Summary
In two observational cohorts of inner-city children with asthma, secondhand smoke exposure was associated with worsened symptoms in overweight/obese compared to normal weight children, suggesting that elevated BMI may increase susceptibility to secondhand smoke, a common indoor pollutant.
TO THE EDITOR —
Asthma in overweight and obese children has been associated with worsened morbidity. Overweight and obese children may also be more susceptible to air pollution. Elevations in particulate matter, sulfur dioxide, and ozone have been associated with greater respiratory symptoms in overweight or obese children compared to normal weight children(1, 2).
Secondhand smoke (SHS) is a common indoor air pollutant. Approximately 37% of children in the United States were still exposed in 2011-2012, amid encouraging secular declines(3). If SHS exposure is associated with worsened asthma control in overweight and obese children, this may offer additional insight into the increased asthma morbidity observed in these children, and it would raise important questions about pathways that determine susceptibility. While public health initiatives to reduce obesity and SHS exposure are underway, such a finding would also have clinical relevance.
To investigate possible effect modification of the effects of SHS by overweight/obesity, we analyzed data from two observational cohorts of inner-city, predominantly African American children with asthma, the Mouse Allergen and Asthma Cohort Study (MAACS) and the Discover Study. In MAACS, 150 children were followed every three months for one year. SHS exposure was assessed by urine cotinine and asthma morbidity by caregiver recall of the number of days of symptoms in the prior two weeks. Symptoms included: days with cough, wheeze, chest tightness; slowed activity due to asthma; exercise-related symptoms; trouble speaking due to wheeze; cough without cold; nocturnal awakening; and a composite maximum asthma-days (days with slowed activity, exercise-related symptoms, or nocturnal symptoms due to asthma). In Discover, 162 children were followed every three months for nine months. SHS exposure was assessed by in-home airborne nicotine, and asthma morbidity by daily diary that asked if the participant had symptoms, used a rescue inhaler, had an unanticipated acute care need, or missed school due to asthma(4). Symptoms included trouble breathing; limited activities due to asthma; bother due to asthma; and nocturnal awakening. Full details are in the online repository.
Normal weight, overweight, and obesity were defined as BMI ≥5th, ≥85th, and ≥95th percentile by U.S. Centers for Disease Control criteria. Underweight participants were excluded. Baseline characteristics were statistically compared across BMI categories with Fisher’s exact and ANOVA/Kruskal-Wallis tests. Binomial generalized estimating equation regression models were constructed to estimate the association between SHS exposure and the daily odds for each outcome, utilizing an exchangeable correlation structure and robust variance estimator. Models controlled for age, gender, race, inhaled corticosteroid use, atopy, caregiver education level, and season of visit. A Wald-type test for interaction by BMI category was performed. Statistical significance was accepted at p<0.05 for main effects and at p<0.1 for interactions as suggested by Selvin and applied previously in modest sample-size environmental studies(5). For MAACS, overweight and obese participants were combined for the outcome of trouble speaking due to low event rates. Cotinine and nicotine were log10 transformed to achieve normality; values below the limit of detection were multiply imputed from a log-normal distribution fit by maximum likelihood estimation(6) and were resampled at the participant level due to its longitudinal structure. Analysis was performed in Stata 13 (StataCorp; College Station, TX). Studies were approved by the Johns Hopkins Institutional Review Board. Assent from participants and informed consent from caregivers were obtained.
Of 289 participants with complete information, 48% were overweight/obese (Table E1). Participant were ages 5-17 years, 92% African American, and 52% male; 80% were atopic by skin test or blood testing, and 53% reported inhaled corticosteroid use. Half (49%) of urine cotinine and the majority (71%) of airborne nicotine measurements were above the limit of detection. With exception of there being fewer obese males within MAACS, there were no significant differences in baseline characteristics, proportion of cotinine or nicotine values above limit of detection, or their measured concentration between BMI categories (Tables E2, E3). In Discover, participants spent on average 15.6 hours/day at home, which was also similar between BMI categories.
There was evidence that overweight and obesity enhanced associations between SHS exposure and asthma outcomes (Table 1). For example, SHS exposure in MAACS was associated with greater odds of a maximum asthma-symptom day among obese (aOR 2.25; 95% CI 1.12-4.50) and overweight (3.40 [1.32-8.75]) compared to normal weight children (1.21 [0.73-2.00]) and in Discover with greater trouble breathing among obese (1.43 [1.02-2.01]) compared to overweight (1.34 [0.81-2.23]) or normal weight children (0.86 [CI 0.61-1.21]) (p-interaction<0.1 for all comparisons, Figure 1).
Table 1.
Association Between Secondhand Smoke Exposure and Asthma Outcomes*
| Study Outcome | BMI Category
|
P-value† | ||
|---|---|---|---|---|
| Normal Weight | Overweight | Obese | ||
| Mouse Allergen and Asthma Cohort Study | ||||
| Maximum asthma symptom-day‡ | 1.25 (0.81-1.92) | 3.40 (1.32-8.75) | 2.25 (1.12-4.50) | 0.096 |
| Cough, wheeze, or chest tightness | 1.34 (0.88-2.03) | 2.76 (1.09-7.03) | 1.92 (1.01-3.65) | 0.331 |
| Slowed activity due to asthma | 1.21 (0.73-2.00) | 3.22 (1.08-9.64) | 2.72 (1.24-5.95) | 0.090 |
| Trouble speaking due to wheeze§ | 1.06 (0.48-2.31) | 14.81 (4.09-53.66) | <0.01 | |
| Nocturnal symptoms | 0.81 (0.46-1.41) | 5.66 (1.12-28.68) | 1.68 (0.81-3.49) | 0.037 |
| Exercise-related symptoms | 1.24 (0.73-2.12) | 4.28 (1.42-12.96) | 1.40 (0.72-2.73) | 0.169 |
| Cough without cold | 1.00 (0.47-2.13) | 3.93 (1.68-9.22) | 1.04 (0.43-2.54) | 0.046 |
| Rescue inhaler use | 1.01 (0.64-1.60) | 1.27 (0.62-2.60) | 1.09 (0.46-2.59) | 0.848 |
| Acute care need | 0.77 (0.50-1.18) | 1.40 (0.68-2.87) | 0.92 (0.55-1.54) | 0.367 |
|
| ||||
| Discover Study | ||||
| Trouble breathing | 0.86 (0.61-1.21) | 1.34 (0.81-2.23) | 1.43 (1.02-2.01) | 0.093 |
| Limiting activities due to asthma | 0.85 (0.64-1.11) | 1.20 (0.54-2.65) | 1.18 (0.84-1.64) | 0.266 |
| Bother due to asthma | 0.81 (0.60-1.10) | 1.06 (0.49-2.32) | 1.49 (1.08-2.04) | 0.019 |
| Woken due to asthma | 0.79 (0.56-1.13) | 1.26 (0.91-1.74) | 0.97 (0.59-1.59) | 0.141 |
| Rescue inhaler use | 0.76 (0.55-1.04) | 1.86 (0.71-4.87) | 0.79 (0.47-1.32) | 0.231 |
| Acute care need | 0.68 (0.37-1.25) | 1.07 (0.34-3.39) | 2.05 (0.94-4.45) | 0.046 |
| Absent from school | 0.86 (0.58-1.28) | 1.03 (0.35-3.07) | 1.72 (0.89-3.31) | 0.216 |
Odds ratio per 10-fold increase in cotinine/nicotine, adjusted for age, sex, race, inhaled corticosteroid use, atopic status, caregiver education level, and season.
P-value for overall interaction between BMI category and cotinine/nicotine.
Highest number of days of slowed activity, nocturnal symptoms, or exercise-related symptoms
Due to low event rate, overweight and obese categories combined
Figure 1.
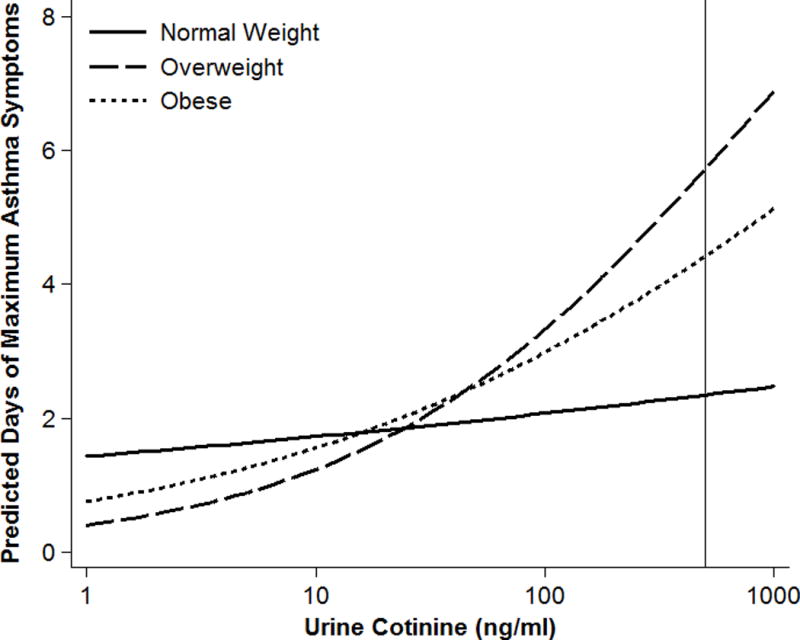
The finding that overweight and obesity may increase susceptibility to SHS has potential clinical and public health relevance. It also adds to existing evidence that elevated BMI increases susceptibility to air pollution(1, 2, 7). Confirmatory and mechanistic investigation of these findings is warranted. Potential pathways may involve interdependent effects of obesity and SHS exposure on airways and systemic oxidative stress and inflammation. Case-control studies have identified additive interactions between asthma and obesity for elevations in inflammatory cytokines and propensity toward non-atopic systemic inflammation(8). Alternatively, pathways may involve differences in respiratory mechanics; overweight and obese children may heightened exposure to SHS due to higher resting minute ventilation(9) or exaggerated increases in minute ventilation as a result of pollutant exposure(10).
The finding in the MAACS study that the overweight group was most affected has been reported previously in studies of outdoor and indoor air pollution(1,2). This could reflect the condition that obese children are impacted by other factors that worsen asthma symptoms beyond air pollution, including more extreme impact of body mass on respiratory mechanics and greater influence of comorbidities. However, this pattern was not seen in Discover. Potential reasons for this heterogeneity between studies include differences in age, atopy prevalence and use of ICS, as well as unmeasured differences in adherence to ICS. Further, SHS exposure in MAACS was ascertained by urine cotinine and in Discover by airborne nicotine; differential accuracy in exposure measurement may contribute to differences between study findings. Finally, studied outcomes were all patient-reported, and our findings may be confounded by differential symptom reporting between BMI categories. While inclusion of two studies addressing the same fundamental question is a strength, we were unable to combine outcomes systematically because of these differences in study design and population. Larger studies that provide greater statistical power are needed to confirm the effects we have observed.
In two well-characterized cohorts, SHS exposure was associated with enhanced asthma symptoms in overweight and obese children compared to normal weight children. This suggests that overweight and obese children are more susceptible to SHS and adds to evidence that elevated BMI increases vulnerability to air pollution. These findings offer new insight into why asthma in overweight and obese children may be more severe.
Supplementary Material
Acknowledgments
Funding: Supported by grants from the National Institutes of Health, National Institute for Environmental Health Sciences under F32ES028578 (TDW), R01ES023447 (ECM), R01ES026170 (ECM), P50ES018176 (NNH), P01ES018176 (GBD), P50ES015903(GBD), K24ES021098 (GBD); the National Institute of Allergy and Infectious Diseases under R01AI081845 (ECM), K24AI114769 (ECM), U01AI083238 (ECM); the National Center for Advancing Translational Sciences under KL2TR001077 (EPB); and the United States Environmental Protection Agency (EPA) under agreements 83615201 (NNH) and 83451001 (GBD). This document has not been formally reviewed by EPA or NIH. The views expressed in this document are solely those of the authors and do not necessarily reflect those of EPA or NIH. No endorsement is made of any products or commercial services mentioned in this publication.
Footnotes
Respective Contributions (ICJME): TDW, ECM, GBD, NNN, and MCM conceived the study and overall design. KK, ECM, GBD, and NNN contributed to the acquisition of data. TDW, EPB, RP, KK, CR, and MCM contributed to analysis of data. TDW drafted the initial manuscript. All authors contributed to the critical revision of the manuscript and approval of the final version to be published.
Conflicts of Interest: The authors report no conflicts of interest relevant to the submitted work. NNH reports grant funding and personal fees from AstraZeneca and GlaxoSmithKline and grant funding from Boehringer Ingelheim and the COPD Foundation outside of the submitted work. MCM reports royalty funding from UpToDate outside of the submitted work.
References
- 1.Lu KD, Breysse PN, Diette GB, Curtin-Brosnan J, Aloe C, Williams DAL, et al. Being overweight increases susceptibility to indoor pollutants among urban children with asthma. J Allergy Clin Immunol. 2013;131(4):1017–23.e3. doi: 10.1016/j.jaci.2012.12.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dong GH, Qian Z, Liu MM, Wang D, Ren WH, Fu Q, et al. Obesity enhanced respiratory health effects of ambient air pollution in Chinese children: the Seven Northeastern Cities study. Int J Obes. 2013;37(1):94–100. doi: 10.1038/ijo.2012.125. [DOI] [PubMed] [Google Scholar]
- 3.Homa DM, Neff LJ, King BA, Caraballo RS, Bunnell RE, Babb SD, et al. Vital signs: disparities in nonsmokers’ exposure to secondhand smoke–United States, 1999-2012. MMWR Morbidity and mortality weekly report. 2015;64(4):103–8. [PMC free article] [PubMed] [Google Scholar]
- 4.Santanello NC, Davies G, Galant SP, Pedinoff A, Sveum R, Seltzer J, et al. Validation of an asthma symptom diary for interventional studies. Arch Dis Child. 1999 May;80(5):414–20. doi: 10.1136/adc.80.5.414. Epub 1999/04/20. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Selvin S. Statistical Analysis of Epidemiologic Data. New York: Oxford University Press; 2004. [Google Scholar]
- 6.Lubin JH, Colt JS, Camann D, Davis S, Cerhan JR, Severson RK, et al. Epidemiologic Evaluation of Measurement Data in the Presence of Detection Limits. Environ Health Perspect. 2004;112(17):1691–6. doi: 10.1289/ehp.7199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bennett WD, Hazucha MJ, Folinsbee LJ, Bromberg PA, Kissling GE, London SJ. Acute Pulmonary Function Response to Ozone in Young Adults As a Function of Body Mass Index. Inhal Toxicol. 2007;19(14):1147–54. doi: 10.1080/08958370701665475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rastogi D, Fraser S, Oh J, Huber AM, Schulman Y, Bhagtani RH, et al. Inflammation, metabolic dysregulation, and pulmonary function among obese urban adolescents with asthma. Am J Respir Crit Care Med. 2015;191(2):149–60. doi: 10.1164/rccm.201409-1587OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bennett WD, Zeman KL. Effect of body size on breathing pattern and fine-particle deposition in children. J Appl Physiol. 2004;97(3):821–6. doi: 10.1152/japplphysiol.01403.2003. [DOI] [PubMed] [Google Scholar]
- 10.Shore SA, Rivera-Sanchez YM, Schwartzman IN, Johnston RA. Responses to ozone are increased in obese mice. J Appl Physiol. 2003;95(3):938–45. doi: 10.1152/japplphysiol.00336.2003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


