Abstract
Soft tissue sarcomas (STS) are heterogeneous mesenchymal malignancies with variable biological behavior. The primary management for localized STS is surgical resection, which may be combined with neoadjuvant or adjuvant radiation therapy to increase the probability of achieving local control. Many patients with large, high-grade STS develop metastatic disease. Several clinical trials of immune checkpoint blockade for STS show promising responses for patients with metastatic disease. In this review, we discuss recent and ongoing clinical trials of immune checkpoint inhibition for STS. We explain the rationale for immune checkpoint inhibition and radiation therapy, and highlight new studies testing this combination in the neoadjuvant setting for patients with high-risk STS. We also describe novel combinations of immunotherapy with targeted therapies and chemotherapies being tested in the metastatic setting, and discuss how these combinations have the potential to be integrated into adjuvant therapy in the future.
Keywords: soft tissue sarcoma, immune checkpoint blockade, anti-PD-1, anti-CTLA-4, immunotherapy, radiation therapy
Soft Tissue Sarcoma: Background and History of Immune Therapy
Soft tissue sarcomas (STS) are a rare and heterogeneous group of mesenchymal malignancies. They affect patients of all ages and can occur anywhere in the body. In the United States, approximately 13,000 STS are reported each year in adults [1], representing approximately 1% of all adult malignancies [2]. STS account for 10-15% of all childhood tumors [3]. Over 50 histologic subtypes of STS have been described [4], with an estimated one-third driven by chimeric fusion genes generated from chromosomal translocations [5]. The remaining two-thirds of STS harbor complex karyotypes and are typically associated with dysfunction of the p53 tumor suppressor pathway [6].
The primary management for localized STS is surgical resection. For patients with large and/or high-grade STS, neoadjuvant or adjuvant radiation therapy is often used to improve local control [8–10]. For many STS subtypes, the value of adjuvant chemotherapy remains controversial [11–13]. Following local treatment, approximately 50% of patients with large, high-grade sarcomas develop metastases, most commonly occurring in the lung [14]. After metastases occur, available systemic therapies can temporarily decrease disease burden, but median survival remains less than 2 years [15,16]. Thus, alternative approaches are needed to reduce the number of sarcoma patients who develop metastases and therefore improve survival.
Sarcoma has a rich history of immuno-oncology research. In 1891, Dr. William Coley, now known as the “Father of Immunotherapy,” first demonstrated the ability of the immune system to reject a malignant tumor in a patient with sarcoma [7]. Dr. Coley injected tumors with a live preparation of streptococcus organisms designated as “Coley's Toxins,” which caused the infection erysipelas and presumably stimulated the immune system [7]. Over his long career, he treated hundreds of patients with inoperable and metastatic sarcomas with immunotherapy, which reportedly had remarkable results [7]. With the development of radiation and chemotherapy, “Coley's Toxins” fell out of favor, and immunotherapy was not used to treat sarcoma patients for many years. The re-emergence of immunotherapy in the context of immune checkpoint blockade has led to the development of clinical trials testing these immunomodulatory agents for the treatment of STS.
Immune Checkpoint Inhibition
The use of immune checkpoint inhibition has become a major focus in oncology due to dramatic and durable responses in patients with multiple tumor types [17–19]. The goal of immunotherapy is to stimulate the immune system to attack malignant tumor cells [20]. Immune checkpoint inhibitors rely on activation of a patient's existing anti-tumor immune cells [20,21]. The best response rates have been observed in patients with lung cancer and melanoma, which are often highly mutated tumors and thus express numerous tumor-specific neoantigens against which the immune system may mount an attack [17,22,23]. Because of the impressive results of immune checkpoint blockade in many cancers, several clinical trials are now testing immune checkpoint inhibitors in STS.
Immune checkpoint blockade is a powerful approach to activate anti-tumor immunity. Immune checkpoints are inhibitory pathways that modulate the duration and magnitude of immune responses and preserve self-tolerance [20]. A major mechanism by which cancers evade the immune system is by exploiting these immune checkpoint pathways [24] to prevent T cells from recognizing tumor-specific antigens and eliminating tumor cells [25,26]. Because many immune checkpoint signaling cascades are initiated by ligand-receptor interactions on the cell surface [20], they can be blocked by antibodies. These antibodies can “release the brakes” on the immune system, which has the potential to unleash an anti-tumor immune response. The major targets of FDA-approved immunotherapeutic antibodies are programmed cell death protein-1 (PD-1), its ligand programmed cell death ligand-1 (PD-L1), and cytotoxic T lymphocyte-associated antigen-4 (CTLA-4). Although inhibitory anti-CTLA-4 and anti-PD-1 antibodies have successfully been used to treat many cancers, they remain relatively understudied in STS. Here we review the current landscape for immune checkpoint inhibitor trials in STS and discuss opportunities for incorporating immunotherapy into the neoadjuvant and adjuvant settings.
Immunologic Profile of Soft Tissue Sarcoma
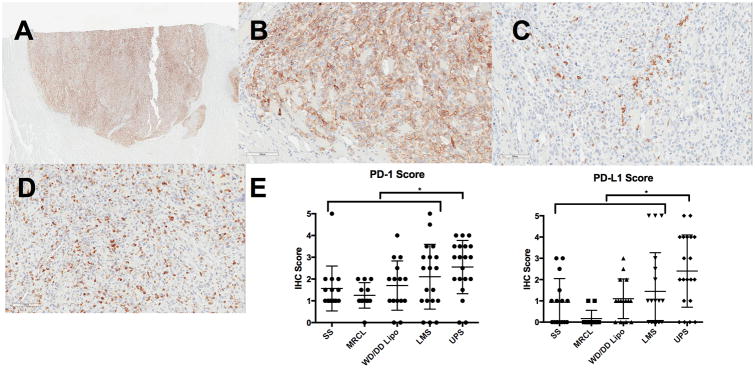
A recent study by Pollack et al. characterized the immunologic profile of five common STS subtypes: leiomyosarcoma, undifferentiated pleomorphic sarcoma (UPS), synovial sarcoma, and liposarcoma (both well-differentiated/dedifferentiated liposarcoma and myxoid/round cell liposarcoma subtypes) [27]. They found that UPS and leiomyosarcoma, two of the more genetically “complex” sarcomas, had high gene expression levels related to antigen presentation and T cell infiltration, as well as a more oligoclonal T cell receptor repertoire. Compared to other sarcoma subtypes, UPS also had the most T cell infiltration and highest expression of PD-1 and PD-L1. Prior radiotherapy or chemotherapy was not associated with T cell infiltration or clonality. Another study examining tumor immune cell infiltrate in 17 UPS patients demonstrated increased T cells after neoadjuvant radiotherapy (2 Gy × 25 fractions) [28]. Median CD4 infiltrate increased significantly (3 to 13 cells/mm2, p = 0.01), and a similar trend was observed for CD8 infiltrate (55 to 111 cells/mm2, p = 0.17). The immune cell infiltrates did not differ significantly between patients receiving neoadjuvant radiotherapy alone versus neoadjuvant chemotherapy and radiotherapy. While no PD-L1 expression was observed at baseline, 21% of tumors exhibited PD-L1 staining after radiotherapy.
Recently, The Cancer Genome Atlas (TCGA) Research Network reported the immune microenvironment signature of 206 STS across 7 histological subtypes [29]. The immune microenvironment, which was inferred from mRNA expression and DNA methylation profiles, revealed three distinct clusters. Interestingly, the three sarcoma subtypes with high levels of copy number alterations (UPS, myxofibrosarcomas (MFS), and dedifferentiated liposarcomas) were found to have a similar immune microenvironment. Of note, in UPS and MFS, the presence of dendritic cells correlated with improved disease-specific survival, suggesting a role for antigen presentation in the response of the immune system to these sarcomas [29].
Taken together, these studies suggest that STS, especially those with high frequency of copy number alterations such as UPS and MFS [27,29], may be capable of eliciting an immune response. Therefore, some histological STS subtypes may be poised to respond to immune checkpoint inhibitors or other immunotherapies. However, the optimal treatment approach will likely be subtype-specific. Further work is necessary to understand differences in immune response for each sarcoma subtype and how current treatments, such as radiotherapy and chemotherapy, affect this response.
Radiation and Immunotherapy
As immune checkpoint blockade usage increased in patients with non-small cell lung cancer and melanoma [17–19], abscopal effect case reports began to appear in the literature [31–34]. The abscopal effect [31], in which local irradiation elicits a systemic immune response leading to regression of distant tumors outside of the radiation field, provides one rationale for combining immune checkpoint blockade and radiation therapy (Figure 1). In preclinical studies, abscopal responses after radiotherapy and immune checkpoint blockade have been reliably reproduced in transplanted tumor models in immunocompetent mice [35,36], but this phenomenon remains relatively uncommon in clinical practice [31]. Numerous ongoing preclinical studies and active clinical trials seek to determine the optimal radiotherapy fractionation and timing relative to immune checkpoint blockade to activate an abscopal response. Although radiation has historically been considered a treatment focused on achieving local tumor control, mounting evidence suggests that radiation alone can elicit an immune response [37] and therefore radiation has the potential to synergize with immune checkpoint blockade to produce a durable antitumor response not only within the radiation field, but also against distant metastatic disease [34,38].
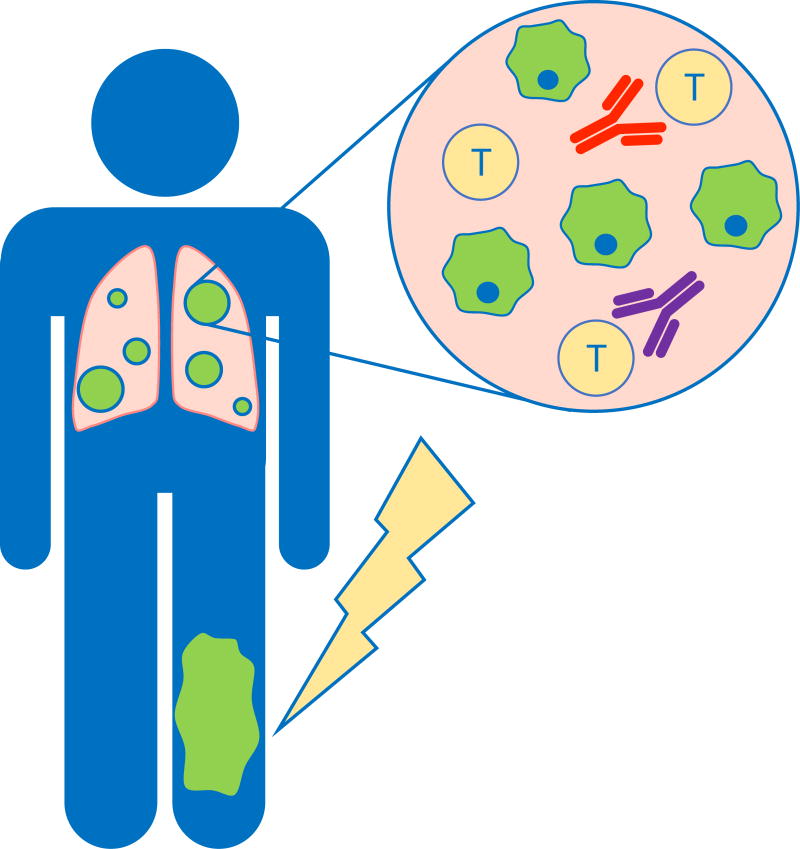
Figure 1. Abscopal response may eliminate micrometastatic disease in soft tissue sarcoma patients.
In an abscopal response, the primary tumor (green) is treated with radiation therapy, which has been shown to generate a systemic immune response. The anti-tumor immune response is mediated by both CD4 and CD8 T cells (yellow). This response may be further enhanced by blocking the inhibitory checkpoints CTLA-4 (orange) and/or PD-1 (purple) on the surface of CD4 and CD8 T cells. By increasing activation and effector function of T cells, immune checkpoint blockade has the potential to eradicate both irradiated and non-irradiated tumor cells. This has the potential to dramatically improve outcomes for patients with soft tissue sarcoma by eradicating micrometastatic disease.
The potential impact of an abscopal response is high in STS, in which approximately 50% of patients with large, high-grade tumors develop metastases [14]. The frequent development of metastases despite local tumor control implies that micrometastatic disease is often present at the time of primary tumor resection. Combining immunotherapy and neoadjuvant radiotherapy has the potential to elicit a systemic immune response to improve long-term survival in sarcoma patients by eradicating micrometastatic disease. In the 1970s, Dr. Helen Stone and her colleagues used a mouse model of sarcoma to demonstrate that the immune system plays an important role in tumor response to radiation therapy [30]. In an allograft STS model, they showed that a higher radiation dose was needed to achieve tumor cure in immunodeficient mice compared to immunocompetent mice, suggesting that the immune system contributes to tumor elimination after radiation therapy [30].
The majority of clinical trials testing the combination of radiation and immunotherapy are in the setting of established metastatic disease [39]. However, lower pre-treatment tumor volume has been correlated with improved response to immune checkpoint blockade [40], suggesting that combining radiation therapy and immunotherapy may be more effective in the definitive setting. For example, adjuvant treatment with the anti-PD-L1 antibody durvalumab after definitive chemoradiation for stage III non-small-cell lung cancer significantly improved progression-free survival compared to chemoradiation followed by placebo [18]. Neoadjuvant and/or adjuvant immune checkpoint blockade would be a paradigm shift for the management of tumors with high risk for developing metastases such as STS—employing immunotherapy to prevent, rather than to treat, metastatic disease.
Although preclinical studies and anecdotal clinical outcomes testing the combination of radiation and immunotherapy have generated significant excitement, to our knowledge, no mature randomized clinical trials in treatment-naive patients have established the superiority of radiation therapy and concurrent immunotherapy to either radiotherapy alone or immunotherapy alone. Many ongoing trials treat patients with combined checkpoint blockade and radiation therapy after they have progressed through chemotherapy or immune checkpoint blockade alone [39]. However, this design makes it challenging to determine if there is synergy from the combination of immune checkpoint blockade and radiation therapy. Even for patients who progress on immune checkpoint blockade alone, but subsequently respond to the same immunotherapy combined with radiotherapy, the response cannot necessarily be attributed to radiotherapy given the potential for delayed responses to immune checkpoint inhibition [41–43].
Clinical Studies of Immune Checkpoint Blockade in Soft Tissue Sarcoma
Several trials have examined the efficacy of immune checkpoint inhibition in metastatic STS with mixed results (Table 1). Maki et al. published the first study to investigate immune checkpoint blockade in STS. This small pilot phase II trial examined the efficacy of targeting CTLA-4 with ipilimumab in synovial sarcoma [44]. Although synovial sarcoma is a translocation-driven sarcoma with relatively low mutational burden [45], it often has high expression of the endogenous cancer testis antigen NY-ESO-1 [46,47]. Six patients with synovial sarcoma received three doses of ipilimumab [44]. Only four patients completed treatment, and all patients showed radiological evidence of disease progression by the third cycle [44]. Emerging data in STS characterizing T-cell infiltration and immune checkpoint molecules, such as PD-1 and PD-L1, suggest that the more genetically “complex” histological subtypes, such as UPS and leiomyosarcoma [27], may be more likely to respond to immune checkpoint inhibitors.
Table 1.
Clinical trials for immune checkpoint blockade in soft tissue sarcoma.
| NCT Identifier | Phase | Title | Sarcoma Subtype(s) | Immune Checkpoint Therapy | Additional Interventions | Sponsor/Collaborators |
|---|---|---|---|---|---|---|
| NCT02428192 | Phase II | Nivolumab Alone or in Combination with Ipilimumab in Treating Patients with Advanced Uterine Leiomyosarcoma | Advanced uterine LMS | Nivolumab +/- Ipilimumab | -- | National Cancer Institute (NCI) |
| NCT02815995 | Phase II | A Phase II Multi-Arm Study to Test the Efficacy of Immunotherapeutic Agents in Multiple Sarcoma Subtypes | Multiple (soft tissue sarcoma and osteosarcoma) | Durvalumab and tremelimumab, followed by druvalumab | -- | MD Anderson Cancer Center, MedImmune |
| NCT02301039 | Phase II | SARC028: A Phase II Study of the Anti-PD1 Antibody Pembrolizumab (MK-3475) in Patients with Advanced Sarcomas | LMS, poorly differentiated/ de-differentiated LPS, high grade UPS/MFH, MPNST, and synovial sarcoma | Pembrolizumab | -- | Sarcoma Alliance for Research through Collaboration, Merck Sharp & Dohme |
| NCT02500797 | Phase II | Randomized Phase II Study of Nivolumab with or without Ipilimumab in Patients with Metastatic or Unresectable Sarcoma | Dedifferentiated/pleomorphic LPS, UPS/MFH, GIST | Nivolumab +/- Ipilimumab | -- | National Cancer Institute (NCI) |
| NCT03317457 | Phase II | A Randomized Phase II Study of Durvalumab (MEDI4736) and Tremelimumab Compared to Doxorubicin in Patients with Advanced or Metastatic Soft Tissue Sarcoma | Fibrosarcoma, UPS, LMS, LPS, malignant glomus tumor, RMS, angiosarcoma, synovial sarcoma, and MPNST | Durvalumab and Tremelimumab | vs Doxorubicin | AIO-Studien-gGmbH, AstraZeneca |
| NCT01643278 | Phase I | Phase I Study of Dasatinib in Combination with Ipilimumab for Patients with Advanced Gastrointestinal Stromal Tumor and Other Sarcomas | GIST and other sarcoma subtypes | Ipilimumab | Dasatinib | National Cancer Institute (NCI) |
| NCT02406781 | Phase II | Combination of MK3475 and Metronomic Cyclophosphamide in Patients with Advanced Sarcomas: Multicentre Phase II Trial (PEMBROSARC) | LMS, UPS, osteosarcoma, GIST, others | Pembrolizumab | Metronomic Cyclophospha-mide (Week on/Week off schedule) | Institut Bergonié, Merck Sharp & Dohme Corp., Ministry of Health, France |
| NCT03138161 | Phase I/II | Phase 1/2 Study of Safety/Efficacy Using TRABECTEDIN, IPILIMUMAB and NIVOLUMAB Triple Therapy as First Line Treatment of Advanced Soft Tissue Sarcoma | Soft Tissue Sarcoma | Ipilimumab, Nivolumab | Trabectedin | Sarcoma Oncology Research Center, Bristol-Myers Squibb |
| NCT02888665 | Phase I/II | A Trial of Pembrolizumab in Combination with Doxorubicin as Treatment for Patients with Advanced Sarcomas | Soft Tissue Sarcoma | Pembrolizumab | Doxorubicin | Fred Hutchinson Cancer Research Center, National Cancer Institute (NCI) |
| NCT03123276 | Phase I/II | A Phase I Study to Assess the Safety and Tolerability of Pembrolizumab in Combination with Fixed Rate Gemcitabine Chemotherapy in Patients with Leiomyosarcoma and Undifferentiated Pleomorphic Sarcoma | LMS and UPS | Pembrolizumab | Gemcitabine | Royal Marsden NHS Foundation Trust, Merck Sharp & Dohme |
| NCT03116529 | Phase I/II | Neoadjuvant Anti-PD-L1 (Durvalumab/ MEDI4736) Plus Anti-CTLA-4 (Tremelimumab) and Radiation for High Risk Soft-Tissue Sarcoma | Intermediate- or high-grade Soft Tissue Sarcoma | Durvalumab and Tremelimumab | Radiation (50 Gy, 1.8-2 Gy per fraction plus 15 Gy GRID radiation for bulky disease) | University of Maryland, AstraZeneca |
| NCT03307616 | Phase II | Phase II Study of Neoadjuvant Checkpoint Blockade in Patients with Surgically Resectable Undifferentiated Pleomorphic Sarcoma and Dedifferentiated Liposarcoma | UPS and dedifferentiated LPS | Nivolumab, Ipilimumab | Radiation therapy | M.D. Anderson Cancer Center, Bristol-Myers Squibb |
| NCT03092323 | Phase II | SU2C-SARC032: A Phase II Randomized Controlled Trial of Neoadjuvant Pembrolizumab With Radiotherapy and Adjuvant Pembrolizumab in Patients with High-Risk, Localized Soft Tissue Sarcoma of the Extremity | UPS and dedifferentiated/pleomorphic LPS | Pembrolizumab | Radiation therapy (50 Gy, 2 Gy per fraction) | Sarcoma Alliance for Research through Collaboration, Stand Up to Cancer, Merck Sharp & Dohme |
Abbreviations: LMS Leiomyosarcoma, UPS Undifferentiated Pleomorphic Sarcoma, MFH Malignant Fibrous Histiocytoma, LPS Liposarcoma, MPNST Malignant Peripheral Nerve Sheath Tumor, GIST Gastrointestinal Stromal Tumor, RMS rhabdomyosarcoma, GRID Spatially Fractionated Radiation Therapy
Uterine leiomyosarcoma has also demonstrated resistance to immune checkpoint inhibition. In a phase II study of twelve patients with previously treated advanced uterine leiomyosarcoma (NCT02428192), no patients responded to the anti-PD-1 antibody nivolumab, as measured by progression free survival (PFS) [48]. By contrast, one report describes a treatment-naive patient with metastatic uterine leiomyosarcoma who experienced an impressive response to the anti-PD-1 antibody pembrolizumab [49]. After 9 months of pembrolizumab, all lesions showed significant regression except for a single mass, which was resected. After resection, the patient has experienced > 2 years of complete tumor remission. Intriguingly, the treatment-resistant lesion harbored biallelic PTEN loss and decreased expression of two neoantigens expressed in the primary tumor [49], which suggest potential mechanisms of resistance to PD-1 blockade.
Early results from an ongoing study examining combined anti-PD-L1 and anti-CTLA-4 immune checkpoint blockade for metastatic sarcoma (NCT02815995) demonstrate activity in some histological subtypes. In this phase II multi-arm study, patients with previously treated soft tissue or bone sarcoma receive anti-PD-L1 (durvalumab) and anti-CTLA-4 (tremelimumab) therapy for four cycles, followed by durvalumab for 12 weeks. By Immune-Related Response Criteria (irRC), one of four patients with metastatic UPS showed a partial response to combined immune checkpoint inhibition [50].
Another clinical trial testing anti-PD-1 therapy in sarcomas is SARC028 (NCT02301039), which is a phase II trial of pembrolizumab in 86 patients with unresectable, metastatic, or recurrent soft tissue or bone sarcoma. The primary outcome measure is objective response rate (ORR) by RECIST version 1.1 criteria, and secondary endpoints are adverse events, PFS, overall survival (OS), and response rates by irRC. For the initial cohort, 7 of 40 patients with STS had an objective response, with promising response rates for specific histological subtypes. In particular, 1 complete response and 3 partial responses were observed among 10 patients with UPS and 2 partial responses were observed among 10 patients with dedifferentiated liposarcoma [51]. Response rates were 1 of 10 and 0 of 10 for synovial sarcoma and leiomyosarcoma, respectively. Patients who responded to pembrolizumab had higher tumor-infiltrating lymphocytes at baseline [52]. Median PFS was 18 weeks among the 40 evaluable patients with STS. For patients with UPS or dedifferentiated liposarcoma, median PFS was 30 weeks and 25 weeks, respectively. Median OS was 49 weeks among all STS patients, but median OS had not been reached for UPS patients [51]. Enrollment to SARC028 was recently expanded for the UPS and dedifferentiated liposarcoma cohorts.
The Alliance for Clinical Trials in Oncology conducted a randomized phase II trial (Alliance A091401; NCT02500797) of immune checkpoint blockade in patients with metastatic or unresectable bone or soft tissue sarcoma with progressive disease after alternative regimens [53]. Patients received either nivolumab alone or nivolumab in combination with ipilimumab. The primary endpoint was objective tumor response rate (ORR; confirmed complete or partial response lasting at least four weeks). Secondary outcome measures included adverse events, clinical benefit rate, response duration, PFS, and OS. Included histologies among the first 85 patients were as follows: 4% angiosarcoma, 34% leiomyosarcoma, 6% liposarcoma, 13% UPS, 13% spindle cell sarcoma, 5% synovial sarcoma, 10% bone sarcoma, and 15% other. Interim results showed low response with nivolumab monotherapy (5% ORR), with partial responses observed in patients with alveolar soft part sarcoma, leiomyosarcoma, and sarcoma not otherwise specified. Combined nivolumab and ipilimumab appeared to have better antitumor activity (16% ORR), with complete responses observed in one patient with myxofibrosarcoma and one patient with uterine leiomyosarcoma. Partial responses to nivolumab and ipilimumab were observed in two patients with UPS, one patient with uterine leiomyosarcoma and one with non-uterine leiomyosarcoma, one patient with myxofibrosarcoma, and one patient with angiosarcoma. Median PFS was 1.7 months for nivolumab and 4.1 months for nivolumab and ipilimumab. Enrollment is closed, although the study is ongoing.
No trials to date have compared immune checkpoint therapy to anthracyclines, which are standard first-line chemotherapy for many subtypes of metastatic sarcoma. MEDISARC is an upcoming German phase II clinical trial (NCT03317457) that will randomize patients with metastatic or locally advanced sarcoma to receive durvalumab and tremelimumab versus six cycles of doxorubicin. Eligible histologic subtypes include fibrosarcoma, UPS, leiomyosarcoma, liposarcoma (dedifferentiated, pleomorphic or myxoid), malignant glomus tumor, rhabdomyosarcoma (alveolar or pleomorphic), angiosarcoma, synovial sarcoma, and malignant peripheral nerve sheath tumors. The primary outcome is OS, with secondary endpoints including adverse events, ORR, PFS, duration of response, and quality of life. Estimated enrollment is 100 patients, and study opening is anticipated in early 2018.
Combinations of Targeted Therapy or Chemotherapy with Immune Checkpoint Blockade
Novel combinations of immunotherapy with targeted therapies and chemotherapies in STS have been tested primarily in the metastatic setting [54]. If regimens active against metastatic disease are identified, then they have the potential to be tested as adjuvant therapy in the future. One early retrospective study of 28 patients with relapsed metastatic or unresectable soft tissue or bone sarcoma examined safety and efficacy of nivolumab [55]. Many patients, including some responders, were concurrently receiving pazopanib, a multikinase inhibitor approved for sarcoma in 2012 [56]. Response was assessed using RECIST 1.1 criteria by comparing baseline imaging to PET/CT taken after at least 4 doses of nivolumab. Three partial responses were observed, each in one patient with dedifferentiated chondrosarcoma, epithelioid sarcoma, and maxillary osteosarcoma. Nine patients had stable disease, including 3 patients with leiomyosarcoma. This retrospective study suggested clinical benefit (partial response or stable disease) in 50% of sarcoma patients after > 4 cycles of nivolumab. The most common severe adverse events (grade 3-4) were liver function test elevation, colitis, and pneumonitis, and all five severe adverse events occurred in patients who were treated with concomitant pazopanib.
A recent phase IB study (NCT01643278) examined the safety and efficacy of the tyrosine kinase inhibitor dasatinib in combination with ipilimumab in twenty patients with gastrointestinal stromal tumor (GIST) and eight patients with other sarcoma subtypes [57]. Patients received a one-week dasatinib lead-in, then 3 or 10 mg/kg ipilimumab every 3 weeks with dasatinib followed by maintenance dasatinib (70 mg daily, 100 mg daily, or 70 mg twice daily). While this regimen was well-tolerated, efficacy was poor. Eighteen patients were evaluable for radiographic response, and no partial or complete responses were observed based on RECIST 1.1 or irRC.
Results were recently reported for a French multicenter phase II clinical trial (NCT02406781) assessing pembrolizumab with metronomic cyclophosphamide in patients with metastatic STS, including leiomyosarcoma, UPS, GIST, and several other histologies [58]. While treatment was well tolerated, response was limited with 3 of 50 patients free from progression at 6 months. Median PFS was 1.4 months across all histologies. Tumor sample evaluation revealed high expression levels of indoleamine 2,3-dioxygenase 1 (IDO1) in infiltrating immune cells, and the kynurenine to tryptophan ratio in plasma increased significantly after pembrolizumab. Given the role for the IDO1 product kynurenine in regulatory T cell expansion, the authors posit that the IDO1 pathway may be contributing to pembrolizumab resistance, providing rationale for combining anti-PD-1 therapy with IDO1 inhibitors for STS.
Multiple dose-escalation studies are ongoing to test the combination of chemotherapy and dual anti-CTLA-4 and anti-PD-1 immune checkpoint blockade in STS. The Sarcoma Oncology Research Center is conducting an open-label phase 1/2 trial (NCT03138161) testing trabectedin, ipilimumab, and nivolumab in patients with STS [59]. In Phase I, previously treated patients will receive ipilimumab, nivolumab, and escalating doses of trabectedin (1 mg/m2, 1.2 mg/m2, then 1.5 mg/m2, 3-6 patients per dose) to identify the maximum tolerated dose (MTD). Following dose escalation, 22-28 previously untreated patients will receive trabectedin at the MTD in combination with ipilimumab and nivolumab, with possible surgical resection after the first treatment cycle. The primary outcome measure is trabectedin MTD, and the secondary outcome measures are objective response rate (24 months), 6-month PFS, and 6-month OS.
An ongoing phase I/II trial at the University of Washington (NCT02888665) is assessing the safety and efficacy of pembrolizumab with doxorubicin for patients with metastatic or unresectable sarcoma. Patients receive pembrolizumab every 3 weeks with concurrent doxorubicin for cycles 2-7. The primary outcomes are doxorubicin MTD and ORR compared to historical control rates. Secondary outcomes include response duration, incidence of adverse events, PFS, OS, and time to response. Completed data collection for primary outcome measures is expected in August 2018.
The Royal Marsden NHS Foundation Trust is conducting an open-label trial (NCT03123276) of pembrolizumab and gemcitabine for leiomyosarcoma and UPS [60]. This is a two-part phase I, single-center dose escalation and dose expansion study in 24 patients with newly diagnosed metastatic or inoperable leiomyosarcoma or UPS. The first twelve patients will be in the dose escalation cohort: 6 patients will receive 800 mg/m2 of gemcitabine in combination with 200 mg of pembrolizumab given every 3 weeks. If no dose-limiting toxicities are noted, then gemcitabine will be increased to 1000 and 1200 mg/m2. The next 12 patients will be enrolled in the MTD cohort to study safety and tolerability, as well as to preliminarily assess response to therapy. The primary endpoint is response evaluation by RECIST 1.1 at 2 months after the last dose. Secondary outcome measures include immunophenotyping of tumor samples and response stratification according to tumor PD-L1 expression.
Clinical Studies of Neoadjuvant Radiotherapy and Immune Checkpoint Blockade in Soft Tissue Sarcoma
While a subset of patients with metastatic, treatment-refractory sarcoma respond to immune checkpoint blockade, it is conceivable that efficacy would be improved if administered to treatment-naive patients with less tumor burden [40]. The goal of neoadjuvant and/or adjuvant immunotherapy is to trigger immune clearance of clinically undetectable metastases. Decreased rates of metastasis would significantly improve outcomes for STS patients. Neoadjuvant radiation therapy is often administered for sarcoma and has been reported to contribute to an anti-tumor immune response with immune checkpoint blockade in many preclinical and clinical studies with various tumor types [35,38,61,62]. To date, no clinical studies combining radiation therapy with immunotherapy to treat STS have been presented or published, but ongoing work is reviewed below.
NEXIS (NCT03116529) is a single-arm study in which 35 patients with intermediate- or high-grade STS > 5 cm in the trunk (non-retroperitoneal) or extremity receive neoadjuvant and adjuvant durvalumab and neoadjuvant tremelimumab with preoperative radiation therapy (at least 50 Gy at 1.8-2 Gy/fraction) [63]. This study will evaluate the safety, tolerability, and efficacy of durvalumab and tremelimumab in combination with radiation prior to surgical resection of high-risk STS. Patients with no evidence of disease following surgery will receive four additional doses of durvalumab, and patients with evidence of residual disease following surgery will receive nine additional doses of durvalumab unless there is clear disease progression. Patients with bulky sarcomas (>10 cm) will also receive a single 15 Gy fraction of high-dose spatially fractionated (GRID) radiation therapy 1-3 days prior to standard fractionated radiation therapy. The endpoints are histopathologic response in the surgical resection specimen and the number of patients experiencing high-grade toxicity. Secondary outcome measures include OS, disease-specific survival rate, relapse-free survival rate, and radiologic response to treatment (RECIST 1.1 and irRC). NEXIS opened in June 2017, and the estimated completion date is June 2022.
MD Anderson Cancer Center recently opened a randomized phase II clinical trial (NCT03307616) to compare neoadjuvant nivolumab alone versus neoadjuvant nivolumab and ipilimumab in patients with surgically resectable UPS or retroperitoneal dedifferentiated liposarcoma. Patients with UPS of the trunk or extremities receive concurrent radiation therapy starting two weeks after the first cycle of nivolumab with or without ipilimumab. The primary endpoint is pathologic response measured as percent hyalinization in the surgical resection specimen. Secondary measures include immunologic response, change in immune infiltrate relative to baseline, ORR, recurrence-free survival, OS, and safety. The study opened in October 2017, and estimated enrollment is 40 patients.
SU2C-SARC032 (NCT03092323) is an ongoing multi-center randomized clinical trial to examine the safety and efficacy of neoadjuvant pembrolizumab and radiation therapy in patients with clinically localized, high-risk STS of the extremity. This trial tests whether addition of neoadjuvant PD-1 immune checkpoint blockade with pembrolizumab to radiation therapy and adjuvant pembrolizumab can activate a systemic anti-tumor response to eliminate micrometastatic disease and improve disease-free survival. SU2C-SARC032 opened in July 2017 with a planned accrual of 110 patients.
Based on promising results of SARC028 in specific sarcoma subtypes, enrollment for SU2C-SARC032 is restricted to patients with UPS or dedifferentiated/pleomorphic liposarcoma. Patients are randomized to neoadjuvant radiation therapy (50 Gy in 25 fractions) followed by surgical resection (standard of care) versus neoadjuvant radiotherapy with 3 cycles of concurrent pembrolizumab (once before, during and after radiotherapy) followed by surgical resection and adjuvant pembrolizumab. In the experimental arm, patients receive up to one year of pembrolizumab (3 cycles of neoadjuvant and 14 cycles of adjuvant pembrolizumab). The primary endpoint is 2-year disease-free survival. Secondary endpoints include toxicity, local control, metastasis-free survival, and OS. Correlative studies from NEXIS, the MD Anderson trial, and SU2C-SARC032 may improve the understanding of immune checkpoint inhibition and radiotherapy in STS, inform patient selection for future clinical trials, and potentially identify novel targets for immunotherapy of STS.
Conclusions
Preclinical evidence suggests a role for the immune system in the therapeutic response of sarcomas, but clinical data remain limited. The need for hypothesis-driven clinical trials is clear, and ongoing phase II trials are examining immune checkpoint blockade in patients with high-risk, localized disease, either alone or in combination chemotherapy or radiation therapy. If successful, immune checkpoint inhibition could represent a paradigm shift for immunotherapy in the treatment of sarcoma from treating established metastases to preventing development of metastatic disease (Figure 1). Correlative studies will be essential to inform patient selection for future trials and to optimize this therapeutic approach. Much remains to be learned from these ongoing trials that have the potential to change the way we treat patients with soft tissue sarcoma.
Figure 2.
Pollack SM, He Q, Yearley JH, Emerson R, Vignali M, Zhang Y, et al. T-cell infiltration and clonality correlate with programmed cell death protein 1 and programmed death-ligand 1 expression in patients with soft tissue sarcomas. Cancer. 2017; doi:10.1002/cncr.30726
Acknowledgments
AJW is supported by F30CA221268. DGK is supported by 1R35-CA197616-02 from the NCI and DGK and YMM are supported by a Stand Up To Cancer (SU2C) Catalyst award.
Conflict of Interest: Dr. Mowery and Dr. Kirsch receive research funding from a SU2C Catalyst Research Grant supported by Merck for a clinical trial testing immune checkpoint inhibition and radiation therapy in sarcomas. Dr. Riedel receives clinical research funding for trials exploring immune checkpoint inhibition in sarcoma from Immune Design and Merck. He has served on an advisory board for Immune Design.
Funding: This work was supported by the National Cancer Institute of the US NIH under award number F30CA221268 (AJW) and R35CA197616 (DGK).
Footnotes
Author Contributions: AJW, YMM, RFR, and DGK contributed to conceptualization, investigation, writing the original draft, and editing. AJW and DGK were responsible for visualization and data presentation. DGK was responsible for supervision and project administration.
References
- 1.Howlader N, Noone AM, Krapcho M, Garshell J, Miller D, Altekruse SF, et al. SEER cancer statistics review, 1975-2012 [Internet] Bethesda, MD: National Cancer Institute; 2013. [cited 2015 Jan 28]. [Google Scholar]
- 2.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 3.Scheinberg Tahlia, Lomax Anna, Tattersall Martin HN, Thomas David Morgan, McCowage Geoffrey Brian, Sullivan Michael James, Bhadri Vivek Chris O'brien Lifehouse, University of Sydney, Garvan Institute of Medical Research, Darlinghurst, Australia, The Children's Hospital at Westmead, Westmead, Australia, Royal Children's Hospital. PD-1 blockade using pembrolizumab in adolescent and young adult patients with advanced bone and soft tissue sarcoma. [cited 18 May 2017];2017 ASCO Annual Meeting Abstracts. Meeting Abstracts [Internet] Available: http://abstracts.asco.org/199/AbstView_199_184854.html.
- 4.Fletcher CDM, Krishnan Unni K, Mertens F. Pathology and Genetics of Tumours of Soft Tissue and Bone. IARC. 2002 [Google Scholar]
- 5.Mitelman F. Recurrent chromosome aberrations in cancer. Mutat Res. 2000;462:247–253. doi: 10.1016/s1383-5742(00)00006-5. [DOI] [PubMed] [Google Scholar]
- 6.Borden EC, Baker LH, Bell RS, Bramwell V, Demetri GD, Eisenberg BL, et al. Soft tissue sarcomas of adults: state of the translational science. Clin Cancer Res. 2003;9:1941–1956. [PubMed] [Google Scholar]
- 7.McCarthy EF. The toxins of William B. Coley and the treatment of bone and soft-tissue sarcomas. Iowa Orthop J. 2006;26:154–158. [PMC free article] [PubMed] [Google Scholar]
- 8.Wang D, Zhang Q, Eisenberg BL, Kane JM, Li XA, Lucas D, et al. Significant Reduction of Late Toxicities in Patients With Extremity Sarcoma Treated With Image-Guided Radiation Therapy to a Reduced Target Volume: Results of Radiation Therapy Oncology Group RTOG-0630 Trial. J Clin Oncol. 2015;33:2231–2238. doi: 10.1200/JCO.2014.58.5828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Larrier NA, Czito BG, Kirsch DG. Radiation Therapy for Soft Tissue Sarcoma: Indications and Controversies for Neoadjuvant Therapy, Adjuvant Therapy, Intraoperative Radiation Therapy, and Brachytherapy. Surg Oncol Clin N Am. 2016;25:841–860. doi: 10.1016/j.soc.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 10.O'Sullivan B, Davis AM, Turcotte R, Bell R, Catton C, Chabot P, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet. 2002;359:2235–2241. doi: 10.1016/S0140-6736(02)09292-9. [DOI] [PubMed] [Google Scholar]
- 11.Adjuvant chemotherapy for localised resectable soft-tissue sarcoma of adults: meta-analysis of individual data. Sarcoma Meta-analysis Collaboration. Lancet. 1997;350:1647–1654. [PubMed] [Google Scholar]
- 12.Judson I, Verweij J, Gelderblom H, Hartmann JT, Schöffski P, Blay JY, et al. Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced or metastatic soft-tissue sarcoma: a randomised controlled phase 3 trial. Lancet Oncol. 2014;15:415–423. doi: 10.1016/S1470-2045(14)70063-4. [DOI] [PubMed] [Google Scholar]
- 13.Pervaiz N, Colterjohn N, Farrokhyar F, Tozer R, Figueredo A, Ghert M. A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft-tissue sarcoma. Cancer. 2008;113:573–581. doi: 10.1002/cncr.23592. [DOI] [PubMed] [Google Scholar]
- 14.Brennan MF, Antonescu CR, Moraco N, Singer S. Lessons learned from the study of 10,000 patients with soft tissue sarcoma. Ann Surg. 2014;260:416–21. doi: 10.1097/SLA.0000000000000869. discussion 421–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maki RG, Wathen JK, Patel SR, Priebat DA, Okuno SH, Samuels B, et al. Randomized phase II study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft tissue sarcomas: results of sarcoma alliance for research through collaboration study 002 [corrected] J Clin Oncol. 2007;25:2755–2763. doi: 10.1200/JCO.2006.10.4117. [DOI] [PubMed] [Google Scholar]
- 16.Tap WD, Jones RL, Van Tine BA, Chmielowski B, Elias AD, Adkins D, et al. Olaratumab and doxorubicin versus doxorubicin alone for treatment of soft-tissue sarcoma: an open-label phase 1b and randomised phase 2 trial. Lancet. 2016;388:488–497. doi: 10.1016/S0140-6736(16)30587-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med. 2017 doi: 10.1056/NEJMoa1709937. [DOI] [Google Scholar]
- 19.Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med Massachusetts Medical Society. 2016;375:1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 20.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14:1014–1022. doi: 10.1038/ni.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science American Association for the Advancement of Science. 2015;348:124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SAJR, Behjati S, Biankin AV, et al. Signatures of mutational processes in human cancer. Nature. 2013;500:415–421. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 25.Fourcade J, Sun Z, Benallaoua M, Guillaume P, Luescher IF, Sander C, et al. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J Exp Med. 2010;207:2175–2186. doi: 10.1084/jem.20100637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE, et al. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114:1537–1544. doi: 10.1182/blood-2008-12-195792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pollack SM, He Q, Yearley JH, Emerson R, Vignali M, Zhang Y, et al. T-cell infiltration and clonality correlate with programmed cell death protein 1 and programmed death-ligand 1 expression in patients with soft tissue sarcomas. Cancer. 2017 doi: 10.1002/cncr.30726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keung EZ, Tsai JW, Ali AM, Cormier JN, Bishop AJ, Guadagnolo BA, et al. Analysis of the immune infiltrate in undifferentiated pleomorphic sarcoma of the extremity and trunk in response to radiotherapy: Rationale for combination neoadjuvant immune checkpoint inhibition and radiotherapy. Oncoimmunology. 2018;7:e1385689. doi: 10.1080/2162402X.2017.1385689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cancer Genome Atlas Research Network. Electronic address: elizabeth.demicco@sinaihealthsystem.ca, Cancer Genome Atlas Research Network. Comprehensive and Integrated Genomic Characterization of Adult Soft Tissue Sarcomas. Cell. 2017;171:950–965.e28. doi: 10.1016/j.cell.2017.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stone HB, Peters LJ, Milas L. Effect of host immune capability on radiocurability and subsequent transplantability of a murine fibrosarcoma. J Natl Cancer Inst. 1979;63:1229–1235. [PubMed] [Google Scholar]
- 31.Siva S, MacManus MP, Martin RF, Martin OA. Abscopal effects of radiation therapy: A clinical review for the radiobiologist. Cancer Lett Elsevier. 2015;356:82–90. doi: 10.1016/j.canlet.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 32.Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. 2012;366:925–931. doi: 10.1056/NEJMoa1112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stamell EF, Wolchok JD, Gnjatic S, Lee NY, Brownell I. The abscopal effect associated with a systemic anti-melanoma immune response. Int J Radiat Oncol Biol Phys. 2013;85:293–295. doi: 10.1016/j.ijrobp.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chandra RA, Wilhite TJ, Balboni TA, Alexander BM, Spektor A, Ott PA, et al. A systematic evaluation of abscopal responses following radiotherapy in patients with metastatic melanoma treated with ipilimumab. Oncoimmunology. 2015;4:e1046028. doi: 10.1080/2162402X.2015.1046028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dovedi SJ, Cheadle EJ, Popple AL, Poon E, Morrow M, Stewart R, et al. Fractionated Radiation Therapy Stimulates Antitumor Immunity Mediated by Both Resident and Infiltrating Polyclonal T-cell Populations when Combined with PD-1 Blockade. Clin Cancer Res. 2017;23:5514–5526. doi: 10.1158/1078-0432.CCR-16-1673. [DOI] [PubMed] [Google Scholar]
- 36.Dewan MZ, Galloway AE, Kawashima N, Dewyngaert JK, Babb JS, Formenti SC, et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res. 2009;15:5379–5388. doi: 10.1158/1078-0432.CCR-09-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee Y, Auh SL, Wang Y, Burnette B, Wang Y, Meng Y, et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood. 2009;114:589–595. doi: 10.1182/blood-2009-02-206870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Twyman-Saint Victor C, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520:373–377. doi: 10.1038/nature14292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kang J, Demaria S, Formenti S. Current clinical trials testing the combination of immunotherapy with radiotherapy. J Immunother Cancer. 2016;4:51. doi: 10.1186/s40425-016-0156-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang AC, Postow MA, Orlowski RJ, Mick R, Bengsch B, Manne S, et al. T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature. 2017;545:60–65. doi: 10.1038/nature22079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ott PA, Hodi FS, Robert C. CTLA-4 and PD-1/PD-L1 blockade: new immunotherapeutic modalities with durable clinical benefit in melanoma patients. Clin Cancer Res. 2013;19:5300–5309. doi: 10.1158/1078-0432.CCR-13-0143. [DOI] [PubMed] [Google Scholar]
- 42.Chiou VL, Burotto M. Pseudoprogression and Immune-Related Response in Solid Tumors. J Clin Oncol. 2015;33:3541–3543. doi: 10.1200/JCO.2015.61.6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wolchok JD, Hoos A, O'Day S, Weber JS, Hamid O, Lebbé C, et al. Guidelines for the Evaluation of Immune Therapy Activity in Solid Tumors: Immune-Related Response Criteria. Clin Cancer Res American Association for Cancer Research. 2009;15:7412–7420. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 44.Maki RG, Jungbluth AA, Gnjatic S, Schwartz GK, D'Adamo DR, Keohan ML, et al. A Pilot Study of Anti-CTLA4 Antibody Ipilimumab in Patients with Synovial Sarcoma. Sarcoma. 2013;2013:168145. doi: 10.1155/2013/168145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Joseph CG, Hwang H, Jiao Y, Wood LD, Kinde I, Wu J, et al. Exomic analysis of myxoid liposarcomas, synovial sarcomas, and osteosarcomas. Genes Chromosomes Cancer. 2014;53:15–24. doi: 10.1002/gcc.22114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jungbluth AA, Antonescu CR, Busam KJ, Iversen K, Kolb D, Coplan K, et al. Monophasic and biphasic synovial sarcomas abundantly express cancer/testis antigen NY-ESO-1 but not MAGE-A1 or CT7. International journal of cancer Wiley Online Library. 2001;94:252–256. doi: 10.1002/ijc.1451. [DOI] [PubMed] [Google Scholar]
- 47.Lai JP, Robbins PF, Raffeld M, Aung PP, Tsokos M, Rosenberg SA, et al. NY-ESO-1 expression in synovial sarcoma and other mesenchymal tumors: significance for NY-ESO-1-based targeted therapy and differential diagnosis. Mod Pathol Nature Publishing Group. 2012;25:854. doi: 10.1038/modpathol.2012.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ben-Ami E, Barysauskas CM, Solomon S, Tahlil K, Malley R, Hohos M, et al. Immunotherapy with single agent nivolumab for advanced leiomyosarcoma of the uterus: Results of a phase 2 study. Cancer. 2017;123:3285–3290. doi: 10.1002/cncr.30738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.George S, Miao D, Demetri GD, Adeegbe D, Rodig SJ, Shukla S, et al. Loss of PTEN Is Associated with Resistance to Anti-PD-1 Checkpoint Blockade Therapy in Metastatic Uterine Leiomyosarcoma. Immunity. 2017;46:197–204. doi: 10.1016/j.immuni.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Somaiah N, Conley A, Lin H, Sanchez-Espiridion B, Amini B, Ravi V, et al. A phase II multi-arm study to test the efficacy of durvalumab and tremelimumab in multiple sarcoma subtypes. Connective Tissue Oncology Society. 2017:2804799. [Google Scholar]
- 51.Tawbi HA, Burgess M, Bolejack V, Van Tine BA, Schuetze SM, Hu J, et al. Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): a multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol. 2017;(17):30624–1. doi: 10.1016/S1470-2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Burgess MA, Bolejack V, Van Tine BA, Schuetze S, Hu J, D'Angelo SP, et al. Multicenter phase II study of pembrolizumab (P) in advanced soft tissue (STS) and bone sarcomas (BS): Final results of SARC028 and biomarker analyses. J Clin Orthod American Society of Clinical Oncology. 2017;35:11008–11008. [Google Scholar]
- 53.D'Angelo SP, Mahoney MR, Van Tine BA, Atkins J, Milhem MM, Jahagirdar BN, et al. Nivolumab with or without ipilimumab treatment for metastatic sarcoma (Alliance A091401): two open-label, non-comparative, randomised, phase 2 trials. Lancet Oncol. 2018;19:416–426. doi: 10.1016/S1470-2045(18)30006-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pollack SM, Ingham M, Spraker MB, Schwartz GK. Emerging Targeted and Immune-Based Therapies in Sarcoma. J Clin Oncol. 2018;36:125–135. doi: 10.1200/JCO.2017.75.1610. [DOI] [PubMed] [Google Scholar]
- 55.Paoluzzi L, Cacavio A, Ghesani M, Karambelkar A, Rapkiewicz A, Weber J, et al. Response to anti-PD1 therapy with nivolumab in metastatic sarcomas. Clin Sarcoma Res. 2016;6:24. doi: 10.1186/s13569-016-0064-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.National Cancer Institute; [cited 16 Jan 2018]. FDA Approval for Pazopanib Hydrochloride. Internet. Available: https://www.cancer.gov/about-cancer/treatment/drugs/fda-pazopanibhydrochloride. [Google Scholar]
- 57.D'Angelo SP, Shoushtari AN, Keohan ML, Dickson MA, Gounder MM, Chi P, et al. Combined KIT and CTLA-4 Blockade in Patients with Refractory GIST and Other Advanced Sarcomas: A Phase Ib Study of Dasatinib plus Ipilimumab. Clin Cancer Res. 2017;23:2972–2980. doi: 10.1158/1078-0432.CCR-16-2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Toulmonde M, Penel N, Adam J, Chevreau C, Blay JY, Le Cesne A, et al. Use of PD-1 Targeting, Macrophage Infiltration, and IDO Pathway Activation in Sarcomas: A Phase 2 Clinical Trial. JAMA Oncol. 2018;4:93–97. doi: 10.1001/jamaoncol.2017.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Trabectedin, Ipilimumab and Nivolumab as First Line Treatment for Advanced Soft Tissue Sarcoma - Full Text View - ClinicalTrials.gov [Internet] [cited 19 Jan 2018]; Available: https://clinicaltrials.gov/ct2/show/NCT03138161.
- 60.Pembrolizumab and Gemcitabine Chemotherapy in Leiomyosarcoma and Undifferentiated Pleomorphic Sarcoma - Full Text View - ClinicalTrials.gov [Internet] [cited 19 Jan 2018]; Available: https://clinicaltrials.gov/ct2/show/study/NCT03123276.
- 61.Zheng W, Skowron KB, Namm JP, Burnette B, Fernandez C, Arina A, et al. Combination of radiotherapy and vaccination overcomes checkpoint blockade resistance. Oncotarget. 2016;7:43039–43051. doi: 10.18632/oncotarget.9915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim JE, Patel MA, Mangraviti A, Kim ES, Theodros D, Velarde E, et al. Combination Therapy with Anti-PD-1, Anti-TIM-3, and Focal Radiation Results in Regression of Murine Gliomas. Clin Cancer Res. 2017;23:124–136. doi: 10.1158/1078-0432.CCR-15-1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Neoadjuvant Durvalumab and Tremelimumab Plus Radiation for High Risk Soft-Tissue Sarcoma (NEXIS) [cited 21 Oct 2017];Clinicaltrials.gov [Internet] Available: https://clinicaltrials.gov/ct2/show/NCT03116529.