Abstract
Rationale and Objectives
This study compares the performance of T2 maps in detection of prostate cancer (PCa) in comparison with T2-weighted (T2W) MR images.
Materials and Methods
The prospective study was IRB approved. Consenting patients (n=45) with histological confirmed PCa underwent preoperative 3T MRI, with or without endorectal coil. Two radiologists working independently, marked regions of interests (ROIs) on PCa lesions separately on T2W images, and T2 maps. Each ROI was assigned a score of 1–5 based on the confidence in accurately detecting cancer, with 5 being the highest confidence. Subsequently, the histologically confirmed PCa (n=112) on whole mount sections were matched with ROIs to calculate sensitivity, positive predictive value (PPV) and radiologist’s confidence score. Quantitative T2 values of PCa and benign tissue ROIs were measured.
Results
Sensitivity and confidence score for PCa detection were similar for T2W images (51%, 4.5±0.8) and T2 maps (52%, 4.5±0.6). However, PPV was significantly higher (p = 0.001) for T2 maps (88%) compared to T2W (72%) images. The use of endorectal coil nominally improved sensitivity (T2W: 55 vs 47%, T2 map: 54 vs 48%) compared to non-endorectal coil use, but not PPV and confidence score. Quantitative T2 values for PCa (105±28ms) was significantly (p=9.3×10−14) lower than benign PZ tissue (211±71ms), with moderate significant correlation with Gleason score (ρ=−0.284).
Conclusion
Our study shows that review of T2 maps by radiologists has similar sensitivity but higher PPV compared to T2W images. Additional quantitative information obtained from T2 maps is helpful in differentiating cancer from normal prostate tissue and determining its aggressiveness.
Keywords: T2-weighted, T2 map, prostate cancer, confidence score
Introduction
Prostate cancer (PCa) is the most common non-cutaneous cancer and second leading cause of death among men in the United States (1, 2). Conventional prostate specific antigen (PSA) screening and transrectal ultrasound (TRUS) guided biopsies have low sensitivity in PCa detection (3, 4). In addition, distinguishing low risk tumors from potentially life-threatening tumors using PSA and TRUS is inadequate, and many patients with low risk tumors elect to undergo radical prostatectomy, a major procedure with potential quality of life-altering complications. These limitations emphasize the need for non-invasive imaging techniques for detection, staging and risk stratification of PCa, which may enable better choice of treatment and reduce the overtreatment of indolent diseases (5).
Multiparametric MRI (mpMRI) is increasingly being used for prostate cancer diagnosis and guiding biopsies, and has high sensitivity, negative predictive value and specificity in PCa diagnosis (6, 7). mpMRI has the potential to provide reliable information about the cancer grade, location and volume for the selection of optimum therapy (7). T2-weighted (T2W) imaging is an integral part of mpMRI protocol recommended by American College of Radiology and European Society of Urogenital Radiology in the consensus guidelines: Prostate Imaging Reporting and Data System (PI-RADS) (8). PCa shows hypointensity in T2-weighted images compared to benign tissue and have a sensitivity of around 50–70% for PCa detection (6, 9–12). T2 mapping techniques requires acquisition of multiple T2W images at various echo times (TEs). The signal from various TEs is then fitted an exponential MR signal decay model to generate an estimate of the quantitative T2 values. Numerous studies have found that quantitative T2 values are significantly lower in PCa compared to benign prostate tissue (13–17). The interpretation of conventional T2W images is subjective as signal intensities in T2W images are not comparable between patients and contrast is highly dependent on imaging parameters. However, quantitative T2 values from T2 maps are absolute and comparable. The use of T2 maps may help mitigate the subjective nature of T2W image interpretation, and allow improved PCa detection based on subtle difference in T2 values between different tissue.
There is no study to our knowledge that has compared the diagnostic performance of T2W images with T2 maps in the PCa detection. This study compares the performance of T2 maps in detection of prostate cancer in comparison with conventional T2W images.
Materials and Methods
Study patients
This prospective study was conducted after institutional review board approval, informed patient consent and was compliant with Health Insurance Portability and Accountability Act. Forty-five consecutive patients were included for this study, with prior biopsy proven prostate cancer that were scheduled to undergo radical prostatectomy that agreed to participate in the study. Among the contraindications to inclusion in the study was prior receipt of radiation or hormonal replacement therapy (leading to alterations in prostatic signal on MRI). The mean age of patient was 59 years (range 42–76 years), and mean PSA was 9.5 ng/mL (range 0.8 – 66.1 ng/mL) prior to MR imaging.
MR Imaging
Patients underwent preoperative MRI with a 3T Philips Achieva MR system with a 6-channel cardiac phased array coil placed around the pelvis, and with or without endorectal coil (Medrad, Bayer Healthcare). For patients undergoing imaging using an endorectal coil, a 1mg dose of glucagon (Glucagon, Eli Lilly & Co., Indianapolis) was injected prior to MR imaging to limit peristalsis of the rectal wall. T2W images and multi echo T2W images for T2 maps were acquired. The patients were randomly assigned to one of three groups (Group A–C). Group A (n = 15) was imaged without any endorectal coil using turbo spin echo (TSE) imaging pulse sequence for both T2W images and T2 mapping. Group B (n = 15) was imaged with an endorectal coil using TSE for both T2W images and T2 mapping. Group C (n = 15) was imaged with an endorectal coil using TSE for T2W images and the recently developed k-t-T2 sequence (18) for T2 mapping. Table 1 provides details of the imaging parameters used.
Table 1.
MR Imaging parameters
| Imaging sequence | Pulse sequence | FOV (mm) | Matrix size | In plane Resolution (mm) | TE (ms) | TR (s) |
|---|---|---|---|---|---|---|
| Group A: TSE without endorectal coil | TSE (T2W) | 180 | 240 × 240 | 0.75 × 0.75 | 115 | 4.5 |
| TSE (T2 map) | 200 | 250 × 250 | 0.8 × 0.8 | 38,88,138, 188,238,288 | 8.6 | |
| Group B: TSE with endorectal coil | TSE (T2W) | 160 | 400 × 400 | 0.4 × 0.4 | 115 | 4.2 |
| TSE (T2 map) | 160 | 212× 212 | 0.75 × 0.75 | 30, 60, 90, 120, 150, 180, 210, 240, 270 | 8.8 | |
| Group C: TSE (T2W) and k-t-T2 (T2 map) with endorectal coil | TSE (T2W) | 160 | 400 × 400 | 0.4 × 0.4 | 115 | 4.2 |
| k-t-T2 (T2 map) | 160 | 160 × 160 | 1.0 × 1.0 | 24, 36, 48, 60, 72, 84, 96, 108, 120, 132, 144, 156, 168, 180, 192, 204, 216, 228, 240, 252, 264, 276, 288, 300, 312, 324, 336, 348, 360, 382, 396 | 3.3 |
TSE - turbo spin echo, T2W - T2 weighted
Slice thickness = 3 mm, Flip angle = 90°
Scan time for T2 mapping using TSE sequence = 492 sec (without endo-rectal coil) and 436 sec (with endo-rectal coil), k-t-T2 sequence= 316 sec
Scan time for T2 weighted imaging using TSE sequence = 270 sec (without endo-rectal coil) and 381 sec (with endo-rectal coil)
T2 maps were calculated using an in-house MATLAB (MathWorks, Natick, MA) program on a voxel by voxel basis from multi-echo T2-weighted images using a mono-exponential signal decay model:
where S is the signal at each echo time (TE) and S0 is the extrapolated signal at TE = 0 ms.
MR image Analysis
The T2W images and T2 maps for all 45 patients were loaded on custom module PCampReview in 3D Slicer (19) and radiologists were blinded to results from histology. Two expert radiologists (R1: XX with 15 years’ experience, R2: YY with 5 years’ experience) working independently, freely evaluated the entire image set and marked regions of interests (ROIs) where they were suspicious for PCa lesions separately on T2W images, and after a 2-week gap on T2 maps. Each ROI was assigned a score of 1–5 based on the confidence in accurately detecting cancer, with 5 being the highest confidence.
The individuals underwent radical prostatectomy and whole prostates were fixed in formalin and serially sectioned approximately in the same plane as MR images. Whole mount tissue sections were embedded in paraffin, hematoxylin and eosin-stained and cancers were marked on histological slides by an expert pathologist (ZZ, 10 years’ experience). The specimens were compared to the preoperative MR imaging in a retrospective fashion with the sites of known malignancy identified on MR images. The MR and histology were matched by the consensus of the senior radiologist (XX, 15 years’ experience) and a medical physicist (XX, 5 years of experience with prostate MRI and pathology). This was done 3 months prior to the radiologist reading all the T2W images and T2 maps. The subsequent analysis was done by the medical physicist (XX) rather than a radiologist to avoid any potential conflict of interest. The matching was performed on a lesion basis. The lesions drawn on MRI by radiologists and cancer outlined by pathologist on histology were considered to be overlapping if they were marked in the same prostate sector. Only cancer lesions with a dimension greater than 5 mm were included in the analysis. Subsequently, the histologically confirmed PCa on whole mount sections were matched with ROIs drawn on T2W images and T2 maps by the radiologists. In case radiologists marked multiple ROIs for the same cancer lesion on multiple MR slices, the highest confidence score was used in the subsequent analysis. Sensitivity, precision or positive predictive value (PPV) and radiologist’s mean confidence score for PCa detection using T2-weighted imaging and T2 mapping were calculated and compared using z-test (hypothesis testing). Wilcoxon signed-rank test was performed to assess the difference in radiologist’s confidence score for detection of histologically confirmed PCa lesions using T2 map and T2W images. Cohen’s kappa (κ) was calculated using SPSS (IBM Corporation, Armonk, NY) for measuring inter-observer agreement between the two radiologists.
MR images were analyzed again by a radiologist (XX) and regions of interest (ROIs) were placed on T2 maps on sites of prostatectomy verified malignancy and normal peripheral zone (PZ) tissue to measure T2 values. Quantitative T2 values of all confirmed PCa and benign PZ tissue ROIs were measured. A two tail t-test was performed to assess the difference between means of calculated T2 value of cancer and normal prostate tissue. Spearman correlation coefficient (ρ) was calculated between Gleason score and quantitative T2 values to investigate whether quantitative T2 values can determine the aggressiveness of cancer for deciding the optimal treatment option.
Results
A total of 112 histologically confirmed PCa with dimension greater than 5 mm were found on whole mount sections. Detailed pathology results (Gleason score and pathological stage of PCa) are provided in Table 2. The histologically confirmed PCa on whole mount sections were matched with ROIs drawn on T2W and T2 maps by the 2 radiologists. Representative T2W images and T2 maps and corresponding histology sections with cancer lesions marked are shown in Figure 1. The detailed radiologist performance in PCa detection is provided in Table 3. The sensitivity, PPV and confidence score for PCa detection using T2W images and T2 maps, T2 maps obtained using TSE vs. k-t- T2 sequences, and effect of using endorectal coil are listed.
Table 2.
Histological Confirmed Cancers
| Overall | Group A: TSE without endorectal coil | Group B: TSE with endorectal coil | Group C: TSE (T2W) and k-t-T2 (T2 map) with endorectal coil | ||
|---|---|---|---|---|---|
| n | 112 | 43 | 32 | 37 | |
| Gleason score | 3+3 | 54 | 19 | 13 | 22 |
| 3+4 | 42 | 19 | 13 | 10 | |
| 4+3 | 15 | 4 | 6 | 5 | |
| 4+5 | 1 | 1 | - | - | |
| Stage | T2 | 87 | 38 | 20 | 29 |
| T2c | 8 | 1 | 2 | 5 | |
| T3 | 1 | 1 | - | - | |
| T3a | 14 | 1 | 10 | 3 | |
| T3b | 2 | 2 | - | - | |
| Mean Tumor Size (range) (cm2) | 1.6 × 0.8 (0.5–5.3 × 0.2–3.5) | 1.5 × 0.8 (0.5–4.3 × 0.2–3.5) | 1.7 × 0.9 (0.5–5.3 × 0.2–2.0) | 1.5 × 0.7 (0.5–3.5 × 0.2–1.7) | |
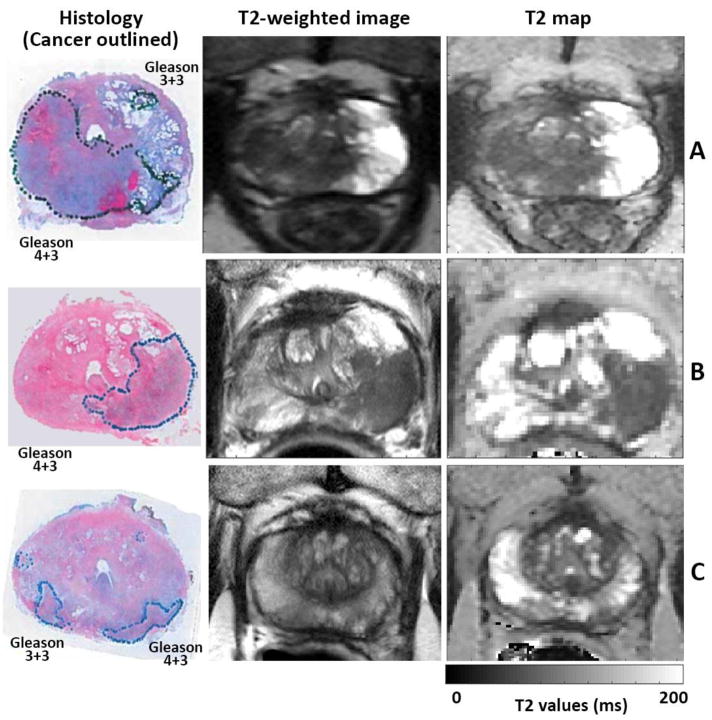
Figure 1.
Whole mount histology from prostatectomy specimen with cancers markers along with corresponding T2-weighted image and T2 map. Prostate cancers show hypo-intensity in T2-weighted images and T2 maps compared to benign tissue. (A) Group A - No endorectal coil was used while TSE sequence was used for T2 mapping, (B) Group B - Endorectal coil was used and TSE sequence was used for T2 mapping (C) Group C - Endorectal coil was used and k-t-T2 sequence was used for T2 mapping.
Table 3.
Radiologist performance statistics
| MRI sequence | Sensitivity | Positive Predictive Value | Confidence Score | ||
|---|---|---|---|---|---|
| T2-weighted vs. T2 maps | |||||
| T2W | Overall | 51 % | 72 % | 4.5 ± 0.8 | |
| Radiologist 1 | 52 % | 81 % | 4.5 ± 0.8 | ||
| Radiologist 2 | 51 % | 65 % | 4.5 ± 0.9 | ||
| T2 map | Overall | 52 % | 88 % | 4.5 ± 0.6 | |
| Radiologist 1 | 57 % | 88 % | 4.7 ± 0.5 | ||
| Radiologist 2 | 47 % | 88 % | 4.2 ± 0.5 | ||
| Endorectal coil usage | |||||
| T2W | No endorectal coil | Overall | 47 % | 71 % | 4.4 ± 1.0 |
| Radiologist 1 | 47 % | 80 % | 4.4 ± 0.9 | ||
| Radiologist 2 | 47 % | 65 % | 4.3 ± 1.0 | ||
| Endorectal coil | Overall | 54 % | 72 % | 4.5 ± 0.8 | |
| Radiologist 1 | 55 % | 81 % | 4.6 ± 0.7 | ||
| Radiologist 2 | 54 % | 65 % | 4.5 ± 0.8 | ||
| T2 map | No endorectal coil | Overall | 48 % | 89 % | 4.4 ± 0.9 |
| Radiologist 1 | 53 % | 88 % | 4.8 ± 0.4 | ||
| Radiologist 2 | 44 % | 90 % | 4.2 ± 0.5 | ||
| Endorectal coil | Overall | 54 % | 87 % | 4.4 ± 0.5 | |
| Radiologist 1 | 59 % | 87 % | 4.8 ± 0.5 | ||
| Radiologist 2 | 49 % | 87 % | 4.2 ± 0.5 | ||
Qualitative results
Sensitivity for PCa detection were similar (Z = 0.095, p = 0.925) for T2W images (overall: 51%, radiologist 1: 52%, radiologist 2: 51%) and T2 maps (overall: 52%, radiologist 1: 57%, radiologist 2: 47%). Wilcoxon signed-rank test showed no significant difference (Z = −0.013, p = 0.990) in radiologist assigned confidence score for PCa detection using T2 maps (overall: 4.5 ± 0.6; radiologist 1: 4.7 ± 0.5; radiologist 2: 4.2 ± 0.5) and T2W images (overall: 4.5 ± 0.8; radiologist 1: 4.5 ± 0.8; ra diologist 2: 4.5 ± 0.9). While radiologist 1 showed significantly higher (Z = −2.127, p = 0.033) confidence score for lesion detection using T2 maps over T2W images, radiologist 2 showed significantly lower scores (Z = 2.107, p = 0.035). However, PPV was significantly higher (Z = 3.318, p = 0.001) for T2 maps (overall: 88%, radiologist 1: 88%, radiologist 2: 88%) compared to T2W (overall: 72%, radiologist 1: 81%, radiologist 2: 65%) images.
Use of endorectal coil
The use of endorectal coil nominally improved sensitivity (T2W: overall: 54 vs 47%, radiologist 1: 55 vs 47%, radiologist 2: 54 vs 47%; T2 map: overall: 54 vs 48%, R1: 59 vs 53%, radiologist 2: 49 vs 44%) compared to non-endorectal coil use, but not PPV and confidence score. However, no statistical difference was found.
Inter-observer agreement
There was moderate inter-observer agreement in confidence scores between the two radiologists with a calculated Cohen’s kappa (κ) of 0.46 (p = 3.5×10−26) overall. While there was good agreement in confidence scores assigned individually by the two radiologists for the T2W images (κ = 0.61, p = 3.1×10 −23), only a fair agreement for T2 maps (κ = 0.34, p = 5.6×10−11) was found.
Quantitative results
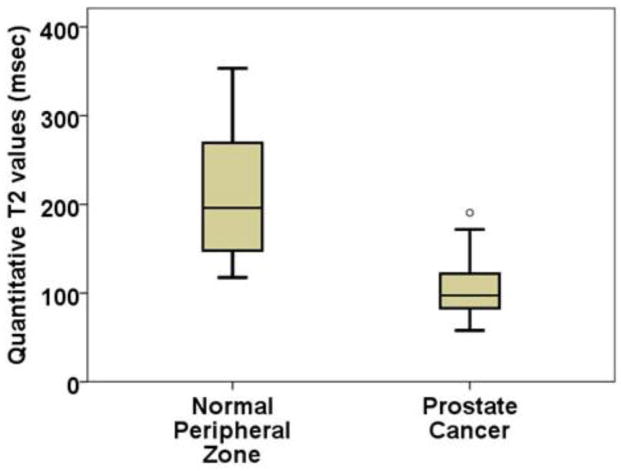
Quantitative T2 values of all confirmed PCa (n = 112) and benignPZ tissue (n = 42) ROIs were measured. Quantitative T2 values for PCa (105 ± 28 ms) was significantly (t = 11.908, p = 9.3×10−14) lower than benign PZ tissue (211 ± 71 ms). Figure 2 shows a box-plot comparing quantitative T2 values of PCa and benign PZ tissue. There was moderate Spearman correlation (ρ = −0.284, p = 0.009) between T2 values and cancer Gleason score.
Figure 2.
Quantitative T2 values from T2 maps: T2 values are significantly (p=9.3×10−14) lower for prostate cancer (105±28ms) compared to normal peripheral zone tissue (211±71ms).
Discussion
T2 weighted imaging along with diffusion weighted imaging (DWI) of the prostate remains the backbone for prostate cancer diagnosis. DWI and T2W-weighted are recommended as the primary sequences for PCa detection in the peripheral and transition zones respectively by PIRADS consensus guidelines (8). However, the results of this study shows that review of traditional T2W-weighted MR images by radiologists has low sensitivity for PCa detection. Similar low sensitivity for PCa detection has been shown using T2W in other previous studies (6, 9–12). These limitations, specifically the low sensitivity in PCa detection using T2W MR images emphasize the need for better imaging technique for improved PCa detection.
Our study shows that review of T2 maps by radiologists has similar sensitivity and radiologists’ confidence score compared to traditional T2W MR images. Importantly, PPV was higher for T2 maps compared to T2W images in this study. An increase in PPV implies a combination of higher numbers of true positives and lower number of false positives. The increase in PPV with comparable sensitivity suggests the T2-maps may provide better contrast between benign and cancer tissue. In addition, quantitative T2 values can be standardized and compared between patients unlike T2W imaging. Therefore, improved PPV would increase the yield of targeted biopsies. The sensitivity are similar to values found in previous observer studies looking at T2W images (6, 9–12). While some studies have shown higher sensitivity and PPV using T2W images, it should be noted that in these studies either the index or dominant lesions were considered in the analysis (11), or the cancer lesions dimensions were significantly larger (20) than those analyzed in this study. The PPV using T2 maps is higher than values found for T2W in these studies. In addition, PPV using T2 maps alone is comparable to results from mpMRI. It might be interesting for future work to investigate how T2-mapping would affect diagnosis when radiologists are using PIRADS-based scoring.
Radiologists are trained and more experienced in looking at T2W images than T2 maps for PCa detection. Despite this fact, T2 maps provided similar or better performance for diagnosis of prostate cancer. The diagnostic performance for PCa detection using T2 maps may be expected to improve with each radiologist’s increased experience with T2 maps. This is evidenced by the fact that while there was good agreement in confidence scores assigned individually by the two radiologists for the T2W images, while only a fair agreement for T2 maps was found. In addition, sensitivity and confidence score was higher for the more experienced radiologist (radiologist 1), who also had more experience looking at T2 maps for research purposes, compared to radiologist 2.
The use of endorectal coil nominally improved sensitivity compared to non-endorectal coil use, but not PPV and confidence score. These results are consistent with previous studies that have shown that image quality and prostate cancer detection rate is improved with the use of endorectal coil (20–22). Sensitivity of T2W images in PCa detection improved by 7% with the use endorectal coil in this study, which is consistent with the results of a previous study that also showed a 7% improvement (22). Similarly, the use of endorectal coil did not improve the PPV for PCa detection in our study and the previous study mentioned above. However, the use of endorectal coil improved the PPV in another previous study (20). The spatial resolution is lower in patients imaged without endorectal coil due to lower signal to noise ratio. This trend is seen in previous studies as well. Additionally, the radiologists’ confidence scores were similar for cancerous lesions imaged using an endorectal coil over those imaged without it. However, this result could be biased as radiologists could not be blinded to presence of endorectal coil, and this may have influenced the radiologists’ assignment of confidence score. The use of endorectal coil also improved sensitivity for T2 maps suggesting the use of endorectal coil may be recommended to maximize the potency of T2 mapping.
Quantitative T2 values for PCa were significantly lower than benign PZ tissue in this study. These results are consistent with previous studies (15, 17, 23). While the interpretation of T2W images is subjective as signal intensities in T2W images are not comparable between patients and contrast is highly dependent on imaging parameters, quantitative T2 values from T2 maps are absolute and comparable. This additional quantitative information obtained from T2 maps is helpful in differentiating cancer from normal prostate tissue. However, it should be noted that there may be inter patient, short and long term repeatability and reproducibility across multiple imaging sequences and scanner variability of T2 values. Additionally, T2 values are also dependent on the imaging parameters.
A limitation of this study was the use of different techniques for T2 mapping in these cases. T2 mapping in fifteen cases were done using k-t-T2 sequence (18), while conventional TSE sequence was used for T2 mapping in other patients. However, it has been shown that the T2 values using the k-t-T2 sequence has excellent correlation with traditional T2 values (17, 18, 24), and as a result the different imaging sequences used should not cause measurement variability. The time required to acquire T2 maps with k-t-T2 (316 msec) was significantly lower than TSE (436–492 msec). The reduced imaging time using k-t-T2 sequence is possible through k-space under-sampling resulting in accelerated image acquisition. In addition, more echo times were sampled with the k-t-T2 sequence (32 TE’s) than with the TSE sequence (6–9 TE’s). Imaging time and motion artifacts in T2 mapping could be reduced further using k-t-T2 sequence with a smaller number of echo times, similar to the TE’s sampled with the TSE sequence (6–9 TE’s).
The same radiologist matched MR and pathology 3 months prior to the radiologist reading all the T2W images and T2 maps. The 3-month washout period is deemed adequate to avoid any potential bias, and the subsequent analysis was done by the medical physicist rather than a radiologist to avoid any potential conflict of interest. Since T2W images were used for the MR-pathology match, any bias, if any would improve performance of T2W images, while performance with T2 maps will be unaffected. Since T2 maps showed better performance (similar sensitivity with higher PPV) compared to T2W images, we believe this potential bias is negligible and not detrimental to the results of this study comparing the performance of T2W images and T2 maps in PCa diagnosis.
The use of longer TR (TR ~ 8 sec) for T2 mapping using TSE (with or without endo rectal coil) could produce modest bias by increasing the contribution from water protons with long T1, relative to T2W images acquired with TR~4 seconds. This may have emphasized mobile water protons, e.g. protons from luminal fluid. It should also be noted that the TSE images used in this study, and generally in clinical practice, were acquired with refocusing pulses that were significantly less than 180 degrees – therefore the contrast in the TSE images is not the same as contrast in traditional spin echo images. This may explain some of the differences between T2 maps and T2W images. Moreover, the acquisition time for T2 mapping is longer than T2W imaging. In addition, T2 mapping entails more complex post-processing. Another potential bias is due to the difference in spatial resolution between images between T2W and T2 mapping. T2W images have been obtained with higher resolution than that used to obtain T2-mapping and this certainly affects the identification of PCa by the radiologists.
Due to the lesion based correlation method used, specificity and negative predictive value could not be determined in this study. However, this inherent limitation is not unique to our study, as previous observer studies with similar setup have described this limitation (11, 20).
Another limitation of this study is the small sample size and limited readers. In addition, the two readers were aware of the fact that the patients that were imaged underwent subsequent radical prostatectomy for prostate cancer. Therefore, there could be a bias during assigning confidence score for PCa presence. However, this should not affect the comparison between the confidence score assigned by radiologist for T2W images and T2 maps.
Conclusion
Our study shows that review of T2 maps by radiologists has similar sensitivity and radiologists’ confidence score compared to traditional T2-weighted MR images. Importantly, PPV was higher for T2 maps compared to T2W images in this study. Quantitative information obtained from T2 maps is also helpful in differentiating cancer from normal prostate tissue and to determine its aggressiveness. Therefore, further investigation is needed to explore the utility of using T2 mapping as a possible primary diagnostic MR sequence for diagnosis of prostate cancer.
Acknowledgments
Grant sponsor: This work was supported by Philips Healthcare, Guerbet LLC and National Institutes of Health (NIH R01 CA172801 and NIH 1S10OD018448-01).
Abbreviations
- mpMRI
multiparametric MRI
- MRI
Magnetic Resonance Imaging
- PCa
Prostate Cancer
- PI-RADS
Prostate Imaging – Reporting and Data System
- PPV
Positive Predictive Value
- PZ
Peripheral Zone
- ROI
Region of Interest
- T2W
T2 weighted
- TE
Echo Time
- TR
Repetition Time
- TRUS
Transrectal Ultrasound
- TSE
Turbo Spin Echo
- TZ
Transition Zone
Footnotes
Disclosures: Dr Aytekin Oto has the following disclosures. Research Grant, Koninklijke Philips NV Research Grant, Guerbet SA Research Grant, Profound Medical Inc. Medical Advisory Board, Profound Medical Inc Speaker, Bracco Group
Potential conflict of interest: One of our authors: Ajit Devaraj is an employee of Philips Healthcare, a commercial entity. However, the data was analyzed and controlled by authors who were not employed by or a consultant for a company in the medical industry. In addition, the current study compares the clinical utility of T2 mapping vs. T2-weighted MRI in the detection of prostate cancer. Both of these are general MR imaging approaches not tied to a particular sequence or technique that Philips Healthcare intends to promote. While the study was carried out on a single vendor, the results are not vendor specific or dependent. The above aspects of the study are considered sufficient to addresses any potential conflicts of interest.
IRB statement: The study was conducted after institutional review board approval, informed patient consent and was compliant with Health Insurance Portability and Accountability Act.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA: A Cancer Journal for Clinicians. 2012;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Johnson LM, Choyke PL, Figg WD, Turkbey B. The Role of MRI in Prostate Cancer Active Surveillance. BioMed Research International. 2014;2014:6. doi: 10.1155/2014/203906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stephan C, Rittenhouse H, Hu X, Cammann H, Jung K. Prostate-Specific Antigen (PSA) Screening and New Biomarkers for Prostate Cancer (PCa) EJIFCC. 2014;25(1):55–78. [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmed HU, El-Shater Bosaily A, Brown LC, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. The Lancet. 389(10071):815–22. doi: 10.1016/S0140-6736(16)32401-1. [DOI] [PubMed] [Google Scholar]
- 5.Gupta RT, Kauffman CR, Polascik TJ, Taneja SS, Rosenkrantz AB. The state of prostate MRI in 2013. Oncology. 2013;27(4):262. [PubMed] [Google Scholar]
- 6.Baur AD, Maxeiner A, Franiel T, et al. Evaluation of the prostate imaging reporting and data system for the detection of prostate cancer by the results of targeted biopsy of the prostate. Invest Radiol. 2014;49(6):411–20. doi: 10.1097/RLI.0000000000000030. [DOI] [PubMed] [Google Scholar]
- 7.Wysock JS, Lepor H. Multi-parametric MRI imaging of the prostate-implications for focal therapy. Transl Androl Urol. 2017;6(3):453–63. doi: 10.21037/tau.2017.04.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weinreb JC, Barentsz JO, Choyke PL, et al. PI-RADS Prostate Imaging – Reporting and Data System: 2015, Version 2. European Urology. 2016;69(1):16–40. doi: 10.1016/j.eururo.2015.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vilanova JC, Barceló-Vidal C, Comet J, et al. Usefulness of prebiopsy multifunctional and morphologic MRI combined with free-to-total prostate-specific antigen ratio in the detection of prostate cancer. AJR American journal of roentgenology. 2011;196(6):W715–W22. doi: 10.2214/AJR.10.5700. [DOI] [PubMed] [Google Scholar]
- 10.Turkbey B, McKinney YL, Trivedi H, et al. Multiparametric 3T prostate magnetic resonance imaging to detect cancer: histopathological correlation using prostatectomy specimens processed in customized magnetic resonance imaging based molds. The Journal of urology. 2011;186(5):1818–24. doi: 10.1016/j.juro.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosenkrantz AB, Deng F-M, Kim S, et al. Prostate Cancer: Multiparametric MRI for Index Lesion Localization—A Multiple-Reader Study. American Journal of Roentgenology. 2012;199(4):830–7. doi: 10.2214/AJR.11.8446. [DOI] [PubMed] [Google Scholar]
- 12.Greer MD, Brown AM, Shih JH, et al. Accuracy and agreement of PIRADSv2 for prostate cancer mpMRI: A multireader study. Journal of Magnetic Resonance Imaging. 2017;45(2):579–85. doi: 10.1002/jmri.25372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foltz WD, Chopra S, Chung P, et al. Clinical prostate T2 quantification using magnetization-prepared spiral imaging. Magnetic Resonance in Medicine. 2010;64(4):1155–61. doi: 10.1002/mrm.22492. [DOI] [PubMed] [Google Scholar]
- 14.Liney GP, Turnbull LW, Lowry M, Turnbull LS, Knowles AJ, Horsman A. In vivo quantification of citrate concentration and water T2 relaxation time of the pathologic prostate gland using 1H MRS and MRI. Magnetic Resonance Imaging. 1997;15(10):1177–86. doi: 10.1016/s0730-725x(97)00182-3. [DOI] [PubMed] [Google Scholar]
- 15.Hoang Dinh A, Souchon R, Melodelima C, et al. Characterization of prostate cancer using T2 mapping at 3 T: A multi-scanner study. Diagnostic and Interventional Imaging. 2015;96(4):365–72. doi: 10.1016/j.diii.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 16.Gibbs P, Tozer DJ, Liney GP, Turnbull LW. Comparison of quantitative T2 mapping and diffusion-weighted imaging in the normal and pathologic prostate. Magnetic Resonance in Medicine. 2001;46(6):1054–8. doi: 10.1002/mrm.1298. [DOI] [PubMed] [Google Scholar]
- 17.Liu W, Turkbey B, Sénégas J, et al. Accelerated T(2) Mapping for Characterization of Prostate Cancer. Magnetic resonance in medicine: official journal of the Society of Magnetic Resonance in Medicine/Society of Magnetic Resonance in Medicine. 2011;65(5):1400–6. doi: 10.1002/mrm.22874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sénégas J, Liu W, Dahnke H, Song H, Jordan EK, Frank JA. Fast T2 relaxometry with an accelerated multi-echo spin-echo sequence. NMR in Biomedicine. 2010;23(8):958–67. doi: 10.1002/nbm.1521. [DOI] [PubMed] [Google Scholar]
- 19.Fedorov A, Beichel R, Kalpathy-Cramer J, et al. 3D Slicer as an Image Computing Platform for the Quantitative Imaging Network. Magnetic Resonance Imaging. 2012;30(9):1323–41. doi: 10.1016/j.mri.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Turkbey B, Merino MJ, Gallardo EC, et al. Comparison of endorectal coil and nonendorectal coil T2W and diffusion-weighted MRI at 3 Tesla for localizing prostate cancer: Correlation with whole-mount histopathology. Journal of Magnetic Resonance Imaging. 2014;39(6):1443–8. doi: 10.1002/jmri.24317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gawlitza J, Reiss-Zimmermann M, Thörmer G, et al. Impact of the use of an endorectal coil for 3 T prostate MRI on image quality and cancer detection rate. Scientific Reports. 2017;7:40640. doi: 10.1038/srep40640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heijmink SWTPJ, Fütterer JJ, Hambrock T, et al. Prostate Cancer: Body-Array versus Endorectal Coil MR Imaging at 3 T—Comparison of Image Quality, Localization, and Staging Performance. Radiology. 2007;244(1):184–95. doi: 10.1148/radiol.2441060425. [DOI] [PubMed] [Google Scholar]
- 23.Gibbs P, Liney GP, Pickles MD, Zelhof B, Rodrigues G, Turnbull LW. Correlation of ADC and T2 measurements with cell density in prostate cancer at 3.0 Tesla. Invest Radiol. 2009;44(9):572–6. doi: 10.1097/RLI.0b013e3181b4c10e. [DOI] [PubMed] [Google Scholar]
- 24.Wang S, Agarwal H, Karczmar GS, Oto A. Two-compartment T2 Decay for Prostate Cancer Diagnosis. Proc Intl Soc Mag Reson Med; Toronto, Canada. 2015. p. 0944. [Google Scholar]