Abstract
Pain is significantly impacted by the rising epidemic of obesity and metabolic syndrome. Our understanding of how these features impact pain is only beginning to be developed. Here, we investigated how small genetic differences among C57BL/6 mice from two different commercial vendors leads to important differences in the development of high-fat diet-induced mechanical sensitivity. Two sub strains of C57BL/6 mice from Jackson laboratories (C57BL/6J and C57BL/6NIH), as well as C57BL/6 from Charles Rivers (C57BL/6CR) were placed on high-fat diets and analyzed for changes in metabolic features influenced by high-fat diet and obesity, as well as measures of pain related behaviors. All three substrains responded to the high-fat diet, however C57BL/6CR mice had the highest weights, fat mass, and impaired glucose tolerance of the three substrains. In addition, the C57BL/6CR mice were the only strain to develop significant mechanical sensitivity over the course of 8 weeks. Importantly, the C57BL/6J mice were protected from mechanical sensitivity, which may be based on increased physical activity compared to the other two substrains. These findings suggest that activity may play a powerful role in protecting metabolic changes associated with a high-fat diet and these may also be protective in pain associated changes as a result of a high-fat diet. These findings also emphasize the importance of selection and transparency in choosing C57BL/6 substrains in pain-related research.
Keywords: Pain, Obesity, Metabolism, C57BL/6, Diet, Insulin Resistance
Introduction
Obesity and metabolic syndrome are increasing in an alarming fashion worldwide, and increasing attention is being paid to the strong relationship between obesity, metabolic syndrome and pain19. This growing correlation of obesity and pain has been investigated using numerous animal models, many utilizing differing strains of animals, diets, and obesity phenotypes, and it’s clear that the genetic component underlying pain is a key contributor. Animal studies have identified important differences in pain associated with different strains of rodents20. However, there remains a significant gap in our knowledge related to the interplay of obesity, insulin resistance, metabolic syndrome, and pain, and there remains a lack of animal models in which to explore this important problem. These features are key to understanding how this important health change in our communities will impact the treatment of pain13.
Three widely-used strains of C57BL/6 mice utilized in many basic research studies include the C57BL/6J mouse (J) from Jackson Laboratory, the C57BL/6NJ (NJ) from Jackson Laboratory, and the C57BL/6 mouse from Charles River (CR). Because of their heavy usage, new information has emerged related to variations between substrains of C57BL/6 mice, which may affect outcomes utilizing them6, 12, 27. One important mutation among the C57BL/6 substrains is the mutation within nicotinamide nucleotide transhydrogenase (NNT), a mitochondrial protein which strongly influences glucose metabolism, insulin secretion, and mitochondrial function. The NNT mutation is only found in C57BL/6J (J) mice at Jackson Laboratories and not within C57BL/6 NJ mice from Jackson Laboratories or C57BL/6 CR mice from Charles River 7, 26, 29,27.
Consumption of various high-fat diets to induce prediabetes and metabolic syndrome in rodent models leads to reliable phenotypic changes that similarly occur in humans 5, 8, 10, 22, 24. Different strains of mice are known to develop individualized characteristics of small fiber function, including allodynia or hyperalgesia to mechanical and thermal stimuli. These known and unknown genetic differences likely contribute to current problems with reproducibility and rigor associated with key phenotypic differences that alter peripheral nerve sensitivity 1, 4, 5, 8, 24.
Prior research has shown that feeding C57BL/6 CR mice a high-fat diet for 12 weeks induces significant mechanical sensitivity following 8 weeks of diet alteration 5, 8. However, this was not consistent with other studies in which C57BL/6J mice fed a high-fat diet will sometimes develop mechanical allodynia and will demonstrate thermal hypoalgesia 22, 23, 30. Though less often utilized, the NJ mouse has shown alterations in nociceptive measures in response to a high-fat diet as well21. Beyond alterations in behavioral sensitivity measures, the two studies reported discrepancies in large fiber function related to conduction velocity. High-fat fed C57BL/6 J mice also develop slowed motor and sensory nerve conduction speeds 22; while high-fat fed C57BL/6 CR mice on high-fat diets only develop altered motor nerve conduction velocities 9.
The current study identifies key differences within three popular C57BL/6 sub-strains, and examines alterations in behavioral physiological, and anatomical changes in the peripheral nervous system displayed by these mice. C57BL/6J mice are unique from other strains in their mechanical sensitivity, physical activity, glucose tolerance, and other measures of metabolic syndrome compared to C57BL/6 CR or C57BL/6 NJ mice, suggesting importantly, that C57BL/6 sub-strain is a criterion in understanding the relationship of metabolic status and pain. The ability to build a scientific consensus about the role of obesity and metabolic syndrome outcomes, will prove difficult until we better understand the variable phenotypic and genetic features that regulate pain. This information will also improve rigor and reproducibility, leading to better models in which to predict drug and lifestyle interventions to treat pain.
Methods
Diet and Animals
Seven-week old male C57BL/6 #027 mice were purchased from Charles River (Wilmington, Mass), C57BL/6J and C57BL/6NJ mice were purchased from Jackson Laboratory’s (Bar Harbor, ME) and maintained in pair housed cages on a 12:12h light/dark cycle in the research support facility at the University of Kansas Medical Center. All mice were given ad libitum access to food and water and were fed either a standard chow diet (8604; Envigo, Madison Wisconsin; 14% kcals from fat, 32% protein, and 54% carbohydrate) or a high-fat diet (07011; Envigo; 54% kcals from vegetable shortening (hydrogenated) and corn oil fat, 21% protein and 24% carbohydrate).
Daily food intake was measured by monitoring the weight of the remaining food after an initial food bolus. New food boluses were given every 3–4 days. Energy intake was calculated by
Standard diet energy was calculated by multiplying the intake per day by 3.0kcal/g, high-fat diet energy was calculated by multiplying intake per day by 4.9kcal/g. The combined mean energy intake from each mouse was used to calculate the group means.
Animals were fed the standard diet through all baseline testing. After baseline behavioral testing was complete, animals were separated and the groups were given different diets. All animals were 8 weeks of age at the start of diet. All animal use was in accordance with NIH guidelines and conformed to the principles specified in a protocol approved by the University of Kansas Medical Center Institutional Animal Care and Use Committee.
Blood Measurements
Animal weight and blood glucose (glucose diagnostic reagents; Sigma, St. Louis, MO) was measured bi-weekly after a 3 hour fast as previously described 8. Additionally, at the time of sacrifice following a 3 hour fast, blood was drawn from the chest cavity and allowed to clot for 30 min on ice, then spun at 3,000g for 30 min at 4 °C and serum drawn off and frozen at −80 °C until insulin was analyzed from this sample.
After 7 weeks, an intraperitoneal glucose tolerance test (IPGTT) was performed after a 6 hour fast. Animals were given 1g glucose/kg body weight. Blood glucose levels were measured via tail clip immediately before glucose injection, and 15, 30, 60, and 120 minutes thereafter.
Body Composition
Body composition to assess fat mass was measured by MRI using the EchoMRI-100 (EchoMRI, Houston, TX). Fat mass and lean mass composition was determined biweekly from baseline testing until sacrifice at the completion of 8 weeks of diet.
Metabolic Testing
A metabolic monitoring system (Promethion, Sable Systems Int., Las Vegas, NV) measuring oxygen consumption, carbon dioxide production, and a multi-dimensional infrared beam break system was employed to assess the activity (meters traveled), energy intake (kcal consumed), total energy expenditure (TEE) and respiratory quotient (RQ) over a 48-hr period, 4–6 weeks after implementation of diet. Animals were singly housed in the metabolic chamber system and allowed to acclimate to the chamber environment for two days prior to data collection. Data were analyzed as two 12-hour cycle averages (12 hours ambient light [07:00–19:00] and 12 hours of dark [19:00–07:00]) and were calculated per animal; these light and dark averages were then used to calculate group means.
Sensory Behavior Testing
Behavior testing to assess signs of diet-induced sensitivity was carried out at baseline and biweekly time points. For all behavioral tests, animals were allowed to acclimate to the testing equipment in two separate sessions prior to the initial testing day. Before each behavior test, animals were allowed to acclimate to the behavior testing room for 30 minutes followed by a 30-minute acclimation to the testing equipment.
Mechanical Sensitivity
Mice were placed in individual clear plastic cages on a wire mesh table 55 cm above the table. Von Frey monofilaments (0.07–4.0g) were applied perpendicularly to the plantar surface of the hind paw until the filament bent. Testing began with the 0.6g filament. If the animals withdrew their paw, it was counted as a positive withdrawal and the next lowest filament was applied. If the animal did not respond, the next larger filament was applied. Filaments were applied until there was an initial change in response followed by four additional filament applications. The 50% withdrawal threshold was calculated using the formula from the up-down method previously described3.
Thermal Sensitivity
Mice were placed in individual clear plastic cages on a Hargreaves’s apparatus and a 4.0 V radiant heat source was applied three times to the hind paw. Time elapsed for each animal to withdraw the hind paw was counted as withdrawal latency (sec). Latencies from three applications were used to calculate the mean latency per animal and mean latencies were combined to calculate group means.
Chemogenic Sensitivity
Mice were assessed for chemogenic nociception sensitivity as previously described 11. The amount of time devoted to the injected foot (licking and biting) was recorded in 5-minute intervals for 1 hour. Two windows of time, acute (Phase 1; 0–30 min post-injection) and inflammatory (Phase 2; 30–60 min post-injection) phases of the formalin test were examined.
Intraepidermal Nerve Fiber Density (IENFD)
Footpads were collected and processed using standardized protocols previously described for intraepidermal nerve fiber density 8.
Statistical Analysis
All data is presented as mean ± SEM. Data was analyzed using a one way ANOVA, two-factor ANOVA, 3-factor ANOVA, or repeated measures ANOVA with post hoc comparisons analyzed using Fisher’s test of least square difference where appropriate. Statistical significance was defined as p <0.05 and statistics were run using GraphPad Prism 7.0 (GraphPad Software Inc., La Jolla, CA), and SPSS24 (IBM,).
Results
Obesity and Prediabetes Associated Measures
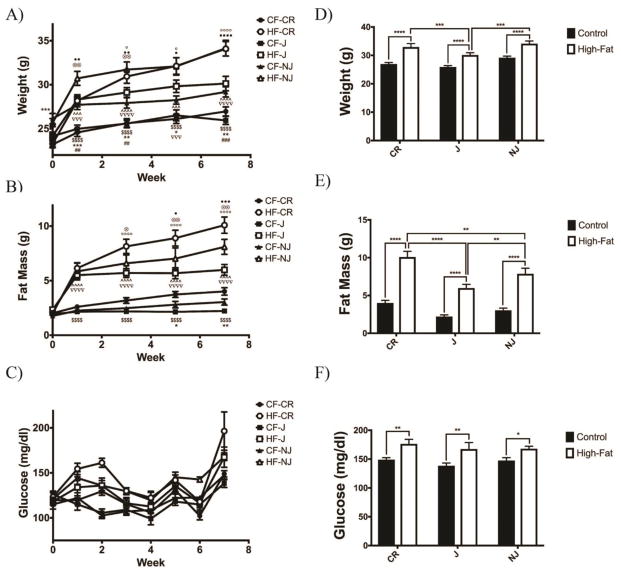
All mouse strains had increased body mass following 3 weeks of a high-fat diet (J p<0.01, NJ p<0.05, CR p<0.01) (ANOVA p<0.0001) (Fig. 1a). All strains displayed increases in fat mass following just 1 week of a high-fat diet (p<0.0001) relative to control-fed mice (ANOVA p=0.022) (Fig. 1b). Throughout the study high-fat fed mice display slightly increased blood glucose; however all failed to reach overt hyperglycemia (>200mg/dl glucose) (ANOVA p=0.027) (Fig. 1c). Of the high-fat fed strains, C57BL/6NJ and C57BL/6CR mice displayed a larger increase in body mass as compared to C57BL/6J mice (NJ p<0.001; CR p<0.0001). C57BL/6NJ mice were larger than C57BL/6J and C57BL/6CR mice on a control diet throughout the study suggesting these mice are larger at the same relative age. At 8 weeks all high-fat fed mice had significantly more body mass than their control-fed counterparts (Fig. 1d). At 8 weeks all strains displayed increased fat mass compared to control-fed mice (p<0.0001), though C57BL/6CR mice display the greatest increase relative to control-fed mice (Fig. 1e). At 8 weeks, all strains displayed slightly increased blood glucose in fat-fed mice (J p<0.01; NJ p<0.05; CR p<0.01) (Fig. 1f).
Figure 1. All C57BL/6 Mice Display Increased Body Weight, Fat Mass, and Glucose When Fed a High-Fat Diet.
A) All high fat-fed groups had increased bodyweight relative to control-fed groups beginning at 3 weeks (n=10 for all groups). B) Beginning after 1 week on a high fat diet all groups displayed an increase in fat mass. C) All high fat groups display slightly elevated blood glucose beginning 1 week after the start of a high fat diet. D) After 7 weeks of a high fat diet all groups display an increase in body weight, with C57BL/6J animals displaying the smallest increase in weight. E) Following 7 weeks of a high fat diet C57BL/6CR animals have the greatest increase in fat mass. F) After 7 weeks of a high fat diet all groups display mildly elevated blood glucose. All data presented as mean ± SEM * p<0.05; ** p<0.01; *** p<0.001; **** p<0.0001 CF: Control-fed HF: High fat-fed
Glucose Tolerance and Insulin Measures
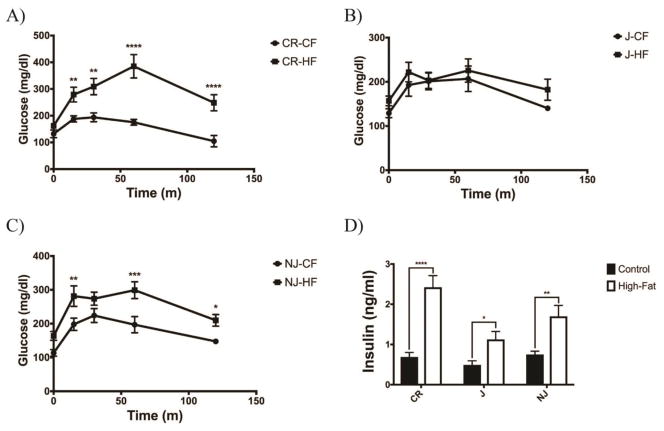
Both high-fat C57BL/6CR and C57BL/6NJ mice developed glucose intolerance relative to their control-fed counterparts (p<0.0001), where as C57BL/6J mice did not develop glucose intolerance following 6 weeks of high-fat feeding (Fig. 2a, b, c). All strains displayed increased fasting insulin compared to control-fed mice (J p<0.05; NJ p<0.01; CR p<0.0001) (Fig. 2d).
Figure 2. C57BL/6CR and C57BL/6NJ Mice Develop Glucose Intolerance When Fed a High Fat Diet.
A) High fat-fed C57BL/6CR animals display significantly reduced glucose tolerance following 6 weeks on a high fat diet (n=6 for all groups). B) High fat-fed C57BL/6J mice showed no change in glucose tolerance following 6 weeks of a high fat diet (n=6 for all groups). C) High fat-fed C57BL/6NJ mice display slightly reduced glucose tolerance 6 weeks on a high fat diet (n=6 on all groups). D) Fasting insulin was increased in all groups on a high fat diet, with C57BL/6CR mice having the highest levels of hyperinsulinemia (n=10 for all groups). All data presented as mean ± SEM * p<0.05; ** p<0.01; *** p<0.001; **** p<0.0001 CF: Control-fed HF: High fat-fed
Metabolic Testing
All metabolic cage data was analyzed, as separate 12-hour light cycles to take in to affect the differences in activity mice will display during their primary sleep versus active times.
Activity Level
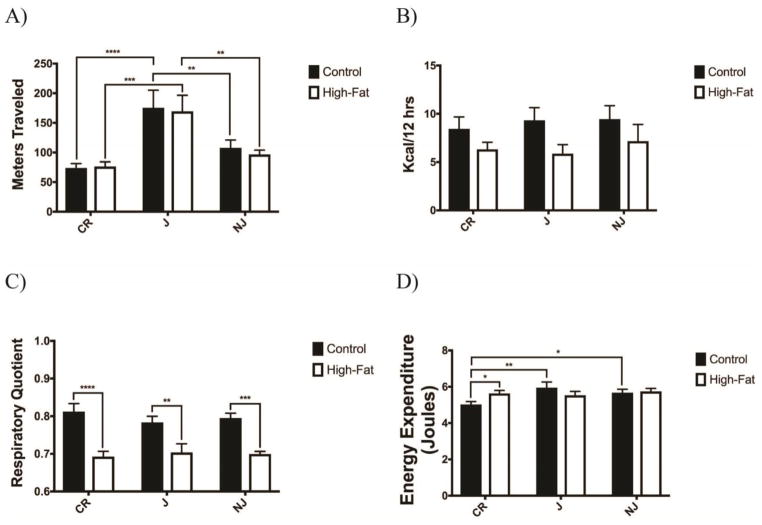
Both control and high-fat fed C57BL/6J mice have significantly increased activity levels during the dark cycle as compared to C57BL/6CR (p<0.001) and C57BL/6NJ strains (p<0.01) (Fig. 3a).
Figure 3. C57BL/6 Sub-strains Have Different Intrinsic Activity and Energy Expenditure on a High Fat Diet.
A) Control-fed and high fat-fed C57BL/6J mice display significantly greater activity during dark cycle than C57BL/6CR or C57BL/6NJ mice (n=8 for all groups). B) No sub-strain or diet group displayed an alteration in energy intake during active dark cycle time. C) All high fat-fed substrains displayed a reduced respiratory quotient associated with increased fat fuel utilization during their active dark cycle. D) Control-fed C57BL/6CR showed reduced energy expenditure relative to high fat-fed C57BL/6CR and control-fed C57BL/6J and C57BL/6NJ animals. All data presented as mean ± SEM * p<0.05; ** p<0.01; *** p<0.001; **** p<0.0001 CF: Control-fed HF: High fat-fed
Energy Intake
No groups or strains showed any differences in energy intake during active dark cycle, though all control fed animals trend towards an increase in intake (Fig. 3b).
Respiratory Quotient
Traditionally, utilization of a high-fat diet increases whole body lipid oxidation as seen by a respiratory quotient (RQ) near 0.7 (VCO2/VO2) 14. In all strains high-fat fed mice displayed a reduced RQ (dark cycle CR p<0.0001; J p<0.01; NJ p<0.001); while all control-fed mice showed a traditional mixed diet RQ of ~0.8 (Fig. 3c). The light cycle showed similar though non-significant alterations as expected due to the utilization of less fuel when sleeping, as only C57BL/6NJ animals show a significantly increased RQ in control-fed mice (p<0.05).
Energy Expenditure
Control-fed C57BL/6CR mice had reduced energy expenditure relative to control-fed C57BL/6J and C57BL/6NJ mice, likely due to reduced activity levels (Fig. 3d). Only high-fat fed C57BL/6CR mice had increased energy expenditure relative to control-fed mice.
Changes in Peripheral Sensation
Mechanical
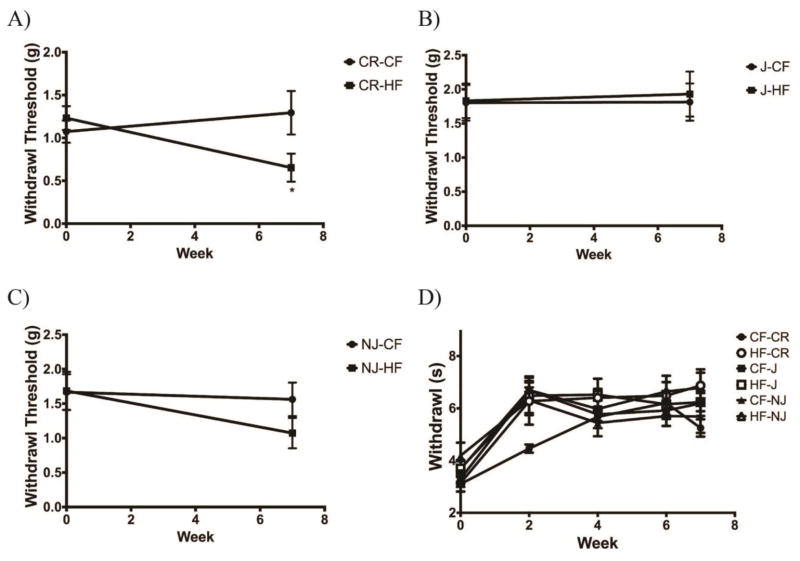
High-fat fed C57BL/6CR mice developed mechanical sensitivity following 7 weeks of diet (p<0.05) (Fig. 4a). There were no changes in mechanical threshold in C57BL/6J mice when fed a high-fat diet (Fig. 4b). While not significant, C57BL/6NJ animals also display a small decrease in mechanical threshold when fed a high-fat diet (Fig. 4c).
Figure 4. High Fat-Fed C57BL/6CR mice Develop Mechanical Allodynia.
A) Following 7 weeks of a high fat diet C57BL/6CR mice have increased mechanical sensitivity relative to control-fed mice (n=10 for all groups). B) C57BL/6J mice had no changes in mechanical sensitivity after 7 weeks of a high fat diet. C) High fat-fed C57BL/6NJ mice did not develop significant alterations in mechanical sensitivity after 7 weeks. D) No diet group or substrain showed a significant change in thermal sensitivity across 7 weeks when fed a high fat diet. All data presented as mean ± SEM * p<0.05; ** p<0.01; *** p<0.001; **** p<0.0001 CF: Control-fed HF: High fat-fed
Thermal
Following 7 weeks of a high-fat diet, no group or strain displayed changes in thermal sensitivity (Fig. 4d).
Chemogenic
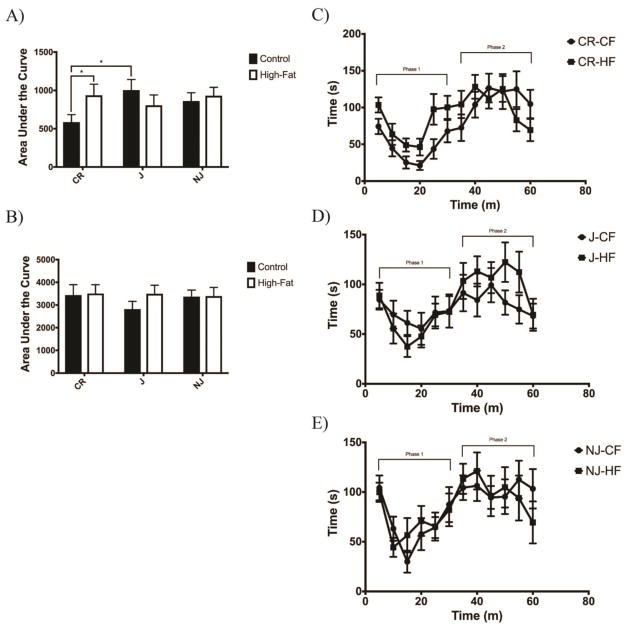
C57BL/6CR high fat-fed mice displayed a larger phase 1 response to formalin injection compared to control-fed mice (p=0.0468) (Fig. 5a&c). No groups displayed any alterations in phase 2 of the formalin test (Fig. 5b). C57BL/6NJ and C57BL/6J mice showed no differences in response to formalin when fed a high fat diet (Fig. 5d&e).
Figure 5. C57BL/6CR Animals Display Heighted Chemogenic Sensitivity When Fed a High Fat Diet.
A) High fat-fed C57BL/6CR mice were the only group to display increased nociceptive behaviors phase 1 as a response to formalin injection. Control-fed C57BL/6J mice also showed an increased response in phase 1 as compared to C57BL/6CR mice (n=15 for all groups). B) No sub-strain or diet group displayed changes in phase 2 responses following formalin injection. C) Control-fed vs. high fat C57BL/6CR mice D) Control-fed vs. high fat-fed C57BL/6J mice E) Control-fed vs. high fat-fed C57BL/6NJ mice. All data presented as mean ± SEM * p<0.05; ** p<0.01; *** p<0.001; **** p<0.0001 CF: Control-fed HF: High fat-fed
IENFD
Control-fed C57BL/6NJ substrain display an increased epidermal fiber density relative to C57BL/6CR and C57BL/6J mice, reflecting a genetic difference in these mice in epidermal fiber density (Fig. 6a & 6b). None of the mouse strains displayed changes in epidermal fiber density after 8 weeks on a high fat diet (Fig. 6c, d, e).
Figure 6. Epidermal Fiber Density Was Not Altered by a High Fat Diet.
A) Sections of the plantar surface of the hind paw stained with PGP9.5 to identify sensory axons innervating the epidermis B) No substrain displayed differences in their IENFD when fed a high fat diet. On the control diet, C57BL/6NJ mice did have an increased IENFD relative to C57BL/6CR and C57BL/6J mice (n=8 for all groups) C) IENF levels in C57BL/6CR mice following 7 weeks of diet D) IENF levels in C57BL/6J mice following 7 weeks of diet E) IENF levels in C57BL/6NJ mice following 7 weeks of diet. All data presented as mean ± SEM * p<0.05
CF: Control-fed HF: High fat-fed
Discussion
The increasing obesity epidemic mandates we understand how metabolic syndrome affects pain. As part of this, we need more information about key features that add variability to the role of obesity and metabolic syndrome to pain. The C57BL/6 mouse strain has become the most frequently used mouse in biomedical research, including studies of metabolic and high-fat diet associated with pain. The present study reveals important difference between C57BL/6 substrains that impact pain, and illustrates the importance of selecting C57BL/6 substrains to accurately model pain modulation in studies using these mice. These differences include variations in metabolic syndrome associated measures, physical activity, and mechanical sensitivity.
Our analysis of the 3 substrains of C57BL/6 mice revealed different responses in metabolic features to a high-fat diet. Throughout the study, C57BL/6CR and C57BL/6J mice displayed the greatest changes in metabolic measures. C57BL/6J mice experienced the mildest changes in metabolic syndrome measures, including the smallest increase in body weight, fat mass, glucose intolerance, and fasting insulin. Differences in glucose tolerance and insulin levels between C57BL/6CR and C57BL/6J may also be related to genetic differences between the two substrains. It is known that C57BL/6J mice harbor a mutation in the mitochondrial gene NNT7, 26. The NNT mutation in C57BL/6J mice leads to reduced insulin secretion and altered glucose metabolism26. It is plausible that reduced insulin secretion in C57BL/6 J mice due to the NNT mutation contributes through insulin-driven mechanisms (insulin secretion, insulin resistance, and glucose intolerance) to overall differences among the 3 strains.
Recent research in mice is now suggesting that the temperature in which mice are housed plays an important role in the severity of high-fat diet-induced metabolic syndrome28. This research suggests that mice housed at high temperatures (30–32°C, thermoneutrality) leads to greater obesity and metabolic consequences. For our studies, all mice were housed at 22 C, and it is possible that different metabolic complications would be seen comparatively in the 3 substrains, including the C57BL/6J mice that had the mildest change to the high-fat diet. Additionally, our utilization of a 54% high-fat diet while being a slightly lower fat diet than some others who have utilized 60% diets, we anticipation this small alteration in fat does not have profound effects. A key difference from other diets is our diet was nota high sucrose ‘western’ diet as some have used before and future work should examine if this dietary difference alters sensitivity. Future studies should examine the effects of a high-fat diet while housing mice at thermoneutral temperatures to determine if the stratification of metabolic consequences remain among the 3 substrains persists.
An additional important limitation of this work is we chose to utilize only male C57BL/6 mice. Previous work by our groups and many others has shown the important and predictable changes in metabolic syndrome and nociception with a high-fat diet in male mice. Recent work has shown that female mice do show important differences in high-fat diet-induced changes in both obesity related measures and nociception. To better focus on only the differences caused by subtle alterations of this in-bred genetic strain, the effects of sex were not examined. Future work should be sure to examine differences with in strains of mice utilizing female animals to further understand key differences between sexes when utilizing diet-induced models of pain.
Physical activity plays a critically important role in metabolic syndrome and pain4. Reduced obesity and insulin resistance induced by a high-fat diet in C57BL/6J mice may be explained by variations in ambulatory activity levels. Increased activity powerfully reduces obesity and improves glucose tolerance, and insulin resistance. Despite relatively mild differences in activity among the 3 substrains, evidence suggests that even minimal alterations in ambulatory activity levels can drive significant changes in these measures15. The fact that C57BL/6J mice displayed significant elevations in their in-cage activity could explain their modest obesity in response to a high-fat diet, leading to downstream benefits in mechanical sensitivity. This is consistent with the data revealing that C57BL/6CR mice showed the lowest energy expenditure of the substrains examined, leading to greater storage of energy as fat, despite having similar energy intake as C57BL/6J and C57BL/6NJ mice. Alternatively, the increased activity may drive this behavioral modification by itself, as a number of studies in not obese animals reveal powerful benefits of exercise and activity on pain reduction4. Previous work from our lab has shown that physical activity is able to rescue high-fat diet-induced mechanical sensitivity in C57BL/6CR mice independent of a improvements in obesity or glucose tolerance 5, 8. Here, we hypothesize that increased activity in C57BL/6J mice may suppress the development of mechanical sensitivity induced by a high-fat diet. Physical activity-induced analgesia can be mediated by mu-opioid and serotonin transporter modulation, thus the C57BL/6J mice, through their ambient activity levels may be modulating nociceptive responsiveness via increased central nervous system opiate signaling18.
A focal point of this study was to evaluate how a high-fat diet altered pain status of the 3 substrains of mice. C57BL/6CR mice fed a high-fat diet developed consistent changes in mechanical and chemogenic sensitivity, including mechanical sensitivity and an elevated response in phase 1 to formalin, consistent with emerging studies on the role of a high-fat diet in pain modulation5, 8, 9. The lack of changes in nociceptive responses in C57BL/6J and C57BL/6NJ mice could be associated with similar lack of changes in obesity and metabolic measures. This feature is important as it suggests that studies be aware of this variation and design experiments that account for these substrain differences, including diet choice and temperature-controlled rooms that achieve thermal neutrality.
Increased inflammation is an important mechanism associated with metabolic syndrome, and changes in systemic inflammation likely play a role in the current study. A high-fat diet leads to increased pro-inflammatory signaling throughout the body, which then heightens inflammatory-related changes including chemogenic sensitivity2, 31. The reduced fat mass, and metabolic syndrome observed in C57BL/6J and C57BL/6NJ mice merits further study into diet induced changes in inflammation and if nociceptive protection is provided by a reduced inflammatory response. It will be important to incorporate temporal studies to determine if nociceptive changes will occur at later time points in C57BL/6J and C57BL/6NJ mice22.
Diabetes and metabolic syndrome induced neuropathies are commonly associated with reduced epidermal innervation, and recent studies in prediabetic patients with neuropathy reveal these axonal changes occur early in neuropathy progression in human patients16, 17, 25. Research in mice to model these changes has been variable, with some reporting epidermal axon changes and others no changes22. Our current studies revealed no changes in epidermal axons in response to a high-fat diet, or among the 3 substrains of mice in response to the diet. It’s plausible that these changes occur later in the progression and future research should investigate longer-term alterations in IENFD with a high-fat diet10, 22.
Conclusion
The current studies highlight the role of metabolic changes and activity level in regulating nociceptive responsiveness in mice. Small variations within murine genetic backgrounds lead to significant increases in body weight, fat mass, fasting insulin, glucose intolerance, activity level, and nociceptive sensitivity. These key alterations in mouse sub-strains could have profound differences in their utilization in metabolic syndrome associated disease modeling including pain. In addition, the use of a ‘western’ diet high in carbohydrates a C57BL/6J animal kept at thermal neutral temperatures has become a common model in obesity and diabetes research. However, the present work suggests that other substrains may be much more susceptible to metabolic syndrome, and our results may allow for better modeling in studies of pain modulation associated with metabolic alterations. We suggest that researchers examining metabolic syndrome or obesity-induced disease take great care in examining not only the background but also sub-strain differences in their models of disease.
Perspective.
Obesity and metabolic syndrome play an important role in pain. This study identifies key differences in the response to a high-fat diet among substrains of C57BL/6 mice and differences in intrinsic physical activity that may influence pain sensitivity. The results emphasize physical activity as a powerful modulator of obesity-related pain sensitivity.
Highlights.
Substrains of C57/Bl6 mice develop varied metabolic consequences on a high-fat diet
C57/Bl6J mice display increased ambulatory activity in sedentary housing
C57/Bl6CR mice increase metabolic syndrome related measures on a high-fat diet
C57/Bl6 substrains show alterations in the development of diet-induced pain
Footnotes
Disclosures: This work was supported by National Institutes of Health (NIH) grants R01NS43313 (DEW), NIH/NIGMS COBRE grant P30 GM103326, P20 GM103418 from the IDeA Network of Biomedical Research Excellence (INBRE) Program. None of the authors of this work have competing or conflicting interests in the associated work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anderson NJ, King MR, Delbruck L, Jolivalt CG. Role of insulin signaling impairment, adiponectin and dyslipidemia in peripheral and central neuropathy in mice. Dis Model Mech. 2014;7:625–633. doi: 10.1242/dmm.015750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrew D, Greenspan JD. Mechanical and heat sensitization of cutaneous nociceptors after peripheral inflammation in the rat. Journal of neurophysiology. 1999;82:2649–2656. doi: 10.1152/jn.1999.82.5.2649. [DOI] [PubMed] [Google Scholar]
- 3.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 4.Cooper MA, Kluding PM, Wright DE. Emerging Relationships between Exercise, Sensory Nerves, and Neuropathic Pain. Frontiers in neuroscience. 2016;10:372. doi: 10.3389/fnins.2016.00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooper MA, Ryals JM, Wu PY, Wright KD, Walter KR, Wright DE. Modulation of diet-induced mechanical allodynia by metabolic parameters and inflammation. Journal of the peripheral nervous system: JPNS. 2016:39–46. doi: 10.1111/jns.12199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fontaine DA, Davis DB. Attention to Background Strain Is Essential for Metabolic Research: C57BL/6 and the International Knockout Mouse Consortium. Diabetes. 2016;65:25–33. doi: 10.2337/db15-0982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freeman HC, Hugill A, Dear NT, Ashcroft FM, Cox RD. Deletion of nicotinamide nucleotide transhydrogenase: a new quantitive trait locus accounting for glucose intolerance in C57BL/6J mice. Diabetes. 2006;55:2153–2156. doi: 10.2337/db06-0358. [DOI] [PubMed] [Google Scholar]
- 8.Groover AL, Ryals JM, Guilford BL, Wilson NM, Christianson JA, Wright DE. Exercise-mediated improvements in painful neuropathy associated with prediabetes in mice. Pain. 2013;154:2658–2667. doi: 10.1016/j.pain.2013.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guilford BL, Ryals JM, Wright DE. Phenotypic changes in diabetic neuropathy induced by a high-fat diet in diabetic C57BL/6 mice. Experimental diabetes research. 2011;2011:848307. doi: 10.1155/2011/848307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hinder LM, O’Brien PD, Hayes JM, Backus C, Solway AP, Sims-Robinson C, Feldman EL. Dietary reversal of neuropathy in a murine model of prediabetes and metabolic syndrome. Dis Model Mech. 2017;10:717–725. doi: 10.1242/dmm.028530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson MS, Ryals JM, Wright DE. Early loss of peptidergic intraepidermal nerve fibers in an STZ-induced mouse model of insensate diabetic neuropathy. Pain. 2008;140:35–47. doi: 10.1016/j.pain.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keane TM, Goodstadt L, Danecek P, White MA, Wong K, Yalcin B, Heger A, Agam A, Slater G, Goodson M, Furlotte NA, Eskin E, Nellaker C, Whitley H, Cleak J, Janowitz D, Hernandez-Pliego P, Edwards A, Belgard TG, Oliver PL, McIntyre RE, Bhomra A, Nicod J, Gan X, Yuan W, van der Weyden L, Steward CA, Bala S, Stalker J, Mott R, Durbin R, Jackson IJ, Czechanski A, Guerra-Assuncao JA, Donahue LR, Reinholdt LG, Payseur BA, Ponting CP, Birney E, Flint J, Adams DJ. Mouse genomic variation and its effect on phenotypes and gene regulation. Nature. 2011;477:289–294. doi: 10.1038/nature10413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klinck MP, Mogil JS, Moreau M, Lascelles BDX, Flecknell PA, Poitte T, Troncy E. Translational pain assessment: could natural animal models be the missing link? Pain. 2017;158:1633–1646. doi: 10.1097/j.pain.0000000000000978. [DOI] [PubMed] [Google Scholar]
- 14.Krogh A, Lindhard J. The Relative Value of Fat and Carbohydrate as Sources of Muscular Energy: With Appendices on the Correlation between Standard Metabolism and the Respiratory Quotient during Rest and Work. The Biochemical journal. 1920;14:290–363. doi: 10.1042/bj0140290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krogh-Madsen R, Thyfault JP, Broholm C, Mortensen OH, Olsen RH, Mounier R, Plomgaard P, van Hall G, Booth FW, Pedersen BK. A 2-wk reduction of ambulatory activity attenuates peripheral insulin sensitivity. Journal of applied physiology. 2010;108:1034–1040. doi: 10.1152/japplphysiol.00977.2009. [DOI] [PubMed] [Google Scholar]
- 16.Lauria G, Bakkers M, Schmitz C, Lombardi R, Penza P, Devigili G, Smith AG, Hsieh ST, Mellgren SI, Umapathi T, Ziegler D, Faber CG, Merkies IS. Intraepidermal nerve fiber density at the distal leg: a worldwide normative reference study. Journal of the peripheral nervous system: JPNS. 2010;15:202–207. doi: 10.1111/j.1529-8027.2010.00271.x. [DOI] [PubMed] [Google Scholar]
- 17.Lauria G, Holland N, Hauer P, Cornblath DR, Griffin JW, McArthur JC. Epidermal innervation: changes with aging, topographic location, and in sensory neuropathy. Journal of the neurological sciences. 1999;164:172–178. doi: 10.1016/s0022-510x(99)00063-5. [DOI] [PubMed] [Google Scholar]
- 18.Lima LV, DeSantana JM, Rasmussen LA, Sluka KA. Short-duration physical activity prevents the development of activity-induced hyperalgesia through opioid and serotoninergic mechanisms. Pain. 2017;158:1697–1710. doi: 10.1097/j.pain.0000000000000967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McVinnie DS. Obesity and pain. Br J Pain. 2013;7:163–170. doi: 10.1177/2049463713484296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mogil JS, Wilson SG, Bon K, Lee SE, Chung K, Raber P, Pieper JO, Hain HS, Belknap JK, Hubert L, Elmer GI, Chung JM, Devor M. Heritability of nociception I: responses of 11 inbred mouse strains on 12 measures of nociception. Pain. 1999;80:67–82. doi: 10.1016/s0304-3959(98)00197-3. [DOI] [PubMed] [Google Scholar]
- 21.Nealon CM, Patel C, Worley BL, Henderson-Redmond AN, Morgan DJ, Czyzyk TA. Alterations in nociception and morphine antinociception in mice fed a high-fat diet. Brain Res Bull. 2017 doi: 10.1016/j.brainresbull.2017.06.019. [DOI] [PubMed] [Google Scholar]
- 22.O’Brien PD, Sakowski SA, Feldman EL. Mouse models of diabetic neuropathy. ILAR J. 2014;54:259–272. doi: 10.1093/ilar/ilt052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Obrosov A, Shevalye H, Coppey LJ, Yorek MA. Effect of tempol on peripheral neuropathy in diet-induced obese and high-fat fed/low-dose streptozotocin-treated C57Bl6/J mice. Free Radic Res. 2017;51:360–367. doi: 10.1080/10715762.2017.1315767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Obrosova IG, Ilnytska O, Lyzogubov VV, Pavlov IA, Mashtalir N, Nadler JL, Drel VR. High-fat diet induced neuropathy of pre-diabetes and obesity: effects of “healthy” diet and aldose reductase inhibition. Diabetes. 2007;56:2598–2608. doi: 10.2337/db06-1176. [DOI] [PubMed] [Google Scholar]
- 25.Pittenger GL, Ray M, Burcus NI, McNulty P, Basta B, Vinik AI. Intraepidermal nerve fibers are indicators of small-fiber neuropathy in both diabetic and nondiabetic patients. Diabetes care. 2004;27:1974–1979. doi: 10.2337/diacare.27.8.1974. [DOI] [PubMed] [Google Scholar]
- 26.Shimomura K, Galvanovskis J, Goldsworthy M, Hugill A, Kaizak S, Lee A, Meadows N, Quwailid MM, Rydstrom J, Teboul L, Ashcroft F, Cox RD. Insulin secretion from beta-cells is affected by deletion of nicotinamide nucleotide transhydrogenase. Methods in enzymology. 2009;457:451–480. doi: 10.1016/S0076-6879(09)05025-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simon MM, Greenaway S, White JK, Fuchs H, Gailus-Durner V, Wells S, Sorg T, Wong K, Bedu E, Cartwright EJ, Dacquin R, Djebali S, Estabel J, Graw J, Ingham NJ, Jackson IJ, Lengeling A, Mandillo S, Marvel J, Meziane H, Preitner F, Puk O, Roux M, Adams DJ, Atkins S, Ayadi A, Becker L, Blake A, Brooker D, Cater H, Champy MF, Combe R, Danecek P, di Fenza A, Gates H, Gerdin AK, Golini E, Hancock JM, Hans W, Holter SM, Hough T, Jurdic P, Keane TM, Morgan H, Muller W, Neff F, Nicholson G, Pasche B, Roberson LA, Rozman J, Sanderson M, Santos L, Selloum M, Shannon C, Southwell A, Tocchini-Valentini GP, Vancollie VE, Westerberg H, Wurst W, Zi M, Yalcin B, Ramirez-Solis R, Steel KP, Mallon AM, de Angelis MH, Herault Y, Brown SD. A comparative phenotypic and genomic analysis of C57BL/6J and C57BL/6N mouse strains. Genome Biol. 2013;14:R82. doi: 10.1186/gb-2013-14-7-r82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thurlby PL, Trayhurn P. The role of thermoregulatory thermogenesis in the development of obesity in genetically-obese (ob/ob) mice pair-fed with lean siblings. Br J Nutr. 1979;42:377–385. doi: 10.1079/bjn19790127. [DOI] [PubMed] [Google Scholar]
- 29.Toye AA, Lippiat JD, Proks P, Shimomura K, Bentley L, Hugill A, Mijat V, Goldsworthy M, Moir L, Haynes A, Quarterman J, Freeman HC, Ashcroft FM, Cox RD. A genetic and physiological study of impaired glucose homeostasis control in C57BL/6J mice. Diabetologia. 2005;48:675–686. doi: 10.1007/s00125-005-1680-z. [DOI] [PubMed] [Google Scholar]
- 30.Watcho P, Stavniichuk R, Ribnicky DM, Raskin I, Obrosova IG. High-fat diet-induced neuropathy of prediabetes and obesity: effect of PMI-5011, an ethanolic extract of Artemisia dracunculus L. Mediators of inflammation. 2010;2010:268547. doi: 10.1155/2010/268547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. The Journal of clinical investigation. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]