Abstract
Following the collapse of a cavitation bubble cloud, residual microbubbles can persist for up to seconds and function as weak cavitation nuclei for subsequent pulses in a phenomenon known as cavitation memory effect. In histotripsy, the cavitation memory effect can cause bubble clouds to repeatedly form at the same discrete set of sites. This effect limits the efficacy of histotripsy-based tissue fractionation. Our previous studies have shown that low-amplitude bubble coalescing (BC) ultrasound sequences interleaved between high-amplitude histotripsy pulses can coalescence the residual bubbles into one large bubble quickly. This reduces the cavitation memory effect and may increase treatment efficacy. Histotripsy has been investigated for thrombolysis by breaking up clots to debris smaller than red blood cells. However, this treatment has low efficacy for aged or retracted clot. In this study, we investigate the use of histotripsy with BC to improve the treatment efficacy for retracted clots. An integrated histotripsy and bubble coalescing (HBC) transducer system with specialized electronic driving system was built in-house. One high amplitude (32 MPa), 1-cycle histotripsy pulse followed by 36 low amplitude (2.4 MPa), 1-cycle BC pulses formed one HBC sequence. Results show that HBC sequences successfully generated a flow channel through the retracted clots under scan speeds of 0.2 – 0.5 mm/s. The created channel size was 128–480% larger using the HBC sequence compared to using histotripsy alone. The clot debris particles generated during HBC treatments were within the safe range. These results demonstrate the concept that BC improves treatment efficacy of histotripsy thrombolysis for retracted clots.
Keywords: Thrombolysis, Sonothrombolysis, Histotripsy, Cavitation memory effect, Bubble coalescence, Retracted clot
Introduction
Venous thromboembolism
Venous thromboembolism (VT) is a major public health concern. Every year 375,000 to 425,000 new VT cases are diagnosed and medically treated (Grosse, et al. 2016). The most common type of VT is deep vein thrombosis (DVT), which usually occurs in the deep veins of the leg and can result in pulmonary embolism. Current clinical treatment methods include anticoagulation or intravenous administration of thrombolytic drugs (Bates and Ginsberg 2004, Hacke, et al. 2008), catheter-based endovascular techniques (Kim, et al. 2006), and a combination therapy where catheters are used to directly infuse thrombolytic drugs (Semba and Dake 1994). Intravenous administration of thrombolytic drugs has limited effectiveness, requires long treatment times (several hours to days), and carries a risk of major bleeding that can be fatal in a small number of cases (Lansberg, et al. 2009, Leizorovicz, et al. 1994). Catheter-based therapies are invasive with high cost, and also carry risks of hemorrhage, damage to the vessel wall, and infection (Sharafuddin, et al. 2003).
Sonothrombolysis
Therapeutic ultrasound has been explored as a technique to promote clot lysis as both a stand-alone procedure and in conjunction with thrombolytic drugs or microbubbles (Atar and Rosenschein 2004, Siegel and Luo 2008). Substantial work has been done to enhance the efficiency of thrombolytic drugs by applying low-intensity unfocused ultrasound (Alexandrov, et al. 2004, Datta, et al. 2006, Hitchcock, et al. 2011, Pfaffenberger, et al. 2003, Siegel, et al. 2000, Stone, et al. 2007). Moreover, ultrasound combined with microbubbles is able to break down blood clots successfully in both the presence and absence of thrombolytic drugs (Brown, et al. 2011, Datta, et al. 2008, Mizushige, et al. 1999, Molina, et al. 2006, Porter and Xie 2001, Shi, et al. 2016). It has also been shown that therapeutic ultrasound exhibits the ability to induce rapid clot breakdown independently without thrombolytic drugs and exogenous microbubbles (Burgess, et al. 2012, Maxwell, et al. 2009, Maxwell, et al. 2011, Rosenschein, et al. 2000, Wright, et al. 2012, Zhang, et al. 2017, Zhang, et al. 2016, Zhang, et al. 2015).
Aged or Retracted Clot
For DVT cases, patients typically seek medical treatment after symptoms occur, which usually happens several days after initial clot formation. After a clot is formed, clot retraction initiated by platelets takes place and changes the structure and composition of the clot dramatically over time (Feghhi and Sniadecki 2011, Fox and Phillips 1983). In the clot retraction process, the fibrin network shrinks, effectively increasing fibrin spatial density and/or decreasing plasminogen concentration, and resulting in lower porosity and reduced permeability (Blinc, et al. 1994, Carr and Hardin 1987, Kirchhof, et al. 2003). Previous studies show that drug-mediated methods and the sonothrombolysis methods are not effective in treating retracted clots, likely due to the clot’s reduced permeability (Sutton, et al. 2013).
Histotripsy
Histotripsy liquefies soft tissue to acellular debris through well controlled action of cavitation bubbles generated by short, high-intensity ultrasound pulses (Parsons, et al. 2006, Xu, et al. 2005, Xu, et al. 2005). Cavitation microbubbles are generated from endogenous cavitation nuclei in the target tissue during histotripsy treatment. Rapid expansion and collapse of these microbubbles produce high strain, thereby fractionating soft tissue. The feasibility of histotripsy thrombolysis has been investigated as a noninvasive, drug-free, and image guided method in vitro (Maxwell, et al. 2009, Zhang, et al. 2015, Zhang, et al. 2015)and in vivo (Ghorbani, et al. 2016, Maxwell, et al. 2011, Zhang, et al. 2017). Multi-cycle histotripsy pulses generating cavitation bubble clouds via a shock scattering mechanism were used to disintegrate blood clots by Maxwell et al. (Maxwell, et al. 2009, Maxwell, et al. 2011). Zhang et al. improved the precision of the histotripsy thrombolysis by using single-cycle histotripsy pulses to produce cavitation clouds via an intrinsic threshold mechanism, and minimized the risk of vessel damage (Zhang, et al. 2017, Zhang, et al. 2016, Zhang, et al. 2015). Effort was also made by Bader et al. to improve DVT treatment through combination of histotripsy and thrombolytic agent (Bader, et al. 2016).
Cavitation memory effect
After collapse of a cavitation cloud, it takes time on the order of seconds for the remnant microbubbles to completely dissolve. The residual microbubbles provide cavitation nuclei for subsequent cavitation in the same locations (Huber, et al. 1999, Lundt, et al. 2017, Wang, et al. 2012). When subsequent pulses arrive before the residual cavitation nuclei dissolve, a cavitation cloud with a similar pattern as the previous cavitation cloud is generated by re-exciting the residual nuclei. Pre-focal cavitation or peripheral cavitation surrounding the focal center zone is also often observed, resulting in distortion and attenuation of histotripsy pulses at the intended focus. The cavitation memory effect has been shown to significantly reduce the treatment efficacy of histotripsy and may increase the peripheral damage outside of the intended target (Duryea, et al. 2015, Wang, et al. 2012).
Passive approach to mitigate cavitation memory effect
The cavitation memory effect can be removed passively by reducing histotripsy pulse repetition frequency (PRF), which allows residual cavitation nuclei enough time to dissolve between successive pulses (Wang, et al. 2012). Our previous study shows that the number of histotripsy pulses required producing a given level of tissue fractionation decreases with a lower PRF. If a histotripsy PRF of 1 Hz or lower is used, the cavitation memory effect can be neglected. The per-pulse damage efficacy reaches its maximized and the lesion shape matches well with the transducer focus. However, the treatment rate over time is very low using such a low PRF and may not be reasonable in a clinical setting.
Bubble coalescing to actively mitigate cavitation memory effect
In previous work by Duryea et al. (Duryea, et al. 2015, Duryea, et al. 2015), the cavitation memory effect at a high PRF was eliminated by using 1000-cycle 1-MPa tone bursts interleaved by high amplitude histotripsy pulses transmitted by a secondary transducer confocal with the histotripsy transducer. The mechanism was hypothesized to be bubble coalescing (BC) induced by secondary Bjerknes forces associated with the tone burst (Duryea, et al. 2014, Duryea, et al. 2015). The residual nuclei were actively coalesced to a large bubble by the low amplitude tone bursts, effectively reducing and removing the cavitation memory effect. This method allowed the high per-pulse efficiency associated with low pulse rates to be maintained at a 100 Hz PRF and produced homogeneous, reproducible lesion shapes.
Histotripsy thrombolysis with BC
To evaluate the feasibility of enhancing the efficacy of histotripsy-induced thrombolysis using a BC sequence, an integrated histotripsy and bubble coalescing (HBC) transducer system was constructed. This HBC system has specialized driving electronics to deliver both high amplitude (P- >= 30 MPa) histotripsy pulses interleaved with low amplitude (~1–2 MPa) BC sequences. The integrated transducer design dispenses with the need for a second BC transducer and driving system. The HBC transducer system was used to restore flow channels through eight retracted clots in an in vitro DVT model. The histotripsy treatment efficacy (size of the flow channel, restored flow rate) using BC was measured and compared to that using histotripsy alone. This is the first study to investigate HBC for thrombolysis.
Materials and methods
In vitro DVT model
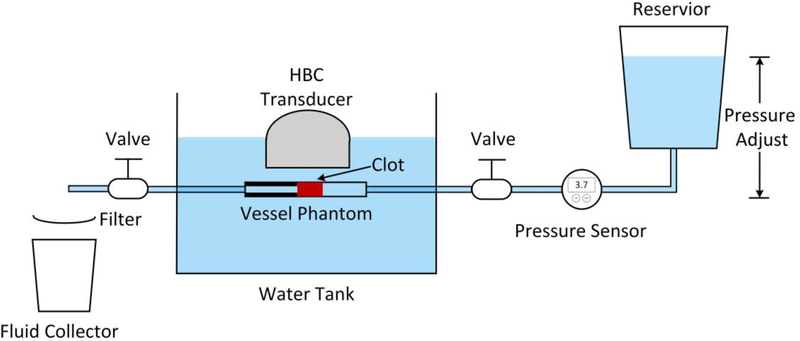
An in vitro flow model (Fig. 1) was designed to mimic an occlusive DVT, which obstructs blood flow completely, but continues to exert hydrostatic pressure on the blood clot. The flow model contained a reservoir, a pressure sensor, a vessel phantom, a filter, and a fluid collector as shown in Fig. 1. Normal saline was poured into the reservoir that was set on a height adjustable holder to apply a constant pressure of 3.7 mmHg on the clots. The pressure was chosen according to reported femoral vein pressure (Albrechtsson, et al. 1981, Negus and Cockett 1967), and was measured by the pressure sensor (MG-9V; SSI Technologies, Janesville, WI, USA). The valves remained opened at all times during each treatment. The vessel phantom with an occlusive clot was connected to the valves through silicone tubes and placed horizontally in degassed deionized water. After the successful generation of a flow channel through the clot, flow would be restored through the vessel phantom thus allowing clot debris to pass through the filter and be retained into the fluid collector.
Fig. 1.
Schematic diagram of in vitro flow model.
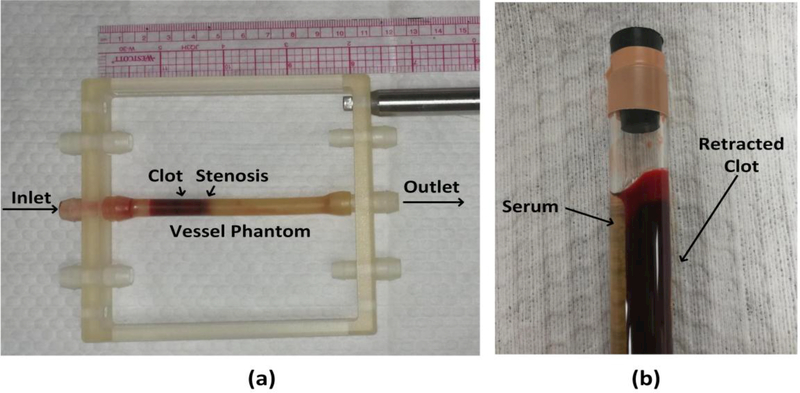
The vessel phantom was developed to mimic a human femoral vein (Fig. 2a). The phantom was made from urethane polymer (Urethane RTV Mold-Making System; Tap Plastics Inc., San Leandro, CA, USA) that has acoustic properties similar to human tissue (Browne, et al. 2003). The outer diameter of the vessel phantom was 8 mm while inner diameters were 6.5 mm on one side and 4.2 mm on the other side forming a 35% stenosis that prevented clots from translating under hydrostatic pressure. The detailed procedure to make the vessel had been described in our previous work (Zhang, et al. 2015).
Fig. 2.
(a) The vessel phantom was held by a 3-D-printed frame. A 35% stenosis in the vessel phantom was used to stabilize the clot so that it did not slip under pressure. The inner diameter was 4.2 mm on the downstream side of the stenosis and 6.5 mm on the upstream side. (b) A clot was retracted in a hydrophilic glass tube after 3 d in 4°C incubation.
Retracted Clot
Retracted clots were made following a protocol described by Sutton et al. (Sutton, et al. 2013). Fresh bovine blood was collected from a local abattoir. A citrate-phosphate-dextrose solution (#C7165; Sigma Aldrich Co., St. Louis, MO, USA) was immediately added to the blood as anti-coagulant at a ratio of 1:9. The blood was used to make clots within the same day it was collected. Bovine blood was first warmed to body temperature (38.6°C) in a water bath. Calcium chloride (#21107; Sigma-Aldrich Co.) was injected into the blood to a final concentration of 20 mmol/L to stimulate clotting. The stimulated blood was carefully poured into a hydrophilic glass tube (Borosilicate Glass Tubing; GSC International Inc., Nixa, MO, USA) with an inner diameter of 8.5 mm to form a retracted clot. After incubation of 3 hours in the water bath, clot and serum were separated (Fig. 2b). The tube with newly formed retracted clot inside was stored at 4 °C for 3 days for further retraction.
After the 3-day incubation, the retracted clots usually had a diameter of 6.5 mm and length of 10 cm. The 10-cm long clot was cut into 2-cm length clots for individual experiments. Before each experiment, three steps were followed to place and fix the clot inside the vessel phantom. First, the clot was warmed up to 38.6°C in fresh bovine blood. Second, the bovine blood with the clot inside mixed with calcium chloride at a final concentration of 20 mmol/L to stimulate clot cross-linking with the vessel phantom. Third, the clot with the stimulated blood coating was inserted into the vessel phantom from the 6.5-mm inner diameter side all the way to the stenosis side. The vessel phantom was then placed in a water bath at 38.6 °C. After 2 hours, the blood coating clotted and cross-linked the retracted clot to the interior of the vessel phantom. The fully-occluded vessel phantom was then connected in-line with the rest of the flow model and the connecting tubes were filled with saline.
HBC system
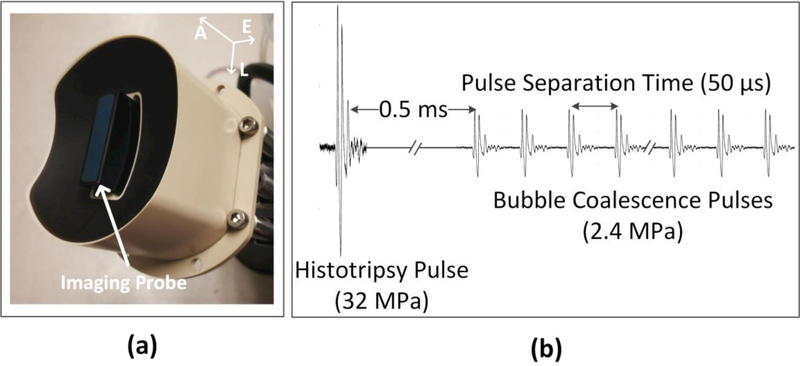
An ultrasound image-guided HBC thrombolysis system was developed in our laboratory. The integrated transducer (Imasonic S.A., Besancon, France) used to generate the HBC pulses is shown in Fig. 3(a). The transducer consisted of an array of 18 elements. The transmitted ultrasound intensity of each element on the integrated transducer array could be individually controlled by a field-programmable gate array (FPGA) and amplified by an amplifier constructed by our group. A custom ultrasound imaging probe (L7.5 MHz; Vermon S.A., Tours, France) was embedded into the central hole of the therapy transducer. SonixTouch imaging machine (BK Ultrasound, Vancouver, British Columbia, Canada) was used to guide and monitor histotripsy thrombolysis treatment on an imaging system.
Fig. 3.
HBC transducer (a) and general pulse scheme (b) used to study the thrombolysis by using HBC sequence. A = axial; L = lateral; E = elevational.
Design of HBC sequence
Fig. 3(b) describes the design of HBC sequence. For histotripsy pulses, all 18 elements were excited simultaneously at a PRF of 100 Hz. Histotripsy pulses at the geometric focus had a center frequency of 1 MHz, and a peak negative pressure (P−) of 32 MPa. The focal beam volume (−6 dB) of the transducer was measured to be 6.5 (axial)×1.3 mm (lateral)×1.5 mm (elevational) at a peak negative pressure of 15 MPa using a fiber-optic probe hydrophone (Parsons, et al. 2006). Peak negative pressures larger than 20 MPa cannot be directly measured and therefore was estimated by linear summation of the peak negative pressure outputs from three separate groups of the transducer elements (six elements per group). For BC pulses, each element was excited individually in sequence to produce a high PRF burst with peak amplitudes of 2.4 MPa. All BC pulses were applied at a delay of 0.5 ms following the histotripsy pulse that allowed the histotripsy bubble cloud to collapse and produce residual nuclei in an unimpeded manner (Duryea, et al. 2015).
BC parameter study
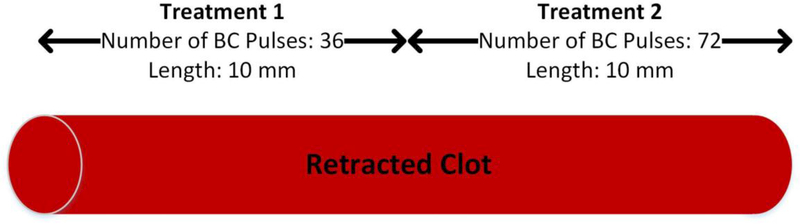
To minimize the output energy while maintaining the highest efficacy of histotripsy thrombolysis, the effect of the number of BC pulses in one HBC sequence was studied. In a recent study (Shi, et al. 2018), a BC sequence consisting of sixty 2.2-MPa pulses was able to remove cavitation memory effect in a gel phantom. In the present study, two BC pulse sequences were tested, one with 36 BC pulses and the other with 72 BC pulses. To reduce the impact of variability across clots, the HBC sequence containing 36 BC pulses was used to treat half of one clot, and the HBC sequence with 72 BC pulses was used to treat the other half of the same clot (Fig. 4). The average peak pressure and the separation time of BC pulses were kept as 2.4 MPa and 50 μs respectively. The time delay of one BC pulse following the previous BC pulse in the same BC sequence was defined as the pulse separation time. The scan speed of the histotripsy focus in the retracted clot was 0.5 mm/s. This trial was repeated 8 times.
Fig. 4.
Schematic diagrams of HBC sequence optimization.
Histotripsy thrombolysis Treatment
The vessel phantom blocked by the retracted clot was connected to the flow model and placed in a tank filled with deionized water. Deionized water contained in the water tank was degassed to 22% of air saturation prior to the experiment at room temperature (~22°C) to remove gas nuclei to mimic in vivo conditions. The therapy transducer with the imaging probe was mounted to a three-axis mechanical positioning system and its lateral axis was oriented perpendicular to the vessel phantom so that the cross section of vessel was imaged during treatment. The treatment path of the transducer in the clot was determined through the following approach. First, for targeting, a bubble cloud was generated in the free field using histotripsy pulses. The center location of the bubble cloud was marked on the ultrasound imaging window as the treatment focus. Second, guided by the real-time ultrasound images, the transducer was moved using the motorized positioner to align the treatment focus at the center of vessel lumen. The transducer was then adjusted to move the treatment focus from one end of the clot to the other end. Third, the movement of the transducer was recorded as a scan path. For treatment, the transducer would move following the pre-determined scan path to apply HBC along the 2-cm clot at a constant speed of 0.2 mm/s and 0.5 mm/s. In this experiment and the large flow channel experiment below, the optimal HBC pulsing scheme from the BC parameter study was employed to treat the entire 2-cm retracted clot. Restored flow and debris size were measured to quantify the treatment results.
Flow channel evaluation
To quantitatively evaluate the flow channels generated during the treatments, each treated clot was scanned using a 20-MHz high-resolution ultrasound probe. The probe was mounted onto the motorized positioner after the treatment. With the imaging plane perpendicular to the vessel axis, the probe was moved by the positioner from one end to the other end of the clot with a step size of 0.2 mm. One cross-sectional image of the clot was taken after waiting for 2 s at each location to allow for image stabilization. One hundred scan images were collected in total from each treated clot. The fractionated clot region was hypoechoic (low brightness on image) when the flow was restored, in comparison to the hyperechoic (high brightness on image) intact clot region. A global image threshold using Otsu’s method was applied to the images. Inside the channel area on each scan image, a region growing method was used to detect the whole cross section of flow channel. To quantify the size of flow channel, the cross-sectional area of the flow channel region was calculated using custom image processing software (Matlab, The MathWorks Inc., Natick, MA, USA).
Restored flow measurement
To evaluate the efficacy of histotripsy thrombolysis, restored flow rates through the re-canalized flow channels were measured. After the flow channel was created, all saline that exited the outlet within 2 min was collected and measured. When the flow was very slow, the restored saline was collected for 5 min. In the large flow channel study, the restored saline was collected for 1 min. The restored flow rate was calculated as the volume of collected saline divided by time (mL/s). Due to the small change in reservoir volume, the pressure was assumed to be constant over this time period.
Debris particle size measurement
As there is a concern that clot debris particles generated from histotripsy thrombolysis treatment may cause hazardous embolization, the debris particles were collected and the size distribution was measured. The size distribution of debris particle induced by histotripsy treatment was measured using microscopic inspection and Coulter counter analysis. Clot debris flushed by the restored flow drained through a filter sheet with a pore size of 300 μm into a fluid collector. Debris particles larger than 300 μm were held by the filter and examined under a microscope. Optical images of the filter sheet with a resolution of 20 μm were captured. The number of distinguishable particles larger than 300 μm was counted. The size distribution of the debris particles in the filtered fluid was analyzed using a Coulter counter (Multisizer 3; Beckman Coulter, Brea, CA, USA). Two aperture tubes of 100 μm and 560 μm were used to measure particles ranged from 2–60 μm and from 60–300 μm. The measurements using these two aperture tubes were combined to generate a distribution of debris particles 2–300 μm.
Large flow channel study
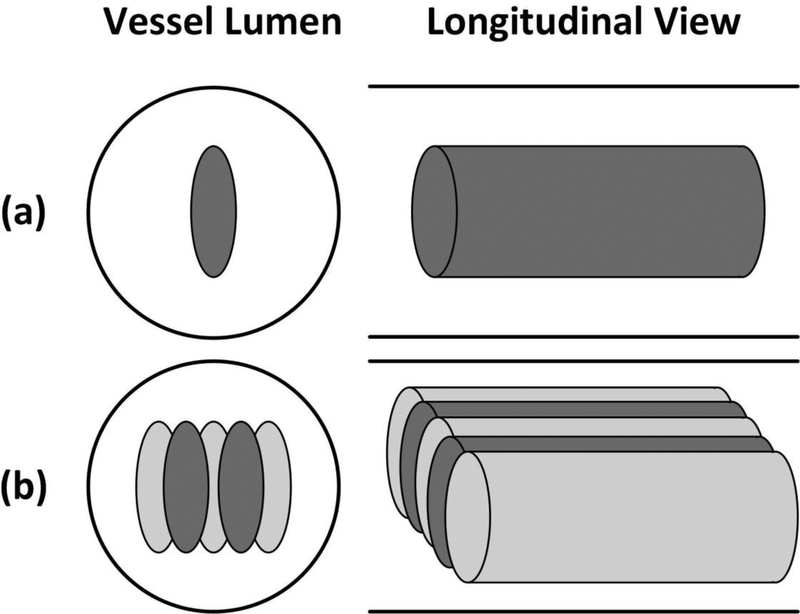
The approximately ellipsoidal focus of the transducer had an axial length much larger than its lateral length. Therefore the channel produced by single-pass treatment was usually too narrow for clinical application and treatment in vivo. To produce a wider flow channel, HBC was combined with a dual-pass multi-focus treatment strategy previously developed by our group. As described in the paper by Zhang et al. (Zhang, et al. 2016), the multi-focus strategy electronically steered the focus of the therapy transducer in the lateral direction. The dual-pass strategy essentially divided the multi-focus treatment into two treatment passes. In the first pass, the treatment zone was scanned through the clot and covered by two foci at each scan location. In the second pass, the treatment zone was scanned through the clot again and covered by three additional foci, resulting in effective five foci treated at each scan location, respectively (Fig. 5). Prior work by Zhang et al. (Zhang, et al. 2016) demonstrated that this dual-pass multi-focus strategy significantly increased the re-canalized flow channel size compared to the single-focus treatments while retaining the treatment speed. The foci treated within one pass were separated by 1 mm in the lateral direction. The scan speed of each pass was 0.2 mm/s.
Fig. 5.
Schematic diagrams of the treatment strategies in the vessel phantom, with the solid lines outlined the inner vessel lumen. The ellipses in the vessel lumen represent the treatment foci. (a) The single-focus strategy; (b) the 2+3-foci dual-pass strategy. The darker foci were treated in the first treatment pass and the light grayish foci were treated in the second treatment pass.
Results
BC parameter study
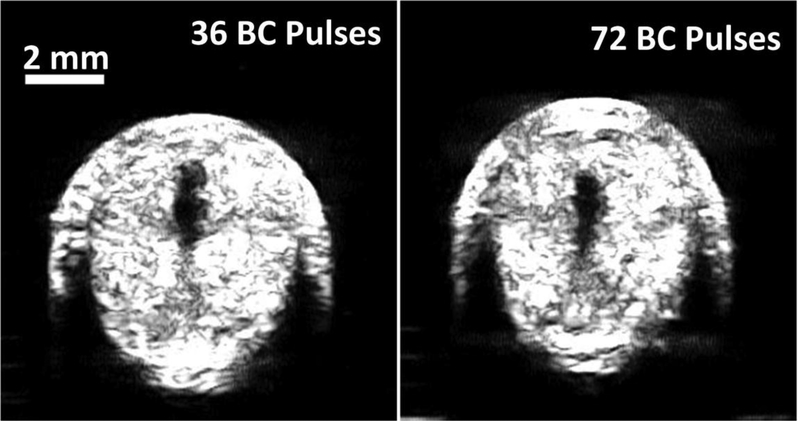
Both the 36- and 72-pulse BC sequences were used to restore flow channels through the occlusive, retracted clots. The sizes of channels produced by the two HBC sequences (36 and 72 BC pulses) were similar in retracted blood clot (Fig. 6). The HBC sequences of 36 BC pulses at scan speed of 0.5 mm/s generated channels with a cross-sectional area of 0.99 mm2 ± 0.23 mm2, measured by the ultrasound image. Similarly, a cross-sectional area of 0.93 mm2 ± 0.19 mm2 through the restored flow channel was generated by HBC sequences of 72 BC pulses. Statistical analyses (two-sided Student’s t-test) demonstrated no significant difference of cross-sectional area generated using the HBC sequence of 36 BC pulses versus 72 BC pulses (p = 0.56). No cavitation or channel production was observed when only BC sequence was used. Additional acoustic energy delivered by 36 BC pulses compared to using histotripsy alone was 20%, while 72 BC pulses added 41% additional energy. To minimize energy deposition to the focus, the HBC sequence of 36 BC pulses was selected.
Fig. 6.
Representative ultrasound images of the cross sections of the flow channels generated using histotripsy pulses followed by 36 BC pulses (left) and 72 BC pulses (right).
Flow channel evaluation
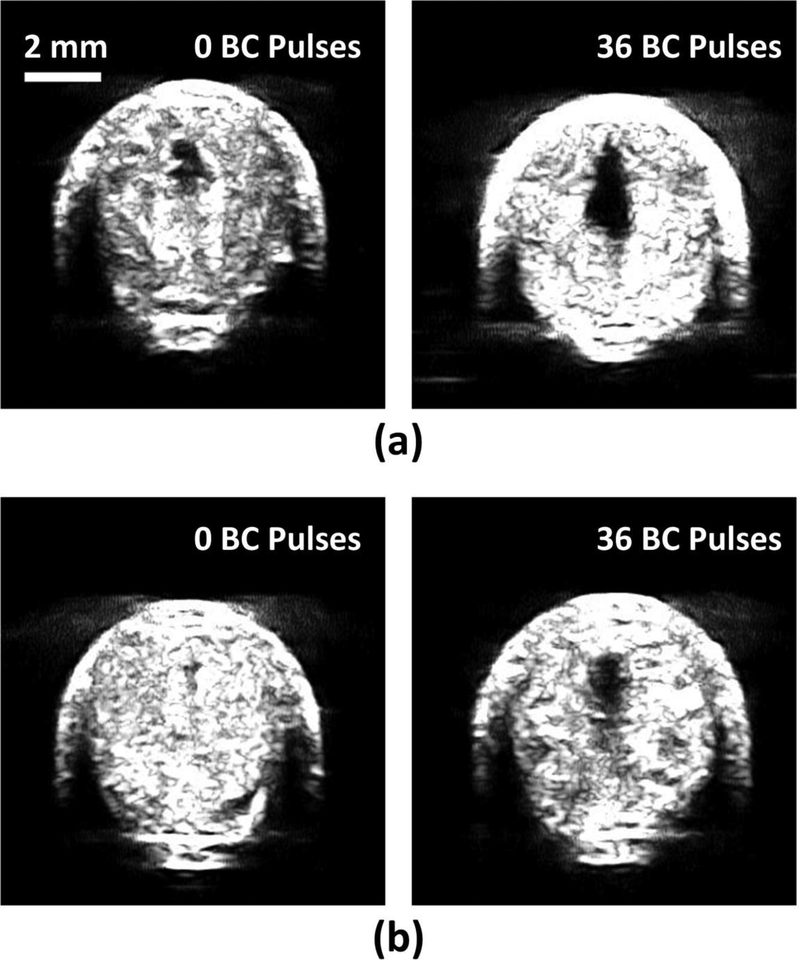
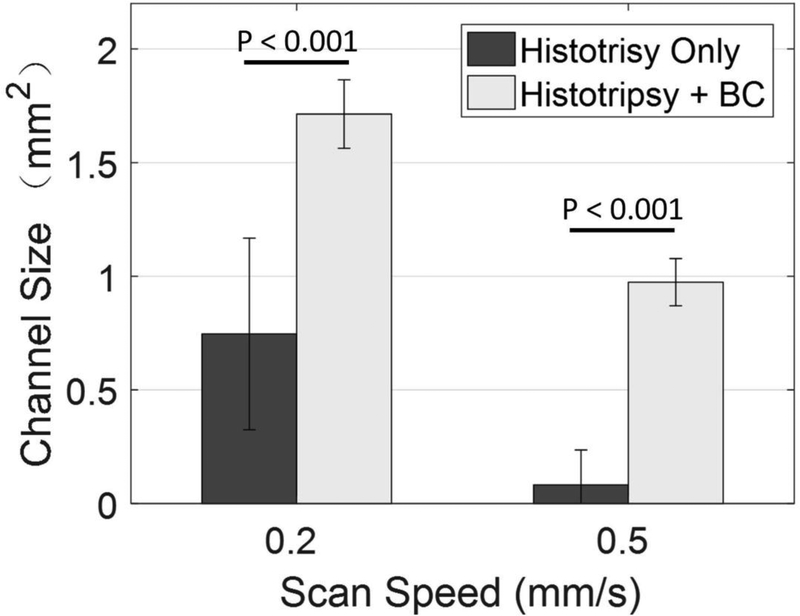
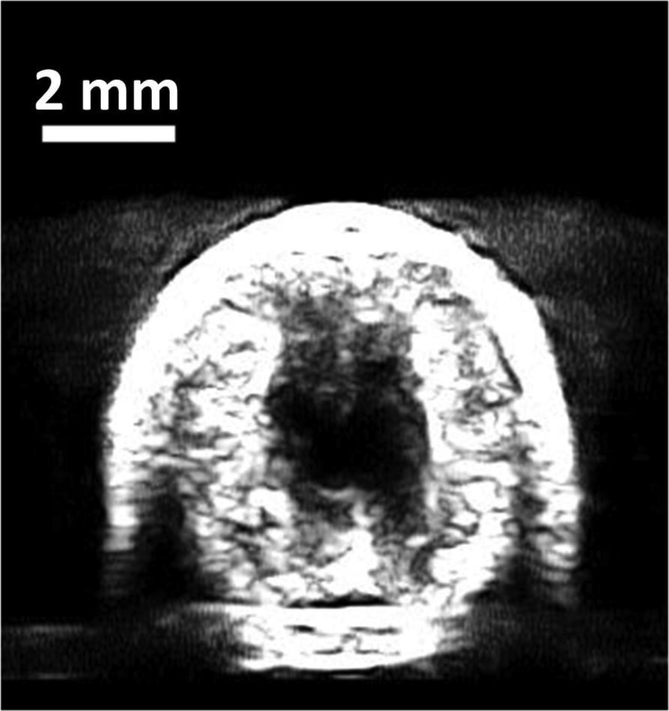
Histotripsy pulses generated flow channels in retracted blood clots with or without BC pulses at the scan speed of 0.2 mm/s. The cross-sectional area of the restored flow channel was 0.75 mm2 ± 0.42 mm2 using histotripsy only, and was 1.71 mm2 ± 0.15 mm2 with histotripsy and BC pulses (Fig. 7a). At a scan speed of 0.5 mm/s, a flow channel was not consistently generated using histotripsy alone, while the HBC sequence consistently generated a flow channel. The cross-sectional areas of the restored flow channel were 0.08 mm2 ± 0.15 mm2 and 0.87 mm2 ± 0.10 mm2, for using histotripsy alone and HBC, respectively (Fig. 7b). Statistical analyses (one-sided Student’s t-test) indicated significant increases in the cross-sectional areas of flow channel generated using the HBC sequence versus histotripsy alone at both scan speeds (p<0.001) as show in Fig. 8. The treatment time to create a flow channel through the 2-cm long retracted clot was 1.67 minutes at 0.2 mm/s scan speed and 0.67 minutes at 0.5 mm/s scan speed.
Fig. 7.
Representative ultrasound images of the cross sections of the flow channels generated using histotripsy pulses alone (left) and HBC sequences (right) at scan speeds of 0.2 mm/s (a) and 0.5 mm/s (b).
Fig. 8.
The mean areas of the cross sections of the flow channels generated using histotripsy pulses alone and HBC sequences at scan speeds of 0.2 mm/s and 0.5 mm/s. N = 8.
Restored flow measurement
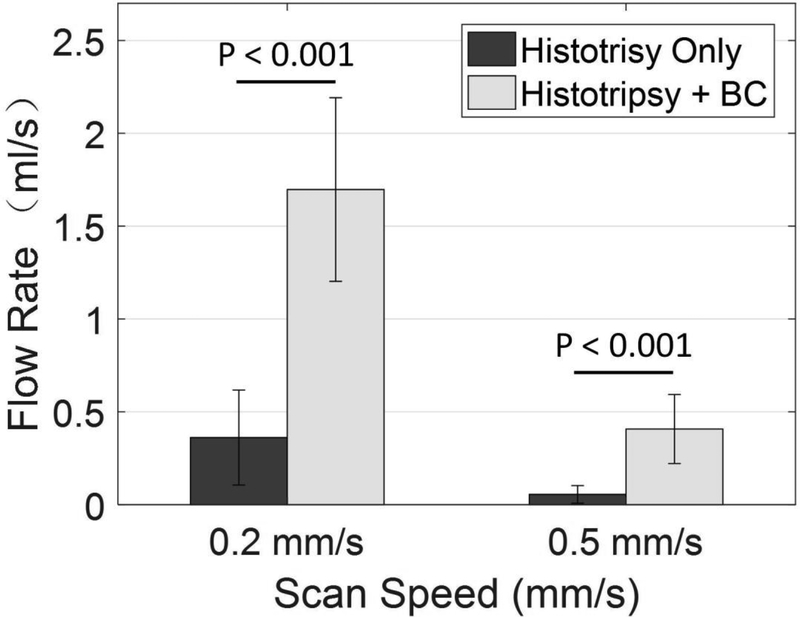
Before any treatment, no flow was observed. Flow was restored after recanalization using histotripsy with and without BC sequences at a scan speed of 0.2 mm/s (Fig. 9). With constant pressure (3.7 mmHg) applied, a larger channel would be expected to permit higher flow rate. The restored flow rate using histotripsy pulses alone was 0.36 ml/s ± 0.26 ml/s, and was 1.70 ml/s ± 0.49 ml/s with BC sequences. The restored flow rate decreased as the scan speed increased from 0.2 mm/s to 0.5 mm/s. The restored flow rate was 0.06 ml/s ± 0.05 ml/s using histotripsy alone, and was 0.41 ml/s ± 0.19 ml/s with BC sequences. The flow rates using HBC sequences were significantly higher than using histotripsy alone at scan speeds of 0.2 mm/s (p<0.001) and 0.5 mm/s (p<0.001), which is similar to the comparison of flow channel in Fig. 8.
Fig. 9.
Restored flow rates after treatments of histotripsy pulses alone and HBC sequences at scan speeds of 0.2 mm/s and 0.5 mm/s. N = 8.
Debris particle size measurement
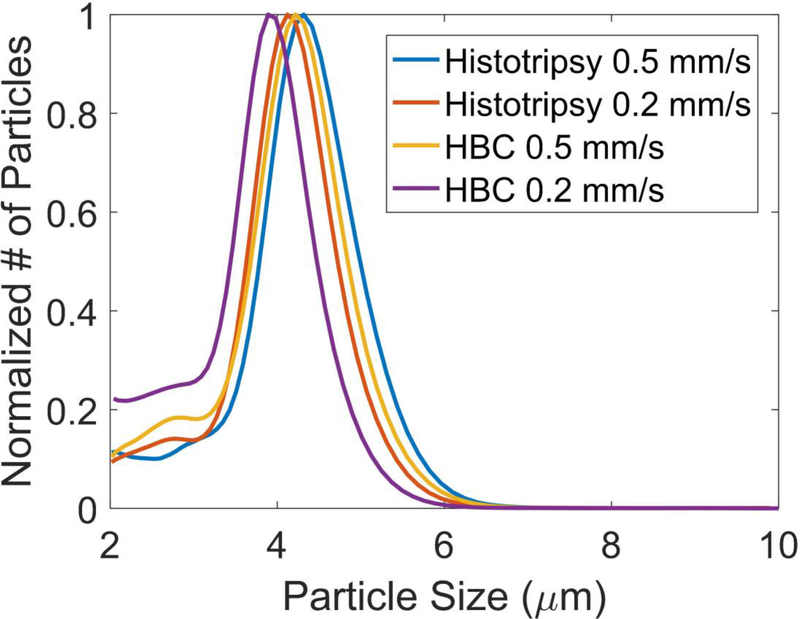
For all treatments, no debris particles larger than 300 μm were detected. The Coulter Counter analysis showed that over 99.9% of the number of the debris particles were less than 10 μm, and the number of the debris particles became orders of magnitude less as the size increased from 10 μm to 100 μm. The clot debris from the four treatment groups (Histotripsy alone/HBC at 0.2 and 0.5 mm/s scan speed) had similar normalized distributions of debris size centered at approximately 4 μm (Fig. 10), and the only difference was the number of debris particles. The debris particles larger than 100 μm generated were calculated and summarized in Table 1. The average numbers of particles larger than 100 μm were similar for histotripsy and HBC treatments at the same scan speed, even though HBC sequence generated much larger channels. In each of the 32 treatments, the number of generated particles with diameter larger than 100 μm was no larger than 10.
Fig. 10.
The normalized distributions of debris particles generated during treatments of histotripsy pulses alone and HBC sequences at scan speeds of 0.2 mm/s and 0.5 mm/s.
Table 1.
Summary of the generated debris > 100 μm (N = 8).
| Particle distribution | Histotripsy at scan speed of 0.2 mm/s | HBC at scan Speed of 0.2 mm/s | Histotripsy at scan speed of 0.5 mm/s | HBC at scan Speed of 0.5 mm/s |
|---|---|---|---|---|
| Treatments with particles > 100 μm | 8/8 | 7/8 | 5/8 | 7/8 |
| Average number of particles > 100 μm | 5.3 | 4.1 | 2.1 | 2.6 |
| Average size of particles between 100–300 μm | 127.2 | 146.3 | 118.9 | 120.6 |
| Maximal particle size > 100 μm | 242.7 | 275.8 | 184.1 | 167.9 |
| Particles > 300 μm | 0 | 0 | 0 | 0 |
Large flow channel study
The feasibility of combining HBC and dual-pass multi-focus strategy to create a larger flow channel was investigated. Fig. 11 shows the flow channel generated through the retracted clot using HBC sequence following the 2+3-foci dual-pass strategy at a scan speed of 0.2 mm/s for each pass. The average cross-sectional area of the flow channel generated was 4.92 mm2 ± 0.58 mm2, compared to the cross-sectional area of 1.71 mm2 ± 0.15 mm2 using HBC and a single focus at the same scan speed. The height of the channels was 3.11 mm ± 0.21 mm. The width of the channels was 2.35 mm ± 0.05 mm. The vessel phantom was completely blocked by the retracted clot before the treatment. After recanalization, the flow was restored to 3.30 ml/s ± 1.20 ml/s by employing the joint strategy. The 2-cm retracted clot was re-canalized in 3.33 minutes using HBC with the dual-pass multi-focus strategy. Thus, the HBC thrombolysis method with dual-pass multi-focus strategy resulted in a thrombolysis speed of 1.67 min/cm and a flow channel with cross-sectional area of 4.92 mm2 ± 0.58 mm2, while our previous study showed a thrombolysis speed of 5.5 min/cm and a flow channel with cross-sectional area of 4.13 ± 1.09 mm2 using only histotripsy with the dual-pass multi-focus strategy. This flow channel would be sufficiently large and considered clinically relevant to yield therapeutic effect within the 6.5 mm diameter vessel lumen. Such a thrombolysis speed is significantly faster than any currently available thrombolysis methods for retracted clots.
Fig. 11.
Representative ultrasound images of the cross sections of the flow channels generated using HBC sequence under 2+3-foci dual-pass treatment strategy.
Discussion
Histotripsy is an effective, noninvasive method for thrombolysis without the requirement of thrombolytic drugs or microbubbles, thus reducing complications associated with thrombolytic drug such as extensive bleeding. Both pharmacotherapy and histotripsy have demonstrated sub-optimal efficacy against retracted clots (Vlaisavljevich, et al. 2013, Vlaisavljevich, et al. 2015). This study demonstrates an effective thrombolysis strategy for retracted clots by combining histotripsy with BC using an integrated HBC transducer. High amplitude histotripsy pulses interleaved with low amplitude BC sequences were applied to retracted clots by a single array transducer with a specialized electronic driving system. The lower amplitude BC sequences were produced by sequentially emitting pulses from each of the 18 elements in the histotripsy array. This new HBC thrombolysis method combined with the dual-pass, multi-focus strategy resulted in significantly faster thrombolysis speed and a larger flow channel compared to histotripsy using dual-pass multi-focus strategy alone without BC. The thrombolysis speed of 1.67 min/cm with a flow channel of a cross-sectional area of 4.92 mm2 ± 0.58 mm2 is expected to produce effective recanalization through a retracted clot at a rate much faster than any existing thrombolysis methods.
It is believed that that the significant improvement in thrombolysis efficiency is attributed to the reduction of the cavitation memory effect by using BC pulses. After collapse of a cavitation cloud generated by histotripsy pulses, the residual bubbles act as seeds for subsequent histotripsy pulses in the same locations. As a result, sites where remnant nuclei persisted became over-treated while those without remnant nuclei were undertreated. In addition to cavitation memory effects, attenuation of histotripsy waveforms by the remnant bubbles are also believed to contribute to the reduction of per-sequence efficiency based on recent studies (Duryea, et al. 2014, Duryea, et al. 2015). The presence of pre-focal residual cavitation bubbles persisting from pulse to pulse shielded and attenuated part of the subsequent histotripsy pulse, distorting the waveform and reducing its amplitude at the intended focus. In general, residual cavitation nuclei are the key factor to both cavitation memory and attenuation effects. By introducing BC pulses, a significant reduction of cavitation memory and attenuation effects was observed. This effect could be due to the combination of the primary Bjerknes force and the secondary Bjerknes force that were generated in the acoustic field by BC pulses. Previous studies suggested that bubble coalescence by secondary Bjerknes forces was the dominant factor (Duryea, et al. 2014). The coalescence of residual cavitation nuclei reduced significantly the number of residual cavitation nuclei and thus removed the effects of cavitation memory and attenuation. As a result the per-sequence acoustic energy efficiency was increased significantly. In addition, BC sequence alone could not generate cavitation or stimulate thrombolysis, which indicated that thrombolysis was generated by histotripsy pulses and that BC sequence only contributed to removal of the cavitation memory effect.
The number of pulses in a BC sequence can be optimized to reduce cavitation memory effect while minimizing the added acoustic energy. Too few BC pulses were not sufficient to achieve sufficient bubble coalescing, while too many pulses led to superfluous acoustic energy deposition to the focus. A previous study in our lab showed that sixty 2.2-MPa BC pulses were sufficient to remove cavitation memory effect in blood cell gel phantoms. As our HBC system has 18 elements, and the BC sequence was generated by exciting each of the 18 elements individually and sequentially, the number of pulses in the BC sequence was programmed to be 18 × N using this HBC system. Thus, we tested 36 and 72 2.4-MPa BC pulses in the parameter study, and the results showed that both sequences achieved a similar thrombolysis enhancement in retracted clots. Preliminary tests (unpublished) suggested that 18 pulses were not sufficient to achieve this BC effect. Therefore, the HBC sequence formed by one histotripsy pulse and 36 2.4-MPa BC pulses was selected to use for this study.
The results from the histotripsy and HBC treatments at both scan speeds of 0.2 mm/s and 0.5 mm/s support the hypothesis that the histotripsy thrombolysis treatment efficacy for retracted clot can be enhanced by applying BC pulses. The HBC treatments showed a statistically and likely clinically significant improvement in flow channel size compared to the treatments using histotripsy pulses alone. In fact, the cross-sectional area of the restored flow channel generated by HBC sequence at scan speed of 0.5 mm/s was similar to the flow channel generated by histotripsy alone at scan speed of 0.2 mm/s. This shows that HBC sequences generated a similar channel utilizing 60% less histotripsy pulses and 48% of the total propagated energy. At the same scan speed, the flow channel size and restored flow rates generated by HBC were more than twice as high as those using histotripsy alone, which further indicated that thrombolysis efficacy was improved in retracted clots by using HBC sequences.
The debris particles generated from the HBC sequence treatments and those from the histotripsy treatments had similar normalized size distribution. The largest particles observed were 275.8 μm for HBC treatment and 242.7 μm in diameter for histotripsy treatment. In the HBC treatments, the average number of debris particles > 100 μm for each treatment was < 5, and the maximum number of debris particles > 100 μm for one treatment was 10. In the absence of a patent foramen ovale, any large particle of clot debris during the DVT treatment would be filtered by the lung first and thus would not reach the brain. In addition, 100 micron mechanical filters are commonly used clinically in cardiovascular surgeries to avoid any hazardous embolization, thus particles around 100 microns are not major clinical concerns. In fact, in mechanical thrombectomy procedures, clot particles up to 1000 μm are generated and no severe embolism has been reported from human studies (MÜller-hÜlsbeck, et al. 2001, Uflacker, et al. 1996, Yasui, et al. 1993). Furthermore, thrombolysis using histotripsy in a porcine DVT model showed no sign of pulmonary or cerebral embolism (Zhang, et al. 2017). Thus, the debris particles generated from the HBC sequence treatments would not cause massive pulmonary embolism and be within the safe range.
In the large flow channel study, the combination of HBC with dual-pass multi-focus treatment strategy showed a statistically significant improvement in channel size from the HBC treatments. With the electronic focal steering, the treatment zone was covered by multiple cavitation foci. A wider flow channel was achieved by using this joint strategy. The total energy of the joint strategy doubled compared to HBC treatment at 0.2 mm/s scan speed for one retracted clot, but the mean channel size increased to 288% of the mean channel size produced by HBC treatment at 0.2 mm/s scan speed. Thus, the combination of HBC with dual-pass multi-focus treatment showed the potential to continue to improve thrombolysis efficiency.
Conclusion
Histotripsy combined with BC was investigated to achieve fast thrombolysis of retracted blood clots. High amplitude histotripsy pulses interleaved with low amplitude BC pulses were delivered by an integrated HBC transducer system with specialized driving electronics built in our lab. BC sequence was demonstrated to improve thrombolysis efficacy of histotripsy by reducing the cavitation memory effect. HBC sequences exhibited a significant improvement in both cross-sectional area and flow rate through the re-canalized flow channel compared to using histotripsy alone. The debris generated during all the treatments remained within a safe range. The results show that histotripsy thrombolysis was significantly improved by incorporating with BC pulses. By incorporating a dual-pass, multi-focus strategy a larger flow channel was created. These results suggest that HBC has tremendous potential to enhance and advance current thrombolysis methods. Hence HBC thrombolysis in vivo will be investigated in future. Moreover, the potential vessel wall damage and pulmonary embolism also belong to this investigation.
Acknowledgements
This work was supported by a grant from National Institute of Biomedical Imaging and Bioengineering (NIBIB) of the National Institutes of Health under Award Number R01EB008998, a grant from National Cancer Institute under Award Number R01-CA211217, and a grant from National Institute of Neurological Disorders and Stroke (NINDS) of the National Institutes of Health under Award Number R21NS093121. Disclosure notice: Drs. Hitinder Gurm, Gabe Owens, Tim Hall, and Zhen Xu have financial interests and/or other relationships with HistoSonics Inc.
Reference
- Albrechtsson U, Einarsson E, Eklöf B. Femoral vein pressure measurements for evaluation of venous function in patients with postthrombotic iliac veins. Cardiovasc Intervent Radiol 1981; 4:43–50. [DOI] [PubMed] [Google Scholar]
- Alexandrov AV, Molina CA, Grotta JC, Garami Z, Ford SR, Alvarez-Sabin J, Montaner J, Saqqur M, Demchuk AM, Moyé LA. Ultrasound-enhanced systemic thrombolysis for acute ischemic stroke. N Engl J Med 2004; 351:2170–78. [DOI] [PubMed] [Google Scholar]
- Atar S, Rosenschein U. Perspectives on the role of ultrasonic devices in thrombolysis. J Thromb Thrombolysis 2004; 17:107–14. [DOI] [PubMed] [Google Scholar]
- Bader KB, Haworth KJ, Shekhar H, Maxwell AD, Peng T, McPherson DD, Holland CK. Efficacy of histotripsy combined with rt-PA in vitro. Phys Med Biol 2016; 61:5253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates SM, Ginsberg JS. Treatment of deep-vein thrombosis. N Engl J Med 2004; 351:268–77. [DOI] [PubMed] [Google Scholar]
- Blinc A, Kennedy S, Bryant R, Marder V, Francis C. Flow through clots determines the rate and pattern of fibrinolysis. Thromb Haemost 1994; 71:230–35. [PubMed] [Google Scholar]
- Brown AT, Flores R, Hamilton E, Roberson PK, Borrelli MJ, Culp WC. Microbubbles improve sonothrombolysis in vitro and decrease hemorrhage in vivo in a rabbit stroke model. Invest Radiol 2011; 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne J, Ramnarine K, Watson A, Hoskins P. Assessment of the acoustic properties of common tissue-mimicking test phantoms. Ultrasound Med Biol 2003; 29:1053–60. [DOI] [PubMed] [Google Scholar]
- Burgess A, Huang Y, Waspe AC, Ganguly M, Goertz DE, Hynynen K. High-intensity focused ultrasound (HIFU) for dissolution of clots in a rabbit model of embolic stroke. PloS One 2012; 7:e42311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr M, Hardin CL. Fibrin has larger pores when formed in the presence of erythrocytes. Am J Physiol Heart Circ Physiol. 1987; 253:H1069–H73. [DOI] [PubMed] [Google Scholar]
- Datta S, Coussios C-C, Ammi AY, Mast TD, de Courten-Myers GM, Holland CK. Ultrasound-enhanced thrombolysis using Definity® as a cavitation nucleation agent. Ultrasound Med Biol 2008; 34:1421–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S, Coussios C-C, McAdory LE, Tan J, Porter T, De Courten-Myers G, Holland CK. Correlation of cavitation with ultrasound enhancement of thrombolysis. Ultrasound Med Biol 2006; 32:1257–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duryea AP, Cain CA, Roberts WW, Hall TL. Removal of residual cavitation nuclei to enhance histotripsy fractionation of soft tissue. IEEE Trans Ultrason Ferroelectr Freq Control 2015; 62:2068–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duryea AP, Cain CA, Tamaddoni HA, Roberts WW, Hall TL. Removal of residual nuclei following a cavitation event using low-amplitude ultrasound. IEEE Trans Ultrason Ferroelectr Freq Control 2014; 61:1619–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duryea AP, Tamaddoni HA, Cain CA, Roberts WW, Hall TL. Removal of residual nuclei following a cavitation event: a parametric study. IEEE Trans Ultrason Ferroelectr Freq Control 2015; 62:1605–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feghhi S, Sniadecki NJ. Mechanobiology of platelets: techniques to study the role of fluid flow and platelet retraction forces at the micro-and nano-scale. Int J Mol Sci. 2011; 12:9009–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J, Phillips D. 1983. Polymerization and organization of actin filaments within platelets Seminars in hematology: Elsevier, 243–60. [PubMed] [Google Scholar]
- Ghorbani M, Oral O, Ekici S, Gozuacik D, Koşar A. Review on lithotripsy and cavitation in urinary stone therapy. IEEE Rev Biomed Eng 2016; 9:264–83. [DOI] [PubMed] [Google Scholar]
- Grosse SD, Nelson RE, Nyarko KA, Richardson LC, Raskob GE. The economic burden of incident venous thromboembolism in the United States: A review of estimated attributable healthcare costs. Thromb Res 2016; 137:3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacke W, Kaste M, Bluhmki E, Brozman M, Dávalos A, Guidetti D, Larrue V, Lees KR, Medeghri Z, Machnig T. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 2008; 359:1317–29. [DOI] [PubMed] [Google Scholar]
- Hitchcock KE, Ivancevich NM, Haworth KJ, Stamper DNC, Vela DC, Sutton JT, Pyne-Geithman GJ, Holland CK. Ultrasound-enhanced rt-PA thrombolysis in an ex vivo porcine carotid artery model. Ultrasound Med Biol 2011; 37:1240–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber P, Debus J, Jöchle K, Simiantonakis I, Jenne J, Rastert R, Spoo J, Lorenz WJ, Wannenmacher M. Control of cavitation activity by different shockwave pulsing regimes. Phys Med Biol 1999; 44:1427. [DOI] [PubMed] [Google Scholar]
- Kim HS, Patra A, Paxton BE, Khan J, Streiff MB. Catheter-directed thrombolysis with percutaneous rheolytic thrombectomy versus thrombolysis alone in upper and lower extremity deep vein thrombosis. Cardiovasc Intervent Radiol 2006; 29:1003–07. [DOI] [PubMed] [Google Scholar]
- Kirchhof K, Welzel T, Mecke C, Zoubaa S, Sartor K. Differentiation of White, Mixed, and Red Thrombi: Value of CT in Estimation of the Prognosis of Thrombolysis—Phantom Study 1. Radiology 2003; 228:126–30. [DOI] [PubMed] [Google Scholar]
- Lansberg MG, Bluhmki E, Thijs VN. Efficacy and safety of tissue plasminogen activator 3 to 4.5 hours after acute ischemic stroke. Stroke 2009; 40:2438–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leizorovicz A, Simonneau G, Decousus H, Boissel J. Comparison of efficacy and safety of low molecular weight heparins and unfractionated heparin in initial treatment of deep venous thrombosis: a meta-analysis. BMJ 1994; 309:299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundt JE, Allen SP, Shi J, Hall TL, Cain CA, Xu Z. Non-invasive, Rapid Ablation of Tissue Volume Using Histotripsy. Ultrasound Med Biol 2017; 43:2834–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell AD, Cain CA, Duryea AP, Yuan L, Gurm HS, Xu Z. Noninvasive thrombolysis using pulsed ultrasound cavitation therapy–histotripsy. Ultrasound Med Biol 2009; 35:1982–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell AD, Owens G, Gurm HS, Ives K, Myers DD, Xu Z. Noninvasive treatment of deep venous thrombosis using pulsed ultrasound cavitation therapy (histotripsy) in a porcine model. J Vasc Interv Radiol 2011; 22:369–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushige K, Kondo I, Ohmori K, Hirao K, Matsuo H. Enhancement of ultrasound-accelerated thrombolysis by echo contrast agents: dependence on microbubble structure. Ultrasound Med Biol 1999; 25:1431–37. [DOI] [PubMed] [Google Scholar]
- Molina CA, Ribo M, Rubiera M, Montaner J, Santamarina E, Delgado-Mederos R, Arenillas JF, Huertas R, Purroy F, Delgado P. Microbubble administration accelerates clot lysis during continuous 2-MHz ultrasound monitoring in stroke patients treated with intravenous tissue plasminogen activator. Stroke 2006; 37:425–29. [DOI] [PubMed] [Google Scholar]
- MÜller-hÜlsbeck S, Brossmann J, Jahnke T, Grimm J, Reuter M, Bewig B, Heller M. Mechanical thrombectomy of major and massive pulmonary embolism with use of the Amplatz thrombectomy device. Invest Radiol 2001; 36:317–22. [DOI] [PubMed] [Google Scholar]
- Negus D, Cockett F. Femoral vein pressures in post‐ phlebitic iliac vein obstruction. Br J Surg 1967; 54:522–25. [DOI] [PubMed] [Google Scholar]
- Parsons JE, Cain CA, Abrams GD, Fowlkes JB. Pulsed cavitational ultrasound therapy for controlled tissue homogenization. Ultrasound Med Biol 2006; 32:115–29. [DOI] [PubMed] [Google Scholar]
- Pfaffenberger S, Devcic-Kuhar B, El-Rabadi K, Gröschl M, Speidl WS, Weiss TW, Huber K, Benes E, Maurer G, Wojta J. 2MHz ultrasound enhances t-PA-mediated thrombolysis: comparison of continuous versus pulsed ultrasound and standing versus travelling acoustic waves. Thromb Haemost 2003; 90:583–89. [PubMed] [Google Scholar]
- Porter TR, Xie F. Ultrasound, microbubbles, and thrombolysis. Prog Cardiovasc Dis 2001; 44:101–10. [DOI] [PubMed] [Google Scholar]
- Rosenschein U, Furman V, Kerner E, Fabian I, Bernheim J, Eshel Y. Ultrasound Imaging–Guided Noninvasive Ultrasound Thrombolysis. Circulation 2000; 102:238–45. [DOI] [PubMed] [Google Scholar]
- Semba CP, Dake MD. Iliofemoral deep venous thrombosis: aggressive therapy with catheter-directed thrombolysis. Radiology 1994; 191:487–94. [DOI] [PubMed] [Google Scholar]
- Sharafuddin MJ, Sun S, Hoballah JJ, Youness FM, Sharp WJ, Roh B-S. Endovascular management of venous thrombotic and occlusive diseases of the lower extremities. J Vasc Interv Radiol 2003; 14:405–23. [DOI] [PubMed] [Google Scholar]
- Shi A, Huang P, Guo S, Zhao L, Jia Y, Zong Y, Wan M. Precise spatial control of cavitation erosion in a vessel phantom by using an ultrasonic standing wave. Ultrason Sonochem 2016; 31:163–72. [DOI] [PubMed] [Google Scholar]
- Shi A, Xu Z, Lundt J, Tamaddoni HA, Worlikar T, Hall TL. Integrated histotripsy and bubble coalescence transducer for rapid tissue ablation. IEEE Trans Ultrason Ferroelectr Freq Control 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel RJ, Atar S, Fishbein MC, Brasch AV, Peterson TM, Nagai T, Pal D, Nishioka T, Chae J-S, Birnbaum Y. Noninvasive, transthoracic, low-frequency ultrasound augments thrombolysis in a canine model of acute myocardial infarction. Circulation 2000; 101:2026–29. [DOI] [PubMed] [Google Scholar]
- Siegel RJ, Luo H. Ultrasound thrombolysis. Ultrasonics 2008; 48:312–20. [DOI] [PubMed] [Google Scholar]
- Stone MJ, Frenkel V, Dromi S, Thomas P, Lewis RP, Li KC, Horne M, Wood BJ. Pulsed-high intensity focused ultrasound enhanced tPA mediated thrombolysis in a novel in vivo clot model, a pilot study. Thromb Res 2007; 121:193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton JT, Ivancevich NM, Perrin SR, Vela DC, Holland CK. Clot retraction affects the extent of ultrasound-enhanced thrombolysis in an ex vivo porcine thrombosis model. Ultrasound Med Biol 2013; 39:813–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uflacker R, Rajagopalan P, Vujic I, Stutley JE. Treatment of thrombosed dialysis access grafts: randomized trial of surgical thrombectomy versus mechanical thrombectomy with the Amplatz device. J Vasc Interv Radiol 1996; 7:185–92. [DOI] [PubMed] [Google Scholar]
- Vlaisavljevich E, Kim Y, Owens G, Roberts W, Cain C, Xu Z. Effects of tissue mechanical properties on susceptibility to histotripsy-induced tissue damage. Phys Med Biol 2013; 59:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlaisavljevich E, Lin K-W, Warnez MT, Singh R, Mancia L, Putnam AJ, Johnsen E, Cain C, Xu Z. Effects of tissue stiffness, ultrasound frequency, and pressure on histotripsy-induced cavitation bubble behavior. Phys Med Biol 2015; 60:2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T-Y, Xu Z, Hall TL, Fowlkes JB, Cain CA. An efficient treatment strategy for histotripsy by removing cavitation memory. Ultrasound Med Biol 2012; 38:753–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright C, Hynynen K, Goertz D. In vitro and in vivo high intensity focused ultrasound thrombolysis. Invest Radiol 2012; 47:217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Fowlkes JB, Ludomirsky A, Cain CA. Investigation of intensity thresholds for ultrasound tissue erosion. Ultrasound Med Biol 2005; 31:1673–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Fowlkes JB, Rothman ED, Levin AM, Cain CA. Controlled ultrasound tissue erosion: The role of dynamic interaction between insonation and microbubble activity. J Acoust Soc Am 2005; 117:424–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasui K, Qian Z, Nazarian GK, Hunter DW, Castañeda-Zúñiga WR, Amplatz K. Recirculation-type Amplatz clot macerator: determination of particle size and distribution. J Vasc Interv Radiol 1993; 4:275–78. [DOI] [PubMed] [Google Scholar]
- Zhang X, Jin L, Vlaisavljevich E, Owens GE, Gurm HS, Cain CA, Xu Z. Noninvasive thrombolysis using microtripsy: A parameter study. IEEE Trans Ultrason Ferroelectr Freq Control 2015; 62:2092–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Macoskey JJ, Ives K, Owens GE, Gurm HS, Shi J, Pizzuto M, Cain CA, Xu Z. Non-Invasive Thrombolysis Using Microtripsy in a Porcine Deep Vein Thrombosis Model. Ultrasound Med Biol 2017; 43:1378–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Miller RM, Lin K-W, Levin AM, Owens GE, Gurm HS, Cain CA, Xu Z. Real-time feedback of histotripsy thrombolysis using bubble-induced color Doppler. Ultrasound Med Biol 2015; 41:1386–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Owens GE, Cain CA, Gurm HS, Macoskey J, Xu Z. Histotripsy Thrombolysis on Retracted Clots. Ultrasound Med Biol 2016; 42:1903–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Owens GE, Gurm HS, Ding Y, Cain CA, Xu Z. Noninvasive thrombolysis using histotripsy beyond the intrinsic threshold (microtripsy). IEEE Trans Ultrason Ferroelectr Freq Control 2015; 62:1342–55. [DOI] [PMC free article] [PubMed] [Google Scholar]