubMed] [Google Scholar]
- 54.Seibert JJ, McCowan TC, Chadduck WM, Adametz JR, Glasier CM, Williamson SL, et al. Duplex pulsed Doppler US versus intracranial pressure in the neonate: clinical and experimental studies. Radiology. 1989;171:155–9. [DOI] [PubMed] [Google Scholar]
- 55.Goh D, Minns RA, Hendry GM, Thambyayah M, Steers AJ. Cerebrovascular resistive index assessed by duplex doppler sonography and its relationship to intracranial pressure in infantile hydrocephalus. Pediatr Radiol. 1992;22:246–50. [DOI] [PubMed] [Google Scholar]
- 56.Nishimaki S, Iwasaki Y, Akamatsu H. Cerebral blood flow velocity before and after cerebrospinal fluid drainage in infants with posthemorrhagic hydrocephalus. J Ultrasound Med. 2004;23:1315–9. [DOI] [PubMed] [Google Scholar]
- 57.Leliefeld PH, Gooskens RHJM, Tulleken CAF, Regli L, Uiterwaal CSPM, Han KS, et al. Noninvasive detection of the distinction between progressive and compensated hydrocephalus in infants: is it possible? J Neurosurg Pediatr. 2010;5:562–8. [DOI] [PubMed] [Google Scholar]
- 58.Kahle KT, Kulkarni AV, Limbrick DD, Warf BC. Hydrocephalus in children. Lancet. 2016;387:788–9. [DOI] [PubMed] [Google Scholar]
- 59.O’Shea TM, Kuban KC, Allred EN, Paneth N, Pagano M, Dammann O, et al. Neonatal cranial ultrasound lesions and developmental delays at 2 years of age among extremely low gestational age children. Pediatrics. 2008;122:e662–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fox LM, Choo P, Rogerson SR, Spittle AJ, Anderson PJ, Doyle L, et al. The relationship between ventricular size at 1 month and outcome at 2 years in infants less than 30 weeks’ gestation. Arch Dis Child Fetal Neonatal Ed. 2014;99:F209–14. [DOI] [PubMed] [Google Scholar]
- 61.Dyet LE, Kennea N, Counsell SJ, Maalouf EF, Ajayi-Obe M, Duggan PJ, et al. Natural history of brain lesions in extremely preterm infants studied with serial magnetic resonance imaging from birth and neurodevelopmental assessment. Pediatrics. 2006;118:536–48. [DOI] [PubMed] [Google Scholar]
- 62.Ment LR, Schneider KC, Ainley MA, Allan WC. Adaptive mechanisms of developing brain: the neuroradiologic assessment of the preterm infant. Clin Perinatal. 2000;27:303–23. [DOI] [PubMed] [Google Scholar]
- 63.Kuban KSK, Leviton A. Cerebral palsy. N Engl J Med. 1994;330:188–94. [DOI] [PubMed] [Google Scholar]
- 64.Saliba E, Bertrand P, Gold F, Marchand S, Laugier J. Area of lateral ventricles measured on cranial ultrasonography in preterm infants: association with outcome. Arch Dis Child. 1990;65: 1033–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pappas A, Adams-Chapman I, Shankaran S, McDonald SA, Stoll BJ, Laptook AR, et al. Neurodevelopmental and behavioral outcomes in extremely premature neonates with ventriculomegaly in the absence of periventricular-intraventricular hemorrhage. JAMA Pediatr. 2018;172:32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guzzetta F, Shackelford GD, Volpe S, Perlman JM, Volpe JJ. Periventricular intraparenchymal echodensities in the premature newborn: critical determinant of neurologic outcome. Pediatrics 1986;78:995–1006. [PubMed] [Google Scholar]
- 67.Jacobson L, Ek U, Femell E, Flodmark O, Broberger U. Visual impairment in preterm children with periventricular leukomalacia-visual, cognitive and neuropaediatric characteristics related to cerebral imaging. Dev Med Child Neurol. 1996;38:724–35. [DOI] [PubMed] [Google Scholar]
- 68.Mirmiran M, Barnes PD, Keller K, Constantinou JC, Fleisher BE, Hintz SR, et al. Neonatal brain magnetic resonance imaging before discharge is better than serial cranial ultrasound in predicting cerebral palsy in very low birth weight preterm infants. Pediatrics. 2004;114:992. [DOI] [PubMed] [Google Scholar]
- 69.Maitre NL, Marshall DD, Price WA, Slaughter JC, O’ Shea TM, Maxfield C, et al. Neurodevelopmental outcome of infants with unilateral or bilateral periventricular hemorrhagic infarction. Pediatrics. 2009;124:e1153–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tully HM, Wenger TL, Kukull WA, Doherty D, Dobyns WB. Anatomical configurations associated with posthemorrhagic hydrocephalus among premature infants with intraventricular hemorrhage. Neurosurg Focus. 2016;41:E5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kidokoro H, Neil JJ, Inder TE. New MR imaging assessment tool to define brain abnormalities in very preterm infants at term. AJNR Am J Neuroradiol. 2013;34:2208–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.van’t Hooft J, van der Lee JH, Opmeer BC, Aarnoudse-Moens CSH, Leenders AGE, Mol BWJ, et al. Predicting developmental outcomes in premature infants by term equivalent MRI: systematic review and meta-analysis. Syst Rev. 2015;4:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Woodward LJ, Anderson PJ, Austin NC, Howard K, Inder TE. Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N Engl J Med. 2006;355:685–94. [DOI] [PubMed] [Google Scholar]
- 74.Woodward LJ, Clark CAC, Bora S, Inder TE. Neonatal white matter abnormalities an important predictor of neurocognitive outcome for very preterm children. PLoS ONE. 2012;7: e51879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vasileiadis GT, Gelman N, Han VKM, Williams L-A, Mann R, Bureau Y, et al. Uncomplicated intraventricular hemorrhage is followed by reduced cortical volume at near-term age. Pediatrics. 2004;114:e367. [DOI] [PubMed] [Google Scholar]
- 76.Thompson DK, Inder TE, Faggian N, Warfield SK, Anderson PJ, Doyle LW, et al. Corpus callosum alterations in very preterm infants: perinatal correlates and 2 year neurodevelopmental outcomes. Neuroimage. 2012;59:3571–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brouwer MJ, De Vries LS, Kersbergen KJ, Van Der Aa NE, Brouwer AJ, Viergever MA, et al. Effects of posthemorrhagic ventricular dilatation in the preterm infant on brain volumes and white matter diffusion variables at term-equivalent age. J Pediatr. 2016;168:41–9. [DOI] [PubMed] [Google Scholar]
- 78.Maunu J, Parkkola R, Rikalainen H, Lehtonen L, Haataja L, Lapinleimu H, et al. Brain and ventricles in very low birth weight infants at term: a comparison among head circumference, ultrasound, and magnetic resonance imaging what’s known on this subject. Pediatrics. 2009;123:617–26. [DOI] [PubMed] [Google Scholar]
- 79.Keunen K, Isgum I, van Kooij BJM, Anbeek P, van Haastert IC, Koopman-Esseboom C, et al. Brain volumes at term-equivalent age in preterm infants: imaging biomarkers for neurodevelop-mental outcome through early school age. J Pediatr. 2016;172: 88–95. [DOI] [PubMed] [Google Scholar]
- 80.Nosarti C, Giouroukou E, Healy E, Rifkin L, Walshe M, Reichenberg A, et al. Grey and white matter distribution in very preterm adolescents mediates neurodevelopmental outcome. Brain. 2008;131:205–17. [DOI] [PubMed] [Google Scholar]
- 81.Cheong JLY, Thompson DK, Spittle AJ, Potter CR, Walsh JM, Burnett AC, et al. Brain volumes at term-equivalent age are associated with 2-year neurodevelopment in moderate and late preterm children. J Pediatr. 2016;174:91–7. [DOI] [PubMed] [Google Scholar]
- 82.Fletcher JM, Brookshire BL, Bohan TP, Brandt ME, Davidson KC. Early hydrocephalus In: Rourke BP, editor. Syndrome of nonverbal learning disabilities. Neurodevelopmental manifestations. New York: Guildford Press; 1995. p. 206–38. [Google Scholar]
- 83.Woodward LJ, Edgin JO, Thompson D, Inder TE, Woodward L. Object working memory deficits predicted by early brain injury and development in the preterm infant. Brain. 2005;128: 2578–87. [DOI] [PubMed] [Google Scholar]
- 84.Bora S, Pritchard VE, Chen Z, Inder TE, Woodward LJ. Neonatal cerebral morphometry and later risk of persistent inattention/hyperactivity in children born very preterm. J Child Psychol Psychiatry. 2014;55:828–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fletcher JM, Bohan TP, Brandt ME, Brookshire BL, Beaver SR, Francis DJ. Cerebral white matter and cognition in hydrocephalic children. Arch Neurol. 1992;49:818–24. [DOI] [PubMed] [Google Scholar]
- 86.Fletcher JM, Bohan TP, Brandt ME, Kramer LA, Brookshire BL, Thorstad K, et al. Morphometric evaluation of the hydrocephalic brain: relationships with cognitive development. Child’ s Nerv Syst. 1996;12:192–9. [DOI] [PubMed] [Google Scholar]
- 87.Dennis M, Fitz CR, Netley CT, Sugar J, Harwood-Nash DC, Hendrick EB, et al. The intelligence of hydrocephalic children. Arch Neurol. 1981;38:607–15. [DOI] [PubMed] [Google Scholar]
- 88.Mataro M, Junque C, Poca MA, Sahuquillo J. Neuropsychological findings in congenital and acquired childhood hydrocephalus. Neuropsychol Rev. 2001;11:169–78. [DOI] [PubMed] [Google Scholar]
- 89.Inder TE, Warfield SK, Wang H, Huppi PS, Volpe JJ. Abnormal cerebral structure is present at term in premature infants. Pediatrics. 2005;115:286–94. [DOI] [PubMed] [Google Scholar]
- 90.Lind A, Parkkola R, Lehtonen L, Munck P, Maunu J, Lapinleimu H, et al. Associations between regional brain volumes at term-equivalent age and development at 2 years of age in preterm children. Pediatr Radiol. 2011;41:953–61. [DOI] [PubMed] [Google Scholar]
- 91.Leliefeld PH, Gooskens RHJM, Braun KPJ, Ramos LMP, Uiterwaal CSP, Regli LPE, et al. Longitudinal diffusion-weighted imaging in infants with hydrocephalus: decrease in tissue water diffusion after cerebrospinal fluid diversion. J Neurosurg Pediatr. 2009;4:56–63. [DOI] [PubMed] [Google Scholar]
- 92.Brouwer MJ, van Kooij BJ, van Haastert IC, Koopman-Esseboom C, Groenendaal F, de Vries LS, et al. Sequential cranial ultrasound and cerebellar diffusion weighted imaging contribute to the early prognosis of neurodevelopmental outcome in preterm infants. PLoS ONE. 2014;9:e109556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Partridge SC, Mukheijee P, Henry RG, Miller SP, Berman JI, Jin H, et al. Diffusion tensor imaging: serial quantitation of white matter tract maturity in premature newborns. Neuroimage. 2004; 22:1302–14. [DOI] [PubMed] [Google Scholar]
- 94.Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17: 1429–36. [DOI] [PubMed] [Google Scholar]
- 95.Takahashi M, Ono J, Harada K, Maeda M, Hackney DB. Diffusional anisotropy in cranial nerves with maturation: quantitative evaluation with diffusion MR imaging in rats. Radiology. 2000;216:881–5. [DOI] [PubMed] [Google Scholar]
- 96.Duerden EG, Taylor MJ, Miller SP. Brain development in infants born preterm: looking beyond injury. Semin Pediatr Neurol. 2013;20:65–74. [DOI] [PubMed] [Google Scholar]
- 97.Rose J, Butler EE, Lamont LE, Barnes PD, Atlas SW, Stevenson DK. Neonatal brain structure on MRI and diffusion tensor imaging, sex, and neurodevelopment in very-low-birth weight preterm children. Dev Med Child Neurol. 2009;51:526–35. [DOI] [PubMed] [Google Scholar]
- 98.Rose J, Vassar R, Cahill-Rowley K, Guzman XS, Stevenson DK, Barnea-Goraly N. Brain microstructural development at nearterm age in very-low-birth-weight preterm infants: an atlas-based diffusion imaging study. Neuroimage. 2014;86:244–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gao W, Lin W, Chen Y, Gerig G, Smith JK, Jewells V, et al. Temporal and spatial development of axonal maturation and myelination of white matter in the developing brain. Am J Neuroradiol. 2008;30:290–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rose J, Vassar R, Cahill-Rowley K, Stecher Guzman X, Hintz SR, Stevenson DK, et al. Neonatal physiological correlates of near-term brain development on MRI and DTI in very-low-birth-weight preterm infants. Neuroimage Clin. 2014;5:169–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Adams E, Chau V, Poskitt KJ, Grunau RE, Synnes A, Miller SP. Tractography-based quantitation of corticospinal tract development in premature newborns. J Pediatr. 2010;156:882–8.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ben-Sira L, Goder N, Bassan H, Lifshits S, Assaf Y, Constantini, S. Clinical benefits of diffusion tensor imaging in hydrocephalus. J Neurosurg Pediatr. 2015;16:195–202. [DOI] [PubMed] [Google Scholar]
- 103.Akbari SH, Limbrick DD Jr, McKinstry RC, Altaye M, Ragan DK, Yuan W, et al. Periventricular hyperintensity in children with hydrocephalus. Pediatr Radiol. 2015;45:1189–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mangano FT, Altaye M, McKinstry RC, Shimony JS, Powell SK, Phillips JM, et al. DTI study of children with congenital hydrocephalus: 1 year post-surgical outcomes. J Neurosurg Pediatr. 2016;18:306–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yuan W, Mangano FT, Air EL, Holland SK, Jones BV, Altaye M, et al. Anisotropic diffusion properties in infants with hydrocephalus: a diffusion tensor imaging study. AJNR Am J Neuroradiol. 2009;30:1792–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yuan W, McKinstry RC, Shimony JS, Altaye M, Powell SK, Phillips JM. Diffusion tensor imaging properties and neurobehavioral outcomes in children with hydrocephalus. AJNR Am J Neuroradiol. 2013;34:439–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Air EL, Yuan W, Holland SK, Jones BV, Bierbrauer K, Altaye M. Longitudinal comparison of pre- and postoperative diffusion tensor imaging parameters in young children with hydrocephalus. J Neurosurg Pediatr. 2010;5:385–39. [DOI] [PubMed] [Google Scholar]
- 108.Berman JI, Mukherjee P, Partridge SC, Miller SP, Ferriero DM, Barkovich AJ, et al. Quantitative diffusion tensor MRI fiber tractography of sensorimotor white matter development in premature infants. Neuroimage. 2005;27:862–71. [DOI] [PubMed] [Google Scholar]
- 109.Rose J, Mirmiran M, Butler EE, Lin CY, Barnes PD, Kermoian R. Neonatal microstructural development of the internal capsule on diffusion tensor imaging correlates with severity of gait and motor deficits. Dev Med Child Neurol. 2007;49:745–50. [DOI] [PubMed] [Google Scholar]
- 110.Arzoumanian Y, Mirmiran M, Barnes PD, Woolley K, Ariagno RL, Moseley ME, et al. Diffusion tensor brain imaging findings at term-equivalent age may predict neurologic abnormalities in low birth weight preterm infants. AJNR Am J Neuroradiol. 2003;24:1646–53. [PMC free article] [PubMed] [Google Scholar]
- 111.Sun M, Yuan W, Hertzler DA, Cancelliere A, Altaye M, Mangano FT. Diffusion tensor imaging findings in young children with benign external hydrocephalus differ from the normal population. Child’s Nerv Syst. 2012;28:199–208. [DOI] [PubMed] [Google Scholar]
- 112.Rose J, Cahill-Rowley K, Vassar R, Yeom KW, Stecher X, Stevenson DK, et al. Neonatal brain microstructure correlates of neurodevelopment and gait in preterm children 18–22 mo of age: an MRI and DTI study. Pediatric Research 2015;78:700–8. [DOI] [PubMed] [Google Scholar]